Introduction
Primary liver cancer is the fourth most common
cancer worldwide. HCC occurs due to liver cirrhosis, hepatitis B/C
virus infection, and alcoholic or nonalcoholic steatohepatitis.
Although liver resection has been performed as an effective and
safe treatment in patients with HCC, the possibility of recurrence
remains high (1,2). The use of combination immune
checkpoint inhibitors for unresectable HCC was recently
approved.
Tumor-infiltrating lymphocytes are a major component
of the host anti-tumor immune response. Cluster of differentiation
(CD)3+, CD8+, CD4+, and FoxP3+ T
lymphocytes are the representative subsets of tumor-infiltrating
lymphocytes. With the growing interest in tumor-infiltrating
lymphocytes, an increase in the number of activated cytotoxic T
lymphocytes has been reported to correlate with better survival in
some malignant tumors, including HCC (3–5).
CD8+ T-cell infiltration in tumors plays an important
role in host immunity against tumor progression. Phase III clinical
trials for various immune checkpoint inhibitors and multikinase
inhibitors have been conducted since the approval of sorafenib for
hepatocellular carcinoma in 2009, all of them failed for 10 years
until the emergence of Lenvatinib. The tumor microenvironment in
HCC is complex, due to crosstalk with tumor components, such as
cancer cells, stromal cells, and immune cells. Phenotypic changes
in cancer cell by genetic and epigenetic alternations affect
anti-cancer immunity and cancer-stromal cell interaction, through
the expression of immune checkpoint molecule, cytokines, and growth
factors, which may affect immune system in the tumor (6).
Although dysregulation of the immune system and
uncontrolled inflammatory responses may also contribute to disease
pathology, immune responses are necessary for clearance of
malignant cells, pathogens, and virus-infected cells (7). Myeloid-derived suppressor cells
(MDSCs), which are immature cells, reportedly play important roles
in tumor immune invasion and have a remarkable ability to suppress
T-cell responses (8). Major
factors in MDSCs-mediated immune suppression include expression of
arginase, inducible nitric oxide synthase (iNOS), transforming
growth factor-β, interleukin-10, and cyclooxygenase 2 (9). In particular, the suppressive
activity has been reported to be associated with the metabolism of
L-arginine. L-arginine is a substrate for two enzymes: iNOS, which
generates nitric oxide (NO) and arginase, which converts L-arginine
into urea and L-ornithine (8).
Both of these enzymes has an ability of direct inhabitation for T
cell function (10,11). In addition, vascular endothelial
growth factor (VEGF) is secreted form tumors and causes MDSCs to
accumulate into tumors. VEGF is produced from MDSCs themselves and
is involved in promoting growth of tumor and MDSCs themselves
(12). The attention for
therapeutic strategies for MDSC is increasing. All-trans retinoic
acid induces the differentiation of MDSCs into functional
macrophages and dendric cells (13,14).
Induction of differentiation into functional macrophage and dendric
cells, as antigen-presenting cells, stimulates effector T cells and
enhance the anti-tumor immune response. In the phase IB study, the
treatment with 25-hydroxyvitamin D3 decrease the ratio of
CD34-positive MDSCs in patients with head and neck cancer (15).
In this study, we investigated the
tumor-infiltrating MDSC and CD8+ T-cell status by
immunohistochemistry and evaluated the prognostic impact of
tumor-infiltrating MDSCs and CD8+ T cells in patients
with HCC. Additionally, we clustered the patients with HCC showing
MDSCs and CD8+ T cells and investigated the prognostic
impact of the clustering.
Patients and methods
Patients
In total, 466 patients with HCC who underwent
initial liver resection at the Department of Surgery and Science,
Kyushu University Hospital from January 2004 to November 2018 were
enrolled in this study. The details of our surgical techniques and
patient selection criteria for liver resection in HCC have been
previously reported (16). The
patients were followed up as outpatients every 1 to 3 months after
discharge. Dynamic computed tomography was performed if recurrence
was suspected. Clinical information and follow-up data were
obtained from the medical records. No patients underwent immune
checkpoint inhibitor treatment for recurrence. Informed consents
were obtained. This study was approved by the Ethics Committee of
Kyushu University (approval code 2020-180). An opt-out approach was
employed to obtain informed consent from our patients and personal
information was protected during data collection.
Immunohistochemical staining
Immunohistochemical staining for CD8 was performed
as previously reported (4). The
samples were fixed with 3.7% formaldehyde solution (Sigma-Aldrich)
in room temperature for 24-48 h. Immunohistochemical examinations
were performed on 4-µm formalin-fixed and paraffin-embedded
sections. The sections were first deparaffinized. After inhibition
of endogenous peroxidase activity for 30 min with 3% hydrogen
peroxidase in methanol, in room temperature, the sections were
pretreated with Target Retrieval Solution (Dako) in a microwave
oven at 99°C for 20 or 10 min for CD33 or CD8, respectively, and
then incubated with monoclonal antibodies at 4°C overnight. Immune
complexes were detected with an EnVision Detection System (Dako),
anti-mouse secondary antibody, for 60 min, in room temperature. The
sections were finally incubated in 3,3′-diaminobenzidine, for 7 min
(CD33) and 4 min (CD8), in room temperature, counterstained with
hematoxylin, and mounted. The primary antibodies used were a CD33
mouse antibody (PA0555, no dilution; Leica Biosystems) and a CD8
mouse antibody (ab75129, 1:50; Abcam). Stained slides were scanned
using the NanoZoomer digital slide scanner (Hamamatsu Photonics
K.K.). Immunohistochemical data for CD33 and CD8 staining were
evaluated by three experienced researchers (T.T., S.I. and K.Y.),
who were blinded to the clinical status of the patients. The final
assessments were achieved by consensus. The cells exhibited plasma
membranous staining for CD33.
The number of cells with cytoplasm or membrane
staining in three high-power fields was counted, and we used the
receiver operative characteristic analysis for overall survival
(OS) as the cutoff value of CD33+ infiltrating cells in
tumors. The cutoff value for CD8+ infiltrating cells in
tumors was previously reported (4). CD33+ cells in tumors were
defined as MDSCs in accordance with previous reports (17).
Statistical analysis
Standard statistical analyses were used to evaluate
descriptive statistics, such as medians, frequencies, and
percentages. Continuous variables without a normal distribution and
variables were compared with the Mann-Whitney U test. A logistic
regression analysis was performed to identify variables associated
with MDSC infiltration. Categorical variables were compared using
the χ2 test or Fisher's exact test. Survival data were
used to establish a univariate Cox proportional hazards model.
Covariates that were significant at P<0.05 were included in the
multivariate Cox proportional hazards model. The cumulative OS and
recurrence-free survival (RFS) rates were calculated using the
Kaplan-Meier method, and differences between the curves were
evaluated using the log-rank test. Differences were considered
statistically significant at P<0.05. All statistical analyses
were performed using JMP15 software (SAS Institute Inc.).
Results
MDSCs, CD8+ T cells and
clinicopathological factors
In our cohort of 466 patients with HCC, 344 (73.8%)
patients were male. The median age of the patients was 69 years
(25–75% quantile, 63-76 years). Among all 466 patients, 73 (15.7%)
and 239 (51.3%) showed positive hepatitis B surface antigen and
hepatitis C virus antibody expression, respectively. The median
observation period was 3.69 years (25–75% quantile, 1.99-6.62
years).
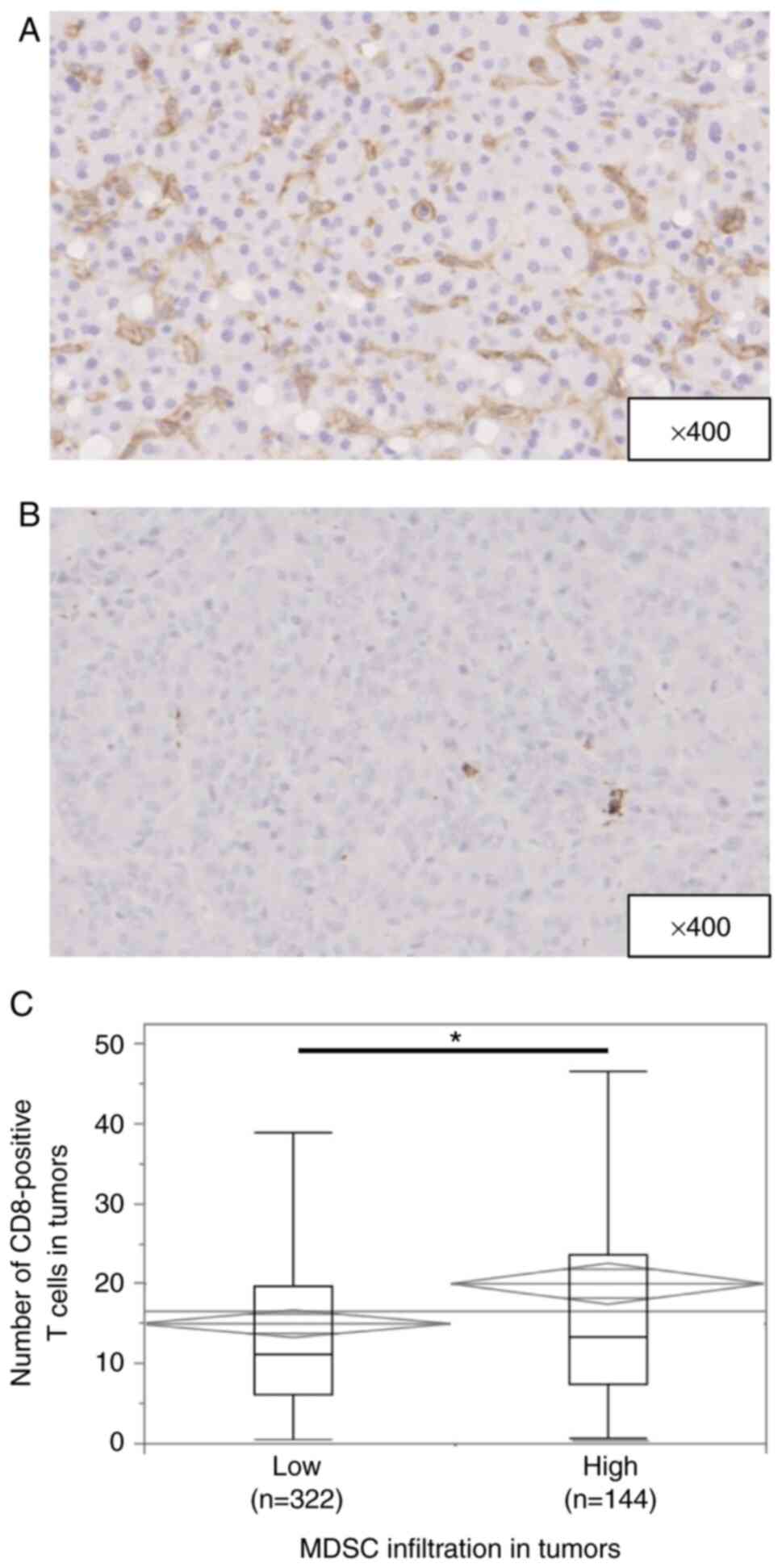
Fig. 1A and B shows
representative immunohistochemical staining of CD33 in HCC tissues.
CD33 expression was observed in the cytoplasm or plasma membrane of
mononuclear cells. The median number of invading CD33+
cells was 73.6 per field (25–75% quantile, 39.6-121 per field).
According to the cut-off value of 108, 144 (30.9%) of 466 patients
had high infiltration of MDSCs. The association between MDSC
infiltration and the patients' clinicopathological characteristics
is shown in Table I. High MDSC
infiltration was observed in patients with larger tumors
(P=0.0216), a poorer Barcelona Clinic Liver Cancer (BCLC) stage
(P=0.0002), more poorly differentiated HCC (P<0.0001), and a
greater presence of microscopic vascular invasion (P=0.0003) and
macroscopic intrahepatic metastasis (P=0.0087).
 | Table I.Association between background
characteristics of patients and intra-tumoral CD33 expression. |
Table I.
Association between background
characteristics of patients and intra-tumoral CD33 expression.
| Variable | CD33 low
(n=322) | CD33 high
(n=144) | P-value |
|---|
| Age, years | 69 (17–87) | 70 (34–86) | 0.5311 |
| Sex (male), n
(%) | 235 (73.0) | 109 (75.7) | 0.5382 |
| BMI,
kg/m2 | 23.0
(16.0-37.9) | 22.5
(14.2-32.6) | 0.8539 |
| HBs-Ag positive, n
(%) | 47 (14.6) | 26 (18.1) | 0.3495 |
| HCV-Ab positive, n
(%) | 166 (51.6) | 73 (50.7) | 0.8640 |
| Child-Pugh
classification, grade B, n (%) | 11 (3.4) | 5 (3.5) | 0.9755 |
| Albumin, g/dl | 4.0 (2.1-5.1) | 3.9 (1.8-4.8) | 0.1193 |
| DCP, mAU/ml | 77 (2–250400) | 109 (9–89477) | 0.9493 |
| AFP, ng/ml | 7.4 (1–693700) | 28.1
(1–994600) | 0.1632 |
| Performing
preoperative TACE or TAE, n (%) | 8 (2.5) | 6 (4.2) | 0.3256 |
| Tumor size, cm | 3.2 (0.9-30) | 3.7 (1–20) | 0.0216 |
| Multiple tumors, n
(%) | 64 (19.9) | 36 (25.0) | 0.2131 |
| BCLC staging (B or
C), n (%) | 45 (14.0) | 42 (29.2) | 0.0001 |
| Gross
classification, single nodular type, n (%) | 208 (65.0) | 80 (55.9) | 0.0633 |
| Poorly
differentiation, n (%) | 80 (24.9) | 63 (43.8) | <0.0001 |
| Microscopic
vascular invasion, n (%) | 82 (25.5) | 61 (42.4) | 0.0003 |
| Microscopic
intrahepatic metastasis, n (%) | 47 (14.6) | 36 (25.0) | 0.0070 |
| F3 or F4, n
(%) | 141 (43.8) | 55 (38.5) | 0.2830 |
Fig. S1A and B
shows representative immunohistochemical staining of CD8 in HCC
tissues. CD8 expression was observed in the cytoplasm or plasma
membrane of mononuclear cells. The median number of invading
CD8+ T cells was 12.0 per field (25–75% quantile,
6.33-21.0 per field). Low CD8+ T-cell infiltration was
observed in male patients and in patients with larger tumors
(P=0.0045), multiple tumors (P=0.0129), a poorer BCLC stage
(P=0.0363), and a greater presence of microscopic vascular invasion
(P=0.0011) and microscopic intrahepatic metastasis (P<0.0001)
(Table SI). The number of
CD8+ T cells in tumors was greater in patients with high
than low MDSC infiltration (P=0.0015) (Fig. 1C).
We also examined the association between MDSC
infiltration and the tumor characteristics. Multivariate analysis
showed that high MDSC infiltration in HCC was significantly
associated with CD8+ T-cell infiltration (P=0.0003) in
addition to poor differentiation (P=0.0068) and microscopic
vascular invasion (P=0.0370) (Table
II).
 | Table II.Univariate and multivariate analyses
of high CD33+ cell infiltration and clinicopathological
factors in patients who underwent hepatic resection for
hepatocellular carcinoma. |
Table II.
Univariate and multivariate analyses
of high CD33+ cell infiltration and clinicopathological
factors in patients who underwent hepatic resection for
hepatocellular carcinoma.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age | 0.99 | 0.98-1.01 | 0.5317 |
|
|
|
| Sex |
|
|
|
|
|
|
| Male
(n=344) | 1.15 | 0.73-1.81 |
|
|
|
|
| Female
(n=122) | 1.00 | (ref.) | 0.5363 |
|
|
|
| HBsAg |
|
|
|
|
|
|
|
Positivity (n=73) | 1.28 | 0.76-2.17 |
|
|
|
|
|
Negativity (n=392) | 1.00 | (ref.) | 0.3544 |
|
|
|
| HCVAb |
|
|
|
|
|
|
|
Positive (n=239) | 0.96 | 0.65-1.43 |
|
|
|
|
|
Negative (n=229) | 1.00 | (ref.) | 0.8640 |
|
|
|
| Alb | 0.71 | 0.46-1.09 | 0.1210 |
|
|
|
| DCP | 1.00 | 1.00-1.00 | 0.9493 |
|
|
|
| AFP | 1.00 | 1.00-1.00 | 0.1820 |
|
|
|
| Tumor size | 1.06 | 1.01-1.12 | 0.0247 | 1.00 | 0.94-1.07 | 0.9683 |
| Macroscopic tumor
numbers |
|
|
|
|
|
|
|
Multiple (n=100) | 1.94 | 1.48-2.55 |
| 0.97 | 0.95-1.87 |
|
| Single
(n=366) | 1.00 | (ref.) | <0.0001 | 1.00 | (ref.) | 0.9347 |
| Poorly
differentiated |
|
|
|
|
|
|
| Present
(n=143) | 2.34 | 1.55-3.55 |
| 1.88 | 1.19-2.97 |
|
| Absent
(n=322) | 1.00 | (ref.) | <0.0001 | 1.00 | (ref.) | 0.0068 |
| Tumor types |
|
|
|
|
|
|
|
Boundary type (n=288) | 1.00 | (ref.) |
|
|
|
|
|
Nonboundary type (n=175) | 1.46 | 0.98-2.19 | 0.0646 |
|
|
|
| Microscopic
vascular invasion |
|
|
|
|
|
|
| Present
(n=143) | 2.15 | 1.42-3.26 |
| 1.67 | 1.03-2.70 |
|
| Absent
(n=323) | 1.00 | (ref.) | 0.0003 | 1.00 | (ref.) | 0.0370 |
| Microscopic
intrahepatic metastasis |
|
|
|
|
|
|
| Present
(n=83) | 1.94 | 1.19-3.17 |
| 1.73 | 0.896-3.34 |
|
| Absent
(n=382) | 1.00 | (ref.) | 0.0076 | 1.00 | (ref.) | 0.1022 |
| Fibrosis |
|
|
|
|
|
|
| F0-2
(n=269) | 1.00 | (ref.) |
|
|
|
|
| F3, 4
(n=196) | 0.80 | 0.54-1.20 | 0.2818 |
|
|
|
| CD8-positive cell
infiltration | 1.02 | 1.01-1.03 | 0.0021 | 1.02 | 1.01-1.04 | 0.0003 |
Univariate and multivariate analyses
of prognostic factors for RFS and OS
We assessed the association between MDSC
infiltration and patient postoperative survival using the
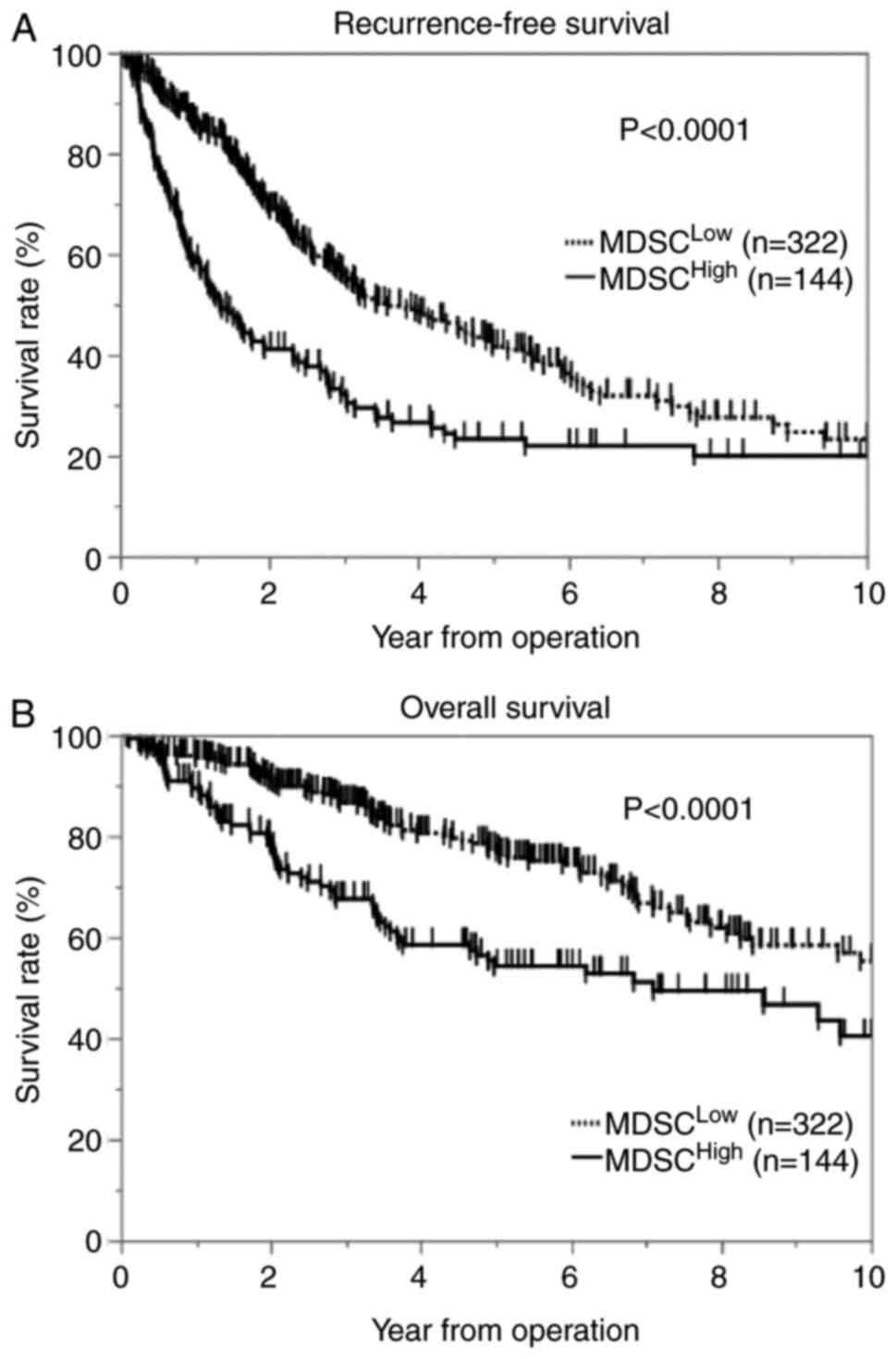
Kaplan-Meier method. The results showed that patients with high
MDSC infiltration in tumors had significantly shorter RFS (log-rank
P<0.0001) and OS (log-rank P<0.0001) after surgery than
patients with low MDSC infiltration (Fig. 2A and B). Next, we assessed the
association between CD8+ T-cell infiltration and patient
postoperative survival using the Kaplan-Meier method. The results
showed that patients with CD8+ T-cell infiltration in
tumors had significantly shorter RFS (log-rank P<0.0001) and OS
(log-rank P<0.0001) after surgery than patients with high
infiltration (Fig. S2A and
B).
Tables III and
IV list the univariate and
multivariate analysis results associated with RFS and OS in
patients with surgically resected HCC. Cox proportional hazard
regression models with multivariate analysis showed that high MDSC
infiltration in tumors was associated with significantly worse RFS
and OS (hazard ratio [HR], 1.98; 95% confidence interval [CI],
1.51-2.60; P<0.0001 and HR, 1.82; 95% CI, 1.27-2.62; P=0.0012)
and that low CD8+ T-cell infiltration in tumors was
associated with significantly worse RFS and OS (HR, 2.31; 95% CI,
1.77-3.01; P<0.0001 and HR, 3.28; 95% CI, 2.20-4.48;
P<0.0001). Age was the only OS factor, but CD8-positive cell
infiltration, microscopic serum albumin, AFP, tumor size, and
intrahepatic metastasis were independent prognostic factors for
both RFS and OS.
 | Table III.Results of univariate and
multivariate analyses of recurrence-free survival. |
Table III.
Results of univariate and
multivariate analyses of recurrence-free survival.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age | 1.01 | 0.97-1.02 | 0.2481 |
|
|
|
| Sex |
|
|
|
|
|
|
| Male
(n=344) | 1.28 | 0.96-1.71 |
|
|
|
|
| Female
(n=122) | 1.00 | (ref.) | 0.0870 |
|
|
|
| HBsAg |
|
|
|
|
|
|
|
Positivity (n=73) | 0.83 | 0.59-1.16 |
|
|
|
|
|
Negativity (n=392) | 1.00 | (ref.) | 0.2709 |
|
|
|
| HCVAb |
|
|
|
|
|
|
|
Positive (n=239) | 0.98 | 0.77-1.25 |
|
|
|
|
|
Negative (n=229) | 1.00 | (ref.) | 0.8607 |
|
|
|
| Alb | 0.55 | 0.42-0.72 | <0.0001 | 0.59 | 0.45-0.78 | 0.0001 |
| DCP | 1.00 | 1.00-1.00 | 0.0001 | 1.00 | 0.99-1.00 | 0.6709 |
| AFP | 1.00 | 1.00-1.00 | <0.0001 | 1.00 | 1.00-1.00 | <0.0001 |
| Tumor size | 1.09 | 1.06-1.12 | <0.0001 | 1.05 | 1.01-1.09 | 0.0133 |
| Macroscopic tumor
numbers |
|
|
|
|
|
|
|
Multiple (n=100) | 1.94 | 1.48-2.55 |
| 1.33 | 0.952-1.87 |
|
|
Solitary (n=366) | 1.00 | (ref.) | <0.0001 | 1.00 | (ref.) | 0.0941 |
| Poorly
differentiated |
|
|
|
|
|
|
| Present
(n=143) | 1.81 | 1.41-2.33 |
| 1.23 | 0.925-1.65 |
|
| Absent
(n=322) | 1.00 | (ref.) | <0.0001 | 1.00 | (ref.) | 0.1524 |
| Tumor types |
|
|
|
|
|
|
|
Boundary type (n=288) | 1.00 | (ref.) |
| 1.00 | (ref.) |
|
|
Nonboundary type (n=175) | 1.50 | 1.17-1.90 | 0.0012 | 1.02 | 0.78-1.33 | 0.9106 |
| Microscopic
vascular invasion |
|
|
|
|
|
|
| Present
(n=143) | 1.90 | 1.48-2.44 |
| 1.19 | 0.89-1.60 |
|
| Absent
(n=323) | 1.00 | (ref.) | <0.0001 | 1.00 | (ref.) | 0.2480 |
| Microscopic
intrahepatic metastasis |
|
|
|
|
|
|
| Present
(n=83) | 3.11 | 2.33-4.17 |
| 1.72 | 1.17-2.55 |
|
| Absent
(n=382) | 1.00 | (ref.) | <0.0001 | 1.00 | (ref.) | 0.0060 |
| Fibrosis |
|
|
|
|
|
|
| F0-2
(n=269) | 1.00 | (ref.) |
|
|
|
|
| F3, 4
(n=196) | 1.11 | 0.87-1.41 | 0.4139 |
|
|
|
| CD33-positive cell
infiltration |
|
|
|
|
|
|
| High
(n=144) | 1.87 | 1.46-2.40 |
| 1.98 | 1.51-2.60 |
|
| Low
(n=322) | 1.00 | (ref.) | <0.0001 | 1.00 | (ref.) | <0.0001 |
| CD8-positive cell
infiltration |
|
|
|
|
|
|
| High
(n=219) | 1.00 | (ref.) |
| 1.00 | (ref.) |
|
| Low
(n=247) | 2.00 | 1.56-2.56 | <0.0001 | 2.31 | 1.77-3.01 | <0.0001 |
 | Table IV.Results of univariate and
multivariate analyses of overall survival. |
Table IV.
Results of univariate and
multivariate analyses of overall survival.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age | 1.02 | 1.00-1.04 | 0.0160 | 1.02 | 1.00-1.04 | 0.0161 |
| Sex |
|
|
|
|
|
|
| Male
(n=344) | 0.99 | 0.69-1.44 |
|
|
|
|
| Female
(n=122) | 1.00 | (ref.) | 0.9763 |
|
|
|
| HBsAg |
|
|
|
|
|
|
|
Positivity (n=73) | 0.94 | 0.60-1.45 |
|
|
|
|
|
Negativity (n=392) | 1.00 | (ref.) | 0.7657 |
|
|
|
| HCVAb |
|
|
|
|
|
|
|
Positive (n=239) | 1.13 | 0.81-1.57 |
|
|
|
|
|
Negative (n=229) | 1.00 | (ref.) | 0.4783 |
|
|
|
| Alb | 0.36 | 0.27-0.51 | <0.0001 | 0.40 | 0.28-0.59 | <0.0001 |
| DCP | 1.00 | 1.00-1.00 | 0.0001 | 1.00 | 0.999-1.00 | 0.9713 |
| AFP | 1.00 | 1.00-1.00 | <0.0001 | 1.00 | 1.00-1.00 | 0.0257 |
| Tumor size | 1.11 | 1.07-1.15 | <0.0001 | 1.04 | 0.98-1.09 | 0.1847 |
| Macroscopic tumor
numbers |
|
|
|
|
|
|
|
Multiple (n=100) | 1.74 | 1.22-2.50 |
| 0.76 | 0.47-1.23 |
|
|
Solitary (n=366) | 1.00 | (ref.) | 0.0025 | 1.00 | (ref.) | 0.2656 |
| Poorly
differentiated |
|
|
|
|
|
|
| Present
(n=143) | 2.20 | 1.58-3.06 |
| 1.45 | 0.98-2.15 |
|
| Absent
(n=322) | 1.00 | (ref.) | <0.0001 | 1.00 | (ref.) | 0.0606 |
| Tumor types |
|
|
|
|
|
|
|
Boundary type (n=288) | 1.00 | (ref.) |
| 1.00 | (ref.) |
|
|
Nonboundary type (n=175) | 1.48 | 1.07-2.05 | 0.0193 | 0.83 | 0.57-1.20 | 0.3182 |
| Microscopic
vascular invasion |
|
|
|
|
|
|
| Present
(n=143) | 2.89 | 1.65-3.18 |
| 1.27 | 0.86-1.87 |
|
| Absent
(n=323) | 1.00 | (ref.) | <0.0001 | 1.00 | (ref.) | 0.2360 |
| Microscopic
intrahepatic metastasis |
|
|
|
|
|
|
| Present
(n=83) | 3.32 | 2.32-4.74 |
| 2.07 | 1.23-3.49 |
|
| Absent
(n=382) | 1.00 | (ref.) | <0.0001 | 1.00 | (ref.) | 0.0063 |
| Fibrosis |
|
|
|
|
|
|
| F0-2
(n=269) | 1.00 | (ref.) |
|
|
|
|
| F3, 4
(n=196) | 1.14 | 0.82-1.59 | 0.4250 |
|
|
|
| CD33-positive cell
infiltration |
|
|
|
|
|
|
| High
(n=144) | 1.94 | 1.40-2.70 |
| 1.82 | 1.27-2.62 |
|
| Low
(n=322) | 1.00 | (ref.) | <0.0001 | 1.00 | (ref.) | 0.0012 |
| CD8-positive cell
infiltration |
|
|
|
|
|
|
| High
(n=219) | 1.00 | (ref.) |
| 1.00 | (ref.) |
|
| Low
(n=247) | 3.37 | 2.33-4.09 | <0.0001 | 3.28 | 2.20-4.88 | <0.0001 |
Stratification of MDSC and
CD8+ T-cell infiltration in HCC
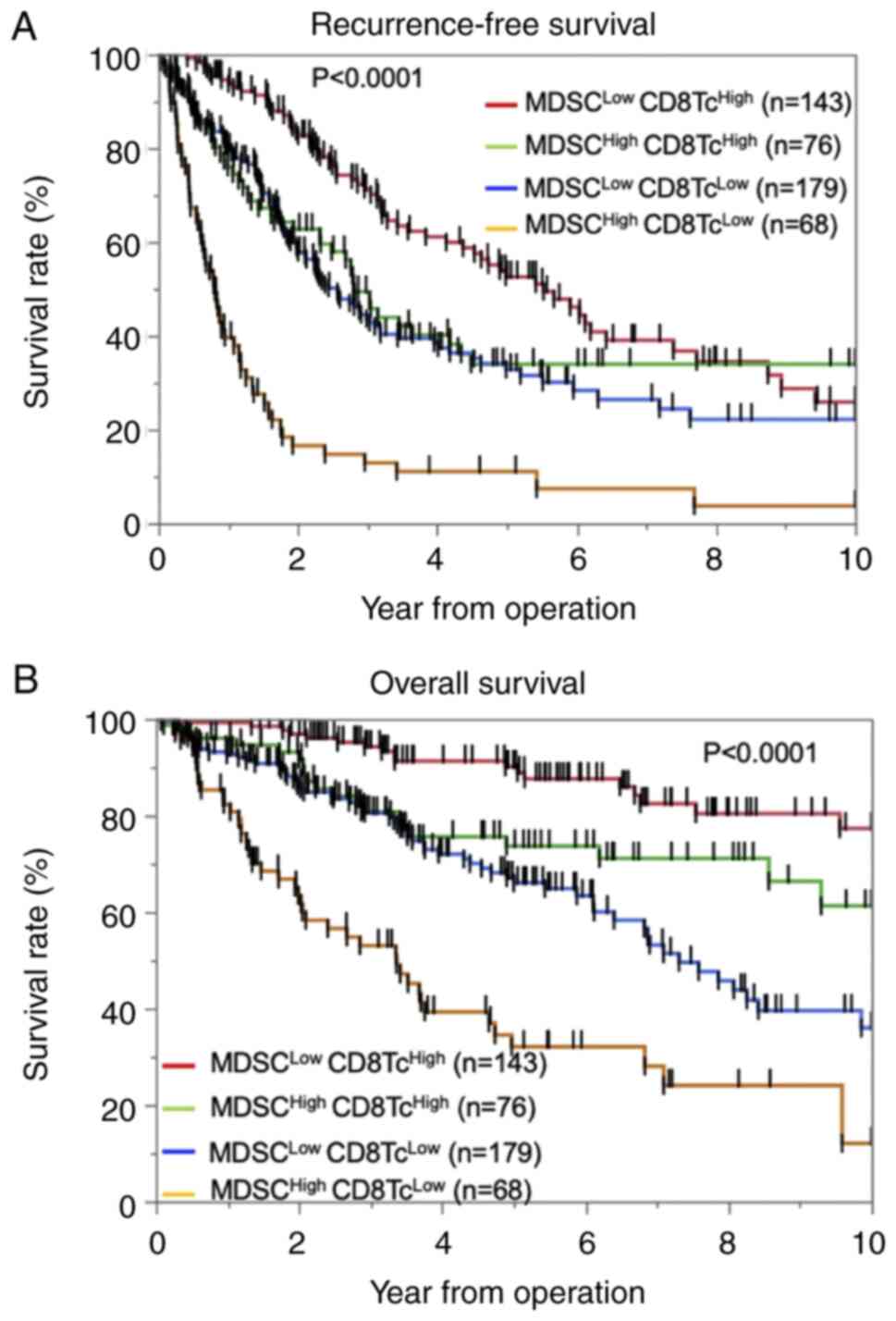
Next, we evaluated the significance of MDSC and
CD8+ T-cell infiltration in predicting OS and RFS. The
patients were divided into the following four groups:
MDSC-Low/CD8-High (n=143), MDSC-High/CD8-High (n=76),
MDSC-Low/CD8-Low (n=179), and MDSC-High/CD8-Low (n=68). We found
that both RFS (log-rank P<0.0001) and OS (log-rank P<0.0001)
were significantly different among the four groups [RFS:
MDSC-Low/CD8-High; 25.9%, MDSC-High/CD8-High; 33.9%,
MDSC-Low/CD8-Low; 22.2%, MDSC-High/CD8-Low 3.68%, OS;
MDSC-Low/CD8-High 77.3%, MDSC-High/CD8-High 61.3%, MDSC-Low/CD8-Low
36.0%, MDSC-High/CD8-Low 12.0%] (Fig.
3A and B). Among the patients with high MDSC infiltration,
those with low CD8+ T-cell infiltration had
significantly poorer RFS (log-rank P<0.0001) and OS (log-rank
P<0.0001) compared with patients with high CD8+
T-cell infiltration. Similarly, among the patients with low MDSC
infiltration, those with low CD8+ T-cell infiltration
had significantly poorer RFS (log-rank P<0.0001) and OS
(log-rank P<0.0001) compared with patients with high
CD8+ T-cell infiltration. The differences in the
clinicopathological characteristics between patients with high MDSC
infiltration and low CD8+ T-cell infiltration are shown
in Table SII. High MDSC
infiltration and low CD8+ T-cell infiltration were
observed in patients with larger tumors (P=0.0001), high serum AFP
leve (P=0.0070), low rate of single nodular type (P=0.0181), a
poorer BCLC stage (P=0.0004), more poorly differentiated HCC
(P<0.0001), and greater presence of microscopic vascular
invasion (P<0.0001) and macroscopic intrahepatic metastasis
(P<0.0001).
Discussion
In the present study, we analyzed the
CD33+ cells in patients with HCC who had undergone
hepatic resection. We demonstrated that high numbers of
tumor-infiltrating CD33+ cells and CD8+ cells
were correlated with a poor prognosis, and we were able to stratify
the prognosis based on the number of tumor-infiltrating
CD33+ cells and CD8+ cells.
MDSCs are the major immunosuppressive population
existing only in pathological conditions such as malignancy and
chronic inflammation (18).
Malignant cells regulate distant sites, such as the bone marrow and
spleen, by secreting soluble factors that cause the accumulation of
myeloid cells; these myeloid cells subsequently promote tumor
metastasis and neovascularization (18). Patients with HCC exhibiting high
numbers of MDSCs reportedly have more vascular invasion than
patients with low numbers of MDSCs (19). In the present study, patients with
high numbers of MDSCs had a significantly higher frequency of
microscopic intrahepatic metastasis and vascular invasion as well
as shorter RFS.
Human MDSCs are phenotypically characterized as
CD11b+ or CD33+ (8,20,21),
and the CD33 myeloid marker can be used instead of CD11b because
the number of CD15+ cells is very low (20). Hence, in this study, we used only
CD33 as an MDSC marker, and the effects of CD33+ cells
other than MDSCs was considered to be very small. Although some
small studies have focused on MDSCs and the prognosis of HCC, we
examined a large number of patients (nearly 500).
Cytotoxic CD8+ T cells play a pivotal
role in anti-tumor immunity. However, in the context of a
suppressive tumor microenvironment and prolonged antigen exposure,
tumor-specific effector CD8+ T cells tend to
differentiate into a condition called T-cell exhaustion (22). Such exhausted CD8+ T
cells are distinguished from functional and memory T cells by their
hierarchical loss of cytokine production ability and killing
capacity (23). Hepatocellular
CCRK/EZH2/NH-kB/IL-6 signaling deteriorates anti-tumor T-cell
responses by induction of MDSC immunosuppression, and HCC with high
mRNA CD11b/CD33/CCRK expression is significantly correlated with
shorter OS and disease-free survival rates (24). In the present study, the
MDSC-High/CD8-Low group had the poorest RFS and OS, although there
were more CD8+ T cells in the tumors in the high than
low MDSC group. In our study, MDSC high group had significantly
poor OS and RFS than MDSC low ones, in CD8+ high group.
MDSC has been reported to decrease mTOR activity in CD8 positive
cells, inhibit T cell differentiation into effector cells, and
reduce the efficacy of immunotherapy (25). Clinical trial in head and neck
cancer have shown that the PI3Kδ/γ, a selective inhibitor of MDSCs,
can enhance the effect of anti-PD-L1 (26). Hence, the stratification of the
patient with HCC by MDSC and CD8+ T cells may predict
the therapeutic effect of immune checkpoint inhibitor, and addition
of anti-MDSCs drugs may bring therapeutic effects to the group that
has not response to immune checkpoint inhibitors. Our analysis
shows that the number of MDSC infiltration is a prognostic factor
independent of CD8+ T cells, and further investigation
is needed.
After encountering tumor antigen, T cells acquire
effector function and traffic to the tumor site to mount an attack
on the tumor (17). Infiltration
of T cells into the tumor microenvironment is the pivotal obstacle
for T cells to initiate an effective anti-tumor response. However,
once T cells have infiltrated the tumor, their success in killing
the tumor is determined by their ability to overcome additional
obstacles and counter-defense mechanisms that they encounter from
the tumor cells, MDSCs, regulatory T cells, stromal cells,
inhibitory cytokines, and other cells in the complex tumor
microenvironment, which act to deteriorate the anti-tumor immune
response (27). Immunotherapy
focusing on immune checkpoint inhibitors has been approved for the
treatment of various cancers (27–30).
Immune checkpoint blockage using anti-programmed death-1
(PD-1)/programmed death ligand 1 (PD-L1) antibodies in HCC has
recently shown favorable results (31,32).
Although a strong prognostic effect of PD-1/PD-L1 expression in HCC
has been reported (4,22,33–36),
the response rate appears to be much lower than that of immunogenic
tumors such as Hodgkin's lymphoma and melanoma, which are
characterized by higher tumorous PD-L1 expression and intratumor
CD8+ cells and a less immunosuppressive microenvironment
in most responding patients (24,27,28).
These clinical observations emphasize the importance of the
compelling need to reverse the non-immunogenic liver tumor
microenvironment for more effective therapeutic responses to immune
checkpoint therapy (24). Although
effective treatment for the non-immunogenic microenvironment in
HCC, such as MDSCs, has not been established, atezolizumab +
bevacizumab may be useful. A recent large phase III study called
IMbrave150 compared atezolizumab + bevacizumab with sorafenib as
the first treatment for patients with unresectable HCC. The study
demonstrated statistically significant and clinically dramatic
improvements in both OS and RFS for atezolizumab + bevacizumab
compared with sorafenib. Bevacizumab, an anti-vascular endothelial
growth factor antibody, has the capability of promoting vascular
normalization, increasing the infiltration of lymphocytes into
tumor tissues, and decreasing the amount and function of MDSCs,
tumor-associated macrophages, and regulatory T cells, thus leading
to synergistic efficacy after combination with PD-1/PD-L1
inhibitors (37). In addition, a
variety of therapeutic agents in other cancers have been reported
for MDSCs. In model mouse, administration of docetaxel decrease
MDSCs and increase the expression of macrophage differentiation
markers (38). Fluorouracil also
selectively damaged MDSCs, which led to an increase response of
tumor specific CD8+ T cell (39).
In conclusion, high infiltration of MDSCs in HCC was
found to be an independent prognostic factor for OS and RFS, and
patients with high infiltration of MDSCs and low infiltration of T
cells in HCC showed a worse prognosis than other patients.
Therefore, MDSC and T-cell infiltration in HCC may be a clinical
biomarker for selection of patients for anti-PD-1/PD-L1 and
anti-vascular endothelial growth factor therapy.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Dr Angela Morben for
editing a draft of this manuscript.
Funding
The present study was supported by the following grant: Japan
Society for the Promotion of Science KAKENHI (no. JP-19K09198).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TTomiy, SI and TY conceived and designed the study.
TTomiy, SI, KY, KK, NI, AM, KM, MM and YO developed the
methodology. TTomiy, SI, NI, AM, KT, YKF, TTomin, TK, YN, KM and NH
acquired data. TTomiy, SI, KT, KY, KK and YO analyzed and
interpreted data. TTomiy, SI, MM and TY wrote, reviewed and/or
revised the manuscript. MM, YO and TY supervised the study. TTomiy,
SI and NI confirm the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present project received ethical approval from
Kyushu University Hospital (approval no. 2020-180; Fukuoka, Japan).
An opt-out approach was employed to obtain informed consent from
our patients and personal information was protected during data
collection.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BCLC
|
Barcelona Clinic Liver Cancer
|
|
CI
|
confidence interval
|
|
HR
|
hazard ratio
|
|
iNOS
|
inducible nitric oxide synthase
|
|
MDSC
|
myeloid-derived suppressor cells
|
|
NO
|
nitric oxide
|
|
OS
|
overall survival
|
|
PD-1
|
programmed death 1
|
|
PD-L1
|
programmed death ligand 1
|
|
RFS
|
recurrence-free survival
|
References
|
1
|
Itoh S, Morita K, Ueda S, Sugimachi K,
Yamashita Y, Gion T, Fukuzawa K, Wakasugi K, Taketomi A and Maehara
Y: Long-term results of hepatic resection combined with
intraoperative local ablation therapy for patients with
multinodular hepatocellular carcinomas. Ann Surg Oncol.
16:3299–3307. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Itoh S, Shirabe K, Taketomi A, Morita K,
Harimoto N, Tsujita E, Sugimachi K, Yamashita Y, Gion T and Maehara
Y: Zero mortality in more than 300 hepatic resections: Validity of
preoperative volumetric analysis. Surg Today. 42:435–440. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yagi T, Baba Y, Ishimoto T, Iwatsuki M,
Miyamoto Y, Yoshida N, Watanabe M and Baba H: PD-L1 expression,
tumor-infiltrating lymphocytes, and clinical outcome in patients
with surgically resected esophageal cancer. Ann Surg. 269:471–478.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Itoh S, Yoshizumi T, Yugawa K, Imai D,
Yoshiya S, Takeishi K, Toshima T, Harada N, Ikegami T, Soejima Y,
et al: Impact of immune response on outcomes in hepatocellular
carcinoma: association with vascular formation. Hepatology.
72:1987–1999. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Unitt E, Marshall A, Gelson W, Rushbrook
SM, Davies S, Vowler SL, Morris LS, Coleman N and Alexander GJ:
Tumour lymphocytic infiltrate and recurrence of hepatocellular
carcinoma following liver transplantation. J Hepatol. 45:246–253.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nishida N: Role of oncogenic pathways on
the cancer immunosuppressive microenvironment and its clinical
implications in hepatocellular carcinoma. Cancers (Basel).
13:36662021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Feehan KT and Gilroy DW: Is resolution the
end of inflammation? Trends Mol Med. 25:198–214. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gabrilovich DI and Nagaraj S:
Myeloid-derived suppressor cells as regulators of the immune
system. Nat Rev Immunol. 9:162–174. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Marvel D and Gabrilovich DI:
Myeloid-derived suppressor cells in the tumor microenvironment:
Expect the unexpected. J Clin Invest. 125:3356–3364. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rodríguez PC and Ochoa AC: Arginine
regulation by myeloid derived suppressor cells and tolerance in
cancer: Mechanisms and therapeutic perspectives. Immunol Rev.
222:180–191. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bronte V and Zanovello P: Regulation of
immune responses by L-arginine metabolism. Nat Rev Immunol.
5:641–654. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tartour E, Pere H, Maillere B, Terme M,
Merillon N, Taieb J, Sandoval F, Quintin-Colonna F, Lacerda K,
Karadimou A, et al: Angiogenesis and immunity: A bidirectional link
potentially relevant for the monitoring of antiangiogenic therapy
and the development of novel therapeutic combination with
immunotherapy. Cancer Metast Rev. 30:83–95. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nefedova Y, Fishman M, Sherman S, Wang X,
Beg AA and Gabrilovich DI: Mechanism of all-trans retinoic acid
effect on tumor-associated myeloid-derived suppressor cells. Cancer
Res. 67:11021–11028. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Almand B, Clark JI, Nikitina E, van Beynen
J, English NR, Knight SC, Carbone DP and Gabrilovich DI: Increased
production of immature myeloid cells in cancer patients: A
mechanism of immunosuppression in cancer. J Immunol. 166:678–689.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lathers DMR, Clark JI, Achille NJ and
Young MRI: Phase 1B study to improve immune responses in head and
neck cancer patients using escalating doses of 25-hydroxyvitamin
D3. Cancer Immunol Immunother. 53:422–430. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Itoh S, Shirabe K, Matsumoto Y, Yoshiya S,
Muto J, Harimoto N, Yamashita Y, Ikegami T, Yoshizumi T, Nishie A
and Maehara Y: Effect of body composition on outcomes after hepatic
resection for hepatocellular carcinoma. Ann Surg Oncol.
21:3063–3068. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ni H, Zhang L, Huang H, Dai S and Li J:
Connecting METTL3 and intratumoural CD33+ MDSCs in predicting
clinical outcome in cervical cancer. J Transl Med. 18:3932020.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gabrilovich DI, Ostrand-Rosenberg S and
Bronte V: Coordinated regulation of myeloid cells by tumours. Nat
Rev Immunol. 12:253–268. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gao X, Tian L, Wu J, Ma XL, Zhang CY, Zhou
Y, Sun YF, Hu B, Qiu SJ, Zhou J, et al: Circulating
CD14+ HLA-DR−/low myeloid-derived suppressor
cells predicted early recurrence of hepatocellular carcinoma after
surgery. Hepatol Res. 47:1061–1071. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bronte V, Brandau S, Chen SH, Colombo MP,
Frey AB, Greten TF, Mandruzzato S, Murray PJ, Ochoa A,
Ostrand-Rosenberg S, et al: Recommendations for myeloid-derived
suppressor cell nomenclature and characterization standards. Nat
Commun. 7:121502016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sanchez-Pino MD, Dean MJ and Ochoa AC:
Myeloid-derived suppressor cells (MDSC): When good intentions go
awry. Cell Immunol. 362:1043022021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ma J, Zheng B, Goswami S, Meng L, Zhang D,
Cao C, Li T, Zhu F, Ma L, Zhang Z, et al: PD1Hi
CD8+ T cells correlate with exhausted signature and poor
clinical outcome in hepatocellular carcinoma. J Immunother Cancer.
7:3312019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Khan O, Giles JR, McDonald S, Manne S,
Ngiow SF, Patel KP, Werner MT, Huang AC, Alexander KA, Wu JE, et
al: TOX transcriptionally and epigenetically programs
CD8+ T cell exhaustion. Nature. 571:211–218. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou J, Liu M, Sun H, Feng Y, Xu L, Chan
AWH, Tong JH, Wong J, Chong CCN, Lai PBS, et al: Hepatoma-intrinsic
CCRK inhibition diminishes myeloid-derived suppressor cell
immunosuppression and enhances immune-checkpoint blockade efficacy.
Gut. 67:9312018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Raber PL, Sierra RA, Thevenot PT, Shuzhong
Z, Wyczechowska DD, Kumai T, Celis E and Rodriguez PC: T cells
conditioned with MDSC show an increased anti-tumor activity after
adoptive T cell based immunotherapy. Oncotarget. 7:17565–17578.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Davis RJ, Moore EC, Clavijo PE, Friedman
J, Cash H, Chen Z, Silvin C, Van Waes C and Allen C: Anti-PD-L1
efficacy can be enhanced by inhibition of myeloid-derived
suppressor cells with a selective inhibitor of PI3Kδ/γ. Cancer Res.
77:2607–2619. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sharma P and Allison JP: The future of
immune checkpoint therapy. Science. 348:56–61. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zou W, Wolchok JD and Chen L: PD-L1
(B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms,
response biomarkers, and combinations. Sci Transl Med.
8:328rv42016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ,
Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al:
Safety and activity of anti-PD-L1 antibody in patients with
advanced cancer. New Engl J Med. 366:2455–2465. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced nonsquamous non-small-cell
lung cancer. New Engl J Med. 373:1627–1639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kudo M: Immune checkpoint inhibition in
hepatocellular carcinoma: basics and ongoing clinical trials.
Oncology. 92 (Suppl 1):S50–S62. 2017. View Article : Google Scholar
|
|
32
|
Prieto J, Melero I and Sangro B:
Immunological landscape and immunotherapy of hepatocellular
carcinoma. Nat Rev Gastroenterol Hepatol. 12:681–700. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yugawa K, Itoh S, Yoshizumi T, Iseda N,
Tomiyama T, Morinaga A, Toshima T, Harada N, Kohashi K, Oda Y and
Mori M: CMTM6 stabilizes PD-L1 expression and is a new prognostic
impact factor in hepatocellular carcinoma. Hepatology Commun.
5:334–348. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu CQ, Xu J, Zhou ZG, Jin LL, Yu XJ, Xiao
G, Lin J, Zhuang SM, Zhang YJ and Zheng L: Expression patterns of
programmed death ligand 1 correlate with different
microenvironments and patient prognosis in hepatocellular
carcinoma. Br J Cancer. 119:80–88. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Calderaro J, Rousseau B, Amaddeo G, Mercey
M, Charpy C, Costentin C, Luciani A, Zafrani ES, Laurent A, Azoulay
D, et al: Programmed death ligand 1 expression in hepatocellular
carcinoma: Relationship with clinical and pathological features.
Hepatology. 64:2038–2046. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Iseda N, Itoh S, Yoshizumi T, Yugawa K,
Morinaga A, Tomiyama T, Toshima T, Kohashi K, Oda Y and Mori M:
ARID1A deficiency is associated with high programmed death ligand 1
expression in hepatocellular carcinoma. Hepatology Commun.
5:675–688. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gao F and Yang C: Anti-VEGF/VEGFR2
monoclonal antibodies and their combinations with PD-1/PD-L1
inhibitors in clinic. Curr Cancer Drug Targets. 20:3–18. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kodumudi KN, Woan K, Gilvary DL, Sahakian
E, Wei S and Djeu JY: A novel chemoimmunomodulating property of
docetaxel: suppression of myeloid-derived suppressor cells in tumor
bearers. Clin Cancer Res. 16:4583–4594. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Vincent J, Mignot G, Chalmin F, Ladoire S,
Bruchard M, Chevriaux A, Martin F, Apetoh L, Rébé C and
Ghiringhelli F: 5-fluorouracil selectively kills tumor-associated
myeloid-derived suppressor cells resulting in enhanced T
cell-dependent antitumor immunity. Cancer Res. 70:3052–3061. 2010.
View Article : Google Scholar : PubMed/NCBI
|