Introduction
Head and neck cancers occur in the oral cavity,
larynx, pharynx and nasal cavity. With ~600,000 new patients
annually, head and neck cancers rank seventh in the global
incidence rate of malignant tumors. However, this cancer type is
difficult to detect in the early stages; thus, patients are often
diagnosed in the late stage. The 5-year overall survival rate is
54.7–65.9%, which has not significantly improved over the years
(1). Furthermore, head and neck
cancer surgery is relatively time consuming and difficult.
Therefore, performing multiple cancer surgeries in a day is
difficult. Consequently, often surgery cannot be performed early,
even after diagnosis. Notably, when surgeons perform an operation
more than a month after the final diagnosis, they often experience
a considerably more advanced lesion than at the time of diagnosis,
especially in patients with advanced head and neck cancer (2).
Meanwhile, β-blockers have been widely used for the
treatment of cardiovascular diseases or anxiety disorders. They may
also exert cancer-treating effects (3–8).
β-blockers inhibit the sympathetic actions of stress-related
catecholamine hormones, such as norepinephrine (NE) (9). This sympathetic mechanism occurs via
β-adrenergic receptors (β-AR), reflecting that β-AR is abundant in
the cancer tissues (9,10). We hypothesized that β-blockers can
slow tumor progression prior to head and neck cancer surgery, which
would confer a great clinical benefit. Additionally, after being
diagnosed with head and neck cancer, patient anxiety is often
amplified. Thus, β-blockers could also help the patients
psychologically before surgery.
According to some experimental evidence, various
cancer cells express β2-ARs, and NE may influence carcinogenesis
through these receptors (8,11). During acute or chronic stress, the
sympathetic adrenal medullary axis is activated, resulting in NE
synthesis. Via β-ARs, NE stimulates cancer growth by upregulating
proangiogenic factors, such as vascular endothelial growth factor
(VEGF) (7). Furthermore,
prostaglandin E2 synthesis is also augmented because of sustained
adrenergic signaling and NE-mediated phosphatase 1 overexpression
(12). However, evidence from
epidemiological and clinical studies remains inconclusive. In a
recent systematic review study about the effect of β-blockers on
cancer recurrence and survival, melanoma was associated with
reduced recurrence [hazard ratio (HR), 0.03; 95% confidence
interval (CI) 0.01–0.17] and increased survival (HR, 0.04; 95% CI,
0.00–0.38). However, endometrial cancer showed increased recurrence
(HR, 1.40; 95% CI, 1.10–1.80) as well as reduced survival (HR,
1.50; 95% CI, 1.12–2.00). In another study, non-selective β-blocker
was associated with improved recurrence and survival in melanoma
and ovarian cancer, but reduced survival in lung cancer (6).
Nevertheless, the effect of β-blockers on head and
neck cancer has remained poorly investigated. A national cohort
study in Taiwan demonstrated that β-blockers could reduce cancer
development risk in head and neck cancer (HR, 0.58, 95% CI,
0.35–0.95) (13). Conversely, a
study on a Korean population observed that β-blocker administration
was associated with decreased survival in head and neck cancer
(14). The majority of research has
focused on assessing the association between NE and β-blockers in
oral cavity cancers (11,15–20).
Other than in oral cavity cancer, only one other study regarding
nasopharyngeal cancer was identified (21). Therefore, the effect of NE and
β-blocker needs to be examined in various head and neck cancers,
including laryngopharyngeal cancer.
Materials and methods
β2-adrenergic receptor expression in
head and neck cancer specimens
In total, 57 head and neck cancer tissue specimens
were obtained from surgically diagnosed patients (median age, 65
years; age range, 33–89 years) by the Department of Pathology in
CHA Bundang Medical Center between January 2009 and December 2018.
The institutional review board of the CHA Medical Center reviewed
and approved our study (IRB approval no. NON2020-003-002). Clinical
staging was based on the TNM staging system of the Union for
International Cancer Control, 8th edition (22). The specimens were fixed in 10% neural
buffered formalin for 24 h at 4°C and embedded in paraffin. Tissue
blocks were divided into 3-µm sections for hematoxylin and eosin
staining and immunohistochemistry of β2-AR. All cases were
diagnosed as squamous cell carcinoma. Patients with neuroendocrine
cancer, sarcoma or preoperative radiotherapy/chemotherapy were
excluded. Tissue microarray sections were dewaxed in xylene,
rehydrated in alcohol and immersed in 3% hydrogen peroxide for 40
min at room temperature. Antigens were retrieved by heating (100°C)
each section in 10 mmol/l of sodium citrate buffer for 10 min.
After being rinsed thrice in phosphate-buffered saline (PBS), each
section was incubated with rabbit polyclonal antibodies to β2-AR
(cat. no. ab61778, 1:100; Abcam) for 18 h at 4°C. Then, they were
diluted and washed thrice in PBS and incubated with horseradish
peroxidase (HRP)-labeled rabbit anti-mouse immunoglobulin for 30
min at room temperature. Subsequently, samples were washed thrice
and stained with diaminobenzidine solution for 5 min at room
temperature to visualize peroxidase activity. For negative
controls, primary antibodies were replaced with PBS. For the
positive control, normal upper eyelid muscles for 3 patients
underwent upper eyelid surgery for ptosis correction were used.
They were 3 females (median age, 58; range, 51–69). The tissue
blocks were stored by the Department of Pathology in CHA Bundang
Medical Center for other research purposes after IRB approval. The
expression of β2-AR protein was assessed by cytoplasmic staining of
the cancer cells. To stratify β2-AR expression, the immunoreactive
score (IRS) system was used because of marked intratumoral
heterogeneous expression in proportion and intensity in cancer
tissues. The IRS system is calculated by multiplying the percentage
of positive cells (0, no positive cells; 1, <10% of positive
cells; 2, 10–50%; 3, 51–80%; 4, >80%) and the intensity of the
staining (0, no color reaction; 1, mild reaction; 2, moderate
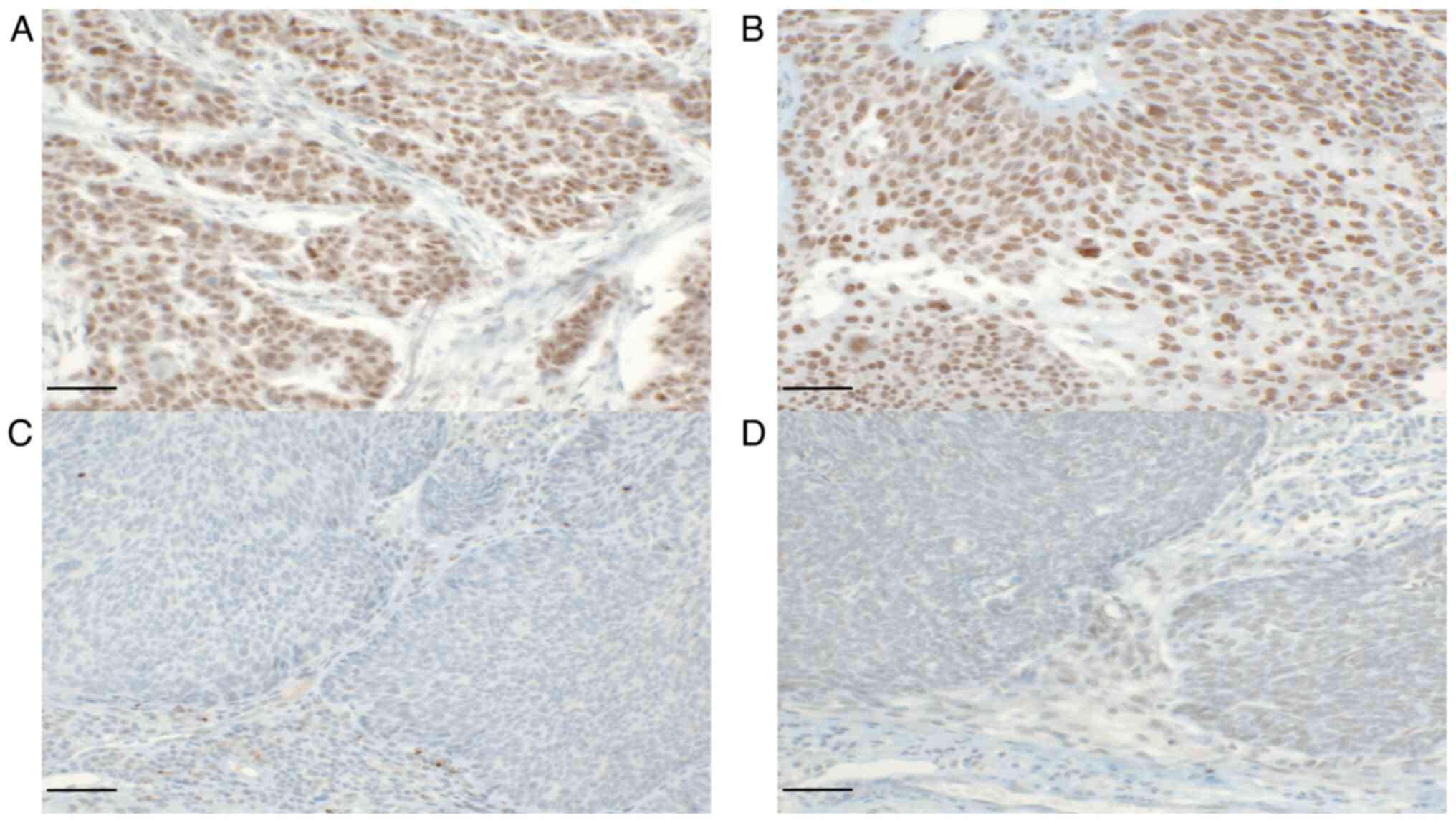
reaction; 3, intense reaction). Thus, the IRS result is between 0
(no staining) and 12 (maximum staining), categorized into negative
(0–1), mild (2–3),
moderate (4–8), and strongly positive (9–12,23). A
light microscope (Olympus System Microscope Model BX43;
magnification, ×200 and ×400) was used to obtain images. The
intensity of the staining was determined by using QuPath version
2.0 freeware (24). It was measured
as the optical density (OD). The staining vectors for all images
were estimated automatically before OD measurement. The samples
with OD <0.2 were considered negative (score, 0), OD ranking
0.2–0.4 was considered mild (score, 1), OD ranking 0.4–0.6 was
considered moderate and OD >0.6 was considered intense (score,
3).
Human papilloma virus (HPV) in head
and neck cancer specimens
DNA was extracted from the specimens using a DNA
isolation kit (ISOLATE II Genomic DNA kit; Meridian Bioscience,
Inc.). HPV 16/18- deoxyribonucleic acid (DNA) was amplified by
polymerase chain reaction (PCR) and nested PCR assays using two
pairs of primers to identify HPV16/18 and the HPV16/18 regions of
the L1 gene and DNA polymerase (DreamTaq Green; Thermo Fisher
Scientific, Inc.). The PCR primer sequences used for each gene were
as follows: HPV 16 forward, 5′-ACCCAGTATAGCTGACAGT-3′ and reverse,
5′-CTCGTTTATAATGTCTACACA-3′; and HPV 18 forward,
5′-ATAGCAATTTTGATTTGTC-3′ and reverse, 5′-AAACTCATTCCAAAATATG-3′.
During the first PCR, the amplification process included 35 cycles
with pre-denaturation at 95°C for 4 min, denaturation at 95°C for
30 sec, annealing at 55°C for 30 sec, first elongation at 72°C for
1 min and 30 sec and additional elongation at 75°C for 10 min.
Using the same reagents, the second PCR was performed on the first
PCR aliquots. Nested PCR results were analyzed by 2% agarose gel
electrophoresis. During the second PCR, the amplification process
was conducted with initial denaturation at 95°C for 4 min,
denaturation at 94°C for 30 sec, annealing at 56.4°C for 30 sec,
first elongation at 75°C for 1 min and 30 sec and additional
elongation at 72°C for 10 min. DNA visualization was conducted
using machine electrophoresis on the Gel Doc EZ System (Bio-Rad
Laboratories, Inc.).
Cell lines and culture conditions
The following four different head and neck cancer
cell lines (HNCCLs) were investigated: oral cavity (YD-10B,
tongue), larynx (SNU-1066, glottis), pharynx (SNU-1041) and nasal
cavity (RPMI-2650) cancers. They were purchased from the Korean
Cell Line Bank and were maintained in a mixture of Dulbecco's
modified Eagle's medium (DMEM; Thermo Fisher Scientific, Inc.) and
Ham's nutrient mixture F12 (Thermo Fisher Scientific, Inc.) at a
3:1 ratio. They were supplemented with 10% fetal bovine serum (FBS;
Thermo Fisher Scientific, Inc.) and 50 U/ml concentration of
penicillin at 37°C in 5% CO2.
Reverse transcription-quantitative
(RT-q) PCR
Total RNA was isolated using TRIzol reagent (Ambion;
Thermo Fisher Scientific, Inc.). For reverse transcription, the
AccuPower® RT premix cDNA synthesis kit was used
according to the manufacturer's instructions (Bioneer Corporation).
For RT-PCR, 1 µg cDNA products were amplified using SYBR Green
Supermix (Bio-Rad Laboratories, Inc.). For SYBR Green assays, β2-AR
and RPS18 primers (for 18S rRNA) were used. The PCR primer
sequences used for each gene were as follows: β2-AR forward,
5′-TTAGCCAGGTGGAGCAGGATG-3′ and reverse,
5′-GCCTAACGTCTTGAGGGCTTTG-3′; and RPS18 forward,
5′-GCAGAATCCACGCCAGTACAAG-3′ and reverse,
5′-GCTTGTTGTCCAGACCATTGGC-3′. Fluorescence was detected with the
CFX Connect™ Real-Time PCR Detection System (Bio-Rad Laboratories,
Inc.). All values were standardized using the 2−∆ΔCq
method (25). Moreover, the level of
β2-AR messenger RNA (mRNA) expression was normalized to 18S rRNA.
PCR conditions were: Initial activation at 95°C for 5 min,
denaturation at 94°C for 40 sec, annealing at 60°C for 30 sec, 35
cycles of extension at 72°C for 60 sec and final elongation at 72°C
for 8 min.
Protein extraction and western blot
analysis
Cell lysates were isolated by adding PRO-PREP™
protein extraction solution (Intron Biotechnology, Inc.) containing
various protease inhibitors, such as phenylmethylsulfonyl fluoride,
ethylenediamine tetraacetic acid, pepstatin A, leupeptin and
aprotinin. Total protein concentration was determined by using a
Pierce BCA Protein Assay kit (Thermo Fisher Scientific, Inc.).
Furthermore, 40 µg of protein/lane was resolved using 10% sodium
dodecyl sulfate-poly acrylamide gel electrophoresis and then
transferred to a nitrocellulose membrane. The nitrocellulose
membranes were blocked in 5% milk/Tris-buffered saline with 0.1%
Tween-20 (TBS-T) at 25°C for 1 h and then incubated with β2-AR
primary antibodies overnight at 4°C. The primary antibody was the
anti-β2-AR rabbit monoclonal antibody (cat. no. ab182136; Abcam) at
a 1:1,000 dilution. After being washed in TBS-T buffer, the
membrane was incubated with HRP-conjugated secondary antibodies for
1 h. The secondary antibody was goat anti-rabbit IgG (cat. no.
ab97051; Abcam) at 1:20,000 dilution. The internal control used was
β-actin. The primary and secondary antibody used for β-actin were
as follows: anti-β-actin mouse antibody (cat. no. sc-47778; Santa
Cruz Biotechnology, Inc.), goat anti-mouse IgG antibody conjugated
to HRP (cat. no. GTX213111-01; GeneTex, Inc.). The immunoreactive
protein bands were visualized with a chemiluminesence solution
enhanced with the LimiFlash™ Ultima Chemiluminescent substrate, HRP
System (Energenesis Biomedical Co., Ltd.). ImageJ version 1.51
software (National Institutes of Health) was used for
densitometry.
Cell viability and proliferation
assays
HNCCLs were seeded in 96-well plates, with
1.5×104 cells (YD-10B), 2.5×104 cells
(SNU-1066) and 2×104 cells (SNU-1041 and RPMI-2650) per
well. They were cultured in RPMI-1640 (Welgene, Inc.) supplemented
with 0.1% FBS and then treated with 0, 1, 5 or 10 µM concentrations
of NE (MilliporeSigma) and 1 µM of propranolol (MilliporeSigma).
For blocking, 1 µM propranolol was added to the HNCCLs 1 h before
adding 10 µM of NE. The treated HNCCLs were then incubated at 37°C
for 24 h. The concentrations of NE and propranolol were selected
based on references describing similar protocols (18,21,26,27).
Furthermore, cell viability and proliferation were evaluated using
the enhanced cell viability assay EZ-CYTOX (DoGen Bio Co., Ltd.)
and the Cytoselect™ BrdU cell proliferation enzyme-linked
immunosorbent assay kits (cat. no. CBA-251; Cell Biolabs, Inc.).
Each experiment was performed thrice independently. The results are
expressed as the mean ± standard error of the mean of three
replicates.
Statistical analysis
Among the variables, significantly associated
clinical factors were identified using the Mann-Whitney U test,
Kruskal-Wallis test and Bonferroni's method. Significant changes in
cell viability and proliferation assays were assessed by
Kruskal-Wallis along with a post hoc test (Mann-Whitney U test with
Bonferroni's method). P<0.05 was considered to indicate a
statistically significant difference. All statistical data were
analyzed using the SPSS version 22.0 software (IBM, Corp.).
Results
Expression of β2-AR in head and neck
cancer specimens and clinical factors
Table I summarizes
the demographics of 57 patients with head and neck cancer. All
patients displayed β2-AR expression, except for two patients with
pharyngeal cancer (55/57; 96.5%). The mean IRS of β2-AR expression
was moderately positive (mean ± standard deviation, 6.63±3.30),
with no significant differences in relation to sex, T stage, N
stage, HPV status, and overall stages. However, it was
significantly associated head and neck cancer subsites (P=0.031;
Table I). In the post-hoc analysis,
oral cavity cancer (P=0.023) had a significantly higher IRS of
β2-AR expression than pharyngeal cancer (Table II and Fig. 1).
 | Table I.Demographics of patients with head and
neck cancer (n=57) and analysis of immunoreactive scores in
immunohistochemistry. |
Table I.
Demographics of patients with head and
neck cancer (n=57) and analysis of immunoreactive scores in
immunohistochemistry.
| Variable | Value | IRS, median
(Q1-Q3) | P-value |
|---|
| Median age, years
(Q1-Q3) | 65 (58–75) | 9 (0–12) |
|
| Sex |
|
| 0.250 |
| Male | 52 (91.2%) | 9 (4–9) |
|
|
Female | 5
(8.8%) | 4 (3–6) |
|
| Subsite |
|
| 0.031 |
| Oral
cavity | 22 (38.6%) | 9 (6–9) |
|
|
Larynx | 16 (28.1%) | 9 (4–9) |
|
|
Pharynx | 11 (19.3%) | 3 (2–8) |
|
| Nasal cavity | 8
(14.0%) | 9 (6–9) |
|
| T stage |
|
| 0.562 |
| T1 | 25 (43.9%) | 9 (3–9) |
|
| T2 | 15 (26.3%) | 8 (4–9) |
|
| T3 | 6
(10.5%) | 6 (3–6) |
|
| T4 | 11 (19.3%) | 9 (4–9) |
|
| N stage |
|
| 0.374 |
| N0 | 34 (59.6%) | 9 (4–9) |
|
| N1 | 6
(10.5%) | 3 (2–9) |
|
| N2 | 17 (39.8%) | 9 (4–9) |
|
| HPV status |
|
| 0.186 |
|
Positive | 13 (22.8%) | 6 (3–9) |
|
|
Negative | 44 (77.2%) | 9 (4–9) |
|
| Stage |
|
| 0.533 |
| I | 22 (38.6%) | 9 (4–9) |
|
| II | 6
(10.5%) | 9 (6–9) |
|
|
III | 7
(12.3%) | 6 (2–9) |
|
| IV | 22 (38.6%) | 9 (4–9) |
|
 | Table II.Demographics of patients with head
and neck cancer (n=57) and analysis of immunoreactive scores in
immunohistochemistry. |
Table II.
Demographics of patients with head
and neck cancer (n=57) and analysis of immunoreactive scores in
immunohistochemistry.
| Variable (median,
Q1-Q3) | Pairwise variable
(median, Q1-Q3) | P-value |
|---|
| Oral cavity (9,
6–9) | Larynx (9,
4–9) | 0.990 |
|
| Pharynx (3,
2–8) | 0.023 |
|
| Nasal cavity (9,
6–9) | 0.904 |
| Larynx (9,
4–9) | Pharynx (3,
2–8) | 0.066 |
|
| Nasal cavity (9,
6–9) | 0.975 |
| Pharynx (3,
2–8) | Nasal cavity (9,
6–9) | 0.309 |
Expression of β2-AR at the mRNA and
protein levels in HNCCLs
All HNCCLs showed the mRNA and protein expression of
β2-AR. Especially, mRNA expression of β2-AR was high in an oral
cavity cancer cell line (Fig. 2A).
In western blot analysis, its protein expression was also strong in
the same cell line (Fig. 2B and
C).
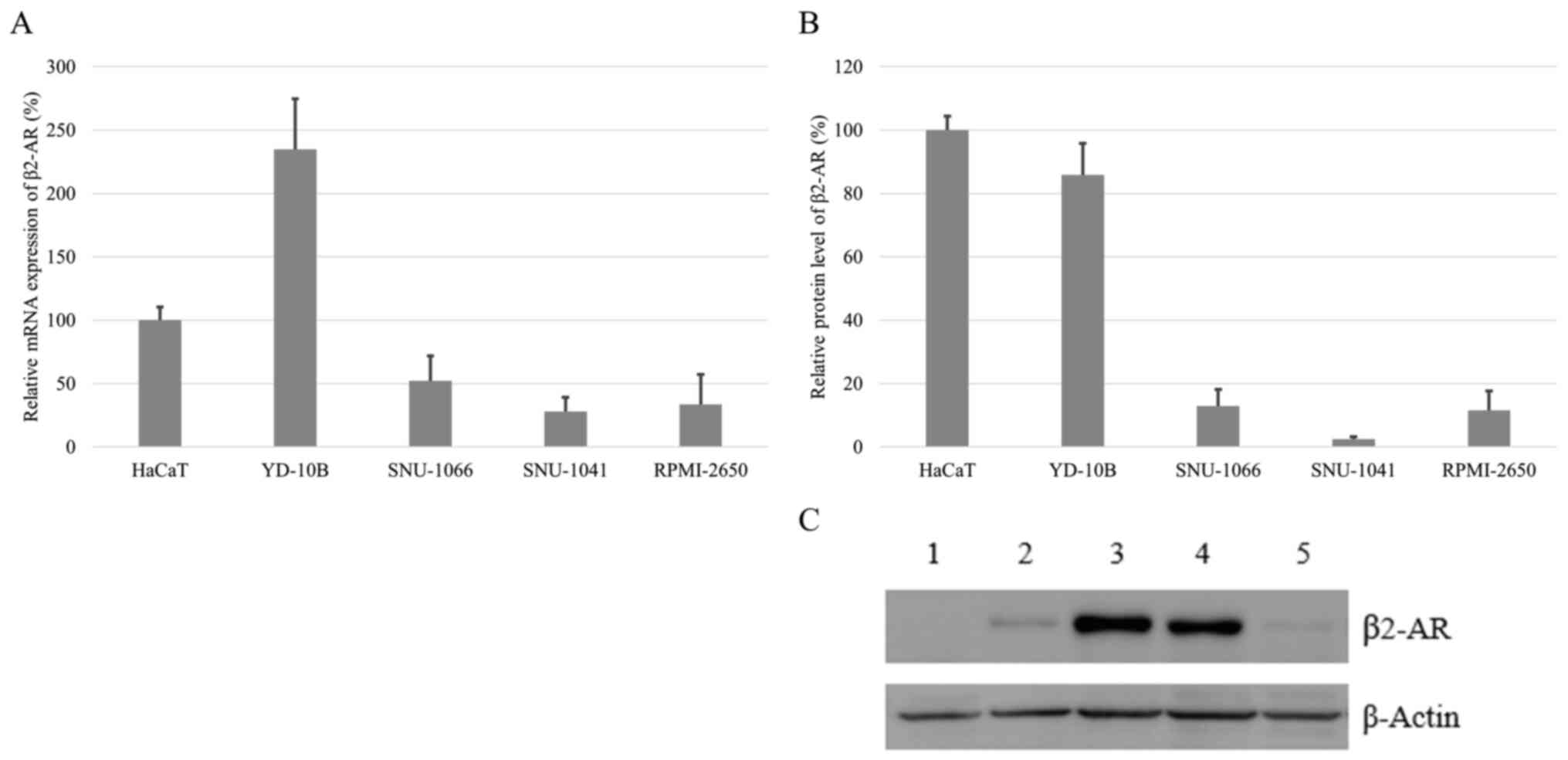 | Figure 2.β2-adrenergic receptor expression in a
normal head and neck cell line, and HNCCLs. (A) Expression of β2-AR
mRNA in HNCCLs relative to a normal head and neck cell line (HaCaT)
assessed via reverse transcription-quantitative PCR. Data are
presented mean ± SD of three different experiments. (B) Expression
of β2-AR protein in the HNCCLs relative to normal head and neck
cell line (HaCaT). Data are presented mean ± SD of three different
experiments. (C) Western blot analysis of β2-AR in the HNCCLs. For
the western blot analysis, β2-AR immunoreactivity was visualized as
a single band that migrated at −47 kDa. Lane 1, pharynx cancer cell
line, SNU-1041; lane 2, larynx cancer cell line, SNU-1066; lane 3,
normal keratinocyte cell line, HaCaT; lane 4, oral cavity cancer
cell line, YD-10; and lane 5, nasal cavity cancer cell line,
RPMI-2650; HNCCLs, head and neck cancer cell lines. |
Cell viability and proliferation of
HNCCLs by NE and propranolol
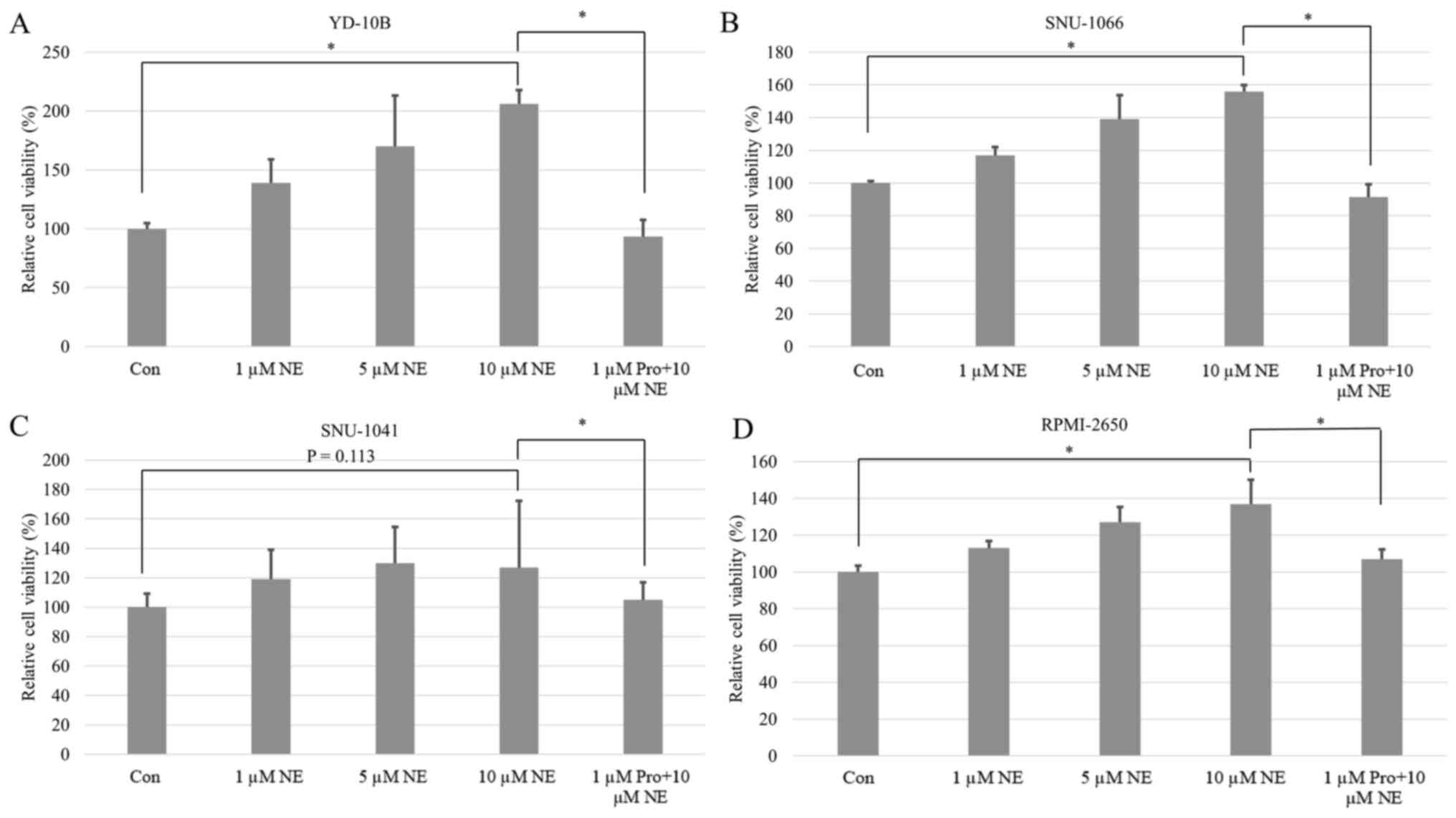
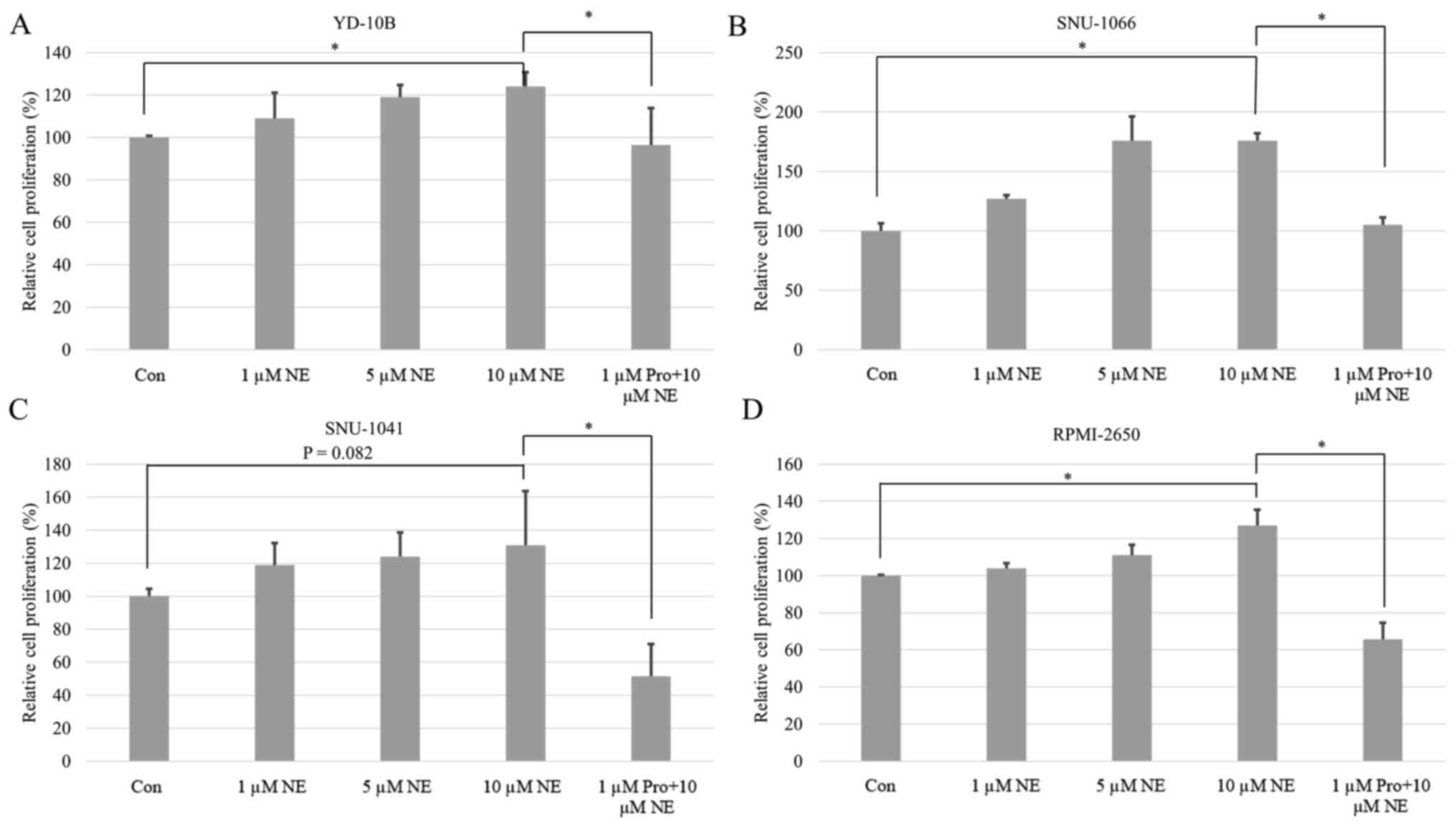
The viability and proliferation of all cancer cells
were induced by NE in a dose-dependent manner. These differences
were all statistically significant except for in pharyngeal cancer
(Figs. 3 and 4). Following post hoc analysis, the cell
viability and proliferation in the 10 µM of NE group were
significantly higher compared with the 0 µM of NE group. The
amplified cell viability and proliferation were significantly
inhibited by propranolol treatment in all cancer types (Figs. 3 and 4).
Discussion
To the best of the authors' knowledge the present
study was the first to investigate and compare β2-AR expression in
various head and neck cancers. Most head and neck cancer tissues
expressed β2-AR moderately. In addition, mRNA and protein
expression levels of β2-AR were detected in all HNCCLs. Notably,
β2-AR expression was higher in oral cavity cancer compared with in
other locations. This may be associated with minor salivary glands
in the oral cavity, as the autonomic nervous system controls
salivation (17). The sympathetic
neurotransmitter NE acts via β-ARs in the salivary gland. The oral
cavity tissue has more minor salivary glands than any other head
and neck subsites. Therefore, it may be the case that there was
higher β2-AR expression in oral cavity tissue compared with other
subsites. Certain studies have explored the role of β2-ARs in head
and neck cancer. Bernabé et al (16) demonstrated that β2-AR mRNAs were
expressed in all 20 oral cavity cancer specimens examined.
Furthermore, Bravo-Calderón et al (17) determined that most of their patients
(77/106, 72.6%) with oral cavity cancer had high expression levels
of β2-AR. Shang et al (11)
also reported that 44/65 (67.7%) oral cavity cancer cases had β2-AR
expression. However, most of the previous studies regarding β-AR in
head and neck cancer tissues only focused on oral cavity cancer. To
the best of our knowledge, the present study was the first to
investigate β2-AR expression in various head and neck cancer tissue
types. The current results revealed that, compared with oral cavity
cancer, pharyngeal cancer had a relatively low expression level of
β2-AR and exhibited no significant difference in the cell viability
and proliferation after NE and β-blocker application. This finding
is likely due to the presence of different β-ARs. Most head and
neck cancer studies have focused on β2-AR; however, the expression
of β1- and β3-AR in head and neck cancer tissues should also been
investigated. There are two main groups of AR; α and β. In humans,
β-ARs can be subdivided into β1, β2 and β3. β1-ARs are
predominantly expressed in the heart, and these account for 70–80%
of the total. The β2-ARs are primarily located in lung bronchioles
and the arteries of skeletal muscles. Meanwhile, β3-AR is primarily
located in the adipose tissue and participates in regulating
lipolysis and thermogenesis (28).
The present results further revealed that NE
stimulates the viability and proliferation of the head and neck
cancer cells in a dose-dependent manner. NE is the neurotransmitter
released during the stress response (7,18,26,29).
According to an animal study using a mouse model, chronic stress
promoted oral cavity cancer growth, with increased epinephrine and
NE levels (19). In addition, Bastos
et al (30) demonstrated that
NE levels were significantly associated with the severity of
anxiety in 93 patients with head and neck cancer. Thus, stress may
influence various head and neck cancers.
In the present study, propranolol attenuated the
viability and proliferation of head and neck cancer cells. Shang
et al (11) observed that
propranolol fully inhibited NE-induced mitogen effect. According to
the study by Bernabé et al (16) study, NE significantly enhanced cell
proliferation and interleukin (IL)-6 expression in oral cavity
cancer cell lines (SCC9 and SCC25); however, these effects were
inhibited by propranolol, supporting the role of β-ARs in IL-6
secretion. In a study by Zhang et al (3) tumor size was significantly different
between mice with tongue cancer administered with a selective β2-AR
blocker and the control group; the β2-AR blocker group also had a
significantly prolonged survival rate.
In addition to the potential benefits of β-blockers
in cancer treatment, they are considered safe and cost-effective
and are already used to treat various other diseases. Numerous
patients with head and neck cancer have accompanying cardiovascular
diseases and complain of stress and anxiety until they are treated.
Thus, after final diagnosis, β-blockers may be administered up to
the time of surgery. This may reduce preoperative anxiety and
stress as well as alleviate the aggravation of head and neck cancer
caused by psychological factors. Additionally, β-blockers may be
used as an adjunct to radiotherapy or chemotherapy after surgery in
various head and neck cancers.
However, the present study had several limitations.
Firstly, during the study planning, evaluation of the expression
levels of β1- and β3-AR was not considered. Moreover, the
expression level of β2-AR in normal head and neck tissue was not
examined. Further research should aim to investigate the expression
of all β-AR subtypes in normal and cancer cell lines and tissues.
Secondly, the selective stimulation of β1- and β2-ARs in cancer
cell lines, other than NE, were not assessed. Thus, future studies
should also assess selective stimulations of β1- and β2-AR.
Thirdly, the present study did not evaluate other types of
β-blockers. Propranolol is a non-selective β-blocker. The effects
of β1- and β2-selective blockers other than propranolol should also
be studied in the future. Fourthly, numerous cell lines were not
studied. Only one cell line per anatomic location may be
insufficient. Fifth, different approaches for elucidating the
related signal pathways must be explored. Sixth, our hypothesis
should also be confirmed using animal models. Finally, although we
performed the cell membrane invasion assay, we did not include the
data in the result. The invasion of all cancer cells were induced
by NE dose-dependently. However, only 1 µM of propranolol did not
attenuate the invasion ability in any HNCCLs (Fig. S1,Fig.
S2,Fig. S3,Fig. S4,Fig.
S5). If we were able to test the propranolol of different
concentrations, we might have had different results.
Despite such limitations, the present study
indicated that the stress-related neurotransmitter NE may directly
stimulate various head and neck cancers, while β-blocker treatment
may attenuate tumor proliferation in various head and neck
cancers.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Research Foundation of Korea (grant no. NRF-2017R1C1B1008842).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
Conceptualization: HGC and SYK. Data curation: KJC,
HKK, NJ, and HAS. Formal analysis: MSK. Funding acquisition: MSK.
Methodology: JJYJ, KHO, and MSK. Figures: NJ and HAS. Writing -
original draft: HAS and MSK. Writing - review and editing: JYJ and
MSK. HAS and MSK confirm the authenticity of all the raw data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All procedures performed in the present were in
accordance with the ethical standards of the institutional research
committee and with the 1964 Helsinki declaration and its later
amendments or comparable ethical standards. The present study was
reviewed and approved by the institutional review board of the CHA
Medical Center (IRB no. NON2020-003-002). The need for informed
consent was waived for both the use of tissues and medical records
because it was practically impossible to obtain consent from the
patients retrospectively and the risk to the patients was extremely
low, even if they were exempted from consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li P, Zhu K, Mo Y, Deng X, Jiang X, Shi L,
Guo C, Zhang W, Zeng Z, Li G, et al: Research Progress of circRNAs
in Head and Neck Cancers. Front Oncol. 11:6162022021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mehanna H, Hardman JC, Shenson JA,
Abou-Foul AK, Topf MC, AlFalasi M, Chan JYK, Chaturvedi P, Chow
VLY, Dietz A, et al: Recommendations for head and neck surgical
oncology practice in a setting of acute severe resource constraint
during the COVID-19 pandemic: An international consensus. Lancet
Oncol. 21:e350–e359. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang C, Liao X, Ma Z, Liu S, Fang F and
Mai H: Overexpression of β-Adrenergic Receptors and the Suppressive
Effect of β(2)-Adrenergic Receptor Blockade in Oral Squamous Cell
Carcinoma. J Oral Maxillofac Surg. 78:1871.e1871–1871.e1823. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Haldar R, Ricon-Becker I, Radin A, Gutman
M, Cole SW, Zmora O and Ben-Eliyahu S: Perioperative COX2 and
β-adrenergic blockade improves biomarkers of tumor metastasis,
immunity, and inflammation in colorectal cancer: A randomized
controlled trial. Cancer. 126:3991–4001. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chaudhary KR, Yan SX, Heilbroner SP,
Sonett JR, Stoopler MB, Shu C, Halmos B, Wang TJC, Hei TK and Cheng
SK: Effects of β-Adrenergic Antagonists on Chemoradiation Therapy
for Locally Advanced Non-Small Cell Lung Cancer. J Clin Med.
8:5752019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yap A, Lopez-Olivo MA, Dubowitz J, Pratt
G, Hiller J, Gottumukkala V, Sloan E, Riedel B and Schier R: Effect
of beta-blockers on cancer recurrence and survival: A meta-analysis
of epidemiological and perioperative studies. Br J Anaesth.
121:45–57. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Weberpals J, Jansen L, Carr PR,
Hoffmeister M and Brenner H: Beta blockers and cancer prognosis -
The role of immortal time bias: A systematic review and
meta-analysis. Cancer Treat Rev. 47:1–11. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wolter NE, Wolter JK, Enepekides DJ and
Irwin MS: Propranolol as a novel adjunctive treatment for head and
neck squamous cell carcinoma. J Otolaryngol Head Neck Surg.
41:334–344. 2012.PubMed/NCBI
|
|
9
|
Silverman DA, Martinez VK, Dougherty PM,
Myers JN, Calin GA and Amit M: Cancer-Associated Neurogenesis and
Nerve-Cancer Cross-talk. Cancer Res. 81:1431–1440. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang B, Wu C, Chen W, Qiu L, Li S, Wang
T, Xie H, Li Y, Li C and Li L: The stress hormone norepinephrine
promotes tumor progression through β2-adrenoreceptors in oral
cancer. Arch Oral Biol. 113:1047122020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shang ZJ, Liu K and Liang DF: Expression
of beta2-adrenergic receptor in oral squamous cell carcinoma. J
Oral Pathol Med. 38:371–376. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schallreuter KU, Lemke KR, Pittelkow MR,
Wood JM, Körner C and Malik R: Catecholamines in human keratinocyte
differentiation. J Invest Dermatol. 104:953–957. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chang PY, Huang WY, Lin CL, Huang TC, Wu
YY, Chen JH and Kao CH: Propranolol Reduces Cancer Risk: A
Population-Based Cohort Study. Medicine (Baltimore). 94:e10972015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim SA, Moon H, Roh JL, Kim SB, Choi SH,
Nam SY and Kim SY: Postdiagnostic use of β-blockers and other
antihypertensive drugs and the risk of recurrence and mortality in
head and neck cancer patients: An observational study of 10,414
person-years of follow-up. Clin Transl Oncol. 19:826–833. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sugiura T, Inoue Y, Matsuki R, Ishii K,
Takahashi M, Abe M and Shirasuna K: VEGF-C and VEGF-D expression is
correlated with lymphatic vessel density and lymph node metastasis
in oral squamous cell carcinoma: Implications for use as a
prognostic marker. Int J Oncol. 34:673–680. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bernabé DG, Tamae AC, Biasoli ÉR and
Oliveira SH: Stress hormones increase cell proliferation and
regulates interleukin-6 secretion in human oral squamous cell
carcinoma cells. Brain Behav Immun. 25:574–583. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bravo-Calderón DM, Oliveira DT, Marana AN,
Nonogaki S, Carvalho AL and Kowalski LP: Prognostic significance of
beta-2 adrenergic receptor in oral squamous cell carcinoma. Cancer
Biomark. 10.51–59. 2011-2012.
|
|
18
|
Vilardi BM, Bravo-Calderón DM, Bernabé DG,
Oliveira SH and Oliveira DT: VEGF-C expression in oral cancer by
neurotransmitter-induced activation of beta-adrenergic receptors.
Tumour Biol. 34:139–143. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xie H, Li C, He Y, Griffin R, Ye Q and Li
L: Chronic stress promotes oral cancer growth and angiogenesis with
increased circulating catecholamine and glucocorticoid levels in a
mouse model. Oral Oncol. 51:991–997. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bravo-Calderón DM, Assao A, Garcia NG,
Coutinho-Camillo CM, Roffé M, Germano JN and Oliveira DT: Beta
adrenergic receptor activation inhibits oral cancer migration and
invasiveness. Arch Oral Biol. 118:1048652020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang EV, Sood AK, Chen M, Li Y, Eubank TD,
Marsh CB, Jewell S, Flavahan NA, Morrison C, Yeh PE, et al:
Norepinephrine up-regulates the expression of vascular endothelial
growth factor, matrix metalloproteinase (MMP)-2, and MMP-9 in
nasopharyngeal carcinoma tumor cells. Cancer Res. 66:10357–10364.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Doescher J, Veit JA and Hoffmann TK: The
8th edition of the AJCC Cancer Staging Manual: Updates in
otorhinolaryngology, head and neck surgery. HNO. 65:956–961.
2017.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang B, Gao J, Zhang Q, Fu Y, Liu G, Shi
J, Li D, Wang F and Guo H: Diagnostic Value of 68Ga-PSMA PET/CT for
Detection of Phosphatase and Tensin Homolog Expression in Prostate
Cancer: A Pilot Study. J Nucl Med. 61:873–880. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bankhead P, Loughrey MB, Fernández JA,
Dombrowski Y, McArt DG, Dunne PD, McQuaid S, Gray RT, Murray LJ,
Coleman HG, et al: QuPath: Open source software for digital
pathology image analysis. Sci Rep. 7:168782017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guo K, Ma Q, Wang L, Hu H, Li J, Zhang D
and Zhang M: Norepinephrine-induced invasion by pancreatic cancer
cells is inhibited by propranolol. Oncol Rep. 22:825–830.
2009.PubMed/NCBI
|
|
27
|
Yang EV, Kim SJ, Donovan EL, Chen M, Gross
AC, Webster Marketon JI, Barsky SH and Glaser R: Norepinephrine
upregulates VEGF, IL-8, and IL-6 expression in human melanoma tumor
cell lines: Implications for stress-related enhancement of tumor
progression. Brain Behav Immun. 23:267–275. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Motiejunaite J, Amar L and Vidal-Petiot E:
Adrenergic receptors and cardiovascular effects of catecholamines.
Ann Endocrinol (Paris). 82:193–197. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Patel PR, Hegde ML, Theruvathu J, Mitra
SA, Boldogh I and Sowers L: Norepinephrine Reduces Reactive Oxygen
Species (ROS) and DNA Damage in Ovarian Surface Epithelial Cells. J
Bioanal Biomed. 7:75–80. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bastos DB, Sarafim-Silva BAM, Sundefeld
MLMM, Ribeiro AA, Brandão JDP, Biasoli ÉR, Miyahara GI, Casarini DE
and Bernabé DG: Circulating catecholamines are associated with
biobehavioral factors and anxiety symptoms in head and neck cancer
patients. PLoS One. 13:e02025152018. View Article : Google Scholar : PubMed/NCBI
|