Introduction
Cancer is the second leading cause of mortality
globally. In 2020, ovarian cancer was the 8th most commonly
diagnosed cancer in the world (1).
There are five subtypes of epithelial ovarian cancer: High-grade
serous carcinoma (HGSC; 75%); clear cell carcinoma (CC; 6%);
endometroid carcinoma (EC; 10%), low-grade serouscarcinoma (3%);
and mucinous carcinoma (MC; 6%) (2). Risk factors for ovarian cancer
include older age at menopause, hormone replacement therapy and
genetic alterations (3). Genomic
variations that have been previously associated with ovarian cancer
include mutations in BRCA1/2 and TP53, high copy number of KRAS,
BRAF, cyclin E1, phosphatidylinositol 3‑kinase (PI3K) catalytic
subunit, β-catenin and HER2, in addition to the loss of the copy
number of PTEN (4,5).
The first-line chemotherapeutic treatment method for
ovarian cancer is platinum-based chemotherapy, including cisplatin,
carboplatin and oxaliplatin (6).
Cisplatin is a platinum drug that interacts directly with DNA,
resulting in the formation of DNA adducts leading to cell death
(7). Although the drug response
rate for ovarian cancer is 60–80%, the majority of patients
eventually become resistant to platinum-based drugs and suffer from
relapses (8). Platinum-based drugs
can cause adverse side effects, including anaphylaxis, cytopenia,
hepatotoxicity, ototoxicity and cardiotoxicity (9). Therefore, novel therapeutic agents
for ovarian cancer treatment that are more effective and with
minimal cytotoxicity are in urgent demand. Over the past decade,
targeted therapy is becoming an important form of ovarian cancer
treatment strategy due to its direct effects on cancer (10). Furthermore, it causes less damage
to normal non-cancerous cells (11). There are various types of targeted
therapy for ovarian cancer treatment, including VEGF (bevacizumab),
EGFR (cetuximab), HER2 (trastuzumab), mTOR (temsirolimus) (12) and poly-ADP ribose polymerase
(PARP1; rucaparib) (13)
inhibitors.
Lignans are secondary plant metabolites that have
been reported to confer a number of biologically active properties,
such as anti-inflammatory, antimicrobial, antiviral and anticancer
effects (14). Lignan-based
compounds etoposide and teniposide have been applied to treat
leukemia, testicular and lung cancer (15). In particular, lignan compounds
isolated from plants have also been found to exert anticancer
effects on ovarian cancer cells. Magnolol has been documented to
inhibit cell proliferation, migration and invasion in
HER2-overexpressing ovarian cancer cells (16). In addition, daurinol and
deoxyschizandrin has been reported to induce cell cycle arrest by
inhibiting topoisomerase IIα (17)
and cyclin E expression (18),
respectively. Arctigenin has also been demonstrated to inhibit
STAT3 phosphorylation and induce caspase-3-dependent apoptosis
(19).
Kusunokinin is a dibenzylbutyrolactone lignan that
can be found in a wide variety of plants, such as Haplophyllum
vulcanicum (20),
Wikstroemia sikokiana (21), Aristolochia malmeana
(22), Aristolochia
cymbifera (23),
Wikstroemia indica (24),
Piper cernuum (25) and
Piper nigrum (26). Other
lignan compounds, including (−)-cubebin, (−)-hinokinin and
(−)-arctigenin, can also be found with (−)-kusunokinin (22,25).
(−)-Cubebin, (−)-hinokinin and (−)-arctigenin have been found to
confer anticancer effects against colon (HT-29) (27), breast (MCF-7 and SKBR-3) (28) and ovarian (OVCAR-3 and SKOV-3)
cancers (19). The anticancer
effects of trans-(−)-kusunokinin isolated from P. nigrum
have been previously evaluated in vitro and in vivo.
Trans-(−)-kusunokinin can exert cytotoxic effects on breast,
colorectal and lung cancer cells. Furthermore, it can reduce breast
tumor growth in rats (29). This
compound can also induce breast cancer cell apoptosis by decreasing
topoisomerase II expression whilst increasing that of Bax,
caspase-8, −7 and −3 (26). In
addition, trans-(−)-kusunokinin can inhibit
N-nitrosomethylurea-induced rat mammary tumor growth by suppressing
c-Src, PI3K, AKT and ERK1/2 signaling and the expression of
proliferative proteins c-Myc, E2F transcription factor-1, CDK1 and
cyclin B1. Trans-(−)-kusunokinin has also been found to decrease
the expression of migratory proteins MMP-2, MMP-9 and E-cadherin
(29). Furthermore, synthetic
trans-(±)-kusunokinin was demonstrated to induce cytotoxicity
against breast cancer and cholangiocarcinoma cells whilst
increasing multi-caspase activity (30). Trans-(−)-kusunokinin can also
interact with colony stimulating factor 1 receptor (CSF1R) and HER,
proteins associated with cancer cell proliferation (31,32)
and aldo‑keto reductase family 1 member B1 (AKR1B1), a protein
associated with migration (33).
Synthetic racemic trans-(±)-kusunokinin, which consists of
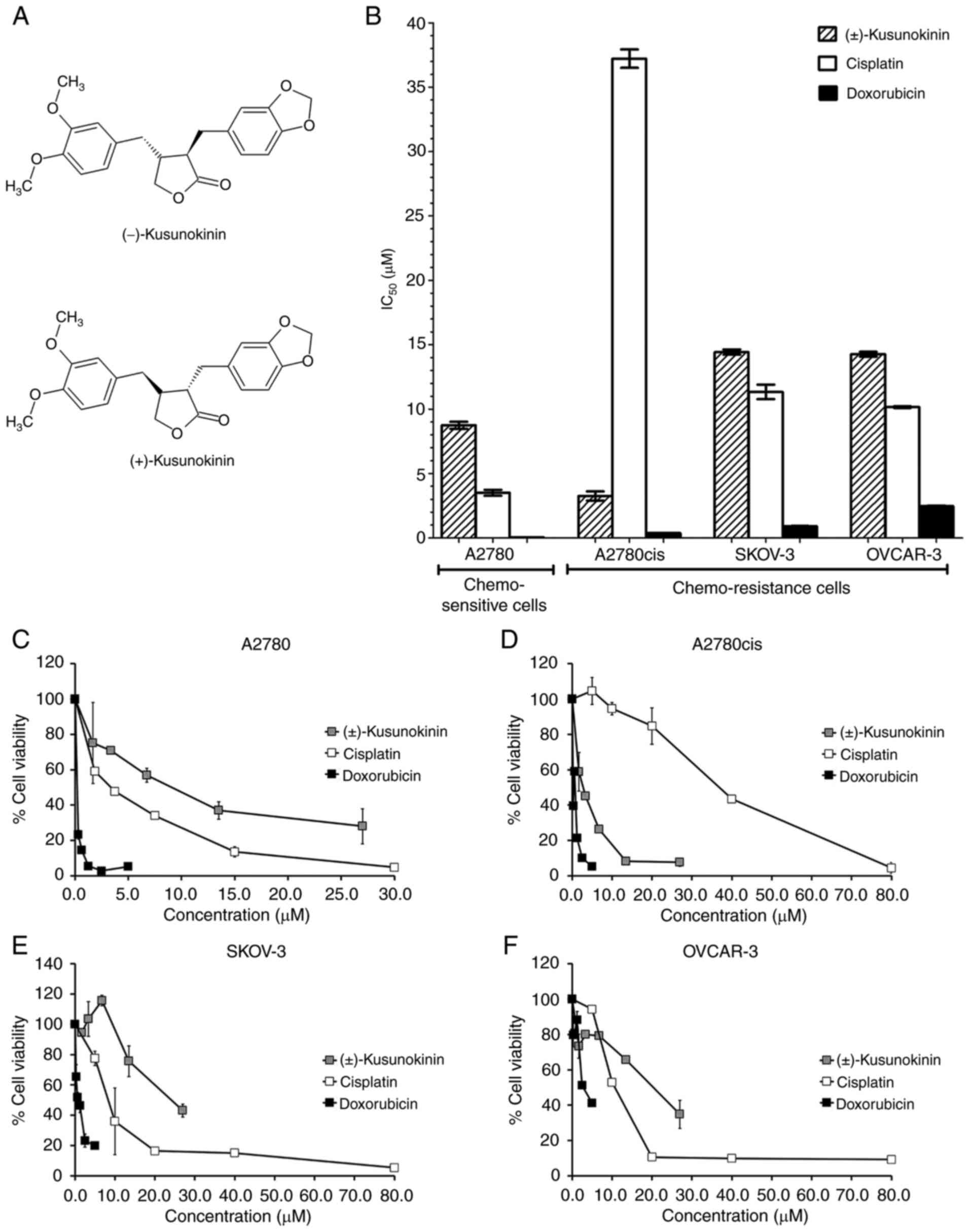
trans-(−)-kusunokinin and trans-(+)-kusunokinin (Fig. 1A), can reduce CSF1R protein
expression and subsequently inhibit AKT and STAT3 activity and the
expression of downstream molecules cyclin D1 and CDK1, leading to
cell cycle arrest at the G2/M phase in MCF-7 cells
(30,31). Additionally, this effective
compound has been demonstrated to decrease Ras, ERK and cyclin B1
expression in breast cancer cells (32).
CSF1R, AKR1B1 and HER2 are overexpressed in ovarian
cancer but not in the normal ovarian surface epithelium (34–36).
In particular, CSF1R and HER2 expression were previously found to
be upregulated upon the induction of cisplatin-resistance in
ovarian cancer cells compared with that in cisplatin-sensitive
cells (34,36). Since trans-(±)-kusunokinin can bind
CSF1R, AKR1B1 and HER2, which causes the inhibition of breast
cancer cell proliferation and induction on programmed cell death,
the present study investigated the potential effects of this
compound on chemosensitive and chemoresistant ovarian cancer
cells.
Materials and methods
Cell culture
SKOV-3 and OVCAR-3 cells were obtained from the
American Type Culture Collection. A2780 and A2780cis cells were
purchased from the European Collection of Authenticated Cell
Cultures and Addexbio Technologies, respectively. A2780 are defined
as chemosensitive (cisplatin-sensitive) cells and as a type of EC
in the epithelial ovarian cancer subtype (37). A2780cis, SKOV-3 and OVCAR-3 are
chemoresistant cells and are classified as EC, CC and HGSC in the
epithelial ovarian cancer subtype, respectively (37,38).
All cells were maintained in RPMI-1640 medium (Thermo Fisher
Scientific, Inc.) with different supplements. Medium for A2780,
A2780cis and SKOV-3 cells was supplemented with 10% FBS (Thermo
Fisher Scientific, Inc.), 1% penicillin/streptomycin (Thermo Fisher
Scientific, Inc.) and 1% L-glutamine (Thermo Fisher Scientific,
Inc.). Medium for OVCAR-3 cells was supplemented with 20% FBS, 1%
penicillin/streptomycin and 1% L-glutamine. For A2780cis cells, 1
µM cisplatin (Sigma-Aldrich; Merck KGaA) was added into RPMI-1640
complete medium every two passages to maintain cisplatin
resistance. All cells were maintained at 37°C in a 5%
CO2 humidified incubator.
Cytotoxicity assay
A2780, A2780cis, SKOV-3 and OVCAR-3 cells were
seeded at 1×104, 2.5×104, 2×104
and 3.7×104 cells/well, respectively, into a 96-well
plate and incubated for 24 h at 37°C with 5% CO2. Next,
cells were treated with the various concentrations of racemic
trans-(±)-kusunokinin (0–26 µM) [synthesis procedure as previously
described (31)], cisplatin (0–80
µM) (Sigma-Aldrich; Merck KGaA) and doxorubicin (0–5 µM;
Sigma-Aldrich; Merck KGaA) for 72 h at 37°C. Cell viability was
then assessed using MTT tetrazolium salt (Invitrogen; Thermo Fisher
Scientific, Inc.). Absorbance of the wells were then detected at
wavelengths of 570 and 650 nm using a Varioskan™ LUX Multimode
Microplate Reader (Thermo Fisher Scientific, Inc.). The cell
viability rate was expressed as a percentage of untreated control
(100% of cell viability), which corresponded to cells treated with
only 0.5% DMSO. The percentage of cell survival was calculated as
previously described (39). The
half maximal inhibitory concentration (IC50) values were
calculated by linear approximation regression of the percentage of
cell survival vs. the compound concentration.
Colony formation assay
A2780 and A2780cis cells were seeded at
1×103 and 2×103 cells/well, respectively,
into a 3.5-cm culture dish and incubated for 24 h at 37°C with 5%
CO2. Next, the cells were treated with 1X or 2X
IC50 concentration of trans-(±)-kusunokinin and
cisplatin for 72 h at 37°C in a 5% CO2. Medium was then
removed and cells were washed with 1X PBS. Fresh complete medium
was added into the plates for further culture. After 5 days of
incubation at 37°C, colonies were stained with 0.5% crystal violet
solution in 25% methanol (Sigma-Aldrich; Merck KGaA) for 10 min at
room temperature and counted under a light inverted microscope
(Olympus Corporation). A colony was defined as a cluster of ≥50
cells (40). The percentage of
colonies was calculated using the following formula: Colonies
(%)=(Number of colonies in treatment group/Number of colonies in
control) ×100.
Apoptosis assay and multi-caspase
activation assay
To investigate the effects of trans-(±)-kusunokinin
on apoptosis and caspase activity in ovarian cancer cells, A2780
and A2780cis cells were seeded into a 12-well plate at cell
densities of 1.5×105 and 2×105 cells per
well, respectively. A2780 and A2780cis cells were treated with the
IC50 concentration of trans-(±)-kusunokinin and
incubated at 37°C. Next, treated cells were harvested at 0, 24, 48
and 72 h.
For apoptosis assay, 2.5×104 treated
cells were stained with 50 µl Muse® Annexin V & Dead
Cell reagent (Muse® Annexin V & Dead Cell kit, cat.
no. MCH100105; EMD Millipore) and incubated at room temperature for
20 min. Apoptotic cells were analyzed using Muse® Cell
Analyzer (EMD Millipore) and results were analyzed by Muse 1.8
analysis software (30). The
low-left quadrant represented the live cells that were not stained
with Annexin V or 7-aminoactinomycin D (7AAD). The low-right
quadrant represented cells in the early stages of apoptosis, which
were stained with only Annexin V. By contrast, the upper-right
quadrant represented cells at the late stages of apoptosis, which
were stained with both Annexin V and 7AAD. The upper-left quadrant
represented dead cells (possibly necrotic), where cells were not
stained with Annexin V but were stained with 7AAD.
For multi-measuring caspase activity,
2.5×104 treated cells were incubated in 50 µl
Muse® MultiCaspase working solution at 37°C for 30 min
for multi-measuring caspase activity. Subsequently, 50 µl caspase
7AAD working solution (Muse® MultiCaspase kit; cat. no.
MCH100109; EMD Millipore) was added and the cells were incubated at
room temperature for 5 min. Multi-caspase activity were analyzed
using Muse® Cell Analyzer (EMD Millipore) and results
were analyzed by Muse 1.8 analysis software (30). In this assay, VAD peptide, an
effective pan-caspase (caspase-1, −3, −4, −5, −6, −7, −8, and −9)
inhibitor, bind to the active sites of the caspases, leading to an
increase in fluorescent intensity in the caspase-positive cell
population. 7AAD was used to detect double-stranded DNA
damaged/dead cells. The lower-left, lower-right, upper-right and
upper-left quadrants represented live cells exhibiting caspase
activity, late stages of caspase activity or are dead following
caspase activiation and necrosis cells, respectively.
Western blot analysis
A2780 and A2780cis cells were treated with the
IC50 concentration of trans-(±)-kusunokinin and
harvested at 0, 24, 48 and 72 h after incubation at 37°C. Cell
pellets were suspended in RIPA buffer (Pierce; Thermo Fisher
Scientific, Inc.). Protein concentration of whole cell lysates was
determined using the Bio-Rad Bradford Protein assay (Bio-Rad
Laboratories, Inc.). In total, 50 µg total protein were then
separated by 12% SDS-PAGE and transferred onto nitrocellulose
membranes (EMD Millipore). Next, membranes were blocked in 5%
non-fat dry milk dissolve in 1X Tris-buffered saline, 0.5% Tween 20
(TBST) for 1 h at room temperature. Afterwards, the membranes were
incubated with primary antibodies against topoisomerase IIα
(1:1,000; rabbit monoclonal antibody; cat. no. 12286; Cell
Signaling Technology, Inc.), cyclin D1 (1:500; rabbit polyclonal
antibody; cat. no. 2922; Cell Signaling Technology, Inc.), CDK1
(1:500; mouse monoclonal antibody; cat. no. sc-53219; Santa Cruz
Biotechnology, Inc.), Bax (1:500; rabbit polyclonal antibody; cat.
no. 2772; Cell Signaling Technology, Inc.), p53-upregulated
modulator of apoptosis (PUMA; 1:500; rabbit polyclonal antibody;
cat. no. 4976; Cell Signaling Technology, Inc.), CSF1R (1:500;
mouse monoclonal antibody; cat. no. sc-46662; Santa Cruz
Technology, Inc.) and GAPDH (internal control; 1:5,000; mouse
monoclonal antibody, cat. no. CB1001; EMD Millipore) for 3 h at
room temperature. For AKR1B1, the membrane was incubated with
rabbit polyclonal antibody (cat. no. AV48180; Sigma-Aldrich; Merck
KGaA) at 4°C overnight. After incubation with topoisomerase IIα,
cyclin D1, CDK1, Bax, PUMA, CSF1R and AKR1B1 antibodies, the
membranes were then incubated with horseradish peroxidase
(HRP)-conjugated goat anti-rabbit (cat. no. 7074; Cell Signaling
Technology, Inc.) or horse anti-mouse IgG antibodies (cat. no.
7076; Cell Signaling Technology, Inc.) at dilution 1:2,500 for 1 h
at room temperature. HRP-conjugated horse anti-mouse IgG antibody
at dilution 1:10,000 was used to detect GAPDH and incubated for 1 h
at room temperature. Immunoreactive bands were detected with
SuperSignal™ West Dura Extended Duration substrate kit (Thermo
Fisher Scientific, Inc.). The intensity of band was analyzed using
the ImageJ software (version 1.5.3; National Institutes of
Health).
Statistical analysis
All data were expressed as the mean ± standard
deviation. Data from the two groups was assessed by the unpaired
Student's t-test, while one-way analysis of variance followed by
Bonferroni's post hoc test (GraphPad Prism8; GraphPad Software,
Inc.) was used to analyze the data in multiple groups. P<0.05
was considered to indicate a statistically significant difference.
All results were determined in ≥3 independent experiments.
Results
Effects of trans-(±)-kusunokinin on
the viability of ovarian cancer cells
To evaluate the effects of trans-(±)-kusunokinin on
chemosensitive (A2780) and chemoresistant (A2780cis, SKOV-3 and
OVCAR-3) ovarian cancer cell lines, the cytotoxicity of
trans-(±)-kusunokinin was detected using MTT assay.
Chemotherapeutic drugs cisplatin and doxorubicin were used as a
positive control. The IC50 values of
trans-(±)-kusunokinin, cisplatin and doxorubicin of the ovarian
cancer cell lines are shown in Fig.
1B. Trans-(±)-kusunokinin exhibited a stronger cytotoxic effect
on A2780cis cells compared with that by cisplatin. In addition,
trans-(±)-kusunokinin exerted the strongest levels of cytotoxicity
against A2780cis (IC50, 3.25±0.62 µM) and A2780
(IC50, 8.75±0.47 µM) cells, which were used as
representatives of chemoresistant and chemosensitive ovarian cancer
cells, respectively. Trans-(±)-kusunokinin showed a cytotoxic
effect on SKOV-3 and OVCAR-3 cells with IC50 values of
14.43±0.34 and 14.26±0.32 µM, respectively. Nevertheless,
trans-(±)-kusunokinin did not show cytotoxicity stronger than
doxorubicin in A2780, A2780cis, SKOV3 and OVCAR-3 cells.
Trans-(±)-kusunokinin also markedly decreased cell viability in all
cell lines tested in a dose-dependent manner (Fig. 1C-F).
Trans-(±)-kusunokinin treatment
inhibits colony formation
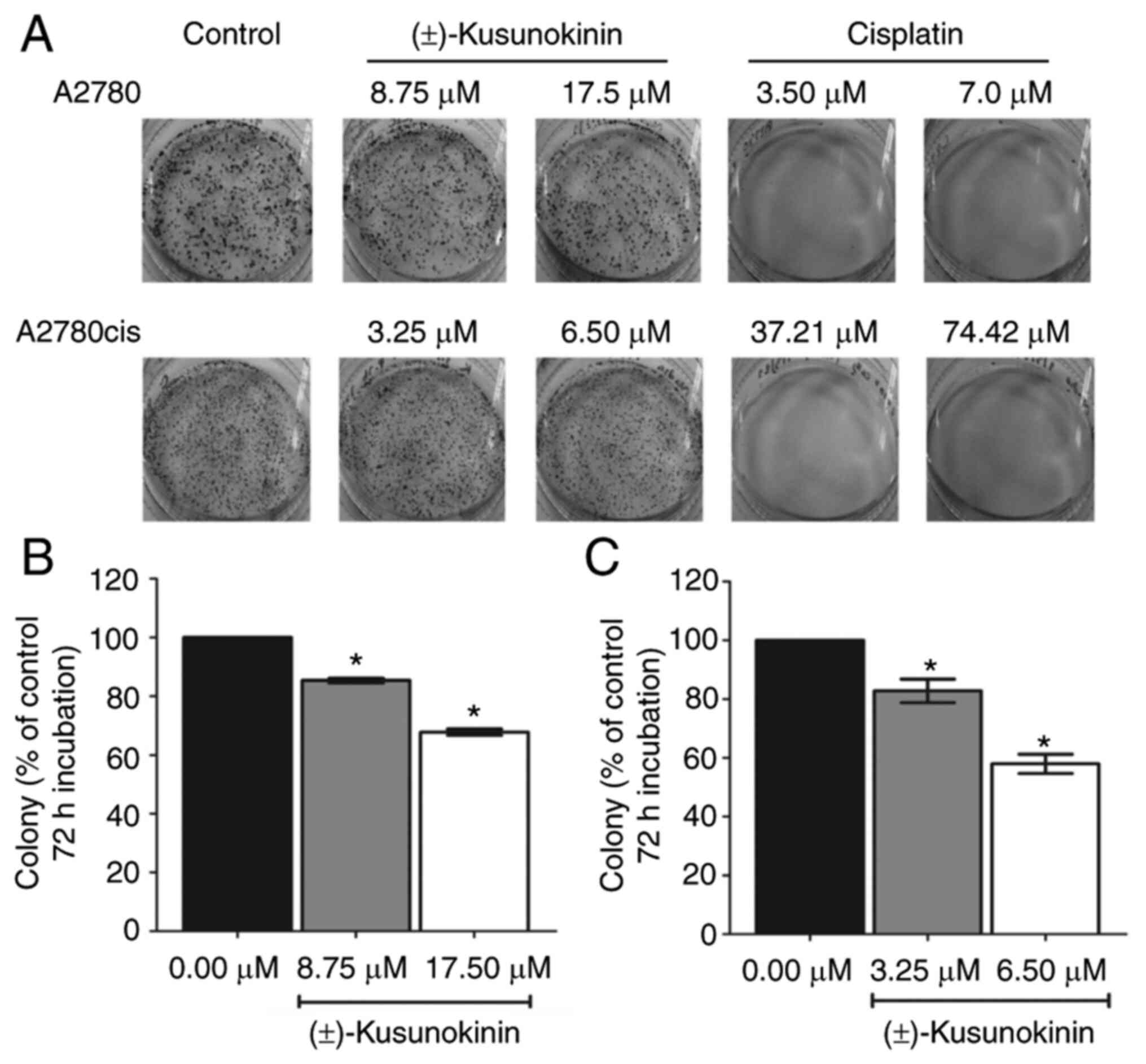
In the present study, the effects of
trans-(±)-kusunokinin on the capacity of the single-cell
proliferation by A2780 and A2780cis cells were next investigated.
The result showed that both IC50 and 2X IC50
concentrations of trans-(±)-kusunokinin significantly inhibited
colony formation by A2780 cells compared with that in the
corresponding control group (Fig. 2A
and B). In addition, IC50 and 2X IC50
concentrations of trans-(±)-kusunokinin significantly inhibited
colony formation by A2780cis cells compared with that in the
corresponding control group (Fig. 2A
and C). However, treatment with both IC50 and 2X
IC50 concentrations of cisplatin almost completely
inhibited the formation of colonies by A2780 and A2780cis cells
(Fig. 2A).
Trans-(±)-kusunokinin induces
apoptosis on both chemoresistant and chemosensitive ovarian cancer
cells
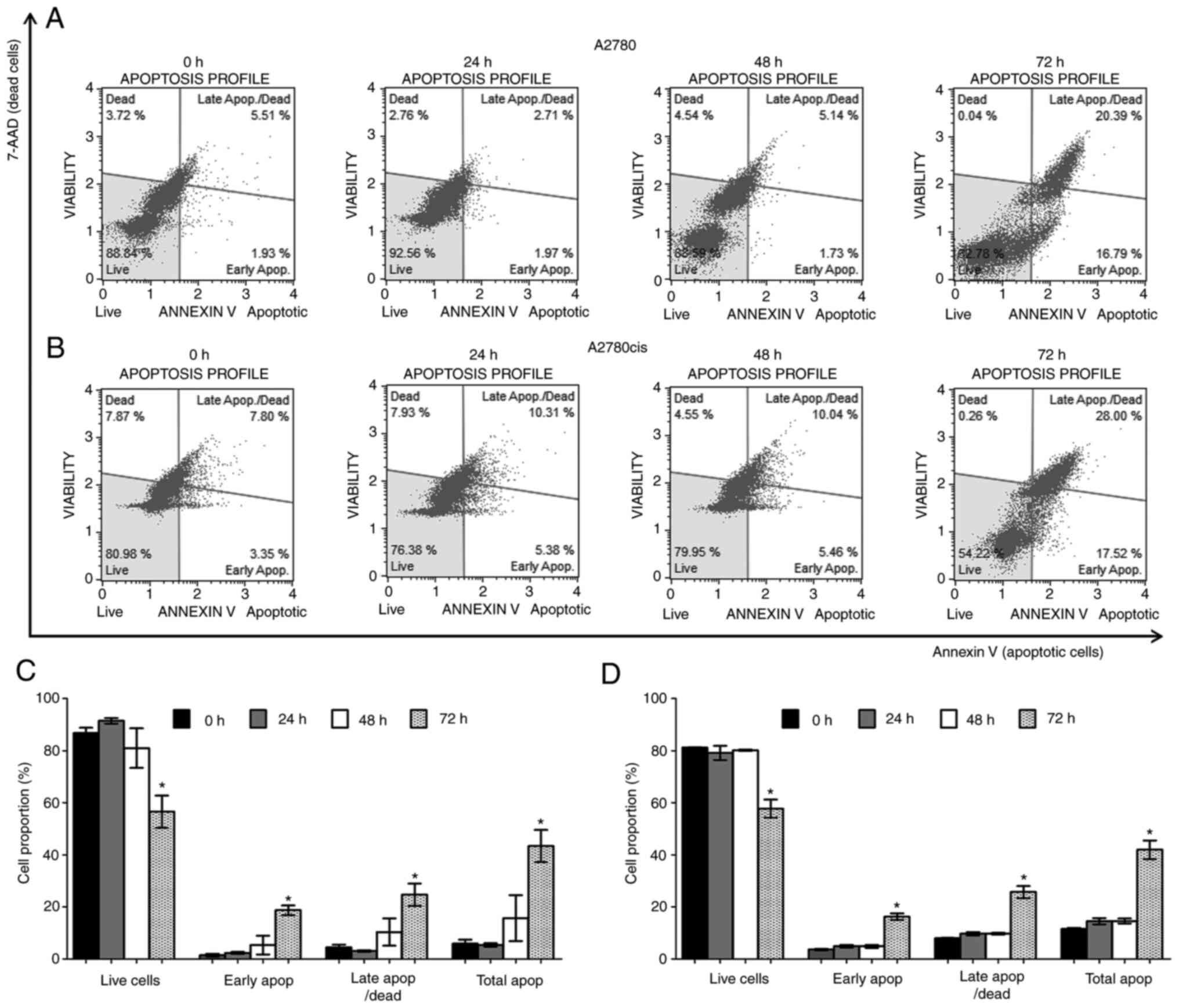
To investigate if the inhibition of cell
proliferation of trans-(±)-kusunokinin was associated with
apoptosis, A2780 and A2780cis cells were incubated with the
IC50 concentration of (±)-kusunokinin at 8.75 and 3.25
µM, respectively. The proportion of apoptotic cells was determined
using Annexin V-FITC staining assay. Double-stranded DNA of damaged
or dead cells was quantified using 7AAD, a fluorescent
intercalator. Results showed that trans-(±)-kusunokinin
significantly decreased the proportion of live cells at 72 h
compared with that in non-treated cells (0 h) for both A2780
(Fig. 3A and C) and A2780cis cells
(Fig. 3B and D). In addition,
trans-(±)-kusunokinin significantly increased the number of early
apoptotic, late apoptotic/dead and total apoptotic A2780 and
A2780cis cells at 72 h compared with that of non-treated cells (0
h; Fig. 3C and D). In total,
>10% of apoptotic cells were found on non-treated A2780cis cells
at 0, 24, 48 and 72 h. However, trans-(±)-kusunokinin significantly
decreased the number of live cells and increased the number of
early apoptotic, late apoptotic/dead and total apoptotic cells at
72 h compared with non-treated cells (72 h) (Fig. S1).
Trans-(±)-kusunokinin enhances
multi-caspases activity
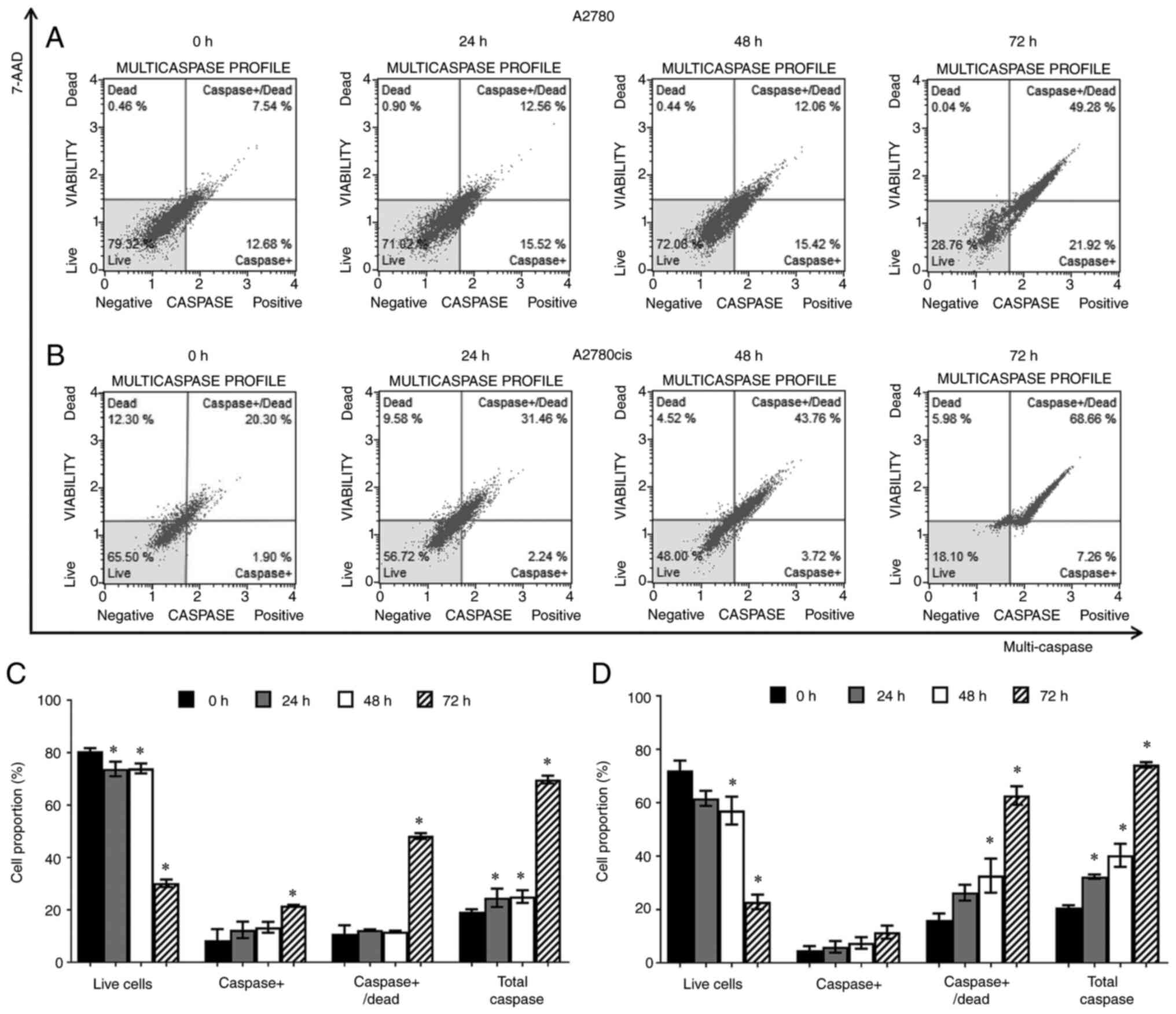
To verify the effects of trans-(±)-kusunokinin on
the apoptotic mechanism, a multi-caspase activity assay (analyzing
caspases −1, −3, −4, −5, −6, −7, −8 and −9) was performed. A2780
and A2780cis cells were incubated with 8.75 and 3.25 µM of
trans-(±)-kusunokinin, respectively, for 24, 48 and 72 h. The
results showed that compared with that in the 0 h group,
trans-(±)-kusunokinin significantly decreased the percentage of
live A2780 (Fig. 4A and C) and
A2780cis (Fig. 4B and D) cells in
a time-dependent manner, but especially at 72 h. In addition,
trans-(±)-kusunokinin increased the percentage of caspase+,
caspase+/dead and total caspase A2780 and A2780cis cells in a
time-dependent manner compared with that in the control 0 h group,
especially at 72 h (Fig. 4C and
D). In total, >10% of caspase+/dead and total caspase cells
were found in the non-treated A2780 and A2780cis groups at 0, 24,
48 and 72 h. Nevertheless, trans-(±)-kusunokinin significantly
decreased the percentage of live cells and increased the percentage
of caspase+/dead and total caspase at 72 h compared with
non-treated cells (72 h). Moreover, the percentage of caspase+,
caspase+/dead and total caspase A2780cis cells were also increased
in a time-dependent manner compared with non-treated cells at 24 to
72 h (Fig. S2).
Trans-(±)-kusunokinin suppresses the
expression of proteins associated with cell proliferation
CSF1R and AKT, proliferation proteins, were found at
significantly higher levels in A2780cis cells compared with A2780
cells (Fig. S3). Due to the
action of trans-(±)-kusunokinin on the inhibition of colony
formation on ovarian cancer cells, the expression of cyclin D1,
CDK1 and topoisomerase II, proteins associated with cell
proliferation (41,42), were determined using western blot
analysis. Chemosensitive (A2780) and chemoresistant (A2780cis)
ovarian cancer cells were treated with 8.75 and 3.25 µM
trans-(±)-kusunokinin, respectively, for 24, 48 and 72 h. The
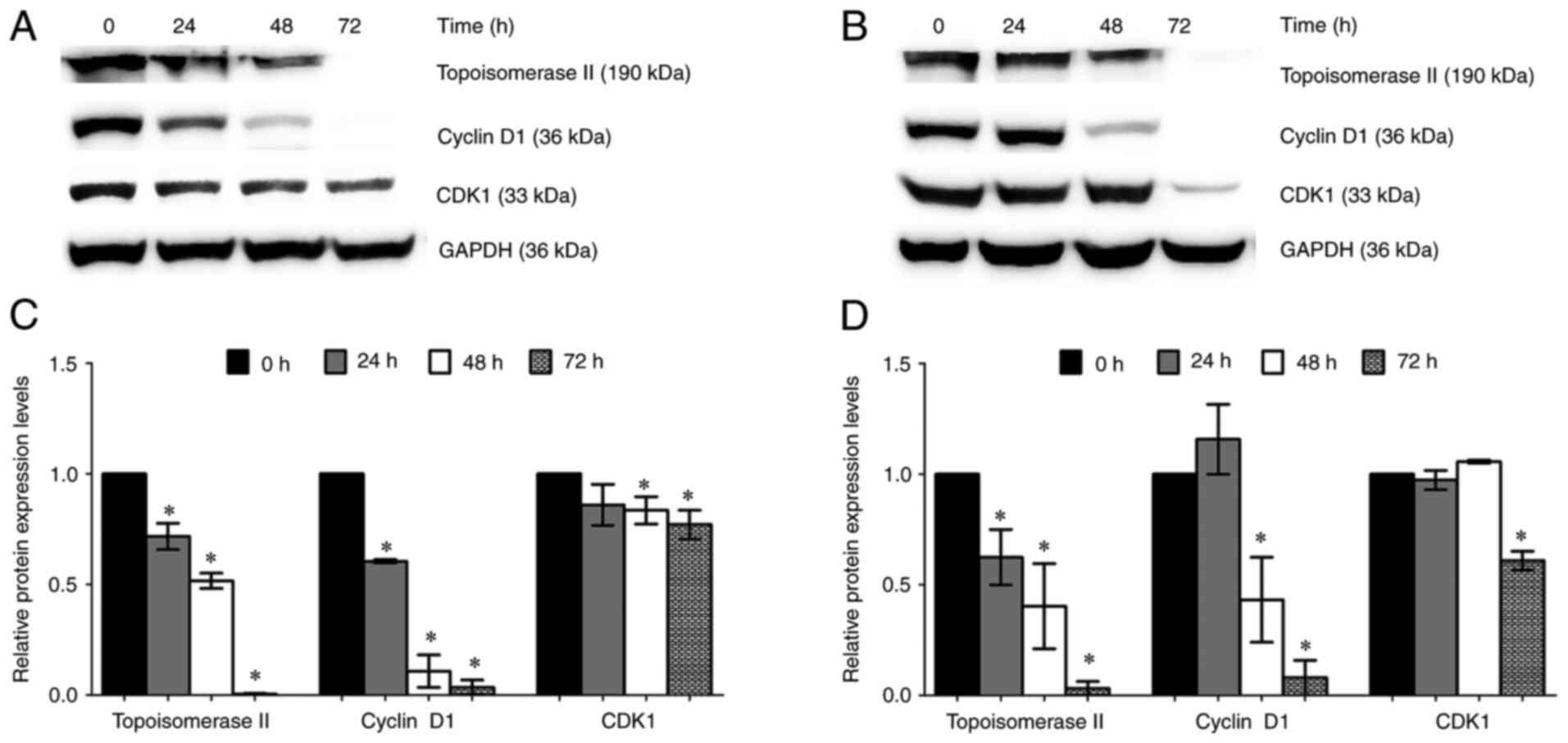
results showed that topoisomerase II and cyclin D1 expression was
significantly decreased in A2780 cells at 24, 48 and 72 h compared
with that in cells in the 0 h control group (Fig. 5A and C). In addition,
trans-(±)-kusunokinin significantly decreased topoisomerase II and
cyclin D1 expression at 48 and 72 h in A2780cis cells compared with
that in cells in the 0 h control group (Fig. 5B and D). CDK1 expression was
significantly suppressed by trans-(±)-kusunokinin treatment at 72 h
in both ovarian cancer cell lines tested (Fig. 5).
Trans-(±)-kusunokinin increases the
expression of apoptotic proteins
To verify the observations of the induction of
apoptosis by trans-(±)-kusunokinin on both chemosensitive (A2780)
and chemoresistant (A2780cis) ovarian cancer cells, proteins
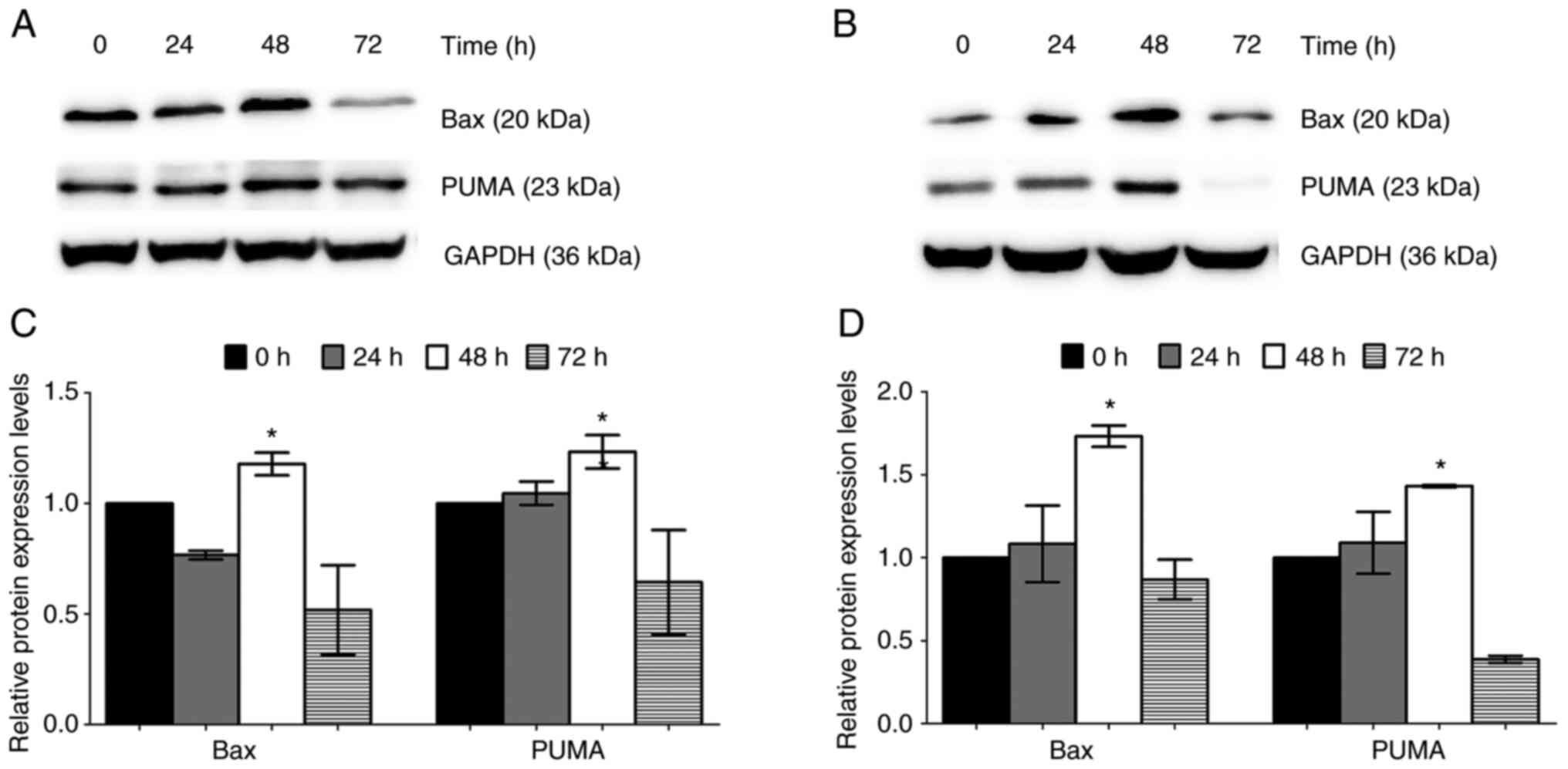
associated with the intrinsic apoptosis pathway Bax and PUMA were
investigated. After treatment with the IC50
concentration of trans-(±)-kusunokinin, Bax and PUMA expression was
significantly increased at 48 h after the treatment of A2780 and
A2780cis cells compared with that in the 0 h control group.
However, Bax and PUMA were downregulated at 72 h in both A2780 and
A2780cis cells (Fig. 6).
Discussion
Trans-(−)-kusunokinin can be extracted from black
pepper (P. nigrum) and has been reported to inhibit breast
(MCF-7, MDA-MB-468 and MDA-MB-231), colon (HT-29 and SW-620) and
lung (A-549) cancer cells (26,29).
In addition, the synthetically derived trans-(±)-kusunokinin has
also been found to inhibit breast cancer (MCF-7, MDA-MB-468 and
MDA-MB-231), colon cancer (HT-29) and cholangiocarcinoma (KKU-M213
and KKU-K100) cells (30). In the
present study, the potential effect of synthetic
trans-(±)-kusunokinin on cisplatin-sensitive (A2780) and
cisplatin-resistant (A2780cis, SKOV-3 and OVAR-3) ovarian cancer
cells was investigated. It was first found that
trans-(±)-kusunokinin exerted particularly potent cytotoxic effects
against A2780cis, even to a higher extent compared with A2780
cells. Ovarian cancer cells were previously demonstrated to exhibit
higher expression levels of CSF1R, AKR1B1 and HER2 compared with
those in the normal ovarian surface epithelium (34–36).
High expression levels of CSF1R and HER2 promote
cisplatin-resistance in ovarian cancer cells (34,36).
EC exhibits a number of genetic features, including the
overexpression of K-ras, HER2 and β-catenin genes and dysfunctions
in PTEN and p53 gene expression (43). Proposed targets for EC therapy
include mTOR, AKT, PI3K, MEK, HER2, VEGF, receptor tyrosine
kinases, CSF1R and PARP (44).
Trans-(±)-kusunokinin acts on a variety of proteins that have been
previously linked to the genetic features of EC, including CSF1R,
AKT, PI3K, RAS and HER2 (29,31–33).
For colony formation, A2780 and A2780cis cells were
incubated with the trans-(±)-kusunokinin for 72 h. Ideally, a
colony should be defined to be >50 cells. Cells incubated with
the IC50 concentrations of trans-(±)-kusunokinin
retained their cell division abilities by ~80% in A2780 and
A2780cis cells. However, 2X IC50 concentrations of
trans-(±)-kusunokinin inhibited colony formation in both cell
lines. In addition, cells incubated with cisplatin were unable to
divide (3–5 cells/colony). Therefore, they could not be counted,
since the staining was too weak due to insufficient cells/colonies.
This suggests that trans-(±)-kusunokinin bound to their target
proteins in a reversible manner, such that after this drug was
removed, the cells returned to their proliferative states through
this process was not as efficient. In addition, the cells did not
show 50% inhibition of colony formation because the cells likely
recovered after the withdrawal of trans-(±)-kusunokinin. By
constrast, cisplatin binds the purine bases of DNA irreversibly,
which causes DNA damage, such that even after the drug was removed,
the cells could not recover. Therefore, few cells in the cisplatin
treatment group remained alive. Hence, in conclusion the ovarian
cancer cells treated with trans-(±)-kusunokinin may have recovered
after trans-(±)-kusunokinin removal, which resumed colony formation
activities in both cell lines.
Trans-(±)-kusunokinin was found to inhibit A2780 and
A2780cis cell proliferation through the induction of apoptosis and
multi-caspase activity. However, >10% apoptosis and dead
A2780cis cells were observed even at 0 h (non-treated cell group).
This effect could be due to cell death during experimental
protocol, which has been previously reported (45,46).
Therefore, additional assays on the apoptosis of non-treated (0 h)
cells at 24, 48 and 72 h were performed to verify the effects of
trans-(±)-kusunokinin on apoptosis at 72 h (Fig. S1). It was observed that >10%
caspase+/dead A2780 and A2780cis cells were also seen at 0 h
(non-treated cell group). This effect may be due to apoptosis
occurring during the experimental process. These results were
previously reported (47–49). Therefore, measurements of
multi-caspase activity in non-treated cells at 24, 48 and 72 h were
performed, which served as an internal control to assess the
function of trans-(±)-kusunokinin on multi-caspase activity at 72 h
(Fig. S2).
In total there are five mechanisms that contribute
to cisplatin resistance: Decreased drug import; increased drug
export; increased drug inactivation by detoxification enzymes;
increased DNA damage repair; and inactivated cell death signaling
(50). Trans-(±)-kusunokinin may
be involved in all key drug resistance mechanisms through the
suppression of CSF1R, AKT, ERK, c-Myc, STAT3 and Bcl-2 signaling,
which was previously reported (29–30).
Overexpression of copper transporter 1 (CTR1) has been found to
increase cisplatin uptake. Therefore, low expression levels of CTR1
promote ovarian cancer resistance to platinum-based drugs (51). Specificity protein (Sp1) is a
transcription factor that can upregulate the expression of CTR1
(52). By contrast, Sp1 can also
be suppressed by c-Myc (53).
c-Myc is involved in the response to oxidative stress. This protein
can activate glutathione-directed survival pathways, which are
involved in cellular detoxification, redox balance and stress
response in tumor cells (54,55).
In addition, nuclear factor-erythroid 2 related factor 2 (Nrf2) has
been found to regulate the expression of AKR1B1 (56). The AKR1B1 enzyme serves an
important role in drug detoxification and can regulate the
development and progression of breast and ovarian cancers (50,56).
Suppression of CSF1R expression by trans-(±)-kusunokinin leads to
the reduction of AKT, ERK and STAT3 signaling, followed by the
reduced expression of Bcl-2, which is associated with the
activation of cell death (55,57,58).
Trans-(±)-kusunokinin may also reverse the mechanism
of doxorubicin resistance through the suppression of AKT in MCF-7
cells (31). Doxorubicin-resistant
cells tend to overexpress Nrf2, which then suppresses ROS
production in cells to negate the effects of doxorubicin (59). One potential upstream signaling
component of Nrf2 was previously found to be AKT (60). Therefore, we hypothesize that the
reduction of CSF1R and AKT may lead to reduced Nrf2 expression.
In the present study, trans-(±)-kusunokinin
inhibited topoisomerase II, cyclin D1 and CDK1 expression,
consistent with a previous finding (30,31).
Both natural trans-(−)-kusunokinin and synthetic
trans-(±)-kusunokinin were previously found to downregulate
topoisomerase II, CDK1 and cyclin D1 in breast cancer cells (MCF-7)
(26,29–31).
These results support the findings from the present study that
trans-(±)-kusunokinin inhibited ovarian cancer cell proliferation
through suppressing the expression of proteins involved in cell
proliferation. Lignan-based compounds, such as daurinol and
(−)-hinokinin, has been found to decrease topoisomerase II and
cyclin D1 expression in ovarian (SNU-840) and breast (MCF-7 and
SKBR-3) cancer cells, respectively (17,28).
During cell proliferation, cyclin D1 and CDKs are downstream
proteins in the CSF1R pathway (61) and are also transcribed by c-Myc
(62). CSF1R translocates into the
nucleus whilst complexed with CSF1 and bind to the promoter region
of cyclin D1, c-Myc and c-Jun (61). Specifically, CSF1R and c-Myc were
previously found to be overexpressed in cisplatin-resistant cells
(SKOV-3/CR, CaoV-3/CR, A2780CP20 and A2780cis), where they served
an important role in the cisplatin resistance mechanism (34,63).
Additionally, activation of the STAT3 signaling pathway is another
potential mechanism in the chemoresistance of ovarian cancer cells
(64). STAT3 is expressed at
higher levels in A2780cis cells compared with A2780 cells (65) and can regulate the expression of
cell cycle (c-Myc and cyclin D1), anti-apoptosis (Bcl-xL, Bcl-2 and
survivin), angiogenesis (VEGF and IL-8) and migration (MMP-2 and
MMP-9) proteins (66). Taken
together, the action of trans-(±)-kusunokinin found in the present
study may have occurred through the suppression of signaling
proteins, especially CSF1R.
The induction of DNA damage by cisplatin induces
apoptosis by activating the intrinsic pathway on ovarian cancer.
p53 is a tumor suppressor protein that responds to DNA damage and
induces apoptotic proteins such as Bax, Bak and PUMA in intrinsic
pathways at the mitochondria (67). In addition, inhibition of
topoisomerase II causes transient breaks in the double-strand DNA
(68). Consequently, ataxia
telangiectasia mutated and ataxia telangiectasia and rad3-related
(a checkpoint protein) can detect the double-stranded breaks in the
DNA and activate checkpoint kinases (CHK)1 and CHK2, in addition to
p53 (69). p53 triggers the
transcription of various apoptotic genes, such as PUMA and
phorbol-12-myristate-13-acetate-induced protein 1. PUMA activates
the pro-apoptotic Bcl-2 family member of proteins, including Bax
and Bak (70). These two proteins
then trigger cytochrome c release from the mitochondria,
followed by the induction of caspases-9 and −3 (71). Topoisomerase II is another
important target for anticancer drugs, including etoposide,
doxorubicin, daunorubicin and mitoxantrone (72). The present study verified the
action of trans-(±)-kusunokinin on the induction of apoptosis,
multi-caspase activity and expression of apoptotic proteins.
Topoisomerase II was found to be decreased at 24 h, followed by
increased PUMA and Bax expression at 48 h (Fig. 6). Bax then induced mitochondrial
dysfunction and caspase activity at 72 h. Consistent with findings
from a previous study, natural trans-(−)-kusunokinin downregulated
topoisomerase II expression whilst upregulating p53 expression at
24 h. The downstream proteins of p53, including p21, Bax,
cytochrome c, cleaved caspases-7 and −8, were sequentially
activated (26). Synthetic
trans-(±)-kusunokinin was also found to induce apoptosis and
multi-caspase activity in breast cancer cells (30). However, the protein levels of PUMA
and Bax were decreased at 72 h, which could be due to the half-life
of the proteins. A previous study showed that Bax expression was
increased at 48 h but was decreased at 72 and 96 h (26). Other natural compounds isolated
from cotton seed (AT101) increased PUMA expression at 12 h but then
decreased at 24 h in A2780cis cells (73).
A2780cis cells have high expression levels of c-Myc
and cyclin D1 expression along with high levels of ERK and AKT
activation, which serve a role in mediating the cisplatin
resistance mechanism (63,74–76).
It was found that CSF1R and AKR1B1 expression was significantly
higher in A2780cis cells compared with that in A2780 cells
(Fig. S3). These results support
the hypothesis on the action of trans-(±)-kusunokinin on
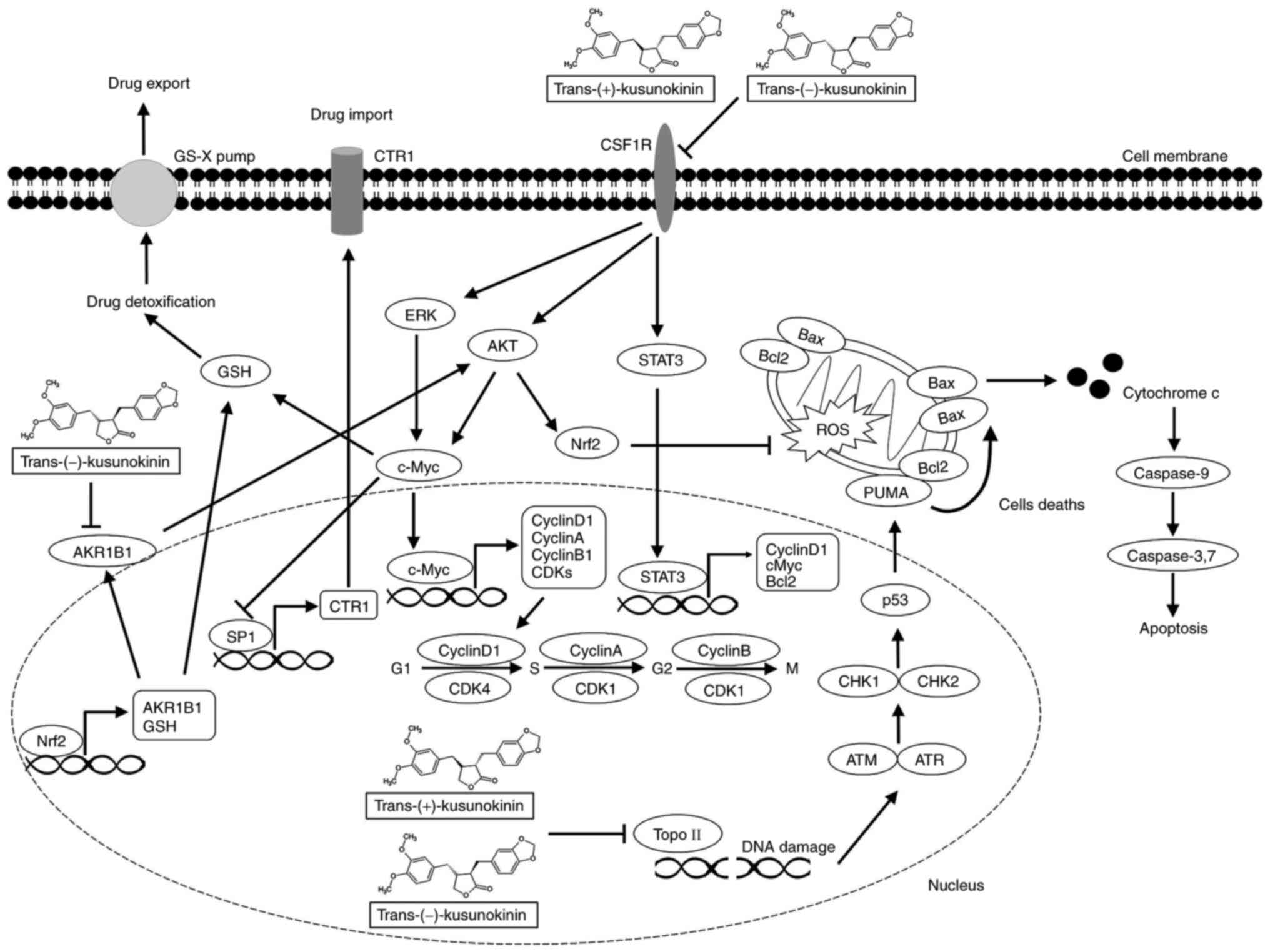
cisplatin-resistant cells. Therefore, the activity of
trans-(±)-kusunokinin in the suppression of cisplatin-resistance in
ovarian cancer cells could be due to its action and binding
activity on the CSF1R and AKR1B1 proteins (Fig. 7). However, the combinatorial
treatment of trans-(±)-kusunokinin with chemotherapeutic drugs
should be evaluated in future studies to confirm the mechanism
underlying drug resistance of ovarian cancer cells. Furthermore, it
is necessary to investigate the underlying molecular mechanisms in
the combination treatment.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present research project was supported by the Agricultural
Research Development Agency (Public Organization; grant no.
CRP6205031700; Bangkok, Thailand), The Graduate scholarship (grant
no. 62-014), Faculty of Medicine, Prince of Songkla University
(Songkla, Thailand), The Research Center for Cancer Control in
Thailand (grant no. MEDRC59036) and Prince of Songkla University
and the Postdoctoral Fellowship, Prince of Songkla University,
Songkhla, Thailand.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Author's contributions
PG contributed to the study conception and design.
NMA performed the majority of the experiments, as well as the
statistical analysis, and drafted the initial manuscript. TR
performed the apoptosis and multi-caspase assays. TT performed the
western blot analysis. PG was responsible for data analysis and
revised the final version of the manuscript. PG and NMA confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Reavis HD and Drapkin R: The tubal
epigenome-An emerging target for ovarian cancer. Pharmacol Ther.
210:1075242020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reid BM, Permuth JB and Sellers TA:
Epidemiology of ovarian cancer: A review. Cancer Biol Med. 14:9–32.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kurman RJ and Shih IeM: Molecular
pathogenesis and extraovarian origin of epithelial ovarian
cancer-shifting the paradigm. Hum Pathol. 42:918–931. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hirst J, Crow J and Godwin A: Ovarian
cancer genetics: Subtypes and risk factors. Ovarian cancer. Pathog
Treat. 1:727052018.
|
|
6
|
Chen X, Wu Y, Dong H, Zhang YC and Zhang
Y: Platinum-based agents for individualized cancer treatment. Curr
Mol Med. 13:1603–1612. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dasari S and Tchounwou PB: Cisplatin in
cancer therapy: Molecular mechanisms of action. Eur J Pharmacol.
740:364–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
van Zyl B, Tang D and Bowden NA:
Biomarkers of platinum resistance in ovarian cancer: What can we
use to improve treatment. Endocr-Relat Cancer. 25:R303–R318. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Oun R, Moussa YE and Wheate NJ:
Correction: The side effects of platinum-based chemotherapy drugs:
A review for chemists. Dalton Trans. 47:78482018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lim HJ and Ledger W: Targeted therapy in
ovarian cancer. Womens Health (Lond). 12:363–378. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Padma VV: An overview of targeted cancer
therapy. Biomedicine (Taipei). 4:192015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Franzese E, Centonze S, Diana A, Carlino
F, Guerrera LP, Di Napoli M, De Vita F, Pignata S, Ciardiello F and
Orditura M: PARP inhibitors in ovarian cancer. Cancer Treat Rev.
73:1–9. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kristeleit R, Shapiro GI, Burris HA, Oza
AM, LoRusso P, Patel MR, Domchek SM, Balmaña J, Drew Y, Chen LM, et
al: A phase I–II study of the oral PARP inhibitor rucaparib in
patients with germline BRCA1/2-mutated ovarian carcinoma or other
solid tumors. Clin Cancer Res. 23:4095–4106. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zálešák F, Bon DJD and Pospíšil J:
Lignans, Neolignans: Plant secondary metabolites as a reservoir of
biologically active substances. Pharmacol Res. 146:1042842019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun J, Wei Q, Zhou Y, Wang J, Liu Q and Xu
H: A systematic analysis of FDA-approved anticancer drugs. BMC Syst
Biol. 11 (Suppl 5):S872017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chuang TC, Hsu SC, Cheng YT, Shao WS, Wu
K, Fang GS, Ou CC and Wang V: Magnolol down-regulates HER2 gene
expression, leading to inhibition of HER2-mediated metastatic
potential in ovarian cancer cells. Cancer Lett. 311:11–19. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kang K, Nho CW, Kim ND, Song D, Park YG,
Kim M, Pan CH, Shin D, Oh SH and Oh HS: Daurinol, a catalytic
inhibitor of topoisomerase IIα, suppresses SNU-840 ovarian cancer
cell proliferation through cell cycle arrest in S phase. Int J
Oncol. 45:558–566. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee K, Ahn JH, Lee KT, Jang DS and Choi
JH: Deoxyschizandrin, isolated from Schisandra berries, induces
cell cycle arrest in ovarian cancer cells and inhibits the
protumoural activation of tumour-associated macrophages. Nutrients.
10:912018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang K, Li LA, Meng YG, You YQ, Fu XY and
Song L: Arctigenin promotes apoptosis in ovarian cancer cells via
the iNOS/NO/STAT3/survivin signaling. Basic Clin Pharmacol Toxicol.
115:507–511. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gözler B, Rentsch D, Gözler T, Ünver N and
Hesse M: Lignans, alkaloids and coumarins from Haplophyllum
vulcanicum. Phytochemistry. 42:695–699. 1996. View Article : Google Scholar
|
|
21
|
Okunishi T, Umezawa T and Shimada M:
Enantiomeric compositions and biosynthesis of Wikstroemia sikokiana
lignans. J Wood Sci. 46:234–242. 2000. View Article : Google Scholar
|
|
22
|
Messiano GB, Vieira L, Machado MB, Lopes
LMX, De Bortoli SA and Zukerman-Schpector J: Evaluation of
insecticidal activity of diterpenes and lignans from Aristolochia
malmeana against Anticarsia gemmatalis. J Agric Food Chem.
56:2655–2659. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sartorelli P, Carvalho CS, Reimao JQ,
Lorenzi H and Tempone AG: Antitrypanosomal activity of a diterpene
and lignans isolated from Aristolochia cymbifera. Planta Med.
76:1454–1456. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kato M, He YM, Dibwe DF, Li F, Awale S,
Kadota S and Tezuka Y: New guaia-type sesquiterpene from
Wikstroemia indica. Nat Prod Commun. 9:1–2. 2014.PubMed/NCBI
|
|
25
|
Morais TR, Costa-Silva TA, Ferreira DD,
Novais BJ, Torrecilhas ACT, Tempone AG and Lago JHG:
Antitrypanosomal activity and effect in plasma membrane
permeability of (−)-bornyl p-coumarate isolated from Piper cernuum
(Piperaceae). Bioorg Chem. 89:1030012019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sriwiriyajan S, Sukpondma Y, Srisawat T,
Madla S and Graidist P: (−)-Kusunokinin and piperloguminine from
Piper nigrum: An alternative option to treat breast cancer. Biomed
Pharmacother. 92:732–743. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Niwa AM, de Paula NA, Vesenick DC, Sartori
D, Maistro EL, Ribeiro LR and Mantovani MS: Evaluation of lignan
(−)-cubebin extracted from Piper cubeba on human colon
adenocarcinoma cells (HT29). J Toxicol Environ Health Part A.
79:92–100. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cunha NL, Teixeira GM, Martins TD, Souza
AR, Oliveira PF, Símaro GV, Rezende KC, Gonçalves Ndos S, Souza DG,
Tavares DC, et al: (−)-Hinokinin induces G2/M arrest and
contributes to the antiproliferative effects of doxorubicin in
breast cancer cells. Planta Med. 82:530–538. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tedasen A, Dokduang S, Sukpondma Y,
Lailerd N, Madla S, Sriwiriyajan S, Rattanaburee T, Tipmanee V and
Graidist P: (−)-Kusunokinin inhibits breast cancer in
N-nitrosomethylurea-induced mammary tumor rats. Eur J Pharmacol.
882:1733112020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rattanaburee T, Thongpanchang T, Wongma K,
Tedasen A, Sukpondma Y and Graidist P: Anticancer activity of
synthetic (±)-kusunokinin and its derivative (±)-bursehernin on
human cancer cell lines. Biomed Pharmacother. 117:1091152019.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rattanaburee T, Tipmanee V, Tedasen A,
Thongpanchang T and Graidist P: Inhibition of CSF1R and AKT by
(±)-kusunokinin hinders breast cancer cell proliferation. Biomed
Pharmacother. 129:1103612020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rattanaburee T, Tanawattanasuntorn T,
Thongpanchang T, Tipmanee V and Graidist P: Trans-(−)-Kusunokinin:
A potential anticancer lignan compound against HER2 in breast
cancer cell lines? Molecules. 26:45372021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tanawattanasuntorn T, Thongpanchang T,
Rungrotmongkol T, Hanpaibool C, Graidist P and Tipmanee V:
(−)-Kusunokinin as a potential aldose reductase inhibitor:
Equivalency observed via AKR1B1 dynamics simulation. ACS Omega.
6:606–614. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yu R, Jin H, Jin C, Huang X, Lin J and
Teng Y: Inhibition of the CSF-1 receptor sensitizes ovarian cancer
cells to cisplatin. Cell Biochem Funct. 36:80–87. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sinreih M, Anko M, Kene NH, Kocbek V and
Rižner TL: Expression of AKR1B1, AKR1C3 and other genes of
prostaglandin F2α biosynthesis and action in ovarian endometriosis
tissue and in model cell lines. Chem Biol Interact. 234:320–331.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Meden H, Marx D, Roegglen T, Schauer A and
Kuhn W: Overexpression of the oncogene c-erbB-2 (HER2/neu) and
response to chemotherapy in patients with ovarian cancer. Int J
Gynecol Pathol. 17:61–65. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tudrej P, Olbryt M, Zembala-Nożyńska E,
Kujawa KA, Cortez AJ, Fiszer-Kierzkowska A, Pigłowski W, Nikiel B,
Głowala-Kosińska M, Bartkowska-Chrobok A, et al: Establishment and
characterization of the novel high-grade serous ovarian cancer cell
line OVPA8. Int J Mol Sci. 19:20802018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Beaufort CM, Helmijr JC, Piskorz AM,
Hoogstraat M, Ruigrok-Ritstier K, Besselink N, Murtaza M, van IJcken
WF, Heine AA, Smid M, et al: Ovarian cancer cell line panel (OCCP):
Clinical importance of in vitro morphological subtypes. PLoS One.
9:e1039882014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sriwiriyajan S, Ninpesh T, Sukpondma Y,
Nasomyon T and Graidist P: Cytotoxicity screening of plants of
genus Piper in breast cancer cell lines. Trop J Pharm Res.
13:921–928. 2014. View Article : Google Scholar
|
|
40
|
Franken NA, Rodermond HM, Stap J, Haveman
J and van Bree C: Clonogenic assay of cells in vitro. Nat Protoc.
1:2315–2319. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hydbring P, Malumbres M and Sicinski P:
Non-canonical functions of cell cycle cyclins and cyclin-dependent
kinases. Nat Rev Mol Cell Biol. 17:280–292. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ali Y and Abd Hamid S: Human topoisomerase
II alpha as a prognostic biomarker in cancer chemotherapy. Tumour
Biol. 37:47–55. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Okuda T, Sekizawa A, Purwosunu Y,
Nagatsuka M, Morioka M, Hayashi M and Okai T: Genetics of
endometrial cancers. Obstet Gynecol Int. 2010:9840132010.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
O'Hara AJ and Bell DW: The genomics and
genetics of endometrial cancer. Adv Genomics Genet. 2012:33–47.
2012.PubMed/NCBI
|
|
45
|
Lee YK, Lim J, Yoon SY, Joo JC, Park SJ
and Park YJ: Promotion of cell death in cisplatin-resistant ovarian
cancer cells through KDM1B-DCLRE1B modulation. Int J Mol Sci.
20:24432019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liu Y, Zhu K, Guan X, Xie S, Wang Y, Tong
Y, Guo L, Zheng H and Lu R: TTK is a potential therapeutic target
for cisplatin-resistant ovarian cancer. J Ovarian Res. 14:1282021.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Magherini F, Fiaschi T, Valocchia E,
Becatti M, Pratesi A, Marzo T, Massai L, Gabbiani C, Landini I,
Nobili S, et al: Antiproliferative effects of two
gold(I)-N-heterocyclic carbene complexes in A2780 human ovarian
cancer cells: A comparative proteomic study. Oncotarget.
9:28042–28068. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mortensen ACL, Mohajershojai T, Hariri M,
Pettersson M and Spiegelberg D: Overcoming limitations of cisplatin
therapy by additional treatment with the HSP90 inhibitor onalespib.
Front Oncol. 10:5322852020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Gorini G, Magherini F, Fiaschi T, Massai
L, Becatti M, Modesti A, Messori L and Gamberi T:
Au2phen and Auoxo6, two dinuclear oxo-bridged gold (III)
compounds, induce apoptotic signaling in human ovarian A2780 cancer
cells. Biomedicines. 9:8712021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chen SH and Chang JY: New insights into
mechanisms of cisplatin resistance: From tumor cell to
microenvironment. Int J Mol Sci. 20:41362019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chen SJ, Kuo CC, Pan HY, Tsou TC, Yeh SC
and Chang JY: Desferal regulates hCtr1 and transferrin receptor
expression through Sp1 and exhibits synergistic cytotoxicity with
platinum drugs in oxaliplatin-resistant human cervical cancer cells
in vitro and in vivo. Oncotarget. 7:49310–49321. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Liang ZD, Long Y, Chen HH, Savaraj N and
Kuo MT: Regulation of the high-affinity copper transporter (hCtr1)
expression by cisplatin and heavy metals. J Biol Inorg Chem.
19:17–27. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Reyes-González JM and Vivas-Mejía PE:
c-MYC and epithelial ovarian cancer. Front Oncol. 11:6015122021.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Benassi B, Fanciulli M, Fiorentino F,
Porrello A, Chiorino G, Loda M, Zupi G and Biroccio A: c-Myc
phosphorylation is required for cellular response to oxidative
stress. Mol Cell. 21:509–519. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sun CY, Nie J, Huang JP, Zheng GJ and Feng
B: Targeting STAT3 inhibition to reverse cisplatin resistance.
Biomed Pharmacother. 117:1091352019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Chen WD and Zhang Y: Regulation of
aldo-keto reductases in human diseases. Front Pharmacol. 3:352012.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kutikhin AG, Yuzhalin AE, Tsitko EA and
Brusina EB: Pattern recognition receptors and DNA repair: Starting
to put a jigsaw puzzle together. Front Immunol. 5:3432014.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wang L, Zhao X, Fu J, Xu W and Yuan J: The
role of tumour metabolism in cisplatin resistance. Front Mol
Biosci. 8:6917952021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Mirzaei S, Zarrabi A, Hashemi F, Zabolian
A, Saleki H, Azami N, Hamzehlou S, Farahani MV, Hushmandi K,
Ashrafizadeh M, et al: Nrf2 signaling pathway in chemoprotection
and doxorubicin resistance: Potential application in drug
discovery. Antioxidants (Basel). 10:3492021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Liu Y, Liu P, Wang Q, Sun F and Liu F:
Sulforaphane attenuates H2O2-induced oxidant
stress in human trabecular meshwork cells (HTMCs) via the
phosphatidylinositol 3-kinase (PI3K)/serine/threonine kinase
(Akt)-mediated factor-E2-related factor 2 (Nrf2) signaling
activation. Med Sci Monit. 25:811–818. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Achkova D and Maher J: Role of the
colony-stimulating factor (CSF)/CSF-1 receptor axis in cancer.
Biochem Soc Trans. 44:333–341. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
García-Gutiérrez L, Delgado MD and León J:
MYC Oncogene contributions to release of cell cycle brakes. Genes
(Basel). 10:2442019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Reyes-González JM, Armaiz-Peña GN, Mangala
LS, Valiyeva F, Ivan C, Pradeep S, Echevarría-Vargas IM,
Rivera-Reyes A, Sood AK and Vivas-Mejía PE: Targeting c-MYC in
platinum-resistant ovarian cancer. Mol Cancer Ther. 14:2260–2269.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Liang F, Ren C, Wang J, Wang S, Yang L,
Han X, Chen Y, Tong G and Yang G: The crosstalk between STAT3 and
p53/RAS signaling controls cancer cell metastasis and cisplatin
resistance via the Slug/MAPK/PI3K/AKT-mediated regulation of EMT
and autophagy. Oncogenesis. 8:592019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Veskimäe K, Scaravilli M, Niininen W,
Karvonen H, Jaatinen S, Nykter M, Visakorpi T, Mäenpää J, Ungureanu
D and Staff S: Expression analysis of platinum sensitive and
resistant epithelial ovarian cancer patient samples reveals new
candidates for targeted therapies. Transl Oncol. 11:1160–1170.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Furtek SL, Backos DS, Matheson CJ and
Reigan P: Strategies and approaches of targeting STAT3 for cancer
treatment. ACS Chem Biol. 11:308–318. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Yuan Z, Cao K, Lin C, Li L, Liu HY, Zhao
XY, Liu L, Deng HX, Li J, Nie CL and Wei YQ: The p53 upregulated
modulator of apoptosis (PUMA) chemosensitizes intrinsically
resistant ovarian cancer cells to cisplatin by lowering the
threshold set by Bcl-x(L) and Mcl-1. Mol Med. 17:1262–1274. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Atkin ND, Raimer HM and Wang YH: Broken by
the cut: A journey into the role of topoisomerase II in DNA
fragility. Genes (Basel). 10:7912019. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Turnell AS and Grand RJ: DNA viruses and
the cellular DNA-damage response. J Gen Virol. 93((Pt 10)):
2076–2097. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Strasser A, Cory S and Adams JM:
Deciphering the rules of programmed cell death to improve therapy
of cancer and other diseases. EMBO J. 30:3667–3683. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Tait SW and Green DR: Mitochondrial
regulation of cell death. Cold Spring Harb Perspect Biol.
5:a0087062013. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Skok Ž, Zidar N, Kikelj D and Ilaš J: Dual
inhibitors of human DNA topoisomerase II and other cancer-related
targets. J Med Chem. 63:884–904. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Hu W, Wang F, Tang J, Liu X, Yuan Z, Nie C
and Wei Y: Proapoptotic protein Smac mediates apoptosis in
cisplatin-resistant ovarian cancer cells when treated with the
anti-tumor agent AT101. J Biol Chem. 287:68–80. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Hahne JC, Honig A, Meyer SR, Gambaryan S,
Walter U, Wischhusen J, Häussler SF, Segerer SE, Fujita N, Dietl J
and Engel JB: Downregulation of AKT reverses platinum resistance of
human ovarian cancers in vitro. Oncol Rep. 28:2023–2028. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Noel EE, Yeste-Velasco M, Mao X, Perry J,
Kudahetti SC, Li NF, Sharp S, Chaplin T, Xue L, McIntyre A, et al:
The association of CCND1 overexpression and cisplatin resistance in
testicular germ cell tumors and other cancers. Am J Pathol.
176:2607–2615. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Kielbik M, Krzyzanowski D, Pawlik B and
Klink M: Cisplatin-induced ERK1/2 activity promotes G1 to S phase
progression which leads to chemoresistance of ovarian cancer cells.
Oncotarget. 9:19847–19860. 2018. View Article : Google Scholar : PubMed/NCBI
|