|
1
|
The Human Protein ATLAS: DCLK1, . Human Protein ATLAS Summary. https://www.proteinatlas.org/ENSG00000133083-DCLK1June 8–2020
|
|
2
|
National Center for Biotechnology Information (NCBI), . DCLK1 doublecortin like kinase 1 [Homo sapiens (human)]. NCBI; Bethesda, MD: 2020, https://www.ncbi.nlm.nih.gov/gene/9201June 8–2020
|
|
3
|
Vreugdenhil E, Kolk SM, Boekhoorn K, Fitzsimons CP, Schaaf M, Schouten T, Sarabdjitsingh A, Sibug R and Lucassen PJ: Doublecortin-like, a microtubule-associated protein expressed in radial glia, is crucial for neuronal precursor division and radial process stability. Eur J Neurosci. 25:635–648. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Walker TL, Yasuda T, Adams DJ and Bartlett PF: The doublecortin-expressing population in the developing and adult brain contains multipotential precursors in addition to neuronal-lineage cells. J Neurosci. 27:3734–3742. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lin PT, Gleeson JG, Corbo JC, Flanagan L and Walsh CA: DCAMKL1 encodes a protein kinase with homology to doublecortin that regulates microtubule polymerization. J Neurosci. 20:9152–9161. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Matsumoto N, Pilz DT and Ledbetter DH: Genomic structure, chromosomal mapping, and expression pattern of human DCAMKL1 (KIAA0369), a homologue of DCX (XLIS). Genomics. 56:179–183. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Burgess HA and Reiner O: Alternative splice variants of doublecortin-like kinase are differentially expressed and have different kinase activities. J Biol Chem. 277:17696–17705. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
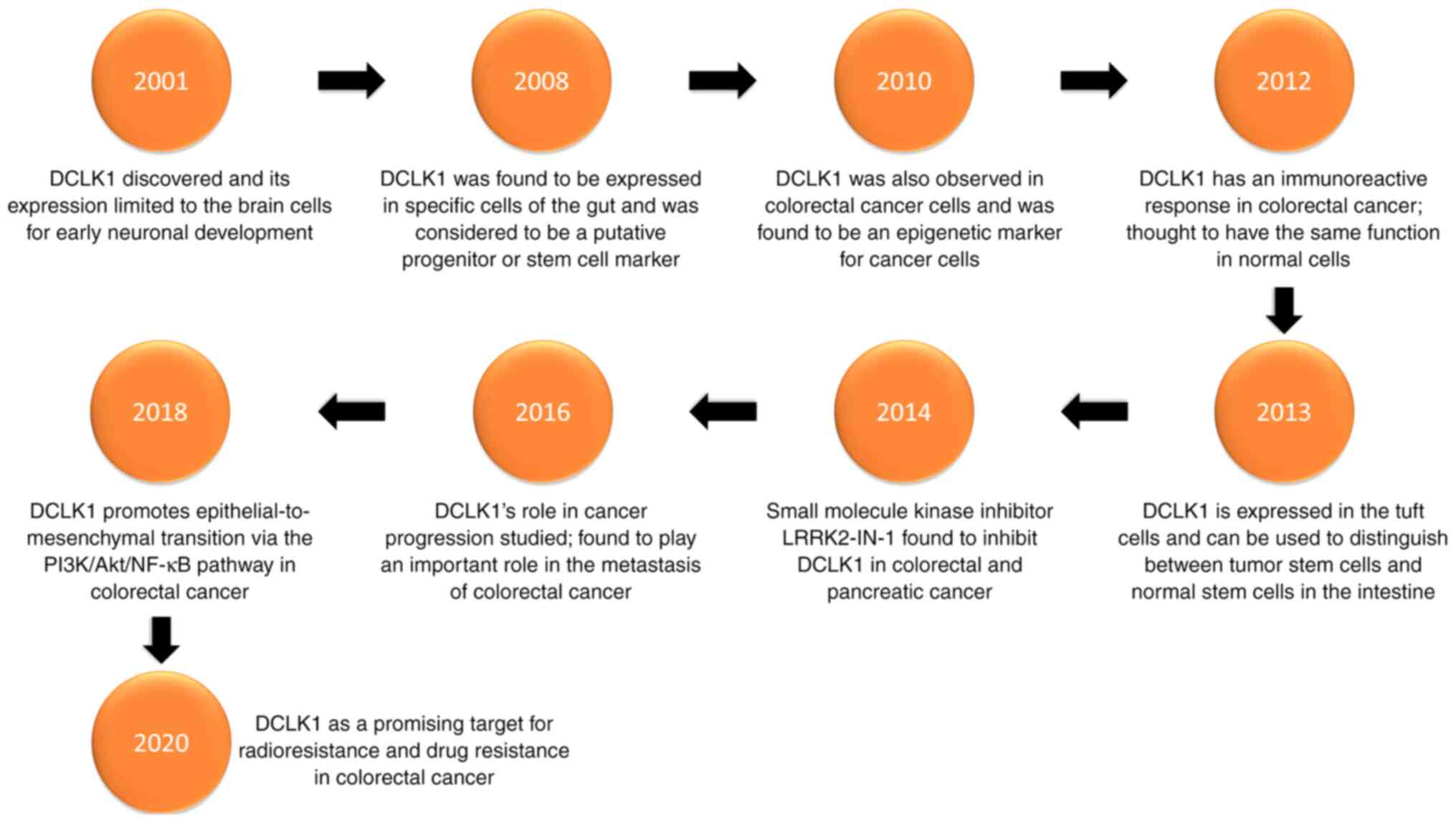
Hirshoren N, Cohen J, Neuman T, Weinberger JM and Eliashar R: DCLK1 expression in gastrointestinal stem cells and neoplasia. J Cancer Ther Res. 1:122012. View Article : Google Scholar
|
|
9
|
Liu H, Wen T, Zhou Y, Fan X, Du T, Gao T, Li L, Liu J, Yang L, Yao J, et al: DCLK1 plays a metastatic-promoting role in human breast cancer cells. Biomed Res Int. 2019:10619792019.PubMed/NCBI
|
|
10
|
Sakaguchi M, Hisamori S, Oshima N, Sato F, Shimono Y and Sakai Y: MIR-137 regulates the tumorigenicity of colon cancer stem cells through the inhibition of DCLK1. Mol Cancer Res. 14:354–362. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chandrakesan P, Yao J, Qu D, May R, Weygant N, Ge Y, Ali N, Sureban SM, Gude M, Vega K, et al: Dclk1, a tumor stem cell marker, regulates pro-survival signaling and self-renewal of intestinal tumor cells. Mol Cancer. 16:302017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chandrakesan P, Panneerselvam J, Qu D, Weygant N, May R, Bronze MS and Houchen CW: Regulatory Roles of Dclk1 in epithelial mesenchymal transition and cancer stem cells. J Carcinog Mutagen. 7:2572016.PubMed/NCBI
|
|
13
|
Liu W, Wang S, Sun Q, Yang Z, Liu M and Tang H: DCLK1 promotes epithelial-mesenchymal transition via the PI3K/Akt/NF-κB pathway in colorectal cancer. Int J Cancer. 142:2068–2079. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sureban SM, May R, Ramalingam S, Subramaniam D, Natarajan G, Anant S and Houchen CW: Selective blockade of DCAMKL-1 results in tumor growth arrest by a Let-7a MicroRNA-dependent mechanism. Gastroenterology. 137:649–59, 659.e1-2. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Roy BC, Ahmed I, Ramalingam S, Jala V, Haribabu B, Ramamoorthy P, Ashcraft J, Valentino J, Anant S, Sampath V and Umar S: Co-localization of autophagy-related protein p62 with cancer stem cell marker dclk1 may hamper dclk1′s elimination during colon cancer development and progression. Oncotarget. 10:2340–2354. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gerbe F, Legraverend C and Jay P: The intestinal epithelium tuft cells: Specification and function. Cell Mol Life Sci. 69:2907–2917. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
DelGiorno KE, Naeem RF, Fang L, Chung CY, Ramos C, Luhtala N, O'Connor C, Hunter T, Manor U and Wahl GM: Tuft cell formation reflects epithelial plasticity in pancreatic injury: Implications for modeling human pancreatitis. Front Physiol. 11:882020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ting HA and von Moltke J: The Immune function of tuft cells at gut mucosal surfaces and beyond. J Immunol. 202:1321–1329. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shu T, Tseng HC, Sapir T, Stern P, Zhou Y, Sanada K, Fischer A, Coquelle FM, Reiner O and Tsai LH: Doublecortin-like kinase controls neurogenesis by regulating mitotic spindles and M phase progression. Neuron. 49:25–39. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu JS, Schubert CR, Fu X, Fourniol FJ, Jaiswal JK, Houdusse A, Stultz CM, Moores CA and Walsh CA: Molecular basis for specific regulation of neuronal kinesin-3 motors by doublecortin family proteins. Mol Cell. 47:707–721. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shin E, Kashiwagi Y, Kuriu T, Iwasaki H, Tanaka T, Koizumi H, Gleeson JG and Okabe S: Doublecortin-like kinase enhances dendritic remodelling and negatively regulates synapse maturation. Nat Commun. 4:14402013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qin J, Mizuguchi M, Itoh M and Takashima S: A novel migration-related gene product, doublecortin, in neuronal migration disorder of fetuses and infants with Zellweger syndrome. Acta Neuropathol. 100:168–173. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lipka J, Kapitein LC, Jaworski J and Hoogenraad CC: Microtubule-binding protein doublecortin-like kinase 1 (DCLK1) guides kinesin-3-mediated cargo transport to dendrites. EMBO J. 35:302–318. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lu Y, Maruyama J, Kuwata K, Fukuda H, Iwasa H, Arimoto-Matsuzaki K, Sugimura H and Hata Y: Doublecortin-like kinase 1 compromises DNA repair and induces chromosomal instability. Biochem Biophys Reports. 16:130–137. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
O'Connell MR, Sarkar S, Luthra GK, Okugawa Y, Toiyama Y, Gajjar AH, Qiu S, Goel A and Singh P: Epigenetic changes and alternate promoter usage by human colon cancers for expressing DCLK1-isoforms: Clinical Implications. Sci Rep. 5:149832015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Singh P, O'Connell M and Shubhashish S: Epigenetic regulation of human DCLK-1 gene during coloncarcinogenesis: Clinical and mechanistic implications. Stem Cell Investig. 3:512016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Barker N and Clevers H: Lineage tracing in the intestinal epithelium. Curr Protoc Stem Cell Biol Chapter. 5:Unit5A.4. 2010.PubMed/NCBI
|
|
28
|
Kretzschmar K and Watt FM: Lineage tracing. Cell. 148:33–45. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Simons BD and Clevers H: Stem cell self-renewal in intestinal crypt. Exp Cell Res. 31:2719–2724. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tan DW and Barker N: Intestinal stem cells and their defining niche. Curr Top Dev Biol. 107:77–107. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Westphalen CB, Asfaha S, Hayakawa Y, Takemoto Y, Lukin DJ, Nuber AH, Brandtner A, Setlik W, Remotti H, Muley A, et al: Long-lived intestinal tuft cells serve as colon cancer-initiating cells. J Clin Invest. 124:1283–1295. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
May R, Qu D, Weygant N, Chandrakesan P, Ali N, Lightfoot SA, Li L, Sureban SM and Houchen CW: Brief report: Dclk1 deletion in tuft cells results in impaired epithelial repair after radiation injury. Stem Cells. 32:822–827. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Qu D, Weygant N, May R, Chandrakesan P, Madhoun M, Ali N, Sureban SM, An G, Schlosser MJ and Houchen CW: Ablation of doublecortin-like kinase 1 in the colonic epithelium exacerbates dextran sulfate sodium-induced colitis. PLoS One. 10:e01342122015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Westphalen CB, Takemoto Y, Tanaka T, Macchini M, Jiang Z, Renz BW, Chen X, Ormanns S, Nagar K, Tailor Y, et al: Dclk1 defines quiescent pancreatic progenitors that promote injury-induced regeneration and tumorigenesis. Cell Stem Cell. 18:441–455. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Middelhoff M, Westphalen CB, Hayakawa Y, Yan KS, Gershon MD, Wang TC and Quante M: Dclk1-expressing tuft cells: Critical modulators of the intestinal niche? Am J Physiol Gastrointest Liver Physiol. 313:G285–G299. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chandrakesan P, May R, Weygant N, Qu D, Berry WL, Sureban SM, Ali N, Rao C, Huycke M, Bronze MS and Houchen CW: Intestinal tuft cells regulate the ATM mediated DNA Damage response via Dclk1 dependent mechanism for crypt restitution following radiation injury. Sci Rep. 6:376672016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sureban SM, May R, Weygant N, Qu D, Chandrakesan P, Bannerman-Menson E, Ali N, Pantazis P, Westphalen CB, Wang TC and Houchen CW: XMD8-92 inhibits pancreatic tumor xenograft growth via a DCLK1-dependent mechanism. Cancer Lett. 351:151–161. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gagliardi G, Goswami M, Passera R and Bellows CF: DCLK1 immunoreactivity in colorectal neoplasia. Clin Exp Gastroenterol. 5:35–42. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Qu D, Weygant N, Yao J, Chandrakesan P, Berry WL, May R, Pitts K, Husain S, Lightfoot S, Li M, et al: Overexpression of DCLK1-AL increases tumor cell invasion, drug resistance, and KRAS activation and can be targeted to inhibit tumorigenesis in pancreatic cancer. J Oncol. 2019:64029252019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Powrózek T, Krawczyk P, Nicoś M, Kuźnar-Kamińska B, Batura-Gabryel H and Milanowski J: Methylation of the DCLK1 promoter region in circulating free DNA and its prognostic value in lung cancer patients. Clin Transl Oncol. 18:398–404. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Whorton J, Sureban SM, May R, Qu D, Lightfoot SA, Madhoun M, Johnson M, Tierney WM, Maple JT, Vega KJ and Houchen CW: DCLK1 is detectable in plasma of patients with barrett's esophagus and esophageal adenocarcinoma. Dig Dis Sci. 60:509–513. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wu X, Qu D, Weygant N, Peng J and Houchen CW: Cancer stem cell marker DCLK1 correlates with tumorigenic immune infiltrates in the colon and gastric adenocarcinoma microenvironments. Cancers (Basel). 12:2742020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ito H, Tanaka S, Akiyama Y, Shimada S, Adikrisna R, Matsumura S, Aihara A, Mitsunori Y, Ban D, Ochiai T, et al: Dominant expression of DCLK1 in human pancreatic cancer stem cells accelerates tumor invasion and metastasis. PLoS One. 11:e01465642016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gao T, Wang M, Xu L, Wen T, Liu J and An G: DCLK1 is up-regulated and associated with metastasis and prognosis in colorectal cancer. J Cancer Res Clin Oncol. 142:2131–2140. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sureban SM, May R, Qu D, Weygant N, Chandrakesan P, Ali N, Lightfoot SA, Pantazis P, Rao CV, Postier RG and Houchen CW: DCLK1 regulates pluripotency and angiogenic factors via microRNA-dependent mechanisms in pancreatic cancer. PLoS One. 8:e739402013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ge Y, Weygant N, Qu D, May R, Berry WL, Yao J, Chandrakesan P, Zheng W, Zhao L, Zhao KL, et al: Alternative splice variants of DCLK1 mark cancer stem cells, promote self-renewal and drug-resistance, and can be targeted to inhibit tumorigenesis in kidney cancer. Int J Cancer. 143:1162–1175. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Fan CB, Yan XH, Tian M, Zhang S, Liu JL, Sheng YX, Dong L and Zhang WL: Long non-coding RNA NEAT1 regulates Hodgkin's lymphoma cell proliferation and invasion via miR-448 mediated regulation of DCLK1. Eur Rev Med Pharmacol Sci. 24:6219–6227. 2020.PubMed/NCBI
|
|
48
|
Ali N, Nguyen CB, Chandrakesan P, Wolf RF, Qu D, May R, Goretsky T, Fazili J, Barrett TA, Li M, et al: Doublecortin-like kinase 1 promotes hepatocyte clonogenicity and oncogenic programming via non-canonical β-catenin-dependent mechanism. Sci Rep. 10:105782020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Gzil A, Zarębska I, Bursiewicz W, Antosik P, Grzanka D and Szylberg Ł: Markers of pancreatic cancer stem cells and their clinical and therapeutic implications. Mol Biol Rep. 46:6629–6645. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Subramaniam D, Angulo P, Ponnurangam S, Dandawate P, Ramamoorthy P, Srinivasan P, Iwakuma T, Weir SJ, Chastain K and Anant S: Suppressing STAT5 signaling affects osteosarcoma growth and stemness. Cell Death Dis. 11:1492020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Fesler A, Liu H and Ju J: Modified miR-15a has therapeutic potential for improving treatment of advanced stage colorectal cancer through inhibition of BCL2, BMI1, YAP1 and DCLK1. Oncotarget. 9:2367–2383. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kantara C, O'Connell M, Sarkar S, Moya S, Ullrich R and Singh P: Curcumin promotes autophagic survival of a subset of colon cancer stem cells, which are ablated by DCLK1-siRNA. Cancer Res. 74:2487–2498. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sarkar S, O'Connell MR, Okugawa Y, Lee BS, Toiyama Y, Kusunoki M, Daboval RD, Goel A and Singh P: FOXD3 regulates csc marker, dclk1-s, and invasive potential: Prognostic implications in colon cancer. Mol Cancer Res. 15:1678–1691. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Weygant N, Ge Y, Qu D, Kaddis JS, Berry WL, May R, Chandrakesan P, Bannerman-Menson E, Vega KJ, Tomasek JJ, et al: Survival of patients with gastrointestinal cancers can be predicted by a surrogate microRNA signature for cancer stem-like cells marked by DCLK1 kinase. Cancer Res. 76:4090–4099. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Mirzaei A, Madjd Z, Kadijani AA, Tavakoli-Yaraki M, Modarresi MH, Verdi J, Akbari A and Tavoosidana G: Evaluation of circulating cellular DCLK1 protein, as the most promising colorectal cancer stem cell marker, using immunoassay based methods. Cancer Biomark. 17:301–311. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Bailey JM, Alsina J, Rasheed ZA, McAllister FM, Fu YY, Plentz R, Zhang H, Pasricha PJ, Bardeesy N, Matsui W, et al: DCLK1 marks a morphologically distinct subpopulation of cells with stem cell properties in pre-invasive pancreatic cancer. Gastroenterology. 146:245–256. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Vedeld HM, Skotheim RI, Lothe RA and Lind GE: The recently suggested intestinal cancer stem cell marker DCLK1 is an epigenetic biomarker for colorectal cancer. Epigenetics. 9:346–350. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Mirzaei A, Tavoosidana G, Modarressi MH, Rad AA, Fazeli MS, Shirkoohi R, Tavakoli-Yaraki M and Madjd Z: Upregulation of circulating cancer stem cell marker, DCLK1 but not Lgr5, in chemoradiotherapy-treated colorectal cancer patients. Tumor Biol. 36:4801–4810. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Sarkar S, Popov VL, O'Connell MR, Stevenson HL, Lee BS, Obeid RA, Luthra GK and Singh P: A novel antibody against cancer stem cell biomarker, DCLK1-S, is potentially useful for assessing colon cancer risk after screening colonoscopy. Lab Invest. 97:1245–1261. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Krishnamachary B, Subramaniam D, Dandawate P, Ponnurangam S, Srinivasan P, Ramamoorthy P, Umar S, Thomas SM, Dhar A, Septer S, et al: Targeting transcription factor TCF4 by γ-Mangostin, a natural xanthone. Oncotarget. 10:5576–5591. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhou B, Sun C, Hu X, Zhan H, Zou H, Feng Y, Qiu F, Zhang S, Wu L and Zhang B: MicroRNA-195 suppresses the progression of pancreatic cancer by targeting DCLK1. Cell Physiol Biochem. 44:1867–1881. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Deng H, Qianqian G, Ting J and Aimin Y: miR-539 enhances chemosensitivity to cisplatin in non-small cell lung cancer by targeting DCLK1. Biomed Pharmacother. 106:1072–1081. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ji D, Zhan T, Li M, Yao Y, Jia J, Yi H, Qiao M, Xia J, Zhang Z, Ding H, et al: Enhancement of sensitivity to chemo/radiation therapy by using miR-15b against DCLK1 in colorectal cancer. Stem Cell Reports. 11:1506–1522. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Li J, Wang Y, Ge J, Li W, Yin L, Zhao Z, Liu S, Qin H, Yang J, Wang L, et al: Doublecortin-like kinase 1 (DCLK1) regulates B cell-specific moloney murine leukemia virus insertion site 1 (Bmi-1) and is associated with metastasis and prognosis in pancreatic cancer. Cell Physiol Biochem. 51:262–277. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Rangarajan P, Subramaniam D, Paul S, Kwatra D, Palaniyandi K, Islam S, Harihar S, Ramalingam S, Gutheil W, Putty S, et al: Crocetinic acid inhibits hedgehog signaling to inhibit pancreatic cancer stem cells. Oncotarget. 6:27661–27673. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
National Institutes of Health (NIH), . DCX gene doublecortin-Genetics Home Reference. NIH; Bethesda, MD: 2020, https://ghr.nlm.nih.gov/gene/DCXAugust 25–2020
|
|
67
|
National Center for Biotechnology Information (NCBI), . DCX doublecortin [Homo sapiens (human)]. NCBI; Bethesda, MD: 2020, https://www.ncbi.nlm.nih.gov/gene/1641August 25–2020
|
|
68
|
Horesh D, Sapir T, Francis F, Wolf SG, Caspi M, Elbaum M, Chelly J and Reiner O: Doublecortin, a stabilizer of microtubules. Hum Mol Genet. 8:1599–1610. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Gleeson JG, Peter TL, Flanagan LA and Walsh CA: Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron. 23:257–271. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Francis F, Koulakoff A, Boucher D, Chafey P, Schaar B, Vinet MC, Friocourt G, McDonnell N, Reiner O, Kahn A, et al: Doublecortin is a developmentally regulated, microtubule- associated protein expressed in migrating and differentiating neurons. Neuron. 23:247–256. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
STRING: DCLK1 protein (human)-STRING coexpression view. https://string-db.org/cgi/network?taskId=bnI9rrwHb9Jm&sessionId=bLIMVpiJ6j48June 29–2020
|
|
72
|
Koizumi H, Tanaka T and Gleeson JG: Doublecortin-like kinase functions with doublecortin to mediate fiber tract decussation and neuronal migration. Neuron. 49:55–66. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Huttlin EL, Ting L, Bruckner RJ, Gebreab F, Gygi MP, Szpyt J, Tam S, Zarraga G, Colby G, Baltier K, et al: The BioPlex Network: A systematic exploration of the human interactome. Cell. 162:425–440. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Huttlin EL, Bruckner RJ, Paulo JA, Cannon JR, Ting L, Baltier K, Colby G, Gebreab F, Gygi MP, Parzen H, et al: Architecture of the human interactome defines protein communities and disease networks. Nature. 545:505–509. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Slepak TI, Salay LD, Lemmon VP and Bixby JL: Dyrk kinases regulate phosphorylation of doublecortin, cytoskeletal organization, and neuronal morphology. Cytoskeleton (Hoboken). 69:514–527. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
National Center for Biotechnology Information (NCBI), . ANK2 ankyrin 2 [Homo sapiens (human)]. NCBI; Bethesda, MD: 2020, https://www.ncbi.nlm.nih.gov/gene?cmd=Retrieve&dopt=full_report&list_uids=287June 29–2020
|
|
77
|
Liu ZQ, He WF, Wu YJ, Zhao SL, Wang L, Ouyang YY and Tang SY: LncRNA SNHG1 promotes EMT process in gastric cancer cells through regulation of the miR-15b/DCLK1/Notch1 axis. BMC Gastroenterol. 20:1562020. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Meng QB, Yu JC, Kang WM, Ma ZQ, Zhou WX, Li J, Zhou L, Cao ZJ and Tian SB: Expression of doublecortin-like kinase 1 in human gastric cancer and its correlation with prognosis. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 35:639–644. 2013.(In Chinese). PubMed/NCBI
|
|
79
|
Cao W, Wei W, Zhan Z, Xie D, Xie Y and Xiao Q: Regulation of drug resistance and metastasis of gastric cancer cells via the microRNA647-ANK2 axis. Int J Mol Med. 41:1958–1966. 2018.PubMed/NCBI
|
|
80
|
Cao W, Wei W, Zhan Z, Xie D, Xie Y and Xiao Q: Role of miR-647 in human gastric cancer suppression. Oncol Rep. 37:1401–1411. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Girotto G, Vuckovic D, Buniello A, Lorente-Cánovas B, Lewis M, Gasparini P and Steel KP: Expression and replication studies to identify new candidate genes involved in normal hearing function. PLoS One. 9:e853522014. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Wells HRR, Newman TA and Williams FMK: Genetics of age-related hearing loss. J Neurosci Res. 98:1698–1704. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Vuckovic D: Identification of the genetic determinants of hearing loss by means of genetic isolates. Università degli studi di Trieste; Trieste: 2015, https://www.openstarts.units.it/handle/10077/10847March 2–2015
|
|
84
|
Cancer Genetics Web: MSI1, . Gene Summary. http://www.cancerindex.org/geneweb/MSI1.htmAugust 29–2020
|
|
85
|
Song X, Zhou C, Zhou S, Zhang L, Feng G, Zhao D and Huang F: The expression patterns of Mis1 is related with the glioma grade and the cytoplasmic Mis1 promotes angiogenesis. Tissue Cell. 45:1–6. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Gao C, Han C, Yu Q, Zhou J, Guan Y, Li N, Zhou J, Tian Y and Zhang Y: Downregulation of Msi1 suppresses the growth of human colon cancer by targeting p21cip1. Int J Oncol. 46:732–740. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Moghbeli M, Forghanifard MM, Sadrizadeh A, Mozaffari HM, Golmakani E and Abbaszadegan MR: Role of Msi1 and MAML1 in regulation of notch signaling pathway in patients with esophageal squamous cell carcinoma. J Gastrointest Cancer. 46:365–369. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Sureban SM, Qu D and Houchen CW: Regulation of miRNAs by agents targeting the tumor stem cell markers DCLK1, MSI1, LGR5, and BMI1. Curr Pharmacol Rep. 1:217–222. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Sakakibara S, Nakamura Y, Yoshida T, Shibata S, Koike M, Takano H, Ueda S, Uchiyama Y, Noda T and Okano H: RNA-binding protein Musashi family: Roles for CNS stem cells and a subpopulation of ependymal cells revealed by targeted disruption and antisense ablation. Proc Natl Acad Sci USA. 99:15194–15199. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Kim H, Lee C, Kim WH, Maeng YH and Jang BG: Expression profile of intestinal stem cell markers in colitis-associated carcinogenesis. Sci Rep. 7:65332017. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Ye F, Zhou C, Cheng Q, Shen J and Chen H: Stem-cell-abundant proteins Nanog, Nucleostemin and Musashi1 are highly expressed in malignant cervical epithelial cells. BMC Cancer. 8:1082008. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Sureban SM, May R, Qu D, Asfa S, Anant S and Houchen CW: Knockdown of Musashi-1 Results in Tumor Growth Arrest Through Inhibition of c-MYC, Notch-1 and EMT by Let-7a, Mir-144 and Mir-200a MicroRNAs dependent mechanisms respectively. Gastroenterology. 140:S482011. View Article : Google Scholar
|
|
93
|
Vo DT, Qiao M, Smith AD, Burns SC, Brenner AJ and Penalva LOF: The oncogenic RNA-binding protein Musashi1 is regulated by tumor suppressor miRNAs. RNA Biol. 8:817–828. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Kim CK, Yang VW and Bialkowska AB: The role of intestinal stem cells in epithelial regeneration following radiation-induced gut injury. Current Stem Cell Rep. 3:320–332. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
GeneMANIA, . DCLK1: H. sapiens. https://genemania.org/September 17–2020
|
|
96
|
GeneCards, . TNIK Gene (Protein Coding). https://www.genecards.org/cgi-bin/carddisp.pl?gene=TNIKSeptember 17–2020
|
|
97
|
GeneCards, . CALM1 Gene (Protein Coding). https://www.genecards.org/cgi-bin/carddisp.pl?gene=CALM1September 17–2020
|
|
98
|
GeneCards, . CDCA8 Gene (Protein Coding). https://www.genecards.org/cgi-bin/carddisp.pl?gene=CDCA8&keywords=CDCA8September 17–2020
|
|
99
|
GeneCards, . DGUOK Gene (Protein Coding). https://www.genecards.org/cgi-bin/carddisp.pl?gene=DGUOK&keywords=DGUOKSeptember 17–2020
|
|
100
|
GeneCards, . FBLIM1 Gene (Protein Coding). https://www.genecards.org/cgi-bin/carddisp.pl?gene=FBLIM1&keywords=FBLIM1September 17–2020
|
|
101
|
GeneCards, . HAX1 Gene (Protein Coding). https://www.genecards.org/cgi-bin/carddisp.pl?gene=HAX1&keywords=Dclk1September 17–2020
|
|
102
|
GeneCards, . NUFIP1 Gene (Protein Coding). https://www.genecards.org/cgi-bin/carddisp.pl?gene=NUFIP1&keywords=NUFIp1September 17–2020
|
|
103
|
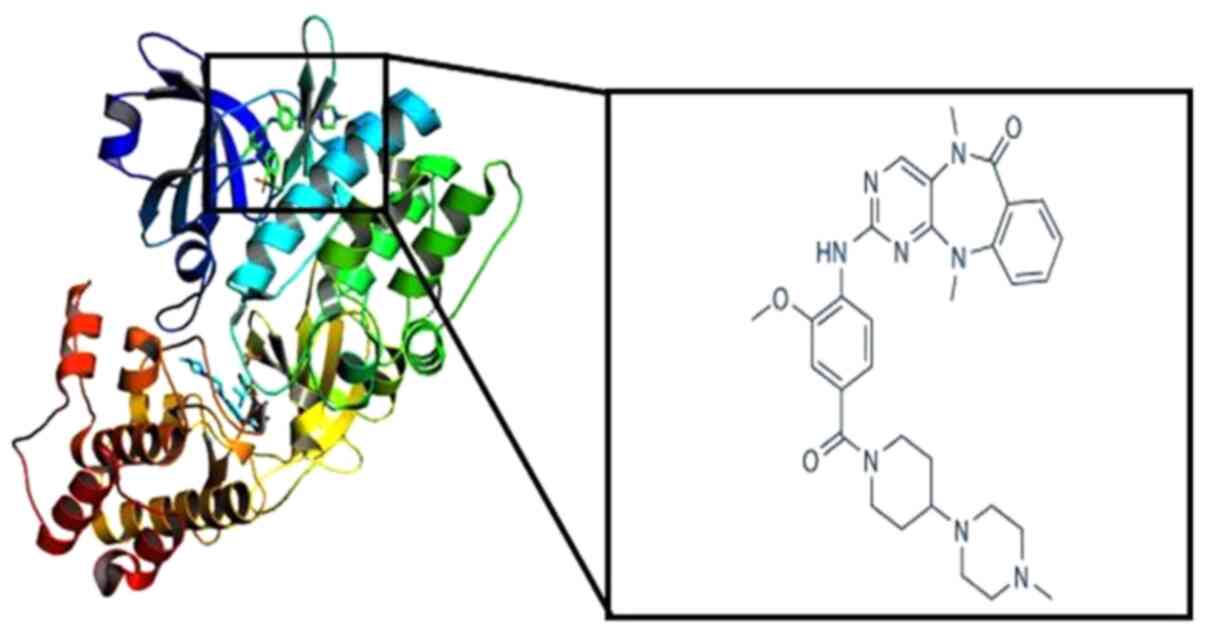
Weygant N, Qu D, Berry WL, May R, Chandrakesan P, Owen DB, Sureban SM, Ali N, Janknecht R and Houchen CW: Small molecule kinase inhibitor LRRK2-IN-1 demonstrates potent activity against colorectal and pancreatic cancer through inhibition of doublecortin-like kinase 1. Mol Cancer. 13:1032014. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Yan R, Li J, Zhou Y, Yao L, Sun R, Xu Y, Ge Y and An G: Inhibition of DCLK1 down-regulates PD-L1 expression through Hippo pathway in human pancreatic cancer. Life Sci. 241:1171502020. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Lorenzo N, Sabina DM, Guido C, Ilaria Grazia Z, Samira S, Valeria A, Daniele C, Diletta O, Antonella G, Marco M, et al: DCLK1, a putative stem cell marker in human cholangiocarcinoma. Hepatology. 73:144–159. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Kumar C, P T V L and Arunachalam A: Structure based pharmacophore study to identify possible natural selective PARP-1 trapper as anti-cancer agent. Comput Biol Chem. 80:314–323. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Fu Y, Ye T, Liu YX, Wang J and Ye F: Based on the virtual screening of multiple pharmacophores, docking and molecular dynamics simulation approaches toward the discovery of novel HPPD inhibitors. Int J Mol Sci. 21:55462020. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Shiri F, Pirhadi S and Ghasemi JB: Dynamic structure based pharmacophore modeling of the Acetylcholinesterase reveals several potential inhibitors. J Biomol Struct Dyn. 37:1800–1812. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Benafqir M, Hsini A, Laabd M, Laktif T, Ait Addi A, Albourine A and Alem NE: Application of Density Functional Theory computation (DFT) and Process Capability Study for performance evaluation of Orthophosphate removal process using Polyaniline@Hematite-titaniferous sand composite (PANI@HTS) as a substrate. Sep Purif Technol. 236:1162862020. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Zhao Z, Li E, Qin Y, Liu X, Zou Y, Wu H and Zhu T: Density functional theory (DFT) studies of vanadium-titanium based selective catalytic reduction (SCR) catalysts. J Environ Sci (China). 90:119–137. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Vafaei-Nezhad M, Ghiasi R and Shafiei F: Conformational Analysis of 2-halo-1,3,2-dioxaphosphinanes: A density functional theory (DFT) Investigation. Chem Methodol. 4:161–171. 2020. View Article : Google Scholar
|
|
112
|
Schaller D, Šribar D, Noonan T, Deng L, Nguyen TN, Pach S, Machalz, Bermudez M and Wolber G: Next generation 3D pharmacophore modeling. WIREs Comput Mol Sci. 10:e14682020. View Article : Google Scholar
|
|
113
|
Lee JY, Krieger JM, Li H and Bahar I: Pharmmaker: Pharmacophore modeling and hit identification based on druggability simulations. Protein Sci. 29:76–86. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Pal S, Kumar V, Kundu B, Bhattacharya D, Preethy N, Reddy MP and Talukdar A: Ligand-based pharmacophore modeling, virtual screening and molecular docking studies for discovery of potential topoisomerase i inhibitors. Comput Struct Biotechnol J. 17:291–310. 2019. View Article : Google Scholar : PubMed/NCBI
|