Introduction
Oral cancer, the sixth most common cancer, is a
severe and growing problem with an estimated incidence of ~275,000
cases annually (1). Oral squamous
cell carcinoma (OSCC), the commonest type of oral cancer, can occur
via numerous processes during which multiple genetic events alter
the normal functioning of oncogenes and tumor suppressor genes.
Cancer-related genes display the following six fundamental
features: Growth signal self-sufficiency, insensitivity to
growth-inhibitory signals, apoptosis evasion, limitless replicative
potential, sustained angiogenesis and the ability to invade and
metastasize (2). Previous studies
have shown that OSCC development is associated with cell
proliferation and apoptosis rates (3–5);
thus, it is necessary to understand the mechanisms underlying
malignant tumors to develop new and effective treatment
strategies.
GTPases of immunity-associated proteins (GIMAPs),
also known as immunity-associated nucleotide binding proteins or
IMAPs, are a family of GTPases found in vertebrates and plants.
Humans have seven GIMAPs clustered on chromosome 7, consisting of
an amino-terminal guanine-nucleotide binding domain (G-domain)
followed by varying C-terminal extensions of 50–100 amino acids
long (6,7). GIMAPs have seven isoforms expressed
in humans (namely, GIMAP1, GIMAP2, GIMAP4-GIMAP8; GIMAP3 is a
pseudogene) (8), regulating T cell
survival during their development, selection and homeostasis and
they may be linked to the onset of T lymphopenia, leukemia and
autoimmunity (9,10). GIMAPs may also be involved in the
mitochondrial regulation of lymphocyte apoptosis by interacting
with the Bcl-2 family proteins (8). In addition, it has been suggested
that GIMAP3 and GIMAP5 are involved in the mitochondria-mediated
apoptosis regulatory pathway (8).
GIMAP3 and GIMAP5 have similar primary structures and they are both
localized in the intracellular membrane fraction, where several
Bcl-2 family proteins are located. Furthermore, both GIMAP3 and
GIMAP5 co-immunoprecipitate with Bcl-2 and Bcl-xL in T cells
(8). Patterson et al
(11) indicate that GIMAP5 is
associated with lymphocyte survival, autoimmunity and colitis and
is essential for the inactivation of glycogen synthase kinase-3β
following T cell activation. The preliminary examination of the
mRNA expression levels of GIMAP1, GIMAP2, GIMAP4-GIMAP8 in OSCC the
present study revealed only GIMAP2 expression to be significantly
(P<0.05; Fig. 1A and B)
increased compared with that in HNOKs with the other isoforms
showing low expression and GIMAP4 and GIMAP7 not being expressed
(Fig. S1).
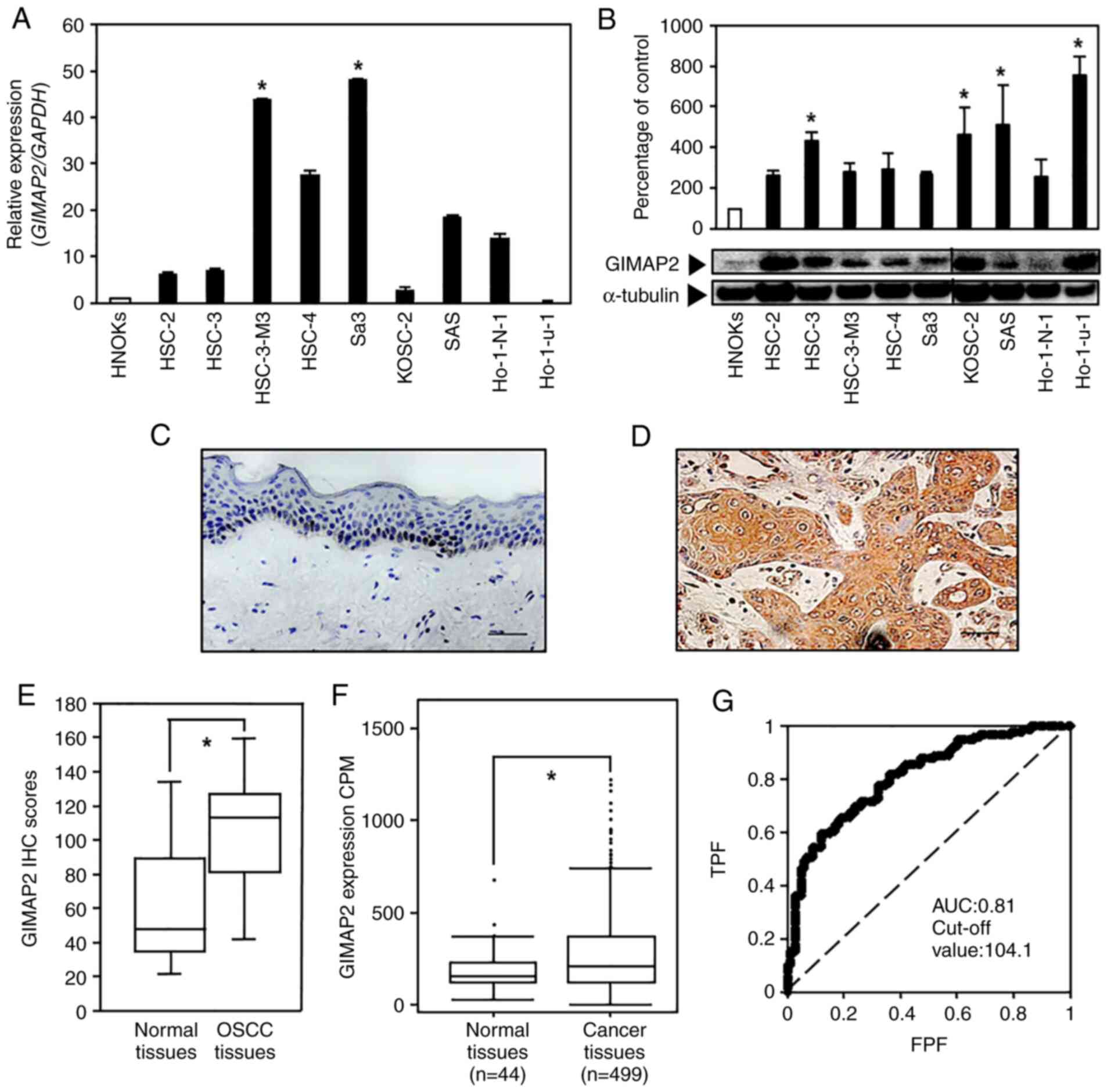 | Figure 1.Evaluation of GIMAP2 expression in
OSCC-derived cell lines and primary OSCC specimens. (A) Reverse
transcription-quantitative PCR analysis showed that GIMAP2 mRNA
expression was significantly upregulated (*P<0.05, Dunnett's
test) in the two OSCC cell lines compared with that in HNOKs. (B)
Western blotting was conducted three times per cell type using
GIMAP2 primer No. 1. The results are expressed as mean ± standard
error of the mean of triplicate data. GIMAP2 expression was
upregulated (*P<0.05, Dunnett's test) in four OSCC cell lines
compared with that in HNOKs. Non-continuous parts of blots probed
on the same membrane are indicated using vertical lines.
Representative IHC results for GIMAP2 expression in (C) the normal
oral tissues (scale bar, 50 µm) and (D) primary OSCC tissues (scale
bar, 50 µm). (E) IHC scores showed GIMAP2 expression in primary
OSCC (n=100) and normal tissue samples. The GIMAP2 IHC scores of
the normal oral tissues and primary OSCC tissues ranged from 21.2
to 134.5 (median, 44.6) and from 30.2 to 148.0 (median, 109.8),
respectively. GIMAP2 expression was considerably (*P<0.05,
Wilcoxon signed-rank test) higher in OSCC tissues compared in
normal oral tissues. (F) The Cancer Genome Atlas data show the
GIMAP2 expression status in primary HNSCC; n=499) and normal
tissue samples (n=44). GIMAP2 expression was considerably higher in
HNSCC tissues than in normal tissues (*P<0.05, Student's
t-test). (G) The ROC curve analysis indicated that the cut-off
value was 104.1 and the AUC was 0.81. GIMAP2, GTPases of
immunity-associated proteins 2; OSCC, oral squamous cell carcinoma;
HNOK, human normal oral keratinocyte; IHC, immunohistochemistry;
ROC, receiver operating characteristic; AUC, area under the curve;
TPF, true-positive fraction; FPF, false-positive fraction. |
GIMAP2, expressed in humans with no orthologs in
mice and rats, is the second-largest protein in the human GIMAP
family containing two C-terminal hydrophobic regions (12). According to BioGPS (https://www.biogps.org), T cells, blood cells,
including platelets and the spleen express GIMAP2. To the best of
the authors knowledge, there are only two studies on GIMAP2. In one
of the studies, Schwefel et al (6) showed that GIMAP2 assembles into a
GTP-dependent scaffold and the C-terminal amino acid stretch
targets GIMAP2 toward lipid droplets. In the other study, Schwefel
et al (7) found that GIMAP2
expression was maintained in all the examined human lymphoma T cell
lines, whereas the expression of other GIMAP members was inhibited
in these tumor cell lines. This is in line with the observations of
the present study and suggests a favorable role of GIMAP2 in cancer
cell survival. Certain GIMAPs may be associated with T lymphopenia
and leukemia (9–11,13),
although the biological functions of most GIMAPs, including GIMAP2,
remain to be elucidated. As the primary function of GIMAP2 in the
progression of solid cancers, such as OSCC, is currently unclear,
remain investigated its expression and molecular mechanisms in
OSCC.
Materials and methods
Cell and tissue samples
Human OSCC-derived cell lines, including HSC-2
(RBRC-RCB1945; oral cavity), HSC-3 (JCRB-0623; tongue), HSC-4
(RBRC-RCB1902, tongue), HSC3-M3 (JCRB-1354, tongue), Sa3
(RBRC-RCB0980, gingiva), Ho-1-N-1 (JCRB-0831, buccal mucosa), KOSC2
(JCRB-0126.1, mouth floor), SAS (RBRC-RCB 1974, tongue) and
Ho-1-u-1 (RBRC-RCB2102, mouth floor), were purchased from the JCRB
Cell Bank and RIKEN BioResource Center. All cancer cells were
cultured in low-glucose Dulbecco's modified Eagle's medium (DMEM;
Sigma-Aldrich; Merck KGaA) supplemented with 10% heat-inactivated
fetal bovine serum (FBS; HyClone; Cytiva) and 50 U/ml of penicillin
and streptomycin at 37°C in a humidified 5% carbon dioxide
atmosphere. Human normal oral keratinocytes (HNOKs) were obtained
from three healthy donors. The donors comprised 2 men and 1 woman.
The donors were 27, 28 and 22 years old, respectively, and were
recruited between April 2017 and June 2017. HNOKs were cultured in
Oral Keratinocyte Medium New Zealand BPE (ScienCell Research
Laboratories, Inc.; cat. no. 2611) as described previously
(14,15). The ethics committee of the Graduate
School of Chiba University approved this study (protocol number
680). All patients provided written informed consent prior to their
inclusion in the study.
mRNA expression analysis
The present study performed reverse transcription
quantitative PCR (RT-qPCR) (14–18).
Cell were grown to 80% confluence in a 10-cm dish. Total RNA was
isolated using TRIzol® Reagent (Invitrogen; Thermo
Fisher Scientific, Inc.; cat. no. 15596018), according to the
manufacturer's instructions. cDNA was generated using ReverTra Ace
qPCR RT Master Mix (Toyobo Life Science; cat. no. FSQ-201)
according to the manufacturers' instructions. RT-qPCR was performed
in a 20-µl reaction volume using FastStart SYBR-Green Master (Roche
Diagnostics; cat. no. 4673492001) according to the manufacturer's
protocol (17,18). The following primers were used to
amplify GIMAP2: GIMAP2 No. 1, forward, 5′-CGATTCAAATGCTTGCTTCC-3′
and reverse, 5′-GGACCAAAATGAACACAGTCAC-3′ (Thermo Fisher
Scientific, Inc., Waltham, MA, USA); GIMAP2 No. 2, forward,
5′-TGGAAGGACCACTGTGAAGC-3′ and reverse, 5′-GTCCTGTGAGGTATAGCGGC-3′
(Greiner Bio-One Co Ltd.); and GIMAP2 No. 3 forward,
5′-GGATGCCATGGGACACACAA-3′ and reverse, 5′-TAAAGGCACAGATTCGCCCA-3′
(Greiner Bio-One Co Ltd.).
In addition, the following primers and universal
probes were used: GIMAP1, forward, 5′-TCGAGCTCCTCTCTGGTTATG-3′ and
reverse, 5′-TGCAGTCTCAGCCTATGCAC-3′ (Thermo Fisher Scientific,
Inc.); GIMAP4, forward, 5′-ACACCAGGGGCCAGTTATG-3′ and reverse,
5′-TGCTGTTTCCTGTTGCACTT-3′ (Thermo Fischer Scientific); GIMAP5,
forward, 5′-TGGGGGACACACTCCATAAT-3′ and reverse, 5′-
GCAGACGCAGTTAAGGAGGA-3′ (Thermo Fisher Scientific, Inc.); GIMAP6,
forward, 5′-GATGGAGGAAGAAGAATATGAACAA-3′ and reverse,
5′-TTCTGTTCTTTCTCCCTTAGACCT-3′ (Thermo Fisher Scientific, Inc.);
GIMAP7, forward, 5′-CTCTAGAACTTAGGCACGTACAAGAC-3′ and reverse
5′-CTCTAGAACTTAGGCACGTACAAGAC-3′ (Thermo Fisher Scientific, Inc.);
GIMAP8, forward, 5′-CAGATATAGTGCCTTCAACTACCG-3′ and reverse,
5′-GGACAATGTTCAGGGTTTCTTT-3′ (Thermo Fisher Scientific, Inc.); and
glyceraldehyde 3-phosphate dehydrogenase (GAPDH), forward,
5′-AGCCACATCGCTCAGACA-3′ and reverse, 5′-GCCCAATACGACCAAATCC-3′
(Thermo Fisher Scientific, Inc.). The transcript amounts for the
target genes were estimated from the respective standard curves and
normalized to the GAPDH.
A LightCycler 480 PCR system (Roche Diagnostics) was
used with the following RT-qPCR conditions: initial denaturation at
95°C for 10 min, 45 amplification cycles of denaturation at 95°C
for 10 sec, annealing at 60°C for 30 sec and extension at 72°C for
1 sec, followed by a cooling step at 40°C for 30 sec. This
experiment was performed in triplicate.
Western blotting
Protein extraction and immunoblotting were conducted
as previously described (16,18,19).
Cells were washed three times with cold phosphate buffered saline
(FUJIFILM Wako Pure Chemical Corporation; cat. no. 045-29795) and
gently and briefly centrifuged (11,000 × g; 4°C; 5 min). The cell
pellets were then incubated at 4°C for 10 min in a lysis buffer (7
M urea, 2 M thiourea, 4% w/v CHAPS, and 10 mM Tris, pH 7.4). The
total protein concentration was measured using a dye-binding method
based on the Bradford assay with Bio-Rad Protein Assay Dye Reagent
Concentrate (Bio-Rad Laboratories, Inc.; cat. no. 5000006JA). A
total of 20 µg of the protein was loaded per lane.
Protein extracts were electrophoresed on 4–12%
Bis-Tris gel (Invitrogen; Thermo Fisher Scientific, Inc.; cat. no.
NP0336BOX) and transferred to nitrocellulose membranes (Invitrogen;
Thermo Fisher Scientific, Inc.; cat. no. 77010) and blocked for 1 h
at room temperature (25°C) with Blocking One (Nacalai Tesque, Inc.;
cat. no. 03953-95). The membranes were then incubated with
polyclonal rabbit anti-GIMAP2 antibody (Rabbit polyclonal antibody
specific for human GIMAP2; cat. no. HPA013589; 1:100; Atlas
Antibodies). In addition, the following antibodies were used: p21
(Mouse monoclonal antibody specific for human p21; cat. no.
sc-6246, 1:200; Santa Cruz Biotechnology, Inc., Inc.),
cyclin-dependent kinase (CDK)4 (Mouse monoclonal antibody specific
for human CDK4; cat. no. sc-23896, 1:500; Santa Cruz Biotechnology,
Inc.), CDK6 (Mouse monoclonal antibody specific for human CDK6;
cat. no. sc-7961, 1:200; Santa Cruz Biotechnology, Inc.), Cyclin D1
(Mouse monoclonal antibody specific for human Cyclin D1; cat. no.
sc-20044, 1:200; Santa Cruz Biotechnology, Inc.), Cyclin E (Mouse
monoclonal antibody specific for human Cyclin E; cat. no.
sc-377100, 1:100; Santa Cruz Biotechnology, Inc.), CDK2 (Mouse
monoclonal antibody specific for human CDK2; cat. no. sc-6248,
1:200; Santa Cruz Biotechnology, Inc.), p53 (Mouse monoclonal
antibody specific for human p53; cat. no. sc-393031, 1:100; Santa
Cruz Biotechnology, Inc.), Rb (Mouse monoclonal antibody specific
for human Rb, NBP2-54476IR, 1:200; Novus Biologicals),
phosphorylated (p)-Rb (Ser780; Rabbit polyclonal antibody specific
for human p-Rb (Ser780); cat. no. ab47763, 1:500; Abcam), Bcl-2
(Mouse monoclonal antibody specific for human Bcl-2; cat. no.
sc-7382, 1:50; Santa Cruz Biotechnology, Inc.), Bak (Mouse
monoclonal antibody specific for human Bak; cat. no. sc-517390,
1:200; Santa Cruz Biotechnology, Inc.), Bax (Mouse monoclonal
antibody specific for human Bax; cat. no. sc-7480, 1:200; Santa
Cruz Biotechnology, Inc.), Bcl-xL (Mouse monoclonal antibody
specific for human Bcl-xL; cat. no. sc-8392, 1:200; Santa Cruz
Biotechnology, Inc.) and mouse α-tubulin (Mouse monoclonal antibody
specific for human α-tubulin; cat. no. sc-5286, 1:1,000; Santa Cruz
Biotechnology, Inc.) overnight at 4°C. The membranes were then
washed with TBS-T (1% Tween) and incubated with horseradish
peroxidase-conjugated anti-rabbit IgG (Promega Corporation; cat.
no. W4011) or anti-mouse IgG as a secondary antibody (Promega
Corporation; cat. no. W4021), for 1 h at room temperature (25°C).
Finally, the membranes were developed using Clarity Western ECL
Substrate (Bio-Rad Laboratories, Inc.; cat. no. 170–5061), and
immunoblotting was visualized with the ChemiDoc XRS Plus system
(Bio-Rad Laboratories, Inc.). The signal intensities were
quantitated using the Image Lab system 6.1 Software (Bio-Rad
Laboratories, Inc.). Densitometric GIMAP2 protein data were
normalized to α-tubulin protein levels.
Immunohistochemistry (IHC)
analysis
IHC analysis was performed using 100 tissue samples
according to a previously described scoring system (18,20–24).
To determine the cut-off value for the GIMAP2 IHC clinical
parameter scores, the scores of 100 samples were evaluated by
receiver operating characteristic (ROC) curve analysis using a bell
curve in Microsoft Excel (Microsoft Corporation) and Excel
Statistics (Social Survey Research Information Co., Ltd.). Samples
with a score above the cut-off value were defined as
GIMAP2-positive. Polyclonal rabbit anti-GIMAP2 antibody (Rabbit
polyclonal antibody specific for human GIMAP2; cat. no. HPA013589,
1:50; Atlas Antibodies) was used as the primary antibody and Dako
EnVision+ System- HRP Labeled Polymer Anti-Rabbit (Agilent
Technologies, Inc.; cat. no. K4003) was used as the secondary
antibody.
Gene expression data for patients with head and neck
squamous cell carcinoma (HNSCC) was downloaded from The Cancer
Genome Atlas (TCGA) project webpage (http://cancergenome.nih.gov). In total, 499 patients
with complete data were selected (i.e., each had a dataset of
microRNA expression and publicized clinical information).
Transfection
In a 6-well tissue culture plate, HSC-2 and HSC-3
cells were cultured to a 50–70% confluency in antibiotic-free DMEM
supplemented with FBS. Stable knockdown transfectants were
established by transfecting the cell lines (HSC-2 and HSC-3) with
GIMAP2-targeting short hairpin (sh)RNA [shGIMAP2; GIMAP2 shRNA
Plasmid(h): cat. no. sc-89424-SH; Santa Cruz Biotechnology, Inc.]
and control shRNA (shMock) (Control shRNA Plasmid-A: cat. no.
sc-108060; Santa Cruz Biotechnology, Inc.) using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's instructions.
The concentration of the shRNA plasmid was 0.1 µg/µl. The
transfection was carried out at 37°C for 48 h. Two to three weeks
after transfection, viable colonies were transferred to new dishes.
shGIMAP2- and shMock-transfected cells were used for further
experiments.
The vector GIMAP2 Human Untagged Clone (OriGene
Technologies, Inc.; cat. no. SC101332) was transiently transfected
into stable transfectants to confirm the effects of GIMAP2
knockdown. The circular untagged cloning vector PCMV6-XL4 (OriGene
Technologies, Inc.; cat. no. PCMV6XL4) was used as the negative
control. Stable transfectants were isolated using low-glucose DMEM
(Sigma-Aldrich; Merck KGaA) supplemented with 10% heat-inactivated
FBS, 50 U/ml penicillin and streptomycin and 1 µg/ml puromycin
(Santa Cruz Biotechnology, Inc.) at 37°C in a humidified 5% carbon
dioxide atmosphere.
Proliferation assay and cell cycle
analysis
Proliferation assays were performed as previously
described (16,18,19,25).
Cell cycle was analyzed by flow cytometry with a BD Accuri C6 Flow
Cytometer (Becton-Dickinson and Company) and FlowJo 10.5.3 software
(FlowJo LLC) as previously described (21,26–28).
Caspase 3/7 activity assay
GIMAP2-knockdown cells (HSC-2 shGIMAP2 and HSC-3
shGIMAP2) and shMock cells (HSC-2 shMock and HSC-3 shMock)
(2×103 cells/well) were seeded in white-walled 96-well
plates and cultured for 2, 4, or 6 days. The activity levels of
caspases-3/7 were analyzed using the Caspase-Glo 3/7 assay system,
according to the manufacturer's instructions (Promega Corporation;
cat. no. G8091). Briefly, the plates were equilibrated to room
temperature (25°C). Caspase-Glo 3/7 reagent (100 µl) was added into
each well. Following incubation at room temperature for 30 min, the
luminescence signal was detected with Synergy HTX (BioTek
Instruments, Inc.).
Statistical analysis
Wilcoxon signed-rank test (P<0.05) was performed
to identify significant associations and ROC curve analysis was
used to define a cut-off value to confirm whether samples were
GIMAP2-positive or -negative for the classified clinical parameters
(Fig. 1E). Wilcoxon signed-rank
test was used to compare the median values of paired samples.
Furthermore, areas under the curve were determined to confirm the
usefulness of this method (Fig.
1G). Dunnett's test was used for the analysis of data shown in
Figs. 1A and B, 2A and B, 3A, 4,
S1, S2, S3
and S5. Dunnett's post hoc test
was performed after the one-way ANOVA. Two-tailed Student's t-test
was used for the analysis of data shown in Figs. 1F, 3B
and C, 5 and S2.
Results
GIMAP2 upregulation in OSCC-derived
cells
To evaluate GIMAP2 expression, RT-qPCR and western
blotting analyses of nine OSCC-derived cell lines and HNOKs was
performed. The expression of GIMAP2 mRNA was significantly
upregulated (P<0.05) in two OSCC cell lines compared with that
in HNOKs (Fig. 1A and S2). The GIMAP2 level was significantly
upregulated (P<0.05, Dunnett's test) in the four OSCC cell lines
compared with that in HNOKs (Fig.
1B). Protein level prediction based on the mRNA level is
inaccurate because the mRNA and protein levels do not strictly
correlate. As one of the reasons is presumably a mutation in the
primer-binding site, the mRNA level was verified in Ho-1-u-1 cells
by PCR using two additional primer sets targeting different coding
regions. As shown in Fig. S2A,
GIMAP2 was not expressed in Ho-1-u-1 cells, resulting in a
significant difference compared with that in HNOKs.
GIMAP2 expression in primary
OSCCs
Representative IHC data for GIMAP2 immunoreactivity
in normal oral tissues and OSCC samples (magnification, ×400) are
shown in Fig. 1C and D; strong
cytoplasmic staining for GIMAP2 was detected in OSCC samples,
whereas the normal oral tissues showed negative immunoreactivity.
The IHC scores of tissue specimens from 100 patients with OSCC were
used to investigate the clinical correlations between GIMAP2
expression and pathological characteristics. GIMAP2 expression was
significantly higher in OSCC tissues compared with normal oral
tissues (P<0.05; Fig. 1E). The
GIMAP2 IHC scores of the adjacent normal tissues ranged from 21.2
to 134.5 (median, 44.6); whereas those of the OSCC tissues ranged
from 30.2 to 148.0 (median, 109.8). Gene expression data analysis
of patients from TCGA revealed that GIMAP2 expression was
significantly higher in HNSCC tissues compared with normal oral
tissues (P<0.05; Fig. 1F). To
determine the cut-off value for the GIMAP2 IHC scores, a ROC curve
analysis was performed, which yielded an area under the curve of
0.81 and a cut-off value of 104.1 (Fig. 1G). The clinical classifications of
GIMAP2-positive OSCC were significantly associated (P<0.05) with
T-primary tumors and the OSCC stage (Table I).
 | Table I.Correlation between GIMAP2 expression
and the clinical classification of oral squamous cell
carcinoma. |
Table I.
Correlation between GIMAP2 expression
and the clinical classification of oral squamous cell
carcinoma.
|
| Results of
immunostaining No. of patients (%) |
|---|
|
|
|
|---|
| Variable | Total | GIMAP2
negative | GIMAP2
positive | P-value |
|---|
| Age at surgery
(years) |
|
|
|
|
|
>70 | 41 | 14 (34) | 27 (66) | 0.580a |
|
60-70 | 35 | 16 (46) | 19 (54) |
|
|
<60 | 24 | 10 (42) | 14 (58) |
|
| Sex |
|
|
|
|
|
Male | 57 | 21 (37) | 36 (63) | 0.458a |
|
Female | 43 | 19 (44) | 24 (56) |
|
| T-primary
tumor |
|
|
|
|
|
T1+T2 | 51 | 32 (63) | 19 (37) | 0.0004a,b |
|
T3+T4 | 49 | 8 (16) | 41 (84) |
|
| N-regional lymph
node |
|
|
|
|
|
Negative | 63 | 24 (38) | 39 (62) | 0.612a |
|
Positive | 37 | 16 (43) | 21 (57) |
|
| Stage |
|
|
|
|
| I | 15 | 10 (67) | 5 (33) | 0.004a,b |
| II | 20 | 12 (60) | 8 (40) |
|
|
III | 18 | 7 (39) | 11 (61) |
|
| IV | 47 | 11 (23) | 36 (77) |
|
| Histopathologic
type |
|
|
|
|
|
Good | 68 | 28 (41) | 40 (59) | 0.811a |
|
Moderate | 28 | 11 (39) | 17 (61) |
|
|
Poor | 4 | 1 (25) | 3 (75) |
|
| Vascular
Invasion |
|
|
|
|
|
Negative | 62 | 24 (39) | 38 (61) | 0.737a |
|
Positive | 38 | 16 (42) | 22 (58) |
|
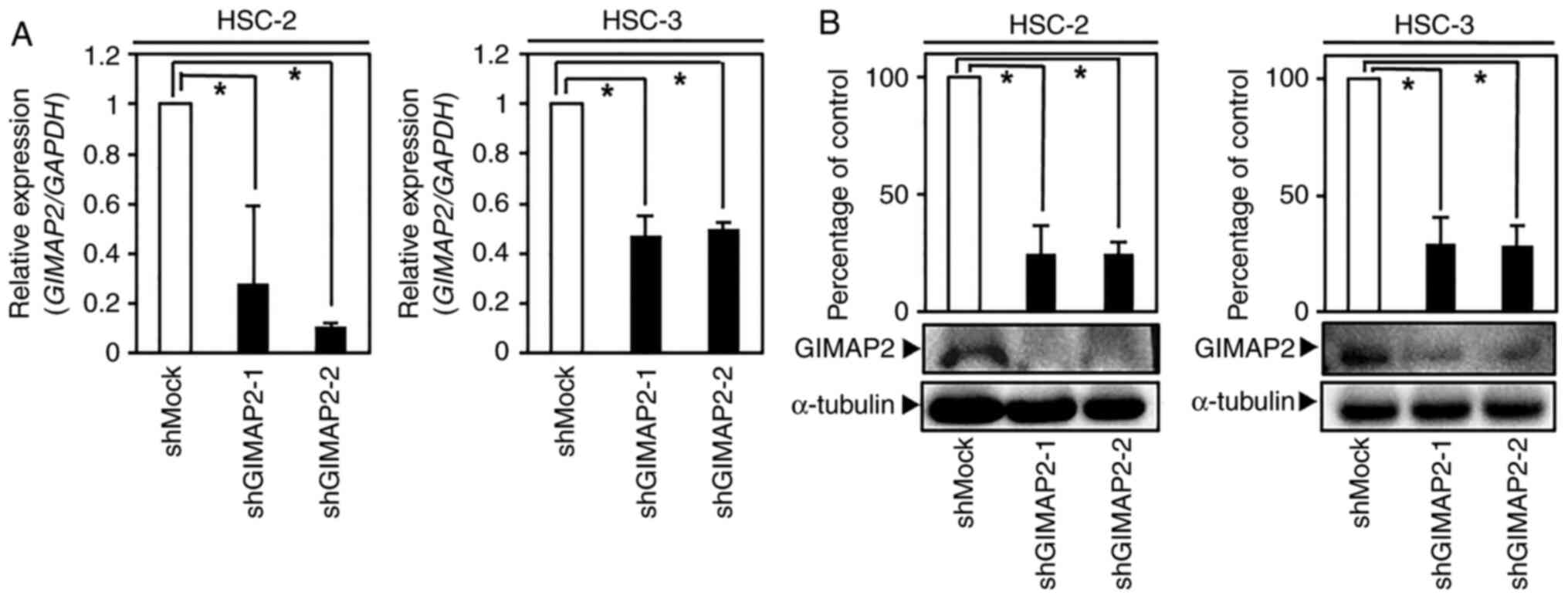
Establishment of GIMAP2-knockdown
cells
As GIMAP2 was significantly upregulated in
OSCC-derived cells (Figs. 1A and B
and S2), its expression in
GIMAP2-knockdown cells (HSC-2 shGIMAP2 and HSC-3 shGIMAP2) was
investigated. GIMAP2 mRNA and protein expressions were
significantly lower in shGIMAP2 cells compared with shMock cells
(P<0.05; Figs. 2A and B and
S3). As other GIMAP isoforms were
not highly expressed in the OSCC cell lines examined, the GIMAP2
shRNA used might not have affected other GIMAP isoforms, as it is
specific for GIMAP2 (P<0.05; Fig.
S1).
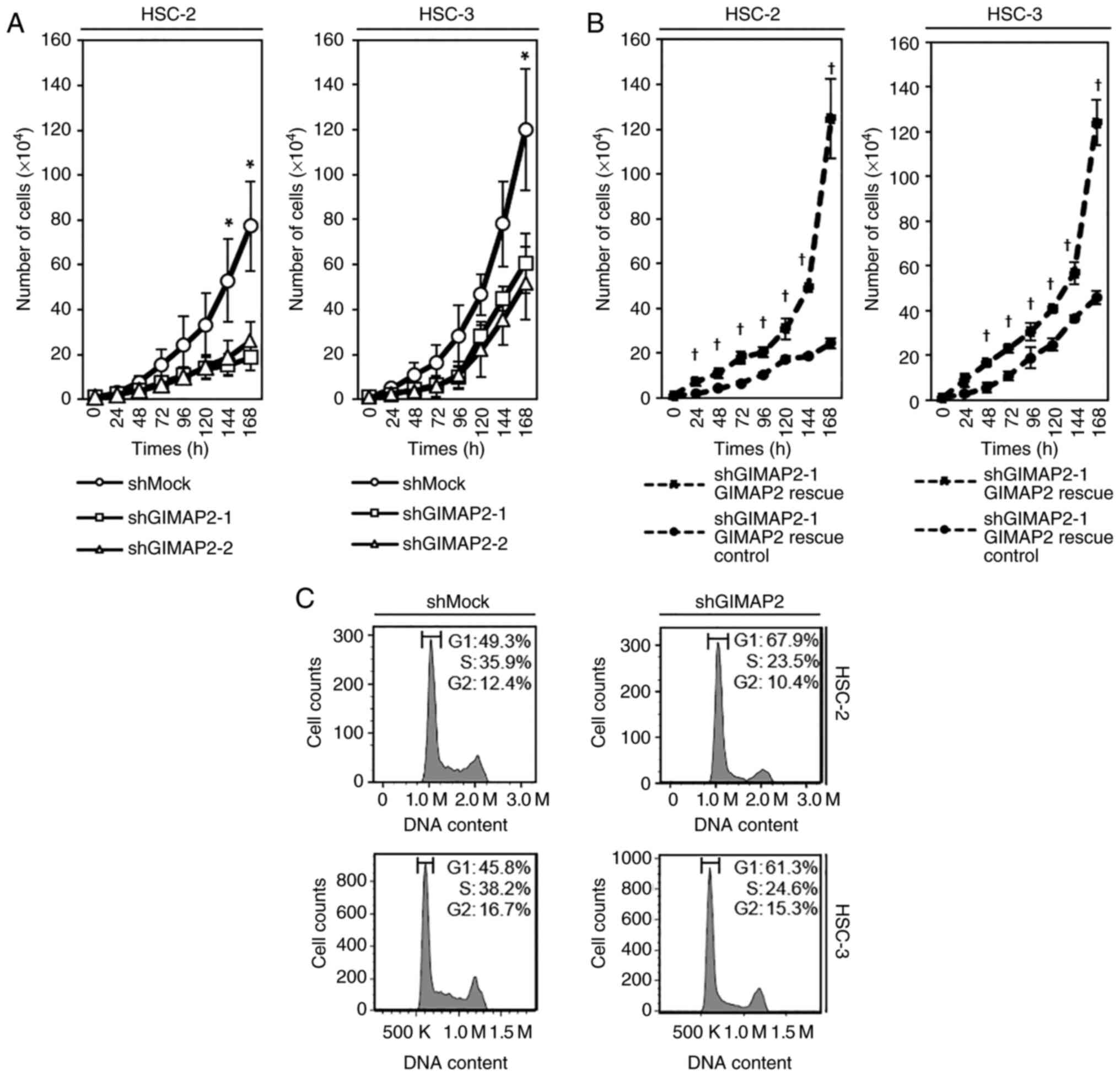
Growth of GIMAP2-knockdown cells
To investigate the effect of GIMAP2 knockdown on
cell growth, a cell proliferation assay was performed and it was
found that cell growth was significantly lower in shGIMAP2 cells
(HSC-2 shGIMAP2 and HSC-3 shGIMAP2) compared with shMock cells
(P<0.05; Fig. 3A). For
validation, the vector GIMAP2 Human Untagged Clone was transiently
transfected into stable transfectants to rescue GIMAP2 expression.
The present study confirmed that the expression of GIMAP2 protein
increased in GIMAP2-transfected shMock cells (HSC-2 shMock GIMAP2
overexpression and HSC-3 shMock GIMAP2 overexpression), but not in
the control shMock cells (HSC-2 shMock control and HSC-3 shMock
control; Fig. S4). In addition,
GIMAP2 was expressed in GIMAP2-transfected cells (HSC-2 shGIMAP2-1
rescue and HSC-3 shGIMAP2-1 rescue), but not in shGIMAP2 cells
(HSC-2 shGIMAP2-1 rescue control and HSC-3 shGIMAP2-1 rescue
control; Fig. S4). In addition,
cell growth was significantly (P<0.05) higher in
GIMAP2-transfected shGIMAP2 cells (HSC-2 shGIMAP2-1 rescue and
HSC-3 shGIMAP2-1 rescue) than in shGIMAP2 cells (HSC-2 shGIMAP2-1
rescue control and HSC-3 shGIMAP2-1 rescue control; Fig. 3B).
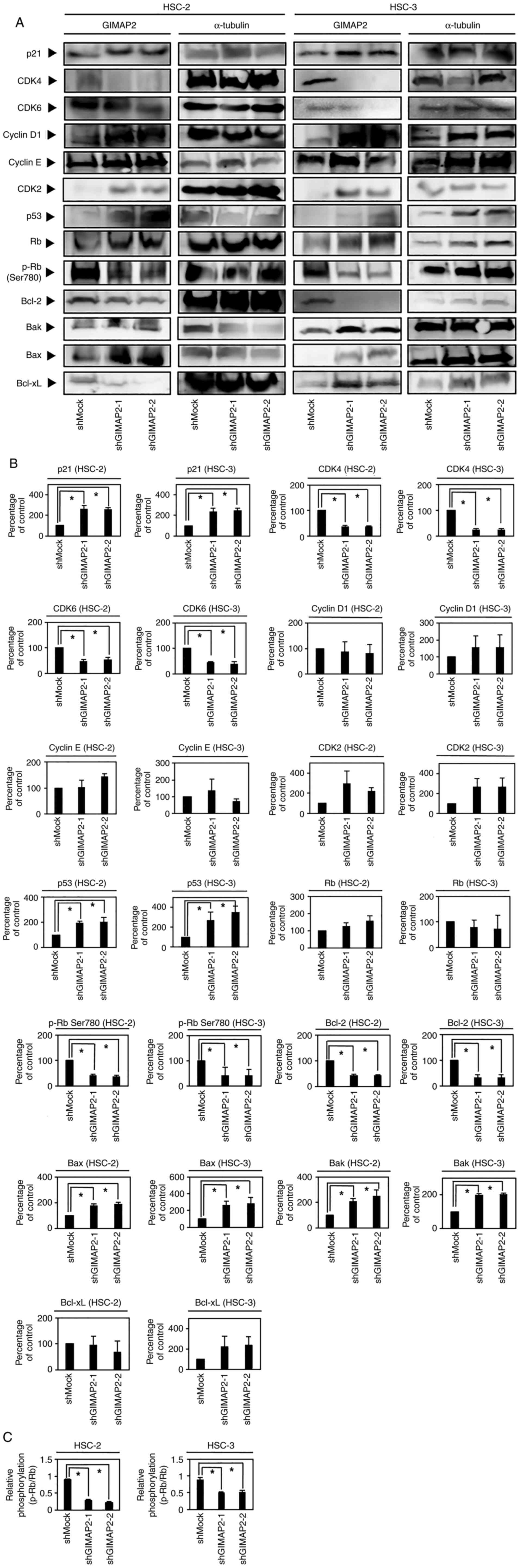
Cell cycle analysis of
GIMAP2-knockdown cells
Cell cycle analysis showed that the percentage of
shGIMAP2 cells in the G1 phase was significantly higher
than that of shMock cells (P<0.05; Fig. 3C). In addition, G1
phase-related protein expression in shGIMAP2 cells revealed CDK4,
CDK6 and phosphorylated (p-)Rb (S780) to be downregulated, whereas
p53 and p21 were upregulated (P<0.05; Figs. 4 and S5), indicating that shGIMAP2 suppressed
proliferation by arresting the cell cycle in the G1
phase.
Apoptosis-related protein
expression
The GIMAP family, including GIMAP2, is hypothesized
to be associated with apoptosis (12). The evaluation of apoptosis-related
protein expression in shGIMAP2 cells showed that Bcl-2 was
significantly downregulated, whereas Bak and Bax were upregulated
(P<0.05; Figs. 4 and S5), suggesting that GIMAP2 may be
associated with apoptosis inhibition.
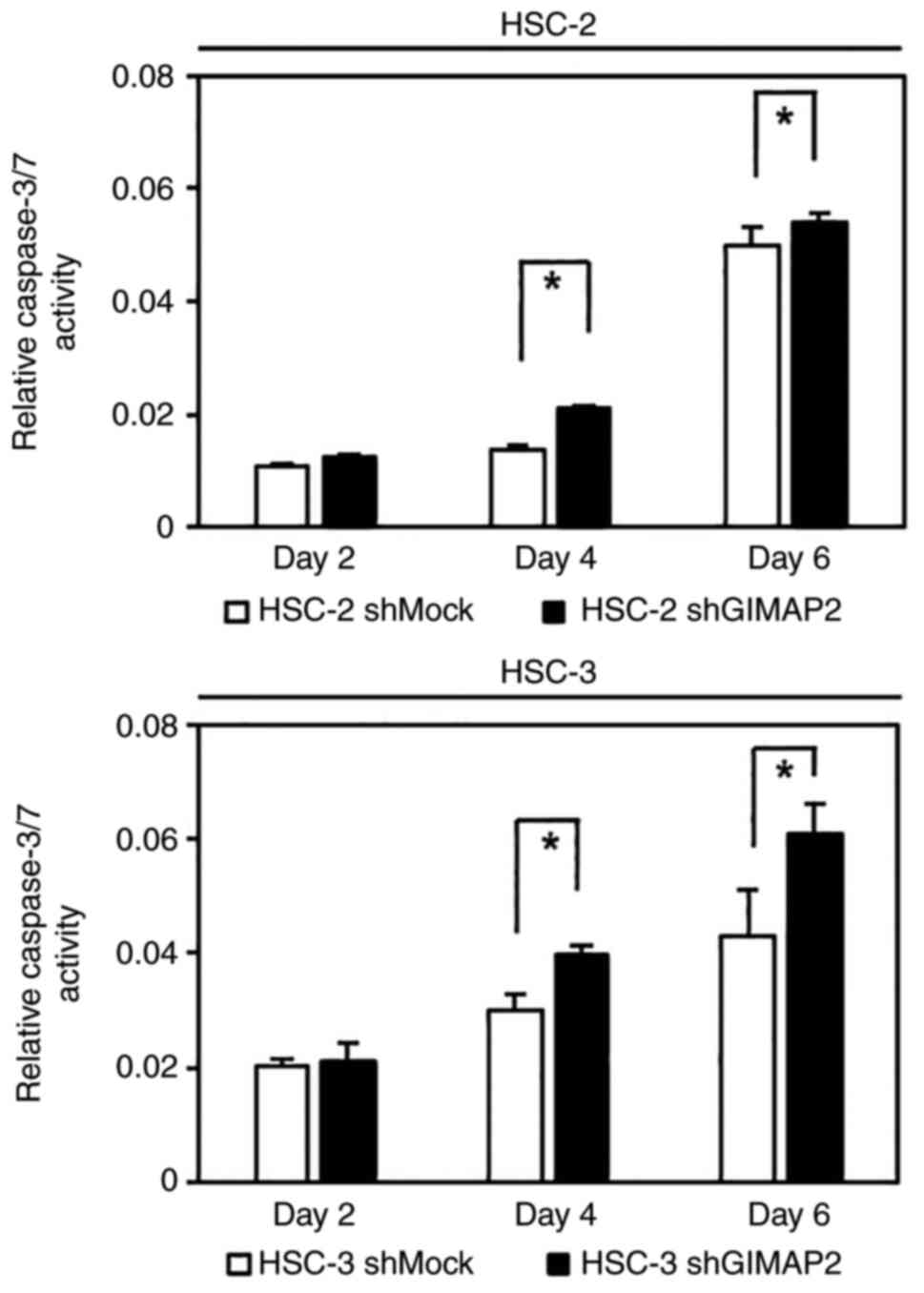
Caspase 3/7 activity assay
To determine whether the expression of GIMAP2 was
associated with the inhibition of apoptosis, the caspase-3/7
activity in shGIMAP2 cells was evaluated. Fig. 5 shows that shGIMAP2 cells exhibited
significant activation of caspase-3/7 compared with that in shMock
cells on days 4 and 6.
Discussion
To the best of the authors' knowledge, the present
study is the first to demonstrate that GIMAP2 is upregulated in
HNSCC and is positively associated with TNM classification. The
knockdown of GIMAP2 revealed that it droves cell proliferation by
arresting the cell cycle in the G1/S phase and that it
may inhibit apoptosis via Bcl-2 upregulation and Bak and Bax
downregulation, suggesting that GIMAP2 serves an important role in
TNM classification in human OSCC. The cell cycle analysis showed
that GIMAP2 knockdown induced G1/S phase arrest in OSCC
cells and decreased CDK4/CDK6 and p-Rb activities. Recently, the
changes in Rb phosphorylation via CDK4/CDK6 signaling have been
reported to control tumor cell proliferation by dysregulating cell
cycle progression in several types of tumors, including lung,
prostate, head and neck cancers (29–33).
Accordingly, the present study confirmed that the expression of
GIMAP2 activated the cyclin D1/CDK4/CDK6 complex, which
phosphorylates Rb and results in OSCC proliferation. In addition,
increased p53 and p21 expression was observed in GIMAP2-knockdown
cells; a study reports that abnormal p53 and p21 signaling
correlates with tumor cell proliferation via the Rb/E2F pathway
(34), in which E2F activation
suppresses the function of p53 and p21 and induces excessive OSCC
proliferation. Thus, the data of the present study suggested that
GIMAP2 may be associated with cell cycle progression via the Rb/E2F
pathway. In addition to cell cycle arrest, p53 regulates apoptosis
via the release of cytochrome c and the transcriptional regulation
of pro-apoptotic genes (35).
Thus, GIMAP2-knockdown OSCC cells exhibited decreased cell growth,
which was associated with CDK4, CDK6 and p-Rb downregulation and
p53 and p21 upregulation. The present study found that not only the
pro-apoptotic proteins Bak and Bax, but also caspase-3/7 was
upregulated in GIMAP2-knockdown cells, whereas the anti-apoptotic
protein Bcl-2 was downregulated, consistent with previous findings
for other GIMAP family proteins (35). The Bcl-2 family members are
associated with cancer development (36), whereas GIMAP2 may be associated
with apoptotic signals (37) and
inhibit Bcl-2 family-mediated apoptotic signals to support cancer
cell survival and development.
Therefore, GIMAP2 may regulate OSCC tumor growth
partly via the inhibition of apoptosis; however, further studies
are warranted to elucidate the mechanism underlying GIMAP2
anti-apoptotic effects. GIMAP2 expression in OSCC cells was
associated with high CDK4, CDK6 and p-Rb expression and low p53 and
p21 expression, demonstrating an essential role of GIMAP2 in growth
regulation. In addition, GIMAP2 expression was positively
associated with Bcl2 and inversely associated with Bak and Bax
expression, which may be indicative of a secondary function of
GIMAP2 in controlling apoptosis. Thus, GIMAP2 expression could be a
biomarker of OSCC progression and apoptosis inhibition.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Ms. Lynda C.
Charters for editing and reviewing this manuscript for English
language.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
MK and HT conceived and designed the study. DN and
AK analyzed and interpreted the patient data. MK, IM, KK and MI
performed the histological experiments and IHC scoring. MK, KS, KU
and MS performed bioinformatics analysis and experiments and
interpreted the data. MK and KS drafted and revised the manuscript.
KU and HT confirm the authenticity of all the raw data. All authors
provided their opinions on the article and data. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
The ethics committee of the Graduate School of Chiba
University approved this study (protocol number 680). All patients
provided written informed consent prior to their inclusion in the
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
GIMAP2
|
GTPases of immunity-associated
proteins 2
|
|
OSCC
|
oral squamous cell carcinoma
|
|
HNOK
|
human normal oral keratinocyte
|
|
RT-qPCR
|
reverse transcription quantitative
PCR
|
References
|
1
|
Warnakulasuriya S: Global epidemiology of
oral and oropharyngeal cancer. Oral Oncol. 45:309–316. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sarkis SA, Abdullah BH, Abdul Majeed BA
and Talabani NG: Immunohistochemical expression of epidermal growth
factor receptor (EGFR) in oral squamous cell carcinoma in relation
to proliferation, apoptosis, angiogenesis and lymphangiogenesis.
Head Neck Oncol. 2:132010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yu T, Liu K, Wu Y, Fan J, Chen J, Li C,
Yang Q and Wang Z: MicroRNA-9 inhibits the proliferation of oral
squamous cell carcinoma cells by suppressing expression of CXCR4
via the Wnt/β-catenin signaling pathway. Oncogene. 33:5017–5027.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hu J, He Y, Yan M, Zhu C, Ye W, Zhu H,
Chen W, Zhang C and Zhang Z: Dose dependent activation of retinoic
acid-inducible gene-I promotes both proliferation and apoptosis
signals in human head and neck squamous cell carcinoma. PLoS One.
8:e582732013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Uchida F, Uzawa K, Kasamatsu A, Takatori
H, Sakamoto Y, Ogawara K, Shiiba M, Tanzawa H and Bukawa H:
Overexpression of cell cycle regulator CDCA3 promotes oral cancer
progression by enhancing cell proliferation with prevention of
G1 phase arrest. BMC Cancer. 12:3212012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schwefel D, Fröhlich C, Eichhorst J,
Wiesner B, Behlke J, Aravind L and Daumke O: Structural basis of
oligomerization in septin-like GTPase of immunity-associated
protein 2 (GIMAP2). Proc Natl Acad Sci USA. 107:20299–20304. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schwefel D, Arasu BS, Marino SF, Lamprecht
B, Köchert K, Rosenbaum E, Eichhorst J, Wiesner B, Behlke J, Rocks
O, et al: Structural insights into the mechanism of GTPase
activation in the GIMAP family. Structure. 21:550–559. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nitta T and Takahama Y: The lymphocyte
guard-IANs: Regulation of lymphocyte survival by IAN/GIMAP family
proteins. Trends Immunol. 28:58–65. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liau WS, Tan SH, Ngoc PC, Wang CQ,
Tergaonkar V, Feng H, Gong Z, Osato M, Look AT and Sanda T:
Aberrant activation of the GIMAP enhancer by oncogenic
transcription factors in T-cell acute lymphoblastic leukemia.
Leukemia. 31:1798–1807. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schnell S, Demolliere C, van den Berk P
and Jacobs H: Gimap4 accelerates T-cell death. Blood. 108:591–599.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Patterson AR, Endale M, Lampe K, Aksoylar
HI, Flagg A, Woodgett JR, Hildeman D, Jordan MB, Singh H, Kucuk Z,
et al: Gimap5-dependent inactivation of GSK3β is required for
CD4(+) T cell homeostasis and prevention of immune pathology. Nat
Commun. 9:4302018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nitta T, Nasreen M, Seike T, Goji A,
Ohigashi I, Miyazaki T, Ohta T, Kanno M and Takahama Y: IAN family
critically regulates survival and development of T lymphocytes.
PLoS Biol. 4:e1032006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Patterson AR, Bolcas P, Lampe K, Cantrell
R, Ruff B, Lewkowich I, Hogan SP, Janssen EM, Bleesing J, Hershey
GK and Hoebe K: Loss of GTPase of immunity-associated protein 5
(Gimap5) promotes pathogenic CD4(+) T-cell development and allergic
airway disease. J Allergy Clin Immunol. 143:245–257.e6. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kasamatsu A, Uzawa K, Nakashima D, Koike
H, Shiiba M, Bukawa H, Yokoe H and Tanzawa H: Galectin-9 as a
regulator of cellular adhesion in human oral squamous cell
carcinoma cell lines. Int J Mol Med. 16:269–273. 2005.PubMed/NCBI
|
|
15
|
Endo Y, Uzawa K, Mochida Y, Shiiba M,
Bukawa H, Yokoe H and Tanzawa H: Sarcoendoplasmic reticulum Ca(2+)
ATPase type 2 downregulated in human oral squamous cell carcinoma.
Int J Cancer. 110:225–231. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shiiba M, Ishige S, Saito Y, Shimizu T,
Minakawa Y, Kasamatsu A, Ogawara K, Uzawa K and Tanzawa H:
Down-regulated expression of family with sequence similarity 3,
member B (FAM3B), in oral squamous cell carcinoma. Int J Oral Sci
Int. 9:9–16. 2012. View Article : Google Scholar
|
|
17
|
Shida-Sakazume T, Endo-Sakamoto Y, Unozawa
M, Fukumoto C, Shimada K, Kasamatsu A, Ogawara K, Yokoe H, Shiiba
M, Tanzawa H and Uzawa K: Lysophosphatidylcholine acyltransferase1
overexpression promotes oral squamous cell carcinoma progression
via enhanced biosynthesis of platelet-activating factor. PLoS One.
10:e01201432015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Saito T, Kasamatsu A, Ogawara K, Miyamoto
I, Saito K, Iyoda M, Suzuki T, Endo-Sakamoto Y, Shiiba M, Tanzawa H
and Uzawa K: Semaphorin7A promotion of tumoral growth and
metastasis in human oral cancer by regulation of G1 cell
cycle and matrix metalloproteases: Possible contribution to tumoral
angiogenesis. PLoS One. 10:e01379232015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Takahara T, Kasamatsu A, Yamatoji M, Iyoda
M, Kasama H, Saito T, Takeuchi S, Endo-Sakamoto Y, Shiiba M,
Tanzawa H and Uzawa K: SIPA1 promotes invasion and migration in
human oral squamous cell carcinoma by ITGB1 and MMP7. Exp Cell Res.
352:357–363. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Koide N, Kasamatsu A, Endo-Sakamoto Y,
Ishida S, Shimizu T, Kimura Y, Miyamoto I, Yoshimura S, Shiiba M,
Tanzawa H and Uzawa K: Evidence for critical role of lymphocyte
cytosolic protein 1 in oral cancer. Sci Rep. 7:433792017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Minakawa Y, Kasamatsu A, Koike H, Higo M,
Nakashima D, Kouzu Y, Sakamoto Y, Ogawara K, Shiiba M, Tanzawa H
and Uzawa K: Kinesin family member 4A: A potential predictor for
progression of human oral cancer. PLoS One. 8:e859512013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Baba T, Sakamoto Y, Kasamatsu A, Minakawa
Y, Yokota S, Higo M, Yokoe H, Ogawara K, Shiiba M, Tanzawa H and
Uzawa K: Persephin: A potential key component in human oral cancer
progression through the RET receptor tyrosine
kinase-mitogen-activated protein kinase signaling pathway. Mol
Carcinog. 54:608–617. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Uzawa K, Kasamatsu A, Saito T, Takahara T,
Minakawa Y, Koike K, Yamatoji M, Nakashima D, Higo M, Sakamoto Y,
et al: Long-term culture of human odontoma-derived cells with a Rho
kinase inhibitor. Exp Cell Res. 347:232–240. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Toeda Y, Kasamatsu A, Koike K,
Endo-Sakamoto Y, Fushimi K, Kasama H, Yamano Y, Shiiba M, Tanzawa H
and Uzawa K: FBLIM1 enhances oral cancer malignancy via modulation
of the epidermal growth factor receptor pathway. Mol Carcinog.
57:1690–1697. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kimura Y, Kasamatsu A, Nakashima D,
Yamatoji M, Minakawa Y, Koike K, Fushimi K, Higo M, Endo-Sakamoto
Y, Shiiba M, et al: ARNT2 regulates tumoral growth in oral squamous
cell carcinoma. J Cancer. 7:702–710. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Miyamoto I, Kasamatsu A, Yamatoji M,
Nakashima D, Saito K, Higo M, Endo-Sakamoto Y, Shiiba M, Tanzawa H
and Uzawa K: Kinesin family member 14 in human oral cancer: A
potential biomarker for tumoral growth. Biochem Biophys Rep.
3:26–31. 2015.PubMed/NCBI
|
|
27
|
Uchida F, Uzawa K, Kasamatsu A, Takatori
H, Sakamoto Y, Ogawara K, Shiiba M, Bukawa H and Tanzawa H:
Overexpression of CDCA2 in human squamous cell carcinoma:
correlation with prevention of G1 phase arrest and
apoptosis. PLoS One. 8:e563812013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hayashi F, Kasamatsu A, Endo-Sakamoto Y,
Eizuka K, Hiroshima K, Kita A, Saito T, Koike K, Tanzawa H and
Uzawa K: Increased expression of tripartite motif (TRIM) like 2
promotes tumoral growth in human oral cancer. Biochem Biophys Res
Commun. 508:1133–1138. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rader J, Russell MR, Hart LS, Nakazawa MS,
Belcastro LT, Martinez D, Li Y, Carpenter EL, Attiyeh EF, Diskin
SJ, et al: Dual CDK4/CDK6 inhibition induces cell-cycle arrest and
senescence in neuroblastoma. Clin Cancer Res. 19:6173–6182. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fry DW, Bedford DC, Harvey PH, Fritsch A,
Keller PR, Wu Z, Dobrusin E, Leopold WR, Fattaey A and Garrett MD:
Cell cycle and biochemical effects of PD 0183812. A potent
inhibitor of the cyclin D-dependent kinases CDK4 and CDK6. J Biol
Chem. 276:16617–16623. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Patnaik A, Rosen LS, Tolaney SM, Tolcher
AW, Goldman JW, Gandhi L, Papadopoulos KP, Beeram M, Rasco DW,
Hilton JF, et al: Efficacy and safety of abemaciclib, an inhibitor
of CDK4 and CDK6, for patients with breast cancer, non-small cell
lung cancer, and other solid tumors. Cancer Discov. 6:740–753.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zi X and Agarwal R: Silibinin decreases
prostate-specific antigen with cell growth inhibition via
G1 arrest, leading to differentiation of prostate
carcinoma cells: Implications for prostate cancer intervention.
Proc Natl Acad Sci USA. 96:7490–7495. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hamilton E and Infante JR: Targeting
CDK4/6 in patients with cancer. Cancer Treat Rev. 45:129–138. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nevins JR: The Rb/E2F pathway and cancer.
Hum Mol Genet. 10:699–703. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hickman ES, Moroni MC and Helin K: The
role of p53 and pRB in apoptosis and cancer. Curr Opin Genet Dev.
12:60–66. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Adams JM and Cory S: The Bcl-2 apoptotic
switch in cancer development and therapy. Oncogene. 26:1324–1337.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schwefel D and Daumke O: GTP-dependent
scaffold formation in the GTPase of immunity associated protein
family. Small GTPases. 2:27–30. 2011. View Article : Google Scholar : PubMed/NCBI
|