Introduction
Breast cancer (BC) is the most diagnosed cancer
among women worldwide, with over one million cases and ≥400,000
females succumbing to the disease every year, accounting for 14% of
total cancer deaths (1). BC is the
second leading cause of cancer-associated mortality in women,
following lung cancer (2).
According to data published by the American Cancer Society, as of
January 2019, >3,800,000 women were diagnosed with BC and
>150,000 of them are living with metastatic disease that
severely affects their quality of life (2). It is estimated that 12.8% of women
have a high risk of developing invasive BC in their lifetime, and
the mortality rate is >88.8/100,000 cases (2). Thus, it is important to identify
effective novel therapeutic targets for BC.
MicroRNAs (miRNAs/miRs) function as key regulators
in modulating cell proliferation, invasion, migration and apoptosis
in BC (3–5). miR-337-3p, a member of the miRNA
family, acts as a tumor suppressor in cancer progression. For
example, overexpression of miR-337-3p decreases cell proliferation
and invasion in clear cell renal cell carcinoma (6). Furthermore, miR-337-3p inhibits the
growth of hepatocellular carcinoma (7) and cervical cancer (8). In BC, miR-337-3p inhibits
epithelial-to-mesenchymal transition (EMT) under chronic stress by
targeting STAT3 (9). However, the
complexity of BC progression suggests that the regulatory mechanism
on BC progression may involve multiple genes downstream of
miR-337-3p. Thus, the regulatory mechanism of miR-337-3p in BC
cells requires further investigation.
The cyclin-dependent kinase 1 (CDK1) gene consists
of nine exons and is located on chromosome 10q21.2. CDK1 protein is
a member of the Ser/Thr protein kinase family (10). Previous studies have reported that
CDK1 acts as an oncogene in lung cancer (11), pancreatic ductal adenocarcinoma
(12), colorectal cancer (13), bladder cancer (14) and epithelial ovarian cancer
(15). In BC, CDK1 inhibition has
been associated with favorable treatment outcome, suggesting that
CDK1 may be a target for BC therapy (16). However, the upstream regulators of
CDK1 in BC remain unknown.
The present study aimed to investigate the molecular
regulation of miR-337-3p/CDK1 and further verify its role in BC.
Based on a previous study (9), it
was hypothesized that miR-337-3p binds to 3′-untranslated region
(UTR) of CDK1 to decease its expression and inhibit BC
progression.
Materials and methods
Bioinformatics analysis
GSE139038 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE139038),
an mRNA microarray dataset of BC and normal samples was downloaded
from the Gene Expression Omnibus (GEO) database (17) (https://www.ncbi.nlm.nih.gov/geo/) that stores gene
expression profiles. Genes upregulated in BC samples were screened
out based on the cut-off criteria of an adjusted P<0.05 (adj.P)
and log2 fold-change (log2FC) >2. The
upregulated genes were uploaded to Search Tool for the Retrieval of
Interacting Genes/Proteins (STRING) (18) (https://string-db.org) for Gene Ontology (GO)
enrichment analysis, and the key genes were further screened. The
expression patterns of the key genes in breast invasive carcinoma
(BRCA) were analyzed according to The Cancer Genome Atlas (TCGA)
data (19). Kaplan-Meier Plotter
(20) (http://kmplot.com/analysis) or StarBase (http://starbase.sysu.edu.cn/index.php)
were used to analyze the association between prognosis and the key
genes and miRNAs in BC. GSE143564 (21), a miRNA microarray dataset of BC and
normal samples was also downloaded from the GEO database. Based on
the cut-off criteria P<0.05, and log2FC<-1, miRNAs
downregulated in BC samples were screened out. TargetScan (22) was used to predict miRNA target
genes. The key miRNAs were identified by overlapping the
downregulated miRNAs identified in the GSE143564 dataset and
TargetScan.
Clinical samples
A total of 45 paired BC and adjacent normal tissues
(>3 cm from the tumor) were collected from patients (median age,
50 years; range 38–67 years) who were diagnosed with BC and
underwent mastectomy at the People's Hospital of Zhengzhou
University (Zhengzhou, China) between February 2019 and June 2020.
The inclusion criteria were as follows: i) Pathological
confirmation of BC; ii) normal cognition and no communication
problems; and iii) no radiotherapy or chemotherapy prior to
surgery. Exclusion criteria included: i) Patients with other breast
diseases; ii) patients with other malignances; iii) serious heart,
lung, kidney and other organ complex diseases; iv) patients with
serious infectious diseases; and v) patients who refused to provide
experimental specimens. The specimens were preserved in liquid
nitrogen at −80°C until subsequent experimentation. The present
study was approved by the Ethics Committee of the People's Hospital
of Zhengzhou University (approval no. 2019-10), and performed in
accordance with the Declaration of Helsinki. Written informed
consent was provided by all patients prior to the study start. The
clinical characteristics of all patients are presented in Table I.
 | Table I.Clinical characteristics of patients
with breast cancer (n=45). |
Table I.
Clinical characteristics of patients
with breast cancer (n=45).
| Characteristic | Number of patients,
n | Percentage, % |
|---|
| Age, years |
|
|
|
≤50 | 19 | 42.2 |
|
>50 | 26 | 57.8 |
| Menstrual
status |
|
|
|
Premenopausal | 24 | 53.3 |
|
Postmenopausal | 21 | 46.7 |
| ER status |
|
|
|
Positive | 25 | 55.6 |
|
Negative | 20 | 44.4 |
| Lymph node
metastasis |
|
|
|
Positive | 16 | 35.6 |
|
Negative | 29 | 64.4 |
| TNM stage |
|
|
| I | 9 | 20.0 |
| II | 14 | 31.1 |
|
III | 20 | 44.4 |
| IV | 2 | 4.4 |
Cell culture
The BC cell lines, T-47D, MCF7, MDA-MB-231, SK-BR-3
and BT-474, and human normal mammary cells (MCF10A) were purchased
from the American Type Culture Collection (ATCC). T-47D cells was
maintained in RPMI-1640 (cat. no. 30-2001; ATCC) supplemented with
0.2 U/ml bovine insulin and 10% fetal bovine serum (FBS) (Gibco;
Thermo Fisher Scientific, Inc.). MCF7 and MDA-MB-231 cells were
maintained in DMEM (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS. SK-BR-3 cells were maintained in McCoy's
5A medium (Gibco; Thermo Fisher Scientific, Inc.). BT-474 cells
were maintained in ATCC Hybri-Care Medium (cat. no. 46-X)
supplemented with 10% FBS. MF10A cells were maintained in RPMI-1640
supplemented with 10% FBS. All cells were cultured in an incubator
at 37°C with 5% CO2.
Ttransfection
Small interfering (si)-CDK1, miR-337-3p mimic,
miR-337-3p inhibitor, and their corresponding negative controls
(si-NC, mimic-NC and inhibitor-NC) were purchased from Shanghai
GenePharma Co., Ltd. T-47D and MCF7 cells were transfected at the
exponential growth stage. T-47D and MCF7 cells (1×106)
were seeded into 6-well plates in 2 ml RPMI-1640 medium (Gibco;
Thermo Fisher Scientific, Inc.) supplemented with 10% FBS and
cultured for 24 h until the cells reached 70% confluence. Cells
were subsequently transfected with 50 nM siRNA, mimic, or
inhibitor, using Lipofectamine® 2000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) and cultured in
Opti-MEM serum-free medium (Gibco; Thermo Fisher Scientific, Inc.)
for 6 h at 37°C. After removing the Opti-MEM serum-free medium,
fresh medium (RPMI-1640 or DMEM) supplemented with 10% FBS was
added to the cells and further incubated for 48 h at 37°C.
Transfection efficiency was analyzed via reverse
transcription-quantitative (RT-q)PCR and western blot analyses 48 h
post-transfection. Transfectant sequences are displayed in Table SI.
RT-qPCR
RNA was isolated from BC tissues and cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). A total of 200 ng of extracted RNA was reverse transcribed
into cDNA using the ReverTra Ace qPCR RT kit (Toyobo Life Science).
qPCR was subsequently performed using Thunderbird®
SYBR® qPCR Mix (Toyobo Life Science), according to the
manufacturer's instructions. The following thermocycling conditions
were used for qPCR: 95°C for 60 sec, 40 cycles of 95°C for 15 sec
and 60°C for 30 sec. The internal references for miR-337-3p and
CDK1 were U6 and β-actin, respectively. Table II lists the primer sequences used
for qPCR. The 2−ΔΔCq method (23) was used to detect the relative
expression levels of miR-337-3p and CDK1.
 | Table II.Primer sequences used for
quantitative PCR. |
Table II.
Primer sequences used for
quantitative PCR.
| Gene (accession
no.) | Primer sequence
(5′-3′) |
|---|
| miR-337-3p
(MIMAT0000754) | Forward:
CGCTTCAGCTCCTATATGA |
|
| Reverse:
GCGAGCACAGAATTAATACGAC |
| U6 (NR_004394) | Forward:
GCAAATTCGTGAAGCGTTCCATA |
|
| Reverse:
AACGAGACGACGACAGAC |
| CDK1
(NM_033379) | Forward:
AAATGTGTGTAGGTCTCAC |
|
| Reverse:
ATGATTTAAGCCAACTCAAA |
| β-actin
(EF095209) | Forward:
AGGCACCAGGGCGTGAT |
|
| Reverse:
GCCCACATAGGAATCCTTCTGAC |
Western blotting
Total protein was extracted from BC cells using
western and immunoprecipitation (IP) cell lysis buffers (Sangon
Biotech Co., Ltd.). The BCA Protein Assay kit (Sangon Biotech Co.,
Ltd.) was used to quantify the protein concentration. Equal amounts
of proteins (20 µg) were separated via 10% SDS-PAGE, transferred
onto nitrocellulose membranes (MilliporeSigma) and blocked with
Tris-buffered saline and 0.1% Tween 20 (TBST) containing 5% non-fat
skimmed milk at 25°C for 3 h. The membranes were washed with TBST
followed by incubation with primary antibodies against CDK1
(1:1,000; Abcam; cat. no. ab18) and β-actin (1:2,000; Abcam; cat.
no. ab8226) overnight at 4°C. Subsequently, membranes were
incubated with horseradish peroxidase-conjugated secondary antibody
(1:2,000; cat. no. sc-2357; Santa Cruz Biotechnology, Inc.) for 1 h
at 37°C. β-actin was used as the normalization control. SuperSignal
West Pico Chemiluminescent Substrate (Pierce; Thermo Fisher
Scientific, Inc.) was used to visualize the protein bands, which
were analyzed using ImageJ software (Version 1.44; National
Institutes of Health).
Cell Counting Kit-8 (CCK-8) assay
The CCK-8 (Abcam; cat. no. ab228554) assay was used
to assess cell viability. Cells were seeded into 96-well microtiter
plates at a density of 6.0×103 cells/well. Following
transfection for 0, 24, 48, and 72 h, 10 µl of CCK-8 solution was
added to each well. Following incubation for 2 h, the absorbance
was measured at a wavelength of 450 nm, using a spectrophotometric
microtiter plate reader (Bio-Rad Laboratories, Inc.).
BrdU cell proliferation (ELISA)
The BrdU Cell Proliferation ELISA kit (Abcam; cat.
no. ab126556) was used. T-47D and MCF7 cells were plated at a
density of 2×105 cells/ml in 100 µl/well cell culture
media. Following transfection for 48 h, 20 µl of BrdU reagent was
diluted twice and pipetted into the cells. The plates were
centrifuged for 5 min at 300 × g, 4°C. The media was aspirated and
fixing solution (200 µl/well) was added to the cells. Following
incubation at room temperature for 30 min, the fixing solution was
aspirated, and the plate was washed three times with wash buffer.
Cells were incubated with anti-BrdU monoclonal antibody (100
µl/well; 1:500) for 1 h at room temperature. After washing the
plates, 100 µl/well peroxidase goat anti-mouse IgG conjugate
(1:500) was added to the cells and incubated for 30 min at room
temperature. Subsequently, 100 µl/well TMB peroxidase substrate was
added to the cells and incubated for 30 min at room temperature.
Finally, 100 µl of stop solution was added to each well and a
spectrophotometric microtiter plate reader was used to measure the
absorbance at a wavelength of 450 nm.
Cell adhesion assay
Fibronectin (Sigma-Aldrich; Merck KGaA) was used to
coat 96-well plates overnight, and the plates were subsequently
blocked with 1% BSA (Sigma-Aldrich; Merck KGaA) at 37°C for 2 h.
Cells (3×104) were seeded into 96-well plates with
serum-free RPMI-1640 medium (ATCC). Following incubation for 1 h,
the non-adherent cells were washed and 10 µl/well MTT substrate was
added to the adherent cells and incubated for an additional 2 h.
Then, 150 µl acidic isopropanol (0.1 N HCL and isopropanol) was
added to dissolve the purple formazan crystals, and the plates were
kept in the dark at room temperature for 15 min. Then, 100 µl
supernatant solution from each sample was transferred into a
96-well plate. Absorbance was measured at a wavelength of 570 nm,
using a spectrophotometric microtiter plate reader.
Flow cytometric analysis
The Annexin V-FITC Apoptosis Detection kit (cat. no.
BMS500FI-100; Invitrogen; Thermo Fisher Scientific, Inc.) was used
in the present study. T-47D and MCF7 cells were trypsinized after
48 h of transfection and resuspended in 200 µl of 1X binding buffer
at a cell density of 5×105 cells/ml. Subsequently,
Annexin V-FITC (5 µl) and 10 µl propidium iodide (PI) was added to
the cells and incubated for 10 min at room temperature. Cell
apoptosis was detected using FACS (BD Biosciences) and CellQuest
software (version 5.1; Becton, Dickinson and Company) was used to
analyze the results.
Wound healing assay
Following transfection, 1×105 cells were
seeded into 6-well plates and cultured until they reached 90%
confluence. Subsequently, a 200-µl sterile pipette was used to
scratch the cell monolayers. After removing the non-adherent cells,
cells were cultured for 24 h in serum-free RPMI-1640 medium. The
images of the wounds at 0 and 24 h were observed at ×100
magnification under a light microscope (DM2000; Leica Microsystems,
Inc).
Transwell assay
After coating the Transwell chamber with
Matrigel® (Corning, Inc.), 5×104 cells were
added to the upper chamber in serum-free RPMI-1640 medium.
Subsequently, 500 µl of medium containing 10% FBS was added to the
lower chamber. Following incubation for 24 h at 37°C, the invasive
cells on the lower surface were fixed with methanol at 25°C for 10
min and stained with 0.1% crystal violet at 25°C for 20 min. Cells
were observed at ×200 magnification under a light microscope.
Dual-luciferase reporter assay
Wild-type (WT) CDK1 3′-UTR and mutant (MUT) CDK1
3′-UTR (without the binding sites) were purchased from Shanghai
GenePharma Co., Ltd. The CDK1 3′-UTR WT and MUT sequences were
cloned into the pGL3 basic vector (Promega Corporation) to generate
pGL3-CDK1-3′-UTR WT and pGL3-CDK1-3′-UTR MUT constructs. T-47D and
MCF7 cells were seeded into 6-well plates and cultured until 60–80%
confluence. Cells were subsequently co-transfected with
pGL3-CDK1-3′-UTR WT and pGL3-CDK1-3′-UTR MUT and miR-337-3p
mimic/miR-NC, using Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.). After 24 h, cells were lysed, and
luciferase activity was detected using the Dual-Luciferase Reporter
Assay System (Promega Corporation). Relative luciferase activity
normalized to Renilla luciferase activity.
RNA pull-down assay
Biotinylated miR-377-3p (Bio-miR-377-3p) and the
corresponding NC (Thermo Fisher Scientific, Inc.) were transfected
into T-47D and MCF7 cells, using Lipofectamine® 2000
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Cells were
lysed with 500 µl immunoprecipitation (IP) buffer (20 mM Tris-HCl,
pH 8.0, 200 mM NaCl, 1 mM EDTA, 1 mM EGTA, 0.5% Triton X-100, 0.4
U/µl RNasin, protease inhibitor cocktail) for 1 h at 4°C, and
centrifuged at 14,000 × g for 15 min at 4°C. Then, 500 µl cell
lysates were incubated with 10 µl Dynabeads M-280 Streptavidin
(Invitrogen; Thermo Fisher Scientific, Inc.) according g to the
manufacturer's protocol. In brief, the beads were washed 3 times
using RNase-free solutions (Sigma-Aldrich; Merck KGaA) by
centrifugation at 5,000 × g for 5 min (4°C). After Bio-miR-377-3p
was incubated at room temperature for 10 min on a rotator, 10 mM
EDTA (pH 8.2) with 95% formamide (Sigma-Aldrich; Merck KGaA) was
added to the immobilized miR-377-3p fragment at 65°C for 5 min. The
bound RNAs were isolated using TRIzol® reagent and
RT-qPCR analysis was performed to detect the expression levels of
the bound targets.
Statistical analysis
Statistical analysis was performed using SPSS
software (version 19.0; IBM Corp.). Data are presented as the mean
± SD. Paired or unpaired Student's t-test was used to compare
differences between two groups, while one-way or two-way ANOVA
followed by Dunnett's or Tukey's post hoc test were used to compare
differences between multiple groups. Pearson's correlation analysis
was performed to analyze the correlations between miR-337-3p and
CDK1 expression in BC tissues. Kaplan-Meier plotter analysis was
applied to assess the relationship between gene expression and
individual overall survival. Associations between miR-337-3p/CDK1
expression and clinical characteristics were analyzed by Fisher's
exact test. P<0.05 was considered to indicate a statistically
significant difference.
Results
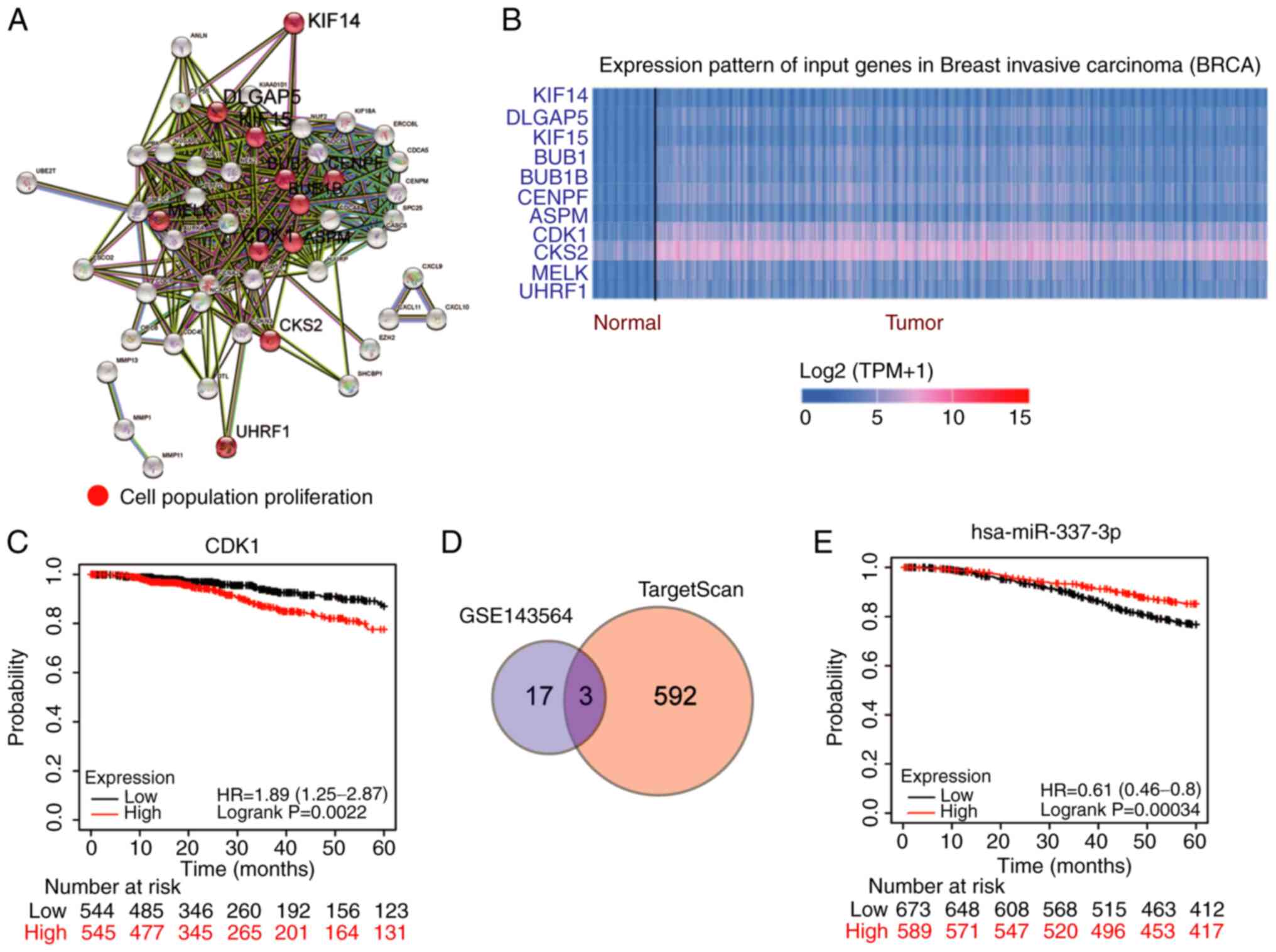
CDK1 and miR-337-3p are key regulators
in BC
Based on the cut-off criteria of adj.P<0.05 and
log2FC>2, 64 key differentially expressed genes were
identified in the GSE139038 dataset (consisting of BC samples)
downloaded from the GEO database. Following STRING analysis, 11
genes were confirmed to be associated with cell proliferation
(Fig. 1A). In TCGA data, CDK1 and
CKS2 were upregulated in BRCA among the 11 genes (Fig. 1B). For prognosis of BC, CDK1 was
closely associated with the prognosis of BC (Fig. 1C) compared with CKS2, that was not
significantly associated with the prognosis of BC (Fig. S1A), according to the data from the
Kaplan-Meier plotter analysis. To identify the key miRNAs in BC,
downregulated miRNAs were screened from the GSE143564 dataset using
the cut-off criteria of P<0.05 and log2FC <-1, and
the miRNAs that bind to CDK1 3′-UTR were predicted using
TargetScan. A total of three miRNAs (miR-548aj-3p, miR-3685 and
miR-337-3p) overlapped in the GSE143564 and TargetScan (Fig. 1D) data. Among these three miRNAs,
miR-337-3p was closely associated with BC prognosis (Fig. 1E), whereas miR-3685 and
miR-548aj-3p were not significantly associated with BC prognosis
(Fig. S1B and C). Therefore, it
was hypothesized that CDK1 and miR-337-3p are key regulators in
BC.
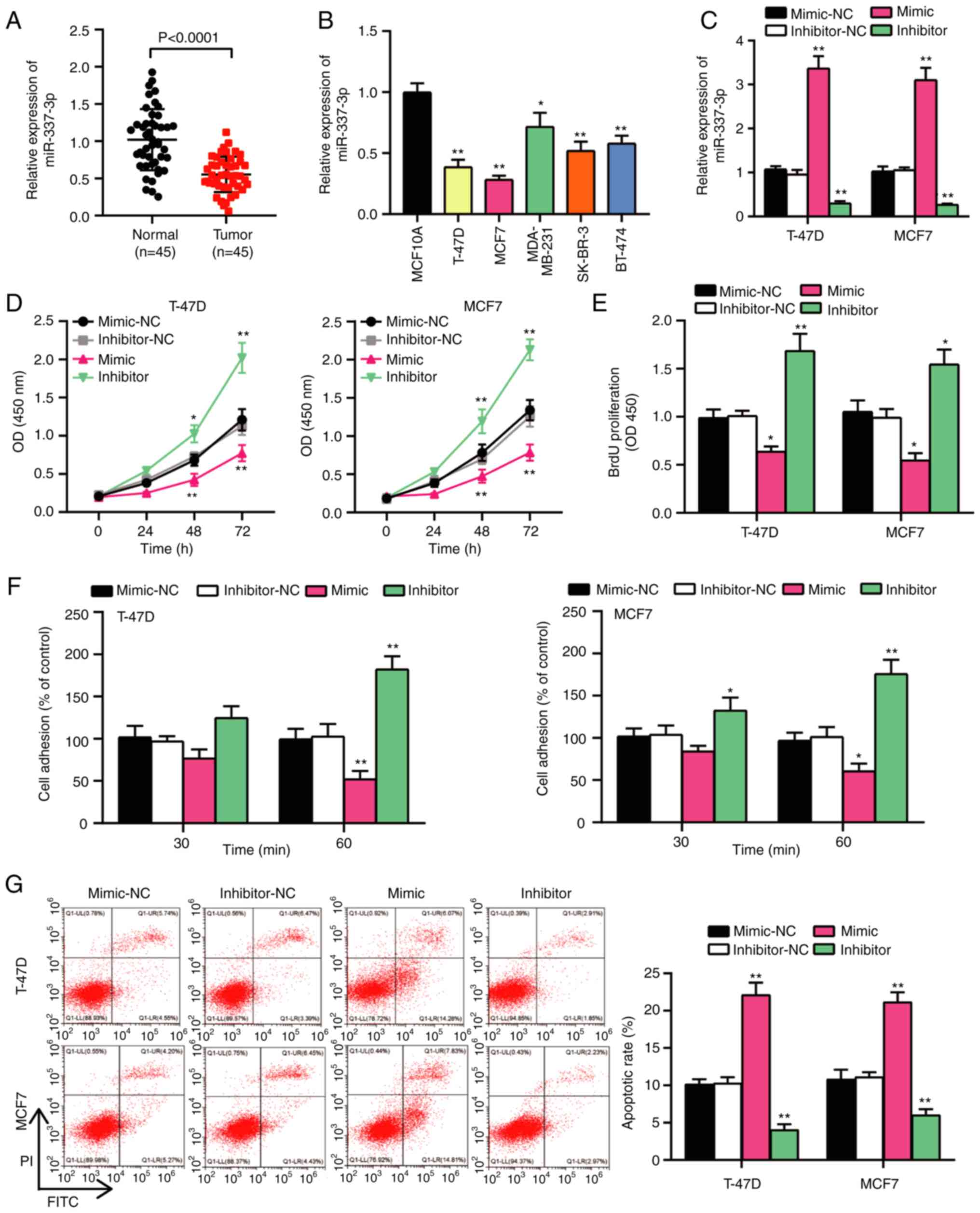
miR-337-3p suppresses BC
progression
To confirm the potential role of miR-337-3p in BC,
its expression was detected in BC tissues and cells. The results
demonstrated that miR-337-3p was expressed at low levels in BC
tissues and cells (Fig. 2A and B).
miR-337-3p expression was associated with estrogen receptor (ER)
status (P=0.013) and lymph node metastasis (P=0.010); however, its
expression was not significantly associated with age, menstrual
status or TNM stage (Table III).
miR-337-3p expression decreased by >50% in T-47D and MCF7 cells
compared with human normal mammary cells (MCF10A) (Fig. 2B). Thus, T-47D and MCF7 cell lines
were selected for subsequent experimentation. The present study
assessed the transfection efficiency of miR-337-3p mimics or
inhibitor in T-47D and MCF7 cells. The results demonstrated that
miR-337-3p expression increased >3-fold following transfection
with miR-337-3p mimics and decreased to 70% following transfection
with the miR-337-3p inhibitor compared with their respective NCs
(Fig. 2C). A series of functional
assays were performed to verify the proliferation, adhesion and
apoptosis of T-47D and MCF7 cells. The results of the CCK-8 and
BrdU assays demonstrated that BC cell viability and proliferation
were inhibited following transfection with miR-337-3p mimic, and
activated following transfection with the miR-337-3p inhibitor
(Fig. 2D and E). Furthermore, the
cell adhesion assay demonstrated that BC cell adhesion was impaired
following overexpression of miR-337-3p and enhanced following
miR-337-3p knockdown (Fig. 2F).
Flow cytometry was performed to assess cell apoptosis, and the
results demonstrated that the apoptotic rate increased >2-fold
following transfection with miR-337-3p mimic and decreased by
almost 50% following transfection with miR-337-3p inhibitor
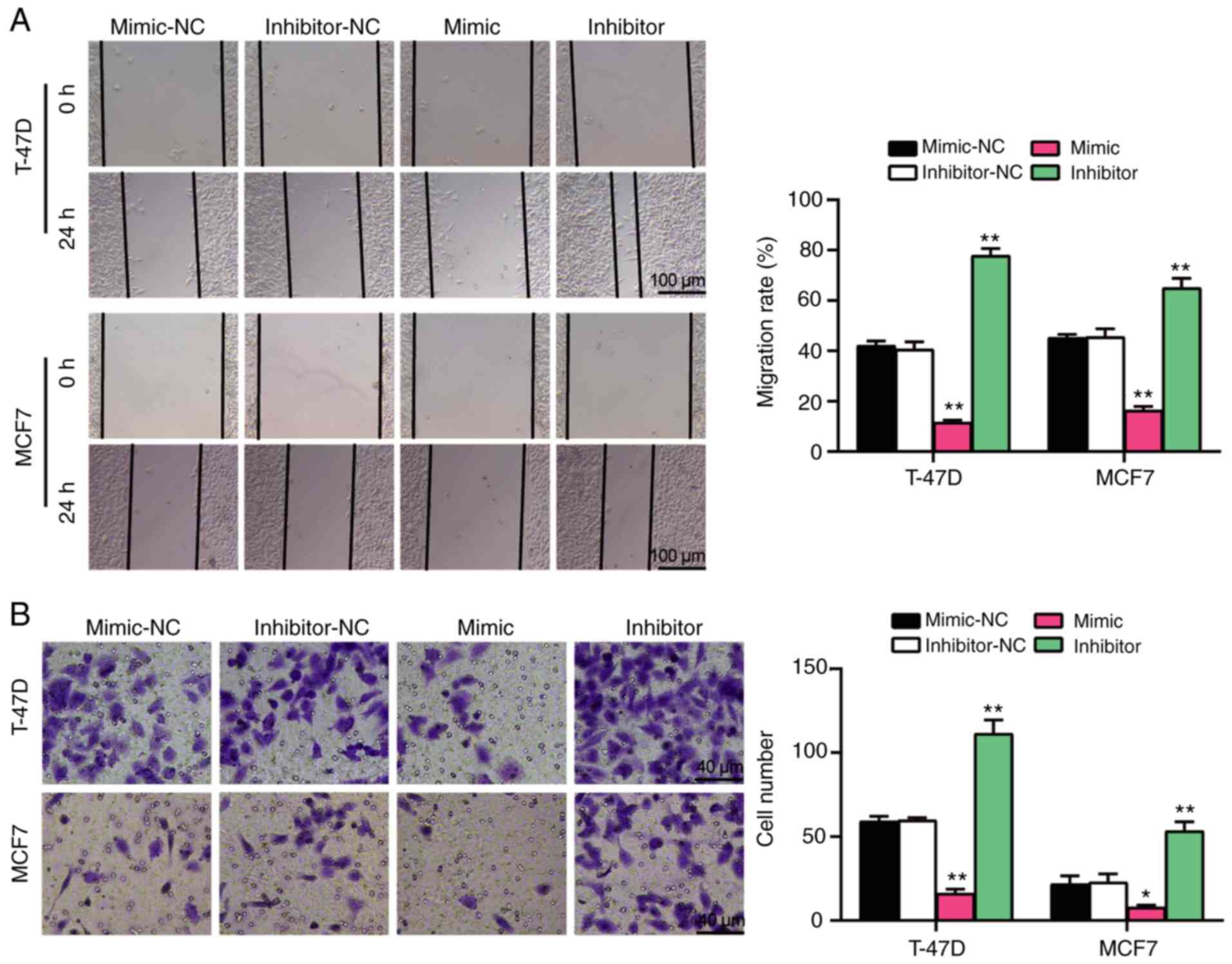
(Fig. 2G). Furthermore,
transfection with miR-337-3p mimic inhibited cell migration and
invasion, whereas transfection with miR-337-3p inhibitor promoted
cell migration and invasion (Fig. 3A
and B). Taken together, these results suggest that miR-337-3p
acts as a tumor suppressor in BC cells.
 | Table III.Association between miR-337-3p/CDK1
expression and clinical characteristics. |
Table III.
Association between miR-337-3p/CDK1
expression and clinical characteristics.
|
| miR-337-3p
expression |
| CDK1
expression |
|
|---|
|
|
|
|
|
|
|---|
| Characteristic | Low (n=25) | High (n=20) | P-value | Low (n=22) | High (n=23) | P-value |
| Age, years |
|
| 0.736 |
|
| 0.436 |
|
≤50 | 10 | 9 |
| 8 | 11 |
|
|
>50 | 15 | 11 |
| 14 | 12 |
|
| Menstrual
status |
|
| 0.423 |
|
| 0.661 |
|
Premenopausal | 12 | 12 |
| 11 | 13 |
|
|
Postmenopausal | 13 | 8 |
| 11 | 10 |
|
| ER status |
|
| 0.013a |
|
| 0.002b |
|
Positive | 18 | 7 |
| 7 | 18 |
|
|
Negative | 7 | 13 |
| 15 | 5 |
|
| Lymph node
metastasis |
|
| 0.010b |
|
| 0.017a |
|
Positive | 13 | 3 |
| 4 | 12 |
|
|
Negative | 12 | 17 |
| 18 | 11 |
|
| TNM stage |
|
| 0.100 |
|
| 0.115 |
| I | 2 | 7 |
| 6 | 3 |
|
| II | 9 | 5 |
| 9 | 5 |
|
|
III | 12 | 8 |
| 7 | 13 |
|
| IV | 2 | 0 |
| 0 | 2 |
|
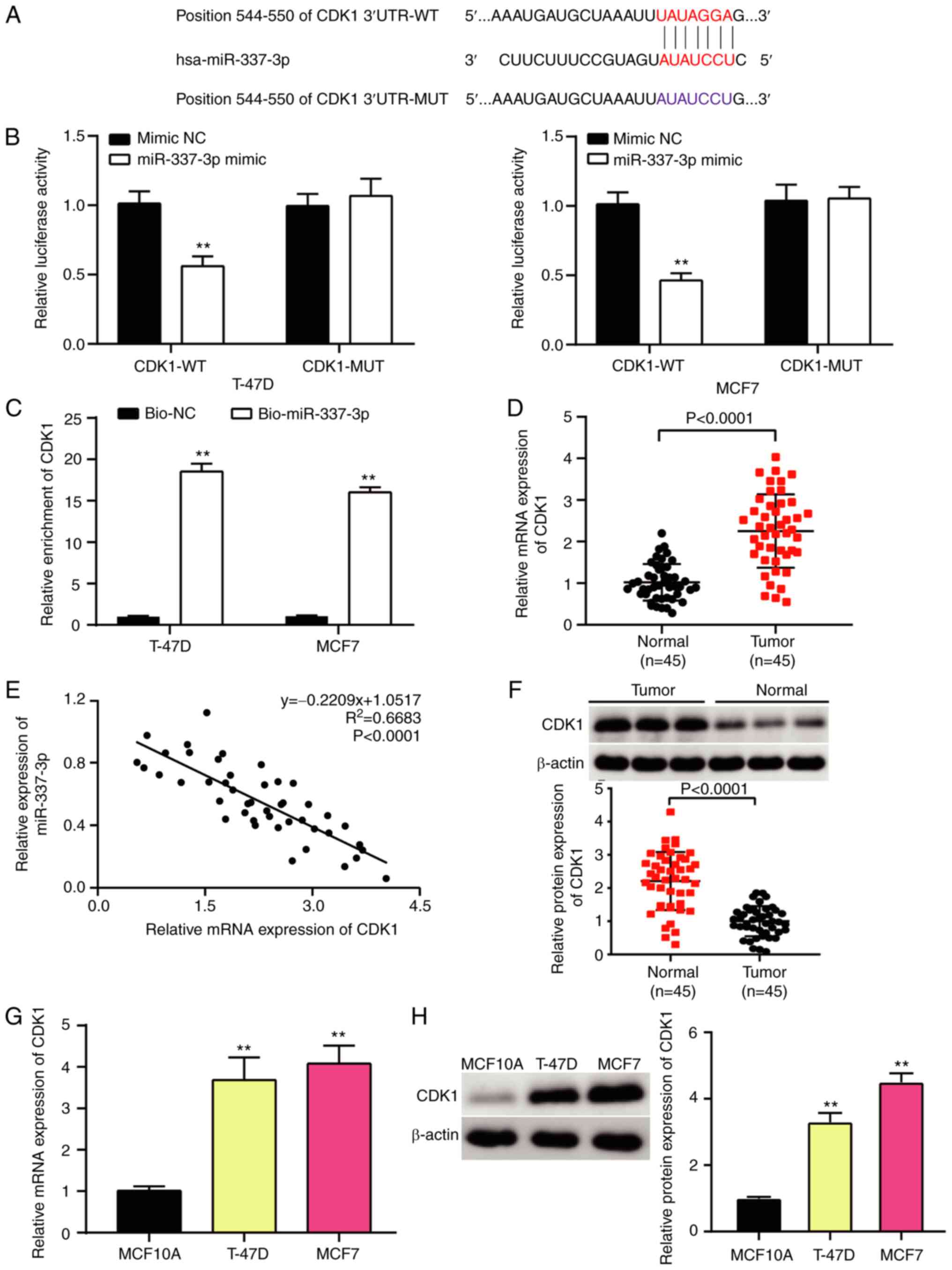
Identification of the miR-337-3p/CDK1
axis in BC cells
The present study investigated the association
between miR-337-3p and CDK1 in BC cells. The TargetScan database
predicted a binding site at position 544–550 of the CDK1 3′-UTR
(Fig. 4A). To confirm the binding
stringency of miR-337-3p and CDK1, the dual-luciferase reporter and
RNA pull-down assays were performed (Fig. 4B and C). The results demonstrated
that the relative luciferase activity of the CDK1 WT sequence
markedly decreased by ~50% compared with the CDK1 MUT type.
Similarly, CDK1 was pulled down in T-47D and MCF7 cells following
transfection with bio-miR-337-3p. Furthermore, miR-337-3p inhibited
CDK1 expression in both time- and dose-dependent manners (Fig. S2). CDK1 expression was detected
via RT-qPCR analysis, and the results demonstrated that CDK1 was
aberrantly upregulated in BC tissues (Fig. 4D). In addition, CDK1 expression was
significantly associated with ER status (P=0.002) and lymph node
metastasis (P=0.017) (Table
III). Pearson's correlation analysis exhibited a negative
correlation between miR-337-3p and CDK1 in BC cells (Fig. 4E). Western blot analysis
demonstrated that CDK1 protein expression was upregulated in BC
tissues (Fig. 4F). Furthermore,
RT-qPCR and western blot analyses demonstrated that CDK1 expression
increased in T-47D and MCF7 cells (Fig. 4G and H). Taken together, these
results suggest that the miR-337-3p/CDK1 axis is involved in BC
cells.
miR-337-3p/CDK1 axis modulates BC
progression
To determine the changes in biological behavior of
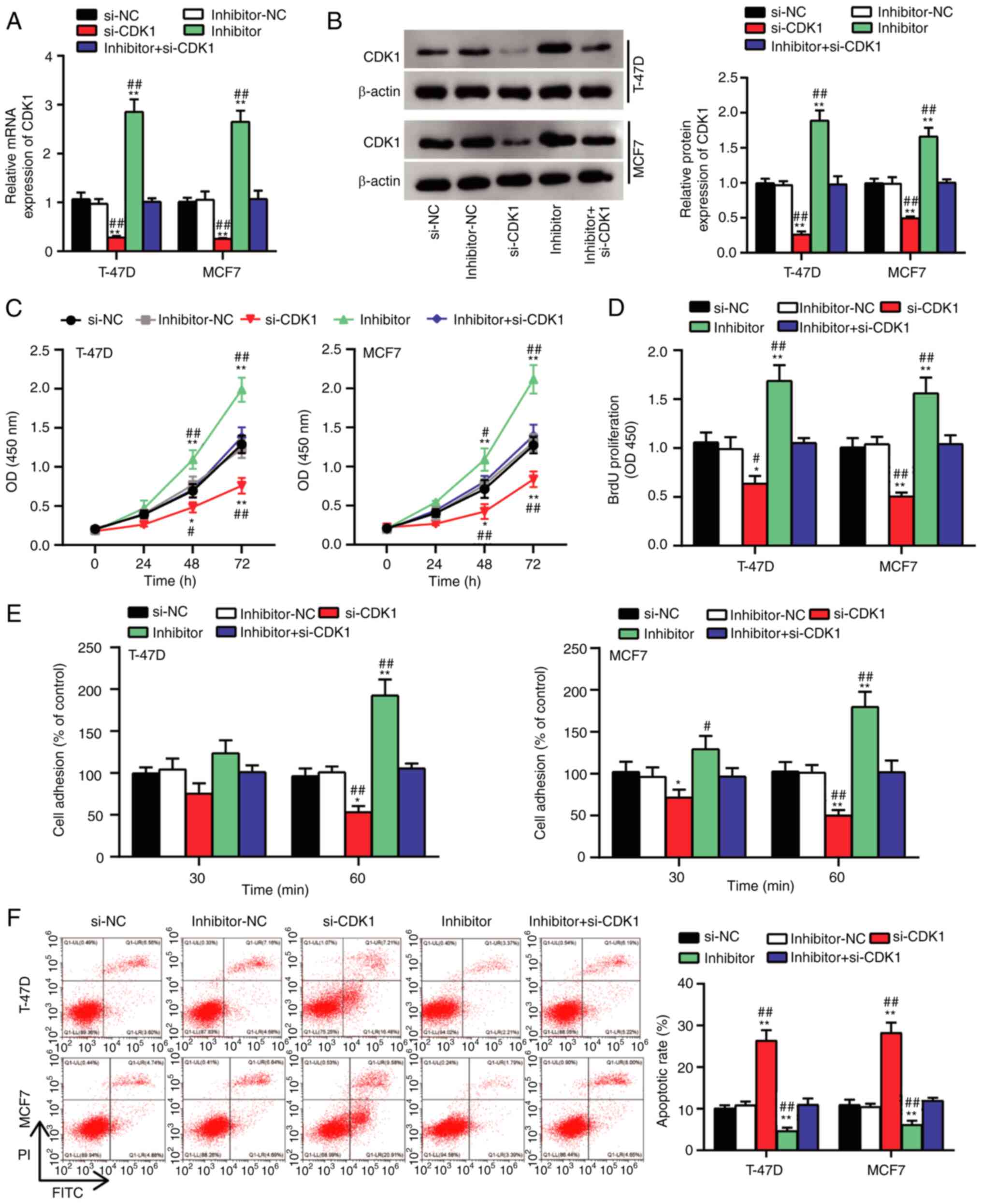
BC cells induced by the miR-337-3p/CDK1 axis, a series of rescue
assays were performed. Changes in CDK1 mRNA and protein expression
levels were detected following overexpression of miR-337-3p or CDK1
knockdown (Fig. 5A and B). The
results demonstrated that CDK1 expression increased following
miR-337-3p inhibition and reduced following CDK1 knockdown.
Notably, these effects were reversed following transfection with
miR-337-3p inhibitor + si-CDK1. The results of the CCK-8 and BrdU
assays demonstrated that cell viability and proliferation increased
and decreased, respectively, when miR-337-3p was knocked down or
CDK1 was inhibited. Notably, the effects on cell proliferation were
reversed following transfection with miR-337-3p inhibitor + si-CDK1
(Fig. 5C and D). Furthermore, cell
adhesion increased following miR-337-3p knockdown and decreased
following CDK1 knockdown, respectively. These effects were reversed
following transfection with miR-337-3p inhibitor + si-CDK1
(Fig. 5E). Cell apoptosis was
enhanced following CDK1 inhibition and reduced following miR-337-3p
knockdown, the effects of which were reversed following
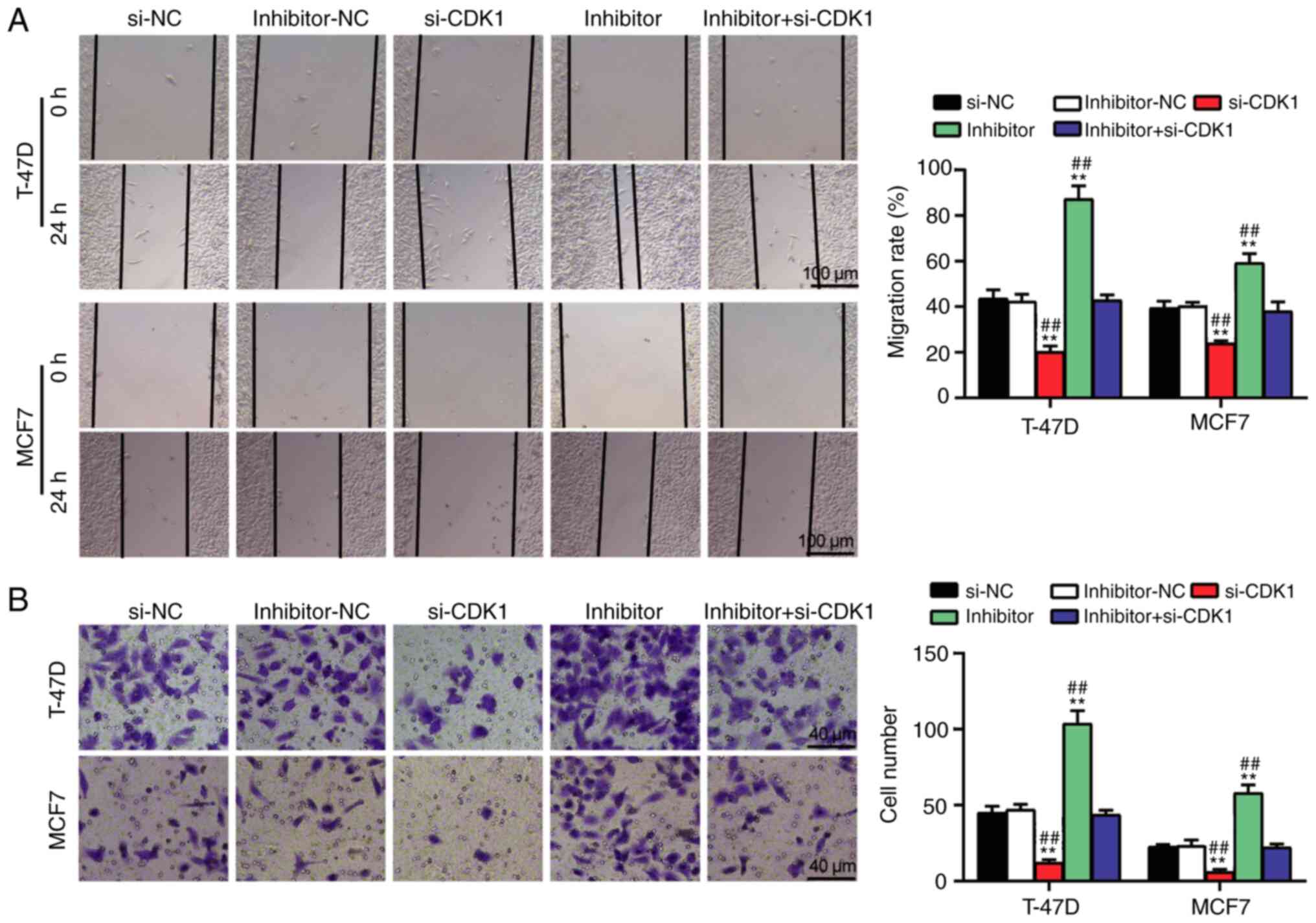
transfection with miR-337-3p inhibitor + si-CDK1 (Fig. 5F). Furthermore, cell migration and
invasion were impaired following transfection with si-CDK1;
however, the negative effect of si-CDK1 on cell migration and
invasion was relieved following transfection with miR-337-3p
inhibitor (Fig. 6A and B). Taken
together, these results suggest that the miR-337-3p/CDK1 axis
modulates the proliferation, adhesion and apoptosis of BC
cells.
Discussion
The present study investigated the function and
molecular mechanism of miR-337-3p in BC. The results demonstrated
that miR-337-3p plays a key role in BC by targeting CDK1.
miR-337-3p decreased the mRNA and protein expression levels of CDK1
in BC cells. In addition, miR-337-3p suppressed the progression of
BC by modulating cellular processes, such as cell proliferation,
adhesion, migration, invasion and cell apoptosis by targeting
CDK1.
miR-337-3p has been extensively studied as a tumor
suppressor in different types of cancer, such as hepatocellular
carcinoma, non-small cell lung cancer, cervical cancer and clear
cell renal cell carcinoma (ccRCC) (6–8,24,25).
miR-337-3p has been reported to be expressed at low levels in ccRCC
cells, and overexpression of miR-337-3p decreases cell
proliferation and invasion in ccRCC and hepatocellular carcinoma
(6,7). A previous study demonstrated that
high miR-337-3p expression is associated with a higher 5-year
survival rate in patients with HCC (7). In BC, Du et al (9) demonstrated that miR-337-3p targets
STAT3, thereby inhibiting EMT in BC with chronic stress (9). The results of the present study were
consistent with previous findings (6,8).
miR-337-3p was aberrantly expressed at low levels in BC cells, and
miR-337-3p overexpression inhibited the proliferation, adhesion,
migration and invasion of BC cells, but activated cell apoptosis.
However, in contrast to the study by Du et al (9), the results of the present study
demonstrated that CDK1 was the downstream target of miR-337-3p and
relieved the inhibitory effect of miR-337-3p on BC cells. Taken
together, these results suggest that miR-337-3p may target multiple
genes to play an antitumor role in BC.
Numerous studies have confirmed that CDK1 plays an
oncogenic role in different types of cancer, including melanoma
(26), gastric cancer (27) and bladder cancer (14). For example, CDK1 activates
tumorigenesis in melanoma (26).
High cytoplasmic CDK1 expression is a key predictor of poor overall
survival in patients with epithelial ovarian cancer (15). Furthermore, CDK1 has been
demonstrated to activate the proliferation of BC cells, and
functions as an effective target in BC therapy (16). In the present study, functional
assays demonstrated that inhibition of CDK1 decreased cell
proliferation, adhesion, migration and invasion, but increased cell
apoptosis in BC, which is consistent with a previous study
(16). To the best of our
knowledge, the present study was the first to demonstrate that
miR-337-3p targets CDK1 in BC cells, thereby regulating the
positive effect of CDK1 on BC cells. A previous study suggested
that CDK1 associated with cyclin B is the primary stimulus for the
entry of cells into mitosis (28);
therefore, the miR-337-3p/CDK1 axis may regulate the cell cycle and
play a key role in regulating the malignancy of BC cells.
The present study only investigated the function and
regulation of the miR-337-3p/CDK1 axis at the cellular level in
vitro; however, animal experiments are essential for further
characterization of its in vivo functions. The process of BC
tumorigenesis and progression is complex. The present study
confirmed the interaction between miR-337-3p and CDK1; however,
further studies are required to determine other downstream
mechanisms in BC.
In conclusion, the results of the present study
demonstrated that miR-337-3p was expressed at low levels in BC
tissues and cells, and plays a tumor suppressive role in the
progression of BC cells by targeting CDK1. CDK1 activates BC cell
progression and miR-337-3p modulates BC progression by decreasing
the mRNA and protein expression levels of CDK1 in BC cells. Taken
together, these results provide insight and potential targets for
the design of effective therapies for the treatment of BC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
SXK and JYL performed the experiments and analyzed
the data. BZ and FL conceived and designed the present study. YY
and TQ acquired the data, and confirmed the authenticity of all the
raw data. YY, TQ and SXK analyzed and interpreted the data. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the People's Hospital of Zhengzhou University
(Zhengzhou, China; approval no. 2019-10), and performed in
accordance with the Declaration of Helsinki. Written informed
consent was provided by all patients prior to the study start.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li T, Mello-Thoms C and Brennan PC:
Descriptive epidemiology of breast cancer in China: Incidence,
mortality, survival and prevalence. Breast Cancer Res Treat.
159:395–406. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
DeSantis CE, Ma J, Gaudet MM, Newman LA,
Miller KD, Sauer AG, Jemal A and Siegel RL: Breast cancer
statistics, 2019. CA Cancer J Clin. 69:438–451. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wei YT, Guo DW, Hou XZ and Jiang DQ:
miRNA-223 suppresses FOXO1 and functions as a potential tumor
marker in breast cancer. Cell Mol Biol (Noisy-le-grand).
63:113–118. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li P, Xu T, Zhou X, Liao L, Pang G, Luo W,
Han L, Zhang J, Luo X, Xie X and Zhu K: Downregulation of miRNA-141
in breast cancer cells is associated with cell migration and
invasion: Involvement of ANP32E targeting. Cancer Med. 6:662–672.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bertoli G, Cava C and Castiglioni I:
MicroRNAs: New biomarkers for diagnosis, prognosis, therapy
prediction and therapeutic tools for breast cancer. Theranostics.
5:1122–1143. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhuang Q, Shen J, Chen Z, Zhang M, Fan M,
Xue D, Lu H, Xu R, He X and Hou J: miR-337-3p suppresses the
proliferation and metastasis of clear cell renal cell carcinoma
cells via modulating Capn4. Cancer Biomark. 23:515–525. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zuo XL, Chen ZQ, Wang JF, Wang JG, Liang
LH and Cai J: miR-337-3p suppresses the proliferation and invasion
of hepatocellular carcinoma cells through targeting JAK2. Am J
Cancer Res. 8:662–674. 2018.PubMed/NCBI
|
|
8
|
Cao XM: Role of miR-337-3p and its target
Rap1A in modulating proliferation, invasion, migration and
apoptosis of cervical cancer cells. Cancer Biomark. 24:257–267.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Du P, Zeng H, Xiao Y, Zhao Y, Zheng B,
Deng Y, Liu J, Huang B, Zhang X and Yang K: Chronic stress promotes
EMT-mediated metastasis through activation of STAT3 signaling
pathway by miR-337-3p in breast cancer. Cell Death Dis. 11:7612020.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou Y, Shen JK, Hornicek FJ, Kan Q and
Duan Z: The emerging roles and therapeutic potential of
cyclin-dependent kinase 11 (CDK11) in human cancer. Oncotarget.
7:40846–40859. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tong W, Han TC, Wang W and Zhao J: LncRNA
CASC11 promotes the development of lung cancer through targeting
microRNA-302/CDK1 axis. Eur Rev Med Pharmacol Sci. 23:6539–6547.
2019.PubMed/NCBI
|
|
12
|
Piao J, Zhu L, Sun J, Li N, Dong B, Yang Y
and Chen L: High expression of CDK1 and BUB1 predicts poor
prognosis of pancreatic ductal adenocarcinoma. Gene. 701:15–22.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sung WW, Lin YM, Wu PR, Yen HH, Lai HW, Su
TC, Huang RH, Wen CK, Chen CY, Chen CJ and Yeh KT: High
nuclear/cytoplasmic ratio of Cdk1 expression predicts poor
prognosis in colorectal cancer patients. BMC Cancer. 14:9512014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tian Z, Cao S, Li C, Xu M, Wei H, Yang H,
Sun Q, Ren Q and Zhang L: LncRNA PVT1 regulates growth, migration,
and invasion of bladder cancer by miR-31/ CDK1. J Cell Physiol.
234:4799–4811. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang W, Cho H, Shin HY, Chung JY, Kang ES,
Lee EJ and Kim JH: Accumulation of cytoplasmic Cdk1 is associated
with cancer growth and survival rate in epithelial ovarian cancer.
Oncotarget. 7:49481–49497. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Izadi S, Nikkhoo A, Hojjat-Farsangi M,
Namdar A, Azizi G, Mohammadi H, Yousefi M and Jadidi-Niaragh F:
CDK1 in breast cancer: Implications for theranostic potential.
Anticancer Agents Med Chem. 20:758–767. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wilhite SE and Barrett T: Strategies to
explore functional genomics data sets in NCBI's GEO database.
Methods Mol Biol. 802:41–53. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Szklarczyk D, Gable AL, Lyon D, Junge A,
Wyder S, Huerta-Cepas J, Simonovic M, Doncheva NT, Morris JH, Bork
P, et al: STRING v11: Protein-protein association networks with
increased coverage, supporting functional discovery in genome-wide
experimental datasets. Nucleic Acids Res. 47:D607–D613. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu J, Lichtenberg T, Hoadley KA, Poisson
LM, Lazar AJ, Cherniack AD, Kovatich AJ, Benz CC, Levine DA, Lee
AV, et al: An integrated TCGA pan-cancer clinical data resource to
drive high-quality survival outcome analytics. Cell.
173:400–416.e411. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nagy Á, Munkácsy G and Győrffy B:
Pancancer survival analysis of cancer hallmark genes. Sci Rep.
11:60472021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu B, Liu G, Jin Y, Yang T, Zhang D, Ding
L, Zhou F, Pan Y and Wei Y: miR-15b-5p promotes growth and
metastasis in breast cancer by targeting HPSE2. Front Oncol.
10:1082020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Agarwal V, Bell GW, Nam JW and Bartel DP:
Predicting effective microRNA target sites in mammalian mRNAs.
ELife. 12:e050052015. View Article : Google Scholar
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tang D, Zhao L, Peng C, Ran K, Mu R and Ao
Y: LncRNA CRNDE promotes hepatocellular carcinoma progression by
upregulating SIX1 through modulating miR-337-3p. J Cell Biochem.
120:16128–16142. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li Q, Huang Q, Cheng S, Wu S, Sang H and
Hou J: Circ_ZNF124 promotes non-small cell lung cancer progression
by abolishing miR-337-3p mediated downregulation of JAK2/STAT3
signaling pathway. Cancer Cell Int. 19:2912019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Menon DR, Luo Y, Arcaroli JJ, Liu S,
KrishnanKutty LN, Osborne DG, Li Y, Samson JM, Bagby S, Tan AC, et
al: CDK1 Interacts with Sox2 and promotes tumor initiation in human
melanoma. Cancer Res. 78:6561–6574. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang L, Kang W, Lu X, Ma S, Dong L and
Zou B: LncRNA CASC11 promoted gastric cancer cell proliferation,
migration and invasion in vitro by regulating cell cycle pathway.
Cell Cycle. 17:1886–1900. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xie D, Song H, Wu T, Li D, Hua K, Xu H,
Zhao B, Wu C, Hu J, Ji C, et al: MicroRNA-424 serves an
anti-oncogenic role by targeting cyclin-dependent kinase 1 in
breast cancer cells. Oncol Rep. 40:3416–3426. 2018.PubMed/NCBI
|