Introduction
Primary vaginal carcinoma (PVC) accounts for 1–2% of
all gynaecological malignancies and mainly affects postmenopausal
women, the majority of whom are diagnosed at an early stage
(1–3). Radiation therapy, with or without
concurrent chemotherapy, is considered to be the treatment of
choice and results in 5-year cancer-specific survival rates of ~70%
(4). Due to its rarity, few
studies have been conducted on PVC, and therefore knowledge
concerning biological factors and biomarkers, both diagnostic and
prognostic, is limited. Established prognostic factors include age,
tumour size, and stage (5–8). Several molecular biomarkers have been
proposed, including p16, Ki67, p53, EGFR, VEGF and human
papillomavirus (HPV) infection, although findings regarding these
markers have been inconsistent (7,9,10).
Therefore, identification of new biomarkers is essential for the
improvement of diagnosis, treatment outcome and prognosis for
patients with PVC.
Cancer cells are able to proliferate constitutively
(11). One important mechanism
that supports endless proliferation is the addition of telomere
repeats, which protect chromosomes from shortening. This process is
carried out by the telomerase enzyme, a ribonucleoprotein complex
consisting of two components: The telomerase reverse transcriptase
(TERT) and the telomerase RNA complex (TERC) (12). Additional factors required for the
catalytical activity of telomerase include dyskerin (also known as
dyskerin pseudouridine synthase 1) and the WD repeat containing
antisense to TP53 β (WRAP53β) protein (also known as telomerase
Cajal body protein 1 or as WD repeat domain 79) (13). Dyskerin serves as a backbone of the
telomerase complex. WRAP53β is required for telomere synthesis in
human cancer cells, as this protein localises telomerase to
telomeres (14). Dyskerin and
WRAP53β are usually localised in nuclear organelles known as Cajal
bodies, where they are involved in both the biogenesis of
telomerase and in spliceosomal machinery (14,15).
Dyskerin, a pseudouridine synthase, is also found in
the nucleoli of cells (16), where
it is responsible for the modification of ribosomal RNA molecules
important to ribosome biogenesis. When dysregulated, this protein
has been associated with various cancer types, including breast
cancer (17), renal cancer
(18) pituitary tumours (19) and glioma (20). In addition, increased expression of
dyskerin has often been associated with worse prognosis (16,18,21–23).
To our knowledge, dyskerin has not previously been studied in
relation to gynaecological malignancies.
WRAP53β, originally identified as an antisense gene
to the TP53 tumour suppressor (24), is a scaffolding protein involved in
the intracellular trafficking of RNA, telomerase and DNA repair
proteins. WRAP53β has been linked to a variety of cellular
processes, including the maintenance of Cajal bodies (24), telomere elongation (14) and DNA repair (25). Loss of WRAP53β is associated with
poor prognosis in head and neck (26), breast (27) and ovarian cancer (28), suggesting a tumour suppressor role
(29,30). Although upregulation of WRAP53β has
also been reported in cancer, correlation with patient survival has
been inconsistent (31–33). Instead, this upregulation, which is
known to occur in precancerous lesions, may reflect WRAP53β
involvement in DNA repair in order to constrain tumour progression
(29,30).
The present study aimed to examine the expression
patterns of dyskerin and WRAP53β in patients with PVC. Moreover, as
part of a search for effective biomarkers to evaluate prognosis in
PVC, the expression of these two proteins and their potential
association with clinical variables and survival was also
evaluated.
Materials and methods
Sample collection
The present study is based on archived diagnostic
PVC tumour samples from a consecutive cohort of 81 women. The
inclusion criteria were women diagnosed with and treated for PVC
between January 1975 and December 2002 at Örebro University
Hospital or at the central hospitals in Eskilstuna, Västerås, and
Karlstad (9). Seven cases were
excluded after immunohistochemical evaluation due to insufficient
tumour samples.
The clinical characteristics of this cohort have
been previously described in a study by Larsson et al
(9), including information on age
at diagnosis (mean, 69.4 years; range, 37–90 years), tumour size,
International Federation of Gynecology and Obstetrics (FIGO) stage,
tumour localisation, histological type (including basaloid squamous
cell carcinoma, non-keratinising squamous cell carcinoma,
keratinising squamous cell carcinoma, verrucous squamous cell
carcinoma, adenocarcinoma, sarcoma and melanoma, based on World
Health Organisation criteria) and tumour grade (34). Treatment and follow-up data for
each patient were obtained through hospital records. All patient
records were subjected to retrospective follow-up from the time of
diagnosis. Median follow-up time for patients who were alive at the
end of the study was 121 months (range, 44–290 months). As in the
previous protocol by Larsson et al (9), complete remission was defined as
disappearance of all clinical evidence of disease after primary
treatment, while tumour recurrence was defined as detection of
cancer after a period of at least 6 months following initial
complete remission.
Information on HPV status was also reported in
Larsson et al (9). Of the
81 tumour samples, 37 were HPV-positive, 34 were HPV-negative, and
10 had insufficient material for HPV detection. Of the 37
HPV-positive cases, 26 (70%) were HPV16-positive, while the
remaining 11 were positive for other high-risk HPV genotypes.
Immunohistochemical staining and
analysis of dyskerin and WRAP53β
In all, 68 of the 81 tumour samples were found to be
appropriate for immunohistochemical staining and analysis of
dyskerin and WRAP53β. The paraffin-embedded tumour samples were cut
into 5-µm sections and established to be representative by two
pathologists (MGK and OG). Paraffin was then dissolved in xylene,
and the tissue samples were rehydrated by stepwise washing with 96
and 70% ethanol in phosphate-buffered saline. The tissue samples
were then immersed in a 2% solution of H2O2
in methanol at room temperature for 30 min to reduce background
staining. Epitopes were retrieved by heating in citrate buffer
(water bath, 96°C for 15 min), and the tumour samples were then
cooled to room temperature. The primary anti-dyskerin (cat. no.
sc-373956; Santa Cruz Biotechnology, Inc.) and anti-WRAP53 (cat.
no. PA-2020-100; Innovagen AB) antibodies, diluted 1:200 in a
blocking buffer (2% bovine serum albumin, 0.2% Tween-20, 10%
glycerol and 0.05% NaN3 in phosphate-buffered saline;
all from Sigma-Aldrich; Merck KGaA) were applied and left to stand
for 60 min at room temperature. Protein signals were visualised
using the secondary antibodies provided in the EnVision™ Detection
Peroxidase/DAB system kit (Dako; Agilent Technologies, Inc.), which
was used according to the manufacturer's protocol. Nuclei were
stained with Mayer's haematoxylin (Dako; Agilent Technologies,
Inc.).
The dyskerin nuclear signal was assessed as: i)
Negative (no positive cells observed); ii) weak (1+); iii) moderate
(2+); and iv) and strong (3+) by an experienced pathologist (OG),
using a light microscope Leica (magnification, ×400). Tumour cells
were only analysed in sections in which the total number of cells
was ≥400 (minimum of 5 fields). The location of the protein signals
detected within the nucleoplasm and/or in nuclear bodies was also
recorded. In the present study, nuclear bodies refer to
nucleoplasmic bodies, excluding nucleoli.
Two experienced pathologists (OG and MGK) carried
out microscopic evaluation of WRAP53β signals based on the fraction
of stained tumour cells and on staining intensity. The percentage
of positive cells was categorised into four, semi-quantitative
groups: i) 0, negative; ii) 1, <25%; iii) 2, 25–50%; and iv) 3,
>50% of cells (MKG). In addition, staining intensity was graded
as: i) 0, negative (no positive cells observed); ii) 1, weak; iii)
2, moderate; and iv) 3, strong.
Statistical analysis
Pearson's χ2 test or Fisher's exact tests
were used to analyse the association between ordinal variables and
IHC parameters. An independent-sample t-test was used to analyse
the differences between the means of the groups. Survival analysis
according to the expression of dyskerin and WRAP53β is presented as
a Kaplan-Meier graph, and a log-rank test was used to compare the
different dichotomised groups. Multivariate analysis of different
prognostic factors, such as age at diagnosis, tumour size,
histology and FIGO stage, was performed using the Cox
proportional-hazards model for survival outcome. Statistical
analysis was carried out using SPSS 19 software (IBM Corp.).
P<0.05 was considered to indicated a statistically significant
difference.
Results
Immunohistochemical analysis of
dyskerin
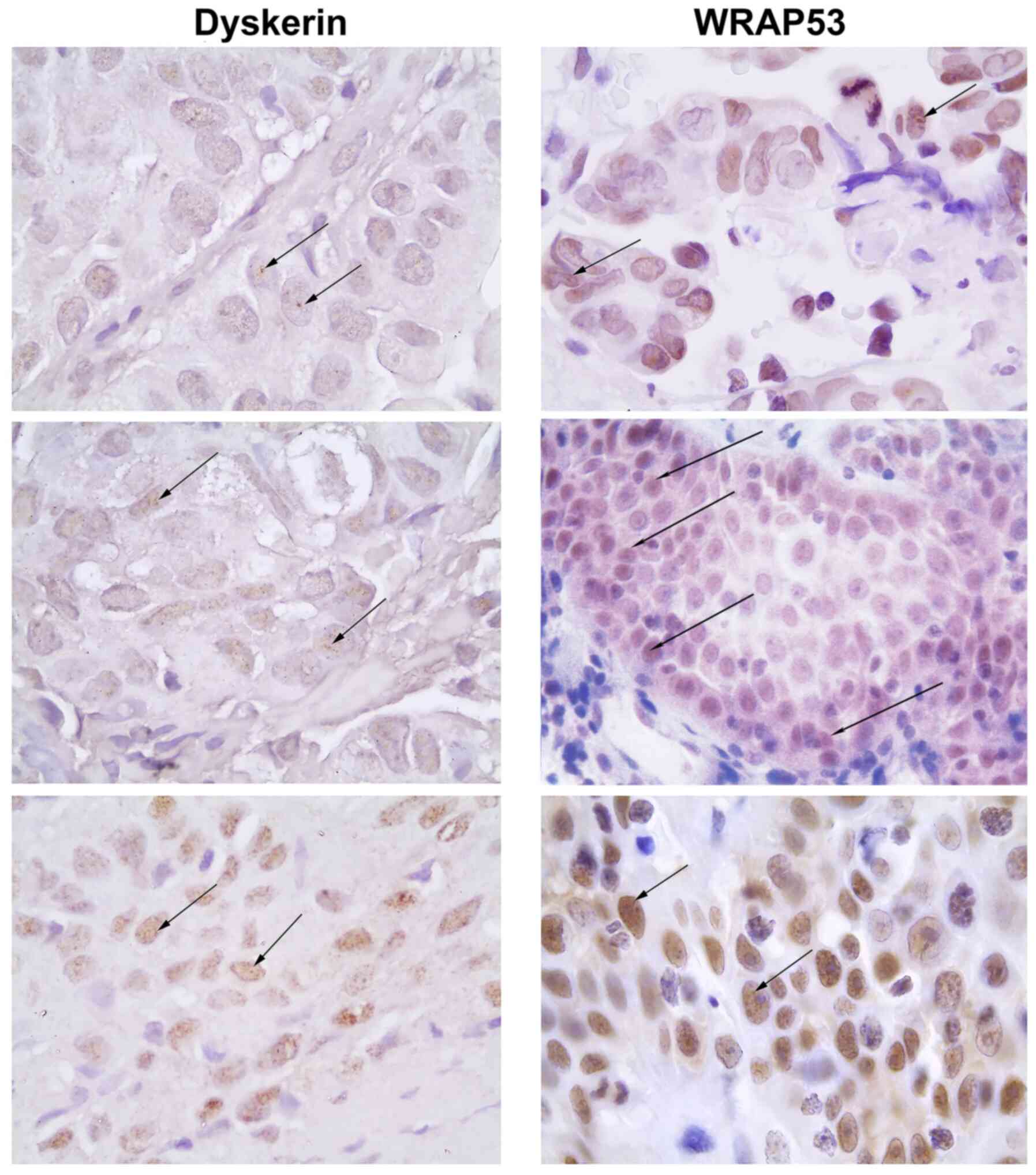
Immunohistochemical analysis of 68 tumour samples
revealed varying degrees of nuclear expression of dyskerin
(Fig. 1 and Table I). The majority of cells (79%)
demonstrated a weak staining intensity of dyskerin, whereas 7%
showed an moderate and 11% a strong staining intensity. Most of the
staining was seen in nuclear bodies + nucleoplasm (57%), not only
in nucleoli.
 | Table I.IHC analysis of dyskerin staining
intensity and localisation pattern in tumour samples of patients
with primary vaginal cancer. |
Table I.
IHC analysis of dyskerin staining
intensity and localisation pattern in tumour samples of patients
with primary vaginal cancer.
| IHC parameter | n (%) |
|---|
| Dyskerin IHC
staining intensity |
|
Negative (no positive
cells) | 1 (1.5) |
| 1+
(weak) | 54 (79.4) |
| 2+
(moderate) | 5 (7.4) |
| 3+
(strong) | 8 (11.7) |
| Dyskerin
localisation |
| Nuclear
bodies (negative nucleoplasm) | 9 (13.2) |
|
Nucleoplasm (negative nuclear
bodies) | 15 (22.1) |
| Nuclear
bodies + nucleoplasm | 39 (57.3) |
| No
staining or very weak | 5 (7.3) |
Examination of dyskerin staining intensity in
relation to clinical variables (Table
II) revealed no association between the expression of dyskerin
and FIGO stage at diagnosis (Pearson's χ2; P=0.509), nor
with tumour localisation within the vagina (P=0.644). Although a
high expression of dyskerin was more frequently observed in
HPV-negative (81%) compared with in HPV-positive tumour samples
(66%), this difference was not statistically significant (P=0.20)
(data not shown). Examination of the relationship between the
expression of dyskerin and histological type revealed that high
expression was more frequently observed in basaloid and
keratinising tumours (87%) than in other histological types (59%)
(P=0.015; data not shown). High expression of dyskerin was also
significantly associated with poorly differentiated tumours
(P=0.032) (Table II).
 | Table II.Correlation analysis of dyskerin
expression and clinical variables and survival. |
Table II.
Correlation analysis of dyskerin
expression and clinical variables and survival.
| Parameter | Group 0 | Group 1 | P-value |
|---|
| Mean age at
diagnosis, yearsa | 70 | 69 | 0.878 |
| Mean tumour size,
mm | 25 | 23 | 0.465 |
| FIGO stage, n
(%) | | | 0.509 |
| I | 7 (43.8) | 12 (28.0) |
|
| II | 6 (37.5) | 19 (44.2) |
|
|
III | 2 (12.5) | 4 (9.3) |
|
| IV | 1 (6.3) | 8 (18.6) |
|
| Tumour
localisation, n (%) | | | 0.644 |
|
Upper | 5 (31.2) | 12 (27.9) |
|
|
Middle | 4 (25.0) | 7 (16.3) |
|
|
Lower | 4 (25.0) | 14 (32.6) |
|
| Entire
vagina | 2 (12.5) | 9 (20.9) |
|
| Middle
+ lower | 0 (0) | 1 (2.3) |
|
|
Urethra | 1 (6.25) | 0 (0) |
|
| Histological type,
n (%) | | | 0.226 |
|
Basaloid | 4 (25) | 18 (42) |
|
|
Non-keratinizing | 9 (56) | 13 (30.2) |
|
|
Keratinizing | 0 (0) | 8 (18.6) |
|
|
Verrucous | 1 (6.2) | 1 (2.3) |
|
|
Adenocarcinoma | 2 (12.5) | 3 (7) |
|
| Tumour grade, n
(%) | | | 0.032 |
| Grade
1 | 2 (12.5) | 6 (14.3) |
|
| Grade
2 | 7 (43.8) | 17 (40.3) |
|
| Grade
3 | 3 (18.8) | 18 (42.9) |
|
|
Unknown | 4 (25) | 1 (2.4) |
|
| HPV status, n
(%) | | | 0.360 |
|
Negative | 5 (31.2) | 21 (48.8) |
|
|
Positive | 11 (69) | 21 (48.8) |
|
|
Unknown | 0 (0) | 1 (2.3) |
|
| Primary cure rate,
n (%) | | | 0.241 |
|
Yes | 15 (93.8) | 35 (81.4) |
|
| No | 1 (6.3) | 8 (18.6) |
|
| Recurrence, n
(%) | | | 0.134 |
|
Yes | 3 (18.9) | 17 (39.5) |
|
| No | 13 (81.3) | 26 (60.5) |
|
| Mean time to
recurrence, months | 29 | 19 | 0.511 |
| Localisation of
recurrent disease (n=20), n (%) | | | 0.619 |
|
Local | 2 (66.7) | 3 (17.6) |
|
|
Regional | 0 (0) | 1 (5.9) |
|
| Distant
metastasis | 1 (33.3) | 9 (52.9) |
|
| Local +
regional + distant metastasis | 0 (0) | 3 (17.6) |
|
|
Regional + distant
metastasis | 0 (0) | 1 (0.6) |
|
| Survival status, n
(%) | | | 0.039 |
|
Alive | 5 (31.3) | 8 (18.6) |
|
| Death
from disease | 3 (18.8) | 24 (55.8) |
|
| Death
from intercurrent disease | 8 (50.0) | 11 (25.6) |
|
| Mean
survival time, months | 50 | 92 | 0.017 |
The primary cure rate was lower in patients with
high expression of dyskerin in their tumour samples and those with
low expression (81% vs. 94%; P=0.241); however, this change was not
statistically significant. Similarly, overall recurrence among
patients with high expression of dyskerin was 40%, compared to 19%
among those with low expression (P=0.134). Moreover, 13/14
recurrent tumours with distant metastasis demonstrated a high
expression of dyskerin (P=0.001) (data not shown).
Examination of dyskerin staining intensity in
relation to survival (Table II)
revealed significant associations with disease progression and
survival. Survival time was significantly shorter in patients with
high expression of dyskerin compared with those with low expression
(50 months vs. 92 months; P=0.017). In addition, 55.8% of patients
with high expression of dyskerin had already died from PVC by the
time this study was undertaken, while the corresponding figure for
patients with low expression was 18.8% (P=0.039).
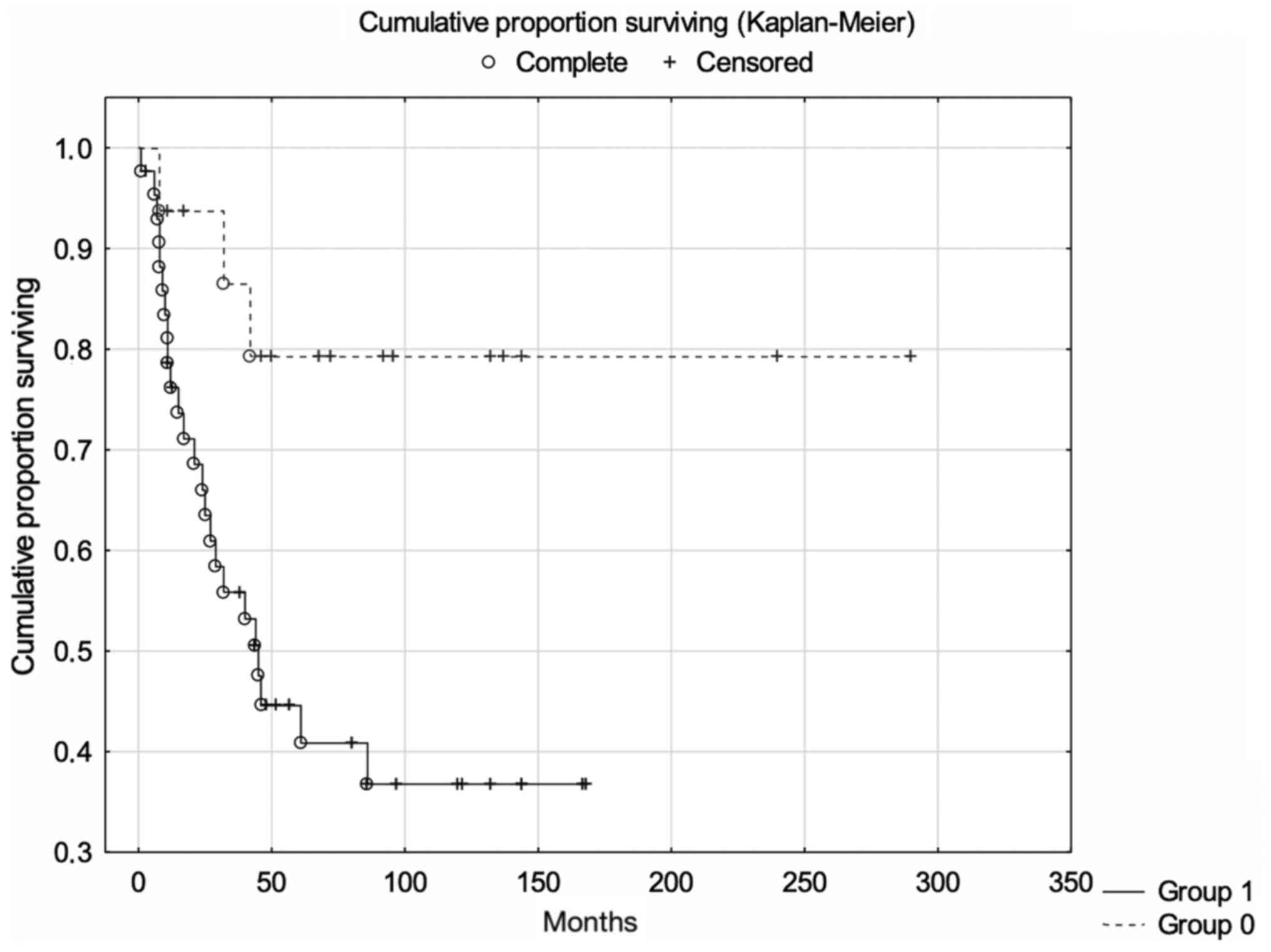
High expression of dyskerin was associated with
significantly lower 5-year cancer-specific survival rates (log-rank
test; P=0.009; Fig. 2). Expression
of dyskerin remained a significant and independent prognostic
factor after correction for age at diagnosis, tumour size,
histological type and FIGO stage (Cox multivariate proportional
regression analysis; P=0.035; Table
III).
 | Table III.Prognostic factors vs.
cancer-specific survival rate. |
Table III.
Prognostic factors vs.
cancer-specific survival rate.
| Factor | HR (95% CI) | P-value |
|---|
| FIGO stage (III–IV
vs. I–II) | 1.669
(0.659-4.224) | ns |
| Age at diagnosis
(per year) | 1.020
(0.980-1.062) | ns |
| Tumour size (per
cm) | 1.099
(0.738-1.636) | ns |
| Histology (squamous
cell carcinoma vs adenocarcinoma) | 1.742
(0.391-7.757) | ns |
| Dyskerin staining
intensity (2–3 vs. 0–1) | 3.701
(1.094-12.517) | 0.035 |
Immunohistochemical analysis of
WRAP53β
As with dyskerin, WRAP53β was expressed in both
nuclear bodies (12%; likely Cajal bodies) and in nucleoplasm (16%)
(Fig. 3; Table IV). The majority of tumour samples
showed expression of WRAP53β, but no significant association was
observed between the percentage of stained cells or staining
intensity of WRAP53β and clinical variables or survival rates (data
not shown).
 | Table IV.IHC analysis of WRAP53β staining
intensity, fraction of stained cells and localisation pattern in
tumour samples of patients with primary vaginal cancer. |
Table IV.
IHC analysis of WRAP53β staining
intensity, fraction of stained cells and localisation pattern in
tumour samples of patients with primary vaginal cancer.
| IHC paramter | n (%) |
|---|
| WRAP53β IHC
staining intensity |
| 0 | 8 (8.5) |
| 1 | 22 (32.4) |
| 2 | 20 (29.4) |
| 3 | 18 (26.5) |
| WRAP53β IHC
fraction of stained cells |
| 0 | 13 (16.0) |
| 1 | 32 (39.5) |
| 2 | 20 (24.7) |
| 3 | 3 (3.7) |
| WRAP53β
localisationa |
| Nuclear
bodies | 8 (13.1) |
|
Nucleoplasm | 11 (18.0) |
| Nuclear
bodies + nucleoplasm | 40 (65.6) |
|
Diffuse | 2 (3.3) |
Discussion
The present study demonstrates for the first time to
the best of our knowledge that high expression of dyskerin, a
protein involved in the modification of nuclear RNA and telomere
elongation, is significantly associated with lower cancer-specific
survival, as well as with lower overall survival in patients with
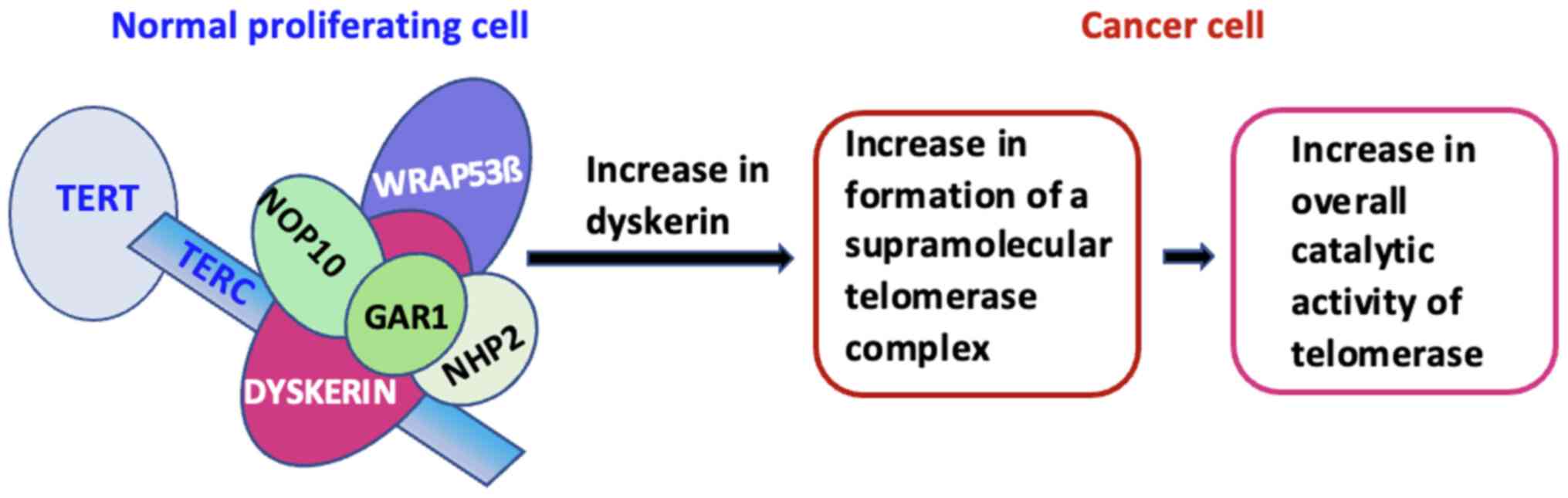
PVC. One explanation is that dyskerin upregulation may lead to an
increase in telomerase supramolecular complex formation, thus
increasing the overall catalytic activity of telomerase (Fig. 4). Moreover, the higher dyskerin
expression was significantly associated with poorly differentiated
tumours. It was also associated with an increased risk of recurrent
disease in terms of distant metastasis (Table II). In agreement with these
findings, elevated dyskerin levels have been linked to progression
and aggressiveness in several tumour types (17,19,20,35)
as well as to worse clinical outcomes in breast cancer (16), lung cancer (22), hepatocellular carcinoma (21), renal cell cancer (18) and neuroblastoma (23). Taken together, these findings
suggest that dyskerin may be a useful prognostic marker for several
types of cancer, including PVC.
A potential limitation of these findings is the
limited number of patients included in this retrospective study.
This reflects the low incidence of PVC (1) as well as the small population size in
Sweden. However, given the non-parametric statistical model used in
this study, the assumed risk of an underpowered study design is
outweighed by the importance of performing exploratory studies on
PVC. Indeed, previous knowledge on the biological factors of PVC
has been based on similar sample sizes (2). In addition, due to the rarity of PVC,
the present study was retrospective and based on archived,
paraffin-embedded tumour samples instead of fresh-frozen material;
the latter would have enabled complementary analyses. Nevertheless,
future studies on a larger cohort with additional medical centres
to confirm the present findings and to analyse the relationship
between the expression of dyskerin and HPV infection in PVC, as
well as in other gynaecological malignancies.
The mechanism by which dysregulation of dyskerin
contributes to cancer development is debated, although it appears
to be linked to both enhanced telomerase activity and protein
biogenesis (17,20). Non-small cell lung cancer provides
one example of how high dyskerin expression is significantly
associated with worse overall survival due to TERC stabilisation.
Such stabilisation has been traced to the overexpression of
dyskerin rather than to TERC gene amplification (22), which is in line with the idea that
dyskerin can modify telomerase activity through the regulation of
TERC levels, and independent of TERT expression (36). In the case of prostate cancer, high
expression of dyskerin mRNA is associated with more advanced
clinical stage and recurrent disease. In this example,
dysregulation of dyskerin is associated with enhanced protein
biosynthesis rather than with telomerase activity (35). Indeed, loss of dyskerin function
has been shown to reduce the amount of pseudouridinylated ribosomal
RNA and thereby impair ribosome function and synthesis of proteins
(16,37).
Conversely, inactivating mutations of dyskerin cause
dyskeratosis congenita, a rare genetic disease associated with a
predisposition to cancer development (mainly haematological
malignancies and head and neck cancer) (38). Similarly, dyskerin has also been
shown to be downregulated in sporadic chronic lymphocytic leukaemia
(39).
In light of the current study, upregulation of
dyskerin might be associated with the poor prognosis of PVC due to
enhancement of telomerase activity and/or altered protein
synthesis. In an attempt to investigate the former, the expression
of the WRAP53β protein, which is known to have a role in
transporting dyskerin and the telomerase complex to telomeres as
required for telomere elongation (14), was examined. The findings suggested
that WRAP53β was expressed to varying degrees in the majority of
PVC tumour samples, but this expression had no significant
association with clinical parameters or patient survival. In PVC,
the sub-cellular localisation of WRAP53β could not be linked to
survival, in contrast with findings for both breast (27) and head and neck cancer (26). It can be concluded that the role of
WRAP53β as a telomere transporter appears to be intact in PVC and
that dyskerin upregulation could therefore result in enhanced
telomere elongation. Upregulation of WRAP53β may also indicate its
involvement in the DNA damage response, as suggested by Bergstrand
et al (29).
Notably, the telomerase complex plays a crucial role
in an important oncogenic pathway that stimulates the development
and progression of HPV-associated cancer, which relates to the E6
oncogene of HPV that regulates TERT activity (40). Our finding of low expression of
dyskerin in HPV-positive PVC tumour samples may suggest that
dyskerin is indispensable to telomerase activity in these samples,
since the E6 protein activates transcription of the human (h)TERT
component of the telomerase complex (41). The association between gene
expression linked to hTERT activity, including dyskerin, and
malignant progression of HPV-induced cervical lesions, has been
previously studied (42), although
no correlation was observed between dyskerin expression at the
protein level and the severity of precursor lesions. In the present
study, dyskerin was detectable in nuclear bodies and/or in
nucleoplasm, but not in the cytoplasm. Previous studies have
detected dyskerin mainly in the nucleus, as well as in the
cytoplasm of cervical precursor lesions (42), renal cell carcinoma (18) and hepatocellular carcinoma cells
(21). One possible explanation
for this discrepancy with our results may be the use of different
dyskerin antibodies.
In summary, the present study demonstrates that
upregulation of dyskerin is significantly associated with poor
prognosis in PVC. This may be explained by the fact that an high
expression of dyskerin may lead to increased telomerase
supramolecular complex formation, thereby increasing the overall
catalytic activity of telomerase. The future studies on PVC cell
lines in vitro, using overexpression and downregulation of
dyskerin with RNA sequencing analysis may clarify the functional
consequences of dyskerin overexpression. In conclusion, the present
findings point to dyskerin as a promising prognostic marker and as
a potential putative therapeutic target in PVC.
Acknowledgements
Not applicable.
Funding
This study was supported by The Swedish Cancer Foundation (grant
nos. 110544 and CAN2011/471), The Karolinska Institute Cancer
Strategic Grants (grant no. 5888-05722), The Swedish Research
Council (521-2008-2899), The Stockholm County Council (grant nos.
20130097 and 20160155), The Gustaf V Jubilee Fund (grant nos.
154022 and 151202) and The Centre of Clinical Research, Västmanland
County Council (grant no. LTV-940144). The funding bodies had no
role in the design of the study, the collection, analysis, or
interpretation of data, or in the manuscript writing.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CR, SA, MF, KH and GLL contributed to study design.
GLL, LK and EK performed the immunohistochemical preparation. LK
and MGK performed immunohistochemical analysis. BS and CR performed
statistical analysis and interpretation of data was performed by
CR, BS, and SA. DL helped with the writing of the manuscript and
was involved in the planning of the study, formulating the
hypothesis and study design. CR drafted the manuscript. BS and CR
confirm the authenticity of all the raw data. All authors
critically reviewed the manuscript. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by The Regional
Ethical Review Board in Uppsala, Sweden (approval no. 2008/294),
who did not request specific informed consent from patients.
Patients were originally orally informed about the clinical
research database. After 2003, they were also informed about tissue
biobanking in accordance with the Swedish Biobank Act 2002:297. The
study was performed in accordance with the Declaration of
Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
Dr Cecilia Ranhem's ORCID-ID is
0000-0002-3743-6217.
Glossary
Abbreviations
Abbreviations:
|
PVC
|
primary vaginal carcinoma
|
|
TERT
|
telomerase reverse transcriptase
|
|
TERC
|
telomerase RNA complex
|
|
HPV
|
human papillomavirus
|
|
IHC
|
immunohistochemical
|
|
WRAP53β
|
WD repeat containing antisense to
TP53
|
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global Cancer Statistics 2020:
GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36
Cancers in 185 Countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hellman K, Silfversward C, Nilsson B,
Hellstrom AC, Frankendal B and Pettersson F: Primary carcinoma of
the vagina: factors influencing the age at diagnosis. The
Radiumhemmet series 1956–96. Int J Gynecol Cancer. 14:491–501.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hacker NF, Eifel PJ and van der Velden J:
Cancer of the vagina. Int J Gynaecol Obstet. 131 (Suppl 2):S84–S87.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ghezelayagh T, Rauh-Hain JA and Growdon
WB: Comparing mortality of vaginal sarcoma, squamous cell
carcinoma, and adenocarcinoma in the surveillance, epidemiology,
and end results database. Obstet Gynecol. 125:1353–1361. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hiniker SM, Roux A, Murphy JD, Harris JP,
Tran PT, Kapp DS and Kidd EA: Primary squamous cell carcinoma of
the vagina: Prognostic factors, treatment patterns, and outcomes.
Gynecol Oncol. 131:380–385. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shah CA, Goff BA, Lowe K, Peters WA III
and Li CI: Factors affecting risk of mortality in women with
vaginal cancer. Obstet Gynecol. 113:1038–1045. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gadducci A, Fabrini MG, Lanfredini N and
Sergiampietri C: Squamous cell carcinoma of the vagina: Natural
history, treatment modalities and prognostic factors. Crit Rev
Oncol Hematol. 93:211–224. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hellman K, Lundell M, Silfversward C,
Nilsson B, Hellstrom AC and Frankendal B: Clinical and
histopathologic factors related to prognosis in primary squamous
cell carcinoma of the vagina. Int J Gynecol Cancer. 16:1201–1211.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Larsson GL, Helenius G, Andersson S, Sorbe
B and Karlsson MG: Prognostic impact of human papilloma virus (HPV)
genotyping and HPV-16 subtyping in vaginal carcinoma. Gynecol
Oncol. 129:406–411. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hellman K, Lindquist D, Ranhem C, Wilander
E and Andersson S: Human papillomavirus, p16(INK4A), and Ki-67 in
relation to clinicopathological variables and survival in primary
carcinoma of the vagina. Br J Cancer. 110:1561–1570. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hayflick L: The illusion of cell
immortality. Br J Cancer. 83:841–846. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Blackburn EH: Switching and signaling at
the telomere. Cell. 106:661–673. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nguyen THD, Collins K and Nogales E:
Telomerase structures and regulation: Shedding light on the
chromosome end. Curr Opin Struct Biol. 55:185–193. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Venteicher AS, Abreu EB, Meng Z, McCann
KE, Terns RM, Veenstra TD, Terns MP and Artandi SE: A human
telomerase holoenzyme protein required for Cajal body localization
and telomere synthesis. Science. 323:644–648. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mahmoudi S, Henriksson S, Weibrecht I,
Smith S, Söderberg O, Strömblad S, Wiman KG and Farnebo M: WRAP53
is essential for Cajal body formation and for targeting the
survival of motor neuron complex to Cajal bodies. PLoS Biol.
8:e10005212010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Montanaro L, Brigotti M, Clohessy J,
Barbieri S, Ceccarelli C, Santini D, Taffurelli M, Calienni M,
Teruya-Feldstein J, Trerè D, et al: Dyskerin expression influences
the level of ribosomal RNA pseudo-uridylation and telomerase RNA
component in human breast cancer. J Pathol. 210:10–18. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Montanaro L, Calienni M, Bertoni S, Rocchi
L, Sansone P, Storci G, Santini D, Ceccarelli C, Taffurelli M,
Carnicelli D, et al: Novel dyskerin-mediated mechanism of p53
inactivation through defective mRNA translation. Cancer Res.
70:4767–4777. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang M, Pan Y, Jiang R, Hou P, Shan H,
Chen F, Jiang T, Bai J and Zheng J: DKC1 serves as a potential
prognostic biomarker for human clear cell renal cell carcinoma and
promotes its proliferation, migration and invasion via the NF-κB
pathway. Oncol Rep. 40:968–978. 2018.PubMed/NCBI
|
|
19
|
Bellodi C, Krasnykh O, Haynes N,
Theodoropoulou M, Peng G, Montanaro L and Ruggero D: Loss of
function of the tumor suppressor DKC1 perturbs p27 translation
control and contributes to pituitary tumorigenesis. Cancer Res.
70:6026–6035. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Miao FA, Chu K, Chen HR, Zhang M, Shi PC,
Bai J and You YP: Increased DKC1 expression in glioma and its
significance in tumor cell proliferation, migration and invasion.
Invest New Drugs. 37:1177–1186. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu B, Zhang J, Huang C and Liu H:
Dyskerin overexpression in human hepatocellular carcinoma is
associated with advanced clinical stage and poor patient prognosis.
PLoS One. 7:e431472012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Penzo M, Ludovini V, Treré D, Siggillino
A, Vannucci J, Bellezza G, Crinò L and Montanaro L: Dyskerin and
TERC expression may condition survival in lung cancer patients.
Oncotarget. 6:21755–21760. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
O'Brien R, Tran SL, Maritz MF, Liu B, Kong
CF, Purgato S, Yang C, Murray J, Russell AJ, Flemming CL, et al:
MYC-Driven Neuroblastomas Are Addicted to a Telomerase-Independent
Function of Dyskerin. Cancer Res. 76:3604–3617. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mahmoudi S, Henriksson S, Corcoran M,
Méndez-Vidal C, Wiman KG and Farnebo M: Wrap53, a natural p53
antisense transcript required for p53 induction upon DNA damage.
Mol Cell. 33:462–471. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Henriksson S, Rassoolzadeh H, Hedström E,
Coucoravas C, Julner A, Goldstein M, Imreh G, Zhivotovsky B, Kastan
MB, Helleday T, et al: The scaffold protein WRAP53β orchestrates
the ubiquitin response critical for DNA double-strand break repair.
Genes Dev. 28:2726–2738. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Garvin S, Tiefenböck K, Farnebo L, Thunell
LK, Farnebo M and Roberg K: Nuclear expression of WRAP53β is
associated with a positive response to radiotherapy and improved
overall survival in patients with head and neck squamous cell
carcinoma. Oral Oncol. 51:24–30. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Silwal-Pandit L, Russnes H, Borgen E,
Skarpeteig V, Moen Vollan HK, Schlichting E, Kåresen R, Naume B,
Børresen-Dale AL, Farnebo M, et al: The Sub-Cellular Localization
of WRAP53 Has Prognostic Impact in Breast Cancer. PLoS One.
10:e01399652015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hedström E, Pederiva C, Farnebo J, Nodin
B, Jirström K, Brennan DJ and Farnebo M: Downregulation of the
cancer susceptibility protein WRAP53β in epithelial ovarian cancer
leads to defective DNA repair and poor clinical outcome. Cell Death
Dis. 6:e18922015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bergstrand S, O'Brien EM and Farnebo M:
The Cajal Body Protein WRAP53β Prepares the Scene for Repair of DNA
Double-Strand Breaks by Regulating Local Ubiquitination. Front Mol
Biosci. 6:512019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Henriksson S and Farnebo M: On the road
with WRAP53β: Guardian of Cajal bodies and genome integrity. Front
Genet. 6:912015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang H, Wang DW, Adell G and Sun XF:
WRAP53 is an independent prognostic factor in rectal cancer- a
study of Swedish clinical trial of preoperative radiotherapy in
rectal cancer patients. BMC Cancer. 12:2942012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rao X and Huang D, Sui X, Liu G, Song X,
Xie J and Huang D: Overexpression of WRAP53 is associated with
development and progression of esophageal squamous cell carcinoma.
PLoS One. 9:e916702014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhu Y, Ding L, Chen BF, Song JG and Yao
YS: Oncogenic Activity of Wrap53 in Human Colorectal Cancer In
Vitro and in Nude Mouse Xenografts. Med Sci Monit. 24:6129–6136.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Herrington SC and Ordi J: Chapter 34:
Tumours of the vagina. In: WHO Classification of Tumours: Female
genital tumours. (5th edition). 4:WHO Classification of Tumours
Editorial Board (ed), IARC Press. (Lyon, France). 2020.
|
|
35
|
Sieron P, Hader C, Hatina J, Engers R,
Wlazlinski A, Müller M and Schulz WA: DKC1 overexpression
associated with prostate cancer progression. Br J Cancer.
101:1410–1416. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Montanaro L, Calienni M, Ceccarelli C,
Santini D, Taffurelli M, Pileri S, Treré D and Derenzini M:
Relationship between dyskerin expression and telomerase activity in
human breast cancer. Cell Oncol. 30:483–490. 2008.PubMed/NCBI
|
|
37
|
Bellodi C, McMahon M, Contreras A, Juliano
D, Kopmar N, Nakamura T, Maltby D, Burlingame A, Savage SA,
Shimamura A, et al: H/ACA small RNA dysfunctions in disease reveal
key roles for noncoding RNA modifications in hematopoietic stem
cell differentiation. Cell Rep. 3:1493–1502. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Heiss NS, Knight SW, Vulliamy TJ, Klauck
SM, Wiemann S, Mason PJ, Poustka A and Dokal I: X-linked
dyskeratosis congenita is caused by mutations in a highly conserved
gene with putative nucleolar functions. Nat Genet. 19:32–38. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Poncet D, Belleville A, t'kint de
Roodenbeke C, Roborel de Climens A, Ben Simon E, Merle-Beral H,
Callet-Bauchu E, Salles G, Sabatier L, Delic J, et al: Changes in
the expression of telomere maintenance genes suggest global
telomere dysfunction in B-chronic lymphocytic leukemia. Blood.
111:2388–2391. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Katzenellenbogen R: Telomerase Induction
in HPV Infection and Oncogenesis. Viruses. 9:E1802017. View Article : Google Scholar
|
|
41
|
Klingelhutz AJ, Foster SA and McDougall
JK: Telomerase activation by the E6 gene product of human
papillomavirus type 16. Nature. 380:79–82. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Koskimaa HM, Kurvinen K, Costa S, Syrjänen
K and Syrjänen S: Molecular markers implicating early malignant
events in cervical carcinogenesis. Cancer Epidemiol Biomarkers
Prev. 19:2003–2012. 2010. View Article : Google Scholar : PubMed/NCBI
|