Introduction
Pancreatic cancer is a major neoplasm with a high
mortality and its prevalence is increasing worldwide. Various
efforts have been made to improve its diagnosis and treatment, but
the prognosis is poor and the 5-year survival rate is only
appproximately 4% (1,2). The reason for the poor prognosis is
that the pancreas is a deeply located organ, symptoms are not
immediately apparent, and early detection therefore is difficult.
Moreover, surgery is impossible due to the invasion into the
surrounding organs at the time of onset. On the other hand, the
biological properties of pancreatic cancer are characteristic and
the tumors have more abundant fibrous stroma than other neoplasms
(3). Despite the high degree of
this so-called ‘desmoplastic change’, pancreatic cancer cells are
characterized by readily invading the fibrous stroma (4).
Various cell signaling pathways are involved in the
invasion mechanism of pancreatic cancer. For example, it has been
suggested that several cytokines belonging to the transforming
growth factor-β family, such as bone morphogenetic protein,
contribute to its regulation; these molecules are involved in the
epithelial-mesenchymal transition (EMT) and cancer-stroma
interaction (5,6). Recent findings suggested that
interleukin-32 (IL-32) is deeply involved in the invasiveness of
pancreatic cancer, based on a comprehensive gene analysis of highly
invasive pancreatic cancer lines subcloned by the invasion assay
(IA) method (7). In this study,
the gene and protein expression levels of IL-32 were distinctly
increased in highly invasive cell lines.
To obtain invasiveness, cancer cells must first be
motile and able to disrupt the surrounding hard interstitium,
resulting in EMT. EMT is one of the processes that occurs in the
embryonic period, as a result of various signaling pathways, but is
also a phenomenon observed in tumor tissues (8). EMT cells lose the expression of
molecules that cause epithelial binding, produce mesenchymal
substances such as vimentin and α-smooth muscle actin, and acquire
invasiveness (9). It is crucial to
elucidate the factors involved in this invasion mechanism that
occur early in pancreatic cancer cells. Although there are a few
reports available on the relationship between IL-32 expression and
pancreatic cancer (10,11), there is, at present, no report that
IL-32 has a direct relationship with invasiveness. Therefore, the
aim of the present study was to determine, by regulating the
expression of the IL-32 gene, whether IL-32 expression
contributes to the invasiveness of pancreatic cancer and through
what molecular mechanism the invasive ability is obtained. Due to
the large number of molecules potentially involved in the invasion
mechanism, representatives from those involved in EMT and
subsequent processes were selected, including E-cadherin,
Claudin-1, Slug, CD44 and CTGF as EMT-related molecules (12–14),
MMP2 and MMP9 as a molecule disrupting the surrounding interstitium
(15), and Wnt5a/b and
transglutaminase (TGM) 2 for cell motility (16–18).
Materials and methods
Cell lines and cell culture
The human pancreatic cancer cell lines (PANC-1, MIA
PaCa-2) were obtained from the American Type Culture Collection.
Cells with high invasive potential were established from PANC-1
cells by the IA method, as described in a previous report (7). The original cell line was used as the
parent (P) and the subcloned cell line that had acquired high
invasiveness was referred to as (S). Each cell line was maintained
in RPMI-1640 culture medium (FUJIFILM Wako Pure Chemical
Corporation) containing 10% (v/v) fetal bovine serum, 100 IU/ml
penicillin and 100 µg/ml streptomycin at the condition of 37°C and
5% CO2.
cDNA cloning and overexpression of
human IL-32
The method used to establish cells in which IL-32 is
continuously highly expressed is mentioned below. Using the
first-strand cDNA derived from MIA PaCa-2 cells with low invasive
potential as a template, human IL-32 cDNA was amplified by PCR with
primers 5′-ggggtaccGCCATGTGCTTCCCGAAGGTC
CTCTCTG-3′ (the KpnI site is underlined) and 5′-gggcggccgc
TCATTTTGAGGATTGGGGTTCAGAG-3′ (the NotI site is underlined)
and subcloned into pLITMUS 28 plasmid vector (New England Biolabs).
After confirmation of the cDNA sequence using an ABI3500 Genetic
Analyzer (Thermo Fischer Scientific, Inc.), the KpnI- and
NotI-digested IL-32β and IL-32ε cDNA fragments were cloned
between the KpnI and NotI sites of the pEBMulti-Neo
vector (FUJIFILM Wako Pure Chemical Corporation), to yield the
plasmids pEB-IL-32β and pEB-IL-32ε. Then, the pEB-IL32β, pEB-IL32ε
and control pEB Multi-Neo plasmids were transfected into P cells
with Lipofectamine 3000 reagent (Thermo Fischer Scientific, Inc.),
according to the manufacturer's protocol. After 48 h, the
transfected P cells were selected with G418 (500 µg/ml) for 2
weeks, yielding IL-32β-overexpressing (β), IL-32ε-overexpressing
(ε) and control cells with the unmodified plasmid (MOCK).
Small interfering (si) RNA-mediated
downregulation of IL-32 expression
For the RNA interference assay, a targeted small
interfering RNA (siRNA) oligonucleotide was obtained from the
Nippon gene. The sequences of the IL-32 siRNA were as
follows: Sense, 5′-GGGAGAGCUUUUGUGACAAdTdT-3′; anti-sense,
5′-UUGUCACAAAAGCUCUCCCdTdT-3′. As a negative control, the si
sequences for Luciferase were as follows: sense,
5′-CGUACGCGGAAUACUUCGATT-3′; anti-sense,
5′-UCGAAGUAUUCCGCGUACGTT-3′. In particular, S and P cells were
transfected with 20 nM of each siRNA using Lipofectamine™ RNAiMAX
Transfection Reagent (Thermo Fischer Scientific, Inc.) to knock
down the IL-32 gene, and then incubated for a further 24 h
at 37°C. S cells transfected with IL-32 siRNA were referred to as
S-si cells and the negative control cells were denoted as S-siLuci,
and they were used for the invasion assay. P cells transfected with
IL-32 siRNA were referred to as P-si cells and the negative control
cells were denoted as P-siLuci cells and were used for the
measurement of mRNA and protein expression levels.
Construction of genome editing
plasmids to induce mutations in IL-32 loci by CRISPR/Cas9
pX362 was used for genome editing of the IL-32 locus
(19). After digestion of pX362
with BbsI, the oligonucleotides
5′-caccTGATGACATGAAGAAGCTGA-3′ and 5′-aaacTCAGCTTCTTCATGTCATCA-3′
(capitals and small letters are protospacer sequences and
additional sequences to clone into the BbsI site,
respectively) corresponding to the single guide RNA target sequence
in the exon 3 of IL-32, were annealed and subcloned into the
BbsI site of pX362 to yield pX362IL-32-1.
Establishment of IL-32 knockout cell
lines
To establish knockout cells in which the expression
of IL-32 was continuously arrested the pX362IL32-1 plasmid, in
which the IL-32 sequence was mutated, was transfected into P cells
using Lipofectamine 3000 reagent (Thermo Fischer Scientific, Inc.).
The DNA-transfected P cells were selected with puromycin (Thermo
Fischer Scientific, Inc.; 2 µg/ml) for 48 h and incubated for 2
weeks. Then, these were cloned individually by limiting dilution
and clones in which IL-32 mRNA expression was appropriately
suppressed were selected. Finally, a knockout cell line of IL-32
(ko) was established.
RNA isolation
Cytoplasmic RNA was extracted using a
PureLink® RNA Mini Kit (Thermo Fischer Scientific, Inc.)
from sub-confluent cells. Concentrations of RNA were measured with
a NanoDrop 1000 (Thermo Fisher Scientific, Inc.).
Selection of the downstream molecules related to
IL-32 and validation of expression changes by western blot analysis
(WB) and reverse transcriptase-quantitative polymerase chain
reaction (RT-qPCR)
WB
Protein extraction was performed using a Complete
Lysis-M (Roche) from sub-confluent cells. Concentrations were
calculated using the Bradford method. After adjusting 18 µg of each
protein sample was separated by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis with 4–15% gradient
gels, and transfected to PVDF membrane Trans-Blot Turbo Transfer
Pack (Bio-Rad) using Trans-Blot Turbo Blotting System (Bio-Rad).
The membranes were blocked with 5% skim milk at room temperature
for 1 h, and then treated with each primary antibody at 4°C
overnight. Primary antibodies were as follows: anti-IL-32 (1:500;
cat. no. 11079-1-AP; ProteinTech Group, Inc.), anti-E-cadherin
(cat. no. 3195), anti-CD44 (cat. no. 3570), anti-GAPDH (cat. no.
5174), anti-β-actin (cat. no. 3700), anti-Wnt5a/b (cat. no. 2530)
(all 1:1,000; all from Cell Signaling Technology) and anti-Claudin
(1:100; cat. no. ab15098; Abcam) and anti-MMP2 (cat. no. ab92536),
anti-MMP9 (cat. no. ab137867), anti-Slug (cat. no. ab27568),
anti-CTGF (cat. no. ab6992) (all 1:1000 dilution; and all from
Abcam) and anti-TGM2 (diluted 1:2000; cat. no. ab2386; Abcam).
After washing, the membranes were reacted with the secondary
antibody at room temperature for 1 h. Secondary antibodies were
horseradish peroxidase-conjugated sheep anti-rabbit IgG (cat. no.
7074S) or anti-mouse IgG (cat. no. 7076S; 1:10000; Cell Signaling
Technology). Detection and visualization were performed with an
ImageQuant LAS500 (GE Healthcare) system using ECL Select Western
Blotting Detection Reagent (GE Healthcare) as the chemiluminescence
detection reagent.
RT-qPCR
RNA was extracted as aforementioned. cDNA was
obtained from the extracted RNA using a Transcriptor Universal cDNA
Master Kit (Roche). Next, quantitative PCR was conducted using a
FastStart Essential DNA Green Master (Roche). Primer sequences for
IL-32 amplification were as follows: sense
5′-AGCTGGAGGACGACTTCAAA-3′; anti-sense 5′-AGAGCAGCAGAAACTCTGGA-3′.
Primer sequences for internal reference (β-actin) were as follows:
sense 5′-CTGGAACGGTGAAGGTGACA-3′; anti-sense
5′-AAGGGACTTCCTGTAACAATGCA-3′. Each cDNA and specific primer were
adjusted to final concentrations of 2.5 ng/µl and 500 nM,
respectively, and then mixed in a total volume of 20 µl of
FastStart Essential DNA Green Master reagent. The qPCR reaction was
carried out in a LightCycler® 480 (Roche) with setting
denaturation at 95°C for 10 sec, annealing at 60°C for 10 sec, and
extension at 72°C for 15 sec per cycle, with 45 cycles. The
analysis was conducted using LightCycler Nano Software (Roche). The
relative expression levels of mRNA were calculated based on
quantitative cycles (Cq) and using the relative Cq
(2−ΔΔCq) method (20).
Real-time monitoring of invasion
ability
To evaluate the invasive ability of the cells (MOCK,
β, ε, S-siLuci, S-si, P, ko), invasion was measured in real-time
using the xCelligence system (ACEA Biosciences), as reported
previously (7).
Morphological and immunohistochemical
studies
Studies of the morphological changes of P, ε, β, and
ko cells were carried out by observing living cells in culture with
an inverted microscope (Olympus IX70; Olympus Corporation). An
immunohistochemical study analyzed 10% buffered formalin-fixed for
<2 days at room temperature and paraffin-embedded sections of
pancreatic ductal adenocarcinoma tumors, surgically resected and
obtained from University Hospital of Toyama. The samples comprised
normal pancreatic tissue and invasive lesions. Immunohistochemistry
was performed using a BenchMark GX automated IHC/ISH slide staining
system (Roche). Deparaffinized sections (4 µm) sections were
heat-treated for antigen retrieval at 98°C for 30 min and then
incubated with 1:100 IL-32 antibody at room temperature for 1 h.
Incubated sections were visualized by diaminobenzidine reaction for
2 min.
Ethics approval was obtained from the University
Hospital of Toyama Ethics Committee (no. R2020054). Although
consent was not obtained from each patient, the patients were
notified of the details of the study by opt-out consent and had the
right to refuse participation in the study.
Results
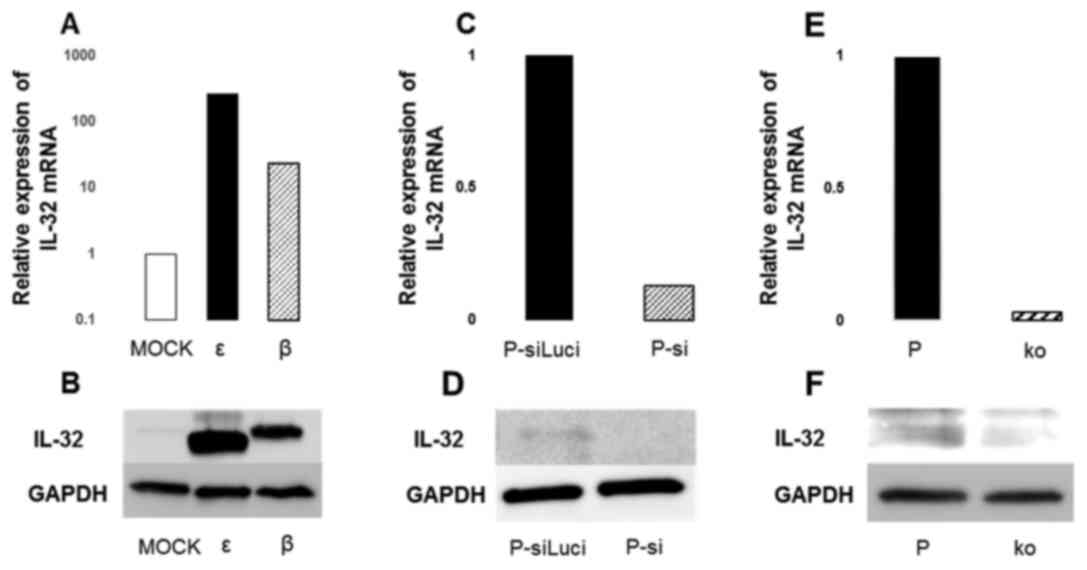
mRNA and protein expression levels of
IL-32 in the cells overexpressing IL-32
Cells constitutively expressing IL-32 were prepared
by transfection of the splice variants of IL-32, IL-32ε and IL-32β,
into P cells. The expression level of IL-32 mRNA was markedly
increased in both β and ε cells, compared with MOCK cells (Fig. 1A). At the protein level, it was
confirmed that both ε and β cells had enhanced expression,
reflecting the difference in the molecular weights of the
translated proteins (Fig. 1B).
The mRNA and protein expression levels
of IL-32 with siRNA transfection and knockout of IL-32
The mRNA expression in P cells was knocked down
using IL-32 siRNA transfection or knockouts following the
CRISPR-cas9 method. The expression of IL-32 mRNA was reduced in
both P-si and ko cells (Fig. 1C and
E). In P-si cells, the expression level of IL-32 mRNA was
reduced to ~1/5 of that of P-siLuci (Fig. 1C). However, the effect of the siRNA
decreased after approximately 4 days and the expression levels
gradually recovered (data not shown). By contrast, the expression
of IL-32 mRNA was suppressed to ~1/32 in ko cells, compared with P
cells (Fig. 1E). Furthermore, the
IL-32 mRNA expression was persistently suppressed and did not
recover, even after more than one week (data not shown). Protein
expression of IL-32 was almost completely suppressed in ko cells
(Fig. 1F).
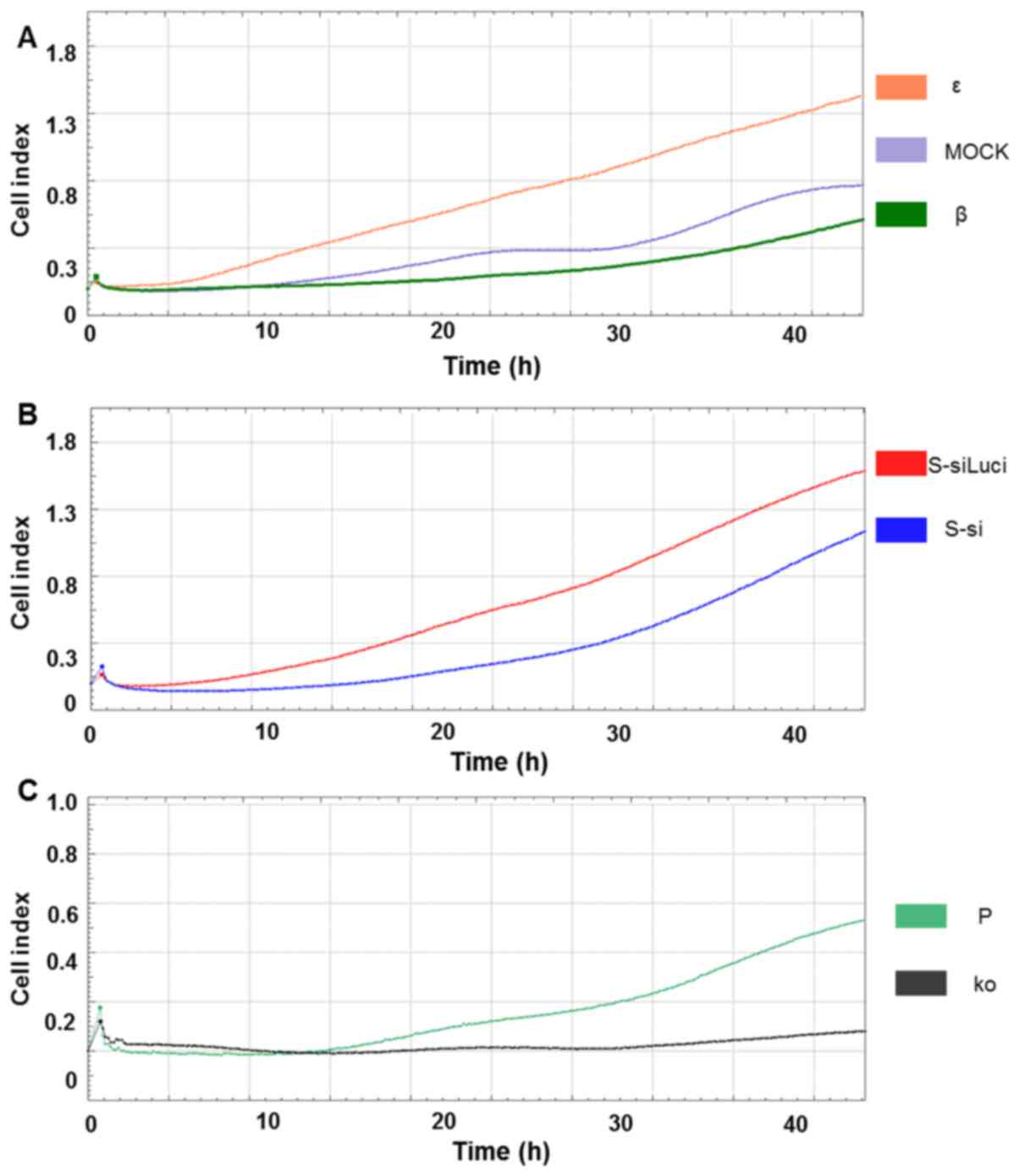
Changes in invasiveness with
overexpression and suppression of IL-32
The number of invading ε cells gradually increased
immediately after the beginning of the analysis and they continued
to be invasive even after 45 h. β cells invaded slowly, compared to
ε cells and their tendency for invasion was similar to MOCK cells
(Fig. 2A). S-si cells began to
invade slowly after 20 h and the number of invading cells was lower
than that of the S-siLuci control (Fig. 2B). Suppression of IL-32 expression
in S-si is shown in Fig. S1. The
ko cells had considerably reduced invasiveness and almost no
invasive cells were observed, even after 50 h (Fig. 2C).
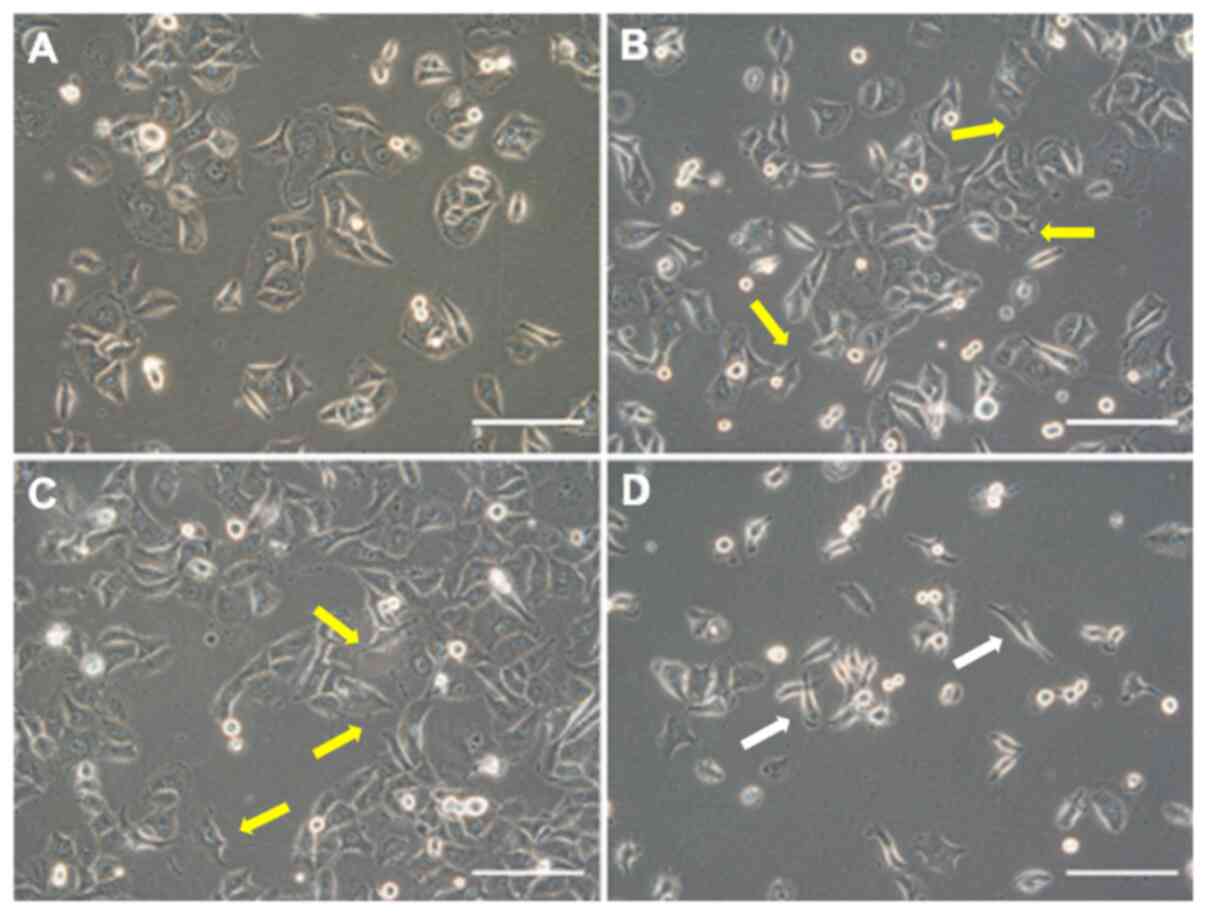
Morphological and immunohistochemical
study of the IL-32 overexpressing and suppressed cells
Morphological changes to the overexpressing and
suppressed cells are shown in Fig.
3. β and ε cells had a more abundant cytoplasm than P cells and
cells with a polygonal shape were predominant. Furthermore,
prominent lamellipodia or filopodia were observed in many cells
(Fig. 3A-C). By contrast, ko cells
had a narrowed cytoplasm and the morphology was spindle-shaped.
Furthermore, the formation of lamellipodia was poor, these being
composed of cells with one or two filopodia (Fig. 3D).
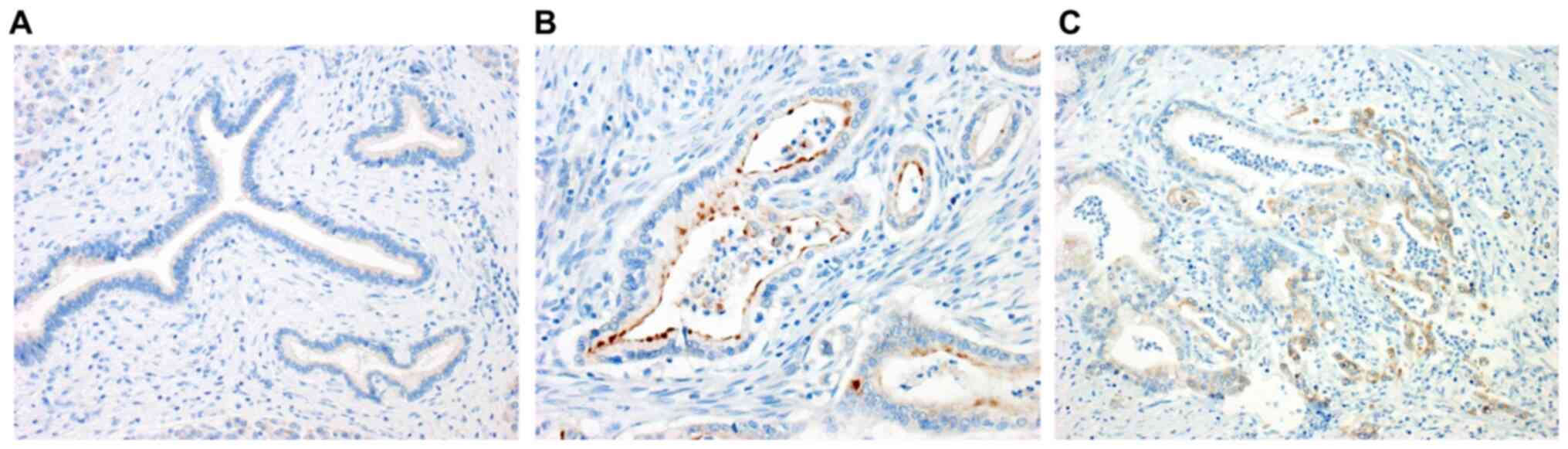
To confirm the expression of IL-32 in pancreatic
cancer tissues, an immunohistochemical study was performed using
surgically resected materials (Fig.
4A-C). No staining of IL-32 was observed in normal pancreatic
tissue (Fig. 4A). By contrast,
immunostaining of IL-32 was evident in the cytoplasm of the
differentiated adenocarcinoma cells, mainly along the luminal side
(Fig. 4B). As a characteristic
finding, many tumor cells, predominantly in the invasive front,
showed a positive reaction (Fig.
4C).
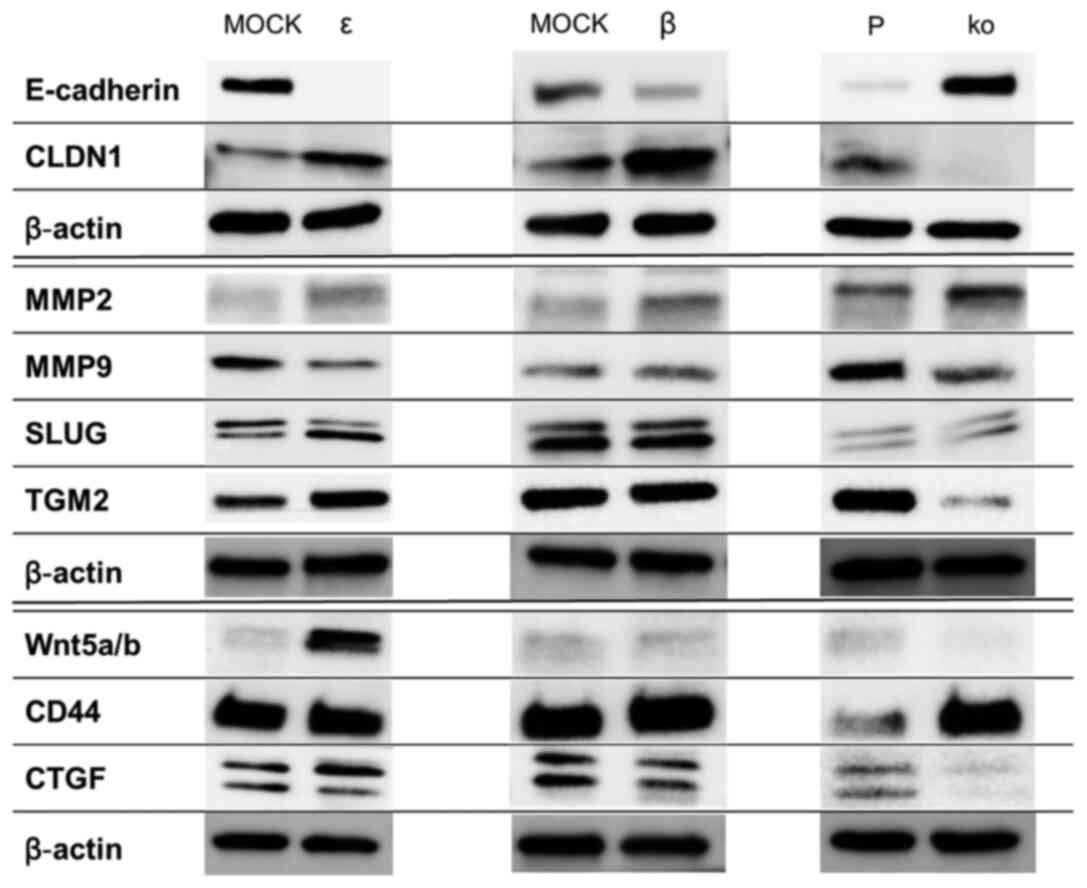
Molecules affected by IL-32
To determine the signaling pathway through which
IL-32 exerts its function, the expression of several proteins was
examined by WB analysis. Distinct differential expression of some
molecules was observed (Fig. 5).
The most apparent change was in E-cadherin, an adherent junction
factor. Its expression was markedly decreased in ε and β cells,
while an increased expression was found in ko cells. Thus, the
expression patterns of IL-32 and E-cadherin were opposed. By
contrast, Claudin-1, a tight junction protein, tended to be
upregulated in ε and β cells and suppressed in ko cells, showing a
pattern correlated with the expression of IL-32. MMP2 expression
tended to be increased in ε, β and ko cells, while MMP9 was
decreased in ε and ko cells. The low molecular component of Slug
was increased only in ε cells. Although the expression of CD44 was
similarly enhanced in ko cells, no distinct difference in
expression was observed in ε and β cells. There was no distinct
difference in the expression of Wnt 5a/b in the ko and β cells but
a marked upregulation was observed in ε cells. The expression of
CTGF and TGM2 was suppressed in ko cells, which did not differ
distinctly from ε and β cells.
Discussion
IL-32 is a cytokine that has been attracting
attention in recent years for its association with the development
of various tumors (21–26). Previously, we established a highly
invasive human pancreatic cancer cell line and investigated
comprehensively the factors that enhance its invasiveness (7). Findings of that study showed that
IL-32 is particularly involved in invasiveness (7). Therefore, the aim of the present
study was to determine in more detail the relationship between
IL-32 and invasiveness in pancreatic cancer. In particular, it was
of interest whether the invasiveness was altered by enhancing or
suppressing the expression of IL-32. IL-32 is known to have several
splice variants (23). In this
study, cells overexpressing IL-32 were established using the IL-32ε
and IL-32β isoforms and it was confirmed that their expression was
distinctly enhanced in the respective transfected cells, compared
with P cells. However, siRNA-transfected, P-si, and ko cells, were
generated with suppression of IL-32. For the ko cells, a mutant
plasmid constructed for the exon 3 region, which is present in all
of these isoforms of IL-32, was designed and transfected into the
cells. Comparing ko and P-si cells to P cells, the expression level
of IL-32 mRNA was reduced to <20% in P-si cells but was reduced
to 5% or less in ko cells. The suppression of IL-32 expression was
maintained for one week or longer in the ko cells but for only
about 4 days in the siRNA-transfected cells. The ko cells were
judged to be capable of prolonged maintenance of IL-32
suppression.
A comparison of the invasiveness of these IL-32
high-expressing and suppressed cells is shown in Fig. 2A-C. IL-32 high-expressing ε cells,
which were transfected with the IL-32ε isoform, showed a tendency
for increased invasiveness compared with MOCK. The ε cells invaded
from an early-stage and this property increased rapidly (Fig. 2A). Conversely, β cells showed less
invasiveness, similar to MOCK. This difference in invasive ability
may be due to the lack of exon 4 in the structure of the IL-32 ε
isoform (23), or it may be the
difference in the ability of either isoform to regulate downstream
factors. That is, exon 4 may have a function of suppressing
invasion ability. However, currently, there are few reports on ε
and invasion; thus, an experimental system in which exon 4 is
selectively deleted must be prepared and examined. By contrast,
S-si and ko cells have reduced or an absence of invasion, compared
with the original cells. From these experimental results, the
different invasiveness of these highly expressing and suppressed
cells also support the concept that IL-32 is a key molecule
involved in invasiveness.
Differences were also observed among ε, β, and ko
cells in terms of cell morphology. Both ε and β cells had abundant
cytoplasm, compared with P cells, and the cells had a polygonal
shape and actively formed lamellipodia and filopodia. These
characteristic morphological changes are observed not only in tumor
cells but also in normal cells when they migrate. When the cells
move, the protrusions elongate by the polymerization of
intracellular actin (27). It is
presumed that lamellipodia play a key role in the advance of
invasive cancer cells in vivo (27); thus, in that respect, it may be
considered that this motility is the reason ε cells have become
highly invasive. The findings that lamellipodia and filopodia were
observed in ε cells with high invasiveness may also support these
speculations. The morphological changes in ko cells, which showed
almost no invasion, also may provide complementary evidence for the
involvement of IL-32 in motility. The immunohistochemical analysis
also indicated that IL-32 was involved in the invasiveness of
pancreatic cancer cells, in that it was expressed only in
pancreatic cancer cells and it was not detected in normal
pancreatic tissue. Furthermore, the expression of IL-32 was
observed in many tumor cells at the invasive fronts, where
invasiveness is considered to be most active, supporting the
concept that IL-32 is expressed mainly in actively invading
cells.
Next, the involvement of downstream factors under
the control of IL-32 was investigated. This comparative study of ε,
β, and ko cells focused on several molecules that have been
suggested to be involved in invasion and changes were detected in
the expression of some of the molecules. The expression of
E-cadherin and IL-32 showed an inverse correlation. Expression of
E-cadherin mRNA and protein was decreased in ε and β cells but was
increased in ko cells. Thus, IL-32 may be an upstream factor that
regulates the expression of E-cadherin (21). Tumor cell budding from the cell
nest is generally seen in the early stages of the invasion of
epithelial tumors, due to the loss of adhesion between the cells.
E-cadherin is an adherent junction factor and plays an important
role in cell-to-cell adhesion. When this molecule is lost or
attenuated, the adherence between the tumor cells is reduced and
tumor cells are released from the cell nest (27). From these findings, it seems that
expression of IL-32 is important at the early stage of invasion and
reduces the expression of E-cadherin. In addition, there are cell
biological features of pancreatic cancer that are rarely seen in
other malignant tumors. Although tumor cells may also infiltrate
the desmoplastic stroma as solitary cells, another characteristic
finding is that the tumor cells may invade in a glandular
arrangement, with cell polarity (4). Claudin-1, a tight junction protein,
may be responsible for these changes; it is essential for the cell
polarity that maintains the glandular structure, as well as the
barrier mechanism (28). This
study also showed a positive correlation of the expression of IL-32
and Claudin-1.
Molecules such as MMPs, that destroy the basement
membrane and stroma, play a major role in invasion. Among the many
MMPs, it has been reported that MMP2 and MMP9 are important in the
invasion process and are highly expressed by many invading tumor
cells. Although there are many molecules that regulate MMPs, the
present study has shown a partial correlation between these MMPs
and IL-32 (24,26,29).
It is possible that the splice variant isoforms of IL-32 regulate
their expression via another pathway. A similar tendency was
observed for Slug, which is usually expressed during transformation
into mesenchymal cells in the EMT phenomenon (27). Herein, only the ε isoform of IL-32
increased Slug expression. Therefore, only this isoform regulates
Slug expression in the mechanism of invasion and/or EMT in
pancreatic cancer.
IL-32 is believed to act upstream of the STAT3
(30), NF-κB (29), and Akt pathways (26) in guiding EMT and angiogenesis. In
the present study, the focus was on the Wnt signaling pathway,
which may be heavily involved in invasiveness, cell motility and
cell polarity. At present, there are no reports of the relationship
between IL-32 and Wnt in neoplastic disease. If IL-32 acts upstream
of Wnt, the Wnt pathway may be involved in the regulation of
invasiveness, cell motility and cell polarity by IL-32. However,
Wnt5a/b was induced only in ε cells. This finding suggests that the
highly invasive ε isoform of IL-32 may regulate the Wnt pathway. In
the future, it will be important to study changes of β-catenin and
RhoA, which form part of this pathway. It was reported previously
that β-catenin was also highly expressed in gastric cancer cells
overexpressing IL-32 (24). In
particular, considering that β-catenin is also markedly involved
with E-cadherin, this may be the key to explaining the present
finding of a negative correlation between IL-32 and E-cadherin.
Regarding other molecules involved in tumor cell
invasion, CD44 also has various splice variants and is involved in
cell-to-cell adhesion or cell-to-cell matrix adhesion (31). Although a relationship with IL-32
has not been reported, speculating from the finding that CD44
expression is enhanced in ko cells, IL-32 may suppress CD44
expression, resulting in decreased adhesion. CTGF also has
essential roles in many biological processes, including cell
adhesion, migration, proliferation and angiogenesis, and is
critically involved in several neoplasms (32). TGM is also involved in tumor cell
migration, invasion, and proliferation (33). Both molecules were also slightly
increased when ε of IL-32 was highly expressed, whereas the
expression of the two molecules was decreased in ko cells. This
finding suggests that IL-32 may play a role as an upstream factor
regulating these molecules.
In conclusion, IL-32 was found to be an important
molecule that regulates the invasiveness of pancreatic cancer.
Previously, we reported the establishment of a highly invasive cell
line from pancreatic cancer and a comprehensive expression gene
analysis revealed that IL-32 is highly expressed in these cells.
Herein, more detail is provided of IL-32 function and its role in
the invasion process. The results showed that the invasiveness of
pancreatic cancer cells could be controlled by regulating the
expression of IL-32. Furthermore, it was revealed that IL-32 is an
important cytokine, as an upstream factor that regulates various
molecules involved in cell adhesion, motility, and invasion. If
low-molecular-weight chemical compounds or antibodies that
specifically bind to IL-32 could be created in the future, it may
be possible to suppress tumor cell invasion by their local
administration into the lesion. As a result, metastasis may be
suppressed, and this may be an approach leading to an improvement
in patient prognosis.
Supplementary Material
Supporting Data
Acknowledgements
pSpCas9n(BB)-2A-Puro (PX462) was a gift from
Professor Feng Zhang (Broad Institute of Massachusetts Institute of
Technology and Harvard, Cambridge, MA, USA) (Addgene plasmid no.
48141). The authors would like to thank Ms. Noriko Matsugi
(Department of Molecular Neuroscience, Faculty of Medicine,
Academic Assembly, University of Toyama, Japan) for their technical
help.
Funding
This research was supported by the Japan Society For The
Promotion Of Science (JSPS) Grants-in-Aid for Scientific Research
(KAKENHI), grant no. 16K08707.
Availability of data and materials
The analyzed data sets derived from this study are
available from the corresponding author on reasonable request.
Authors' contributions
KT, AS and JI designed the study, performed the
experiment and analyzed data. AS and JI confirm the authenticity of
all the raw data. HM provided advice and technical support
concerning construction of IL-32 overexpression strain and knockout
strain. AN, ST, TM and TNa advised on the construction of the study
design and added critical review of the manuscript. TNi and HH
performed collection, analysis and interpretation of some
experimental data. KT and JI wrote the manuscript.
Ethics approval and consent to
participate.
This research was approved by the University
Hospital of Toyama Ethics Committee (no. R2020054). Consent was not
obtained from each patient, albeit the patients were notified of
the details of the study by opt-out consent and had the right to
refuse participation in the study. All research procedures were
carried out in accordance with the Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vincent A, Herman J, Schulick R, Hruban RH
and Goggins M: Pancreatic cancer. Lancet. 378:607–620. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kleeff J, Korc M, Apte M, La Vecchia C,
Johnson CD, Biankin AV, Neale RE, Tempero M, Tuveson DA, Hruban RH,
et al: Pancreatic cancer. Nat Rev Dis Primers. 2:160222016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pelosi E, Castelli G and Testa U:
Pancreatic Cancer: Molecular Characterization, Clonal Evolution and
Cancer Stem Cells. Biomedicines. 5:652017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hruban RH, Pitman MB and Kimstra DS:
Tumors of the Pancreas, Afip Atlas of Tumor Pathology. (6th
edition). American Registry of Pathology. (Washington, DC).
1201–126. 2007.
|
|
5
|
Luo J, Chen XQ and Li P: The Role of TGF-β
and Its Receptors in Gastrointestinal Cancers. Transl Oncol.
12:475–484. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miyazono K, Katsuno Y, Koinuma D, Ehata S
and Morikawa M: Intracellular and extracellular TGF-β signaling in
cancer: Some recent topics. Front Med. 12:387–411. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takagi K, Imura J, Shimomura A, Noguchi A,
Minamisaka T, Tanaka S, Nishida T, Hatta H and Nakajima T:
Establishment of highly invasive pancreatic cancer cell lines and
the expression of IL-32. Oncol Lett. 20:2888–2896. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nisticò P, Bissell MJ and Radisky DC:
Epithelial-mesenchymal transition: General principles and
pathological relevance with special emphasis on the role of matrix
metalloproteinases. Cold Spring Harb Perspect Biol. 4:42012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fuxe J, Vincent T and Garcia de Herreros
A: Transcriptional crosstalk between TGF-β and stem cell pathways
in tumor cell invasion: Role of EMT promoting Smad complexes. Cell
Cycle. 9:2363–2374. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen J, Wang S, Su J, Chu G, You H, Chen
Z, Sun H, Chen B and Zhou M: Interleukin-32α inactivates JAK2/STAT3
signaling and reverses interleukin-6-induced epithelial-mesenchymal
transition, invasion, and metastasis in pancreatic cancer cells.
OncoTargets Ther. 9:4225–4237. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nishida A, Andoh A, Inatomi O and Fujiyama
Y: Interleukin-32 expression in the pancreas. J Biol Chem.
284:17868–17876. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kyuno D, Takasawa A, Kikuchi S, Takemasa
I, Osanai M and Kojima T: Role of tight junctions in the
epithelial-to-mesenchymal transition of cancer cells. Biochim
Biophys Acta Biomembr. 1863:1835032021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen C, Zhao S, Karnad A and Freeman JW:
The biology and role of CD44 in cancer progression: Therapeutic
implications. J Hematol Oncol. 11:642018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jacobson A and Cunningham JL: Connective
tissue growth factor in tumor pathogenesis. Fibrogenesis Tissue
Repair. 5 (Suppl 1):S82012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Scheau C, Badarau IA, Costache R, Caruntu
C, Mihai GL, Didilescu AC, Constantin C and Neagu M: The Role of
Matrix Metalloproteinases in the Epithelial-Mesenchymal Transition
of Hepatocellular Carcinoma. Anal Cell Pathol (Amst).
2019:94239072019.PubMed/NCBI
|
|
16
|
Pukrop T, Klemm F, Hagemann T, Gradl D,
Schulz M, Siemes S, Trümper L and Binder C: Wnt 5a signaling is
critical for macrophage-induced invasion of breast cancer cell
lines. Proc Natl Acad Sci USA. 103:5454–5459. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takeshita A, Iwai S, Morita Y,
Niki-Yonekawa A, Hamada M and Yura Y: Wnt5b promotes the cell
motility essential for metastasis of oral squamous cell carcinoma
through active Cdc42 and RhoA. Int J Oncol. 44:59–68. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Agnihotri N, Kumar S and Mehta K: Tissue
transglutaminase as a central mediator in inflammation-induced
progression of breast cancer. Breast Cancer Res. 15:2022013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ito T, Hayashida M, Kobayashi S, Muto N,
Hayashi A, Yoshimura T and Mori H: Serine racemase is involved in
d-aspartate biosynthesis. J Biochem. 160:345–353. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee J, Kim KE, Cheon S, Song JH, Houh Y,
Kim TS, Gil M, Lee KJ, Kim S, Kim D, et al: Interleukin-32α induces
migration of human melanoma cells through downregulation of
E-cadherin. Oncotarget. 7:65825–65836. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sloot YJE, Rabold K, Ulas T, De Graaf DM,
Heinhuis B, Händler K, Schultze JL, Netea MG, Smit JWA, Joosten
LAB, et al: Interplay between thyroid cancer cells and macrophages:
Effects on IL-32 mediated cell death and thyroid cancer cell
migration. Cell Oncol (Dordr). 42:691–703. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sloot YJE, Smit JW, Joosten LAB and
Netea-Maier RT: Insights into the role of IL-32 in cancer. Semin
Immunol. 38:24–32. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tsai CY, Wang CS, Tsai MM, Chi HC, Cheng
WL, Tseng YH, Chen CY, Lin CD, Wu JI, Wang LH, et al:
Interleukin-32 increases human gastric cancer cell invasion
associated with tumor progression and metastasis. Clin Cancer Res.
20:2276–2288. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yousif NG, Al-Amran FG, Hadi N, Lee J and
Adrienne J: Expression of IL-32 modulates NF-κB and p38 MAP kinase
pathways in human esophageal cancer. Cytokine. 61:223–227. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou Y, Hu Z, Li N and Jiang R:
Interleukin-32 stimulates osteosarcoma cell invasion and motility
via AKT pathway-mediated MMP-13 expression. Int J Mol Med.
35:1729–1733. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Weinberg RA: The Biology of Cancer. (2nd
edition). Garland Science. (Cambridge, MA). 658–691. 2013.
|
|
28
|
Ikenouchi J, Matsuda M, Furuse M and
Tsukita S: Regulation of tight junctions during the
epithelium-mesenchyme transition: Direct repression of the gene
expression of claudins/occludin by Snail. J Cell Sci.
116:1959–1967. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zeng Q, Li S, Zhou Y, Ou W, Cai X, Zhang
L, Huang W, Huang L and Wang Q: Interleukin-32 contributes to
invasion and metastasis of primary lung adenocarcinoma via
NF-kappaB induced matrix metalloproteinases 2 and 9 expression.
Cytokine. 65:24–32. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao WB, Wang QL, Xu YT, Xu SF, Qiu Y and
Zhu F: Overexpression of interleukin-32α promotes invasion by
modulating VEGF in hepatocellular carcinoma. Oncol Rep.
39:1155–1162. 2018.PubMed/NCBI
|
|
31
|
Klingbeil P, Marhaba R, Jung T, Kirmse R,
Ludwig T and Zöller M: CD44 variant isoforms promote metastasis
formation by a tumor cell-matrix cross-talk that supports adhesion
and apoptosis resistance. Mol Cancer Res. 7:168–179. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tsai HC, Su HL, Huang CY, Fong YC, Hsu CJ
and Tang CH: Correction: CTGF increases matrix metalloproteinases
expression and subsequently promotes tumor metastasis in human
osteosarcoma through down-regulating miR-519d. Oncotarget.
11:4922020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang X, Yu Z, Zhou Q, Wu X, Chen X, Li J,
Zhu Z, Liu B and Su L: Tissue transglutaminase-2 promotes gastric
cancer progression via the ERK1/2 pathway. Oncotarget. 7:7066–7079.
2016. View Article : Google Scholar : PubMed/NCBI
|