Introduction
Hepatic carcinomas are among the most common
malignant tumors in China, and cause serious threat to individuals'
health (1). The fatality rate of
Hepatic carcinoma ranks second among malignant tumors. At present,
surgical resection and liver transplantation are effective
treatments for early hepatic carcinoma (2). With advancements in research on various
surgical and drug treatments, the 5-year survival rate of hepatic
carcinoma patients has increased (3). However, advanced stage hepatic
carcinoma lacks effective treatment and intervention, and it is
prone to metastasis at the early stages (4). During the first clinical visit, most
patients with hepatic carcinoma already have micrometastases; the
various complex molecular mechanisms in the occurrence and
development of liver cancer are still unclear (5). Clinically, there is an urgent need to
identify the effective molecular biological markers for the
treatment and prognosis of hepatic carcinoma.
Long non-coding (lnc)RNAs play important roles in
the development, differentiation, proliferation and apoptosis of
cells (6,7). For instance, Deng et al
(8) compared 66 pairs of hepatic
carcinoma tissues and normal tissues and found that CCAT1
expression was increased in liver cancer tissues, and that the
degree of upregulation was associated with tumor size, vascular
invasion and serum alpha-fetoprotein level. Further, Zamani et
al (9) investigated the
epigenetic effects of solute carrier family 25 member 19 in hepatic
carcinoma and confirmed that DNA methylation in the functional
region of maternally expressed 3 (MEG3) was associated with its
loss of expression in hepatic carcinoma. Interestingly, ZNFX1
antisense RNA 1 (ZFAS1) could abolish its tumor suppressor effect
by binding microRNA (miR)-150, while miR-150 could inhibit the
invasion of hepatic carcinoma cells by inhibiting zinc finger E-box
binding homeobox 1 (ZEB1) and matrix metalloproteinases (MMP)14 and
MMP16 (10). H19 was also found to
induce p-glycoprotein expression and MDR1-associated drug
resistance in hepatic carcinoma cells by regulating the
demethylation of the MDR1 promoter (11). In this way, the discovery of the
tumor-associated protein lncRNA and elucidation of its associated
mechanisms will provide new insights in terms of the prognosis and
treatment of hepatic carcinoma.
As reported in previous studies, lncRNA XIST plays
the role of an oncogene in most tumors, the level of which was
associated with short survival and poor prognosis (12,13). In
cases of breast cancer, patients with the low expression of lncRNA
XIST were more sensitive to chemotherapy upon receiving high doses
of alkylating agents, suggesting that this protein can be used as a
biomolecular marker for breast cancer (14). Furthermore, the highly expressed
lncRNA XIST promoted the cell proliferation and invasion of
pancreatic cancer cells by negatively regulating miR-34a-5p
expression. In addition, it was also found that the regulatory
mechanism of XIST in lung cancer was associated with the activation
of the enhancer Of zeste 2 polycomb repressive complex 2 subunit,
leading to the silencing of Kruppel-like factor 2 (KLF2) (15). Various studies have focused on the
role of lncRNA XIST in the development of hepatic carcinoma
(16,17), but the associated molecular mechanism
was complicated. In the present study, the molecular mechanism of
lncRNA XIST in the development of hepatic carcinoma was explored;
moreover, the regulated association of lncRNA XIST/miR-320a/PIK3CA
was also shown. This might provide a new experimental basis for
further revealing the association between lncRNA XIST and the
associated biomarkers of hepatic carcinoma.
Materials and methods
Patients and tissues
The samples were obtained from the surgical tissues
of 69 patients with hepatic carcinoma at the Lianyungang First
People's Hospital. Samples >3 cm from the edge of the cancerous
tissue were used as the adjacent-cancerous tissues. All patients
signed an informed consent form approved by the Lianyungang No. 1
People's Hospital ethics committee (permit no. LW-20140322001),
which complied with medical ethics regulations. Of the enrolled
patients, 39 were male and 30 were female. The patients' age ranged
from 20–71 years, with a median age of 52 years. None of the
patients received adjuvant treatments such as radiotherapy,
chemotherapy or radiofrequency ablation, and the postoperative
specimens were confirmed to be hepatocellular carcinoma by
pathological examination. The specimens of the control group were
adjacent tissues located at least 3 cm from the surgical margin,
and no cancer cells were found on postoperative pathological
examination. All specimens were quickly frozen in liquid nitrogen
after being obtained and stored in a refrigerator at −80°C.
Cell culture and transfection
Hepatoma cell line (SUN449), hepatoblastoma cell
line (Huh-6), liver cancer cell line (HepG2) and transformed human
liver epithelial-2 cells (normal hepatocytes; THLE-2) were
purchased from the American Type Culture Collection. The short
tandem repeat method was used for authentication. The cells were
cultured in Dulbecco's Modified Eagle's Medium (DMEM) containing
10% fetal calf serum (both Invitrogen; Thermo Fisher Scientific,
Inc.) and placed in an incubator at 37°C containing 5%
CO2. After 0.25% trypsin digestion, the cells were
passaged, and the cells in the logarithmic growth phase were used
for the experiments.
A total of 3 short hairpin RNA (sh)-lncRNA XIST
sequences, overexpression vector (oe)-lncRNA XIST, miR-320a mimic,
miR-320a inhibitor, PIK3CA inhibitor, and their corresponding
controls were designed and synthesized by IBSBIO Co. Ltd. Cells
were co-transfected with shlncRNA XIST (2 µg; Ibsbio Co., Ltd.) and
the viral packaging plasmid (2 µg) (Nanjing Cobioer Gene Technology
Co., Ltd.) The expression was detected after incubation at 37°C for
72–96 h. After 72 h, G418 was added for screening. After 48 h of
transfection, G418 was added to each group to achieve a final
concentration of 0, 200, 400, 600, 800 and 1,000 mg·l−1,
respectively. After continuous observation for 4–7 days, the G418
concentration, which can lead to complete cell death in the
untransfected group, was selected as the optimal working
concentration for the subsequent screening of transfected cells.
According to the selected optimal G418 concentration, the
transfected cells were maintained in the culture until a stable
viable cell line was obtained. Lipofectamine® 2000
reagent (Thermo Fisher Scientific, Inc.) was also used to transfect
oe-lncRNAXIST. The corresponding empty vector was used as a
control. Similarly, the miR-320a mimic, miR-320a inhibitor, PIK3CA
inhibitor, and their corresponding controls were also transfected
by Lipofectamine 2000 at 37°C, as aforementioned. Non-targeting
sequence was used as shNC, inhibitor NC, siNC and NC for negative
control experiments. A non-targeting sequence was the negative
control for transfections of miR-320a mimic. After 24 or 48 h, the
following experiments were conducted.
Reverse-transcription-quantitative
polymerase chain reaction (RT-qPCR)
The TRIzol® method was used to extract
the total RNA from hepatic carcinoma tissues and cells according to
the manufacturer's instructions (Invitrogen; Thermo Fisher
Scientific, Inc.). The primers were designed using Primer 5
software (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi)
and synthesized by the Shanghai Yingwei Jieji Company. For cDNA
synthesis, a 20 µl reaction solution was prepared according to the
reverse transcription kit (Roche Diagnostics). The amplification
conditions of the thermal cycler were as follows: 25°C for 10 min;
55°C for 30 min; and 85°C for 5 sec. RT-qPCR was used to
quantitatively detect the expression of each gene. A total of 2 µl
cDNA of each clinical specimen was obtained; then, 10 µl SYBR qPCR
mix (Toyobo Life Science) and 2 µl primer mixture were added, and
dH2O was added to the total volume of 20 µl. The
LightCycler®480 was used to detect relative expression.
Gene expression was quantified using the 2−ΔΔCq method
(18). The primer sequences were as
follows: lncRNA-XIST forward, 5′-ACGCTGCATGTGTCCTTAG-3′;
lncRNA-XIST reverse, 5′-GAGCCTCTTATAAGCTGTTTG-3′; miR-320a forward,
5′-CAACAGAAGGCTCGAGGGAAGTCTGCGTGGCAGG-3′; miR-320a reverse,
5′-ATTCTGATCAGGATCCGAGGCGAATCCTCACATTG-3′; PIK3CA forward,
5′-GGTGACTGTGTGGGACTTATTG-3′; PIK3CA reverse,
5′-TGATGTAGTGTGTGGCTGTTG-3′; GAPDH forward,
5′-CATCACCATCTTCCAGGAGCG-3′; GAPDH reverse,
5′-TGACCTTGCCCACAGCCTTG-3′; U6 forward, 5′-CTCGCTTCGGCAGCACA-3′; U6
reverse, 5′-AACGCTTCACGAATTTGCGT-3′.
Cell count assay
Following treatment, the cells in each group were
trypsinized and collected, centrifuged at 300 × g (4°C) for 5 min
and the supernatant was removed, and the complete medium was added
to adjust the cell concentration to 5×103/ml. The cells
were seeded in a 96-well plate at 100 µl cells per well, with three
replicate wells. After culturing for 12, 24, 48 and 72 h, a total
of 10 µl Cell Counting Kit (CCK)-8 reagent (Beyotime Institute of
Biotechnology) was added to each well, and incubated at 37°C for
1.5 h in the incubator. A microplate reader was used to determine
the absorbance value of each well; the wavelength was 450 nm, and
the proliferation curve was drawn. The cell count assay is an
indirect form that can be used to measure cell proliferation.
Flow cytometry for cell apoptosis
According to the instructions of the apoptosis kit
(Dojindo Molecular Technologies, Inc.), the cells in each group
were trypsinized and collected and washed twice with phosphate
buffered saline (PBS). The cells were centrifuged at 300 × g for 5
min (4°C) and then 100 µl Annexin V buffer was added to suspend the
cells. Finally, PI and Annexin V staining solutions were added
separately and mixed by pipetting. After 15 min of staining at room
temperature and in the dark, 400 µl Annexin V buffer was added to
the sample, and cell apoptosis was detected by flow cytometry.
Transwell assay
After the cells from each group were digested with
trypsin, the cell concentration was adjusted to 1×108/l.
Matrigel on the Transwell membrane was used to detect cell invasion
ability, but Matrigel was not required to measure cell migration
ability. A total of 100 µl DMEM without FBS was added to the upper
chamber of each Transwell, while 500 µl of DMEM was added to the
lower chamber. The Transwell chamber was incubated in an incubator
with 5% CO2 at 37°C for 24 h. The cells were fixed with
4% paraformaldehyde for 15 min at room temperature, and washed
thrice with PBS. Then, the cells were stained with 1% crystal
violet at room temperature for 10 min. After washing away the
crystal violet staining solution, cell migration and invasion were
observed under a light microscope (Leica Microsystems GmbH). Ten
fields were selected to calculate the average number of cells that
migrated and invaded with a magnification of ×400.
Luciferase reporter experiment
The binding regions of lncRNA XIST and PIK3CA with
miR-320a were predicted based on the StarBase (http://starbase.sysu.edu.cn/) and TargetScan databases
(http://www.targetscan.org/vert_72/).
Wild-type lncRNA XIST (lncRNA XIST-wt), wild-type PIK3CA non-coding
region (PIK3CA-WT), mutant lncRNA XIST (lncRNA XIST-Mut) sequence,
and mutant PIK3CA (PIK3CA-Mut) missing the miR-320a binding region,
as well as mutations missing the miR-320a binding region were
designed and the luciferase reporter vector was constructed by
Nanjing Cobioer Gene Technology Co., Ltd. The lncRNA XIST-wt,
lncRNA XIST-Mut, PIK3CA-WT and PIK3CA-Mut luciferase reporter
vectors were transfected into the control and experimental cells at
37°C for 48 h (using Lipofectamine® 2000), and the
Promega dual-luciferase reporter gene kit (Promega Corporation) was
used to test the luciferase activity after transfection.
Fluorescent enzyme activity was recorded as the ratio of firefly
luciferase to Renilla luciferase activity.
Pull-down assay
The RNA pull-down experiment was performed according
to the instructions of the RNA pull-down kit (Thermo Fisher
Scientific, Inc.). The brief steps were as follows: The labeled
target RNA was combined with streptavidin magnetic beads, and 30
Ult of streptavidin magnetic beads were added to a 1.5-ml
centrifuge tube. The magnetic beads were collected, and an equal
volume of RNA capture buffer was used for resuspension and gentle
pipetting. A total of 50 pmol biotin-labeled RNA was added, mixed
well, and incubated for 30 min at room temperature. The magnetic
beads were collected and 100 µl RNA binding buffer was added and
mixed. Then, the liquid was discarded and 100 µl of the mixed RNA
binding reaction solution was combined with the magnetic beads.
Elution buffers (50 µl) were used to elute the complex. The eluate
was collected, RNA was extracted with TRIzol® and stored
at −80°C, and the amount of enriched miR-320a was detected.
Western blot analysis
Western blotting was performed in accordance with
conventional methods. Protein extraction buffer (Shanghai Kanglang
Biotechnology Co., Ltd.) was used to extract the total protein of
each group of hepatocellular carcinoma cells. The concentration of
each histone was detected by the bicinchoninic acid (BCA) protein
quantitative method, and all proteins were boiled and denatured at
100°C. A total of 50 µg of each protein group was subjected to 10%
acrylamide gel electrophoresis, and transferred to a polyvinylidene
fluoride membrane at a constant current. The membrane was sealed in
5% skim milk at room temperature for 1 h, washed with PBS, and
incubated with the primary antibody PIK3CA (1:1,000; cat. no.
ab40776; Abcam) at 4°C overnight. After washing with TPBS, the
membrane was incubated with Alexa Fluor® 488 goat
anti-rabbit IgG (H+L) (ab150077; 1:2,000; cat. no. ab150077; Abcam)
at room temperature for 1 h. Finally, the protein bands were
illuminated, developed and fixed by enhanced chemiluminescence
(Pierce; Thermo Fisher Scientific Inc.). Signals were quantified
using ImageJ 1.63 software (National Institutes of Health).
Statistical analysis
For any quantified parameter, each group had three
parallels, and the experiment was repeated three times. SPSS 21.0
(IBM Corp.) was the statistical software used for the data
analysis. The data are represented by the mean ± standard
deviation. Two-way ANOVA (two-way analysis of variance) with post
hoc test was used. Kaplan-Meier survival analysis was applied to
examine the association between gene expression and survival rate,
and statistical significance was assessed using the Mantel-Cox log
rank test. Pearson's correlation was used to test the gene
expression correlation. P<0.05 was used to indicate a
statistically significant difference.
Results
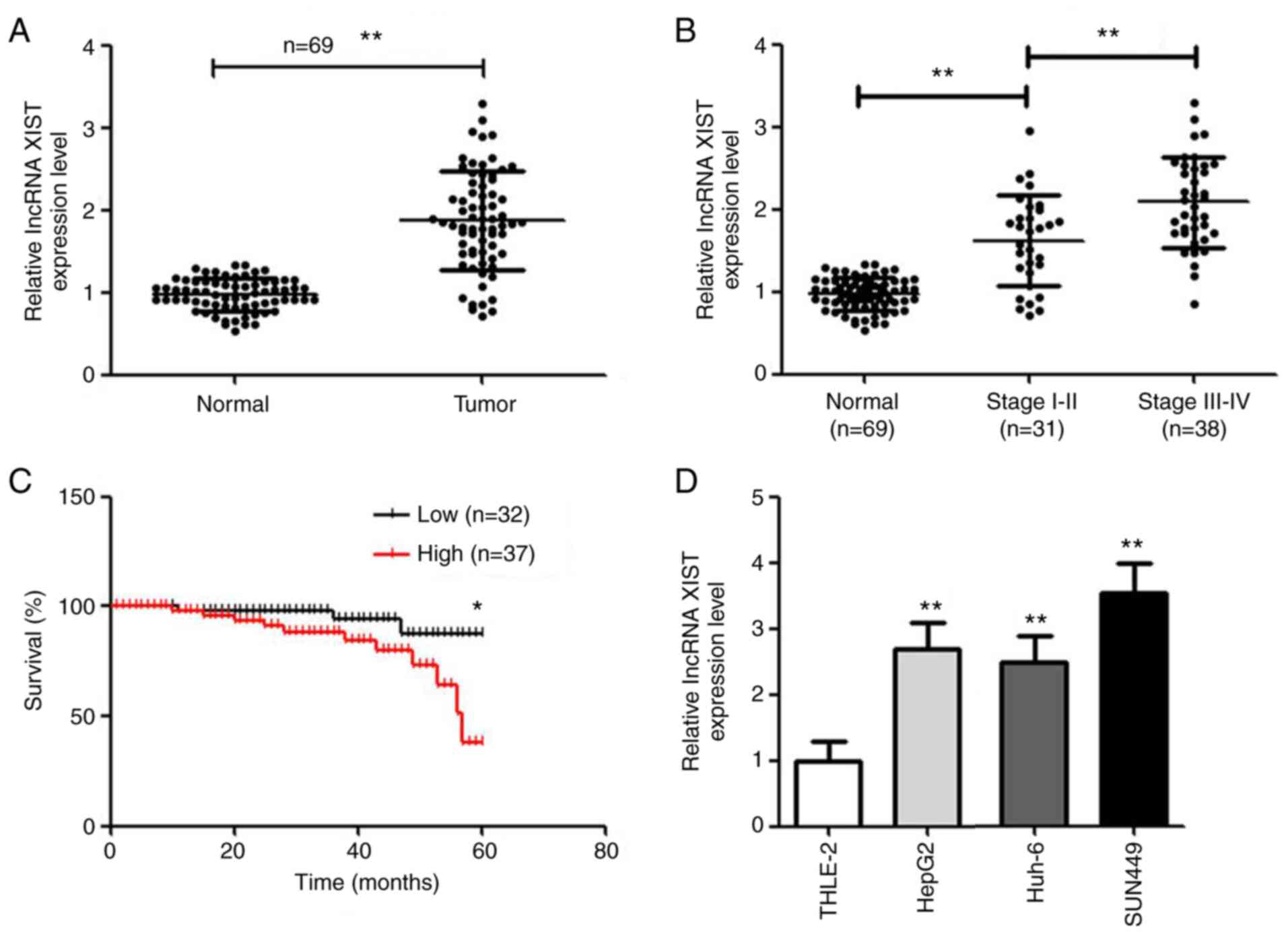
lncRNA XIST expression is higher in
hepatic carcinoma tissues and cells
RT-qPCR was used to detect the expression level of
lncRNA XIST in hepatic carcinoma tissues and cells. Compared with
normal tissues, lncRNA XIST was highly expressed in hepatic
carcinoma tissues (P<0.01; Fig.
1A). Moreover, with increasing tumor stage, the expression of
lncRNA XIST also increased (Fig.
1B). Based on the median value of lncRNA XIST expression
(cut-off value, 1.815), the 69 patients were divided into the
highly expressed lncRNA XIST group (n=37) and low-expressed lncRNA
XIST group (n=32). As shown in Fig.
1C, the survival rate was significantly lower in the highly
expressed lncRNA XIST group. Moreover, tumor size, vascular
invasion and classification were significantly different between
the two groups (Table I). The lncRNA
XIST was highly expressed in all hepatic carcinoma cells compared
with the THLE-2 cell line (Fig. 1D).
Among the hepatic carcinoma cell lines, lncRNA XIST was expressed
the highest in the HepG2 and SUN449 cell lines. Thus, the HepG2 and
SUN449 cell lines were used in the following experiments.
 | Table I.Association between long non-coding
RNA XIST expression and general clinical parameters. |
Table I.
Association between long non-coding
RNA XIST expression and general clinical parameters.
|
|
| lncRNA XIST |
|
|---|
|
|
|
|
|
|---|
|
Characteristics | Number of
patients | Low expression
(< median) | High expression (≥
median) | P-value |
|---|
| Number | 40 | 20 | 20 |
|
| Sex |
|
|
| 0.342 |
|
Male | 19 | 11 | 8 |
|
|
Female | 21 | 9 | 12 |
|
| Age, years |
|
|
| 0.337 |
|
<60 | 23 | 13 | 10 |
|
|
≥60 | 17 | 7 | 10 |
|
| Tumor size, cm |
|
|
| 0.008 |
|
<5 | 26 | 17 | 9 |
|
| ≥5 | 14 | 3 | 11 |
|
| Tumor site |
|
|
| 0.311 |
|
Colon | 13 | 5 | 8 |
|
|
Rectum | 27 | 15 | 12 |
|
| Depth of
invasion |
|
|
| 0.168 |
|
T1-T2 | 12 | 8 | 4 |
|
|
T3-T4 | 28 | 12 | 16 |
|
| Lymph node
metastasis |
|
|
| 0.011 |
| N0 | 22 | 15 | 7 |
|
|
N1-N2 | 18 | 5 | 13 |
|
| Distant
metastasis |
|
|
| 0.212 |
| M0 | 33 | 18 | 15 |
|
| M1 | 7 | 2 | 5 |
|
| TNM stage |
|
|
| 0.004 |
|
I–II | 19 | 14 | 5 |
|
|
III–IV | 21 | 6 | 15 |
|
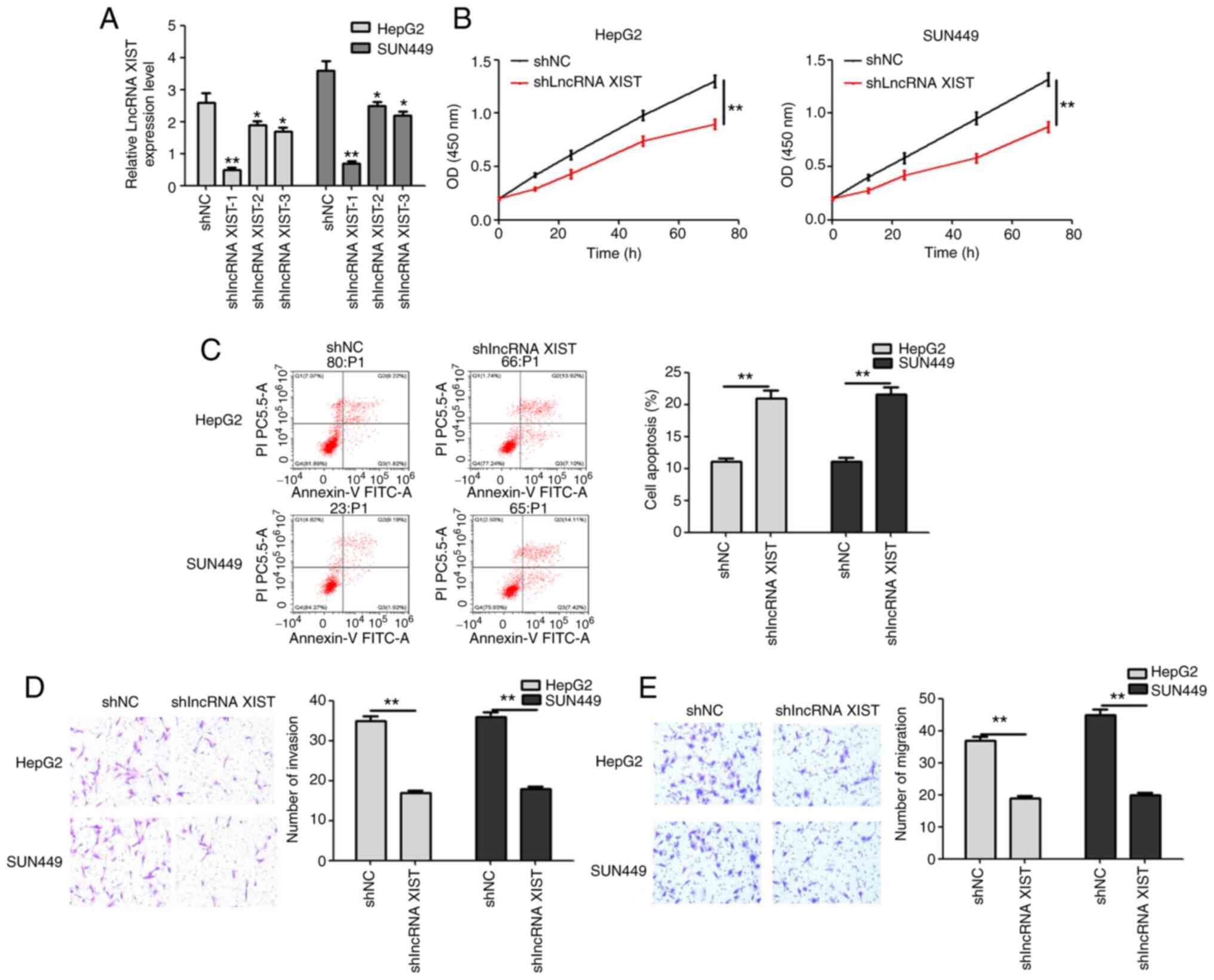
shlncRNA XIST inhibits the
proliferation, invasion and migration of hepatic carcinoma
cells
After 3 shlncRNA XIST transfection, the transfection
efficiency was detected. In terms of the results, the relative
lncRNA XIST level was significantly decreased, and shlncRNA XIST-1
was associated with the highest transfection efficiency (Fig. 2A). For the following experiments,
shlncRNA XIST-1 was used and defined as shlncRNA XIST. Following
shlncRNA XIST transfection for 72 h, the proliferation of both
HepG2 and SUN449 cell lines significantly decreased (Fig. 2B). Moreover, the apoptosis rate was
significantly higher in the shlncRNA XIST group in both cell lines
(Fig. 2C). The invasion and
migration of hepatic carcinoma cells were also inhibited by
shlncRNA XIST (Fig. 2D and E). Thus,
shlncRNA XIST inhibited cell proliferation, invasion and migration,
while promoting the apoptosis of hepatic carcinoma cells.
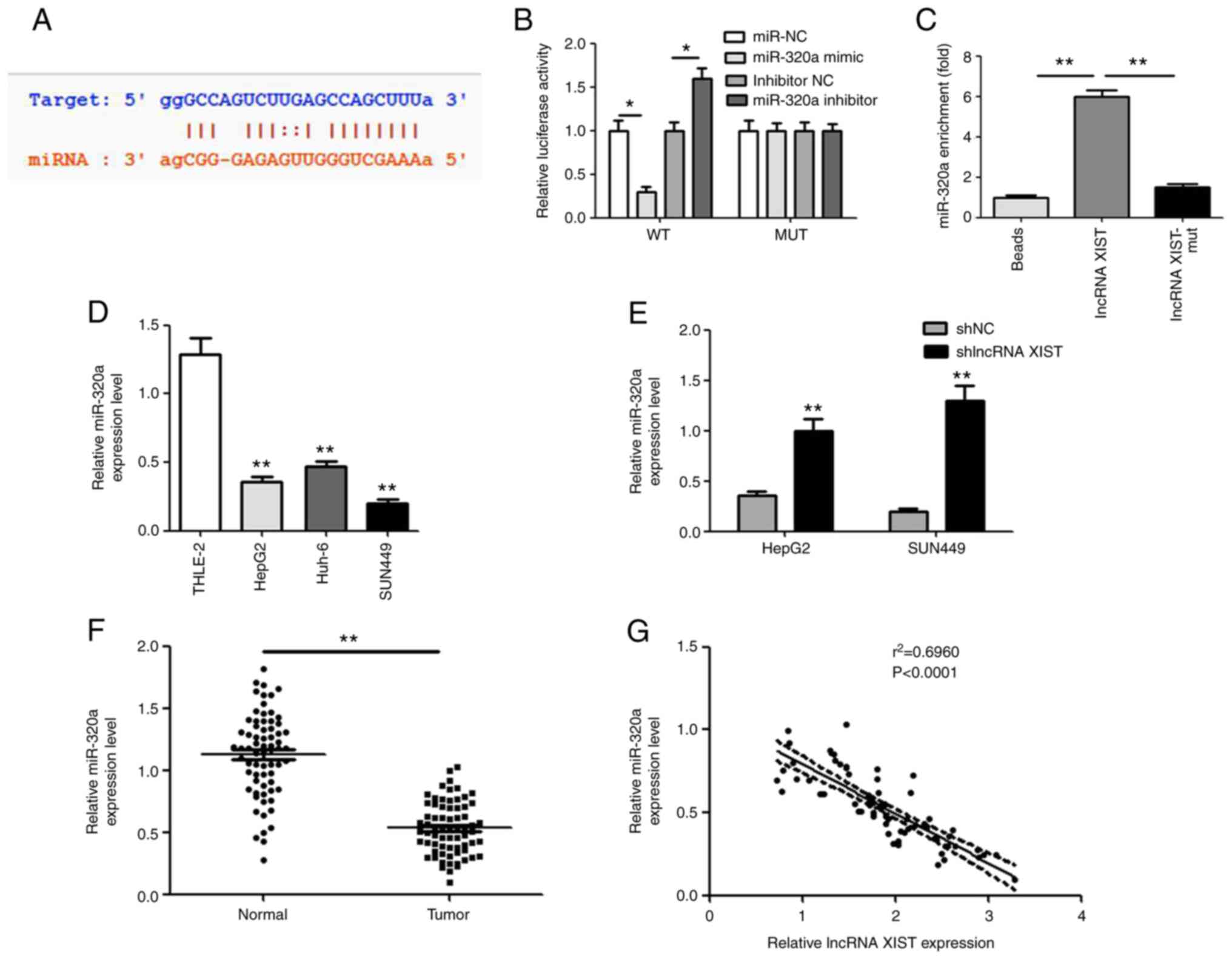
lncRNA XIST negatively regulates
miR-320a
Starbase was applied to predict the targeting
association between lncRNA XIST and miR-320a. The binding site is
shown in Fig. 3A. In the lncRNA
XIST-WT group, the miR-320a mimic resulted in significantly
decreased luciferase activity, while the miR-320a inhibitor
increased its activity. However, both the miR-320a mimic and
miR-320a inhibitor had no effect on luciferase activity in the
lncRNA XIST-MUT group (Fig. 3B).
Compared with the empty vectors and mutants, biotin-labeled lncRNA
XIST was used to pull down miR-320a by affinity in vitro,
and the results confirmed the endogenous binding between miR-320a
and lncRNA XIST (Fig. 3C). In
addition, the expression level of miR-320a was also detected in
hepatocellular carcinoma cells and tissues. As shown in Fig. 3D and E, the miR-320a demonstrated a
lower expression level in hepatocellular carcinoma cells and
tissues. Pearson's correlation analysis also confirmed that
miR-320a and lncRNA XIST demonstrated a negative association in
patients with hepatocellular carcinoma (Fig. 3F). Notably, the relative miR-320a
expression level was significantly increased following shlncRNA
XIST transfection (Fig. 3G).
miR-320a negatively targets
PIK3CA
The Starbase online tool was used to predict the
targeting association between PIK3CA and miR-320a. The binding site
of PIK3CA and miR-320a is shown in Fig.
4A. miR-320a mimic, miR-320a inhibitor and miR-NC were
transfected in cells; the transfections were successful (Fig. 4B). The luciferase reporter gene
experiment was undertaken to verify whether binding occurred. In
the PIK3CA-WT group, the miR-320a mimic significantly decreased the
luciferase activity, while the miR-320a inhibitor increased the
activity. However, both the miR-320a mimic and miR-320a inhibitor
had no effect on luciferase activity in the PIK3CA-MUT group
(Fig. 4C). The expression of PIK3CA
in hepatocellular carcinoma cells was detected by RT-qPCR. As shown
in Fig. 4D, the level of PIK3CA was
significantly higher in hepatocellular carcinoma cells compared
with the THLE-2 cell line. In hepatocellular carcinoma and normal
tissues, the expression of PIK3CA exhibited a similar trend
(Fig. 4E). Following transfection
with miR-320 mimic, the expression level of PIK3CA significantly
decreased (Fig. 4F). Pearson's
correlation analysis was used to confirm the correlation between
PIK3CA and lncRNA XIST or miR-320a expression. The results showed
that PIK3CA was negatively correlated with miR-320a, while PIK3CA
was positively correlated with lncRNA XIST (Fig. 4G and H).
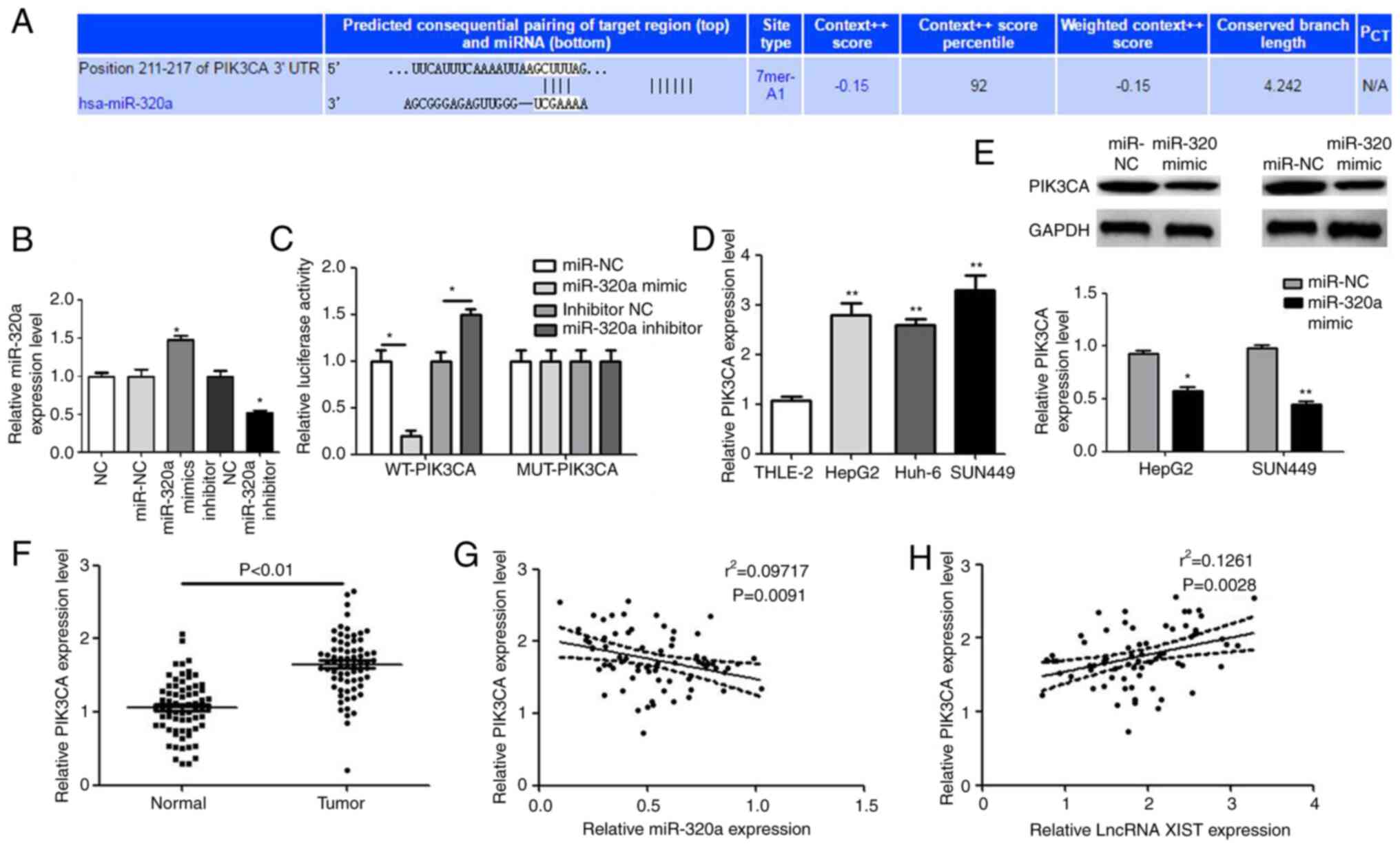 | Figure 4.miR-320a negatively targets PIK3CA.
(A) The binding site of PIK3CA and miR-320a, as based on the
Targetscan database. (B) Transfection efficiency of miR-320a mimic,
inhibitor and NC. *P<0.05 vs. NC group. (C) Fluorescent reporter
assay to verify the binding of PIK3CA and miR-320a. *P<0.05
miR-320a mimic vs. miR-320a NC group, *P<0.05 miR-320a inhibitor
vs. inhibitor NC group. (D) The expression of PIK3CA in
hepatocellular carcinoma cells. *P<0.05 vs. THLE-2l group. (E)
The effect of miR-320a on PIK3CA levels. *P<0.05 and **P<0.01
vs miR-NC group. (F) The expression of PIK3CA in hepatocellular
carcinoma and normal tissues. The expression correlation between
(G) PIK3CA and miR-320a and (H) between PIK3CA and lncRNA XIST,
Compared with NC or normal group, *P<0.05 vs. NC or THLE-2l
group, **P<0.01. miR, microRNA; NC, negative control; lncRNA,
long non-coding RNA; WT, wild type; MUT, mutant; UT3, untranslated
region. |
miR-320a mimic and PIK3CA inhibitor
rescues the effect of oelncRNA XIST
The rescue assay was performed in SUN449 cell lines.
Small interfering RNA (si)PIK3CA significantly decreased PIK3CA
expression, indicating the transfection was successful (Fig. 5A). Based on transfection, the cells
were divided into MOCK, oelncRNA XIST, oelncRNA XIST+miR-320a mimic
and oelncRNA XIST+PIK3CA inhibitor groups. After transfection,
oelncRNA XIST significantly increased lncRNA XIST expression level
(Fig. 5B). OelncRNA XIST
significantly increased PIK3CA, while miR-320a mimic and PIK3CA
inhibitor decreased the expression of PIK3CA at the gene and
protein level (Fig. 5C). With
respect to the effects of miR-320a, PIK3CA and lncRNA XIST levels
on hepatocellular carcinoma development, CCK-8, flow cytometry and
the Transwell assay were processed. When examining the results,
oelncRNA XIST increased the cell number, invasion and migration,
while it decreased the apoptosis of SUN449 cells. The cell number
is an indirect measure of proliferation. More importantly, the
miR-320a mimic and PIK3CA inhibitor demonstrated the recovery
effects of oelncRNA XIST in the aforementioned processes (Fig. 5D-G). The aforementioned findings
indicate that lncRNA XIST accelerates hepatic carcinoma progression
by targeting the miR-320a/PIK3CA axis.
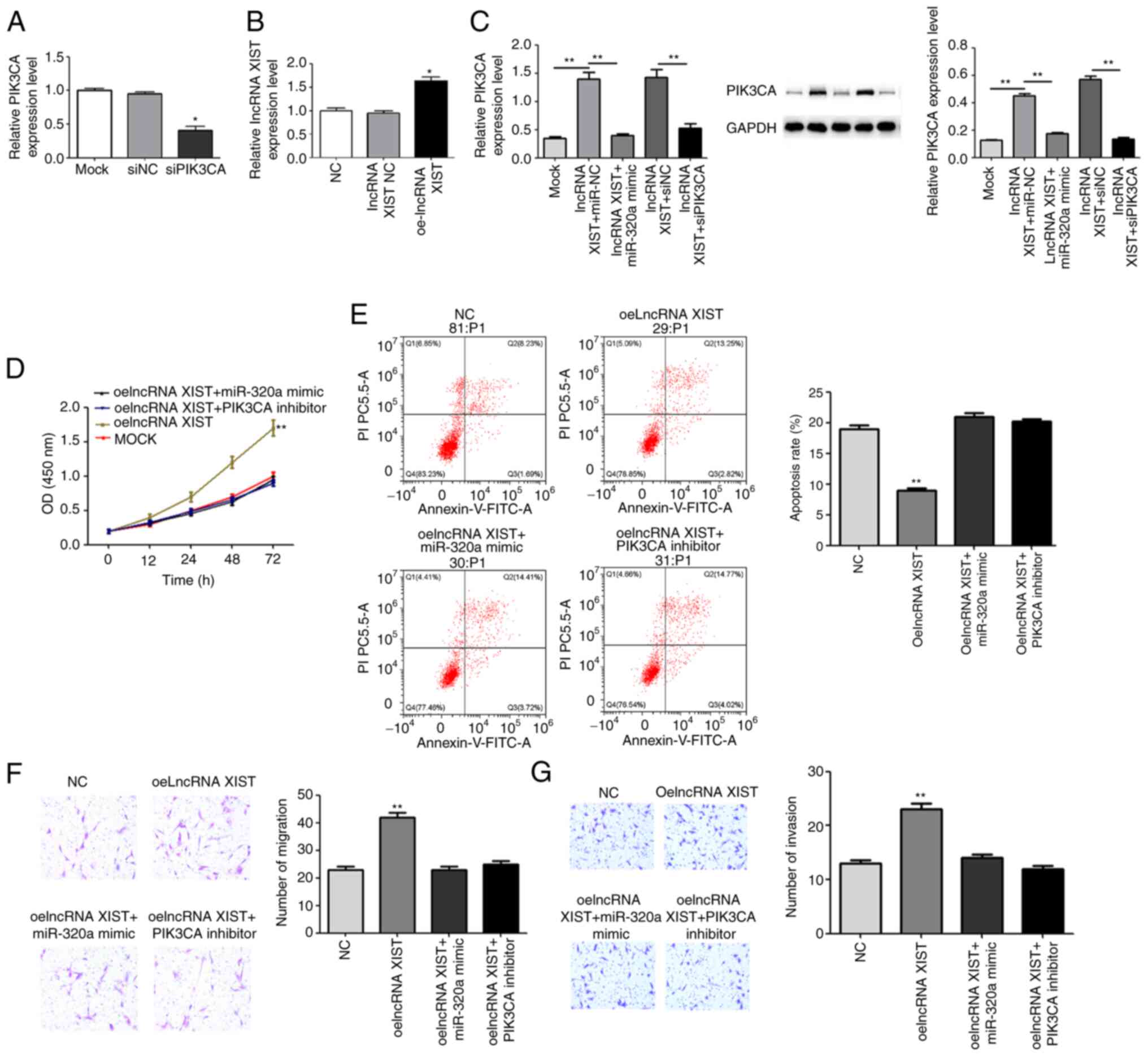 | Figure 5.miR-320a mimic and PIK3CA inhibitor
recover the effect of oelncRNA XIST. The rescue assay was performed
in the SUN449 cell line. Based on transfection, the cells were
divided into the MOCK, oelncRNA XIST, oelncRNA XIST+miR-320a mimic
and oelncRNA XIST+PIK3CA inhibitor groups. (A) Transfection
efficiency of siPIK3CA. (B) Transfection efficiency of oelncRNA
XIST. (C) The relative PIK3CA expression in each group was detected
using reverse transcription-quantitative PCR and western blotting.
(D) The Cell Counting Kit-8 assay was used to measure
proliferation. (E) Flow cytometry for apoptosis. (F) Transwell
assay to assess invasion; magnification, ×400. (G) Transwell assay
for migration assay; magnification, ×400, *P<0.05 and
**P<0.01 vs. NC or mock group. miR, microRNA; oe, overexpression
vector; lncRNA, long non-coding RNA; si, small, interfering RNA;
NC, negative control. |
Discussion
Hepatocellular carcinoma is the most common primary
malignant liver tumor (19). Due to
its high incidence rate, early metastasis and postoperative
recurrence, the patient survival rate is low (20). At present, there are few effective
diagnostic markers of hepatocellular carcinoma. In the present
study, lncRNA XIST was found to be highly expressed in hepatic
carcinoma tissues and cells. Moreover, the expression of lncRNA
XIST was closely associated with disease stage and the patient
survival rate. In order to provide a potential biomarker to
determine the prognosis of hepatic carcinomas, the molecular
mechanism of lncRNA XIST was explored in the present study.
LncRNA XIST was the first non-coding gene identified
within the X inactivation center (XIC) (21). In previous studies, it has been
confirmed to regulate various cancer types, such as gastric cancer,
osteosarcoma, colorectal cancer and non-small cell lung cancer
(22–25). Moreover, lncRNA XIST participated in
different pathways, including the DNA mismatch repair, NF-κB/NLRP3
inflammasome, HIF-1A/AXL signaling and iASPP pathways (13,26–28).
Thus, the DNA mismatch repair defects are involved in a variety of
gastrointestinal and other tumors. In hepatic carcinoma, there is a
loss of heterozygosity at the hMSH2 and/or hMLH1 gene locus, as
well as the instability of associated microsatellites in liver
tissues prior to malignant transformation (29). Wu et al (30) found that inhibiting the activation of
NF-κB/NLRP3 inflammasomes could decrease the immune-inflammatory
response, thereby preventing the disorder and accumulation of lipid
metabolism in the liver under high-fructose conditions. It is worth
noting that lncRNA XIST was found to interact with miR-497-5p and
target programmed cell death 4 (PDCD4), and it was further involved
in the migration and proliferation of hepatic cancer cells
(16). Similar results were also
obtained in the present study, as shlncRNA XIST attenuated the
proliferation, invasion and migration of hepatic cancer cells,
while it accelerated apoptosis.
Although lncRNA XIST does not encode proteins, it
could still function at the RNA level to regulate gene expression.
In the present study, lncRNA XIST negatively targeted miR-320a.
miR-320a is a member of the miR-320 family, which is widely present
in various tissues and organs of the human body. In recent years,
many studies have found that the expression level of miR-320a was
high in the stomach, liver, colorectal and other tumor cells, and
it was also confirmed that miR-320a is closely associated with
tumor occurrence, development, metastasis and prognosis (31,32). Wen
et al (33) detected the
expression of miRNA in the blood and tissues of patients with
hepatocellular carcinoma, and found that the expression of miR-320a
was significantly increased. The aforementioned findings further
confirmed that miR-320a could be used as a biological indicator for
the early detection of hepatocellular carcinoma. Additionally, Yao
et al (34) investigated the
biological functions of miRNA in hepatocellular carcinoma cells,
and found that the overexpression of miR-320a significantly
enhanced the migration and invasion of hepatocellular carcinoma
cells, while downregulating miR-320a inhibited the cells' migration
and invasion ability. It may be that miR-320a decreased the
expression of the G protein subunit alpha I1 (GNAI1), thereby
promoting the metastasis and invasion of tumor cells. Besides,
miR-320a might participate in the epithelial-mesenchymal transition
pathway, and affect the progression of hepatocellular carcinoma
(35). In SMMC-7221 cell lines,
miR-320a was also found to have a lower expression compared with
normal cells, which further influenced migration and apoptosis
(36). Interestingly, the miR-320a
mimic recovered the effect of lncRNA XIST in the present study.
Based on the aforementioned findings, lncRNA XIST might promote
hepatic carcinoma progression by regulating miR-320a
expression.
The results in the present study also confirmed that
miR-320a negatively targeted PIK3CA. PIK3CA is a
phosphatidylinositol kinase that phosphorylates the third hydroxyl
group of the inositol ring (37).
PIK3CA activation was associated with the production of the second
messenger PIP3 on the plasma membrane. PIP3 bound to the signal
proteins AKT serine/threonine kinase (AKT) and pyruvate
dehydrogenase kinase 1 (PDK1), containing the PH domain in the
cell. This prompted PDK1 to phosphorylate the AKT protein Set308,
leading to the activation of AKT (38,39).
Activated AKT promoted or inhibited its downstream target proteins
(Bad, Caspase, NF-KB, GSK-3 and FKHR) through phosphorylation,
thereby regulating cell proliferation, differentiation, apoptosis
and migration (40). In addition,
the PIK3CA inhibitor could recover the effect of lncRNA XIST in the
present study. As a result, lncRNA XIST accelerated hepatic
carcinoma progression by regulating PIK3CA.
According to the obtained results, lncRNA XIST
accelerates hepatic carcinoma progression by targeting the
miR-320a/PIK3CA axis, which might serve as the theoretical basis
underlying targeted therapy for hepatic carcinomas.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LY, JJ and YZ made substantial contributions to the
study design. FX, XT, WX and TX conducted the experiments. WX, YN
and TX analyzed and interpreted the data. All authors have read and
approved the final manuscript. LY and JJ confirm the authenticity
of all the raw data.
Ethics approval and consent to
participate
The study was approved by the ethics committee of
Lianyungang No. 1 People's Hospital (permit no. LW-20140322001),
which complied with medical ethics regulations. All patients signed
an informed consent form.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li B, Liu H, Shang HW, Li P, Li N and Ding
HG: Diagnostic value of glypican-3 in alpha fetoprotein negative
hepatocellular carcinoma patients. Afr Health Sci. 13:703–709.
2013.PubMed/NCBI
|
|
2
|
Akoad ME and Pomfret EA: Surgical
resection and liver transplantation for hepatocellular carcinoma.
Clin Liver Dis. 19:381–399. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yamamoto J, Kosuge T, Saiura A, Sakamoto
Y, Shimada K, Sano T, Takayama T, Sugawara Y, Yamaguchi T, Kokudo N
and Makuuchi M: Effectiveness of hepatic resection for early-stage
hepatocellular carcinoma in cirrhotic patients: Subgroup analysis
according to Milan criteria. Jpn J Clin Oncol. 37:287–295. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Itoh A, Sadamori H, Yabushita K, Monden K,
Tatsukawa M, Hioki M, Hyodo T, Omonishi K, Ueki T, Ohno S, et al:
Advanced hepatocellular carcinoma with hepatic vein tumor
thrombosis and renal dysfunction after hepatic arterial infusion
chemotherapy effectively treated by liver resection with active
veno-venous bypass: Report of a case. BMC Cancer. 16:7052016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lotersztajn S, Julien B, Teixeira-Clerc F,
Grenard P and Mallat A: Hepatic fibrosis: Molecular mechanisms and
drug targets. Annu Rev Pharmacol Toxicol. 45:605–628. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang X, Zhi X, Gao Y, Na T and Zheng J:
LncRNAs in pancreatic cancer. Oncotarget. 7:57379–57390. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu YY and Song X: Advances in research on
circulating miRNAs and lncRNAs in early diagnosis and treatment of
lung cancer. Tumor. 35:584–591. 2015.(In Chinese).
|
|
8
|
Deng L, Yang SB, Xu FF and Zhang JH: Long
noncoding RNA CCAT1 promotes hepatocellular carcinoma progression
by functioning as let-7 sponge. J Exp Clin Cancer Res. 34:182015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zamani M, Sadeghizadeh M, Behmanesh M and
Najafi F: Dendrosomal curcumin increases expression of the long
non-coding RNA gene MEG3 via up-regulation of epi-miRs in
hepatocellular cancer. Phytomedicine. 22:961–967. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li T, Xie J, Shen C, Cheng D, Shi Y, Wu Z,
Deng X, Chen H, Shen B, Peng C, et al: Amplification of long
noncoding RNA ZFAS1 promotes metastasis in hepatocellular
carcinoma. Cancer Res. 75:3181–3191. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tsang WP and Kwok TT: Riboregulator H19
induction of MDR1-associated drug resistance in human
hepatocellular carcinoma cells. Oncogene. 26:4877–4881. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Du Y, Weng XD, Wang L, Liu XH, Zhu HC, Guo
J, Ning JZ and Xiao CC: LncRNA XIST acts as a tumor suppressor in
prostate cancer through sponging miR-23a to modulate RKIP
expression. Oncotarget. 8:94358–94370. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tang Y, He R, An J, Deng P, Huang L and
Yang W: lncRNA XIST interacts with miR-140 to modulate lung cancer
growth by targeting iASPP. Oncol Rep. 38:941–948. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schouten PC, Vollebergh MA, Opdam M,
Jonkers M, Loden M, Wesseling J, Hauptmann M and Linn SC: High XIST
and low 53BP1 expression predict poor outcome after high-dose
alkylating chemotherapy in patients with a BRCA1-like breast
cancer. Mol Cancer Ther. 15:190–198. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fang J, Sun CC and Gong C: Long noncoding
RNA XIST acts as an oncogene in non-small cell lung cancer by
epigenetically repressing KLF2 expression. Biochem Biophys Res
Commun. 478:811–817. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Y, Zhu Z, Huang S, Zhao Q, Huang C,
Tang Y, Sun C, Zhang Z, Wang L, Chen H, et al: lncRNA XIST
regulates proliferation and migration of hepatocellular carcinoma
cells by acting as miR-497-5p molecular sponge and targeting PDCD4.
Cancer Cell Int. 19:1982019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kong Q, Zhang S, Liang C, Zhang Y, Kong Q,
Chen S, Qin J and Jin Y: LncRNA XIST functions as a molecular
sponge of miR-194-5p to regulate MAPK1 expression in hepatocellular
carcinoma cell. J Cell Biochem. 119:4458–4468. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nolan T, Hands RE and Bustin SA:
Quantification of mRNA using real-time RT-PCR. Nat Protoc.
1:1559–1582. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Inagawa S, Itabashi M, Adachi S, Kawamoto
T, Hori M, Shimazaki J, Yoshimi F and Fukao K: Expression and
prognostic roles of beta-catenin in hepatocellular carcinoma:
Correlation with tumor progression and postoperative survival. Clin
Cancer Res. 8:450–456. 2002.PubMed/NCBI
|
|
20
|
Li YT, Luo SF and Liu JF: Advances in
research on prediction for early postoperative metastasis and
recurrence in patients with hepatocellular carcinoma. Chin Med
Abstracts. 13:259–261. 2013.
|
|
21
|
Lee JT: X-Chromosome Inactivation and Long
Non-Coding RNAs. Presentation at the Annual Meeting of the American
Association for the Advancement of Science Feb 13–17 2014 in
Chicago, IL, USA.
|
|
22
|
Li J, Shi Z, Luo TH, Nie MM, Yuan SJ and
Bi JW: Expression and clinical significance of long non-coding RNA
XIST in tissue and plasma of gastric cancer. Acad J Sec Mil Med
Univ. 38:1403–1409. 2017.(In Chinese).
|
|
23
|
Sun X, Wei B, Peng ZH, Fu QL, Wang CJ,
Zheng JC and Sun JC: Knockdown of lncRNA XIST suppresses
osteosarcoma progression by inactivating AKT/mTOR signaling pathway
by sponging miR-375-3p. Int J Clin Exp Pathol. 12:1507–1517.
2019.PubMed/NCBI
|
|
24
|
Song H, He P, Shao T, Li Y, Li J and Zhang
Y: Long non-coding RNA XIST functions as an oncogene in human
colorectal cancer by targeting miR-132-3p. J BUON. 22:696–703.
2017.PubMed/NCBI
|
|
25
|
Zhang YL, Li XB, Hou YX, Fang NZ, You JC
and Zhou QH: The lncRNA XIST exhibits oncogenic properties via
regulation of miR-449a and Bcl-2 in human non-small cell lung
cancer. Acta Pharmacol Sin. 38:371–381. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Du P, Zhao H, Peng R, Liu Q, Yuan J, Peng
G and Liao Y: LncRNA-XIST interacts with miR-29c to modulate the
chemoresistance of glioma cell to TMZ through DNA mismatch repair
pathway. Biosci Rep. 37:BSR201706962017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ma M, Pei Y, Wang X, Feng J, Zhang Y and
Gao MQ: LncRNA XIST mediates bovine mammary epithelial cell
inflammatory response via NF-κB/NLRP3 inflammasome pathway. Cell
Prolif. 52:e125252019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang LG, Cao MZ, Zhang J, Li XY and Sun
QL: LncRNA XIST modulates HIF-1A/AXL signaling pathway by
inhibiting miR-93-5p in colorectal cancer. Mol Genet Genomic Med.
8:e11122020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Macdonald GA, Greenson JK, Saito K,
Cherian SP, Appelman HD and Boland CR: Microsatellite instability
and loss of heterozygosity at DNA mismatch repair gene loci occurs
during hepatic carcinogenesis. Hepatology. 28:90–97. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu Q, Meng WY, Jie Y and Zhao H: LncRNA
MALAT1 induces colon cancer development by regulating
miR-129-5p/HMGB1 axis. J Cell Physiol. 233:6750–6757. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin Y, Zeng Y, Zhang F, Xue L, Huang Z, Li
W and Guo M: Characterization of MicroRNA expression profiles and
the discovery of novel MicroRNAs involved in cancer during human
embryonic development. PLoS One. 8:e692302013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang B and Pan X: RDX induces aberrant
expression of MicroRNAs in Mouse brain and liver. Environ Health
Perspect. 117:231–240. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wen Y, Han J, Chen J, Dong J, Xia Y, Liu
J, Jiang Y, Dai J, Lu J, Jin G, et al: Plasma miRNAs as early
biomarkers for detecting hepatocellular carcinoma. Int J Cancer.
137:1679–1690. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yao J, Liang LH, Zhang Y, Ding J, Tian Q,
Li JJ and He XH: GNAI1 suppresses tumor cell migration and invasion
and is post-transcriptionally regulated by Mir-320a/c/d in
hepatocellular carcinoma. Cancer Biol Med. 9:234–241.
2012.PubMed/NCBI
|
|
35
|
Chou LF, Chen CY, Yang WH, Chen CC, Chang
JL, Leu YL, Liou MJ and Wang TH: Suppression of hepatocellular
carcinoma progression through FOXM1 and EMT inhibition via
hydroxygenkwanin-induced miR-320a expression. Biomolecules.
10:202019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lv X, Xiong W, Song D, Shi H, Li H, Guo P,
Chen D and Li J: The expression of miR-320a on hepatocellular
carcinoma and the effect of apoptosis and migration on SMMC-7721
cell line. Genomics Appl Biol. 5:2305–2311. 2018.(In Chinese).
|
|
37
|
Auger KR, Carpenter CL, Cantley LC and
Varticovski L: Phosphatidylinositol 3-kinase and its novel product,
phosphatidylinositol 3-phosphate, are present in Saccharomyces
cerevisiae. J Biol Chem. 264:20181–20184. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhao JJ: Targeting breast cancers
featuring activating mutations in PIK3CA by generating a lethal
dose of PIP3. Annual summary rept. 1 Feb 2007-31 Jan 2008. Defense
Technical Information Center, Fort Belvoir, VA. 2008.https://apps.dtic.mil/sti/citations/ADA485990February
1–2008 View Article : Google Scholar
|
|
39
|
Orloff MS, He X, Peterson C, Chen F, Chen
JL, Mester JL and Eng C: Germline PIK3CA and AKT1 mutations in
Cowden and Cowden-like syndromes. Am J Hum Genet. 92:76–80. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ali T, Badshah H, Kim TH and Kim MO:
Melatonin attenuates D-galactose-induced memory impairment,
neuroinflammation and neurodegeneration via RAGE/NF-K B/JNK
signaling pathway in aging mouse model. J Pineal Res. 58:71–85.
2015. View Article : Google Scholar : PubMed/NCBI
|