Introduction
Colorectal cancer was the third-most common cancer worldwide in 2018 after lung and breast cancers, and was characterized by the second highest mortality rate after lung cancer (1). The liver is the most common site of synchronous distant metastasis of colorectal cancer, which appears in 10.9% of patients (2), and the liver is the most common site of recurrence of colorectal cancer, which is observed in 7.1% of patients (2).
The most common metastatic organ is the liver, as most of the intestinal mesenteric drainage spreads to the liver via the portal vein. Hepatectomy may therefore help achieving curative treatment of colorectal liver metastasis (2–5). Curative resection, which is recommended for liver metastases when the primary tumor and extrahepatic metastases have been controlled or can be controlled, has been reported to result in a better prognosis than other treatments (2,6,7).
Previous studies have demonstrated that the tumor microenvironment plays an important role in cancer progression. Tumor-infiltrating lymphocytes (TILs), which represent one type of immune cells in the tumor microenvironment, can reflect the anti-tumor immune status of the patients and are positively correlated with the prognosis of various types of cancer, including colorectal cancer (8–12). Furthermore, it has been reported that the degree of TIL infiltration is positively correlated with the therapeutic outcomes after chemotherapy and radiotherapy and might be considered as a useful predictive marker for treatment success (13–16). TILs may therefore serve a crucial role in cancer progression.
Because primary tumors have been the focus of most previous studies, little is known about the characteristics of the local immune status in metastatic tumors. However, the characteristics of local immunity in metastatic tumor have been gradually elucidated in the recent years. Lee et al (17) reported a positively correlation between the degree of TIL infiltration in primary tumor of colorectal cancer and that in liver and lung metastases. In another study, the activation state of local immunity, including activation marker and suppression marker, in colorectal cancer liver metastases was demonstrated to be similar to that of the primary lesion resected at the same time (18). Furthermore, it has been shown that TILs in liver metastases are positively correlated with tumor doubling time (19). However, the relationship between the local immune status in colorectal cancer liver metastases and patient prognosis remains unclear, although the characteristics of local immunity in colorectal cancer liver metastases have gradually been elucidated. In addition, chemotherapy, which is often given before resection of liver metastases, has been reported to potentially alter the local immune status (20).
The present study aimed to investigate the relationship between the degree of TIL infiltration and the prognosis in patients who underwent curative resection of colorectal cancer liver metastases and to examine the effects of preoperative chemotherapy on the function of immune cells.
Materials and methods
Patients
A total of 108 patients with colorectal cancer who underwent curative resection of colorectal cancer liver metastases at the department of Surgery, Osaka City University (Osaka, Japan) between May 1996 and January 2017 were enrolled in the present study. Cases with hepatic arterial infusion therapy as preoperative treatment before hepatectomy were excluded from this study. According to the definition of a multidisciplinary international consensus (21), liver metastases found at the time of the diagnosis were defined as synchronous, and those found after treatment of the primary lesion were defined as metachronous.
The patients were classified into three groups as follows: i) Patients who did not receive preoperative chemotherapy; ii) patients who received short-term preoperative chemotherapy for <6 months; and iii) patients who received long-term preoperative chemotherapy for ≥6 months.
The present study was performed in compliance with the principles expressed in the Declaration of Helsinki and was approved by the Ethics committee of Osaka City University (approval no. 3853). Written informed consent was obtained from all participants prior to the study.
Methods
Peripheral blood samples were obtained from patients within two weeks before surgery ans were used to calculate the absolute peripheral lymphocyte count. The differential white blood cell count was analyzed using an XE-5000 hematology analyzer (Sysmex Corporation) according to the manufacturer's protocol. CD3, a pan-T lymphocyte marker, and CD8, a cytotoxic T lymphocyte marker, which both serve key role in antitumor immunity, were used as targets for the evaluation of TIL infiltration. Immunohistochemistry staining was performed using surgically resected specimens of liver metastases as previously described (14). The outline of the evaluation method is as follows. All specimens treated with 10% formalin fixation for 24–48 h at room temperature and paraffin embedding were cut into 4-µm-thick sections to perform immunohistochemistry staining. Sections were deparaffined and rehydrated, and subjected to endogenous peroxidase blocking in 1% H2O2 solution in methanol for 15 min at room temperature. Antigen retrieval was carried out by autoclaving the sections at 105°C for 10 min in Dako Target Retrieval Solution (Dako; Agilent Technologies, Inc.). Serum blocking was performed with antibody in 10% normal rabbit serum (Histofine SAB-PO (M) kit; cat. no. 424022; Nichirei Biosciences) for 10 min at room temperature. After H2O2 and serum blocking, the slides were incubated with the primary monoclonal mouse anti-human antibodies anti-CD3 (1:400; clone F7.2.38; Dako; Agilent Technologies, Inc.) and anti-CD8 (1:200; clone C8/144B; Dako; Agilent Technologies, Inc.) at 4°C overnight. The secondary antibody was biotin-labeled rabbit anti-mouse IgG + IgA + IgM antibody (1:500; Histofine SAB-PO (M) kit; cat. no. 424022; Nichirei Biosciences). Signal detection was performed with a DAB kit (Histofine simple stain kit; Nichirei Biosciences). The sections were counterstained with hematoxylin. TILs in the inner part of the tumor margin of liver metastases were observed with an optical microscope, and the number of TILs was counted in a randomly selected field at a magnification of ×400. The mean values obtained in five different areas were used for the data analysis. Examples of CD8+TILs and CD3+TILs are presented in Fig. 1A and B.
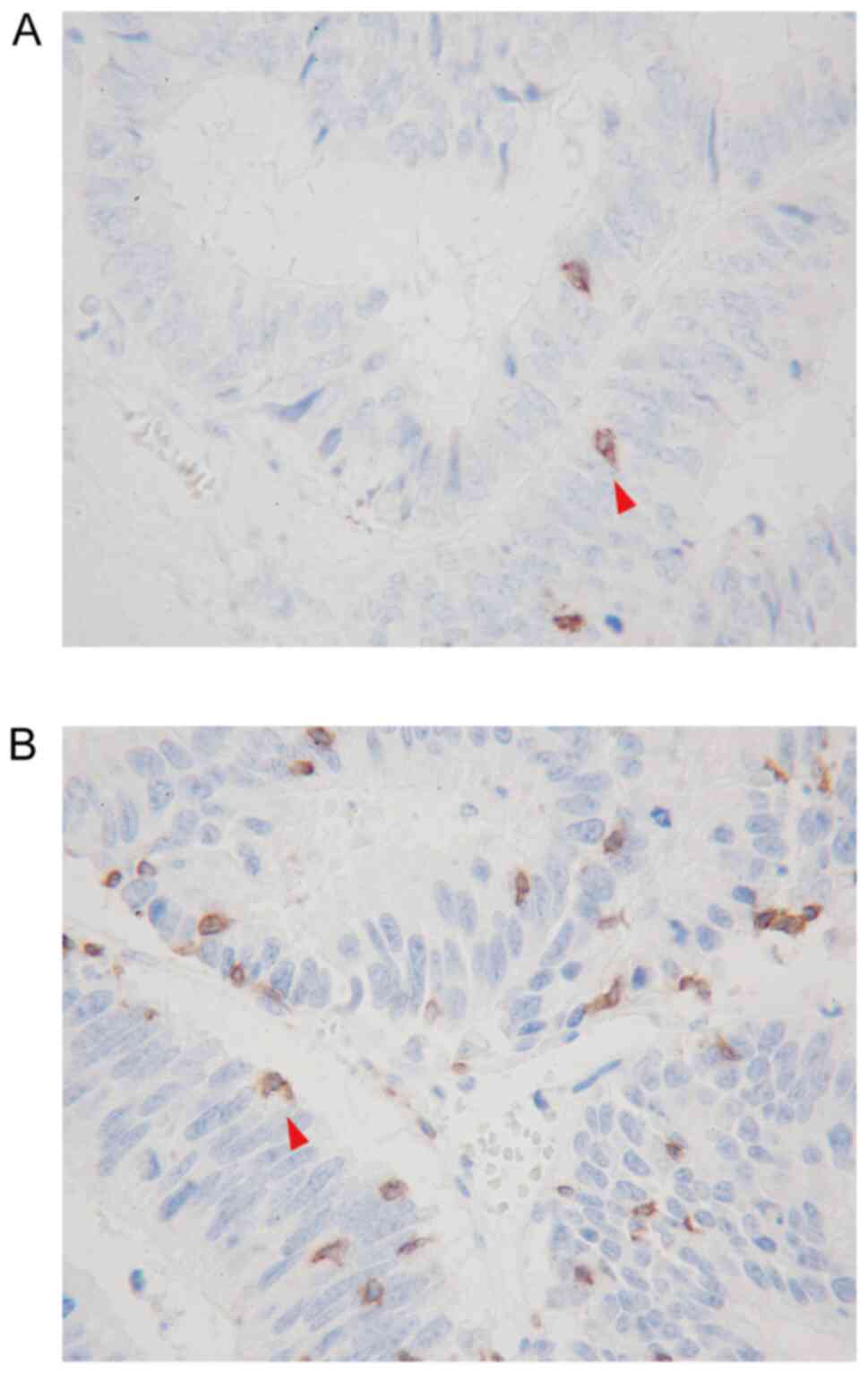 |
Figure 1.
Immunohistochemical detection of (A) CD8-positive TILs (red arrow in A) and (B) CD3-positive TILs (red arrow in B) by ×400 high-power field. TILs, tumor-infiltrating lymphocytes.
|
The specimens were pathologically classified according to the Union for International Cancer Control Tumor-Node-Metastasis classification of malignant tumors (8th edition) (https://www.uicc.org/resources/tnm/publications-resources). All patients were followed up until death or November 30, 2018. The relapse-free survival (RFS) was determined from the date of hepatectomy to the date of relapse, the date of death from any cause, the date of loss to follow-up, or the 30th of November 2018, whichever occurred first. The overall survival (OS) was determined from the date of hepatectomy to the date of death, the date of loss to follow-up, or the 30th of November 2018, whichever occurred first.
Statistical analyses
Analyses were performed using the JMP13 software (version 13.2.0; SAS Institute, Inc.) and the SPSS 25.0 statistical software program (IBM Corp.). Differences between the density of TILs and absolute peripheral lymphocyte count were analyzed using Pearson's correlation coefficient, and differences in the density of TILs between the recurrence group and the recurrence-free group after hepatectomy were analyzed using Wilcoxon's rank sum test. A receiver operating characteristic (ROC) curve was used to determine the appropriate cut-off value for the density of TILs. All patients were then classified into two groups according to the numbers of CD8+TILs and CD3+TILs. The significance of the correlations between the density of TILs and the clinicopathological characteristics was analyzed using Yates' Chi-square test and Fisher's exact test (only these two tests were analyzed using the SPSS software program). The duration of the survival was estimated using the Kaplan-Meier method and differences in the survival curves were analyzed using the log-rank test. P<0.05 was considered to indicate a statistically significant difference.
Results
Relationship between CD8+/CD3+TIL infiltration in colorectal cancer liver metastases and the absolute peripheral lymphocyte count
In patients with resected colorectal liver metastases, there was a positive correlation between the density of CD8+TILs and the density of CD3+TILs in liver metastases (r=0.6842; P<0.0001; Fig. 2A). The density of CD8+/CD3+TILs in liver metastases was not correlated with the absolute peripheral lymphocyte count (CD8, r=−0.0721, P=0.4850; CD3, r=−0.0794, P=0.4421; Fig. 2B and C, respectively).
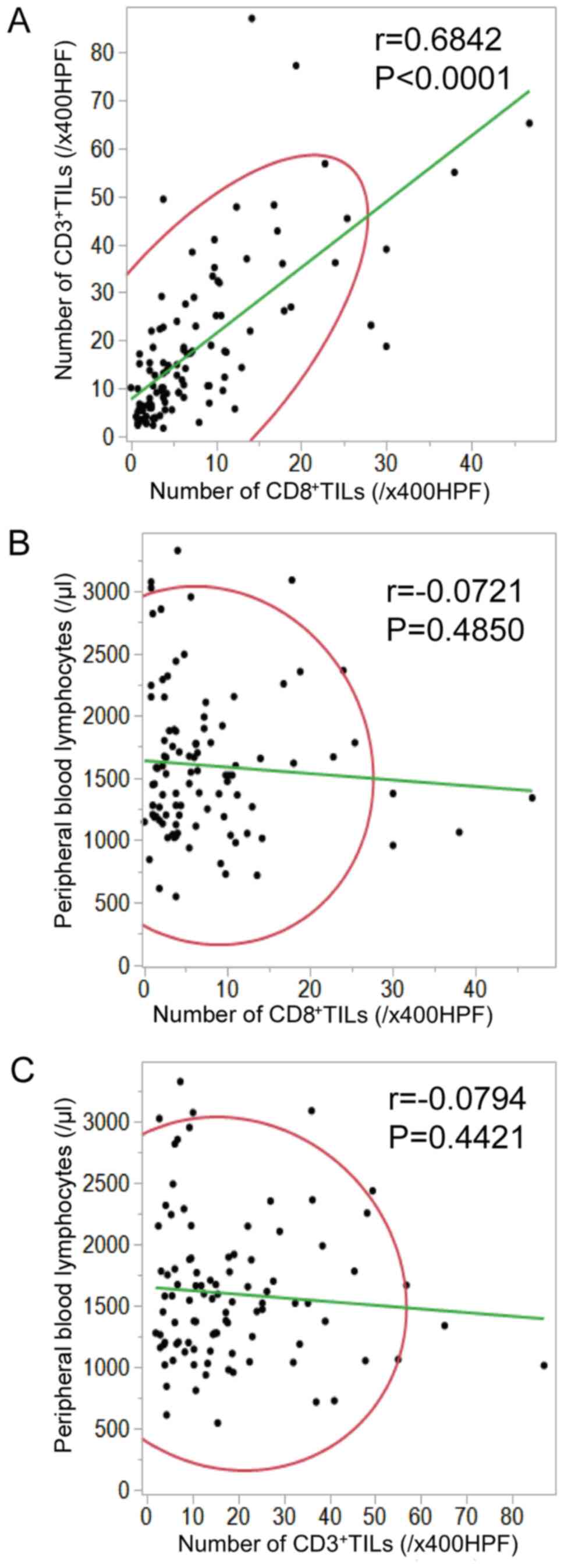 |
Figure 2.
Relationship between CD8+/CD3+TIL infiltration in colorectal cancer liver metastases and the absolute peripheral lymphocyte count. (A) In patients with resected colorectal liver metastases, there was a positive correlation between the density of CD8+TILs and that of CD3+TILs in liver metastases (r=0.6842, P<0.0001). (B and C) The density of TILs in liver metastases did not correlate with the absolute peripheral lymphocyte count (CD8, r=−0.0721, P=0.4850; CD3, r=−0.0794, P=0.4421). TILs, tumor-infiltrating lymphocytes.
|
Comparison of TIL infiltration in colorectal cancer liver metastases between the recurrence group and the recurrence-free group after hepatectomy
In patients with resected colorectal liver metastases, the density of CD8+TILs in liver metastases in the recurrence-free group was significantly higher than that in the recurrence group [median values: recurrence-free group 8.0 vs. recurrence group 3.8; /x400 high-power field (HPF); Fig. 3A]. Conversely, there was no significant difference between the density of CD3+TILs in the recurrence-free group and the recurrence group (Fig. 3B).
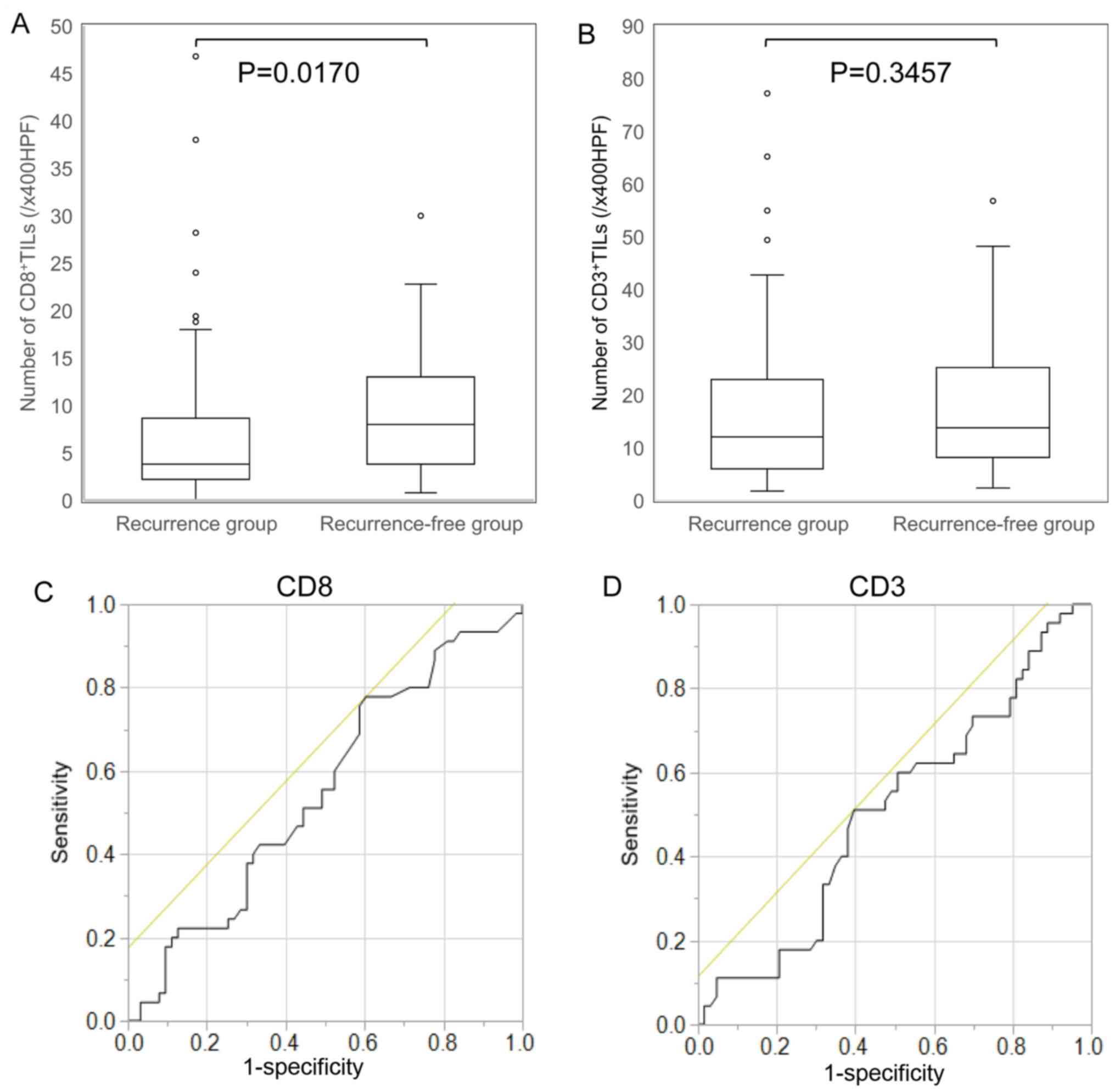 |
Figure 3.
Comparison of TIL infiltration in colorectal cancer liver metastases between the recurrence group and the recurrence-free group after hepatectomy, and ROC curve for determination of the cut-off value of the density of TILs for predicting the prognosis. (A) In patients with resected colorectal liver metastases, the density of CD8+TILs in liver metastases in the recurrence-free group was significantly higher than that in the recurrence group (P=0.017). (B) There were no significant differences between the density of CD3+TILs in the recurrence-free group and the recurrence group (P=0.3457). (C and D) ROC curve analysis of the density of TILs. The five-year overall survival was treated as the state variable, and the density of TILs was treated as the test variable. The ROC curve showed that 3.4/x400HPF was the most appropriate cut-off value for the density of CD8+TIL (area under the curve, 0.5460; sensitivity, 0.7778; specificity, 0.3906) and 10.6/x400HPF was the most appropriate cut-off value for the density of CD3+TIL (area under the curve, 0.5090; sensitivity, 0.5111; specificity, 0.6032). TILs, tumor-infiltrating lymphocytes; ROS, receiver operating characteristic; HPF, high-power field.
|
Cut-off value of the density of TILs for predicting the prognosis of patients with colorectal cancer liver metastasis
We considered the five-year OS as the state variable and the density of CD8+/CD3+TILs as the test variable. The ROC curve showed that 3.4/x400HPF was the most appropriate cut-off value for the density of CD8+TIL (area under the curve, 0.5460; sensitivity, 0.7778; specificity, 0.3906; Fig. 3C) and that 10.6/x400HPF was the most appropriate cut-off value for the density of CD3+TIL (area under the curve, 0.5090; sensitivity, 0.5111; specificity, 0.6032; Fig. 3D). Subsequently, we classified the patients into a high-TIL group and low-TIL group.
Relationship between TIL infiltration in colorectal cancer liver metastases and clinicopathological factors or the prognosis after hepatectomy.
Analyses of patients with colorectal liver metastases. The high-CD8+TIL group was significantly associated with CEA≤5 ng/ml (P=0.025) but not with other clinicopathological characteristics (Table I). The high-CD8+TIL group exhibited a significantly better RFS than the low-CD8+TIL group [high-CD8+TIL median survival time (MST), 24.8 months; low-CD8+TIL MST, 11.5 months; P=0.0045; Fig. 4A]. Similarly, the high-CD8+TIL group exhibited a better OS than the low-CD8+TIL group (high-CD8+TILs MST, 145.9 months; low-CD8+TILs MST, 68.4 months; P=0.0074; Fig. 4B).
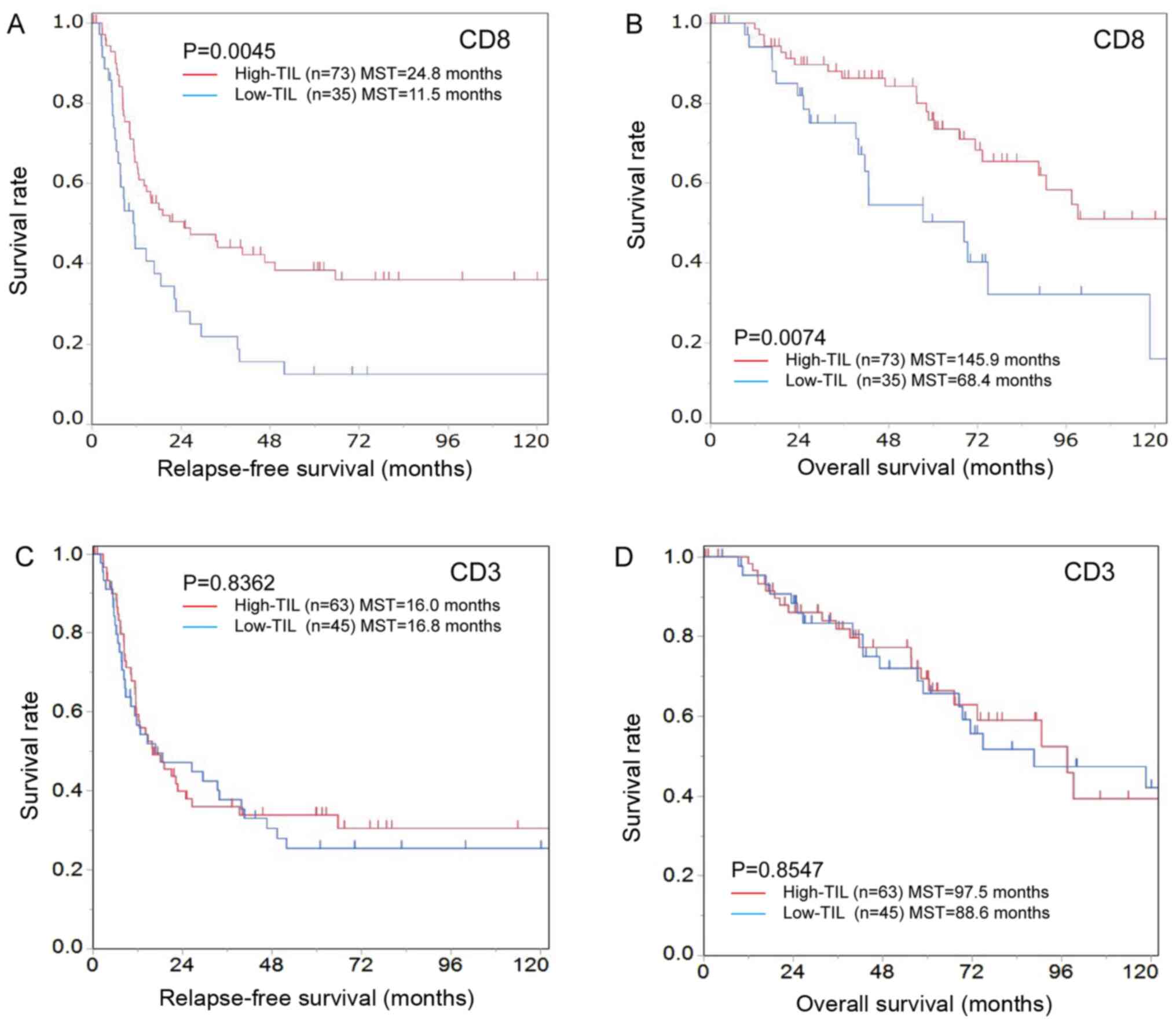 |
Figure 4.
Kaplan-Meier survival curves for the relapse-free survival and overall survival according to the number of CD8-positive TILs in patients with colorectal cancer liver metastases. (A) Relapse-free and (B) overall survival were significantly better in the high-CD8+TIL group compared with the low-CD8+TIL group (relapse-free survival, P=0.0045; overall survival, P=0.0074). (C) There were no significant differences between the high- and low-CD3+TIL groups in relapse-free (P=0.8362) or (D) overall survival (P=0.8547). TILs, tumor-infiltrating lymphocytes.
|
 |
Table I.
Association between CD8+TILs and the clinicopathological characteristics of patients with colorectal cancer liver metastases treated or not with chemotherapy before hepatectomy.
|
Table I.
Association between CD8+TILs and the clinicopathological characteristics of patients with colorectal cancer liver metastases treated or not with chemotherapy before hepatectomy.
| |
Overall patients (n=108) |
No chemotherapy before hepatectomy (n=63) |
Short-term chemotherapy before hepatectomy (n=22) |
Long-term chemotherapy before hepatectomy (n=23) |
| |
|
|
|
|
| Characteristic |
High density of TIL |
Low density of TIL |
P-value |
High density of H TIL |
Low density of TIL |
P-value |
High density of TIL |
Low density of TIL |
P-value |
High density of TIL |
Low density of TIL |
P-value |
| Sex |
|
|
|
|
|
|
|
|
|
|
|
|
| Male |
43 |
20 |
|
27 |
10 |
|
10 |
1 |
|
6 |
9 |
|
| Female |
30 |
15 |
1.000a |
16 |
10 |
0.493a |
8 |
3 |
0.586b |
6 |
2 |
0.193b |
| Age, years |
|
|
|
|
|
|
|
|
|
|
|
|
| <65 |
35 |
18 |
|
20 |
9 |
|
10 |
3 |
|
5 |
6 |
|
| ≥65 |
38 |
17 |
0.894a |
23 |
11 |
1.000a |
8 |
1 |
0.616b |
7 |
5 |
0.842a |
| Location of primary tumor |
|
|
|
|
|
|
|
|
|
|
|
|
| Left |
56 |
21 |
|
32 |
11 |
|
14 |
3 |
|
10 |
7 |
|
| Right |
17 |
14 |
0.116a |
11 |
9 |
0.211a |
4 |
1 |
1.000b |
2 |
4 |
0.371b |
| Tumor depth of primary tumor |
|
|
|
|
|
|
|
|
|
|
|
|
| T≤3 |
41 |
17 |
|
25 |
11 |
|
10 |
0 |
|
6 |
6 |
|
| T4 |
32 |
18 |
0.593a |
18 |
9 |
1.000a |
8 |
4 |
0.096b |
6 |
5 |
1.000a |
| Lymph node metastasis of primary tumor |
|
|
|
|
|
|
|
|
|
|
|
|
| N≤1 |
63 |
27 |
|
40 |
15 |
|
14 |
3 |
|
9 |
9 |
|
| N≥2 |
10 |
8 |
0.358a |
3 |
5 |
0.097b |
4 |
1 |
1.000b |
3 |
2 |
1.000b |
| Numbers of liver metastasis |
|
|
|
|
|
|
|
|
|
|
|
|
| 1 |
46 |
18 |
|
31 |
12 |
|
8 |
0 |
|
7 |
6 |
|
| ≥2 |
27 |
17 |
0.348a |
12 |
8 |
0.503a |
10 |
4 |
0.254b |
5 |
5 |
1.000b |
| Sizes of liver metastasis, cm |
|
|
|
|
|
|
|
|
|
|
|
|
| <5 |
62 |
27 |
|
37 |
17 |
|
17 |
3 |
|
8 |
7 |
|
| ≥5 |
11 |
8 |
0.468a |
6 |
3 |
1.000b |
1 |
1 |
0.338b |
4 |
4 |
1.000b |
| CEA, ng/ml |
|
|
|
|
|
|
|
|
|
|
|
|
| ≤5 |
22 |
3 |
|
13 |
1 |
|
6 |
0 |
|
3 |
2 |
|
| >5 |
51 |
32 |
0.025a |
30 |
19 |
0.027b |
12 |
4 |
0.541b |
9 |
9 |
1.000b |
| Detection of liver metastases |
|
|
|
|
|
|
|
|
|
|
|
|
| Synchronous |
34 |
15 |
|
18 |
9 |
|
12 |
4 |
|
4 |
2 |
|
| Metachronous |
39 |
20 |
0.875a |
25 |
11 |
1.000a |
6 |
0 |
0.541b |
8 |
9 |
0.640b |
The high-CD3+TIL group was significantly associated with left-sided colorectal cancer (P=0.048) and CEA ≤5 ng/ml (P=0.006) but not with other clinicopathological characteristics (Table II). There were no significant differences between the high- and low-CD3+TIL groups regarding the RFS or OS (Fig. 4C and D).
 |
Table II.
Association between CD3+TILs and the clinicopathological characteristics of patients with colorectal cancer liver metastases treated or not with chemotherapy before hepatectomy.
|
Table II.
Association between CD3+TILs and the clinicopathological characteristics of patients with colorectal cancer liver metastases treated or not with chemotherapy before hepatectomy.
| |
Overall patients (n=108) |
No chemotherapy before hepatectomy (n=63) |
Short-term chemotherapy before hepatectomy (n=22) |
Long-term chemotherapy before hepatectomy (n=23) |
| |
|
|
|
|
| Characteristic |
High density of TIL |
Low density of TIL |
P-value |
High density of TIL |
Low density of TIL |
P-value |
High density of TIL |
Low density of TIL |
P-value |
High density of TIL |
Low density of TIL |
P-value |
| Sex |
|
|
|
|
|
|
|
|
|
|
|
|
| Male |
37 |
26 |
|
18 |
19 |
|
9 |
2 |
|
10 |
5 |
|
| Female |
26 |
19 |
1.000a |
14 |
12 |
0.881a |
8 |
3 |
1.000b |
4 |
4 |
0.657b |
| Age, years |
|
|
|
|
|
|
|
|
|
|
|
|
| <65 |
30 |
23 |
|
13 |
16 |
|
9 |
4 |
|
8 |
3 |
|
| ≥65 |
33 |
22 |
0.871a |
19 |
15 |
0.534a |
8 |
1 |
0.360b |
6 |
6 |
0.400b |
| Location of primary tumor |
|
|
|
|
|
|
|
|
|
|
|
|
| Left |
50 |
27 |
|
24 |
19 |
|
14 |
3 |
|
12 |
5 |
|
| Right |
13 |
18 |
0.048a |
8 |
12 |
0.369a |
3 |
2 |
0.548b |
2 |
4 |
0.162b |
| Tumor depth of primary tumor |
|
|
|
|
|
|
|
|
|
|
|
|
| T≤3 |
38 |
20 |
|
21 |
15 |
|
10 |
0 |
|
7 |
5 |
|
| T4 |
25 |
25 |
0.151a |
11 |
16 |
0.259a |
7 |
5 |
0.040b |
7 |
4 |
1.000b |
| Lymph node metastasis of primary tumor |
|
|
|
|
|
|
|
|
|
|
|
|
| N≤1 |
51 |
39 |
|
29 |
26 |
|
13 |
4 |
|
9 |
9 |
|
| N≥2 |
12 |
6 |
0.600a |
3 |
5 |
0.474b |
4 |
1 |
1.000b |
5 |
0 |
0.116b |
| Numbers of liver metastasis |
|
|
|
|
|
|
|
|
|
|
|
|
| 1 |
40 |
24 |
|
24 |
19 |
|
8 |
0 |
|
8 |
5 |
|
| ≥2 |
23 |
21 |
0.389a |
8 |
12 |
0.369a |
9 |
5 |
0.115b |
6 |
4 |
1.000b |
| Sizes of liver metastasis, cm |
|
|
|
|
|
|
|
|
|
|
|
|
| <5 |
54 |
35 |
|
27 |
27 |
|
16 |
4 |
|
11 |
4 |
|
| ≥5 |
9 |
10 |
0.417a |
5 |
4 |
1.000b |
1 |
1 |
0.411b |
3 |
5 |
0.179b |
| CEA, ng/ml |
|
|
|
|
|
|
|
|
|
|
|
|
| ≤5 |
21 |
4 |
|
11 |
3 |
|
6 |
0 |
|
4 |
1 |
|
| >5 |
42 |
41 |
0.006a |
21 |
28 |
0.040a |
11 |
5 |
0.266b |
10 |
8 |
0.611b |
| Detection of liver metastases |
|
|
|
|
|
|
|
|
|
|
|
|
| Synchronous |
28 |
21 |
|
13 |
14 |
|
11 |
5 |
|
4 |
2 |
|
| Metachronous |
35 |
24 |
0.974a |
19 |
17 |
0.913a |
6 |
0 |
0.266b |
10 |
7 |
1.000b |
Subgroup analyses by preoperative chemotherapy. i) Subgroup analyses in the group without preoperative chemotherapy. Among patients who did not receive preoperative chemotherapy, the high-CD8+TIL group was significantly associated with CEA≤5 ng/ml (P=0.027) but not with other clinicopathological characteristics (Table I). The degree of CD8+TIL infiltration was significantly associated with the RFS (high-CD8+TIL MST, 40.6 months; low-CD8+TIL MST, 11.5 months; P=0.0031; Fig. 5A). Furthermore, a high CD8+TIL presence tended to have a better effect on the OS than a low CD8+TIL presence (high-CD8+TIL MST, not available; low-CD8+TIL MST, 68.4 months; P=0.0537; Fig. 5B).
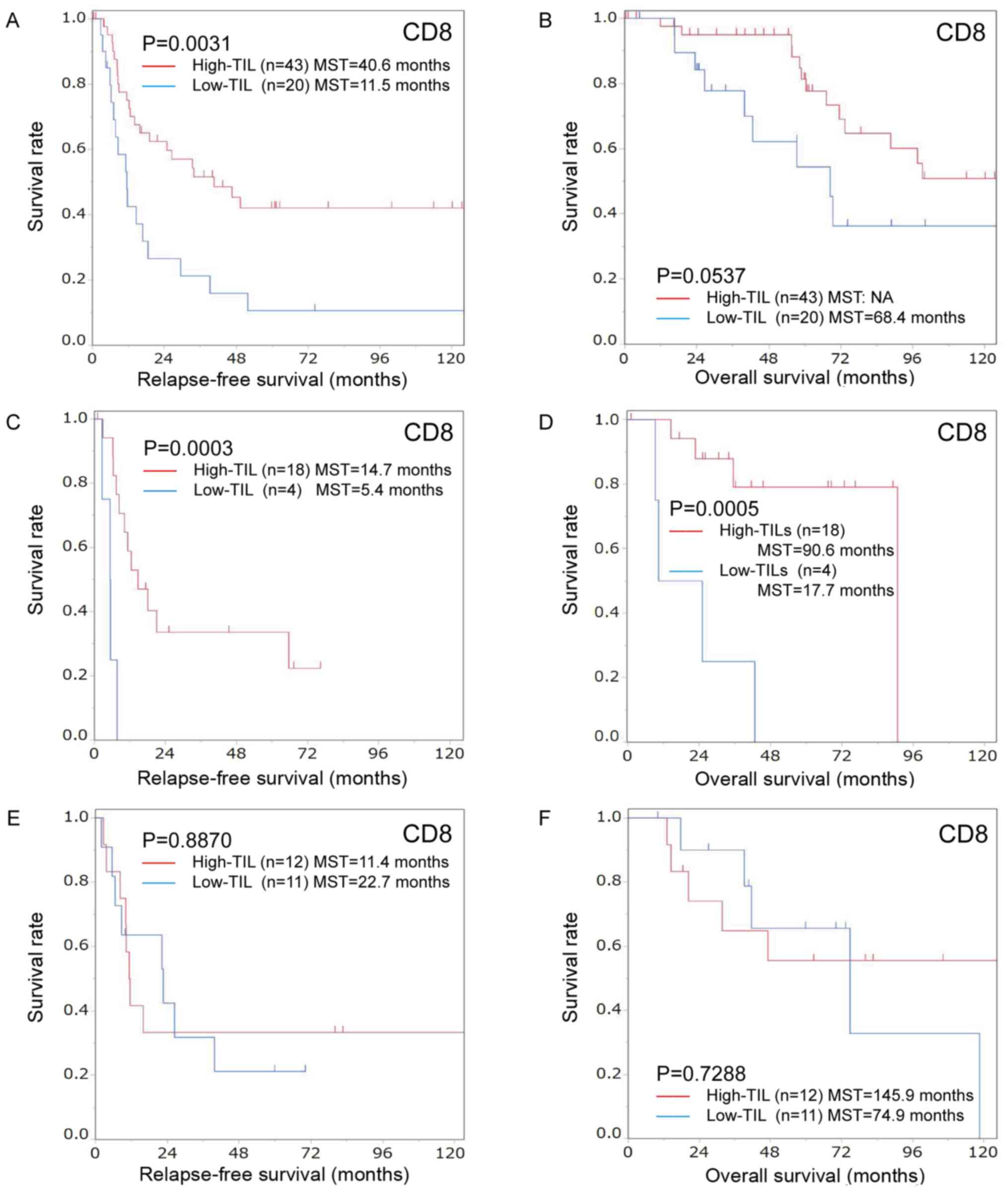 |
Figure 5.
Kaplan-Meier survival curves for the relapse-free survival and overall survival according to the number of CD8-positive TILs in patients with colorectal cancer liver metastasis with/without chemotherapy before hepatectomy. Kaplan-Meier survival curves for the (A) relapse-free survival and (B) overall survival in the group that did not receive chemotherapy before hepatectomy. Relapse-free survival was significantly better in the high-CD8+ TIL group compared with the low-CD8+ TIL group (P=0.0031). High CD8-positive TIL infiltration tended to have a better effect on overall survival than low infiltration (P=0.0537). Kaplan-Meier survival curves for the (C) relapse-free survival and (D) overall survival in the group treated with short-term chemotherapy before hepatectomy. The relapse-free and overall survival were significantly better in the high-CD8+ TIL group than in the low-CD8+ TIL group (relapse-free survival, P=0.0003; overall survival, P=0.0005). Kaplan-Meier survival curves for the (E) relapse-free survival and (F) overall survival in the group treated with long-term chemotherapy before hepatectomy. There were no significant differences between the high- and low-CD8+ TIL groups (relapse-free survival, P=0.8870; overall survival, P=0.7288). TILs, tumor-infiltrating lymphocytes.
|
The high-CD3+TIL group showed a significant association with CEA≤5 ng/ml (P=0.040) but not with other clinicopathological characteristics (Table II). The RFS and OS of the high- and low-CD3+TIL groups did not differ to a statistically significant extent (Fig. 6A and B).
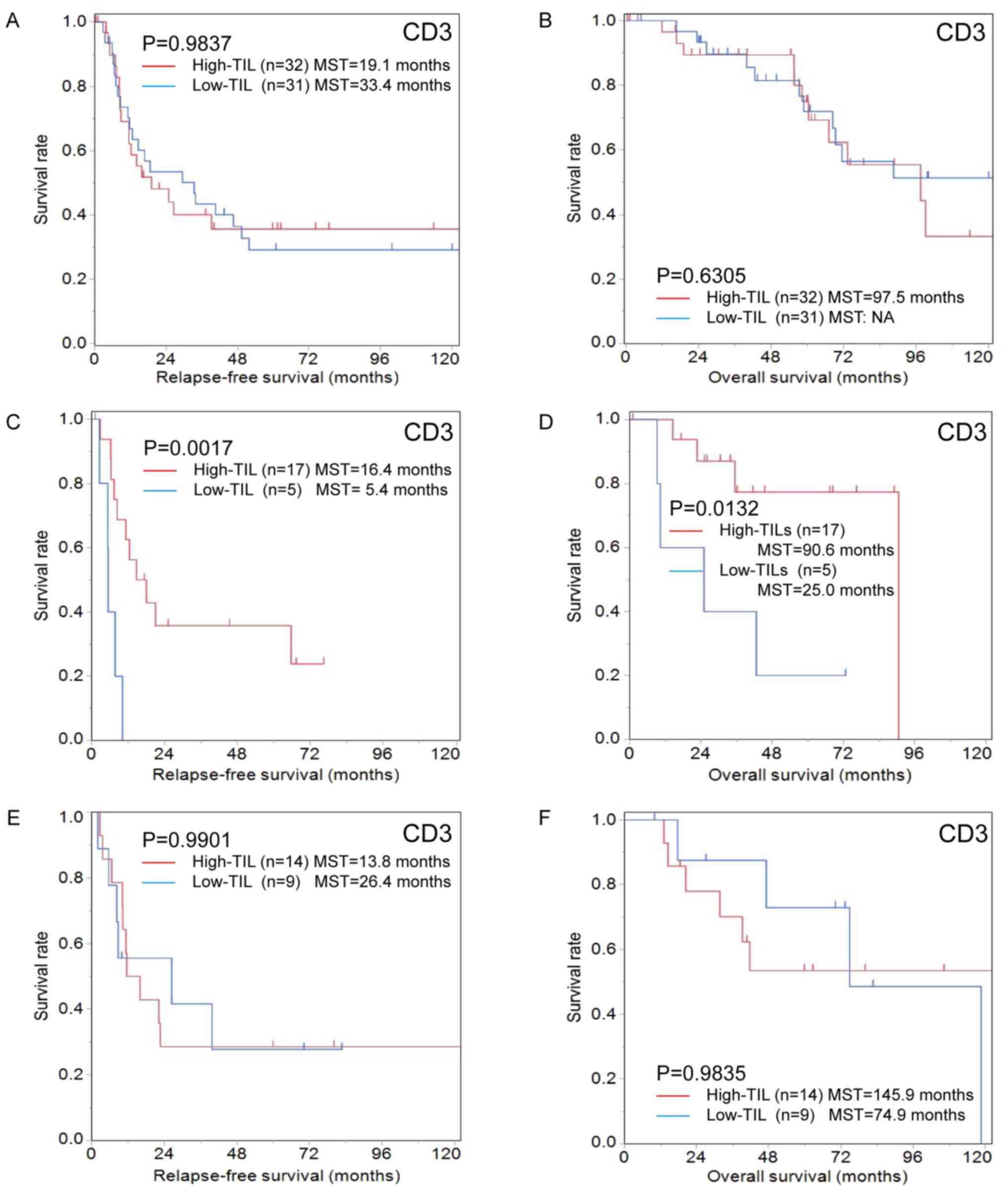 |
Figure 6.
Kaplan-Meier survival curves for relapse-free survival and overall survival according to the number of CD3-positive TILs in patients with colorectal cancer liver metastasis with/without chemotherapy before hepatectomy. Kaplan-Meier survival curves for (A) relapse-free survival and (B) overall survival in the group that did not receive chemotherapy before hepatectomy. There were no significant differences between the high- and low-CD3+ TIL groups (relapse-free survival, P=0.9837; overall survival, P=0.6305). Kaplan-Meier survival curves for (C) relapse-free survival and (D) overall survival in the group treated with short-term chemotherapy before hepatectomy. The relapse-free and overall survival in the high-CD3+ TIL group were significantly better than in the low-CD3+ TIL group (relapse-free survival, P=0.0017; overall survival, P=0.0132). Kaplan-Meier survival curves for (E) relapse-free survival and (F) overall survival in the group treated with long-term chemotherapy before hepatectomy. There were no significant differences between the high- and low-CD3+ TIL groups (relapse-free survival, P=0.9901; overall survival, P=0.9835). TILs, tumor-infiltrating lymphocytes
|
ii) Subgroup analyses in the short-term chemotherapy group
The degree of CD8+TIL infiltration was not correlated with the clinicopathological characteristics in the short-term chemotherapy group (Table I). The degree of CD8+TIL infiltration was significantly associated with the RFS (high-CD8+TIL MST, 14.7 months; low-CD8+TIL MST, 5.4 months; P=0.0003) and OS (high-CD8+TIL MST, 90.6 months; low-CD8+TIL MST, 17.7 months; P=0.0005; Fig. 5C and D).
The high-CD3+TIL group showed a significant association with the tumor depth of the primary tumor (T≤3; P=0.040) but not with other clinicopathological characteristics (Table II). The degree of CD3+TIL infiltration was significantly associated with RFS (high-CD3+TIL MST, 16.4 months; low-CD3+TIL MST, 5.4 months; P=0.0017) and OS (high-CD3+TIL MST, 90.6 months; low-CD3+TIL MST, 25.0 months; P=0.0132; Fig. 6C and D).
iii) Subgroup analyses in the long-term chemotherapy group
The degree of CD8+TIL infiltration was not correlated with clinicopathological characteristics in the long-term chemotherapy group (Table I). There were no significant differences between the high- and low-CD8+TIL groups regarding the RFS or OS (Fig. 5E and F).
The degree of CD3+TIL infiltration was not associated with clinicopathological characteristics (Table II). The RFS and OS of the high- and low-CD3+TIL groups did not differ to a statistically significant extent (Fig. 6E and F).
Discussion
The present study demonstrated that the degree of CD8+TIL infiltration in colorectal cancer liver metastases was significantly lower in the recurrence group compared with the recurrence-free group following hepatectomy, indicating that the density of CD8+TILs may strongly influence the therapeutic effect on and prognosis of colorectal liver metastasis. Furthermore, the local immune status in colorectal cancer liver metastases was correlated with the prognosis after curative hepatectomy in the group without preoperative chemotherapy as well as the group with short-term preoperative chemotherapy. These findings were in accordance with the results from previous studies regarding the primary tumor of colorectal cancer (10–12). Conversely, the degree of TILs was not correlated with patient prognosis in the long-term preoperative chemotherapy group.
Previous studies have reported that the prognosis of various types of carcinoma, including colorectal cancer, is strongly influenced by the cancer microenvironment, including TILs (22–24). The degree of TIL infiltration in the primary tumor of colorectal cancer is positively correlated with recurrence and survival after curative resection (22,25,26) as well as with the effects of radiotherapy and chemotherapy (13,14,27). While relatively few studies have focused on local immunity in distant metastases of colorectal cancer compared to that in the primary tumor of colorectal cancers, a high degree of TIL infiltration in liver metastases reportedly leads to good OS (28). The present study also demonstrated that a large number of CD8+TILs in colorectal cancer liver metastasis was significantly correlated with a good RFS and OS in patients without preoperative chemotherapy or with short-term preoperative chemotherapy.
Most TILs are T cells (10), which mediate adaptive immune responses by targeting antigens expressed by tumor cells (29). However, there are various subsets of T cells with different functions. Th1 cells have been reported to activate CD8+T cells and enhance the anti-tumor immune response, while Th2 cells seem to suppress anti-tumor immunity by activating B cells or producing IL-10 (14,23). The functions of Th17 and Treg cells in antitumor immunity have been controversial, but they reportedly activate anti-tumor immunity or promote tumor growth (14,23). CD8+ T cells have been reported to kill tumor cells directly via granzymes/perforin and the tumor necrosis factor superfamily and to play a key role in anti-tumor immunity, influencing therefore patient prognosis (25,30,31).
In the present study, in patients who did not undergo preoperative chemotherapy before hepatectomy or in those who underwent short-term preoperative chemotherapy, the high-CD8+ TIL group in colorectal cancer liver metastasis was associated with a prolonged OS and RFS. Conversely, the degree of TIL infiltration of colorectal cancer liver metastases was not associated with the prognosis in patients with a long-term preoperative chemotherapy. According to previous studies, long-term chemotherapy may change the local immunity in liver metastases and reduce the prognostic value of the density of TILs (20,32–38). There may be some possible causes to this observation. Firstly, the dysfunction of lymphocytes may be involved. Long-term chemotherapy induces a reduction in the absolute number of lymphocytes and prevents the induction of lymphocytes to secondary lymphoid organs, resulting in decreased lymphocyte activation and thereby weakening the T cell immune response (32). A study by Wu et al (20) using an experimental mouse model demonstrated that the short-term administration of fluorouracil can increase the proliferation and cytotoxicity of CD8+ T cells, while repeated administration causes a decrease in the proliferation and cytotoxicity of CD8+ T cells. Furthermore, the absolute number of immune cells may recover relatively quickly after the discontinuation of chemotherapy; however, the antitumor immune function of immune cells remains diminished for a prolonged period with repeated chemotherapy (20,33). Therefore, it was speculated that the number of T cells in the local tumor microenvironment may not always be associated with the prognosis in cases treated with long-term chemotherapy, as the function of T cells decreases after repeated chemotherapy. Secondly, immune escape mechanisms may be involved. Repeated chemotherapy may increase the expression of immune checkpoint molecules, such as programmed death-ligand 1 (PD-L1) in tumor cells, dendritic cells or macrophages, resulting in immune exhaustion and consequently impaired lymphocyte-mediated immunity (34,35). PD-L1 is known to bind to PD-1 expressed on T cells and suppress the function of TILs. However, while chemotherapy activates local immunity, including TILs, an immunosuppressive mechanism called adaptive immune resistance occurs when PD-L1 expression is upregulated in response to interferon-γ secreted by CD8+ T cells and suppresses the function of TILs (36–38). In long-term chemotherapy, the activation of this mechanism results in the overexpression of PD-L1 and counteracts the immunological effects of TILs (37,38), thus the degree of TIL infiltration may not necessarily correlate with therapeutic effects.
The present study reported no correlation between the degree of TIL infiltration in liver metastases and the absolute peripheral lymphocyte count. Regarding the reason for this absence of correlation, the absolute peripheral lymphocyte count was not evaluated for subgroups with different functions, including T cells, B cells and NK cells. Furthermore, various factors, such as suppressive immune cells, structural abnormalities in the wall of newly formed microvessels, chemokines and cytokines and intratumoral fibrosis, which may affect the recruitment of peripheral lymphocytes into tumors (39–41), were not under consideration in the present study. Therefore, the local immune status of the tumor may not necessarily be associated with the absolute peripheral lymphocyte count. Similarly, we demonstrated that the prognostic ability of CD3 was lower than that of CD8. CD3, a pan-T lymphocyte marker, includes various subsets, such as Th1, Th2, Th17, regulatory T cell and cytotoxic T lymphocyte. These subsets have different functions, resulting in the poor prognostic ability of CD3+ TILs.
This study presented several limitations. Firstly, this study was a retrospective, single-center study with a relatively small number of patients. Secondly, we examined only CD8+ TILs as immune factors that may affect patient prognosis; however, other immunocompetent cells, including other TILs subtypes, tumor-associated macrophages, dendritic cells and myeloid-derived suppressor cells, antigen presentation capability, immune activation markers and immune checkpoint markers and chemokines involved in the local recruitment of immunocompetent cells were not considered in the present study. Thirdly, chemotherapy for at least six months was defined as long-term chemotherapy in this study; however, how long chemotherapy should be continued for to reduce lymphocyte immunity is unclear.
In summary, the present study demonstrated that the degree of CD8+TIL infiltration in colorectal cancer liver metastases may be correlated with that in the primary lesion as well as with patient prognosis. However, in patients who received long-term chemotherapy prior to surgery, the degree of TIL infiltration was not necessarily associated with the prognosis because the function of TILs may be decreased.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request
Authors' contributions
EW and MS conceived and designed the experiments. EW performed the experiments and was a major contributor in analyzing the data. EW and MS contributed to reagents, materials and analytical tools. EW and MS wrote the paper. HN, TF, YI, YO, SK, HT and KM were contributors in revising the design of the experiments and in analyzing the data. MS, HN, TF, YI, YO, SK, HT and KM reviewed and validated the manuscript. EW and MS confirm the authenticity of all the raw data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study was performed in compliance with the principles expressed in the Declaration of Helsinki and was approved by the Ethics Committee of Osaka City University (approval no. 3853). Written informed consent was obtained from all participants in this study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Glossary
Abbreviations
Abbreviations:
|
HPF
|
high-power field
|
|
MST
|
median survival time
|
|
OS
|
overall survival
|
|
RFS
|
relapse-free survival
|
|
ROC
|
receiver operating characteristic
|
|
TILs
|
tumor-infiltrating lymphocytes
|
References
|
1
|
World Health Organization (WHO), . WHO report on cancer: setting priorities, investing wisely and providing care for all. World Health Organization; Geneva: 2020
|
|
2
|
Watanabe T, Muro K, Ajioka Y, Hashiguchi Y, Ito Y, Saito Y, Hamaguchi T, Ishida H, Ishiguro M, Ishihara S, et al: Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol. 23:1–34. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Niederhuber JE: Colon and rectum cancer. Patterns of spread and implications for workup. Cancer. 71 (Suppl 12):4187–4192. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wakabayashi T, Hibi T, Yoneda G, Iwao Y, Sawada Y, Hoshino H, Uemura S, Ban D, Kudo A, Takemura Y, et al: Predictive model for survival after liver resection for noncolorectal liver metastases in the modern era: A Japanese multicenter analysis. J Hepatobiliary Pancreat Sci. 26:441–448. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zarour LR, Anand S, Billingsley KG, Bisson WH, Cercek A, Clarke MF, Coussens LM, Gast CE, Geltzeiler CB, Hansen L, et al: Colorectal Cancer Liver Metastasis: Evolving Paradigms and Future Directions. Cell Mol Gastroenterol Hepatol. 3:163–173. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Abdalla EK, Vauthey JN, Ellis LM, Ellis V, Pollock R, Broglio KR, Hess K and Curley SA: Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 239:818–825; discussion 825-827. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kato T, Yasui K, Hirai T, Kanemitsu Y, Mori T, Sugihara K, Mochizuki H and Yamamoto J: Therapeutic results for hepatic metastasis of colorectal cancer with special reference to effectiveness of hepatectomy: Analysis of prognostic factors for 763 cases recorded at 18 institutions. Dis Colon Rectum. 46 (Suppl 10):S22–S31. 2003.PubMed/NCBI
|
|
8
|
Gooden MJ, de Bock GH, Leffers N, Daemen T and Nijman HW: The prognostic influence of tumour-infiltrating lymphocytes in cancer: A systematic review with meta-analysis. Br J Cancer. 105:93–103. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang D, He W, Wu C, Tan Y, He Y, Xu B, Chen L, Li Q and Jiang J: Scoring System for Tumor-Infiltrating Lymphocytes and Its Prognostic Value for Gastric Cancer. Front Immunol. 10:71. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ropponen KM, Eskelinen MJ, Lipponen PK, Alhava E and Kosma V-M: Prognostic value of tumour-infiltrating lymphocytes (TILs) in colorectal cancer. J Pathol. 182:318–324. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Canna K, McArdle PA, McMillan DC, McNicol AM, Smith GW, McKee RF and McArdle CS: The relationship between tumour T-lymphocyte infiltration, the systemic inflammatory response and survival in patients undergoing curative resection for colorectal cancer. Br J Cancer. 92:651–654. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mei Z, Liu Y, Liu C, Cui A, Liang Z, Wang G, Peng H, Cui L and Li C: Tumour-infiltrating inflammation and prognosis in colorectal cancer: Systematic review and meta-analysis. Br J Cancer. 110:1595–1605. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shibutani M, Maeda K, Nagahara H, Fukuoka T, Iseki Y, Matsutani S, Kashiwagi S, Tanaka H, Hirakawa K and Ohira M: Tumor-infiltrating Lymphocytes Predict the Chemotherapeutic Outcomes in Patients with Stage IV Colorectal Cancer. In Vivo. 32:151–158. 2018.PubMed/NCBI
|
|
14
|
Matsutani S, Shibutani M, Maeda K, Nagahara H, Fukuoka T, Nakao S, Hirakawa K and Ohira M: Significance of tumor-infiltrating lymphocytes before and after neoadjuvant therapy for rectal cancer. Cancer Sci. 109:966–979. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Teng F, Mu D, Meng X, Kong L, Zhu H, Liu S, Zhang J and Yu J: Tumor infiltrating lymphocytes (TILs) before and after neoadjuvant chemoradiotherapy and its clinical utility for rectal cancer. Am J Cancer Res. 5:2064–2074. 2015.PubMed/NCBI
|
|
16
|
Anitei MG, Zeitoun G, Mlecnik B, Marliot F, Haicheur N, Todosi AM, Kirilovsky A, Lagorce C, Bindea G, Ferariu D, et al: Prognostic and predictive values of the immunoscore in patients with rectal cancer. Clin Cancer Res. 20:1891–1899. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee WS, Kang M, Baek JH, Lee JI and Ha SY: Clinical impact of tumor-infiltrating lymphocytes for survival in curatively resected stage IV colon cancer with isolated liver or lung metastasis. Ann Surg Oncol. 20:697–702. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shibutani M, Maeda K, Nagahara H, Fukuoka T, Matsutani S, Kashiwagi S, Tanaka H, Hirakawa K and Ohira M: A comparison of the local immune status between the primary and metastatic tumor in colorectal cancer: A retrospective study. BMC Cancer. 18:3712018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kito A, Tanaka K, Fujimaki H, Nakazawa M, Togo S, Minami M and Shimada H: Tumor doubling time and local immune response to hepatic metastases from colorectal cancer. J Surg Oncol. 96:525–533. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu Y, Deng Z, Wang H, Ma W, Zhou C and Zhang S: Repeated cycles of 5-fluorouracil chemotherapy impaired anti-tumor functions of cytotoxic T cells in a CT26 tumor-bearing mouse model. BMC Immunol. 17:292016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Adam R, de Gramont A, Figueras J, Kokudo N, Kunstlinger F, Loyer E, Poston G, Rougier P, Rubbia-Brandt L, Sobrero A, et al: Managing synchronous liver metastases from colorectal cancer: A multidisciplinary international consensus. Cancer Treat Rev. 41:729–741. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, et al: Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 313:1960–1964. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fridman WH, Pagès F, Sautès-Fridman C and Galon J: The immune contexture in human tumours: Impact on clinical outcome. Nat Rev Cancer. 12:298–306. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Galon J, Angell HK, Bedognetti D and Marincola FM: The continuum of cancer immunosurveillance: Prognostic, predictive, and mechanistic signatures. Immunity. 39:11–26. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pagès F, Kirilovsky A, Mlecnik B, Asslaber M, Tosolini M, Bindea G, Lagorce C, Wind P, Marliot F, Bruneval P, et al: In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol. 27:5944–5951. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Eriksen AC, Sørensen FB, Lindebjerg J, Hager H, dePont Christensen R, Kjær-Frifeldt S and Hansen TF: The Prognostic Value of Tumor-Infiltrating lymphocytes in Stage II Colon Cancer. A Nationwide Population-Based Study. Transl Oncol. 11:979–987. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Halama N, Michel S, Kloor M, Zoernig I, Pommerencke T, von Knebel Doeberitz M, Schirmacher P, Weitz J, Grabe N and Jäger D: The localization and density of immune cells in primary tumors of human metastatic colorectal cancer shows an association with response to chemotherapy. Cancer Immun. 9:12009.PubMed/NCBI
|
|
28
|
Katz SC, Pillarisetty V, Bamboat ZM, Shia J, Hedvat C, Gonen M, Jarnagin W, Fong Y, Blumgart L, D'Angelica M, et al: T cell infiltrate predicts long-term survival following resection of colorectal cancer liver metastases. Ann Surg Oncol. 16:2524–2530. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Boon T, Cerottini JC, Van den Eynde B, van der Bruggen P and Van Pel A: Tumor antigens recognized by T lymphocytes. Annu Rev Immunol. 12:337–365. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cullen SP, Brunet M and Martin SJ: Granzymes in cancer and immunity. Cell Death Differ. 17:616–623. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Medema JP, de Jong J, Peltenburg LT, Verdegaal EM, Gorter A, Bres SA, Franken KL, Hahne M, Albar JP, Melief CJ, et al: Blockade of the granzyme B/perforin pathway through overexpression of the serine protease inhibitor PI-9/SPI-6 constitutes a mechanism for immune escape by tumors. Proc Natl Acad Sci USA. 98:11515–11520. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu L, Zhao L, Yang Y, Gao J, Hu C, Guo B and Zhu B: Cytotoxic chemotherapy reduces T cell trafficking to the spleen by downregulating the expression of C-C motif chemokine ligand 21 and C-C motif chemokine ligand 19. Oncol Lett. 16:5013–5019. 2018.PubMed/NCBI
|
|
33
|
Mackall CL: T-cell immunodeficiency following cytotoxic antineoplastic therapy: A review. Stem Cells. 18:10–18. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang P, Ma Y, Lv C, Huang M, Li M, Dong B, Liu X, An G, Zhang W, Zhang J, et al: Upregulation of programmed cell death ligand 1 promotes resistance response in non-small-cell lung cancer patients treated with neo-adjuvant chemotherapy. Cancer Sci. 107:1563–1571. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Guo L, Song P, Xue X, Guo C, Han L, Fang Q, Ying J, Gao S and Li W: Variation of Programmed Death Ligand 1 Expression After Platinum-based Neoadjuvant Chemotherapy in Lung Cancer. J Immunother. 42:215–220. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Khairallah AS, Genestie C, Auguste A and Leary A: Impact of neoadjuvant chemotherapy on the immune microenvironment in advanced epithelial ovarian cancer: Prognostic and therapeutic implications. Int J Cancer. 143:8–15. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xiao B, Peng J, Wang Y, Deng Y, Ou Q, Wu X, Lin J, Pan Z and Zhang L: Prognostic value of tumor infiltrating lymphocytes combined with PD-L1 expression for patients with solitary colorectal cancer liver metastasis. Ann Transl Med. 8:12212020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Thompson ED, Zahurak M, Murphy A, Cornish T, Cuka N, Abdelfatah E, Yang S, Duncan M, Ahuja N, Taube JM, et al: Patterns of PD-L1 expression and CD8 T cell infiltration in gastric adenocarcinomas and associated immune stroma. Gut. 66:794–801. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Joyce JA and Fearon DT: T cell exclusion, immune privilege, and the tumor microenvironment. Science. 348:74–80. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Salmon H, Franciszkiewicz K, Damotte D, Dieu-Nosjean MC, Validire P, Trautmann A, Mami-Chouaib F and Donnadieu E: Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J Clin Invest. 122:899–910. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Slaney CY, Kershaw MH and Darcy PK: Trafficking of T cells into tumors. Cancer Res. 74:7168–7174. 2014. View Article : Google Scholar : PubMed/NCBI
|




















