Introduction
Osteosarcoma (OS) is the most common primary
malignant bone tumor, occurring primarily during childhood and
adolescence. It is characterized by cell invasion and an early and
high metastasis rate (1). Despite
decades of OS research, surgery and chemotherapy are still the main
treatment strategies for OS; the 5-year overall survival rate for
OS patients is 70–80% (2). Moreover,
the long-term survival rate is still low amongst patients with OS,
due to the high frequency of metastasis and recurrence. Indeed, the
5-year recurrence survival rate is only 17.7% and is associated
with age at diagnosis, disease severity, recurrence site and the
time of recurrence (3). Therefore,
OS treatment has entered a clinical bottleneck, with further
breakthroughs required to improve patient prognosis (4). Thus, OS pathogenesis must be
comprehensively unraveled, with a concomitant strategy towards the
development of new therapeutic drugs.
In the 1960's, paclitaxel (PTX) was identified from
the common Pacific Yew (Taxus chinensis). With its cytotoxic
properties, the molecule attracted attention from researchers, who
rapidly discovered its cytotoxic properties could be used for the
development of new anticancer drugs (5). PTX promotes microtubule polymerization
and stabilization in cells, where it regulates the spindle in
mitosis, antagonizes the abnormal division of chromosomes and halts
cells in the metaphase stage of mitosis, so as to reduce excessive
proliferation and division of anticancer cells (6,7).
Monopolar spindle kinase 1 (Mps1) was identified in 1991 in a yeast
genetic screen of genes involved in spindle pole replication
(8) and plays a central role in
ensuring error-free chromosome segregation (9). Mps1 is critical for the recruitment of
spindle assembly checkpoint (SAC) proteins to unattached
centromeres, formation of the mitotic checkpoint complex (MCC)
formation and inhibition of the multi-subunit E3 ubiquitin ligase
(APC/C) (10). Inhibiting Mps1
activity leads to premature cell separation from mitosis, resulting
in severe chromosome mismatching, non-integer ploidy and eventually
cell death (11). The inhibition of
Mps1 using specific inhibitors reduces SAC signaling inhibition and
returns cells to normal mitotic processes, underscoring its
feasibility as a potential new anticancer strategy (11). Currently, Mps1 inhibitors are used in
basic research, with excellent anti-cancer effects (12–14).
Equally, the synergistic effects of Mps1 inhibitors combined with
PTX has been shown to promote tumor cell death by enhancing cell
division errors (15,16).
The SAC is the primary cell cycle control mechanism
during mitosis, preventing chromosome mis-sets, and regulating the
attachment of all sister chromatids to microtubules radiating from
the poles of the spindle, thereby inhibiting cell entry into
anaphase (17). The PTX regulation
of cell mitosis appears to be inextricably linked to SAC regulation
(18,19). A previous study showed that taxanes,
as microtubule regulators, play apoptotic roles via SAC (20). Therefore, it is important to clarify
the relationship between PTX and SAC and determine whether SAC
regulation is mediated by Mps1.
The AKT/mTOR signaling pathway plays an important
role in the regulation of normal cell growth, metabolism and
survival. Activation or mutation of proteins involved in this
pathway in human cells commonly occurs in different cancer types
and causes a series of cell overgrowth syndrome with potential
transit risk (21,22). In recent studies, the role of the
AKT/mTOR pathway in OS regulation has been documented (23–25).
AKT/mTOR signaling pathway has also been used to investigate the
synergistic use of PTX in anticancer mechanisms and confirm that
the regulation of AKT/mTOR pathway is expected to address the
issues with PTX anti-tumor resistance (26,27).
However, whether PTX inhibits OS cell migration and invasion
through the AKT/mTOR pathway remains unclear. In the present study,
in vivo and in vitro experiments were performed to
demonstrate the role and mechanism of PTX and Mps1 with regard to
OS.
Materials and methods
Sample and clinical data
collection
Between January 2009 and August 2019, 100
intraoperative tissue specimens from 50 patients undergoing OS
surgery in the Department of Pathology, Affiliated Hospital of
Youjiang Medical University for Nationalities (Baise, China). These
included 50 OS samples and 50 normal tissue samples adjacent to the
OS samples. The normal tissue in this study refers to the normal
bone tissue adjacent to the enlarged resection of the tumor with a
distance >5 cm from the tumor edge during surgical resection of
osteosarcoma. Patients (28 male and 22 female) were aged between 11
and 80 years, with an average age of 51±5 years. Preoperative
National nosocomial infections surveillance system grade (28,29) was
observed in five patients, and grade 0 in 45 patients. All study
patients were newly diagnosed OS patients, who had received no
other treatment before surgery. Postoperative pathological
examination results indicated OS, with no other systematic history
of malignant tumors. Inclusion criteria were as follows: i)
Patients undergoing osteosarcoma surgery admitted to the Affiliated
Hospital of Youjiang Medical University for Nationalities from
January 2009 to August 2019 were selected and osteosarcoma
specimens were collected; ii) patients have a clear pathological
diagnosis; iii) patients with primary osteosarcoma; iv) patients
were between 10 and 80 years old; v) patients included both men and
women; vi) all patients received the same standard treatment
regimen for osteosarcoma; and vii) the clinical cases and follow-up
data of the patients were complete. The exclusion criteria were: i)
Patients who had been treated with high-dose chemotherapy and other
anti-tumor drugs before specimen collection; ii) cases complicated
with other malignant tumors; iii) patients with severe heart
disease; iv) liver and kidney failure before treatment; and v)
perioperative death. A log-rank test was used to analyze Mps1
expression and survival rates of 50 clinical patients with OS data.
Prior to sample collection, the study was approved by the Medical
Ethics Committee of the Affiliated Hospital of Youjiang Medical
University for Nationalities (approval no. YYFy-LL-2018-003). All
patients or their parents/legal guardians (for patients <18
years old) provided written informed consent and agreed to the use
of their samples in this study.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA from adjacent normal and tumor tissue from
patients with OS was isolated using TRIzol® (Invitrogen;
Thermo Fisher Scientific, Inc.) according to manufacturer's
instructions. Total RNA was then reverse transcribed to cDNA using
the PrimeScript™ RT-PCR kit (Takara Bio, Inc.). The RT reaction
conditions were 25°C for 10 min, 42°C for 30 min and 85°C for 5
min. Subsequently, qPCR was performed using SYBR-Green reagent
(Yeasen Biotechnology Shanghai) using the following primers: i)
Mps1 forward, 5′-CGCAGCTTTCTGTAGAAATGGA-3′ and reverse,
5′-GAGCATCACTTAGCGGAACAC−3′; and ii) GAPDH forward,
5′-CATGTACGTTGCTATCCAGGC−3′ and reverse,
5′-CTCCTTAATGTCACGCACGAT−3′. The GenBank accession number for Mps1
is NM_003318. PCR thermocycling conditions were as follows: 95°C
for 2 min, 94°C for 15 sec, 60°C for 15 sec and 72°C for 20 sec,
for a total of 40 cycles. Comparative quantification was assessed
using the 2−ΔΔCq method (30), using GAPDH as the endogenous
control.
Histopathology and
immunohistochemistry (IHC)
OS and normal adjacent tissue samples were collected
during intraoperative surgical resection. Specimens were fixed in
10% formalin for 24 h at room temperature and embedded in paraffin,
and were subsequently sectioned into 4-µm sections. Hematoxylin and
eosin staining was performed as follows: The slides were incubated
with hematoxylin solution in a staining jar for 10 min to stain the
nuclei, and then were transferred to a staining jar with eosin
solution for 3 min at room temperature. For IHC analysis,
deparaffinized sections were pretreated with 0.4% pepsin for 60 min
at 37°C, and endogenous peroxidase activity was blocked by
treatment with 3% H2O2 for 10 min at 37°C and
incubated with 50 µl of normal 5–10% goat serum blocking antigen
for 15 min at room temperature. The following primary antibodies
were used: Mps1 (cat. no. ab11108; 1:150; Abcam), MAD1 (cat. no.
ab175245; 1:100; Abcam), Bub1 (cat. no. ab54893; 1:200; Abcam), AKT
(cat. no. 9272S; 1:200; Cell Signaling Technology, Inc.) and mTOR
(cat. no. ab2732; 1:150; Abcam). Tissue sections were incubated
with primary antibodies at 4°C overnight, and then incubated with
horseradish peroxidase-labeled secondary antibody (1:200; cat. no.
KXX0022; Dako; Agilent Technologies, Inc.) at room temperature for
1 h. 3′,3′-Diaminobenzidine was used as a chromogen and the slides
were incubated for 5 min. The sections were counterstained in
Meyer's hematoxylin, washed again and dehydrated with ethanol and
xylene prior to mounting. For IHC, brown particle staining was
determined in OS tissues and normal tissues as a measure of
positive staining. Tissue sections were assessed using an Olympus
BX-41 light microscope to obtain high-resolution images
(magnification, ×200; Olympus Corporation). Aperio Cytoplasma 2
software (Leica Microsystems, Inc.) was used to evaluate the
intensity of IHC staining as follows: i) 0–25 points, (−); ii)
25–50 points, (+); iii) 50–75 points, (++); and iv) >130 points,
(+++).
Cell culture
As for the selection of cell lines for constructing
OS model in nude mice, 143B and MG63 cells are widely used OS
cells. Several relevant studies have shown that 143B and MG63 cells
are mostly used in antitumor experiments for the occurrence and
development of OS (31,32). The human OS cell lines, 143B and
MG63, were purchased from ScienCell Laboratories. Cells were
cultured in high-glucose Dulbecco Modified Eagle's Medium (DMEM;
HyClone; Cytiva) supplemented with 10% fetal bovine serum (FBS;
HyClone; Cytiva), and 100 U/ml penicillin/streptomycin (Gibco;
Thermo Fisher Scientific, Inc.) Cells were maintained in a
humidified atmosphere at 37°C with 5% CO2. MG63 and 143B
OS cells treated with PTX were used for subcutaneous tumor-forming
studies in nude mice. Briefly, cells were treated with 1 µmol/l PTX
for 24 h then transfected with 1 µg short hairpin RNA (shRNA)
plasmid targeting Mps1 or with 1 µg Mps1 overexpression plasmid.
The shRNA plasmid was constructed using the GV248 vector (Shanghai
GeneChem Co., Ltd.). The overexpression plasmid (Mps1 Genbank ID,
NM_003318) was constructed using the GV358 vector (Shanghai
GeneChem Co., Ltd.). Both plasmids were transfected into cells
using Lipofectamine® 2000 for 60 h (with the medium
replaced every day) following the manufacturer's instructions
(Invitrogen; Thermo Fisher Scientific, Inc.) and then maintained in
a humidified atmosphere at 37°C with 5% CO2. Subsequent
experiments were performed at 48 h post-transfection.
Nude mouse tumor model
BALB/c-nu nude mice (male, 5-weeks-old; n=36;
provided by Hunan Changsha Tianqin Biotechnology Co., Ltd.) were
purchased from and housed in specific pathogen-free conditions
(temperature 20–26°C; relative humidity, 40–70%; 12-h light-dark
cycle) at Youjiang Medical University for Nationalities. All animal
experiments were approved by the institute's Animal Ethics
Committee (approval no. 20200122). In an initial experiment, mice
were injected with MG63 cells then mice divided into two groups
(n=6/group): The control (saline group) or the PTX-treated group.
In a separate experiment, mice were divided into four groups
(n=6/group): i) Saline (control); ii) PTX-treated; iii)
sh-MPS1-transfected and iv) PTX-treated and sh-Mps1-transfected.
MG63 cells were harvested, counted, and resuspended in phosphate
buffered saline (PBS) at a final concentration of 2×107
cells/ml. Subsequently, 1×106 cells in 50 µl PBS were
injected into the armpits of mice using a 26-gauge needle. Animal
health and behavior were monitored at the same time every day and
tumor volumes were measured in living nude mice every week until
week 5 (endpoint). No mice died during the experiment. The tumors
were removed from the mice and weighed 5 weeks after the
transplantation. Sodium pentobarbital (150 mg/kg; 1%) was injected
into the mice, which were then sacrificed using cervical
dislocation. The death of the mice was confirmed by the cessation
of breathing and cardiac arrest. Tumor volumes were calculated
using the formula: Volume=0.2618 × L × W × (L + W), where W and L
represent average tumor width and length, respectively.
Flow cytometry analysis of cell
apoptosis
After cell transfection and PTX treatment, cells
were collected for apoptosis assays and were resuspended at
107 cells/ml. A volume of 100 µl was added to a 5-ml
flow tube, and apoptosis measured using an PE Annexin V Apoptosis
Detection kit (BD Biosciences) according to manufacturer's
instructions. Cells were incubated at room temperature and in the
dark for 15 min. The data were acquired by flow cytometry using a
FACScan (BD Biosciences) flow cytometer according to the
manufacturer's instructions. FlowJo V10.0.7 (Tree Star, Inc.) was
used for data analysis.
MTT assay
After digestion with 0.25% trypsin, 143B and MG63
cells were seeded in 96-well plates at 5×103 cells/well,
in DMEM containing 10% FBS, at a constant temperature of 37°C and
5% CO2. The MTT assay was performed at 0, 12, 24, 48 and
72 h. At the indicated timepoints, 20 µl MTT solution (5 mg/ml) was
added to each well, and the plate placed into a 5% CO2
incubator at 37°C for 4 h. The supernatant was then discarded, 150
µl DMSO added to each well, and the plate incubated at room
temperature for 10 min. Absorbance values were measured on an
spectrophotometer at 490 nm. A growth curve was obtained by
plotting the absorbance for each time point.
Scratch assay
The 143B and MG63 cells were seeded into 6-well
plates at a density of 1×105 cells/well, to assess
migration. When cells covered 80–90% of the dish, a sterile 100-µl
pipette tip was used to mark four scratches in each well. Cells
were washed twice in PBS and cultured in serum-free medium, and
images were taken at 0 and 24 h (magnification, ×4). Adobe
Illustrator software (version CC 2019; Adobe Systems, Inc.) was
used to measure the width of the scratches. Cell mobility=(0 h
scratch width-48 h scratch width)/0 h scratch width ×100. The assay
was performed three times.
Transwell migration assay
Transwell migration assays were performed using a
Transwell chamber (Corning, Inc.). The cells were prepared into a
suspension using the medium and then inoculated into a six-well
plate at a rate of 1×104 cells/well. Next, 150 µl
suspended cells in serum-free medium were seeded into the upper
Transwell chamber, and 500 µl DMEM medium supplemented with 20% FBS
was added to the lower chamber. After 24 h, the cells were fixed
with 4% paraformaldehyde at room temperature for >10 min and
stained with 0.5% crystal violet at room temperature for 10 min. An
inverted light microscope was used to examine the cells, and three
random fields were observed to obtain average data.
Western blot analysis
Cells and tissues were lysed in 1X RIPA buffer
(Beyotime Institute of Biotechnology) containing protease and
phosphatase inhibitors. The protein extraction product was
quantified by bicinchoninic acid assay (Beyotime Institute of
Biotechnology). Proteins (50 µg) were loaded and separated on 10%
SDS-polyacrylamide gels, then transferred to polyvinylidene
difluoride membrane (Bio-Rad Laboratories, Inc.) using the wet
transfer method. Each membrane was blocked with TBST (100 mM
Tris-HCl, pH 7.5; 150 mM NaCl; 0.05% Tween-20) with 5% non-fat
dried milk for 1 h at room temperature, then incubated with primary
antibodies at 4°C overnight. The membranes were then further
incubated with HRP-conjugated secondary antibodies (anti-rabbit
IgG, cat. no. 7074, 1:2,000; or anti-mouse IgG, cat. no. 7076,
1:2,000; both Cell Signaling Technology, Inc.) for 1 h at room
temperature. Protein bands were visualized with ECL kit (Amersham;
Cytiva) and the protein expression levels were analyzed using
ImageJ software (v1.48; National Institutes of Health). The
following primary antibodies were used: i) Mps1 (Abcam; cat. no.
ab11108; 1:1,000); ii) MAD1 (Abcam; cat. no. ab175245; 1:1,000);
iii) Bub1 (Abcam; cat. no. ab54893; 1:1,000); iv) Ki67 (Abcam; cat.
no. ab231172; 1:1,000); v) mTOR (Abcam; cat. no. ab2732; 1:2,000);
vi) AKT (Cell Signaling Technology, Inc.; cat. no. 9272S; 1:1,000);
and vii) β-actin (Abcam; cat. no. ab8226; 1:5,000).
Statistical analysis
Graph Pad Prism version 6.0 software (GraphPad
Software, Inc.) was used for statistical analysis and plotting. The
data are presented as the mean ± SD. Tukey's post hoc test was used
following one-way ANOVA. Three replicates were carried out for cell
experiments and six for animal experiments. P<0.05 was
considered significantly different.
Results
Mps1 expression is upregulated in OS
samples and associated with patient survival rate
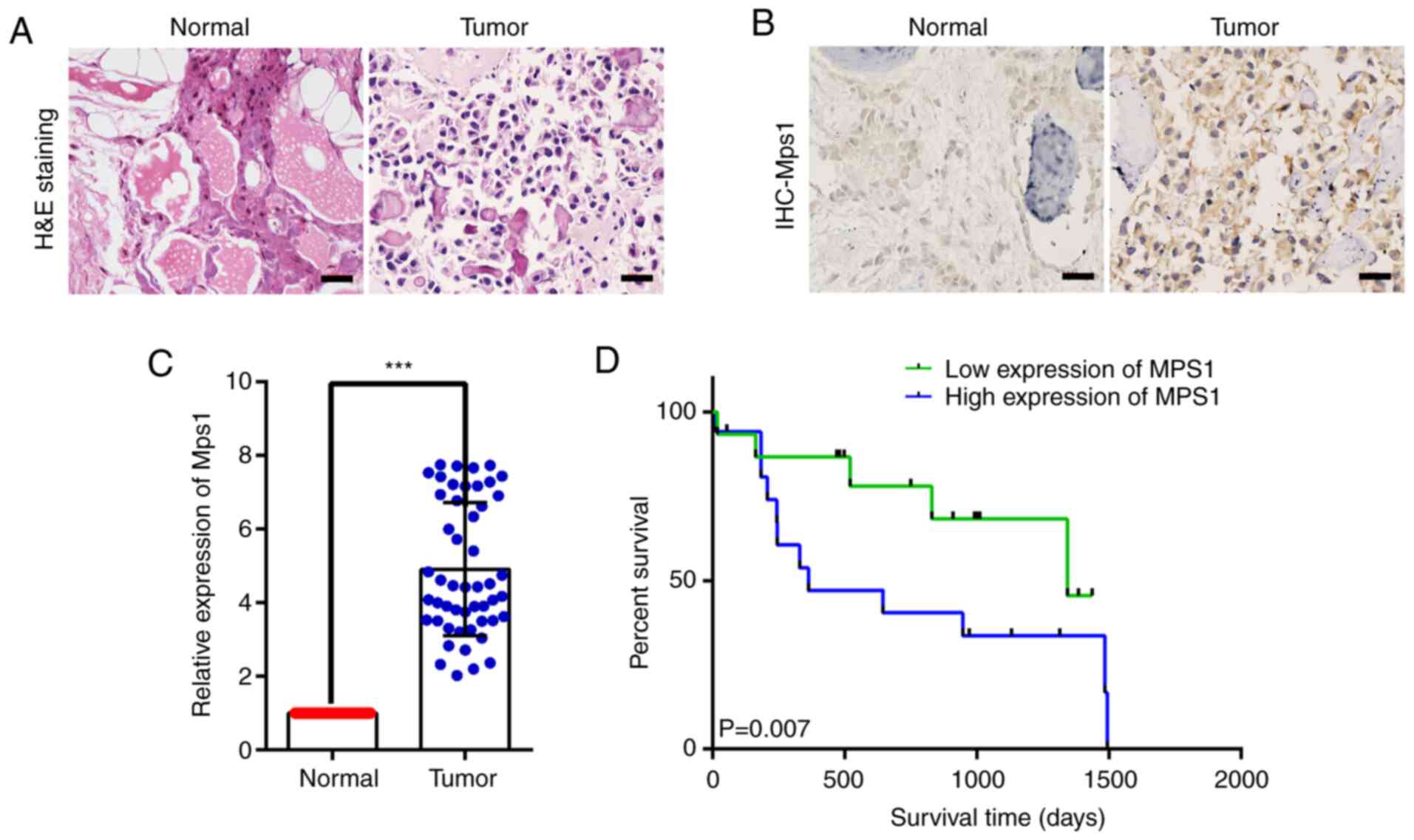
Mps1 expression in OS tissue was analyzed using HE
staining and IHC. HE staining revealed that compared with normal
tissue, OS tissue with obvious neoplastic osteoid tissue appeared
in the background of malignant sarcoma cells, presenting typical
neoplastic osteoid tissue, and OS tissue cells were full of
distinctly heterogeneous neoplastic osteoblasts (Fig. 1A). IHC showed that Mps1 was localized
to the cytoplasm and nuclei, although expression in OS tissue
appeared higher than that in normal bone tissue (Fig. 1B).
Mps1 mRNA expression was analyzed using RT-qPCR in
50 clinical OS and adjacent normal tissue samples. Mps1 mRNA
expression in OS tissue was significantly higher than that in
adjacent normal bone tissue (Fig.
1C). In addition, a log-rank test was used to analyze Mps1
expression and survival rates of 50 clinical patients with OS. The
overall survival rate of patients with high Mps1 expression was
significantly lower than that of patients with low Mps1 expression
(Fig. 1D). Taken together, these
findings indicated that Mps1 expression was significantly
upregulated in OS tissue and may be associated with overall
survival of patients with OS.
Mps1 regulates SAC and Akt/mTOR
signaling in OS
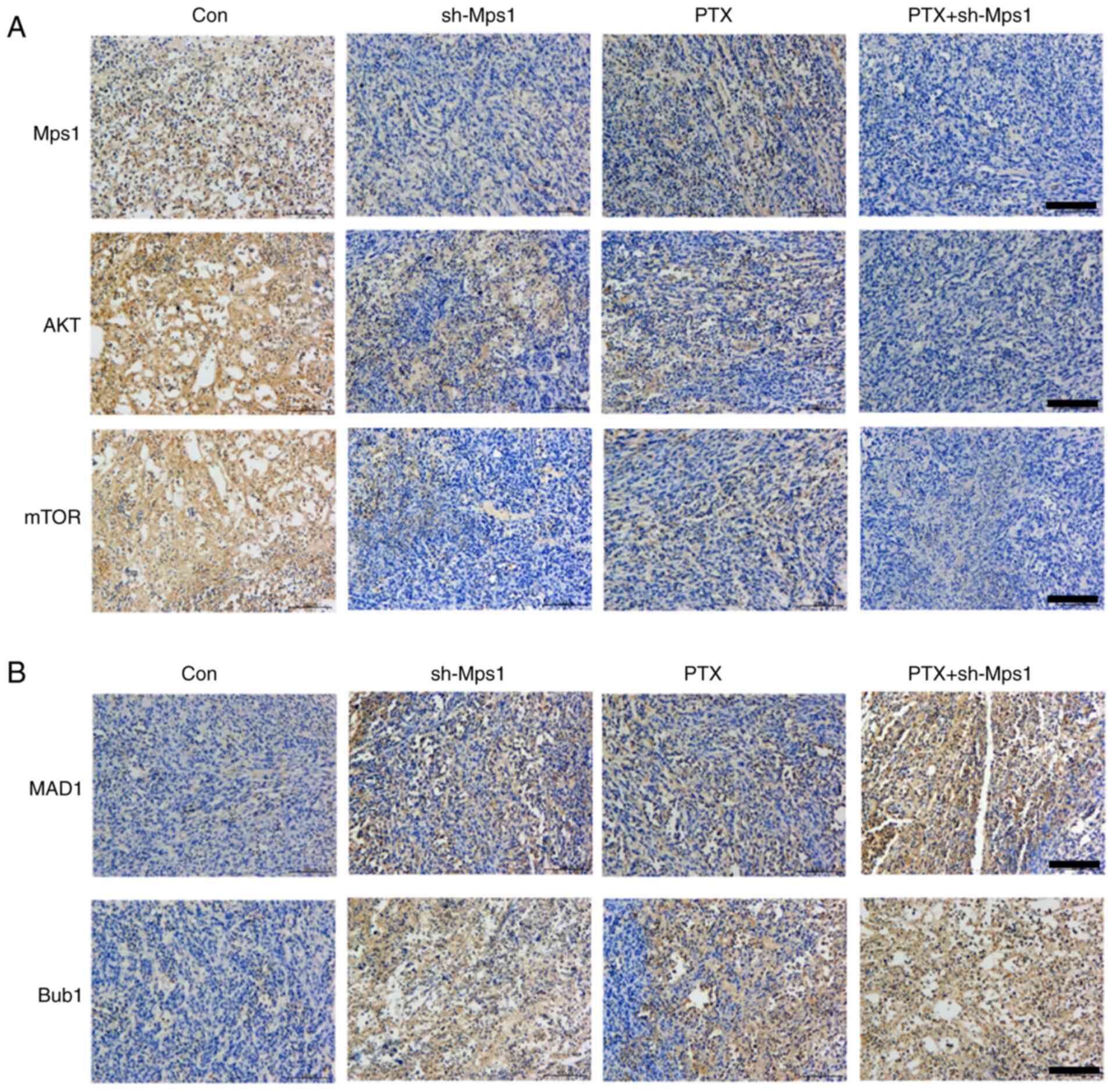
To determine the role of Mps1 and PTX in modulating
OS, SAC and Akt/mTOR signaling were examined. IHC of OS tissue
revealed that expression levels of the SAC components, MAD1 and
Bub1 in OS tissues were lower than in adjacent tissues (Fig. 2A). In contrast, Akt and mTOR
expression levels in OS tissue were significantly higher than those
of adjacent tissue samples (Fig.
2B). These results suggested that SAC may be impaired, whereas
components of Akt/mTOR signaling were upregulated in OS tissue.
Thus, it was hypothesized that SAC and the Akt-mTOR signaling
pathway may play important roles in OS development. Next, the
expression of SAC and Akt/mTOR signaling markers were analyzed
following genetic manipulation of Mps1 expression in vitro.
143B and MG63 cells were transfected with an Mps1 overexpression
vector (OE-Mps1) or shRNA-Mps1 lentiviral vector. Western blot
analysis confirmed overexpression of Mps1 in the OE-Mps1 group and
significantly decreased Mps1 expression in the shRNA-Mps1 group.
Compared with the respective controls, MAD1 and Bub1 expression
were downregulated and AKT and mTOR expression upregulated in the
OE-Mps1 group, whereas the shRNA-Mps1 group showed the opposite
results (Fig. 2C and D). Thus, Mps1
may regulate SAC and Akt/mTOR signaling in OS.
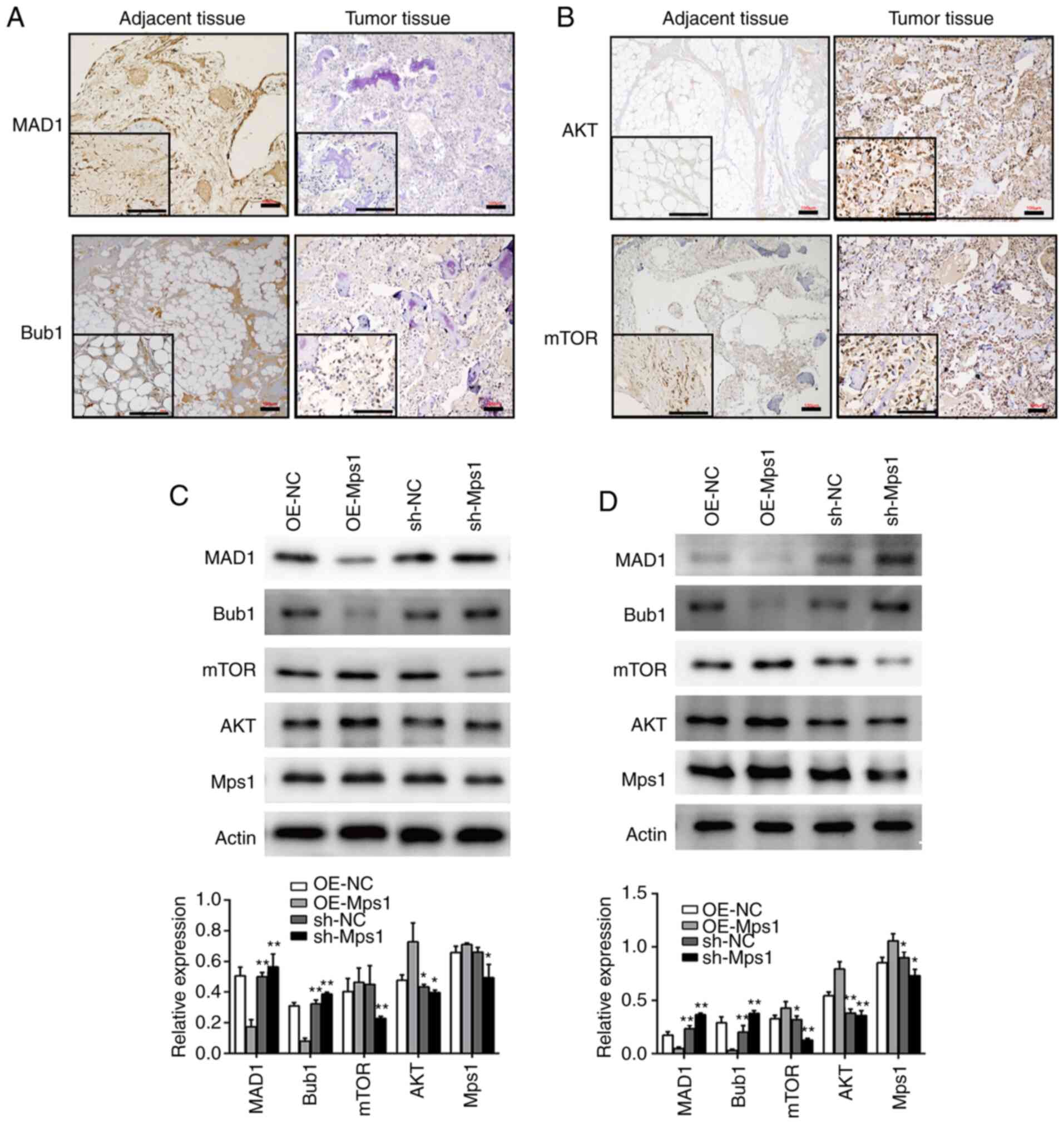 | Figure 2.Mps1 regulates the spindle assembly
checkpoint and Akt/mTOR signaling in osteosarcoma cells. (A and B)
Representative images of (A) MAD1 and Bub1 or (B) AKT and mTOR
immunohistochemical staining in tumor or normal adjacent tissue
samples from patients with OS. Scale bar, 100 µm. (C and D) Western
blot analysis with semi-quantified results of MAD1, Bub1, mTOR, AKT
and Mps1 protein expression in (C) 143B or (D) MG63 cells
transduced with OE-NC, OE-Mps1, sh-NC or sh-Mps1 lentivirus.
*P<0.05, **P<0.01 vs. control. Mps1, monopolar spindle 1
kinase; OE, overexpression; sh, short hairpin RNA; NC, negative
control. |
PTX inhibits Mps1 expression, impairs
migration of OS cells and promotes cell apoptosis
To investigate the impact of PTX on Mps1 expression,
the OS cell lines, 143B and MG63, were treated with PTX for 12, 24,
48 or 72 h. Western blot analysis showed that Mps1 expression
significantly decreased in cells cultured with PTX for 24 and 72 h
(Fig. 3A). PTX also inhibited OS
cell viability in a time-dependent manner (Fig. 3B). From the scratch assay data, it
was observed that cell migration was inhibited by PTX treatment in
both 143B and MG63 cells (Fig. 3C).
Transwell results showed that PTX inhibited cell migration, and
flow cytometry data showed PTX induced cell apoptosis (Fig. 3D-F) compared with the control. Next,
MG63 cells treated with 1 µmol/l PTX were used to construct a nude
mouse OS model, and tumor volume growth was recorded in nude mice
over 5 weeks. Tumor histology and Mps1 expression in OS tissue was
analyzed using HE staining and IHC, respectively. Tumor volumes of
PTX exposed nude mice were significantly smaller than those of the
control group (Fig. 3G). HE staining
revealed no significant differences in the morphology of MG63 tumor
tissues, and IHC confirmed a significant decrease in Mps1
expression (Fig. 3H) compared with
the control. In summary, these data suggested that PTX
downregulated Mps1 expression both in vitro and in
vivo, inhibited OS cell proliferation, migration and promoted
OS cell apoptosis.
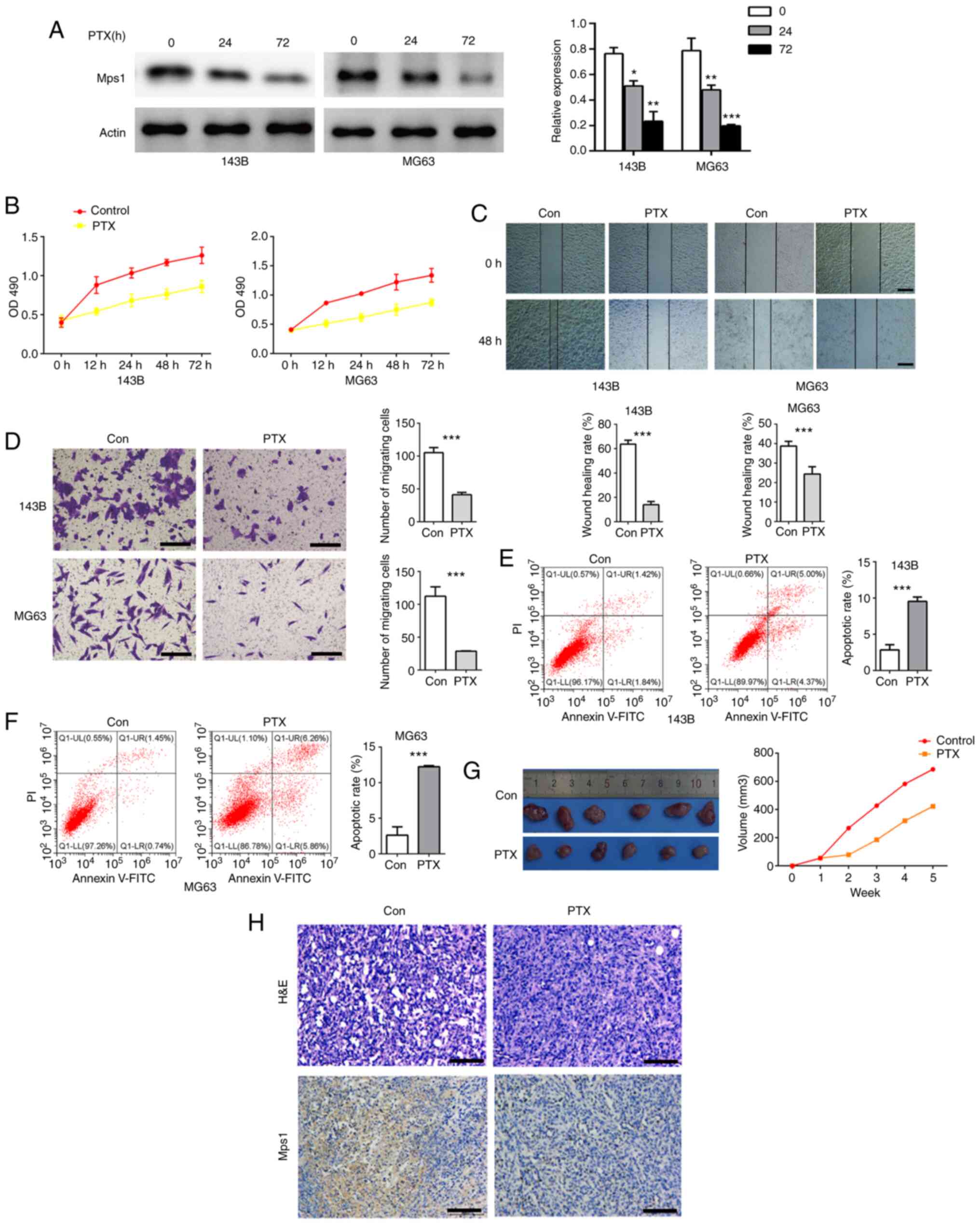 | Figure 3.PTX inhibits Mps1 expression and
migration of osteosarcoma cells and promotes their apoptosis. (A)
Western blot analysis with semi-quantified results of Mps1 protein
expression in 143B or MG63 cells after treatment with PTX for 0, 24
or 72 h. (B) Proliferation of 143B and MG63 cells after treatment
with PTX for 0, 12, 24, 48 and 72 h. (C) Representative
photomicrographs of wound healing assays in 143B and MG63 cells at
0 and 24 h (magnification, ×100). (D) Transwell assay results of
143B and MG63 cells treated with PTX for 24 h. (E and F) Flow
cytometry analysis of apoptosis in (E) 143B and (F) MG63 cells
treated with PTX for 24 h. (G) Representative images and
quantification of the volume of the xenograft tumors in the control
and PTX-treated groups. (H) H&E and immunohistochemical
staining of Mps1 in tumors from the control and PTX-treated groups.
Scale bar, 100 µm. *P<0.05, **P<0.01, ***P<0.001 vs.
control. Data are presented as the mean ± SD. Mps1, monopolar
spindle 1 kinase; PTX, paclitaxel; OD, optical density; H&E,
hematoxylin and eosin. |
PTX combined with Mps1 knockdown
exhibits stronger anti-tumor effects in OS
To determine whether PTX in combination with Mps1
knockdown would have an improved effect against OS compared with
PTX alone, the 143B and MG63 cell lines were transfected with the
shRNA-Mps1 lentiviral vector and treated with PTX. The OS cells
were divided into four groups: i) Control (saline); ii) shRNA-Mps1;
iii) PTX alone; and iv) PTX + shRNA-Mps1. The MTT assay revealed
that PTX + shRNA-Mps1 showed stronger cytotoxicity than PTX alone
in OS cell, and this inhibitory effect increased with longer
exposure times (Fig. 4A).
Furthermore, cell migration was reduced, and the apoptosis rate was
higher in PTX + shRNA-Mps1cells compared with the sh-Mps1 or PTX
alone groups (Fig. 4B and C). The
cells from different groups were then injected into nude mice. The
tumor volume was smaller in mice in the PTX+ shRNA-Mps1 group
compared with the shMps1 or PTX alone groups (Fig. 4D). Thus, these data indicated that
PTX in combination with shRNA-Mps1 exerted a stronger antitumor
effect on OS than shMps1 or PTX alone.
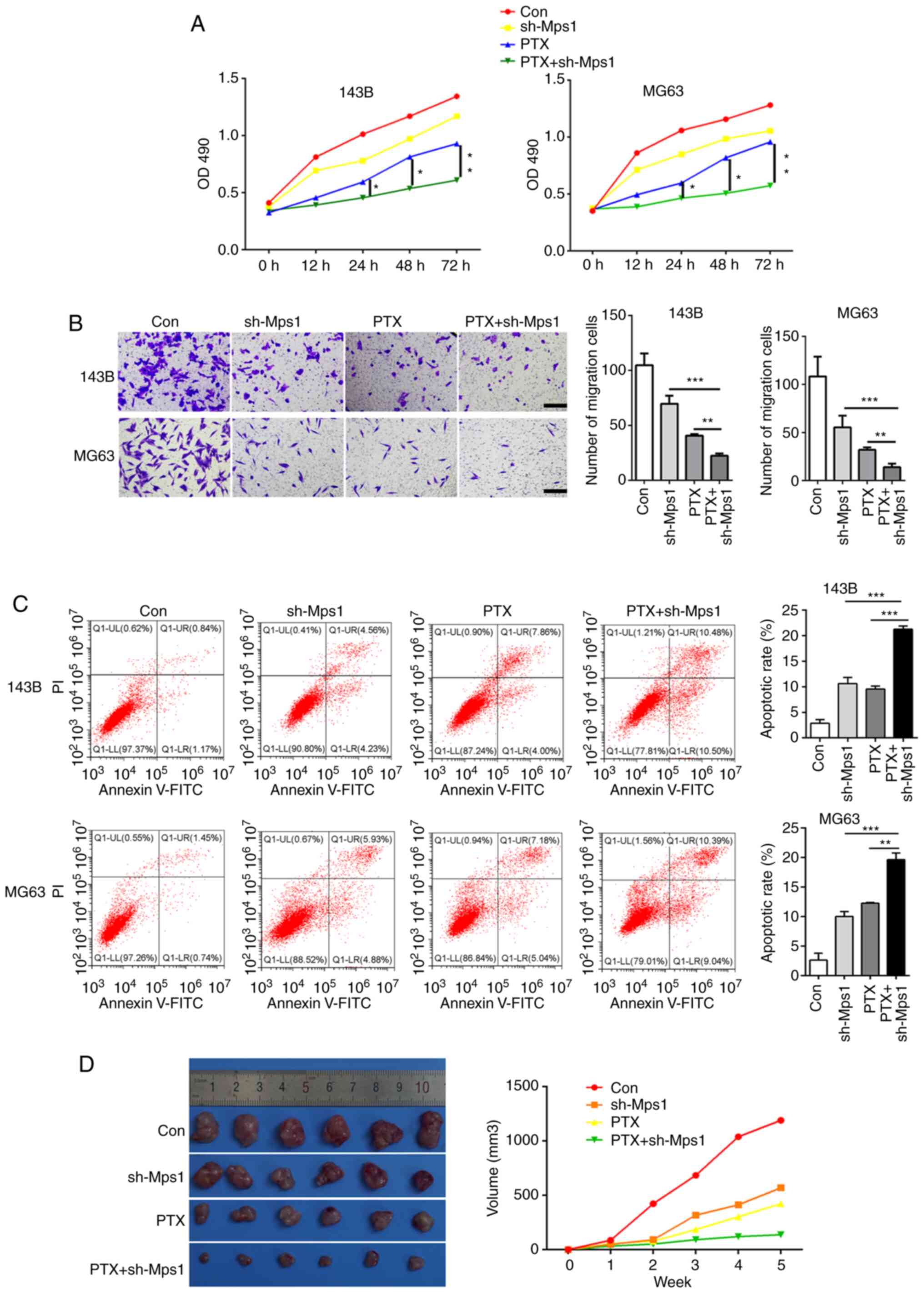 | Figure 4.PTX combined with Mps1 knockdown
exhibits strong anti-tumor effects in osteosarcoma cells. (A) Cell
proliferation curve of 143B and MG63 cells in the con, sh-Mps1, PTX
and PTX + sh-Mps1 groups. (B) Transwell assay results of 143B and
MG63 cells in the con, sh-Mps1, PTX and PTX + sh-Mps1 for 24 h. (C)
Flow cytometry analysis of apoptosis in 143B and MG63 cells in the
con, sh-Mps1, PTX and PTX + sh-Mps1 groups. (D) Representative
images and quantification of the volume of the xenograft tumors in
the con, sh-Mps1, PTX and PTX + sh-Mps1 groups. *P<0.05,
**P<0.01, ***P<0.001. Data are presented as the mean ± SD.
Mps1, monopolar spindle 1 kinase; PTX, paclitaxel; sh, short
hairpin RNA; OD, optical density; con, control. |
PTX in combination with Mps1 knockdown
mediates SAC and Akt/mTOR signaling
To examine the effects of PTX in combination with
Mps1 knockdown on SAC and Akt/mTOR signaling in OS, key biomarkers
were examined in all groups. IHC results suggested that Mps1, Akt
and mTOR expression levels were markedly lower, whereas those of
MAD1 and Bub1 were higher in the shRNA-Mps1 group compared with the
control group. The data from the PTX alone group were comparable
with shRNA-Mps1 alone. In addition, the PTX + shRNA-Mps1 groups
showed the lowest Akt and mTOR expression levels and the highest
MAD1 and Bub1 expression levels (Fig.
5). In conclusion, PTX affected SAC, as well as Akt and mTOR
expression. In addition, PTX in combination with Mps1 knockdown
exerted a stronger effect than either condition alone on SAC, and
the Akt/mTOR pathways.
Discussion
OS is caused by several factors, including aberrant
differentiation of mesenchymal cells and tumor suppressor genes,
oncogene activation, epigenetic events and cytokine production
(33). Although OS therapeutics have
improved after decades of research, long-term survival rates and
prognosis for OS are still low due to early metastasis and high
malignancy (2,34). These facts indicate the requirement
for more mechanistic studies and the development of novel and more
effective treatment strategies to elucidate disease pathogenesis,
in order to improve patient survival times and prognosis.
Currently, OS treatment strategies include neoadjuvant
chemotherapies combined with surgical treatments and postoperative
adjuvant chemotherapies (35). PTX
is an effective anticancer drug that has been widely used to
clinically combat several cancer types (36). PTX is currently used for the
treatment of OS in preclinical research settings, and has shown
promising clinical applications (37). In the present study, PTX exerted
antitumor effects on OS, both in vivo and in
vitro.
As a novel target and biomarker for cancer, Mps1
inhibitors effectively inhibit cancer cell proliferation and result
in significantly improved survival (38). In the present study, high Mps1
expression was associated with poor patient survival. Mps1 was
significantly upregulated in OS compared with normal adjacent
tissues. Moreover, Mps1 modulation appeared to interfere with OS
cell proliferation and development. Thus, to further investigate
the anti-tumor effects of PTX in combination with Mps1 modulation
in OS, several experiments were conducted, which showed that PTX
combined with Mps1 inhibition exerted improved effects against OS
compared with PTX alone. Mps1 is a bispecific protein kinase
upstream of SAC that plays a key role in the precise separation of
chromosomes during mitosis (13). In
the present study, the downstream SAC markers, MAD1 and Bub1 were
downregulated in OS tissue. Regulating Mps1 expression affected
MAD1 and Bub1 expression, and was related to OS cell growth
characteristics. Thus, the combination of PTX and Mps1 inhibition
in OS cells appeared to upregulate SAC, and inhibit the oncogenic
characteristics of OS cells. However, whether these mechanisms are
directly or indirectly regulated by Mps1 to MAD1 and Bub1 has not
yet been clearly established, and requires more research.
The Akt/mTOR pathway has been widely studied in
various tumor cells, with its role in OS attracting considerable
attention (23,24,39). In
this study, Akt and mTOR expression levels were upregulated in OS
tissue and affected by PTX treatment. Moreover, PTX downregulated
the expression of Akt and mTOR to inhibit cancer-related OS
characteristics. PTX combined with Mps1 knockdown significantly
downregulated Akt and mTOR in OS cells.
The present study had some limitations. The
occurrence and development of OS is not regulated by a single
signaling pathway, but more likely regulated via multiple signaling
pathways. The possible relationship between the Mps1 gene and other
signaling pathways implicated in OS occurrence and development were
not examined. The failure to establish a lung metastatic model of
OS in nude mice is also a limitation of this study. Due to the
limitation of research conditions in Guangxi province, OS samples
are relatively rare, so it is difficult to draw reliable
conclusions by distinguishing different subtypes of OS. In
addition, the relationship between the Mps1 gene and the degree of
differentiation, type and tumor size of OS tumors were not
statistically analyzed.
In summary, the present findings showed that PTX had
effective antitumor effects, and that PTX combined with Mps1
inhibition improved the anti-OS effects. Mps1 inhibition
significantly enhanced the tumor inhibition effects of PTX. This
treatment strategy not only prevented OS cell proliferation and
development in vitro, but also inhibited the growth of OS
transplanted tumors in nude mice. Additionally, PTX in combination
with Mps1 inhibition upregulated MAD1 and Bub1 expression,
potentially enhancing SAC in tumor cells. Moreover, the Akt/mTOR
pathway may also represent a target pathway potentially regulated
by PTX and Mps1, and that Akt/mTOR pathway downregulation could be
of great significance in inhibiting OS development. Therefore, PTX
in combination with Mps1 inhibition may have broad application
prospects for molecular therapeutics against OS.
Acknowledgements
Not applicable.
Funding
This study was supported by The National Nature
Science Foundation of Guangxi (grant no. 2018GXNSFAA294091).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KX conceived and designed the study. LL, YW and JC
conducted the in vitro experiments and provided suggestions
for the project. LL, YL and QL conducted the in vivo
experiments. CZ and FL collected and analyzed experimental data. JC
and YW drafted the manuscript and KX revised it. LL performed the
analysis of data. All authors read and approved the final
manuscript. LL and JC confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
This study was approved by The Medical Ethics
Committee of the Affiliated Hospital of Youjiang Medical University
for Nationalities (approval no. YYFy-LL-2018-003). All patients
provided written informed consent and agreed to the use of their
samples in this study. All animal experiments were approved by the
institute's Animal Ethics Committee of the Affiliated Hospital of
Youjiang Medical University for Nationalities (approval no.
20200122).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu K, Ren T, Huang Y, Sun K, Bao X, Wang
S, Zheng B and Guo W: Apatinib promotes autophagy and apoptosis
through VEGFR2/STAT3/BCL-2 signaling in osteosarcoma. Cell Death
Dis. 8:e30152017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Smeland S, Bielack SS, Whelan J, Bernstein
M, Hogendoorn P, Krailo MD, Gorlick R, Janeway KA, Ingleby FC,
Anninga J, et al: Survival and prognosis with osteosarcoma:
Outcomes in more than 2000 patients in the EURAMOS-1 (European and
American Osteosarcoma Study) cohort. Eur J Cancer. 109:36–50. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Spraker-Perlman HL, Barkauskas DA, Krailo
MD, Meyers PA, Schwartz CL, Doski J, Gorlick R, Janeway KA and
Isakoff MS: Factors influencing survival after recurrence in
osteosarcoma: A report from the Children's Oncology Group. Pediatr
Blood Cancer. 66:e274442019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huang H, Han Y, Chen Z, Pan X, Yuan P,
Zhao X, Zhu H, Wang J, Sun X and Shi P: ML264 inhibits osteosarcoma
growth and metastasis via inhibition of JAK2/STAT3 and
WNT/beta-catenin signalling pathways. J Cell Mol Med. 24:5652–5664.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Perdue RE Jr: Procurement of plant
materials for antitumor screening. Cancer Treat Rep. 60:987–998.
1976.PubMed/NCBI
|
|
6
|
van Vuuren RJ, Visagie MH, Theron AE and
Joubert AM: Antimitotic drugs in the treatment of cancer. Cancer
Chemotherapy Pharmacol. 76:1101–1112. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Weaver BA: How Taxol/paclitaxel kills
cancer cells. Mol Biol Cell. 25:2677–2681. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Winey M, Goetsch L, Baum P and Byers B:
MPS1 and MPS2 novel yeast genes defining distinct steps of spindle
pole body duplication. J Cell Biol. 114:745–754. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pachis ST and Kops G: Leader of the SAC:
Molecular mechanisms of Mps1/TTK regulation in mitosis. Open Biol.
8:1801092018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lauzé E, Stoelcker B, Luca FC, Weiss E,
Schutz AR and Winey M: Yeast spindle pole body duplication gene
MPS1 encodes an essential dual specificity protein kinase. EMBO J.
14:1655–1663. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dominguez-Brauer C, Thu KL, Mason JM,
Blaser H, Bray MR and Mak TW: Targeting mitosis in cancer: Emerging
strategies. Mol Cell. 60:524–536. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Han Y, Wu Y, Xu Y, Guo W, Zhang N and Wang
X: Molecular mechanism of point mutation-induced Monopolar spindle
1 (Mps1/TTK) inhibitor resistance revealed by a comprehensive
molecular modeling study. PeerJ. 7:e62992019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mason JM, Wei X, Fletcher GC, Kiarash R,
Brokx R, Hodgson R, Beletskaya I, Bray MR and Mak TW: Functional
characterization of CFI-402257, a potent and selective Mps1/TTK
kinase inhibitor, for the treatment of cancer. Proc Natl Acad Sci
USA. 114:3127–3132. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Choi M, Min YH, Pyo J, Lee CW, Jang CY and
Kim JE: TC Mps1 12, a novel Mps1 inhibitor, suppresses the growth
of hepatocellular carcinoma cells via the accumulation of
chromosomal instability. Br J Pharmacol. 174:1810–1825. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Maia ARR, Linder S, Song JY, Vaarting C,
Boon U, Pritchard CEJ, Velds A, Huijbers IJ, van Tellingen O,
Jonkers J and Medema RH: Mps1 inhibitors synergise with low doses
of taxanes in promoting tumour cell death by enhancement of errors
in cell division. Br J Cancer. 118:1586–1595. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wengner AM, Siemeister G, Koppitz M,
Schulze V, Kosemund D, Klar U, Stoeckigt D, Neuhaus R, Lienau P,
Bader B, et al: Novel Mps1 kinase inhibitors with potent antitumor
activity. Mol Cancer Ther. 15:583–592. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lara-Gonzalez P, Westhorpe FG and Taylor
SS: The spindle assembly checkpoint. Cur Biol. 22:R966–R980. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chong T, Sarac A, Yao CQ, Liao L, Lyttle
N, Boutros PC, Bartlett JMS and Spears M: Deregulation of the
spindle assembly checkpoint is associated with paclitaxel
resistance in ovarian cancer. J Ovarian Res. 11:272018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sudo T, Nitta M, Saya H and Ueno NT:
Dependence of paclitaxel sensitivity on a functional spindle
assembly checkpoint. Cancer Res. 64:2502–2508. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McGrogan B, Phelan S, Fitzpatrick P,
Maguire A, Prencipe M, Brennan D, Doyle E, O'Grady A, Kay E,
Furlong F and McCann A: Spindle assembly checkpoint protein
expression correlates with cellular proliferation and shorter time
to recurrence in ovarian cancer. Hum Pathol. 45:1509–1519. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Y, Kwok-Shing Ng P, Kucherlapati M,
Chen F, Liu Y, Tsang YH, de Velasco G, Jeong KJ, Akbani R,
Hadjipanayis A, et al: A Pan-cancer proteogenomic atlas of
PI3K/AKT/mTOR pathway alterations. Cancer Cell. 31:820–832.e3.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Keppler-Noreuil KM, Parker VE, Darling TN
and Martinez-Agosto JA: Somatic overgrowth disorders of the
PI3K/AKT/mTOR pathway & therapeutic strategies. Am J Med Genet
C Semin Med Genet. 172:402–421. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yue Z, Guan X, Chao R, Huang C, Li D, Yang
P, Liu S, Hasegawa T, Guo J and Li M: Diallyl disulfide induces
apoptosis and autophagy in human osteosarcoma MG-63 cells through
the PI3K/Akt/mTOR pathway. Molecules. 24:26652019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cheng DD, Li SJ, Zhu B, Zhou SM and Yang
QC: EEF1D overexpression promotes osteosarcoma cell proliferation
by facilitating Akt-mTOR and Akt-bad signaling. J Exp Clin Cancer
Res. 37:502018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao GS, Gao ZR, Zhang Q, Tang XF, Lv YF,
Zhang ZS, Zhang Y, Tan QL, Peng DB, Jiang DM and Guo QN: TSSC3
promotes autophagy via inactivating the Src-mediated PI3K/Akt/mTOR
pathway to suppress tumorigenesis and metastasis in osteosarcoma,
and predicts a favorable prognosis. J Exp Clin Cancer Res.
37:1882018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ho CM, Huang CJ, Huang SH, Chang SF and
Cheng WF: Demethylation of HIN-1 reverses paclitaxel-resistance of
ovarian clear cell carcinoma through the AKT-mTOR signaling
pathway. BMC Cancer. 15:7892015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen D, Lin X, Zhang C, Liu Z, Chen Z, Li
Z, Wang J, Li B, Hu Y, Dong B, et al: Dual PI3K/mTOR inhibitor
BEZ235 as a promising therapeutic strategy against
paclitaxel-resistant gastric cancer via targeting PI3K/Akt/mTOR
pathway. Cell Death Dis. 9:1232018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Horan TC and Emori TG: Definitions of key
terms used in the NNIS System. Am J Infection Control. 25:112–116.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Emori TG, Culver DH, Horan TC, Jarvis WR,
White JW, Olson DR, Banerjee S, Edwards JR, Martone WJ, Gaynes RP,
et al: National nosocomial infections surveillance system (NNIS):
Description of surveillance methods. Am J Infect Control. 19:19–35.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Luu HH, Kang Q, Park JK, Si W, Luo Q,
Jiang W, Yin H, Montag AG, Simon MA, Peabody TD, et al: An
orthotopic model of human osteosarcoma growth and spontaneous
pulmonary metastasis. Clin Exp Metastasis. 22:319–329. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pautke C, Schieker M, Tischer T, Kolk A,
Neth P, Mutschler W and Milz S: Characterization of osteosarcoma
cell lines MG-63, Saos-2 and U-2 OS in comparison to human
osteoblasts. Anticancer Res. 24:3743–3748. 2004.PubMed/NCBI
|
|
33
|
de Azevedo JWV, de Medeiros Fernandes TAA,
Fernandes JV Jr, de Azevedo JCV, Lanza DCF, Bezerra CM, Andrade VS,
de Araújo JMG and Fernandes JV: Biology and pathogenesis of human
osteosarcoma. Oncol Lett. 19:1099–1116. 2020.PubMed/NCBI
|
|
34
|
Ferguson JL and Turner SP: Bone cancer:
Diagnosis and treatment principles. Am Fam Physician. 98:205–213.
2018.PubMed/NCBI
|
|
35
|
Biazzo A and De Paolis A:
Multidisciplinary approach to osteosarcoma. Acta Orthop Belg.
82:690–698. 2016.PubMed/NCBI
|
|
36
|
Stage TB, Bergmann TK and Kroetz DL:
Clinical pharmacokinetics of paclitaxel monotherapy: An updated
literature review. Clin Pharmacokinetics. 57:7–19. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Houghton PJ, Kurmasheva RT, Kolb EA,
Gorlick R, Maris JM, Wu J, Tong Z, Arnold MA, Chatterjee M,
Williams TM and Smith MA: Initial testing (stage 1) of the tubulin
binding agent nanoparticle albumin-bound (nab) paclitaxel
(Abraxane(®)) by the pediatric preclinical testing
program (PPTP). Pediat Blood Cancer. 62:1214–1221. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xie Y, Wang A, Lin J, Wu L, Zhang H, Yang
X, Wan X, Miao R, Sang X and Zhao H: Mps1/TTK: A novel target and
biomarker for cancer. J Drug Target. 25:112–118. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cai AL, Zeng W, Cai WL, Liu JL, Zheng XW,
Liu Y, Yang XC, Long Y and Li J: Peroxiredoxin-1 promotes cell
proliferation and metastasis through enhancing AktmTOR in human
osteosarcoma cells. Oncotarget. 9:8290–8302. 2017. View Article : Google Scholar : PubMed/NCBI
|