Introduction
Cervical intraepithelial neoplasia (CIN) is an
abnormality in the squamous cells of the cervix that, if left
untreated, may progress to cervical cancer (1). This condition is asymptomatic and is
histopathologically diagnosed when cervical punch biopsies are
taken from women referred to colposcopy because of an abnormal
cervical cytology test detected by screening (1). Depending on how many thirds of the
thickness of the cervical surface layer are affected by the
abnormal cells the severity of CIN is graded as CIN1, CIN2 or CIN3
(2). Treatment is usually offered
to the patients when there is CIN2 or 3 because there is a high
chance of progression to cervical cancer (1).
Cold coagulation is a method of ablation to treat
CIN lesions, and thus, prevent progression to cervical cancer. It
is a method that was first introduced by Semm in 1966 in the United
Kingdom (3). Using this method the
cold coagulator probe is electrically heated to a temperature of
100–120°C, which is then applied to the cervix for 20–45 sec per
application, allowing for the ablation of cervical lesions
(4,5). Compared with excisional methods of
cervical treatment, such as large loop excision of the
transformation zone (LLETZ), there is evidence suggesting that cold
coagulation has comparable cure rates (4,5). In
a systematic review and meta-analysis performed by Dolman et
al (4) in 2014 involving 4,569
women from 13 studies who were treated with cold coagulation for
CIN lesions, the cure rate was found to be ~95% for CIN2+ lesions
(4). In the updated systematic
review and meta-analysis conducted by Randall et al
(5) in 2018 with 6,371 women from
23 studies who were treated with cold coagulation for biopsy-proven
CIN2+ lesions, the cure rate was ~93.8% (5). In addition, there have been reports
that have directly compared cold coagulation with LLETZ cervical
treatment, which demonstrated comparable cytology and virological
cure rates after treatment (6,7).
Previous separate Cochrane database systematic
reviews published in 2015 and 2017 have shown that excisional and
ablative cervical treatment is associated with a significantly
increased risk of miscarriage in the second trimester (1,8).
Furthermore, there is a higher risk of preterm birth for women
treated using excisional methods compared with that in women who
underwent ablative methods, with this risk increasing as the
quantity of cervical tissue removed increases (1,8).
However, neither of these two previous systematic reviews managed
to identify any studies of cold coagulation in the literature to
include in the ablative methods arm of treatment. Other
observational studies have nevertheless shown that cold coagulation
when compared to cervical LLETZ treatment appeared to be more
‘cervix friendly’ in terms of obstetric outcomes in a future
pregnancy, since it was reported to be associated with reduced risk
of spontaneous preterm birth and miscarriage rates (9).
The biological mechanism that leads to increased
spontaneous preterm birth and miscarriage rates after excisional
and ablative cervical treatment remains unclear (10). It has been hypothesized that this
may be associated with the lack of mechanical support due to a
shortened cervix, changes in the immunological properties of the
endocervix and/or alterations in the quality of the healed tissue
of the cervix (11). Furthermore,
there have been efforts to scientifically quantify the cervical
dimensions after cervical treatment, which revealed that pregnancy
outcomes after excisional treatment of the cervix are correlated
with the proportion of cervix removed and the remaining cervical
volume (12–14).
According to the literature, there has only been one
study published in 2011 reporting the healing pattern of the cervix
after excisional treatment for CIN lesions (15). This previous study had a small
sample size (n=17) and showed increased collagen expression in the
regenerated cervical tissues of six women, reduced collagen
expression in five women and an equivocal pattern of collagen
distribution in six women (15).
It was then concluded that there was no overall change in the
collagen distribution during the regeneration process of the cervix
following excisional treatment for CIN, but further research is
necessary with a larger sample size (15).
In the present report, the healing pattern in the
cervix after cold coagulation treatment in two women was presented.
The objective was to map the collagenisation profile of the
thermally-ablated cervix in relation to the healthy deep stromal
structure of the cervix.
Case report
Cases
Case A is a 24-year-old nulliparous woman who is
also a non-smoker and underwent her first ever cervical cytology
screening test, which revealed severe dyskaryosis. In September
2014, she underwent colposcopy at the Shrewsbury and Telford
Hospitals NHS Trust (Telford, UK), revealing a high grade
colposcopic appearance of the cervix, following which two
colposcopically-directed cervical punch biopsies were taken. The
biopsies uncovered CIN3 and she underwent a single cold coagulation
cervical treatment. Her first follow-up cytology test was at 6
months after treatment, which again demonstrated severe
dyskaryosis. Therefore, she subsequently underwent LLETZ cervical
treatment. The histopathology results of the cervical excision
specimen showed CIN3, with dimensions of the excised tissue being
15×10×10 mm. The endocervical and deep lateral margins were clear,
though complete excision at the ectocervical margin could not be
confirmed. The patient is disease-free at follow-up ever since. The
first follow-up cytology test (test of cure) was at 6 months after
LLETZ excision and was cytology negative and HPV negative. The
patient was then discharged to 3-yearly cytology screening. The
last cytology screening test, which was negative, was in October
2021.
Case B is a 30-year-old nulliparous woman who does
not smoke and underwent a cytology screening test, showing severe
dyskaryosis. In January 2014, she underwent colposcopy at the
Shrewsbury and Telford Hospitals NHS Trust which showed that she
had a high grade colposcopic appearance of the cervix, where
subsequently two colposcopically-directed cervical punch biopsies
were taken. Her biopsies also showed CIN3 and she underwent cold
coagulation cervical treatment. Her first follow-up cytology test
was scheduled at 6 months after treatment, but she was pregnant and
her appointment was therefore deferred until 3 months after
childbirth following a term pregnancy. Her cervical cytology
screening test then revealed a negative result but was tested
positive for high-risk human papilloma virus (HPV). She
subsequently underwent colposcopy, demonstrating a low grade
colposcopic appearance of the cervix before two cervical punch
biopsies were taken revealing CIN3. She then had LLETZ cervical
treatment at 18 months after her initial cold coagulation. The
histopathology result of the cervical excision specimen showed
CIN3, with dimensions of the excised tissue being 22×20×10 mm. The
endocervical margins were clear, but complete excision at the
ectocervical margin could not be confirmed. The patient is
disease-free at follow-up ever since. The first follow-up cytology
test (test of cure) was at 6 months after LLETZ excision and was
cytology negative and HPV negative. The patient was then discharged
to 3-yearly cytology screening. The last cytology screening test,
which was negative, was in December 2018.
Both women were fully counselled on the risks and
benefit of ablative compared with excisional techniques. Cold
coagulation treatment was performed in accordance with the National
Health Service Cervical Screening Programme guidance (16,17).
This means that cold coagulation was offered to women with no
suspicion of cervical cancer according to their colposcopy
examination results and those who had cervical punch biopsies taken
prior to treatment. The colposcopy examinations and cervical
treatments in these two women were undertaken by British Society of
Colposcopy and Cervical Pathology (BSCCP) accredited colposcopists
in the colposcopy unit of the Shrewsbury and Telford Hospitals NHS
Trust in England (DP, MU and WPS). The Semm cold coagulator machine
(model 60001; WISAP Medical Technology GmbH) was used and prior to
treatment the cervix was injected with a local anesthetic (Citanest
3% with Octapressin; 2.2 ml/vial). The cold coagulator probe was
then applied to the cervix with a temperature of 110–120°C for a
minimum of 20 sec, where three applications were recorded per each
woman.
Histopathological staining
The histopathological review of the slides was
performed using H&E, Sirius red and van Giessen staining. The
cervical excision tissues of these two women were stored as wax
blocks in routine storage at ambient temperature prior to staining.
The fixation protocol involved the use of 10% neutral buffered
formalin as the fixant, with the duration of fixation being between
24 and 36 h at room temperature. The tissues were embedded in
paraffin. Dehydration was performed with 3×100% xylene dips
followed by 3×100% alcohol dips to visibly clear the wax from the
slide. H&E staining was performed on a Leica Autostainer XL
(Leica Microsystems GmbH) machine at room temperature throughout
and according to the manufacturer's protocol. Normal light
microscopy was used to image the H&E staining at a
magnification of ×2 and ×4.
In the van Giessen protocol, the tissue sections
were fixed, embedded, sectioned, cleared (xylene and alcohol dips
as aforementioned) and then taken to distilled water. Then, they
were treated with 4% aqueous ammonium iron (III) sulphate for 4 min
at room temperature. They were subsequently washed thoroughly with
running tap water and then rinsed with distilled water. They were
stained with Mayers haematoxylin for 4 min at room temperature and
then washed again in running tap water. They were differentiated
with 1% acid alcohol at room temperature for a few seconds as dark
staining happens quickly and is visible. Subsequently, it was
checked microscopically that the nuclei were darkly stained and the
background was clear. They were washed again in running tap water
and rinsed with distilled water. Next, they were stained with Van
Giesson's solution for 90 sec at room temperature and washed with
distilled water. Finally, they were rapidly dehydrated, cleared and
mounted for microscopical review. Dehydration was performed with
3×100% xylene dips followed by 3×100% alcohol dips. Normal light
microscopy was used to image the staining at a magnification of ×2
and ×4.
The Sirius red protocol initially involved de-waxing
the paraffin sections. Dewaxing was performed with 3×100% xylene
dips followed by 3×100% alcohol dips to visibly clear the wax from
the slide. Next, the nuclei were stained with Weigerts haematoxylin
for 8 min at room temperature and then washed for 10 min. The
sections were then stained for 1 h at room temperature in
picro-sirius red solution (Sirius red F3B (C.I. 35782) 0.5 g,
saturated aqueous solution of picric acid 500 ml; Atom Scientific).
Subsequently, they were washed in two changes of acidified water (5
ml acetic acid in 1 l distilled water). Most of the water was
physically removed from the slides by shaking and they were then
dehydrated in three changes of 100% ethanol. Finally, the tissue
sections were cleared in xylene and mounted for microscopical
review. Normal light microscopy was used to image the Sirius red
staining at a magnification of ×2 and ×4.
Clinical observations
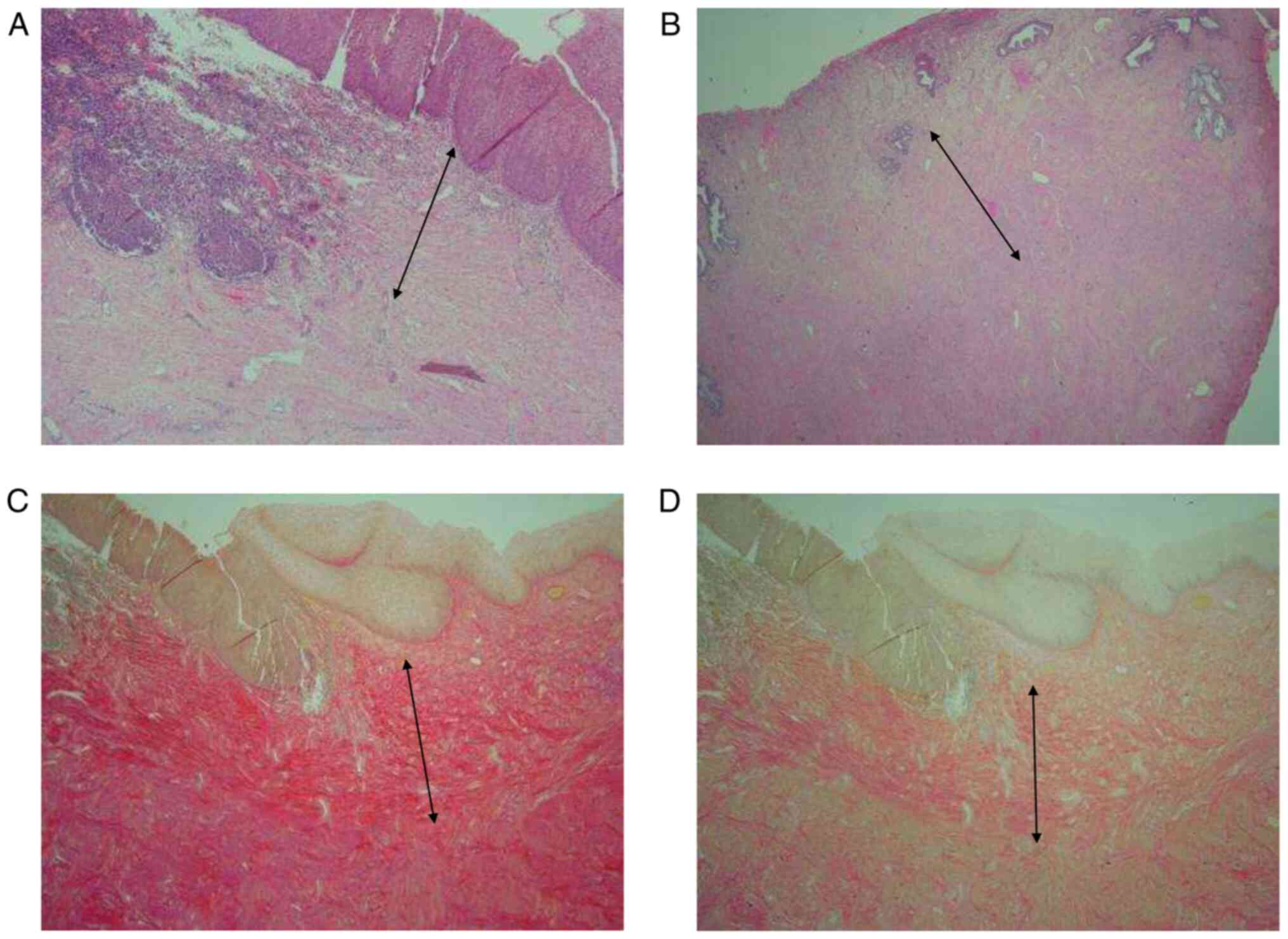
Histopathological analysis showed a well-demarcated
collagenisation of the superficial stroma, which appears to replace
the more loosely organized superficial stroma and stop at the
junction with the healthy muscular stroma (Fig. 1). For cases A and B, the
collagenised superficial stroma depth, which represents the area
that was thermally ablated and has now healed, measured 1.6 and 1.5
mm, respectively. In addition, the normal healthy background
superficial stroma depth was 1.8 and 3.5 mm, respectively. The
collagenisation pattern appeared to be similar between the two
women.
Discussion
The present report described two cases of cold
coagulation treatment of the cervix due to CIN3, where the case A
underwent subsequent LLETZ excision at 7 months after her initial
ablative treatment whereas case B had LLETZ at 18 months after her
initial ablative treatment, since she was pregnant in between. The
excised cervical specimen represents the healed cervix, therefore
histopathological analysis revealed information about the healing
pattern after initial cold coagulation. To the best of our
knowledge, the present study design has been implemented only once
previously by Phadnis et al (15) in 2011, where a histopathological
review was undertaken in women who underwent initial excisional
treatment and then underwent another excisional treatment due to
treatment failure or had a hysterectomy.
The present two cases show that cold coagulation
appears to affect the superficial stroma of the cervix and does not
disrupt the deeper stroma architecture. The collagenised
superficial stroma depth, which represents the area that was
thermally ablated but has now healed, measured 1.6 and 1.5 mm,
respectively. This is a depth that is lower compared with what is
recommended to be removed by LLETZ. Current recommendations state
that cervical excisions should be performed at the depth of ≥7 mm
but <10 mm in reproductive-aged women, since the endocervical
glands extend to a maximum depth of 5.22 mm from the surface of the
cervix (17–19). There have been reports that even
though the colposcopist may opt for the recommended excision depth
of 10 mm, >50% women experienced actual excision depths that
exceeded this limit (9,20,21).
During the cold coagulation procedure no cervical
tissue is removed (4,22). Instead, it is thermally ablated
with reports of an ablation depth of 4–7 mm (4,22).
The two cases in the present report are preliminary
histopathological findings in support of the notion that cold
coagulation does not appear to physiologically disrupt the deep
tissue architecture of the cervix. Therefore, this possibly
explains the reduced rates of adverse obstetric morbidity of these
patients treated because of CIN previously reported (9). It has been repeatedly reported that
there is a ‘dose-response’ effect, where the less cervical tissue
is removed, the lower the risk of obstetric morbidity in a future
pregnancy (23,24).
However, certain limitations should be considered
regarding the findings in the present report. Although the
collagenised superficial stroma depth measured 1.6 and 1.5 mm in
the two cases, after the cervix is thermally ablated a thin,
unquantifiable layer of tissue is always vaporized, which should be
added to the thickness of the healed superficial stroma to provide
the total ablation depth. In addition, the two cases in the present
report were actually unsuccessful in terms of the treatment aim,
similar to the findings of Phadnis et al (15). It is therefore possible that the
deep cervical stroma remains unaltered whilst only the superficial
cervical stroma is collagenized as a result of suboptimal thermal
ablation to a shallow depth. As previously mentioned, a precise
estimate of the total ablation depth is difficult due to the
vaporization of the superficial layer of the cervix during the
thermal ablation. However, cold coagulation can be performed in a
highly standardized manner in the colposcopy unit in the present
report, such that there is no reason to suspect that the treatment
protocols for the two patients were inappropriate. In addition, the
overall cytology cure rates in the present colposcopy unit after
cold coagulation are comparable compared with those after LLETZ
(6). In the two present cases
there were three applications of the cold coagulator onto the
ectocervix, which is the standard procedure frequently applied in
the present colposcopy unit (6).
It has been previously shown in patients treated
with cervical excision that CIN recurrence or treatment failure
does not always depend on the endocervical depth of excision but
other risk factors may play a role, such as the ectocervical margin
involved (20,25). A disadvantage of cold coagulation,
similar to all other ablative methods, is that there is no cervical
specimen to determine the quantity of cervical tissue removed and
to examine the margins of the removed tissue (5). In the present two cases, it was known
that following LLETZ excision the ectocervical margins of the
excision were not clear but were involved by CIN, despite the
relatively large ectocervical surface area excised. The area of
ectocervix that was removed with LLETZ in the present two cases
were 15×10 and 22×20 mm, respectively. This suggests that the
lesion size was relatively widely spread on the ectocervix, which
raises the hypothesis that it may have been the ectocervical
component of the initial thermally-ablated lesion and not the
endocervical depth that is the factor that led to recurrence. If
this hypothesis is correct, then the ablation depth was appropriate
and the deep cervical stromal tissue may not be disrupted by cold
coagulation, thereby explaining the reduced risk of adverse
obstetric outcomes in a future pregnancy compared with that after
LLETZ excision.
Another previous report suggested that the ‘crypt
theory’ could potentially explain the reason for treatment failure
(26). This theory was based on a
small case series of cervical excisions, where it was suggested
that dysplastic cells entrapped deep within the crypts continuously
undergo proliferation and initially remain undetectable by cytology
and colposcopy before they invade the cervical stroma and surface
much later (26). It has also been
suggested that heavy cauterization of the cervical crater post
excision may be another potential culprit of this entrapment
(26). A similar mechanism may
exist in cases of treatment failure after cold coagulation but
further research is warranted to investigate this.
The ideal study design to be considered for future
research would be to include women with a thermally-ablated cervix,
with a test of cure that is cytology and HPV negative at follow-up
to confirm that they are cured. This would indicate that an optimal
thermally ablated depth was reached and then subsequently undergo
hysterectomy for benign reasons. In the present case, the
hysterectomy specimen will represent the healed cervix after the
thermal ablation, where histopathological examination can then be
used to determine the collagenisation pattern in the cervical
stroma. In addition, a separate series of women with a first
excisional cervical treatment could be added to serve as a
reference group to identify any differences on the healing pattern
in the cervix between cold coagulation and cervical excision.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DP conceived and designed this case report. DP, JW,
MU and WPS collected the clinical data. JW performed the
histopathological analysis and provided the related images. DP, JW,
MU and WPS wrote the initial draft of the report and performed
analysis and interpretation of data. DP and JW confirm the
authenticity of all the raw data. All authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patients gave written informed consent for
publication of the case details and associated images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kyrgiou M, Athanasiou A, Kalliala IEJ,
Paraskevaidi M, Mitra A, Martin-Hirsch PP, Arbyn M, Bennett P and
Paraskevaidis E: Obstetric outcomes after conservative treatment
for cervical intraepithelial lesions and early invasive disease.
Cochrane Database Syst Rev. 11:CD0128472017.PubMed/NCBI
|
|
2
|
Fabrizii M, Moinfar F, Jelinek HF,
Karperien A and Ahammer H: Fractal analysis of cervical
intraepithelial neoplasia. PLoS One. 9:e1084572014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Semm K: New apparatus for the
‘cold-coagulation’ of benign cervical lesions. Am J Obstet Gynecol.
95:963–966. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dolman L, Sauvaget C, Muwonge R and
Sankaranarayanan R: Meta-analysis of the efficacy of cold
coagulation as a treatment method for cervical intraepithelial
neoplasia: A systematic review. BJOG. 121:929–942. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Randall TC, Sauvaget C, Muwonge R, Trimble
EL and Jeronimo J: Worthy of further consideration: An updated
meta-analysis to address the feasibility, acceptability, safety and
efficacy of thermal ablation in the treatment of cervical cancer
precursor lesions. Prev Med. 118:81–91. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Papoutsis D, Underwood M, Parry-Smith W
and Panikkar J: Comparison of cure rates in women treated with
cold-coagulation versus LLETZ cervical treatment for CIN2-3 on
pretreatment cervical punch biopsies: A retrospective cohort study.
Arch Gynecol Obstet. 295:979–986. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tadesse WG, Oni AAA and Hickey KPW:
Effectiveness of cold coagulation in treating high-grade cervical
intraepithelial neoplasia: The human papillomavirus evidence of
cure. J Obstet Gynaecol. 39:965–968. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kyrgiou M, Mitra A, Arbyn M, Paraskevaidi
M, Athanasiou A, Martin-Hirsch PP, Bennett P and Paraskevaidis E:
Fertility and early pregnancy outcomes after conservative treatment
for cervical intraepithelial neoplasia. Cochrane Database Syst Rev.
2015:CD0084782015.PubMed/NCBI
|
|
9
|
Papoutsis D, Underwood M, Parry-Smith W
and Panikkar J: Early and late pregnancy outcomes in women treated
with cold-coagulation versus LLETZ cervical treatment for cervical
intraepithelial neoplasia; a retrospective cohort study. Arch
Gynecol Obstet. 297:1015–1025. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Paraskevaidis E, Kyrgiou M and
Martin-Hirsch P: Have we dismissed ablative treatment too soon in
colposcopy practice? BJOG. 114:3–4. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kyrgiou M, Mitra A, Arbyn M, Stasinou SM,
Martin-Hirsch P, Bennett P and Paraskevaidis E: Fertility and early
pregnancy outcomes after treatment for cervical intraepithelial
neoplasia: Systematic review and meta-analysis. BMJ. 349:g61922014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Founta C, Arbyn M, Valasoulis G, Kyrgiou
M, Tsili A, Martin-Hirsch P, Dalkalitsis N, Karakitsos P, Kassanos
D, Prendiville W, et al: Proportion of excision and cervical
healing after large loop excision of the transformation zone for
cervical intraepithelial neoplasia. BJOG. 117:1468–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Papoutsis D, Daskalakis G, Antonakou A,
Rodolakis A, Mesogitis S and Antsaklis A: Sonographic measurement
of cervical volume in nonpregnant women using the geometric formula
for a cylinder versus the three-dimensional automated virtual organ
computer-aided analysis (vocal). J Clin Ultrasound. 39:322–328.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kyrgiou M, Valasoulis G, Stasinou SM,
Founta C, Athanasiou A, Bennett P and Paraskevadis E: Proportion of
cervical excision for cervical intraepithelial neoplasia as a
predictor of pregnancy outcomes. Int J Gynaecol Obstet.
128:141–147. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Phadnis SV, Atilade A, Bowring J, Kyrgiou
M, Young MP, Evans H, Paraskevaidis E and Walker P: Regeneration of
cervix after excisional treatment for cervical intraepithelial
neoplasia: A study of collagen distribution. BJOG. 118:1585–1591.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Luesley D and Leeson S: Colposcopy and
Programme Management. Guidelines for the NHS Cervical Screening
Programme. 2nd edition. NHSCSP Publication No. 20. NHS; Sheffield:
2010
|
|
17
|
NHSCSP, . Colposcopy and Programme
Management. Guidelines for the NHS Cervical Screening Programme.
3rd edition. NHSCSP Publication No. 20. NHSCSP; Sheffield: 2016
|
|
18
|
Anderson MC and Hartley RB: Cervical crypt
involvement by intraepithelial neoplasia. Obstet Gynecol.
55:546–550. 1980.PubMed/NCBI
|
|
19
|
Byrom J, Douce G, Jones PW, Tucker H,
Millinship J, Dhar K and Redman CW: Should punch biopsies be used
when high-grade disease is suspected at initial colposcopic
assessment? A prospective study. Int J Gynecol Cancer. 16:253–256.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ang C, Mukhopadhyay A, Burnley C, Faulkner
K, Cross P, Martin-Hirsch P and Naik R: Histological recurrence and
depth of loop treatment of the cervix in women of reproductive age:
Incomplete excision versus adverse pregnancy outcome. BJOG.
118:685–692. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Papoutsis D, Kandanearachchi P, Antonakou
A, Tzavara C and Sahu B: A method to improve the accuracy between
the presumed depth of excision and the actual depth of excision in
women receiving LLETZ cervical treatment; a single-center,
two-operator experience. Hippokratia. 22:113–121. 2018.PubMed/NCBI
|
|
22
|
Haddad NG, Hussein IY, Blessing K,
Kerr-Wilson R and Smart GE: Tissue destruction following cold
coagulation of the cervix. J Gynecol Surg. 4:23–27. 1988.
View Article : Google Scholar
|
|
23
|
Papoutsis D, Rodolakis A, Mesogitis S,
Sotiropoulou M and Antsaklis A: Regeneration of uterine cervix at 6
months after large loop excision of the transformation zone for
cervical intraepithelial neoplasia. BJOG. 119:678–684. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Castanon A, Landy R, Brocklehurst P, Evans
H, Peebles D, Singh N, Walker P, Patnick J and Sasieni P; PaCT
Study Group, : Risk of preterm delivery with increasing depth of
excision for cervical intraepithelial neoplasia in England: nested
case-control study. BMJ. 349:g62232014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ghaem-Maghami S, Sagi S, Majeed G and
Soutter WP: Incomplete excision of cervical intraepithelial
neoplasia and risk of treatment failure: A meta-analysis. Lancet
Oncol. 8:985–993. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Paraskevaidis E, Athanasiou A, Kalliala I,
Batistatou A, Paraskevaidi M, Bilirakis E, Nasioutziki M,
Paschopoulos M, Lyons D, Arbyn M, et al: Invasive cervical cancer
following treatment of pre-invasive lesions: A potential theory
based on a small case series. Eur J Obstet Gynecol Reprod Biol.
264:56–59. 2021. View Article : Google Scholar : PubMed/NCBI
|