Introduction
Endosialin/CD248/tumor endothelial marker 1 (TEM1)
is classified as a C-type lectin-like transmembrane receptor, found
on the plasma membrane of activated mesenchymal cells, which binds
to fibronectin (FN) (1,2). Endosialin expression is essentially
limited to embryonal development and is expressed at very low
levels in normal adult tissues; however, it is upregulated in
pathological states, including tumor progression and metastasis.
Endosialin does not function as an active metastasis promoting
signaling factor in tumor cells to gain invasive behavior, but
rather as a facilitator, tethering, tumor cells to the matrix and
actively mediating their transmigration through the vascular
basement membrane and underlying endothelial monolayer (3). In sarcomas, endosialin expression is
found in malignant tumor cells, perivascular and stromal cells, and
is speculated to be involved in tumor angiogenesis (4). Another study reported that
endosialin-expressing stem-like cells of osteosarcoma (OS) possess
self-renewing and invasive properties, and are highly drug
resistant. Although endosialin is highly expressed in stem-like
cells of OSs (5), its role has not
been fully uncovered.
MORAb-004/ontuxizumab, a humanized monoclonal
antibody, targets the type C lectin domain of endosialin (4). In preclinical studies, binding of
ontuxizumab to two paired cell lines (Ewing's and synovial sarcoma
cell lines) was confirmed via semi-quantitative immunofluorescence
(6). In addition,
immunofluorescent staining of ontuxizumab-treated human pericytes
exhibited cellular internalization of the antibody, with a
corresponding reduction of surface endosialin. MORAb-004 treated
tumors display overall shortened and distorted blood vessels. The
CD248 levels on cell surfaces of neovasculature pericytes are
significantly reduced due to its internalization. This reduction of
CD248 is also accompanied by reduced α-SMA expression,
depolarization of pericytes and endothelium, and ultimately
dysfunctional microvessels. These findings suggest that MORAb-004
reduces CD248 on pericytes, impairs tumor microvasculature
maturation and ultimately suppresses tumor development (7). The phase II, randomized controlled
trial of MORAb-004/ontuxizumab was performed to evaluate the
safety, as well as the efficacy of ontuxizumab with the combination
of gemcitabine and docetaxel (G/D) in metastatic soft tissue
sarcoma (8,9). This study bases on the hypothesis
that blocking endosialin-mediated tumor angiogenesis with
ontuxizumab can enhance the efficacy of G/D in sarcomas. However,
ontuxizumab with G/D exhibited no enhanced activity compared with
chemotherapy alone in soft tissue sarcomas, whereas the safety of
the combination was consistent with G/D alone. Preclinical
experiments in wild-type and endosialin-deficient mice indicate
that stromal endosialin does not affect primary tumor growth but
strongly promotes metastasis (3).
The present study aimed to determine whether
endosialin expression is associated with tumor progression and
metastasis in OS, and whether endosialin has the potential to be a
novel therapeutic target via MORAb-004/ontuxizumab.
Materials and methods
Human OS specimens
A total of 18 clinical specimens were collected from
patients with OS via biopsy at the Department of Orthopedic
Surgery, Nara Medical University (Kashihara, Japan) prior to any
treatments, including surgery, chemotherapy and radiotherapy,
between December 1991 and July 2017. A total of 18 patients with
osteosarcoma were included in the present study, including 12 men
and six women. The median age of all patients was 23 years (age
range, 8–69 years). Only specimens biopsied for the initial
diagnosis were selected, those at the time of recurrence and during
or after treatments were excluded. Specimens were stored at 4°C
until subsequent experiments. The present study was approved by the
Nara Medical University Certified Review Board (approval no. 2833)
and written informed consent was provided by all patients or family
members prior to the study start. Informed consent was provided in
the form of opt-out on the website (https://naraseikei.com/patients). Patient
characteristics are presented in Table
I.
 | Table I.Baseline characteristics of patients
with osteosarcoma. |
Table I.
Baseline characteristics of patients
with osteosarcoma.
|
|
|
|
|
|
|
| Treatment |
|---|
|
|
|
|
|
|
|
|
|
|---|
| Case | Age, years | Sex | Histological
diagnosis | Site | Metastasis | Metastatic site | Chemotherapy | Surgery | Radiotherapy |
|---|
| 1 | 8 | M | Conventional
osteosarcoma | Lt humerus | - |
| + | + | - |
| 2 | 35 | M | Telangiectatic
osteosarcoma | sacrum | - |
| + | - | + |
| 3 | 16 | F | Malignant fibrous
histiocytoma-like osteosarcoma | Rt fibula | - |
| + | + | - |
| 4 | 14 | F | Conventional
osteosarcoma; osteoblastic type | Rt femur | - |
| + | + | - |
| 5 | 8 | M | Telangiectatic
osteosarcoma | Rt femur | - |
| + | + | - |
| 6 | 14 | M | Conventional
osteosarcoma | Lt femur | - |
| + | + | - |
| 7 | 17 | M | Conventional
osteosarcoma | Rt femur | - |
| + | + | - |
| 8 | 69 | F | Conventional
osteosarcoma; fibroblastic type | Lt femur | - |
| - | + | - |
| 9 | 11 | M | Conventional
osteosarcoma | Lt tibia | - |
| + | + | - |
| 10 | 13 | M | Conventional
osteosarcoma; fibroblastic type | Lt middle
finger | - |
| + | + | - |
| 11 | 15 | F | Giant cell tumor
like osteosarcoma | Lt femur | + | Lung | + | + | - |
| 12 | 17 | M | Conventional
osteosarcoma | Rt humerus | + | Lung | + | + | - |
| 13 | 17 | F | Conventional
osteosarcoma | Lt femur | + | Lung | + | + | - |
| 14 | 16 | M | Conventional
osteosarcoma | Rt tibia | + | Lung | + | + | - |
| 15 | 9 | M | Conventional
osteosarcoma | Rt humerus | + | Lung | + | - | - |
| 16 | 13 | M | Conventional
osteosarcoma; osteoblastic type | Lt tibia | + | Lung | + | + | - |
| 17 | 69 | M | Conventional
osteosarcoma | Lt iliac | + | Lung | - | + | + |
| 18 | 44 | F | Conventional
osteosarcoma; osteoblastic type | Lt tibia | + | Lung, | - | + | + |
|
|
|
|
|
|
| Rt femur |
|
|
|
Immunohistochemistry (IHC)
Specimens were formalin-fixed and paraffin-embedded
immediately after the biopsy. Consecutive 4 µm sections were cut
from each block, and underwent immunohistochemical staining
analysis for CD248. IHC analysis was performed via the
immunoperoxidase technique, as previously described (10). Briefly, following antigen retrieval
with autoclave treatment in citrate buffer for 15 min at 121°C,
specimens were incubated with 3%
H2O2-methanol for 10 min and subsequently
blocked with PBS containing 2% skimmed milk for 30 min at room
temperature. Following incubation with primary antibody against
CD248 (2 µg/ml; 1:100 dilution; cat. no. HPA051856; Cosmo Bio Co.,
Ltd.) at room temperature for 2 h, specimens were briefly washed
with PBS and incubated with secondary antibody conjugated with
peroxidase (1:100 dilution; cat. no. K4061; Envision™+ Dual Link
System-HRP; Dako; Agilent Technologies, Inc.) at room temperature
for 1 h. The specimens were re-washed with PBS, stained with DAB
(Dako; Agilent Technologies, Inc.) for 5 min at room temperature
and subsequently washed lightly in distilled water and running
water. The slides were counterstained with Meyer-hematoxylin for 1
min at room temperature, washed in phosphate buffer for 5 min,
running water for 10 min, dehydrated through graded alcohols,
cleared in xylene and cover slipped.
A total of two experienced pathologists evaluated
the IHC staining to avoid bias. To assess CD248 expression in IHC
staining, staining intensity and staining area (%) were measured at
two hot spots in each specimen using an optical microscope (BX50;
×200 magnification; Olympus Corporation). CD248 staining intensity
was scored as follows: 0, negative; 1+, weak; 2+, moderate and 3+,
strong. The staining intensity of endothelium was defined as 2+ on
the basis that CD248 is a tumor endothelium marker, which is
expressed in vascular endothelial cells of malignant tumors
(11). The staining area (%) at
each staining intensity was measured using ImageJ version 1.52
software (12). The CD248 positive
expression score (IHC score) was calculated as the sum of staining
intensity and staining area (%), which ranged from 0–300. The mean
IHC score in each specimen was used for statistical analysis. CD248
IHC score was determined via receiver operating characteristic
(ROC) curve analysis to determine the cut-off value, and Fisher's
exact test was used to assess the association between CD248
expression and metastasis. An IHC score of CD248 ≥20.5 was
considered positive, while an IHC score <20.5 was negative,
using the cut-off value.
Cell culture and reagents
The OS cell lines, SaOS2, U2OS, HOS and MG63, were
purchased from the American Type Culture Collection and maintained
in (DMEM; Sigma-Aldrich; Merck KGaA) supplemented with 10% fetal
bovine serum (FBS; Sigma-Aldrich; Merck KGaA) and 50 U/ml
penicillin/streptomycin (Nacali Tesque Inc.) at 37°C with 5%
CO2.
FN was purchased from FUJIFILM Wako Pure Chemical
Corporation, while MORAb-004/ontuxizumab was purchased from Eizai
Inc. (https://us.eisai.com).
Cell viability assay
Cells (SaOS2, U2OS and MG63) were seeded into
12-well pates at a density of 10,000 cells/well. The effects of
MORAb-004 (0, 5, 10 and 20 µmol/l) and FN (0 and 10 µg/ml) on cell
proliferation were assessed via the MTS assay after 48 h, as
previously described (13). The
MTS assay was (Promega Corporation) performed according to the
manufacturer's instructions. Briefly, MTS solution was added to
each well for 2 h at 37°C with 5% CO2, and the
absorbance was measured at a wavelength of 490 nm, using a
microplate reader (Multiskan FC; Thermo Fisher Scientific
Inc.).
Apoptosis analysis
Apoptosis was assessed in SaOS and U2OS cells via
(Sigma-Aldrich; Merck KGaA) at room temperature for 10 min, with
MORAb-004 (0 or 20 µmol/l) and FN (0 or 10 µg/ml) for 48 h.
Apoptotic cells were observed under a fluorescence microscope (×200
magnification; Keyence Corporation).
Reverse transcription-quantitative
(RT-q)PCR
RT-qPCR analysis was performed to assess the effect
of MORAb-004 with FN on the expression levels of stem cell markers:
Octamer-binding transcription factor-3 (OCT3), Kruppel-like factor
4 (KLF4), nucleostemin (NS) and clusters of differentiation 44
(CD44), and differentiation markers: Alkaline phosphatase (ALP),
Collagen 1α (COL1α) and runt-related transcription factor 2
(RUNX2), in SaOS2 and U2OS cells. A total of 0.5 µg total RNA was
extracted from ontuxizumab (0 or 20 µmol/l) with FN (10
µg/ml)-treated and untreated (as a control) cells using
TRIzol® RNA Isolation Reagents (Thermo Fisher
Scientific, Inc.) and the RNeasy kit (Qiagen GmbH), followed by
cDNA synthesis. In addition, the expression of endosialin/human
TEM1 in OS cell lines was assessed via RT-qPCR analysis. A total of
0.5 µg of total RNA was extracted from ontuxizumab (20
µmol/l)-treated cells as aforementioned. The primer sequences used
for qPCR were synthesized by Sigma-Aldrich; Merck KGaA and are
listed in Table II. The following
thermocycling conditions were used: 95°C for 5 min; 40 cycles at
95°C for 30 sec, 63.9-71°C for 30 sec and 72°C for 30 sec. PCR
products were electrophoresed using a 2% agarose gel and stained
with ethidium bromide for 20 min at room temperature with shading.
β-actin mRNA was also amplified for use as an internal control. For
the quantification of PCR products, the band quantification was
performed using ImageJ version 1.52 software (National Institutes
of Health). The experiments were performed in triplicate.
 | Table II.Primer sequences used for
quantitative PCR. |
Table II.
Primer sequences used for
quantitative PCR.
| Gene | Primer sequence
(5′-3′) |
|---|
| OCT3 | F
GAAGGATGTGGTCCGAGTGT |
|
| R:
GTGAAGTGAGGGCTCCCATA |
| KLF4 | F:
ATCTTTCTCCACGTTCGCGTCTG |
|
| R:
AAGCACTGGGGGAAGTCGCTTC |
| NS | F:
ATTGCCAACAGTGGTGTTCA |
|
| R:
AATGGCTTTGCTGCAAGTTT |
| CD44 | F:
AAGGTGGAGCAAACACAACC |
|
| R:
AGCTTTTTCTTCTGCCCACA |
| ALP | F:
CGCCTACCAGCTCATGCATA |
|
| R:
GCTCTTCCAGGTGTCAACGA |
| COL1α | F:
CAGGCTGGTGTGATGGGATT |
|
| R:
GGGCCTTGTTCACCTCTCTC |
| RUNX2 | F:
GCGCATTCCTCATCCCAGTA |
|
| R:
GGCTCAGGTAGGAGGGGTAA |
| hTEM1 | F:
GGACACAGATGAGTGCCAGA |
|
| R:
CAGGCCTCGTCTTCATCTTC |
Western blotting
Western blot analysis was performed on the stem cell
markers, CD44V9 and NS, in SaOS2 and U2OS cells, which were treated
with ontuxizumab (20 µmol/l) and FN (10 µg/ml) or untreated (as a
control). Whole cell lysates were prepared using M-PER™ Mammalian
Protein Extraction Reagent (Thermo Fisher Scientific Inc.). The
protein concentrations of cell lysates were evaluated via the
Bradford method, using bovine serum albumin as a standard (14). Proteins (20 µg) were separated from
the cell lysates via SDS-PAGE on 12.5% gels and electrotransferred
onto nitrocellu¬lose membranes. The membranes were blocked with a
solution of 5% skimmed milk at room temperature overnight, and then
incubated with primary antibodies against CD44V9 (1:5,000 dilution;
cat. no. 1370435; Seikagaku Corporation) and NS (1:5,000 dilution;
cat. no. ab70346; Abcam) for 12 h at 4°C (15). Subsequently, the membranes were
washed with PBS and incubated with the secondary antibody,
anti-rabbit imunoglobulins/HRP (1:1,000 dilution; cat. no. P0217;
Dako; Agilent Technologies Inc.) for 1 h at room temperature. A
tubulin antibody (1:5,000 dilution; cat. no. ab4047; Abcam) was
used to measure the amount of protein loaded in each lane. Immune
complexes were visualized using the CSA system (Dako; Agilent
Technologies, Inc.). The intensities of the band were using ImageJ
version 1.52 software.
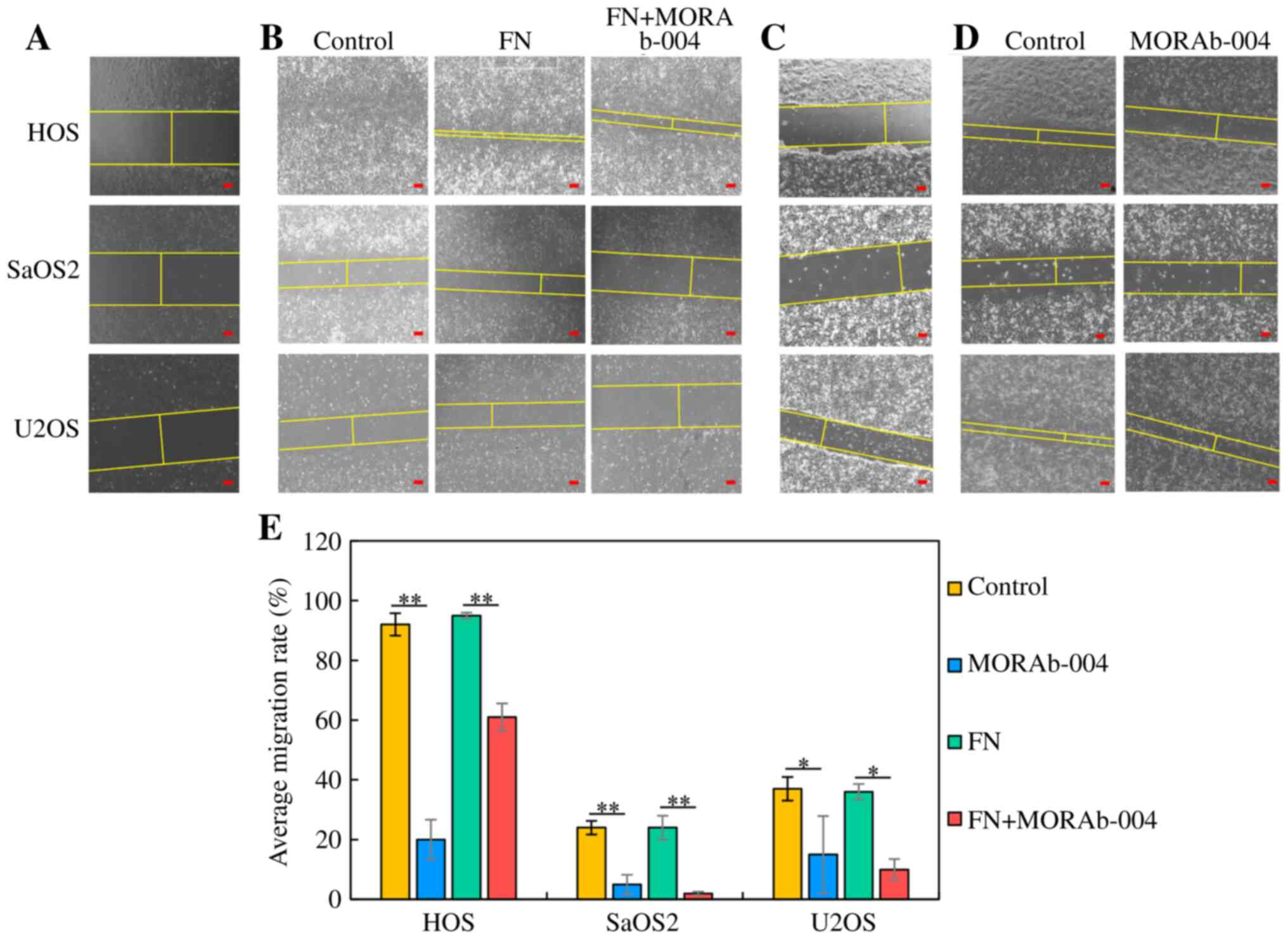
Wound healing assay
The wound healing assay was performed to assess the
effect of MORAb-004 with FN on the migratory ability of OS cell
lines (HOS, SaOS2 and U2OS). Cells were seeded into 3.5 cm culture
dishes and treated with MORAb-004 (0 or 20 µmol/l) and FN (10
µg/ml). Following incubation with 10% FBS at 37°C for 24 h, cells
grown to sub-confluence were scraped to make a cell-free area with
a sharp edge (16–18). Cells migrating into the scraped
area was captured at 0 and 24 h after scraping at ×100
magnification under an optical microscope (ECLIPSE Ti-S100; Nikon
Corporation, Minato Ward) (16).
The width between the edges of each scraped area was measured at 0
and 24 h and the migration rate (MR) was calculated using the
following formula: MR (%) = (Wt=0 - Wt=24) × 100 / Wt=0, Wt=0: the
width at 0 h, Wt=24: The width at 24 h after. The width was showed
by yellow straight line in each figure. The experiments were
performed in triplicate.
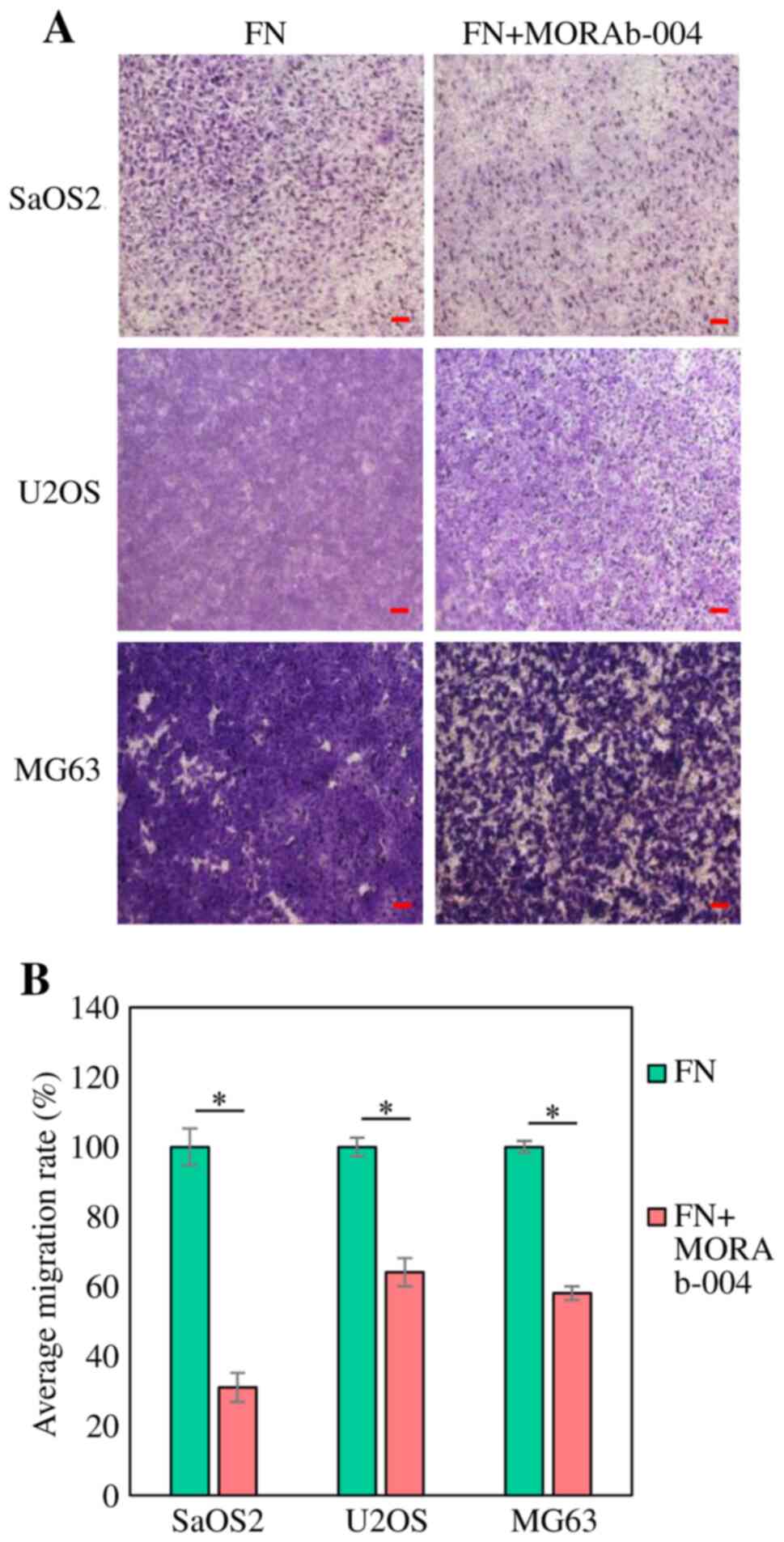
Transwell migration assay
The modified Boyden chamber assay was performed to
assess in vitro migration of the OS cell lines, SaOS2, U2OS
and MG63 treated with MORAb-004. The CytoSelect™ 24-well Cell
Haptotaxis Assay (8 µm) kit (FN-coated, Fluorometric; Cell Biolabs
Inc.) was used, according to the manufacturer's instructions.
0.75×106 cells/ml were suspended in 500 µl of regular
medium (DMEM supplemented with 10% FBS; Sigma-Aldrich; Merck KGaA)
and 2.3×105 cells/ml were placed in the upper part of
the chamber, while the lower part of the chamber was filled with
regular medium. Following incubation for 24 h at 37°C, the filters
were carefully removed from the inserts, stained with hematoxylin
for 10 min at room temperature and mounted on microscopic slides.
Stained cells were counted in whole inserts at ×100 magnification
under an optical microscope (BX50; Olympus Corporation) (16). The area ratio occupied by cells per
field of view in the photomicrograph after 24 h was quantified in
the MORAb-004 treated and the untreated groups using ImageJ version
1.52 software (National Institutes of Health), and the untreated
group was used as the control. The area ratio of the MORAb-004
treated group based on the control was calculated as the migration
rate (%). The experiments were performed in triplicate.
Statistical analysis
Statistical analysis was performed using SPSS
version 26 software (IBM Corp.). Data are presented as the mean ±
SD. IHC analysis was performed once. RT-qPCR, the apoptosis
analysis, western blotting, the wound healing assay, the Transwell
migration assay were performed in triplicate. Fisher's exact and
χ2 tests (two-tailed) were used for IHC analysis. To
assess cell viability, RT-qPCR, wound healing assay and apoptosis
one-way ANOVA followed by Tukey's post hoc test was used. Unpaired
Student's t-test was used to assess Transwell migration assay.
P<0.05 (two-sided) was considered to indicate a statistically
significant difference.
Results
Association between endosialin/CD248
expression and metastasis in human OS
A total of 18 human clinical specimens of OS were
assessed via IHC analysis to determine the association between
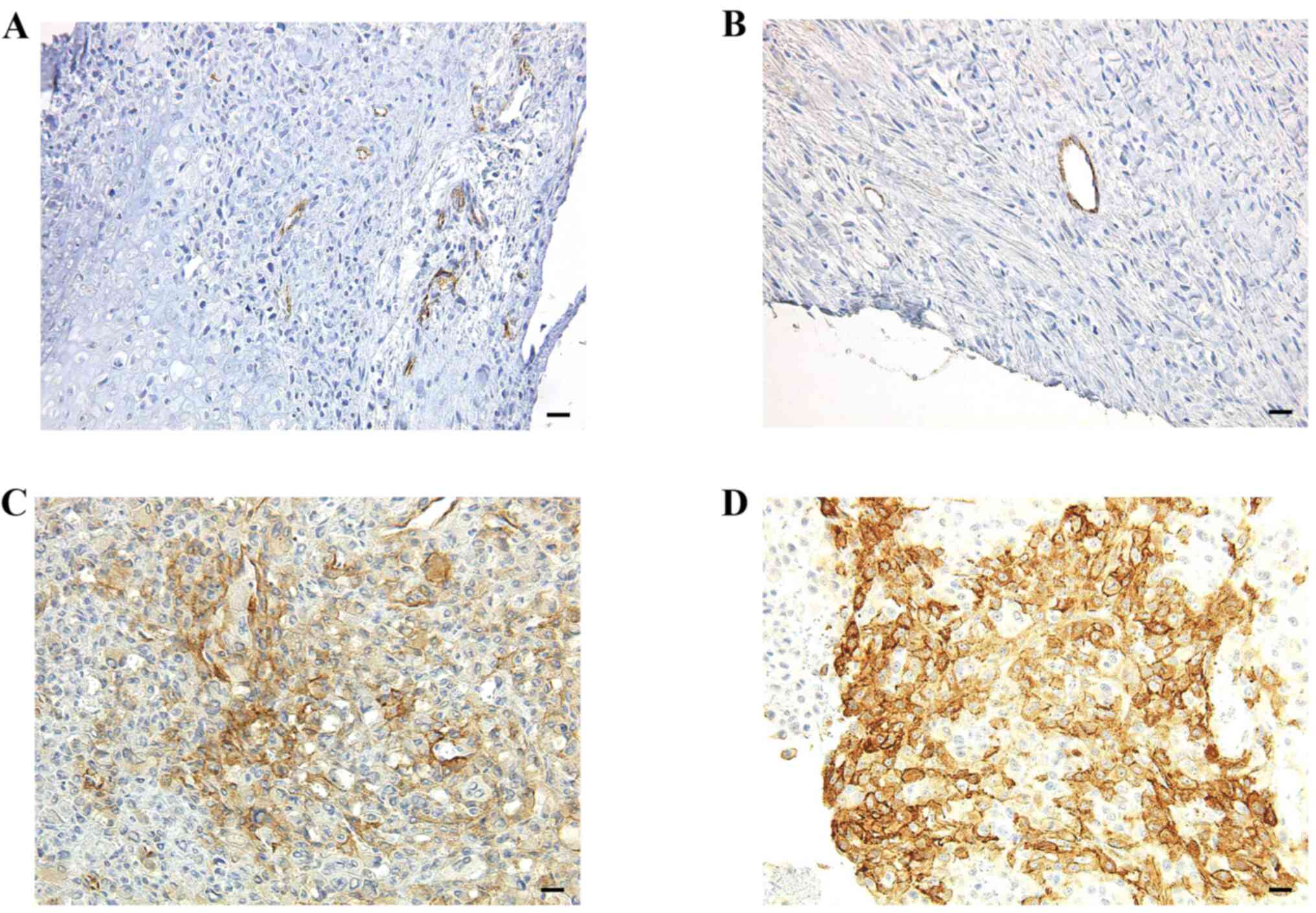
endosialin/CD248 expression and metastasis. Fig. 1 depicts endosialin/CD248 expression
in human OS tissues. The IHC scores were 5, 7, 55 and 82 in
Fig. 1A-D, respectively.
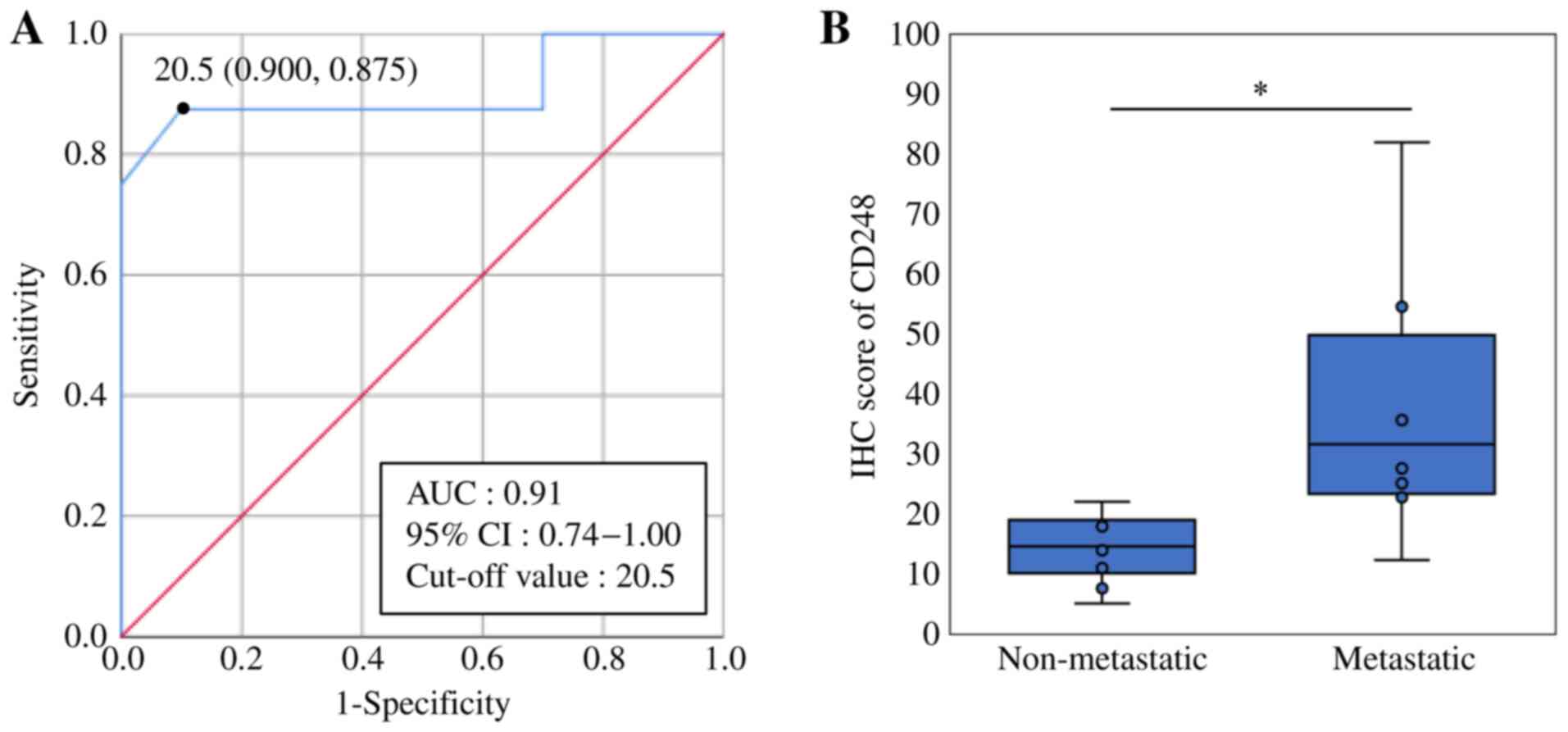
The cut-off value of CD248 was 20.5, with a
sensitivity of 0.875 and a specificity of 0.900 (Fig. 2A). The results demonstrated that
1/10 non-metastatic OSs was positive for CD248 expression, while
7/8 metastatic OSs were positive. The mean IHC score was 14.3 in
the non-metastatic group (range, 5–22) and 36.9 in the metastatic
group (range, 12–82). Taken together, these results suggest that
CD248 is expressed at significantly high levels in OSs with
metastatic disease (Fisher's exact test; P=0.003; Fig. 2B).
Anti-endosialin antibody MORAb-004 has
no cytostatic effect on OS cells
RT-qPCR analysis was performed to detect endosialin
expression in OS cell lines. The expression was confirmed in all
cell lines (Fig. S1). Notably,
endosialin expression was observed in all cell lines used. The
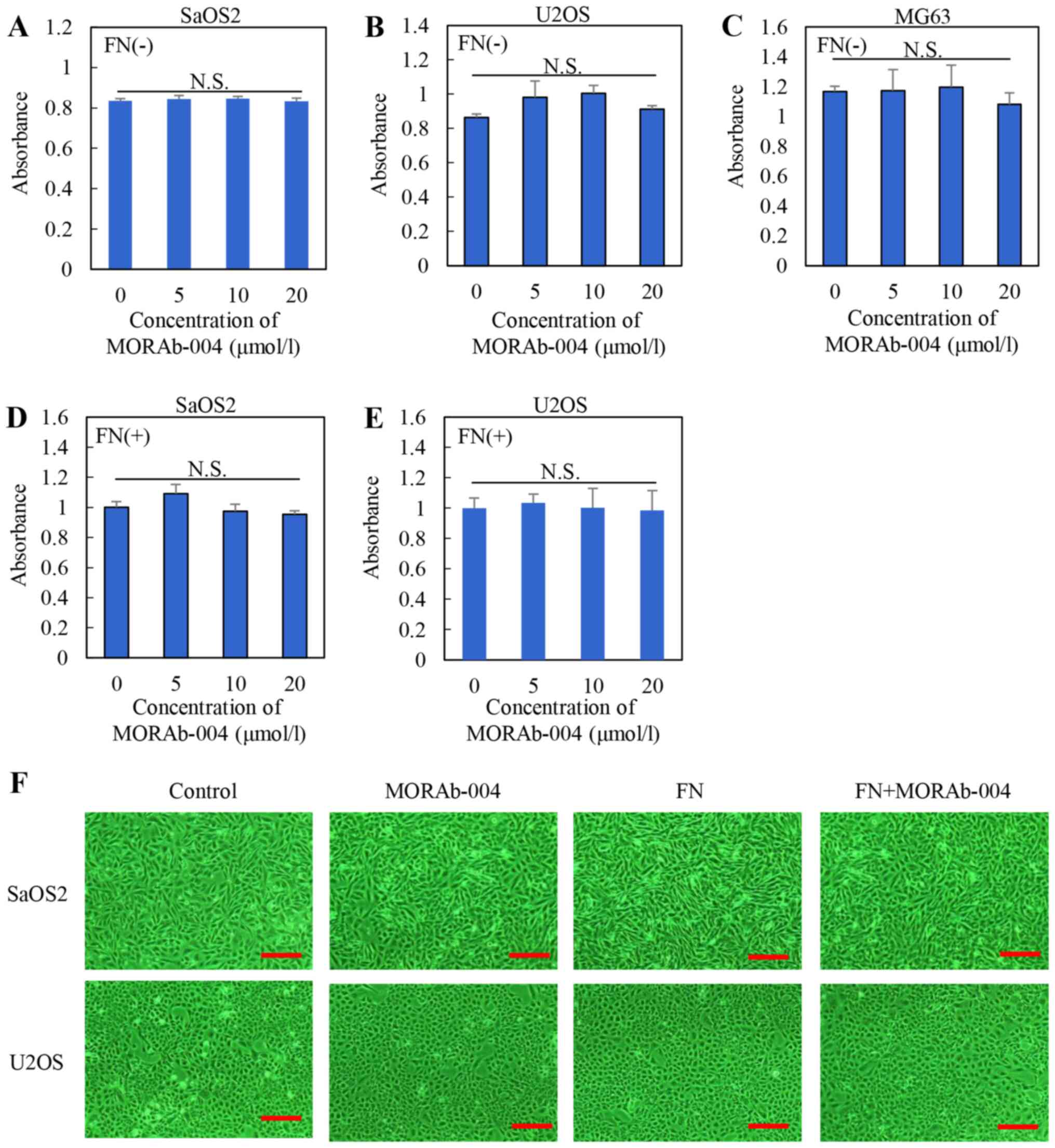
effect of MORAb-004 with or without FN on the viability of SaOS2,
U2OS, MG63 cells was assessed in vitro. OS cells were
treated with different concentrations of MORAb-004 (0, 5, 10 and 20
µmol/l) and FN (0 or 10 µmol/l). The results of the MTS assay
demonstrated no significant differences in cell viability following
treatment with MORAb-004, regardless of the presence of FN
(Fig. 3A-C, treatment without FN;
Fig. 3D and E, treatment with
FN).
In addition, photomicrographs of SaOS2 and U2OS
cells under each condition at ×200 magnification under an inverted
microscope (ECLIPSE Ti-S100; Nikon Corporation; https://www.microscope.healthcare.nikon.com/ja_JP/products/inverted-microscopes/eclipse-ti-series)
are presented in Fig. 3F. The
actual states of cell viability in these cell lines were unaffected
by MORAb-004 or FN. The effect of MORAb-004 with or without FN on
the apoptosis of SaOS2 and U2OS cells was assessed (Fig. S2). No significant differences were
observed in these cell lines under each condition. Collectively,
these results suggest that MORAb-004 has no cytostatic effect and
does not affect the apoptosis of OS cells.
MORAb-004 does not change stemness and
differentiation marker expression in OS
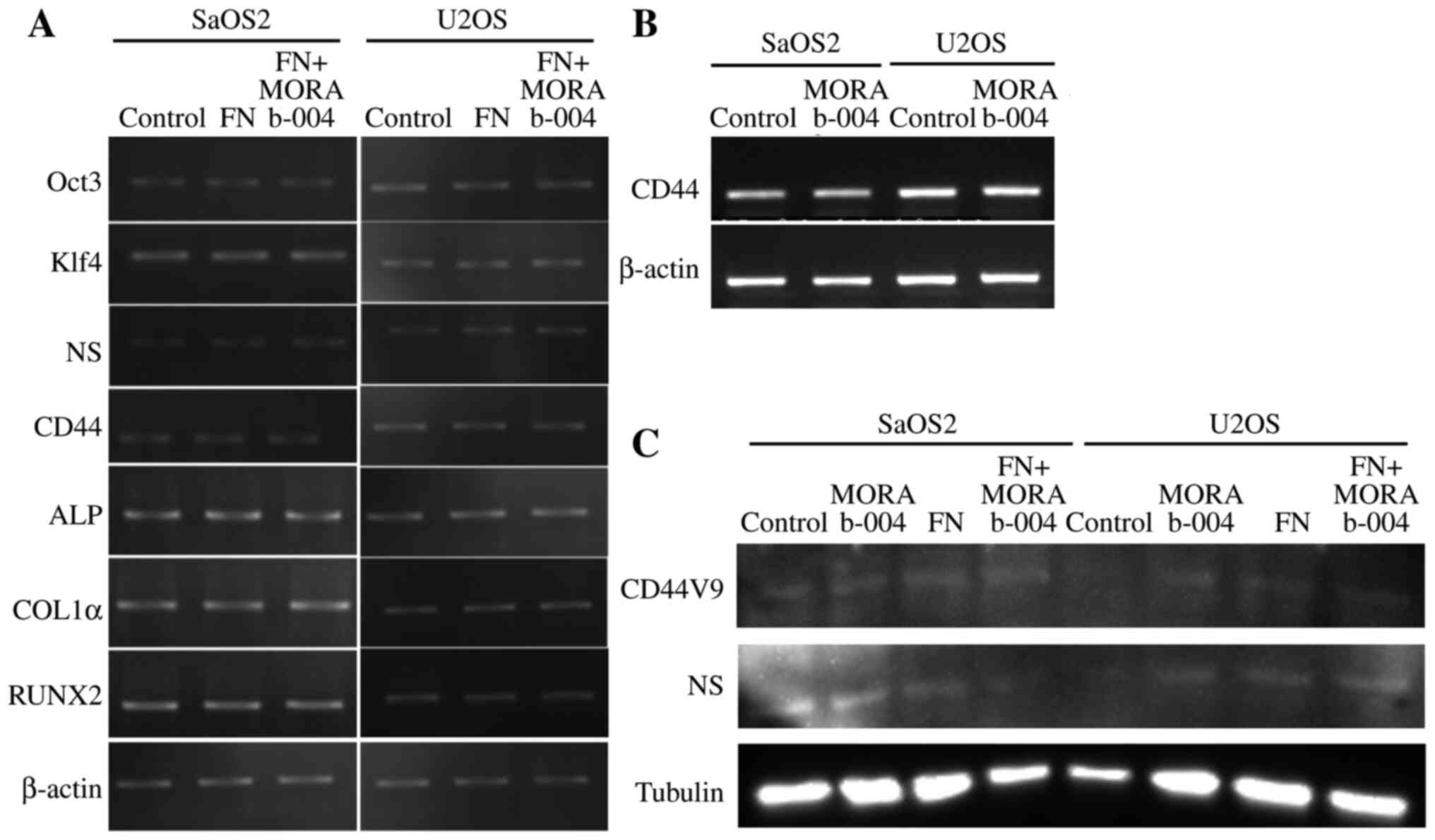
The effect of MORAb-004 in the presence or absence
of FN, one of the specific ligands for CD248 (4), on stem cell and differentiation
markers was assessed via RT-qPCR analysis in the human OS cell
lines, SaOS2 and U2OS (Fig. 4A and
B). Western blot analysis was also performed on the stem cell
markers, CD44V9 and NS, in SaOS2 and U2OS cells (Fig. 4C). The results demonstrated that
MORAb-004 did not change the expression of stem cell or
differentiation markers.
MORAb-004 decreases OS cell migration
in the presence of FN
To determine the efficacy of MORAb-004 in the
presence of FN for sarcoma cell migration, the wound healing assay
and the Transwell migration assays were performed. The results of
the wound healing assay demonstrated that the migratory ability of
OS cells treated with MORAb-004 alone or MORAb-004 and FN decreased
in all cell lines compared with cells treated with control or only
FN by the wound width measured at 24 h (HOS and SaOS2, P<0.001;
U2OS, P=0.010; Fig. 5). The width
is demonstrated by yellow straight line in each figure (Fig. 5A-D). The results of the Transwell
migration assay demonstrated that the number of OS cells which
moved to the lower chamber also decreased following treatment with
MORAb-004 (SaOS2, P=0.00066; U2OS, P=0.00089 and MG63, P=0.00003;
Fig. 6). Taken together, these
results suggest that endosialin may be associated with cell
migration, and MORAb-004 can suppress OS cell migration in the
presence of FN.
Discussion
OS is a highly malignant bone tumor, with frequent
metastasis which disseminates to the lungs and bones (19). The results of the present study
demonstrated that endosialin/CD248 was highly expressed in human OS
with metastatic disease; however, MORAb-004, an
anti-endosialin/CD248 antibody (7), had no cytostatic effect on OS cells
in vitro, and did not change the expression of stem cell and
differentiation markers, suggesting that the antibody maintains
their stemness property and proliferation ability. The wound
healing assay and the Transwell migration assay in vitro
demonstrated that Morab-004 suppressed OS cell migration in the
presence of FN, suggesting that endosialin/CD248 binding to FN can
promote OS cell migration.
Tumor angiogenesis is essential for tumor growth and
metastasis (20). Tomkowicz et
al (4) demonstrated that
endosialin/CD248 mediates the proliferation of primary human
pericytes via the PDGF receptor signaling pathway. In has been
reported that in TEM1 knock-out mice, vessels fail to mature
efficiently, which decreases the number of medium and large
vessels, and increases the number of small vessels (21). This provides evidence that the
endosialin/CD248-1-dependent signaling pathway controls the
proliferation of human pericytes, and is required for the efficient
maturation of vessels within tumors. Thus, a future strategy for
suppressing tumor growth and metastasis is to target this pathway
and mitigate tumor angiogenesis (22). Although blocking
endosialin/CD248-mediated tumor angiogenesis would be expected to
suppress tumor progression, the randomized controlled phase 2 trial
of MORAb-004/ontuxizumab in combination with gemcitabine and
docetaxel in advanced metastatic soft tissue sarcomas exhibited no
improvement in progression-free or overall survival (9). Notably, comparative experiments with
wild-type and endosialin-deficient mice revealed that stromal
endosialin does not affect primary tumor growth but strongly
promotes spontaneous metastasis. Mechanistically,
endosialin-expressing pericytes in the primary tumor promote
metastasis in a cell contact-dependent manner.
Metastasis is a multistep process, including
adhesion to the extracellular matrix (ECM) (3). Tumor cells, including OS cells,
adhere to matrix components via cell-surface receptors, such as
integrins, which bind to the matrix protein FN (20). FN and collagen types I and IV were
identified as specific ligands for endosialin/TEM-1 (4). Notably, cells expressing
endosialin/TEM-1 exhibit enhanced adhesion to FN, as well as
enhanced migration with increases matrix metalloproteinase-9
activity, and these properties can be blocked by a humanized
antibody directed against human endosialin/TEM1 (4).
A predictive biomarker provides information about
the effect of a therapeutic intervention on clinical outcomes, and
can potentially be used to select patients for therapy (9). In the present study, IHC analysis
demonstrated that CD248 expression was significantly higher in
metastatic OS specimens, therefore the IHC score of CD248 may be
feasible as a predictor of prognosis for patients with patients.
Although the detailed mechanisms of promoting invasion and
metastasis through endosialin/CD248 should be elucidated in the
future, it is speculated that upregulated CD248 in stromal and
stem-like tumor initiating cells in OS interacts with FN and
potentially activates proteases in ECM, and subsequently causes
tumor microenvironment changes, which can result in tumor
progression, such as invasion and metastasis (4).
Targeting endosialin/CD248 using antibodies, such as
MORAb-004/ontuxizumab in combination with the conventional
chemotherapeutic agents, can augment the efficacy against a
development of metastatic lesions, potentially by reducing cell
motility and suppressing invasion of OS cells. Further studies
including antibody-drug conjugates are required.
The present study is not without limitations. First,
the sample size for IHC was too small as biopsy specimens at a
single institution were only used. Thus, clinical significance of
endosialin/CD248 in sarcomas should be further evaluated using a
larger sample size. In addition, the effect of MORAb-004 on OS
cells was only evaluated in vitro. To verify the effect on
tumor growth and metastasis, in vivo studies need to be
performed in prospective studies.
In conclusion, the results of the present study
demonstrated that endosialin/CD248 was highly expressed in
metastatic OS, and its binding to FN may promote OS cell migration,
although did not affect tumor growth, suggesting that
endosialin/CD248 may be involved in invasion and metastasis in OS.
Thus, endosialin does not function as an active metastasis
promoting signaling factor in inducing tumor cells to gain invasive
behavior. It is expressed on the surface of tumor cells and
interacts with ECM, such as FN identified as specific ligands for
endosialin and changes the microenvironment. In addition, it
interacts with tumor cells and endothelial cells to promote
metastasis, and may be a selective therapeutic target to prevent
and treat advanced diseases (3).
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Ms. Mari Miyagi and
Ms. Sachiyo Higashimoto for their assistance with the preparation
of this manuscript. They are the secretaries of Nara Medical
University Orthopedic Surgery (Nara, Japan) and contributed to the
data collection and processing of clinical specimens.
Funding
The present study was partly supported by a grant to KH (grant
no. 15K10455) from the Japan Society for the Promotion of Science,
Chiyoda Ward (Tokyo, Japan).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YK performed IHC analysis and prepared the
manuscript. KH supervised the present study, analyzed the data and
prepared the manuscript. SK and SM participated in cell culture,
in vitro experiments and reverse transcription-quantitative
PCR analysis. RFT helped perform the experiments and analyzed the
histopathology in IHC analysis. ST and HF contributed to the
acquisition of data, and the analysis and interpretation of data.
HK supervised the present study and analyzed histopathology in IHC
analysis. YT participated in the analysis and interpretation of
clinical data for IHC, been involved in drafting and revising the
manuscript with critical suggestion, given final approval of the
version to be published and agreed to be accountable for all
aspects of the work. KH and HK confirmed the authenticity of all
the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Nara Medical
University Certified Review Board (approval no. 2833; Nara, Japan)
and written informed consent was provided by all patients or family
members prior to the study start.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Teicher BA: CD248: A therapeutic target in
cancer and fibrotic diseases. Oncotarget. 10:993–1009. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Christian S, Ahorn H, Koehler A,
Eisenhaber F, Rodi H-P, Garin-Chesa P, Park JE, Rettig WJ and
Lenter MC: Molecular cloning and characterization of endosialin, a
C-type lectin-like cell surface receptor of tumor endothelium. J
Biol Chem. 276:7408–7414. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Viski C, König C, Kijewska M, Mogler C,
Isacke CM and Augustin HG: Endosialin-Expressing Pericytes Promote
Metastatic Dissemination. Cancer Res. 76:5313–5325. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tomkowicz B, Rybinski K, Foley B, Ebel W,
Kline B, Routhier E, Sass P, Nicolaides NC, Grasso L and Zhou Y:
Interaction of endosialin/TEM1 with extracellular matrix proteins
mediates cell adhesion and migration. Proc Natl Acad Sci USA.
104:17965–17970. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun D-X, Liao G-J, Liu K-G and Jian H:
Endosialin expressing bone sarcoma stem like cells are highly tumor
initiating and invasive. Mol Med Rep. 12:5665–5670. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lange SE, Zheleznyak A, Studer M,
O'Shannessy DJ, Lapi SE and Van Tine BA: Development of
89Zr-Ontuxizumab for in vivo TEM-1/endosialin PET applications.
Oncotarget. 7:13082–13092. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rybinski K, Imtiyaz HZ, Mittica B,
Drozdowski B, Fulmer J, Furuuchi K, Fernando S, Henry M, Chao Q,
Kline B, et al: Targeting endosialin/CD248 through
antibody-mediated internalization results in impaired pericyte
maturation and dysfunctional tumor microvasculature. Oncotarget.
6:25429–25440. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Diaz LA Jr, Coughlin CM, Weil SC, Fishel
J, Gounder MM, Lawrence S, Azad N, O'Shannessy DJ, Grasso L,
Wustner J, et al: A first-in-human phase I study of MORAb-004, a
monoclonal antibody to endosialin in patients with advanced solid
tumors. Clin Cancer Res. 21:1281–1288. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jones RL, Chawla SP, Attia S, Schöffski P,
Gelderblom H, Chmielowski B, Le Cesne A, Van Tine BA, Trent JC,
Patel S, et al: A phase 1 and randomized controlled phase 2 trial
of the safety and efficacy of the combination of gemcitabine and
docetaxel with ontuxizumab (MORAb-004) in metastatic soft-tissue
sarcomas. Cancer. 125:2445–2454. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kuniyasu H, Yasui W, Shinohara H, Yano S,
Ellis LM, Wilson MR, Bucana CD, Rikita T, Tahara E and Fidler IJ:
Induction of angiogenesis by hyperplastic colonic mucosa adjacent
to colon cancer. Am J Pathol. 157:1523–1535. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bagley RG, Rouleau C, St Martin T, Boutin
P, Weber W, Ruzek M, Honma N, Nacht M, Shankara S, Kataoka S, et
al: Human endothelial precursor cells express tumor endothelial
marker 1/endosialin/CD248. Mol Cancer Ther. 7:2536–2546. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schneider CA, Rasband WS and Eliceiri KW:
NIH Image to ImageJ: 25 years of image analysis. Nat Methods.
9:671–675. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tanabe E, Kitayoshi M, Fujii K, Ohmori H,
Luo Y, Kadochi Y, Mori S, Fujiwara R, Nishiguchi Y, Sasaki T, et
al: Fatty acids inhibit anticancer effects of 5-fluorouracil in
mouse cancer cell lines. Oncol Lett. 14:681–686. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li L, Qiu RL, Lin Y, Cai Y, Bian Y, Fan Y
and Gao XJ: Resveratrol suppresses human cervical carcinoma cell
proliferation and elevates apoptosis via the mitochondrial and p53
signaling pathways. Oncol Lett. 15:9845–9851. 2018.PubMed/NCBI
|
|
15
|
Kishi S, Fujiwara-Tani R, Luo Y, Kawahara
I, Goto K, Fujii K, Ohmori H, Nakashima C, Sasaki T and Kuniyasu H:
Pro-metastatic signaling of the trans fatty acid elaidic acid is
associated with lipid rafts. Oncol Lett. 15:4423–4426.
2018.PubMed/NCBI
|
|
16
|
Luo Y, Fujii K, Ohmori H, Sasahira T,
Moriwaka Y, Isobe M and Kuniyasu H: Antisense phosphorothioate
oligodeoxynucleic acid for CD10 suppresses liver metastasis of
colorectal cancer. Pathobiology. 76:267–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
van der Meer AD, Vermeul K, Poot AA,
Feijen J and Vermes I: A microfluidic wound-healing assay for
quantifying endothelial cell migration. Am J Physiol Heart Circ
Physiol. 298:H719–H725. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Suarez-Arnedo A, Torres Figueroa F,
Clavijo C, Arbeláez P, Cruz JC and Muñoz-Camargo C: An image J
plugin for the high throughput image analysis of in vitro scratch
wound healing assays. PLoS One. 15:e02325652020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lindsey BA, Markel JE and Kleinerman ES:
Osteosarcoma Overview. Rheumatol Ther. 4:25–43. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Broadhead ML, Clark JCM, Myers DE, Dass CR
and Choong PFM: The molecular pathogenesis of osteosarcoma: A
review. Sarcoma. 2011:9592482011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nanda A, Karim B, Peng Z, Liu G, Qiu W,
Gan C, Vogelstein B, St Croix B, Kinzler KW and Huso DL: Tumor
endothelial marker 1 (Tem1) functions in the growth and progression
of abdominal tumors. Proc Natl Acad Sci USA. 103:3351–3356. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tomkowicz B, Rybinski K, Sebeck D, Sass P,
Nicolaides NC, Grasso L and Zhou Y: Endosialin/TEM-1/CD248
regulates pericyte proliferation through PDGF receptor signaling.
Cancer Biol Ther. 9:908–915. 2010. View Article : Google Scholar : PubMed/NCBI
|