Introduction
Hepatocellular carcinoma (HCC) is a common and
lethal malignancy (1). Due to a
late diagnosis and advanced underlying liver cirrhosis, only
limited treatment options with marginal clinical benefits are
available for affected patients (1,2).
Metallotherapeutics, including platinum and other metal-containing
antitumor drugs, is a strategy for treating HCC (3).
Cadmium (Cd) is a toxic heavy metal implicated in
carcinogenesis (4) and the
development of HCC (5). Similar to
the use of chemotherapeutics, such as the carcinogenic and toxic
metalloid arsenic, a metallotherapeutic agent could be applied as a
toxicant to attack the malignancy. Indeed, Cd is also effective
against HCC in experimental animals (6–8) and
in cultured liver tumor SMMC cells and their xenografts (9,10).
Cd-coordinated supramolecules, such as Cd-pyrithione (11), Cd telluride/Cd sulfide (12,13),
Cd-coordinated thiacalix arene tetrasulfate (14), Cd-thiocarbodiazone complex
(15), Cd in combination with
human second mitochondria-derived activator of caspase (16) and a novel binuclear hydrazone-based
Cd(II) complex (17), have shown
potential antitumor effects against chemoresistant malignant
cells.
HCC is often refractory to chemotherapy and
radiotherapy at late stages, and is often associated with the loss
of metallothionein (MT), probably due to hypermethylation of the MT
genes (18–20), and deficiency in MT could make HCC
vulnerable to the necrotic effects of Cd, while surrounding normal
tissues could be protected through MT induction (6). MTs are small, cysteine-rich
cadmium-binding proteins that protect against cadmium toxicity
(21).
The present study was initiated in mice to examine
the effects of cadmium, administered in drinking water, against HCC
formation initiated by diethylnitrosamine (DEN) and promoted by
carbon tetrachloride. Small animal ultrasound was employed to
monitor tumor formation, immunohistochemistry was used to stain for
MT, and the expression levels of the HCC biomarker α- fetoprotein,
MT and tumor necrosis factor-α in the liver were determined via
reverse transcription-quantitative (RT-q)PCR.
Materials and methods
Reagents
Cadmium chloride (CdCl2), DEN and carbon
tetrachloride (CCl4) were obtained from MilliporeSigma.
Other reagents were of analytical grade.
Animals
Male C57BL/6 mice (6 weeks old) were purchased from
the Animal Center Institute of Surgery Research of the Third
Military Medical University (Chongqing, China). Animals were
maintained in specific pathogen-free facilities at Zunyi Medical
University (Zunyi, China), under a controlled environment (22±1°C,
50±2% humidity and a 12:12 h light:dark cycle) and free access to
purified water and standard laboratory chow. Efforts were made to
ameliorate distress and harm to animals by daily monitoring and
humane treatment. Animals were adequately cared for, and
experimental protocols were in compliance with the Animal
Management Guidelines of the Chinese Ministry of Health and
approved by the Animal Use and Care Committee of Zunyi Medical
University (approval no. 2015–01). The study is reported in
accordance with ARRIVE guidelines (https://arriveguidelines.org).
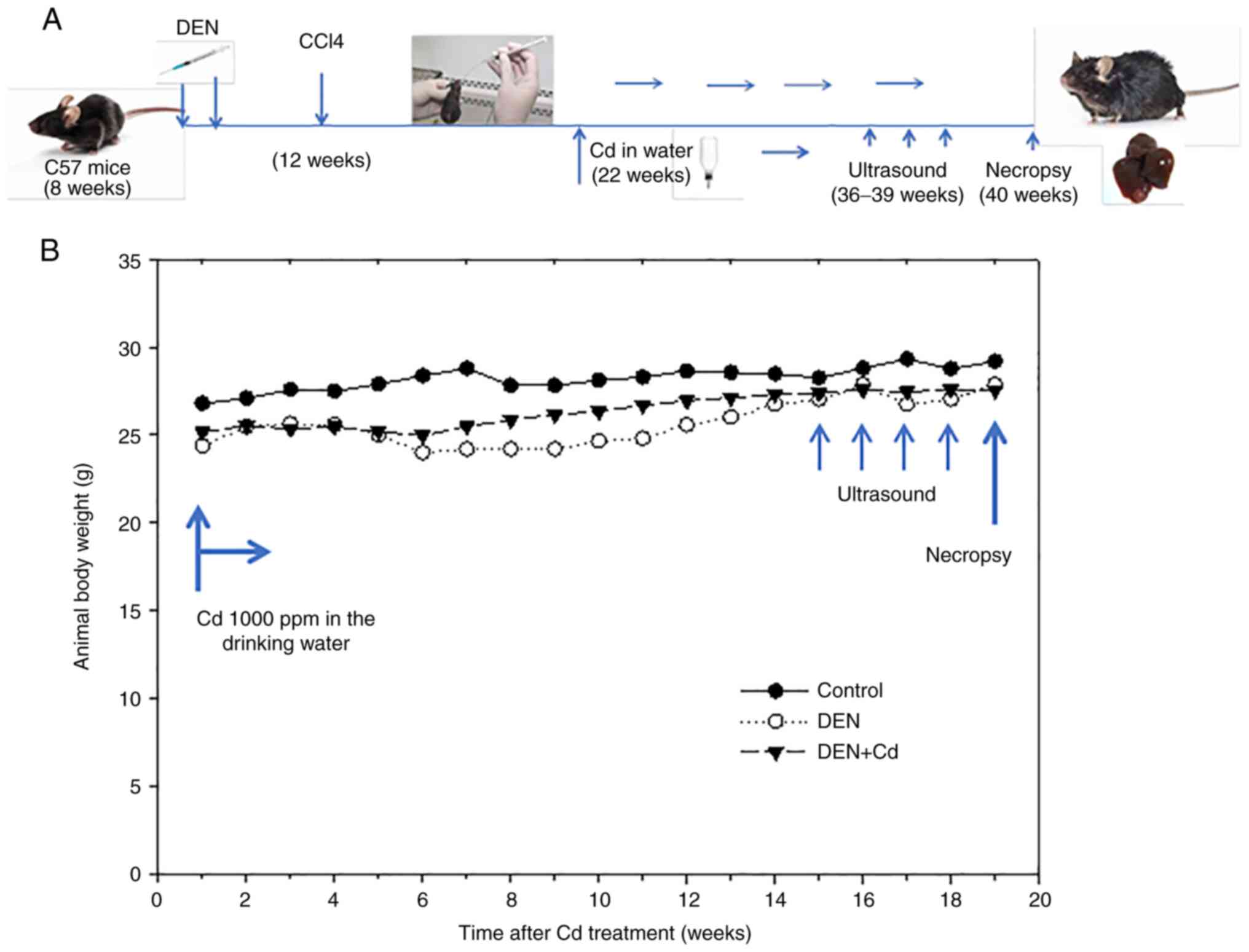
Experimental design
After 2 weeks of acclimation, 40 mice (8 weeks of
age, weighing 16–19 g) were given the first injection of DEN (90
mg/kg, i.p.). Approximately 80% of mice survived the first DEN
injection, and 2 weeks later, the surviving mice were given the
second injection of DEN (50 mg/kg, i.p.), according to the protocol
(22). At 4 weeks after the
initial DEN challenge, mice (12 weeks of age) were orally
administered 20% CCl4, 5 ml/kg, twice a week for 4
months in an attempt to promote liver tumors. At 21 weeks after
initial DEN initiation, the mice were randomly divided into the DEN
(n=14) and DEN + Cd (n=12) group. There was an additional normal
control (n=5) group that did not receive any treatment. Cadmium was
administered via the drinking water (1000 ppm) as CdCl2
from 21–40 weeks according to the previous literature (7,8).
Body weights were monitored weekly, and a single ultrasound
examination of liver tumor formation was performed at 35–39 weeks
after DEN initiation. At the end of the 40-week experiment, the
mice were anesthetized with sodium pentobarbital (65 mg/kg, i.p.)
and subsequently sacrificed by decapitation. The mouse livers were
harvested, and the liver weights and tumor outcomes were recorded.
The treatment process is illustrated in Fig. 1A.
The visible tumors were counted and recorded. The
tumor incidence (number of tumor-bearing mice), tumor numbers
(total tumors found) and tumor size scores (score of 1, tumor size
<1 mm; score of 2, tumor size 1–2 mm; and score of 3, tumor size
>2 mm) were recorded.
Ultrasound detection
Liver tumor formation was measured using B-mode
ultrasound (Vevo® 2100; Fujifilm VisualSonics, Inc.)
with 30-MHz peak frequency linear array transducers (MS400;
Fujifilm VisualSonics, Inc.; mean beam frequency range of 22–55
MHz) in a digitized scale as previously described (23). Ultrasound was used to detect tumor
incidence and size.
Histopathology
Liver tissues were fixed in 10% buffered formalin
for 48 h at room temperature, embedded in paraffin at 60°C and
sliced into 3.5-µm-thick sections using a RM2235 microtome (Leica
Microsystems GmbH). Sections were deparaffinized in xylene and
rehydrated using a gradient of ethanol (100, 95, 85 and 75%). The
histological sections were then stained with hematoxylin and eosin
at room temperature for 8–10 min and 4–5 sec, respectively. The
digitized images of slices were observed via the Olympus image
analysis system (light microscope; Olympus Corporation) at ×4 and
×10 magnifications as previously described (24).
Immunohistochemical analysis
Metallothionein (MT) protein retrieval was performed
in 10 mmol/l citrate buffer (pH 6.0) at 92°C for 15 min. Endogenous
peroxidase was then blocked with 3% H2O2 for
15 min at room temperature. The tissues were then incubated with
10% goat non-immune serum (Invitrogen; Thermo Fisher Scientific,
Inc.) for 1 h at room temperature. Subsequently, sections were
incubated with MT primary antibodies (cat. no. ab12228, 1:100;
Abcam) overnight at 4°C. After washing with PBS for 5 min, slides
were incubated with HRP-conjugated anti-mouse secondary antibody
(1:1,000; cat. no. A0216; Beyotime Institute of Biotechnology) for
1 h at room temperature following the protocol associated with the
SABC Detection System (Beyotime). Finally, sections were stained
with DAB and counterstained with hematoxylin for 5 min at room
temperature. The digitized images of slices were observed via the
LEICA image analysis system (Leica Microsystems GmbH) at ×10
magnification. The expression of proteins was quantitatively
measured by Image ProPlus 6.0 to obtain the positive
staining-integral optical density/area (density mean). A total of
four discontinuous areas were used to analyze the expression levels
of protein in a blinded fashion as previously described (24).
RT-qPCR
Total RNA was extracted from tissues with
TRIzol® (Takara Biotechnology Co., Ltd.) and reverse
transcribed according to the manufacturer's protocol into cDNA
using the PrimeScript™ RT reagent kit (Takara Biotechnology Co.,
Ltd.). qPCR was performed utilizing the iQ™ SYBR Green Supermix
(Bio-Rad Laboratories, Inc.). The PCR cycling conditions were 94°C
for 3 min, followed by 40 cycles of 94°C for 15 sec, 60°C for 20
sec and 72°C for 40 sec. Data were normalized to β-actin and
expressed using the comparative Cq method (23,25,26).
Primers for the mouse genes are shown in Table I.
 | Table I.Primer sequences for reverse
transcription-quantitative PCR. |
Table I.
Primer sequences for reverse
transcription-quantitative PCR.
| Name | Access ID | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| AFP | NM_007423 |
AGCAGGACTGCTCGAAACAT |
AGCGAAATGTAGCAGGAGGA |
| β-actin | NM_007393 |
GATCTGGCACCACACCTTCT |
GGGGTGTTGAAGGTCTCAAA |
| MT-2 | NM_008630 |
CCGATCTCTCGTCGATCTTC |
AGGAGCAGCAGCTTTTCTTG |
| TNF-α | NM_013693 |
TAGCCAGGAGGGAGAACAGA |
TTTTCTGGAGGGAGATGTGG |
Statistical analysis
Data are presented as the mean ± SE and were
analyzed by one-way ANOVA, followed by Ducan's multiple comparison
test using SigmaPlot (Systat Sofeware, Inc.) version 14. For tumor
incidence, Fisher's exact test was performed for the contingency
table, while for tumor number and tumor score, data were analyzed
with the Kruskal-Wallis test, followed by Dunn's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Animal survival and body weight
After the initial DEN (90 mg/kg, i.p.)
administration, 80% of mice survived within 7 days, with a ~20%
reduction in body weight. After the second DEN injection, 95% of
mice survived, with a ~15% reduction in body weight. Animal body
weights slowly recovered afterwards over time. CCl4
promotion (20%, 5 ml/kg, p.o., twice/week) was started at week 4
post-DEN injection and continued for 4 months. Cd was administered
from 21–40 weeks after DEN initiation through the drinking water
(1000 ppm) for 19 weeks. All mice survived Cd treatment. Ultrasound
was randomly performed on a few mice at week 36 post-DEN injection
(15 weeks after Cd treatment), 4 times, until week 40 post-DEN
initiation (19 weeks after Cd treatment). The animal body weights
after Cd intervention are shown in Fig. 1B.
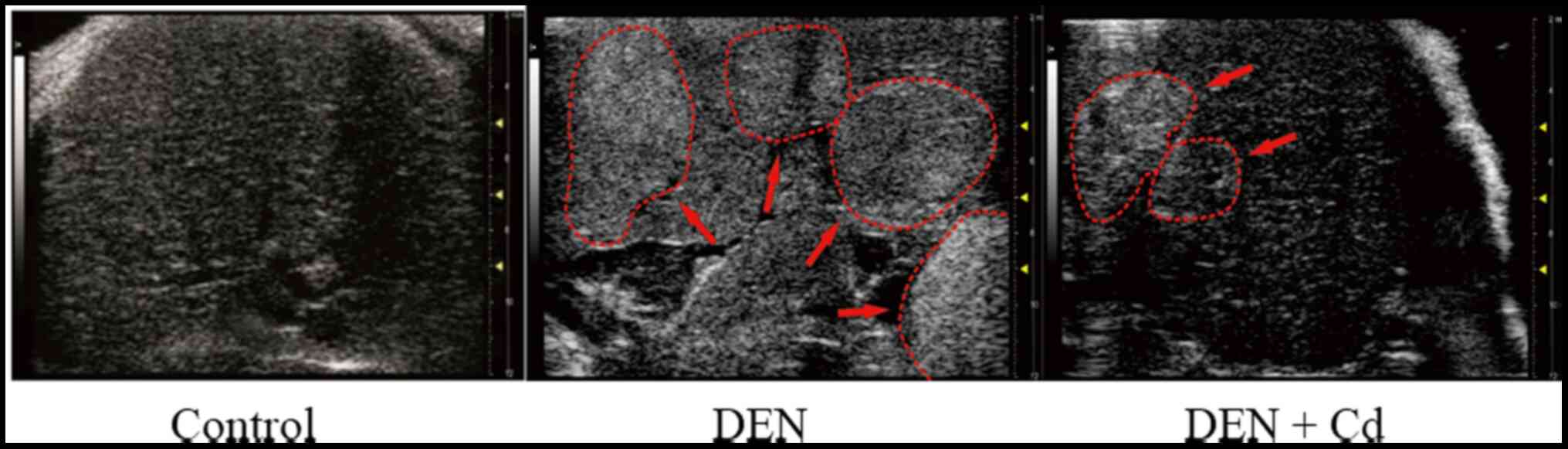
Ultrasound detection of liver tumor
formation
Small animal ultrasound analysis was used to monitor
tumor formation 30 weeks after DEN injection, but no tumors were
detected. At 36 weeks after DEN injection, liver tumors with small
sizes were detected at an ~40% incidence rate. At week 40 after DEN
initiation, a 65% tumor incidence rate was recorded. Fig. 2 shows representative ultrasound
images of DEN-induced liver tumors at 40 weeks after DEN initiation
in control, DEN- and DEN + Cd-treated mice. In the control mice,
the ultrasound showed normal liver, while in the DEN-treated mice,
the liver density was increased (arrows), indicative of liver
tumors. In the DEN + Cd-treated mice, only one mouse showed a
detectable tumor with decreased density compared with non-tumor
tissues.
Animal liver/body weight ratio
At the end of experiment, the mice were euthanized
and their livers were collected and weighed, and the liver/body
ratios were calculated. Fig. 3
shows the liver/body weight ratios of the three groups. DEN
increased the liver/body weight ratio from 48.5 to 53.4 mg/g, while
DEN + Cd further increased the liver/body weight ratio to 55.9
mg/g, which were significantly higher results compared with the
controls.
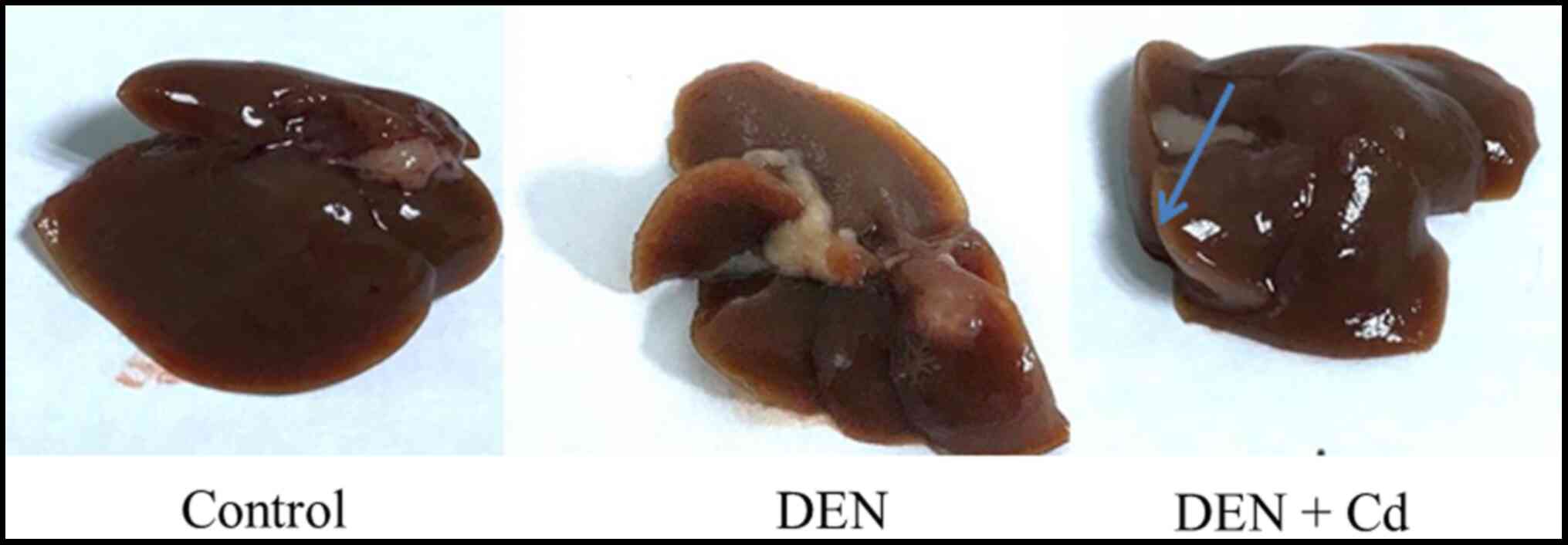
Liver tumor outcome
Representative gross liver images are shown in
Fig. 4, where liver tumors were
detected in DEN-treated mice, no tumors were found in control mice
and only a few tumors were found in DEN + Cd-treated mice.
The tumor outcomes, recorded from blinded
observations, are listed in Table
II. In total, 10 out of 14 DEN-treated mice had tumors, with a
tumor incidence of 71%, and only 2 out of 12 DEN + Cd-treated mice
had tumors, with a tumor incidence of 17%; a total of 15 tumors
were found in DEN-treated mice, but only 2 tumors in DEN +
Cd-treated mice. Some tumors in the DEN-treated mice were large,
and the tumor score was 22, while in DEN + Cd-treated mice, the
score was 3. The tumor number and tumor score of the DEN + Cd group
were statistically significant compared with the DEN group.
 | Table II.Tumor outcomes of DEN and DEN + Cd
groups. |
Table II.
Tumor outcomes of DEN and DEN + Cd
groups.
| Group | n | Tumor incidence, n
(%) | Tumor number | Tumor score |
|---|
| Control | 5 | 0 | 0 | 0 |
| DEN | 14 | 10 (71) | 15 | 22 |
| DEN + Cd | 12 | 2a
(17) | 2a | 3a |
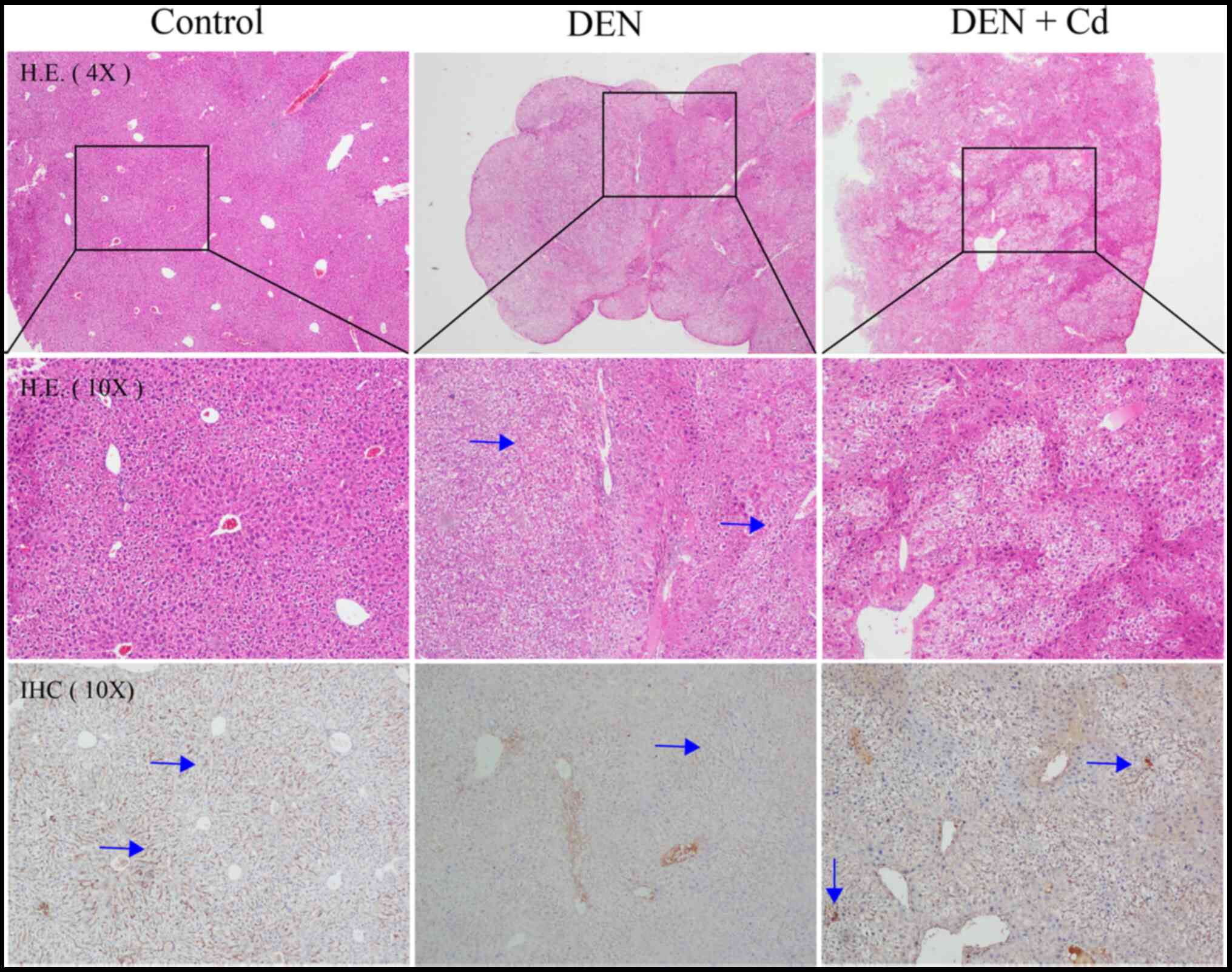
Histology and
immunohistochemistry
Fig. 5 shows
representative histology and immunochemistry images of the tissues
in the different groups. Liver tumors were observed in DEN-treated
mice, and hepatocellular degeneration was evident in both DEN- and
DEN + Cd-treated mice. In DEN-treated livers, MT staining was not
present in the liver tumors, but was strongly present in the
tissues surrounding the tumors. DEN + Cd-treated mice exhibited
increased intensity of the MT stain.
RT-qPCR analysis of liver gene
expression
Fig. 6 shows the
expression of liver genes in the control mice (n=5), and in the
mice treated with DEN (n=14) and DEN + Cd (n=12), with controls set
at 100%. DEN treatment increased the expression of α-fetoprotein
(AFP) over 2-fold (242% of control); however, due to huge
individual variance, the difference was not statistically
significant. DEN + Cd treatment increased the expression to 112% of
the control. The expression of MT-2 was slightly decreased by DEN
treatment (73% of the control), but significantly increased by DEN
+ Cd treatment by almost 3-fold (294% of the control). The
expression of tumor necrosis factor α (TNFα) was slightly increased
by DEN treatment (142% of the control), but significantly increased
by DEN + Cd treatment (224% of the control).
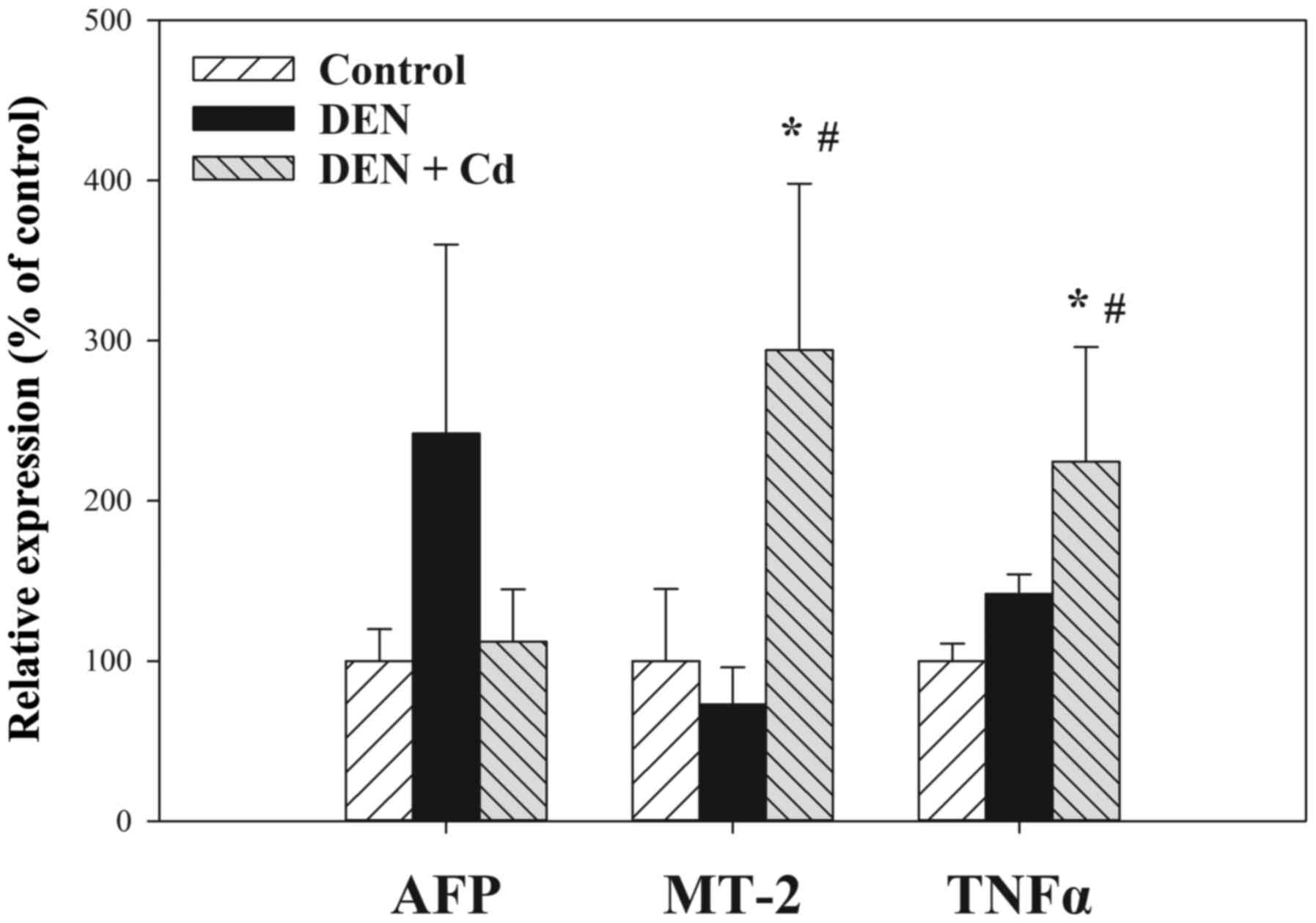 | Figure 6.Expression of AFP, MT-2, and TNFα via
RT-qPCR. At 19 weeks after Cd treatment (500 ppm in the drinking
water), hepatic total RNA was extracted and subjected to RT-qPCR
analysis. Data are presented as the mean ± SEM of the control
(n=5), DEN (n=14) and DEN + Cd (n=12) groups. *P<0.05 vs.
control; #P<0.05 vs. DEN. AFP, α-fetoprotein; MT,
mettalothionein; RT-qPCR, reverse transcription-quantitative PCR;
DEN, dimethylnitrosamine; Cd, cadmium; TNFα, tumor necrosis factor
α. |
Discussion
The present study demonstrated the antitumor effects
of cadmium against DEN-initiated and CCl4-promoted HCC,
as evidenced by ultrasound imaging, tumor incidence, tumor number
and tumor score. Immunohistochemistry showed MT was deficient in
HCC, but was induced in the tissues surrounding the tumor. RT-qPCR
revealed that the HCC biomarker AFP was increased in HCC, but
attenuated by Cd, although the changes were not statistically
significant. Cd treatment also induced MT and TNFα in the liver.
However, histology showed hepatocyte degeneration in the liver in
both DEN- and DEN + Cd-treated groups.
Cadmium has previously been shown to exert antitumor
effects against DEN-induced HCC in B6C3F1 mice (6–8),
including the late stage of HCC by inducing tumor necrosis
(6). The present study replicated
these previous findings in C57/BL mice. Cd exposure is known to
produce tumors in the lungs, liver, prostate, pancreas and
injection sites (4), and long-term
Cd exposure is implicated in HCC development (5). Paradoxically, in an effort to promote
DEN-initiated HCC, Cd was unexpectedly found to produce antitumor
effects (7). This phenomenon is
common for metallo-chemotherapy agents. For example, arsenic is a
well-known human carcinogen, but it is also effective against
hematological malignancies and certain solid tumors with
arsenic-metal complexes (27), and
the toxic effects of Cd towards colorectal carcinoma cells
(28) might contribute to the
antitumor effects of Cd. The use of a toxic metal/metalloid to
treat chemo-resistant malignancies could be a strategy in
metallo-chemotherapy.
Non-invasive imaging of HCC growth in mice with
ultrasound technology (29) would
help monitor HCC growth, the treatment efficacy and the treatment
duration. We have previously successfully applied ultrasound in
cardiovascular studies (23). In
the present study, HCC formation could be detected in mice at 4
months after DEN initiation (at 6 months of age), while at 8 months
of age, 50% tumor incidence was detected, and at 9 months of age,
65% tumor incidence was detected, which was consistent with the
necropsy findings at the end of experiment (at 10 months of age).
Tumor promotion with CCl4 in the current study resulted
in higher tumor incidence compared with that found in previous
studies using Cd alone (7,8). With the aid of ultrasound monitoring,
HCC-bearing mice were sacrificed at 40 weeks of age to examine Cd
antitumor effects, with findings of decreased incidence (71 vs.
17%), decreased tumor numbers (15 vs. 2) and decreased tumor size
scores (22 vs. 3) compared with the DEN-treated group. Ultrasound
appears to be a sensitive means to monitor liver tumor development
and helped to define the Cd treatment regimen for tumor growth
inhibition in the present study.
MTs are small, cysteine-rich cadmium-binding
proteins that protect against cadmium toxicity and are encoded by
MT isoform genes (21,30). In studies on human HCC, MT-1G was
hypermethylated, leading to decreased expression (19), MT-1X and MT-2A tended to decrease
with the progression of HCC (20),
and at the late stage of HCC, MT-1A, MT-2A and metal regulatory
transcription factor 1 levels were decreased (25). In the present study, immunostaining
for MT was not detected in HCC, while the surrounding tissues
exhibited intense staining for MT. This molecular event could be
turned into an advantage for Cd to selectively kill HCC, while
saving normal cells that will be protected from Cd cytotoxicity by
the MT induction.
AFP is a well-known biomarker of HCC that exhibits
increased levels in DEN-treated mouse livers (31). In the present study, the expression
of AFP in the Cd-treated group was attenuated, consistent with the
antitumor effects of Cd. By contrast, the expression of MT-2 and
TNFα in the Cd-treated group was higher compared with that in the
DEN model group. Cd is a potent inducer of the MT genes; both MT-1
and MT-2 are coordinately expressed in the mouse liver (21), and increased MT-2 expression could
protect against Cd toxicity. Induction of TNFα by Cd plays a dual
role in killing tumor cells, as well as in producing liver damage
(32).
It should be noted that the antitumor effects of Cd
should be carefully assessed to ensure the balance between the
benefits and risks from its use. Necrotic effects of Cd on HCC are
a desired therapeutic outcome, but Cd-induced liver injury is an
undesired toxic effect. In the present study, the antitumor effects
of Cd were accompanied by liver damage to various degrees (Figs. 4 and 5). Caution should be taken when using Cd
to treat malignancies over a long period of time, and close
monitoring of the potential adverse effects to balance efficacy and
toxicity is important.
Collectively, the present study demonstrated the
antitumor effects of Cd against DEN-induced HCC in C57/B6 mice; the
mechanism of action appeared to be related to MT-deficiency in HCC,
while normal surrounding tissues were protected by MT. Thus, the
targeting of MT-deficiency in HCC could be a potential therapeutic
strategy.
Acknowledgements
Not applicable.
Funding
This study was supported by the Guizhou Provincial Science and
Technology Program [grant no. QKH(2019)1346], the Science and
Technology Talent Support Project of the Educational Department of
Guizhou Province [grant no. KY(2018)055], the Innovation Talent
Team of Zunyi [grant no. ZSKRC(2019)1], the Innovation Talent Team
of Guizhou Science and Technology Department [grant no.
QKHPTRC(2020)5007] and the Science and Technology Plan of Zunyi
[grant no. ZSKRCPT(2020)7].
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL, JL and YN conceived the experiment. YN performed
the animal studies, including drug administration, body weight
recording and necropsy, and qPCR and histology analyses. BH and YYX
performed the small animal ultrasound imaging, ALH performed the
pathology and immunohistochemistry analyses, and YZ performed the
statistical analysis. YL, YN, BH, YYX and ALH confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
All animal care and experimental protocols complied
with the Animal Management Guidelines of the Chinese Ministry of
Health and were approved by the Animal Use and Care Committee of
Zunyi Medical University (Zunyi, China; approval no. 2015-01).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wörns MA and Galle PR: Future perspectives
in hepatocellular carcinoma. Dig Liver Dis. 42 (Suppl 3):S302–S309.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Javan H, Dayyani F and Abi-Jaoudeh N:
Therapy in Advanced Hepatocellular Carcinoma. Semin Intervent
Radiol. 37:466–474. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kaluderović GN and Paschke R: Anticancer
metallotherapeutics in preclinical development. Curr Med Chem.
18:4738–4752. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Waalkes MP: Cadmium carcinogenesis. Mutat
Res. 533:107–120. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Satarug S: Long-term exposure to cadmium
in food and cigarette smoke, liver effects and hepatocellular
carcinoma. Curr Drug Metab. 13:257–271. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Waalkes MP, Diwan BA, Rehm S, Ward JM,
Moussa M, Cherian MG and Goyer RA: Down-regulation of
metallothionein expression in human and murine hepatocellular
tumors: Association with the tumor-necrotizing and antineoplastic
effects of cadmium in mice. J Pharmacol Exp Ther. 277:1026–1033.
1996.PubMed/NCBI
|
|
7
|
Waalkes MP, Diwan BA, Weghorst CM, Bare
RM, Ward JM and Rice JM: Anticarcinogenic effects of cadmium in
B6C3F1 mouse liver and lung. Toxicol Appl Pharmacol. 110:327–335.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Waalkes MP, Diwan BA, Weghorst CM, Ward
JM, Rice JM, Cherian MG and Goyer RA: Further evidence of the
tumor-suppressive effects of cadmium in the B6C3F1 mouse liver and
lung: Late stage vulnerability of tumors to cadmium and the role of
metallothionein. J Pharmacol Exp Ther. 266:1656–1663.
1993.PubMed/NCBI
|
|
9
|
Du H, Liu X, Liu Y, Jin M, Huang W and Sun
Z: Inhibition effect of cadmium chloride on human hepatocellular
carcinoma cells SMMC7721. Chin J Publ Health. 22:194–195. 2006.
|
|
10
|
Du HJM, Liu Y, Liu X, Wang W and Sun Z:
Study on the anti-tumor effect of calcium chloride in vivo. Mod
Prev Med. 35:3763–3765. 2008.
|
|
11
|
Chen X, Wu J, Yang Q, Zhang X, Zhang P,
Liao S, He Z, Wang X, Zhao C and Liu J: Cadmium pyrithione
suppresses tumor growth in vitro and in vivo through inhibition of
proteasomal deubiquitinase. Biometals. 31:29–43. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu N, Piao M, Arkin K, Ren L, Zhang J, Hao
J, Zheng Y and Shang Q: Imaging of water soluble CdTe/CdS
core-shell quantum dots in inhibiting multidrug resistance of
cancer cells. Talanta. 201:309–316. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang G, Shi L, Selke M and Wang X: CdTe
quantum dots with daunorubicin induce apoptosis of
multidrug-resistant human hepatoma HepG2/ADM cells: In vitro and in
vivo evaluation. Nanoscale Res Lett. 6:4182011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou X, Koizumi Y, Zhang M, Natsui M,
Koyota S, Yamada M, Kondo Y, Hamada F and Sugiyama T:
Cadmium-coordinated supramolecule suppresses tumor growth of T-cell
leukemia in mice. Cancer Sci. 106:635–641. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pérez JM, Cerrillo V, Matesanz AI, Millán
JM, Navarro P, Alonso C and Souza P: DNA interstrand cross-linking
efficiency and cytotoxic activity of novel
cadmium(II)-thiocarbodiazone complexes. ChemBioChem. 2:119–123.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guo C, Li Y, Zhang H, Wang Z, Jin M, Zhang
L, An L, Hu G, Liu X, Liu Y, et al: Enhancement of
antiproliferative and proapoptotic effects of cadmium chloride
combined with hSmac in hepatocellular carcinoma cells.
Chemotherapy. 57:27–34. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bjelogrlić S, Todorović TR, Cvijetić I,
Rodić MV, Vujčić M, Marković S, Araškov J, Janović B, Emhemmed F,
Muller CD, et al: A novel binuclear hydrazone-based Cd(II) complex
is a strong pro-apoptotic inducer with significant activity against
2D and 3D pancreatic cancer stem cells. J Inorg Biochem. 190:45–66.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jacob ST, Majumder S and Ghoshal K:
Suppression of metallothionein-I/II expression and its probable
molecular mechanisms. Environ Health Perspect. 110 (Suppl
5):827–830. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kanda M, Nomoto S, Okamura Y, Nishikawa Y,
Sugimoto H, Kanazumi N, Takeda S and Nakao A: Detection of
metallothionein 1G as a methylated tumor suppressor gene in human
hepatocellular carcinoma using a novel method of double combination
array analysis. Int J Oncol. 35:477–483. 2009.PubMed/NCBI
|
|
20
|
Tao X, Zheng JM, Xu AM, Chen XF and Zhang
SH: Downregulated expression of metallothionein and its
clinicopathological significance in hepatocellular carcinoma.
Hepatol Res. 37:820–827. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Klaassen CD, Liu J and Choudhuri S:
Metallothionein: An intracellular protein to protect against
cadmium toxicity. Annu Rev Pharmacol Toxicol. 39:267–294. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yi X, Long L, Yang C, Lu Y and Cheng M:
Maotai ameliorates diethylnitrosamine-initiated hepatocellular
carcinoma formation in mice. PLoS One. 9:e935992014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang B, Hu P, Hu A, Li Y, Shi W, Huang J,
Jiang Q, Xu S, Li L and Wu Q: Naringenin attenuates carotid
restenosis in rats after balloon injury through its
anti-inflammation and anti-oxidative effects via the RIP1-RIP3-MLKL
signaling pathway. Eur J Pharmacol. 855:167–174. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hu A, Huang J, Li S, Gao Y, Wu L, Deng J,
Liu J, Gong Q, Li L and Xu S: Involvement of stromal cell-derived
factor-1α (SDF-1α), stem cell factor (SCF), fractalkine (FKN) and
VEGF in TSG protection against intimal hyperplasia in rat balloon
injury. Biomed Pharmacother. 110:887–894. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li H, Lu YF, Chen H and Liu J:
Dysregulation of metallothionein and circadian genes in human
hepatocellular carcinoma. Chronobiol Int. 34:192–202. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fei W, Li C, Tao J, Cai X, Yao W, Ye Y,
Zhang Y, Yao Y, Song Q, Li F, et al: Construction of arsenic-metal
complexes loaded nanodrugs for solid tumor therapy: A mini review.
Int J Pharm. 583:1193852020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Iftode A, Drăghici GA, Macașoi I,
Marcovici I, Coricovac DE, Dragoi R, Tischer A, Kovatsi L,
Tsatsakis AM, Cretu O, et al: Exposure to cadmium and copper
triggers cytotoxic effects and epigenetic changes in human
colorectal carcinoma HT-29 cells. Exp Ther Med. 21:1002021.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Anton N, Parlog A, Bou About G, Attia MF,
Wattenhofer-Donzé M, Jacobs H, Goncalves I, Robinet E, Sorg T and
Vandamme TF: Non-invasive quantitative imaging of hepatocellular
carcinoma growth in mice by micro-CT using liver-targeted iodinated
nano-emulsions. Sci Rep. 7:139352017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Klaassen CD, Liu J and Diwan BA:
Metallothionein protection of cadmium toxicity. Toxicol Appl
Pharmacol. 238:215–220. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ghufran H, Azam M, Mehmood A, Butt H and
Riazuddin S: Standardization of diethylnitrosamine-induced
hepatocellular carcinoma rat model with time based molecular
assessment. Exp Mol Pathol. 123:1047152021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kayama F, Yoshida T, Elwell MR and Luster
MI: Role of tumor necrosis factor-alpha in cadmium-induced
hepatotoxicity. Toxicol Appl Pharmacol. 131:224–234. 1995.
View Article : Google Scholar : PubMed/NCBI
|