Introduction
Renal cell carcinoma (RCC) is a common urinary
system malignant tumor and accounts for 2–3% of adult malignant
tumors. The male to female ratio was ~1.5:1 and the 50–70 years old
age group had the highest prevalence (1,2). The
incidence rate of RCC is increasing ~2.5% each year (3,4).
One-third of patients with RCC have metastasis at initial diagnosis
and up to 40% of patients with initially localized RCC eventually
develop metastasis during follow-up (5–7). The
common sites of metastasis from RCC are the lungs (45.2%), followed
by the bone (29.5%), regional lymph nodes (21.8%), the liver
(20.3%), the adrenal gland (8.9%) and the brain (8.1%) (8). Molecular-targeted therapies are the
leading treatment for metastatic RCC (mRCC). However, the objective
response rate (ORR) and complete response of the primary tumor was
only 28 and 2.5%, respectively in patients treated with first-line
targeted therapy (9). In addition,
the prognosis of patients with mRCC was found to be extremely poor,
with a 5-year survival rate of <10% (10). Therefore, it is important to
identify novel prognostic, diagnostic and therapeutic methods to
increase the understanding of the development and progression of
RCC.
Glycoprotein non-metastatic protein B (GPNMB) is a
type I transmembrane protein, which was first isolated and
described in 1995 (11). The
GPNMB gene is located on chromosome 7q15 and encodes a
protein, 572 amino acids in length (12). GPNMB has been found to be expressed
in different tissues and cells, including bone tissues,
osteoclasts, osteoblasts, macrophages and dendritic cells. It plays
diverse and important roles in normal cells and tissues, such as
cell differentiation and migration, and tissue regeneration and
inflammation (13–15). However, the overexpression of GPNMB
has been found in different types of cancer and was found to
increase the invasion and metastasis in several types of tumor
cells. GPNMB promoted the metastasis of melanoma, glioblastoma and
hepatocellular carcinoma (16–18).
In addition, the increased expression of GPNMB, both at the mRNA
and protein expression level, was detected in osteosarcoma tissues
compared with that in the adjacent non-cancerous tissues (19). Furthermore, GPNMB promoted the
development of an aggressive, pro-metastatic phenotype in human
prostate cancer cell lines (20),
and GPNMB overexpression in breast cancer cells was associated with
bone invasion (21). These
previous studies suggested that GPNMB may play an important role in
the bone metastasis (BM) of malignant tumors, which could serve as
a potential therapeutic target.
Therefore, the aim of the current study was to
quantify the protein expression levels of GPNMB in the primary
renal tumor and the matched BM, to investigate the association
between GPNMB expression level and the clinicopathological
parameters and prognosis in patients with RCC and BM. Furthermore,
the results of our previous research found that the expression
level of ERK was significantly increased in the matched BM compared
with that in the primary RCC (22). Therefore, the underlying molecular
mechanism of GPNMB activity in tumor metastasis, particularly via
the activation of the ERK pathway, was also investigated.
Materials and methods
Tissue samples and cell lines
In the present retrospective study, a total of 31
patients with RCC and BM were collected. The present study was
approved by the Ethical Committee of Beijing Jishuitan Hospital
(approval no.2016–16; Beijing, China). Due to the retrospective
design of the study patient consent was not required for data
analysis and all samples were anonymized prior to analysis. The
samples included primary RCC and the matched BM, which were
resected during surgery. The following inclusion criteria were
used: Patients with i) newly diagnosed BM from RCC; ii) BM
diagnosed using a bone scan or positron emission tomography-CT and
iii) definite pathological diagnosis of the BM. The exclusion
criteria included patients with i) concomitant other malignant
tumors; ii) no surgical treatment of BM and iii) targeted therapy
or radiotherapy.
Information regarding the sex, age, time, extent and
the number of BMs, the presence or absence of visceral metastasis,
and the pathological type of BM was also collected from patient
records. All the patients were followed up regularly following
surgery, at 3-month intervals during the first 2 years then, at
6-month intervals thereafter. Chest X-rays, chest CT scans and
serum chemistry analyses (blood biochemistry and routine blood
analysis) was performed for all the patients at every follow-up
visit. Recurrence was evaluated from the patient records at Beijing
Jishuitan Hospital (Beijing China) and the patients were followed
up by their physician until they died or until the date of the last
documented contact.
According to the time of BM, the patients were
divided into 2 groups: RCC with synchronous BM and RCC with
metachronous BM groups. The patients who had BM at initial
diagnosis of RCC were defined as the BM synchronous group. The
patients diagnosed with BM after the diagnosis of RCC were defined
as the BM metachronous group. With respect to the extent of the BM,
the patients were divided into 3 groups: axial only BM,
appendicular only BM, and both axial and appendicular BM
groups.
The human ACHN RCC-derived cell line was purchased
from the National Infrastructure of Cell Line Resource. The cells
were maintained in MEM containing 10% fetal bovine serum (FBS, both
Gibco; Thermo Fisher Scientific, Inc.) at 37°C in a humidified
incubator with 5% CO2.
Immunohistochemistry
All the paraffin-embedded sections (4-µm thick) were
dewaxed in xylene for 5 min three times followed by 100% alcohol
for 5 min, 90% alcohol for 5 min and 80% alcohol for 5 min. The
sections were then rinsed in distilled water for 2 min and the
antigen was retrieved (0.01 M citrate buffer, pH 6.0) at 95°C for
20 min. Endogenous peroxidase activity was blocked with 3% hydrogen
peroxide, then non-specific binding was blocked with 5% normal goat
serum (cat. no. AR1009; Boster Biological Technology) for 30 min at
room temperature. Following which, the sections were incubated with
the primary rabbit anti-human GPNMB (1:500 dilution; cat. no.
ab222109) and ERK antibodies (1:300 dilution; cat. no. ab32537)
(both from Abcam) at 4°C overnight in a wet box. Subsequently, the
sections were incubated with the biotinylated secondary antibody
(1:200 dilution; cat. no. K5007; Dako; Agilent Technologies, Inc.)
for 20 min at room temperature, then with the chromogen,
3,3′-diaminobenzidine for 5 min at room temperature. The sections
were lastly stained with Mayer's hematoxylin (cat. no. H9627;
Sigma-Aldrich; Merck KGaA) for 3 min at room temperature and viewed
under a microscope (Olympus BX53; Olympus Corporation) with ×400
magnification.
At least 500 tumor cells were evaluated for
immunostaining, and the GPNMB or ERK expression level was evaluated
according to the staining intensity and the percentage of cells
expressing GPNMB or ERK. Briefly, the sections were evaluated for
the percentage of stained cells using the following criteria: 0,
0%; 1, <10%; 2,11–50%; 3,51–80% and 4, >80%. At the same
time, the intensity of staining was also evaluated and graded from
1 to 3, as follows: 0, negative; 1, weak; 2, moderate; and 3,
strong. The two values obtained were multiplied together to
calculate a receptor score (maximum value, 12). The tumor samples
that scored ≤6 were considered to have a low expression level of
GPNMB (GPNMB low; n=20), whereas samples which scored >6 were
classified as having a high expression level of GPNMB (GPNMB high;
n=11). To decrease the interobserver variation in evaluating the
staining patterns, the immunohistochemical staining was evaluated
and scored by two independent observers using the semi-quantitative
method. Any discrepancies were resolved by a joint review using a
double-headed light microscope (Axioplan II; Zeiss AG).
RNA interference
Small interfering (si)RNA targeting human GPNMB mRNA
(5′-GGAATACAACCCAATAGA-3′) was ligated into the lentiviral vector
pLVshRNA-EGFP(2A)Puro (0.1 µg; Inovogen Tech) using the restriction
sites, EcoRI and BamHI. The lentivirus was made using
the 293T cells transduced with pLVshRNA-GFP, psPAX2 (cat. no.
12260; Addgene, Inc.) and pMD2.G (cat. no. 12259; Addgene, Inc.) at
a 4:3:2 ratio. The cells were incubated at 37°C in a humidified
incubator with 5% CO2, for 48 h, then the medium was
collected and filtered using a 0.45 µM filter unit. The ACHN cell
line was transduced with a high multiplicity of infection and 10
µg/ml polybrene. The samples were analyzed 72 h following
transduction using flow cytometry (FACS AriaIII; BD Biosciences) to
sort the GFP+ cells. GFP+ cells with
puromycin (cat. no. A1113803; Thermo Fisher Scientific, Inc.)
resistance were used for further analysis. The concentrations of
puromycin used for selection and maintenance were 1 and 0.5 µg/ml,
respectively. A non-targeting sequence (5′-UUCUCCGAACGUGUCACGU-3′;
Invitrogen; Thermo Fisher Scientific, Inc.) was used as the
negative control (NC).
Western blot analysis
The cells were lysed with RIPA buffer, containing 20
mM Tris-HCl (pH 7.4), 150 mM sodium chloride, 1 mM dithiothreitol,
5 mM EDTA and 1% (w/v) Triton X-100 at 4°C for 30 min. The lysates
were then centrifuged at 12,000 × g at 4°C for 20 min. The
supernatant was collected and the protein concentration was
calculated using a BCA assay (Pierce; Thermo Fisher Scientific,
Inc.). Total protein (30 ug) was separated using 10% SDS-PAGE, then
transferred to PVDF membranes (cat. no. IPVH00010; Merck KGaA) and
incubated overnight at 4°C with the primary antibodies (β-actin,
1:5,000; cat. no. sc-47778; Santa Cruz Biotechnology, Inc.; GPNMB,
1:1,000; cat. no. ab227695; Abcam; ERK, 1:1,000; cat. no. 9102;
Cell Signaling Technology, Inc.; and phosphorylated (p)-ERK, 1:500;
cat. no. 3179; Cell Signaling Technology, Inc.). Next, 5% skimmed
milk powder in TBS [25 mM Tris, 0.15M NaCl (pH 7.2-7.5)] was used
for blocking at room temperature for 1 h. The membranes were then
incubated with the HRP-linked secondary antibody (1:5,000 dilution;
cat. no. 7074; Cell Signaling Technology, Inc.) for 1 h at 37°C.
The signal was detected using a SuperSignal West Pico PLUS
Chemiluminescent Substrate (Thermo Fisher Scientific, Inc.).
Cell proliferation assays
In vitro proliferation was analyzed using a
Cell Counting Kit (CCK-8; Shanghai Yeasen Biotechnology Co., Ltd.).
The cells were seeded at a density of 4,000 cells/well in 96-well
plates. Following which, 10 µl/well CCK-8 reagent was added 0, 24,
48, 72 and 96 h later and the cells were incubated for 3 h at 37°C.
The optical density was measured at 450 nm using a microplate
reader (Tecan Group, Ltd.). The experiment was repeated 5
times.
Cell migration and invasion
assays
Matrigel invasion assays were performed at 37°C for
16 h using 24-well Transwell inserts (Corning, Inc.) coated with 30
µg Matrigel (BD Biosciences). The cells (50,000) suspended in 200
µl serum-free DMEM were seeded into the upper chamber and 600 µl
NIH-3T3 conditioned medium (CM) was placed in the lower chamber.
Following incubation at 37°C for 24 h, the cells were fixed with 4%
paraformaldehyde (Sinopharm Chemical Reagent Co., Ltd.) at room
temperature for 10 min, and then stained with 0.5% crystal violet
(Beyotime Institute of Biotechnology) at room temperature for 10
min. The cells that had invaded through the membrane were counted
at ×200 magnification under a light microscope and normalized
relative to the 10,000 seeded cells. Transwell cell migration
assays were performed using the same method; however, the cells
were only incubated for 5 h and without Matrigel. CM from the
NIH-3T3 cell line (cat. no. CRL-1658; American Type Culture
Collection) was maintained in DMEM and cultured in a humidified
incubator at 37°C with 5% CO2, collected, according to
the following process, and used as a chemoattractant: NIH-3T3 cells
were grown to 50% confluency. The medium was then changed to fresh
serum-free medium and the cells were incubated for an additional 48
h. The conditioned media was drawn off the cells and centrifuged at
25°C for 5 min at 200 × g to remove debris, and stored at
−20°C.
Statistical analysis
Measurement data are expressed as mean ± standard
deviation, and enumeration data are shown as n (%). All experiments
were independently repeated three times. Associations between the
clinicopathological parameters and GPNMB expression level were
analyzed using a Fisher's exact test, while the association between
the expression level of ERK and the GPNMB high and low expression
level groups was analyzed using an unpaired Student's t-test, and
the expression level of GPNMB and ERK in the primary renal tumor
and matched BM was analyzed using a paired Student's t-test.
Survival curves were analyzed using the Kaplan-Meier method for
patients with GPNMB high or low expression levels and were
evaluated for statistical significance using the log-rank test.
Overall survival was analyzed in patients separated according to
number of BM, extent of BM and visceral metastasis. Univariate and
multivariate Cox regression analyses were performed to assess the
associations between clinical covariates and survival. P<0.05
was used to indicate a statistically significant difference. All
statistical analyses were performed using the SPSS software program
(v23.0; IBM Corp.).
Results
Clinicopathological features of RCC
with BM
The clinicopathological features of the 31 patients
with RCC and BM, enrolled into the present study are summarized in
Table I. RCC with BM was more
common in males (70.9%; n=22) compared with that in females, and
the male to female ratio of 2.44:1. The median age was 59 years
(range,38–75 years). Synchronous BM was found in 17 patients
(54.8%). A total of 16 patients (51.6%) had solitary BM. There were
13, 12 and 6 patients in the axial only BM, appendicular only BM,
and both axial and appendicular BM groups, respectively. Only 9
patients (29.1%) had visceral metastasis. The most common pathology
was clear cell carcinoma, which was found in 29 patients
(93.5%).
 | Table I.Clinicopathological features in the
patients with renal cell carcinoma and bone metastasis. |
Table I.
Clinicopathological features in the
patients with renal cell carcinoma and bone metastasis.
| Clinicopathological
feature | Number (%) |
|---|
| Sex |
|
|
Male | 22 (70.9) |
|
Female | 9 (29.1) |
| Time of bone
metastasis |
|
|
Synchronous | 17 (54.8) |
|
Metachronous | 14 (45.2) |
| Number of bone
metastasis |
|
|
Solitary | 16 (51.6) |
|
Multiple | 15 (48.4) |
| Extent of bone
metastasis |
|
| Axial
only | 13 (41.9) |
|
Appendicular
only | 12 (38.7) |
| Both
axial and appendicular | 6 (19.4) |
| Visceral
metastasis |
|
|
Yes | 9 (29.1) |
| No | 22 (70.9) |
| Pathology of
primary tumor |
|
| Clear
cell carcinoma | 29 (93.5) |
|
Non-clear cell carcinoma | 2 (6.5) |
| Fuhrman grade of
primary tumor |
|
| II | 12 (38.7) |
|
III | 16 (51.6) |
| IV | 3 (9.7) |
Expression analysis of GPNMB in
patients RCC with BM
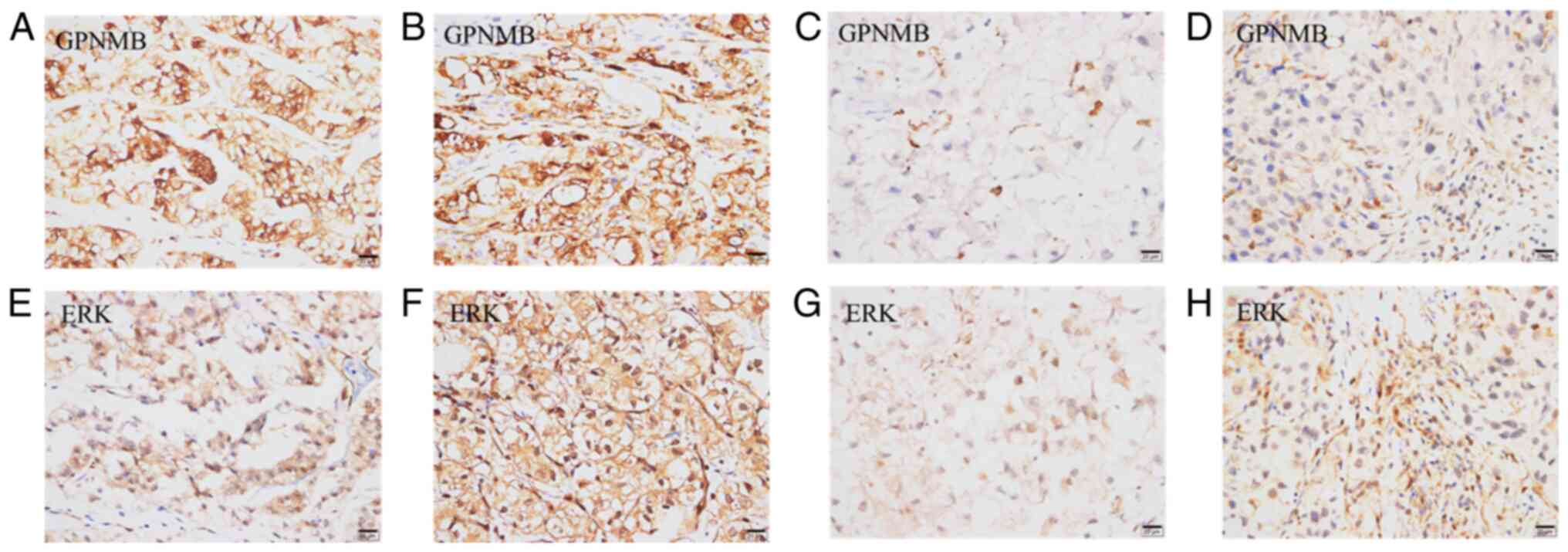
High GPNMB protein expression level in the primary
tumor was detected in 11 (35.5%) out of the 31 patients with RCC
and BM using immunohistochemistry, where it was found in the
membrane (Fig. 1A). The
association between GPNMB expression and the clinicopathological
characteristics was subsequently analyzed (Table II). High GPNMB expression level
was associated with the number (P=0.001) and the extent of BM
(P=0.001), Fuhrman grade (P=0.037), and ERK protein expression
level (P=0.003) in the primary tumor. GPNMB expression was not
associated with sex (P=0.606), time of BM (P=0.707) or visceral
metastasis (P=0.217). The protein expression level of GPNMB and ERK
was significantly increased in the matched BM compared with that in
the primary tumor (P=0.001 and P=0.016, respectively) (Table III; Fig. 1).
 | Table II.Association between GPNMB expression
level in the primary tumor and clinicopathological features. |
Table II.
Association between GPNMB expression
level in the primary tumor and clinicopathological features.
|
| GPNMB expression
level |
|
|---|
|
|
|
|
|---|
| Clinicopathological
feature | High | Low | P-value |
|---|
| Sex, n |
|
| 0.606 |
|
Male | 8 | 14 |
|
|
Female | 3 | 6 |
|
| Time of bone
metastasis, n |
|
| 0.707 |
|
Synchronous | 7 | 10 |
|
|
Metachronous | 4 | 10 |
|
| Number of bone
metastasis, n |
|
| 0.001 |
|
Solitary | 1 | 15 |
|
|
Multiple | 10 | 5 |
|
| Extent of bone
metastasis, n |
|
| 0.001 |
| Axial
only | 4 | 9 |
|
|
Appendicular only | 1 | 11 |
|
| Both
axial and appendicular | 6 | 0 |
|
| Visceral
metastasis, n |
|
| 0.217 |
|
Yes | 5 | 4 |
|
| No | 6 | 16 |
|
| Fuhrman grade of
primary tumor, n |
|
| 0.037 |
| II | 1 | 11 |
|
|
III | 8 | 8 |
|
| IV | 2 | 1 |
|
| ERK expression
levela | 7.25±2.55 | 2.47±0.64 | 0.003 |
 | Table III.Protein expression level of GPNMB and
ERK in the primary renal tumor and matched bone metastasis. |
Table III.
Protein expression level of GPNMB and
ERK in the primary renal tumor and matched bone metastasis.
| Gene name | Primary tumor | Bone
metastasis | P-value |
|---|
| GPNMB | 3.65±1.87 | 6.91±3.68 | 0.001 |
| ERK | 3.96±1.36 | 6.52±3.15 | 0.016 |
Association between GPNMB expression
level and prognosis in patients with RCC and BM
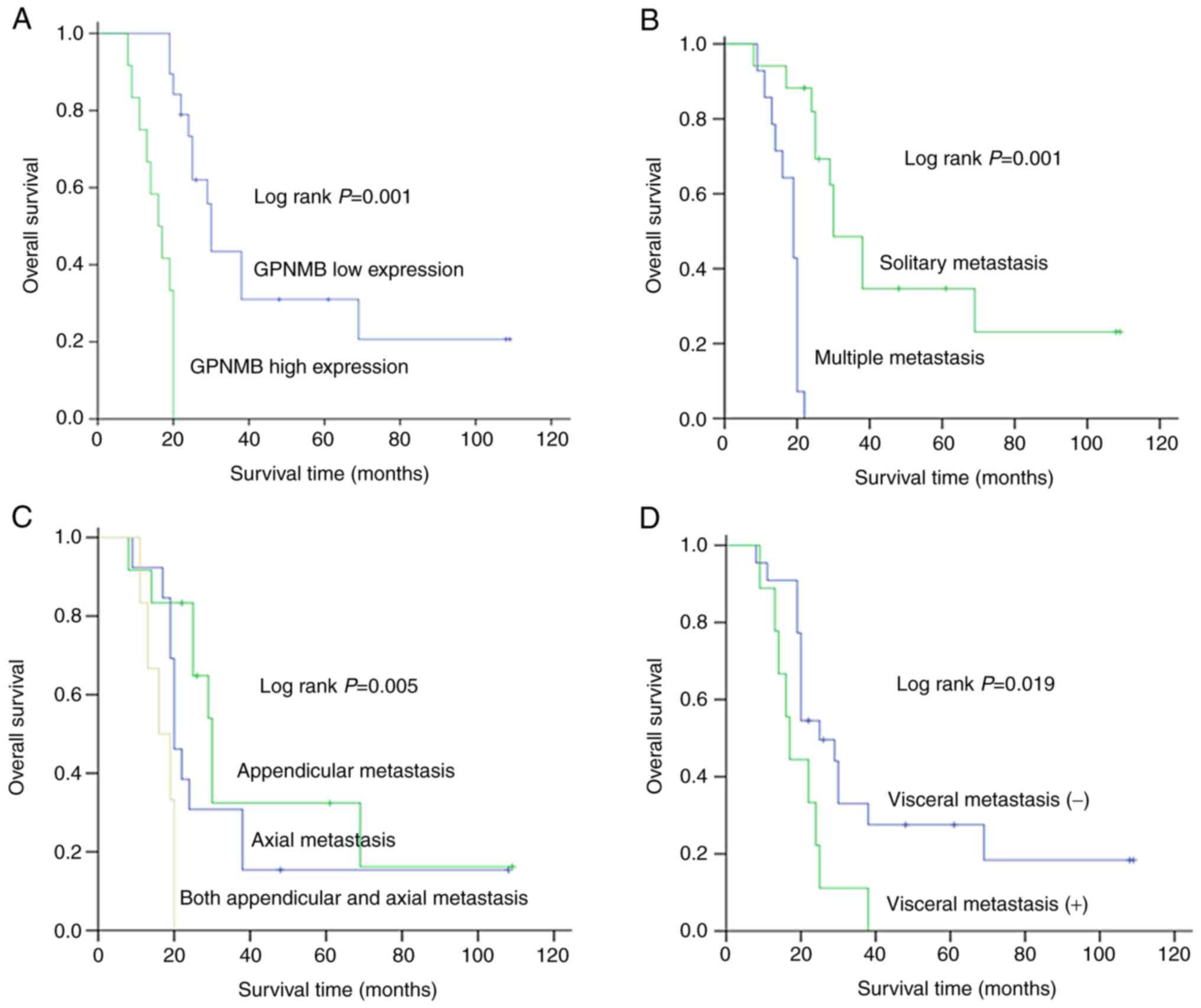
Kaplan-Meier analysis was used to investigate the
association between GPNMB protein expression level and patient
prognosis. High GPNMB protein expression level was significantly
associated with a poor prognosis (log-rank test, P=0.001; Fig. 2A). Overall survival time was also
analyzed using Kaplan-Meier method in patients separated by number
(Fig. 2B) and extent of BM
(Fig. 2C), and visceral metastasis
(Fig. 2D). Univariate and
multivariate analyses were performed using the Cox proportional
hazards model (Table IV) and the
results from the univariate analysis revealed that GPNMB protein
expression level, the number (P=0.001) and extent of BM (P=0.005),
and visceral metastasis (P=0.019) were significant indicators of
poor overall survival time. All the significant variables
identified from the univariate analysis were included in the
multivariant analysis Cox regression model to identify the
independent prognostic factors. The results showed that visceral
metastasis (P=0.029), number of BM (P=0.044) and GPNMB protein
expression level (P=0.039) were independent prognosis factors for
patients with RCC and BM (Table
IV).
 | Table IV.Univariate and multivariate analysis
of factors associated with survival time in 31 patients with renal
cell carcinoma and bone metastasis. |
Table IV.
Univariate and multivariate analysis
of factors associated with survival time in 31 patients with renal
cell carcinoma and bone metastasis.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Clinicopathological
feature | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Sex |
|
|
|
|
|
Male | 1.05
(0.43-2.52) | 0.919 |
|
|
|
Female |
|
|
|
|
| Time of bone
metastasis |
|
|
|
|
|
Synchronous | 1.21
(0.41-2.06) | 0.833 |
|
|
|
Metachronous |
|
|
|
|
| Number of bone
metastasis |
|
|
|
|
|
Solitary | 5.49
(2.14-12.76) | 0.001 | 1.30
(1.02-1.76) | 0.044 |
|
Multiple |
|
|
|
|
| Extent of bone
metastasis |
|
|
|
|
| Axial
only | 10.61
(6.21-18.56) | 0.005 | 1.02
(0.56-1.87) | 0.949 |
|
Appendicular only |
|
|
|
|
| Both
axial and appendicular |
|
|
|
|
| Visceral
metastasis |
|
|
|
|
|
Yes | 2.54
(1.09-5.89) | 0.019 | 3.65
(1.14-11.56) | 0.029 |
| No |
|
|
|
|
| Fuhrman grade of
primary tumor |
|
|
|
|
| II | 2.29
(1.18-4.43) | 0.014 | 1.01
(0.35-2.95) | 0.972 |
|
III |
|
|
|
|
| IV |
|
|
|
|
| GPNMB expression
level | 12.59
(3.52-45.13) | 0.001 | 5.68
(1.09-29.44) | 0.039 |
Effect of GPNMB downregulation on
proliferation in the RCC cells
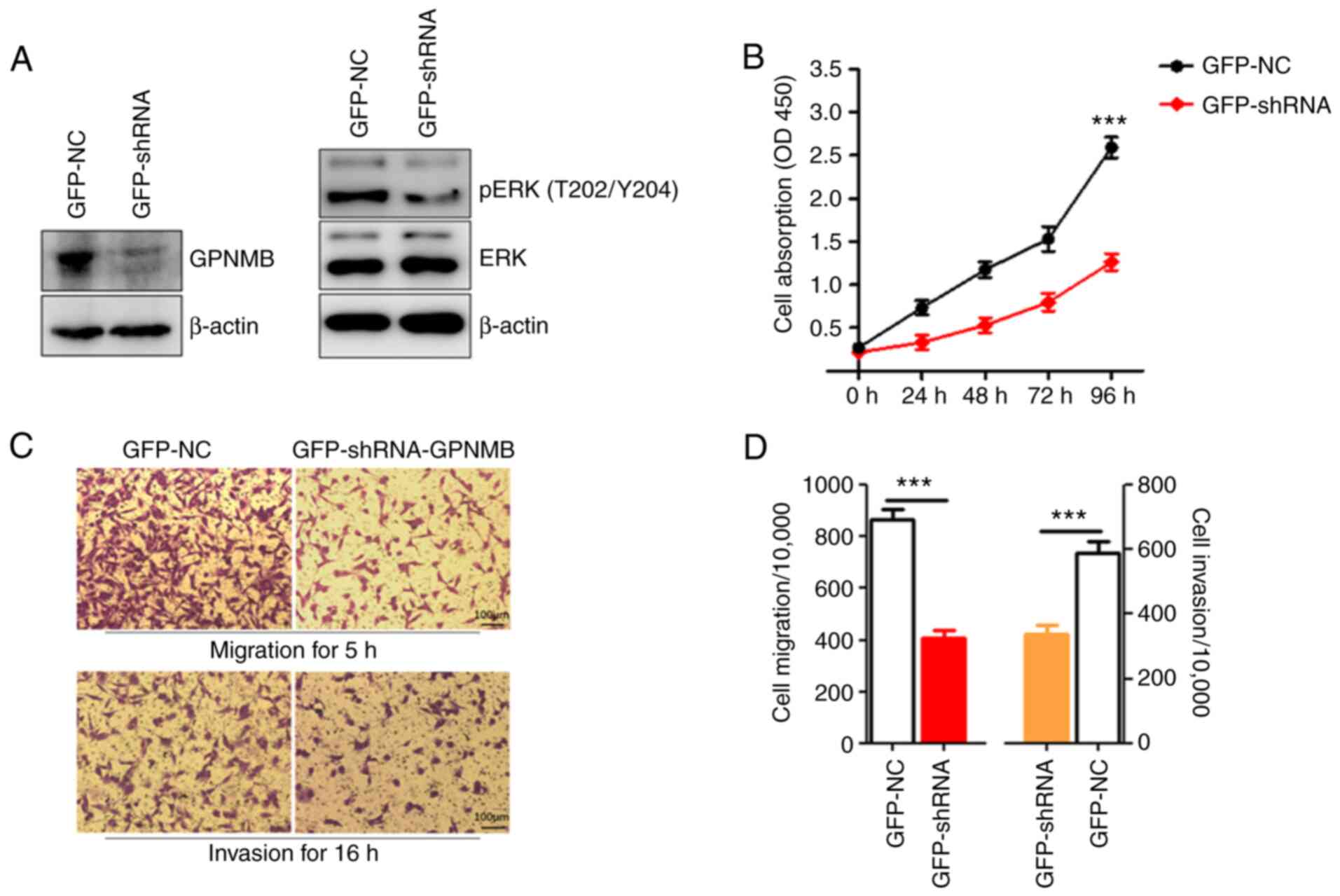
The effects of GPNMB silencing on RCC cell
proliferation were investigated using shRNA. Western blot analysis
showed that the protein expression level of GPNMB was suppressed
following transduction with shRNA (Fig. 3A). To investigate the possible
antiproliferative effects of GPNMB knockdown, a CCK-8 was performed
4 days following the transduction of shRNA. Cell proliferative
ability was significantly decreased in the GPNMB knockdown group
compared with that in the NC group (Fig. 3B).
Effect of GPNMB downregulation on cell
migration and invasion
To investigate the invasion and migration abilities
of the GPNMB knockdown RCC cells, Transwell and Matrigel assays
were performed (Fig. 3C). The
results from the Transwell assay revealed that, following
transduction with GPNMB shRNA, the migration ability of the ACHN
cell line was significantly inhibited (Fig. 3D) compared with that in the cells
transduced with siNC. In addition, the results from the Matrigel
assay revealed that the number of invasive cells in the
GFP-shRNA-GPNMB group was significantly lower compared with that in
the GFP-NC group (Fig. 3D).
Effect of GPNMB inhibition on the ERK
signaling pathway
ERK plays a critical role in tumor cell survival and
proliferation (23). Therefore, it
was investigated whether GPNMB could affect Ras signaling via the
ERK signaling pathway. The results indicated that the expression
levels of phosphorylated ERK were lower in the GPNMB
shRNA-transduced ACHN cells compared with those in the control
cells (Fig. 3A).
Discussion
In the present study, the clinicopathological
significance of GPNMB protein expression level in patients with RCC
and BM was investigated. The immunohistochemical analysis showed
that high protein GPNMB expression level was detected in 11 (35.5%)
out of 31 patients with RCC and BM. In addition, GPNMB protein
expression level was also associated with the number and the extent
of BM. Furthermore, compared with that in the primary renal tumor,
the protein expression level of GPNMB in the matched BM was
significantly increased. These findings are consistent with the
results from the study by Qin et al (24) and validate the hypothesis that bone
metastasized RCC, with a high expression level of GPNMB, could be
the result of a more invasive subclone derived from the primary
tumor. The results of the studies by Rose et al in 2007
(25) and 2010 (26) revealed that overexpression of GPNMB
significantly enhanced the formation of osteolytic bone metastases
from breast cancer; therefore, GPNMB has been identified as a bone
metastatic promotor and a new target for the treatment of breast
cancer. This finding demonstrated that high GPNMB expression level
was associated with enhanced bone metastatic capacities of cancer
cells.
GPNMB has emerged as an immunomodulator and an
important positive mediator of tumor progression and metastasis in
numerous types of solid cancer (13,27).
As shown in the Kaplan-Meier curves, patients with high GPNMB
expression levels had worse overall survival time compared with
that in patients with low GPNMB expression levels. In addition, it
was confirmed that the number and extent of BM, and visceral
metastasis was associated with the survival times of patients with
RCC and BM. Furthermore, multivariate Cox regression analysis
revealed that GPNMB expression, visceral metastasis and the number
of BM were independent prognostic factors for RCC survival. This is
consistent with a previous study, which demonstrated that high
GPNMB expression level was an independent prognostic factor and may
serve as a novel therapeutic target in breast cancer (28). Similarly, overexpression of GPNMB
was also detected in patients with small cell lung cancer and poor
prognosis (29). In addition,
Kaplan-Meier analysis demonstrated that upregulated GPNMB
expression was associated with an unfavorable prognosis for
patients with epithelial ovarian cancer (30). Based on these results, GPNMB
protein expression could be an unfavorable independent prognostic
biomarker for patients with RCC and BM.
The functional significance of GPNMB overexpression
in cancer requires further investigation. GPNMB may promote BM in
cancer cells via a variety of molecular mechanisms. The effect of
GPNMB inhibition on the ERK signaling pathway was analyzed in the
present study. The results indicated that the protein expression
levels of phosphorylated ERK were lower in the cells with GPNMB
knocked out compared with those in the control cells. Furthermore,
the invasive and migratory abilities of the GPNMB-silenced ACHN
cells were also significantly decreased. These findings
demonstrated that GPNMB may promote RCC cell growth and metastasis
via the ERK signaling pathway. However, other mechanisms may also
be involved in GPNMB-mediated metastasis (16,27,31,32).
GPNMB promoted the aggressive phenotypes of prostate cancer cell
lines by inducing MMP-2 and MMP-9, which may represent another
mechanism by which GPNMB promoted tumor metastasis to the bone
(20). In addition, overexpression
of GPNMB in breast epithelial cells induced epithelial-mesenchymal
transition and promoted tumor formation and invasion in mice
(33). GPNMB was also found to
contribute to the acquisition of stem cell-like properties in
dormant breast cancer cells, which could support tumor cell
survival, extravasation, and cause the process of metastasis more
efficient (34). Previously, GPNMB
was identified as a negative regulator of T cell activation. GPNMB
promoted the growth and metastasis of melanoma, and drove tumor
progression and metastasis by downregulating the activation of
melanoma-reactive T cells (18).
Furthermore, GPNMB expression may facilitate the systemic antitumor
responses and mediated effects on angiogenesis in breast cancer
cells (33). Therefore, the
biological roles of GPNMB on RCC progression and BM requires
further investigation.
GPNMB has become an attractive therapeutic target
due to its overexpression in a variety of cancers. Glembatumumab
vedotin, an antibody-drug conjugate, which targets GPNMB, is in
clinical trials as a single agent in multiple types of cancer, such
as advanced melanoma and breast cancer (18,35–37).
The results of several phase II clinical trials showed that
glembatumumab vedotin had modest inhibitory activity in patients
with advanced melanoma. The ORR was 11–33%, the median response
duration was ~6.0 months, the median progression-free survival
(PFS) time was 4.4 months and the median overall survival time was
9.0 months (38,39). The activity of glembatumumab
vedotin in patients with advanced breast cancer and high GPNMB
expression has also been investigated in several phase II studies.
Significantly higher ORR and longer PFS times were found in the
GPNMB overexpression group. The ORR to glembatumumab vedotin
therapy in patients with advanced breast cancer and high GPNMB
expression levels was 30–40%, while the ORR was only 9% in the
chemotherapy group (40). In a
phase II clinical trial where the patients with treatment-resistant
metastatic breast cancer received glembatumumab vedotin, the median
PFS time was 9.1 weeks for all patients and 18.0 weeks for patients
with GPNMB-positive tumors (41).
However, there has been no clinical trial to investigate the
effects of glembatumumab vedotin in patients with RCC and BM;
therefore, further investigation is required.
In conclusion, the results from the current study
suggested that upregulated GPNMB expression was associated with the
extent of BM and poor prognosis, indicating that GPNMB may serve as
a potential prognostic marker for patients with RCC and BM. GPNMB
downregulation suppressed the proliferation, migration and invasion
of the RCC cell line, which may be mediated via the ERK signaling
pathway. However, further studies are required to elucidate the
detailed molecular mechanism of its activity in tumor cell biology,
as well as its potential as a therapeutic target in patients with
RCC and BM.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Beijing Jishuitan
Hospital Nova Program (grant no. XKXX201616).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JPZ and LBM designed the study. ZHL and HDW
performed the experiments. JPZ, ZHL and GLH analyzed the data and
interpreted the results. HDW prepared the figures. JPZ drafted the
manuscript. ZHL and LBM aided in the revisions of the manuscript.
JPZ and LBM confirmed the authenticity of all the raw data. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Beijing Jishuitan Hospital (approval no. 201616).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
GPNMB
|
glycoprotein non-metastatic protein
B
|
|
RCC
|
renal cell carcinoma
|
|
BM
|
bone metastasis
|
|
CCK
|
Cell Counting Kit
|
|
ORR
|
objective response rate
|
|
PFS
|
progression-free survival
|
References
|
1
|
Capitanio U, Bensalah K, Bex A, Boorjian
SA, Bray F, Coleman J, Gore JL, Sun M, Wood C and Russo P:
Epidemiology of renal cell carcinoma. Eur Urol. 75:74–84. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Capitanio U and Montorsi F: Renal cancer.
Lancet. 387:894–906. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zheng T, Zhu C, Bassig BA, Liu S, Buka S,
Zhang X, Truong A, Oh J, Fulton J, Dai M, et al: The long-term
rapid increase in incidence of adenocarcinoma of the kidney in the
USA, especially among younger ages. Int J Epidemiol. 48:1886–1896.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Znaor A, Lortet-Tieulent J, Laversanne M,
Jemal A and Bray F: International variations and trends in renal
cell carcinoma incidence and mortality. Eur Urol. 67:519–530. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guida A, Escudier B and Albiges L:
Treating patients with renal cell carcinoma and bone metastases.
Expert Rev Anticancer Ther. 18:1135–1143. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Molina AMD: A multidisciplinary approach
for the management of earlier stage renal cell carcinoma. Urol
Oncol. 36:15–16. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ravaud A, Motzer RJ, Pandha HS, George DJ,
Pantuck AJ, Patel A, Chang YH, Escudier B, Donskov F, Magheli A, et
al: Adjuvant sunitinib in high-risk renal-cell carcinoma after
nephrectomy. N Engl J Med. 375:2246–2254. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bianchi M, Sun M, Jeldres C, Shariat SF,
Trinh QD, Briganti A, Tian Z, Schmitges J, Graefen M, Perrotte P,
et al: Distribution of metastatic sites in renal cell carcinoma: A
population-based analysis. Ann Oncoly. 23:973–980. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bosse D, Lin X, Simantov R, Lalani AA,
Derweesh I, Chang SL, Choueiri TK and McKay RR: Response of primary
renal cell carcinoma to systemic therapy. Eur Urol. 76:852–860.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Joshi SS, Handorf EA, Zibelman M, Plimack
ER, Uzzo RG, Kutikov A, Smaldone MC and Geynisman DM: Treatment
facility volume and survival in patients with metastatic renal cell
carcinoma: A registry-based analysis. Eur Urol. 74:387–393. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhuo H and Zhou L: Gpnmb/osteoactivin: An
indicator and therapeutic target in tumor and nontumorous lesions.
Pharmazie. 71:555–561. 2016.PubMed/NCBI
|
|
12
|
Rose AAN, Biondini M, Curiel R and Siegel
PM: Targeting GPNMB with glembatumumab vedotin: Current
developments and future opportunities for the treatment of cancer.
Pharmacol Ther. 179:127–141. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Truong DD, Kratz A, Park JG, Barrientos
ES, Saini H, Nguyen T, Pockaj B, Mouneimne G, LaBaer J and Nikkhah
M: A Human organotypic microfluidic tumor model permits
investigation of the interplay between patient-derived fibroblasts
and breast cancer cells. Cancer Res. 79:3139–3151. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Baba M, Furuya M, Motoshima T, Lang M,
Funasaki S, Ma W, Sun HW, Hasumi H, Huang Y, Kato I, et al: TFE3
Xp11.2 translocation renal cell carcinoma mouse model reveals novel
therapeutic targets and identifies GPNMB as a diagnostic marker for
human disease. Mol Cancer Res. 17:1613–1626. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sondag GR, Mbimba TS, Moussa FM, Novak K,
Yu B, Jaber FA, Abdelmagid SM, Geldenhuys WJ and Safadi FF:
Osteoactivin inhibition of osteoclastogenesis is mediated through
CD44-ERK signaling. Exp Mol Med. 48:e2572016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ono Y, Chiba S, Yano H, Nakayama N, Saio
M, Tsuruma K, Shimazawa M, Iwama T and Hara H: Glycoprotein
nonmetastatic melanoma protein B (GPNMB) promotes the progression
of brain glioblastoma via Na(+)/K(+)-ATPase. Biochem Biophys Res
Commun. 481:7–12. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tian F, Liu C, Wu Q, Qu K, Wang R, Wei J,
Meng F, Liu S and Chang H: Upregulation of glycoprotein
nonmetastatic B by colony-stimulating factor-1 and epithelial cell
adhesion molecule in hepatocellular carcinoma cells. Oncol Res.
20:341–350. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tomihari M, Chung JS, Akiyoshi H, Cruz PD
Jr and Ariizumi K: DC-HIL/glycoprotein Nmb promotes growth of
melanoma in mice by inhibiting the activation of tumor-reactive T
cells. Cancer Res. 70:5778–5787. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jin R, Jin YY, Tang YL, Yang HJ, Zhou XQ
and Lei Z: GPNMB silencing suppresses the proliferation and
metastasis of osteosarcoma cells by blocking the PI3K/Akt/mTOR
signaling pathway. Oncol Rep. 39:3034–3040. 2018.PubMed/NCBI
|
|
20
|
Fiorentini C, Bodei S, Bedussi F, Fragni
M, Bonini SA, Simeone C, Zani D, Berruti A, Missale C, Memo M, et
al: GPNMB/OA protein increases the invasiveness of human metastatic
prostate cancer cell lines DU145 and PC3 through MMP-2 and MMP-9
activity. Exp Cell Res. 323:100–111. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nannuru KC, Futakuchi M, Varney ML,
Vincent TM, Marcusson EG and Singh RK: Matrix metalloproteinase
(MMP)-13 regulates mammary tumor-induced osteolysis by activating
MMP9 and transforming growth factor-β Signaling at the tumor-bone
interface. Cancer Res. 70:3494–3504. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jianpo Z, Ning L, Hai W, Haidong W and
Libo M: Expression of GPNMB, ERK, MMP3 and MMP9 in primary lesion
and bone metastasis of renal cell carcinoma and their correlation.
Cancer Res Prev Treat. 47:367–371. 2020.
|
|
23
|
Mayo JC, Hevia D, Quiros-Gonzalez I,
Rodriguez-Garcia A, Gonzalez-Menendez P, Cepas V, Gonzalez-Pola I
and Sainz RM: IGFBP3 and MAPK/ERK signaling mediates
melatonin-induced antitumor activity in prostate cancer. J Pineal
Res. Oct 13–2016.(Epub ahead of print). doi: 10.1111/jpi.12373.
|
|
24
|
Qin C, Liu Z, Yuan Y, Zhang X, Li H, Zhang
C, Xu T and Wang X: Glycoprotein non-metastatic melanoma protein B
as a predictive prognostic factor in clear-cell renal cell
carcinoma following radical nephrectomy. Mol Med Rep. 9:851–886.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rose AA, Pepin F, Russo C, Abou Khalil JE,
Hallett M and Siegel PM: Osteoactivin promotes breast cancer
metastasis to bone. Mol Cancer Res. 5:1001–1114. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rose AA and Siegel PM: Emerging
therapeutic targets in breast cancer bone metastasis. Future Oncol.
6:55–74. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ramani V, Teshima T, Tamura K, Chung JS,
Kobayashi M, Cruz PD Jr and Ariizumi K: Melanoma-derived soluble
DC-HIL/GPNMB promotes metastasis by excluding T-Lymphocytes from
the pre-metastatic niches. J Nvest Dermatol. 138:2443–2451. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rose AA, Grosset AA, Dong Z, Russo C,
Macdonald PA, Bertos NR, St-Pierre Y, Simantov R, Hallett M, Park
M, et al: Glycoprotein nonmetastatic B is an independent prognostic
indicator of recurrence and a novel therapeutic target in breast
cancer. Clin Cancer Res. 16:2147–2156. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li YN, Zhang L, Li XL, Cui DJ, Zheng HD,
Yang SY and Yang WL: Glycoprotein nonmetastatic B as a prognostic
indicator in small cell lung cancer. APMIS. 122:140–146. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ma RQ, Tang ZJ, Ye X, Cheng HY, Sun KK,
Chang XH and Cui H: Overexpression of GPNMB predicts an unfavorable
outcome of epithelial ovarian cancer. Arch Gynecol Obstet.
297:1235–1244. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Arosarena OA, Barr EW, Thorpe R, Yankey H,
Tarr JT and Safadi FF: Osteoactivin regulates head and neck
squamous cell carcinoma invasion by modulating matrix
metalloproteases. J Cell Physiol. 233:409–421. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bhattacharyya S, Feferman L, Sharma G and
Tobacman JK: Increased GPNMB, phospho-ERK1/2, and MMP-9 in cystic
fibrosis in association with reduced arylsulfatase B. Mol Genet
Metab. 124:168–175. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Okita Y, Kimura M, Xie R, Chen C, Shen LT,
Kojima Y, Suzuki H, Muratani M, Saitoh M, Semba K, et al: The
transcription factor MAFK induces EMT and malignant progression of
triple-negative breast cancer cells through its target GPNMB. Sci
Signal. 10:eaak93972017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen C, Okita Y, Watanabe Y, Abe F, Fikry
MA, Ichikawa Y, Suzuki H, Shibuya A and Kato M: Glycoprotein nmb is
exposed on the surface of dormant breast cancer cells and induces
stem cell-like properties. Cancer Res. 78:6424–6435. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kopp LM, Malempati S, Krailo M, Gao Y,
Buxton A, Weigel BJ, Hawthorne T, Crowley E, Moscow JA, Reid JM, et
al: Phase II trial of the glycoprotein non-metastatic B-targeted
antibody-drug conjugate, glembatumumab vedotin (CDX-011), in
recurrent osteosarcoma AOST1521: A report from the Children's
Oncology Group. Eur J Cancer. 121:177–183. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tray N, Adams S and Esteva FJ:
Antibody-drug conjugates in triple negative breast cancer. Future
Oncol. 14:2651–2661. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ott PA, Hamid O, Pavlick AC, Kluger H, Kim
KB, Boasberg PD, Simantov R, Crowley E, Green JA, Hawthorne T, et
al: Phase I/II study of the antibody-drug conjugate glembatumumab
vedotin in patients with advanced melanoma. J Clin Oncol.
32:3659–3666. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ott PA, Pavlick AC, Johnson DB, Hart LL,
Infante JR, Luke JJ, Lutzky J, Rothschild NE, Spitler LE, Cowey CL,
et al: A phase 2 study of glembatumumab vedotin, an antibody-drug
conjugate targeting glycoprotein NMB, in patients with advanced
melanoma. Cancer. 125:1113–1123. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rose AA, Annis MG, Frederick DT, Biondini
M, Dong Z, Kwong L, Chin L, Keler T, Hawthorne T, Watson IR, et al:
MAPK pathway inhibitors sensitize BRAF-mutant melanoma to an
antibody-drug conjugate targeting GPNMB. Clin Cancer Res.
22:6088–6098. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yardley DA, Weaver R, Melisko ME, Saleh
MN, Arena FP, Forero A, Cigler T, Stopeck A, Citrin D, Oliff I, et
al: EMERGE: A randomized phase II study of the antibody-drug
conjugate glembatumumab vedotin in advanced glycoprotein
NMB-expressing breast cancer. J Clin Oncol. 33:1609–1619. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bendell J, Saleh M, Rose AA, Siegel PM,
Hart L, Sirpal S, Jones S, Green J, Crowley E, Simantov R, et al:
Phase I/II study of the antibody-drug conjugate glembatumumab
vedotin in patients with locally advanced or metastatic breast
cancer. J Clin Oncol. 32:3619–3625. 2014. View Article : Google Scholar : PubMed/NCBI
|