Introduction
Breast cancer (BC) occurs due to the uncontrolled
proliferation of breast epithelial cells, ultimately becoming
malignant, as a result of various carcinogens (1). BC treatment strategies include
surgery, radiotherapy and systemic therapy, which are usually based
on a multimodal approach, depending on the stage and biology of the
tumor, as well as patient acceptance and tolerance (2). Advances in the early detection of
novel effective biomarkers, technologies of oncoplastic surgery,
and improvement in patients' lifestyle have contributed to
improvements in the outcomes of patients with BC (3,4);
however, patients who develop metastatic BC and drug resistance
continue to have a poor prognosis (5). Therefore, novel approaches for BC
therapeutics, including the reduction of BC relapse and the
improvement of the mortality rate are urgently required.
The kinesin superfamily (KIFs) is a group of
proteins characterized by their instrumental role in the
intracellular transport of microtubule-based chromosomes during
mitosis (6). The dysregulation of
KIFs may result in uncontrolled cell proliferation, resulting from
premature sister chromatid separation, highlighting their
importance in tumorigenesis. In total, 45 KIFs with various
functions have been discovered and identified in humans, among
which numerous family members have been reported to play a crucial
role in tumor pathobiology (7).
Furthermore, a previous study demonstrated the involvement of KIFs
in BC metastasis, prognosis and resistance to chemotherapy
(8). KIF member 18B (KIF18B) is a
KIF superfamily member. Recent studies have suggested that KIF18B
expression may be significantly upregulated in BC tissue and is
closely associated with a worse overall, relapse-free and distant
metastasis-free survival in BC (9,10).
It has also been demonstrated in another study that KIF18B
silencing may restrict cell proliferation and invasion, and enhance
BC chemosensitivity by modulating the Akt/GSK-3β/β-catenin
signaling pathway (11). Thus, it
was hypothesized that KIF18B may exert a tumor-promoting effect on
BC. However, the potential regulatory mechanisms of KIF18B in BC
have not yet been fully elucidated.
The thyroid hormone receptor-interacting protein 13
(TRIP13) is an ATPase protein associated with various cellular
activities, playing a crucial role in cell progression,
particularly in checkpoint signaling (12). Over the past few decades, the
oncogenic role of TRIP13 has attracted considerable attention. It
has been reported that TRIP13 may be upregulated in numerous types
of cancer and is usually associated with a poor prognosis (13). Moreover, TRIP13 expression is
upregulated in BC and is significantly associated with numerous
clinicopathological features, also indicating a poor prognosis of
patients with BC (14,15).
In the present study, KIF18B expression was
predicted to positively correlate with TRIP13 expression in BC via
analysis using The Cancer Genome Atlas (TCGA) database. The aim of
the present study was therefore to investigate whether KIF18B
promotes the malignant progression of BC by regulating TRIP13.
Materials and methods
Cell culture
The human normal breast epithelial MCF-10A and
MCF-12A cell lines, which have been widely used as control cell
lines for BC cell lines (16,17),
and BC MCF-7, ZR-75-1, HCC-1937 and MDA-MB-436 cell lines, were
purchased from the American Type Culture Collection (ATCC). Cells
were cultured at 37°C in DMEM (Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.),
in a humid atmosphere with 5% CO2.
Cell transfection
The KIF18B-targeting short hairpin RNA (shRNA)
oligonucleotide sequences and TRIP13 cDNA sequences were designed
and synthesized by GenScript and were cloned into the pSilencer
2.1-U6 Neo and pcDNA3.1 vectors (Thermo Fisher Scientific, Inc.),
respectively, which were consequently named shRNA-KIF18B-1
(5′-GAGACCTCAATGCCACCTTTG-3′), shRNA-KIF18B-2
(5′-CCAGTTTCCATGAATGCATTG-3′) and overexpressed (Ov)-TRIP13.
shRNA-negative control (NC; 5′-ATGGCGATTCGTATCATGGCAT-3′) and empty
pcDNA3.1 vector were used as NCs. Transfection was performed using
Lipofectamine 2000® (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol at 37°C for 24 h. At 48 h
following transfection, the transfection efficiency was measured
using reverse transcription-quantitative PCR (RT-qPCR) and western
blot analysis.
RT-qPCR
Total RNA was extracted from the HCC-1937 cells
using the Total RNA Extraction kit (Beijing Solarbio Science &
Technology Co., Ltd.; R1200) according to the manufacturer's
protocol. Total RNA was reverse transcribed using the PrimeScript
RT Reagent kit (Takara Biotechnology Co., Ltd.; cat. no. RR047A)
according to the manufacturer's protocol. qPCR was performed using
the TB Green PrimeScript RT-PCR kit (Takara Biotechnology Co.,
Ltd.; RR086A) and a StepOnePlus Real-Time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. The following thermocycling conditions
were used for the qPCR: initial denaturation at 95°C for 30 sec;
and 40 cycles of denaturation at 95°C for 10 sec, annealing at 60°C
for 20 sec and extension at 70°C for 10 sec. The primer pair
sequences are listed in Table I.
mRNA expression levels were quantified using the 2−ΔΔCq
method (18) and normalized to the
internal reference gene GAPDH.
 | Table I.Primer sequences for RT-quantitative
PCR. |
Table I.
Primer sequences for RT-quantitative
PCR.
| Gene name | Forward (5′-3′) | Reverse (5′-3′) |
|---|
| KIF18B |
GGAGAACCGACGAAAGGTGT |
AGGGTTAAACACCAGCACC-C |
| TRIP13 |
TGCTGATTGATGAGGTGGAGAG |
GGTTGCACAAGTATCACGCA |
| GAPDH |
ATGGGCAGCCGTTAG-GAAAG |
CCCAATACGACCAAATCAGAGAA |
Western blot analysis
Transfected or untransfected HCC-1937 cells were
lysed using RIPA lysis buffer (Vazyme Biotech Co., Ltd.) according
to the manufacturer's protocol. The total protein concentration was
determined using a BCA Protein Quantification kit (Beyotime
Institute of Biotechnology). Total protein (50 µg protein/lane)
from each sample was separated via SDS-PAGE on a 12% gel and
transferred onto PVDF membranes. Following blocking with 5% non-fat
milk at room temperature for 2 h, all membranes were subsequently
incubated with primary antibodies at 4°C overnight. Following
primary antibody incubation, the membranes were incubated with
HRP-conjugated anti-rabbit secondary antibody (1:10,000; ab6721,
Abcam) for a further 2 h at room temperature. Protein bands were
visualized using ChemiGlow detection reagents (ProteinSimple). The
blots were then visualized using a FluorChem 8900 Imager and
semi-quantified using ImageJ software (V1.8.0; National Institutes
of Health). The following primary antibodies were purchased which
were obtained from Abcam: KIF18B (1:5,000; ab168812), TRIP13
(1:5,000; ab128153), MMP12 (1:10,000; ab52897), MMP9 (1:10,000;
ab76003), β-catenin (1:5,000; ab32572), c-Myc (1:1,000; ab32072),
cyclin D1 (1:200; ab16663) and GAPDH (1:10,000; ab181602). GADPH
was used as the loading control.
Cell proliferation assay
The Cell Counting Kit-8 (CCK-8) assay (Beyotime
Institute of Biotechnology) was used to detect cell proliferation.
Briefly, transfected or untransfected cells were seeded into a
96-well plate (1×103 cells/well) and cultured at 37°C in
5% CO2. Following incubation for 24, 48 and 72 h, 10 µl
CCK-8 reagent were added to the culture solution and incubated for
a further 1 h. Cell proliferation was assessed using a microplate
reader (Bio-Rad Laboratories, Inc.), based on the absorbance at 450
nm.
Colony formation assay
In order to observe cell proliferation, the HCC-1937
cells were seeded into a 6-cm plate at a density of 500 cells/plate
and cultured for 24 h. Subsequently, the culture medium was
replaced with fresh DMEM and cultured for a further 14 days.
Colonies were infiltrated with methanol for 10 min, stained with
crystal violet for 10 min at room temperature, imaged using a
fluorescence microscope (magnification, ×100; Olympus Corporation)
and counted using ImageJ software (V1.8.0; National Institutes of
Health).
Wound healing assay
To evaluate cell migration, transfected and
untransfected HCC-1937 cells were seeded into 6-well plated at a
density of 1×104 cells/well. When cells had reached
almost 100% confluency, wounds were created in the cell monolayers
using a sterile pipette tip. Subsequently, the cells were incubated
in serum-free medium for 24 h at 37°C. The wounds were imaged at 0
and 24 h, using an inverted microscope (magnification, ×200;
Olympus Corporation). ImageJ software (V1.8.0; National Institutes
of Health) was used to determine wound widths.
Transwell invasion assay
A 24-well Transwell System (Costar; Corning, Inc.)
was employed to evaluate the invasive ability of the HCC-1937 cells
following transfection. Cells were seeded into the upper chambers
which were pre-coated with 5 µl Matrigel™ (BD Biosciences) at 37°C
for 30 min at a density of 1×105 cells/chamber with
serum-free medium. The lower chamber was filled with 500 µl DMEM
containing 10% FBS. Following incubation for 24 h at 37°C, invading
cells in the lower chamber were fixed using formalin for 30 min and
stained with 0.5% crystal violet (MilliporeSigma) for 15 min at
room temperature. Cells were imaged using a light microscope and
counted using a high-powered microscope (magnification, ×200).
Co-immunoprecipitation (Co-IP)
The HCC-1937 cells were harvested and lysed using
RIPA lysis buffer (Beyotime Institute of Biotechnology). Protein
lysates were incubated with anti-KIF18B (1:1,000; ab168812, Abcam)
and anti-TRIP13 antibodies (1:1,000; ab128153, Abcam) or normal
rabbit IgG (1:1,000; ab172730, Abcam) and rotated overnight at 4°C
for the formation of an immune-complex. The reaction mixture was
then incubated with protein A/G plus-agarose beads (Santa Cruz
Biotechnology, Inc.) for 2 h at 4°C. After being washed five times
with cold washing buffer, the agarose beads were heated with 40 µl
10% SDS buffer to 100°C for 5 min. The samples were analyzed using
western blot analysis, as described above.
Statistical analysis
Data were represented by at least three independent
experimental repeats and are presented as the mean ± SD.
Statistical analysis was performed using GraphPad Prism 7.0
(GraphPad Software, Inc.). Comparisons between two groups were
analyzed using the unpaired Student's t-test. One-way ANOVA
followed by Tukey's post hoc test was used to calculate the
statistical differences between ≥3 groups. Pearson's Correlation
analysis was used to analyze the correlation between two genes
expression in BC. P<0.05 was considered to indicate a
statistically significant difference.
Results
KIF18B expression is upregulated in
BC
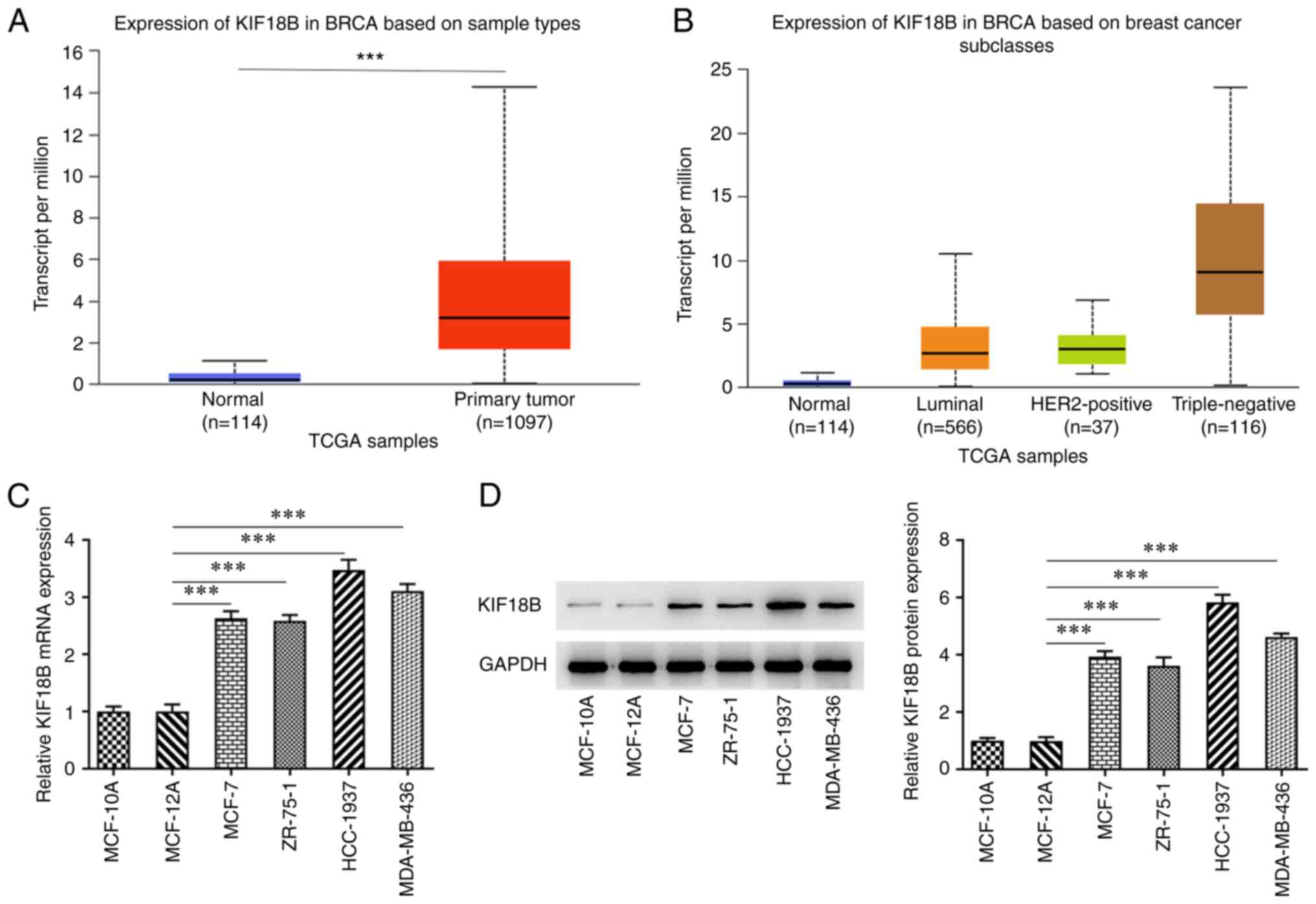
KIF18B expression profile in BC was analyzed by
using TCGA data and the UALCAN database. The results demonstrated
that KIF18B expression was upregulated in patients with BC,
particularly in patients with the triple-negative BC (TNBC)
subtype, in comparison with healthy individuals (Fig. 1A and B). Moreover, the KIF18B
expression levels in human normal breast cells (MCF-10A and
MCF-12A), and in the BC cell lines, MCF-7, ZR-75-1, HCC-1937 and
MDA-MB-436, were also evaluated. The results demonstrated that the
BC cell lines had significantly higher KIF18B mRNA and protein
expression levels in comparison with the normal breast epithelial
cells (Fig. 1C and D). The TNBC
HCC-1937 cell line displayed the highest KIF18B expression levels
and was thus selected for use in subsequent experiments.
Knockdown of KIF18B inhibits the
malignant progression of BC cells
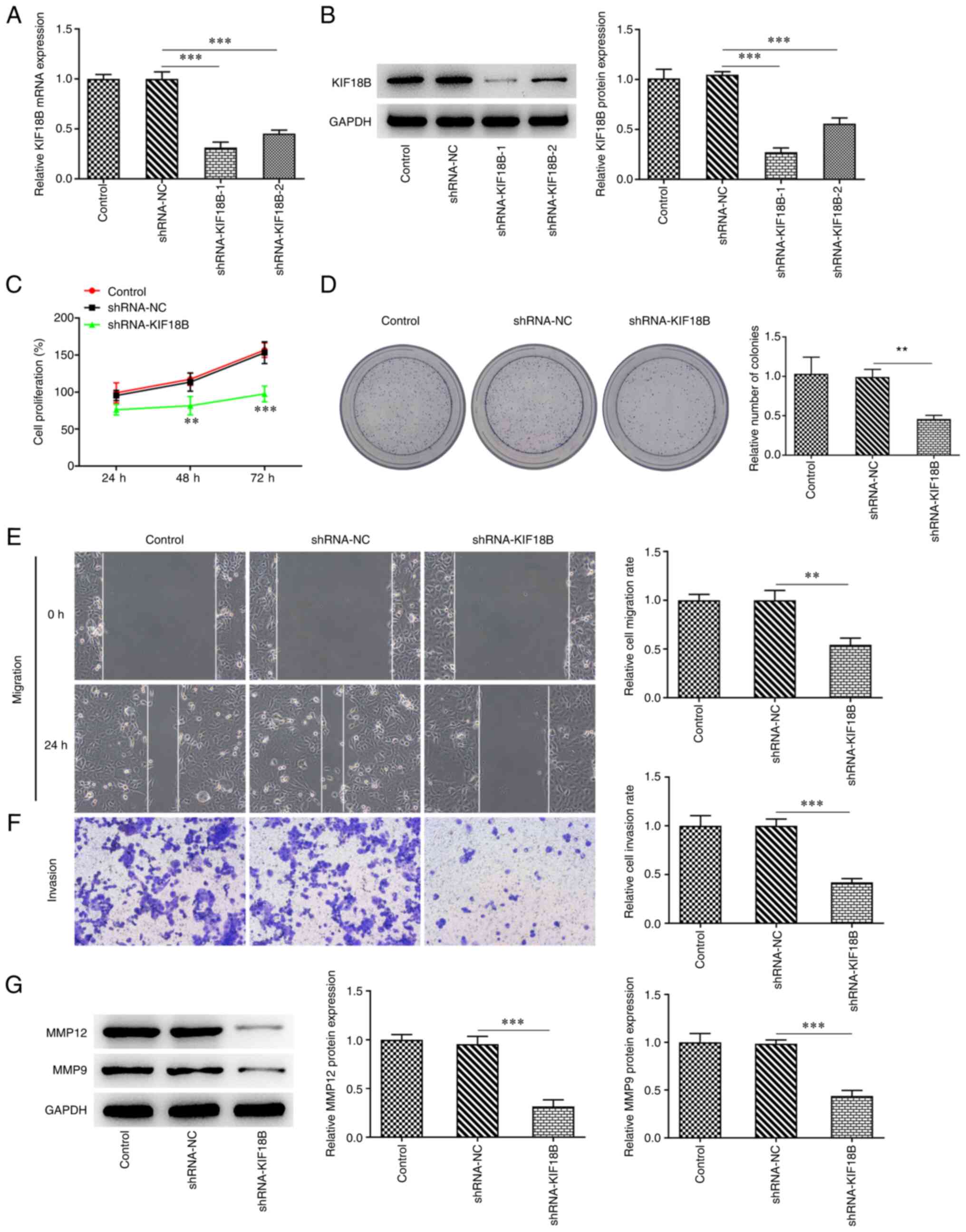
KIF18B expression was knocked down in HCC-1937 BC
cells using shRNAs. shRNA-KIF18B-1 was selected for use in
following experiments as a result of its greater transfection
efficiency (Fig. 2A and B).
Following KIF1B knockdown, the results demonstrated the decreased
viability of the HCC-1937 cells (Fig.
2C). The colony formation assay demonstrated that the KIF1B
knockdown group exhibited a markedly reduced number of colonies
compared with the NC group (Fig.
2D). Furthermore, HCC-1937 cell migratory and invasive
abilities were markedly suppressed as a result of KIF18B knockdown
(Fig. 2E and F). Moreover, KIF18B
knockdown decreased the MMP12 and MMP9 protein expression levels
(Fig. 2G), which have both been
reported to be involved in tumor cell invasion and metastasis
(19).
TRIP13 expression is upregulated in BC
and is positively regulated by the direct binding of KIF18B
Following TCGA data analysis using the UALCAN
database, the results demonstrated that KIF18 expression positively
correlated with TRIP13 expression in BC, with a Pearson correlation
coefficient of 0.65 (Fig. 3A).
Furthermore, TRIP13 expression was found to be upregulated in
patients with BC, particularly in patients with TNBC (Fig. 3B and C). To further verify this
finding, TRIP13 expression in normal breast epithelial MCF-10A and
MCF-12A cells and BC MCF-7, ZR-75-1, HCC-1937 and MDA-MB-436 cells
was investigated. Results demonstrated that TRIP13 exhibited higher
expression levels in BC cell lines, especially in the HCC-1937 cell
line, indicating the potential role of TRIP13 in BC progression
(Fig. 3D and E). Moreover, KIF18B
knockdown significantly decreased the TRIP13 expression levels in
HCC-1937 cells (Fig. 3F). The
direct interaction between TRIP13 and KIF18B was determined using a
Co-IP assay (Fig. 3G). These
findings indicated that KIF18B may play an oncogenic role in BC by
binding to TRIP13 and positively regulating TRIP13 expression
levels.
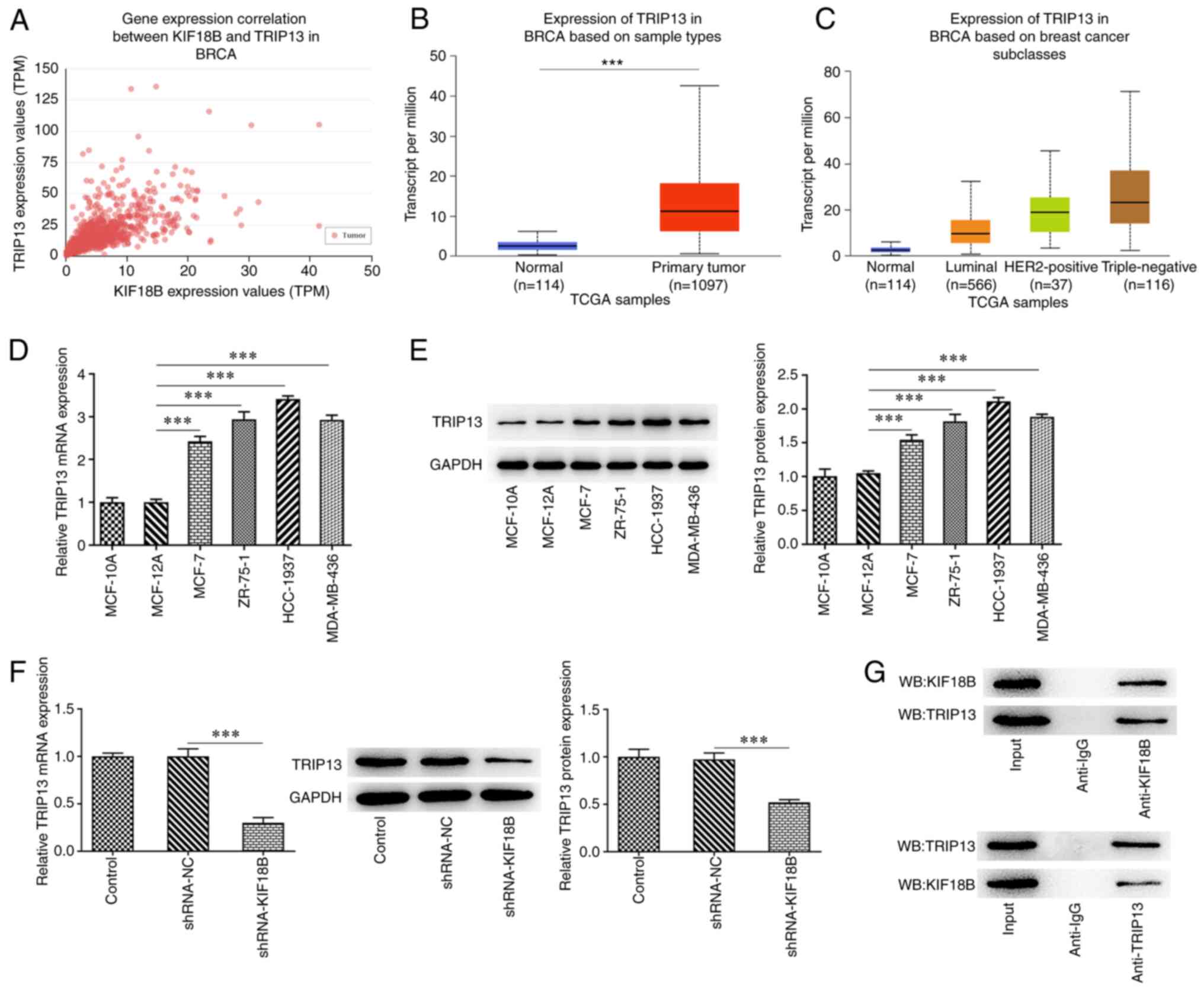 | Figure 3.TRIP13 is overexpressed in BC and is
positively regulated by KIF18B via direct binding. (A) Correlation
between KIF18B and TRIP13 expression in BC. (B) TRIP13 mRNA level
evaluation in BC, based on sample types, by analyzing TCGA data
using online databases UALCAN and subclasses. (C) TRIP13 mRNA level
evaluation in BC, based on subclasses, by analyzing TCGA data using
the UALCAN online database. (D) TRIP13 mRNA expression evaluation
and (E) TRIP13 protein expression evaluation using reverse
transcription-quantitative PCR and western blot analysis,
respectively, in MCF-10A and MCF-12A normal breast epithelial cell
lines, and the BC cell lines, including MCF-7, ZR-75-1, HCC-1937
and MDA-MB-436. (F) TRIP13 mRNA and protein expression evaluation
in HCC-1937 cells with or without KIF18B. (G) Co-IP assay was used
to determine the protein interaction between KIF18B and TRIP13.
***P<0.001. KIF18B, KIF member 18B; TRIP13, thyroid hormone
receptor-interacting protein 13; BC, breast cancer; Co-IP,
co-immunoprecipitation. TCGA, The Cancer Genome Atlas. |
Overexpression of TRIP13 attenuates
the inhibitory effects of KIF18B knockdown on BC tumorigenesis
To verify the interaction between TRIP13 and KIF18B,
TRIP13 overexpression was induced in the HCC-1937 cells via
transfection with Ov-TRIP13 vector (Fig. 4A and B). The control HCC-1937 cell
group or the KIF18B knockdown group were co-transfected with
Ov-TRIP13. Transfection with shRNA-KIF18B markedly decreased cell
viability in the control cell group; however, as compared with the
Ov-NC group, cells that were co-transfected with Ov-TRIP13
exhibited an increased cell viability (Fig. 4C). Similarly, the decreased number
of colonies induced by KIF18B knockdown was reversed by Ov-TRIP13
(Fig. 4D). Furthermore, the
impaired migratory and invasive abilities induced by KIF18B
knockdown were attenuated by Ov-TRIP13 (Fig. 4E and F). KIF18B knockdown also
resulted in decreased MMP12 and MMP9 protein expression levels,
whereas Ov-TRIP13 reversed this effect (Fig. 4G).
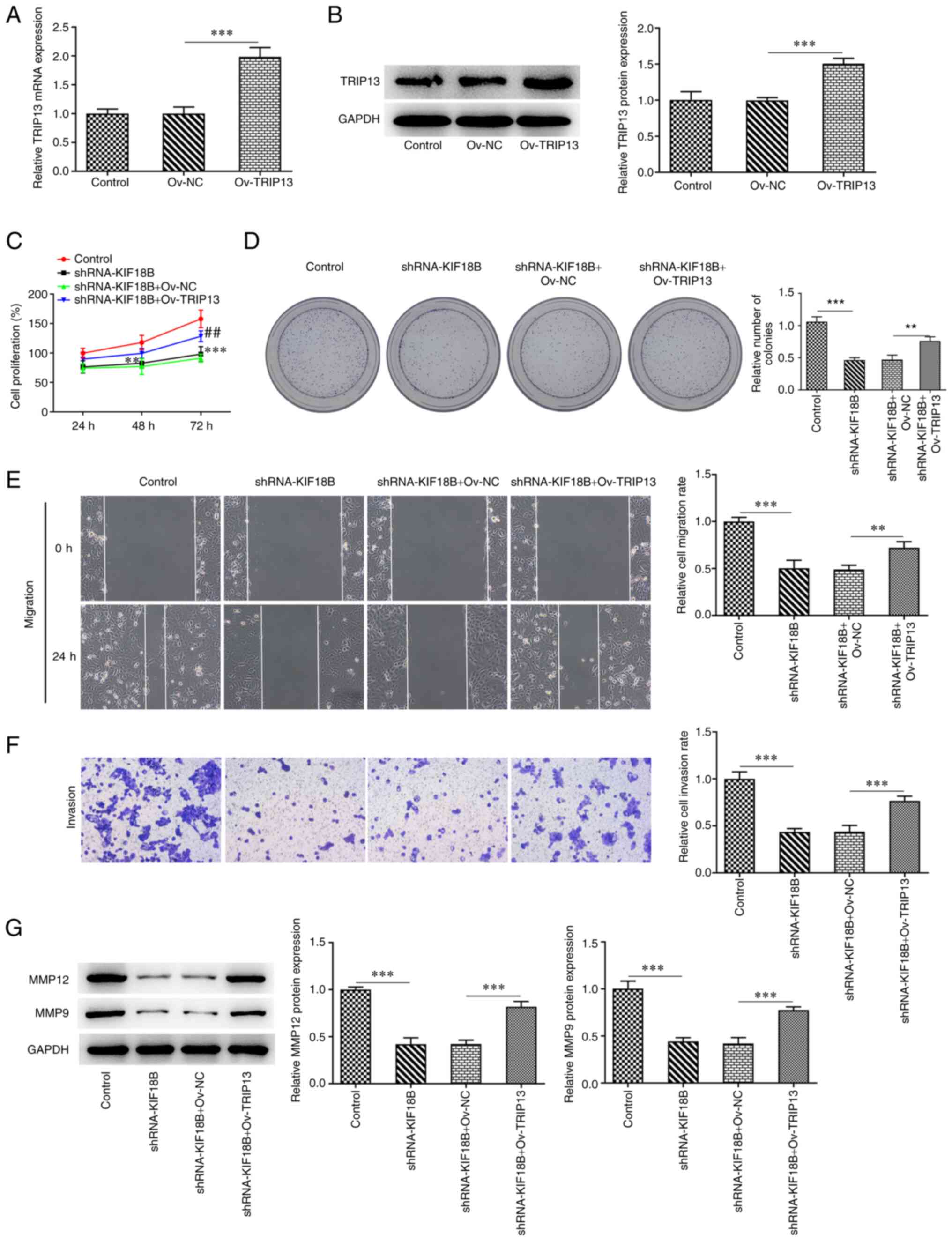 | Figure 4.TRIP13 overexpression attenuates the
inhibitory effects of KIF18B knockdown on HCC-1937 cells
progression. (A) TRIP13 mRNA level evaluation using reverse
transcription-quantitative PCR in transfected HCC-1937 cells. (B)
TRIP13 protein level evaluation using western blot analysis, in
transfected HCC-1937 cells. ***P<0.001. (C) Cell viability
evaluation in transfected HCC-1937 cells at 24, 48 and 72 h
post-transfection, using CCK-8 assay. **P<0.01 and
***P<0.001, vs. control; ##P<0.01, vs.
shRNA-KIF18B + Ov-NC. (D) Cell proliferation evaluation using
colony formation assay. (E) Cell migration evaluation, using wound
healing assay. (F) Cell invasion evaluation, using Transwell assay.
(G) MMP12 and MMP9 protein expression evaluation using western blot
analysis. **P<0.01 and ***P<0.001. KIF18B, KIF member 18B;
TRIP13, thyroid hormone receptor-interacting protein 13; ov-NC,
overexpression negative control. |
Wnt/β-catenin signaling pathway is
involved in the effects of the KIF18B/TRIP13 axis on BC
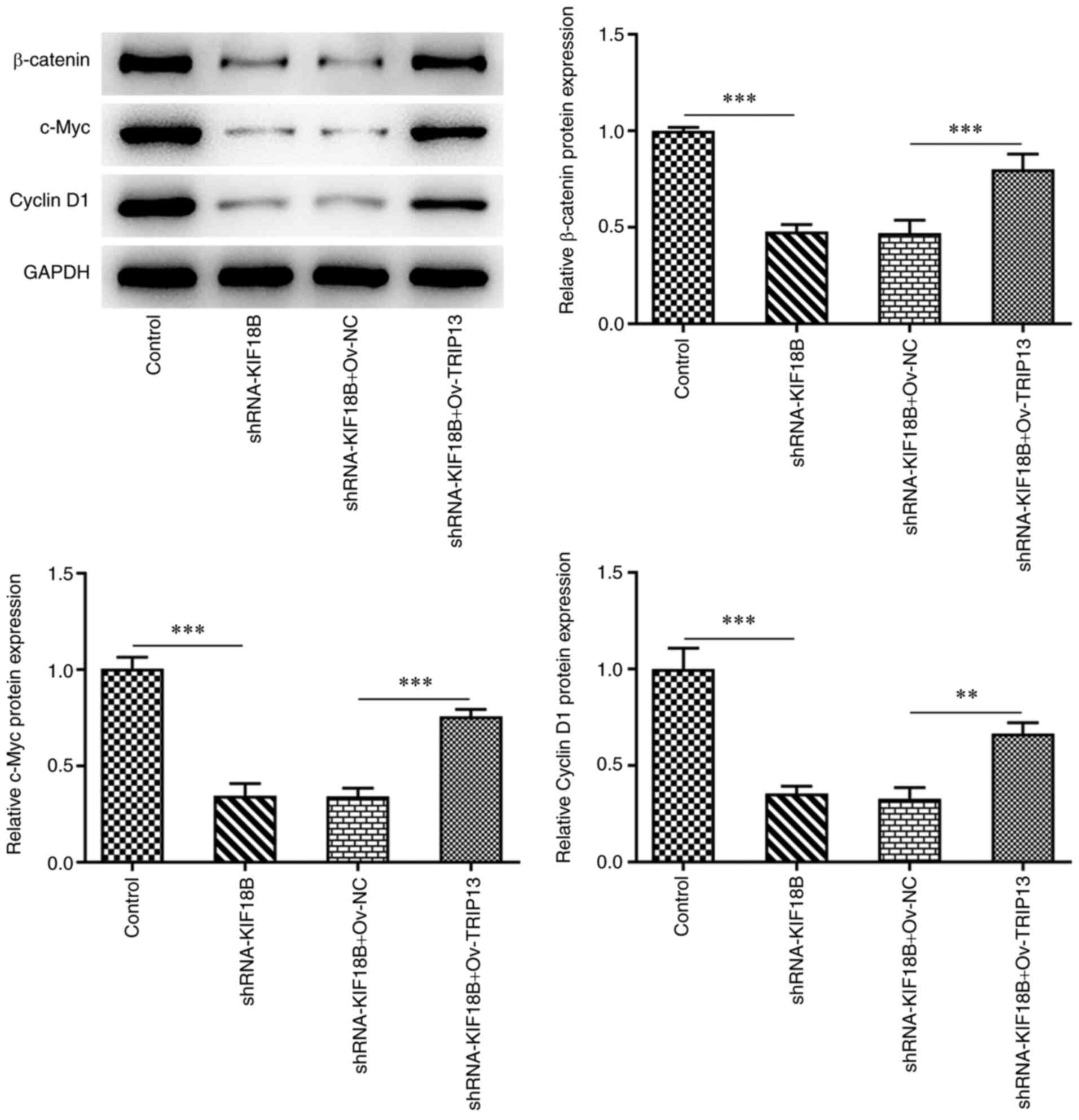
β-catenin signaling pathway protein expression
levels were also determined (Figure
5). The results demonstrated that KIF18B knockdown resulted in
the decreased expression of β-catenin, c-Myc and cyclin D1, in
comparison with the negative control. However, compared with the
shRNA-KIF18B + Ov-NC cell group, the shRNA-KIF18B + Ov-TRIP13 cell
group exhibited significantly higher Wnt/β-catenin signaling
pathway protein expression levels. These results therefore
indicated that KIF18B knockdown induced the inactivation of the
Wnt/β-catenin signaling pathway, whereas TRIP13 overexpression
re-activated this signaling pathway in HCC-1937 BC cells.
Discussion
BC is a common type of cancer that affects women
globally. In the present study, the role of the KIF18B/TRIP13 axis
in BC was explored. The results demonstrated that the expression
levels of KIF18B and TRIP13 were increased in BC. It was
demonstrated that KIF18B promoted BC cell malignant progression by
targeting TRIP13, positively regulating TRIP13 expression levels,
thereby activating the Wnt/β-catenin signaling pathway. Therefore,
the present study indicated a novel mechanism, to the best of our
knowledge, involving KIF18B into driving BC progression.
KIF18B has been reported to be upregulated in BC
samples and has been associated with reduced survival rates
(11). In line with the previously
published results, it was demonstrated in the present study that
KIF18B expression was upregulated in BC cell lines. Furthermore, it
was determined in silico that KIF18B expression was
particularly upregulated in patients with the TNBC subtype, and
this finding was further validated by in vitro experiments,
with KIF18B displaying the highest expression levels in the TNBC
HCC-1937 cell line. BC is a heterogeneous disease and can be
categorized into three groups: i) Hormone receptor-expressing
[estrogen receptor (ER+) or progesterone receptor
(PR+)]; ii) HER2-expressing (HER2+); and TNBC
(ER−, PR− and HER2−), which is
particularly aggressive and is associated with a poor prognosis and
high heterogeneity (20,21). The role of KIF18B in HCC-1937 cell
aggression was therefore investigated. Subsequent functional
analysis revealed that KIF18B knockdown effectively inhibited
HCC-1937 cell proliferation, migration and invasion. The invasion
of BC cells into the surrounding extracellular matrix and their
subsequent migration into the bloodstream and lymphatic system are
pivotal steps in BC metastasis (22). The results of the present study
suggested the potential value of KIF18B knockdown in the treatment
of BC, particularly TNBC.
To explore the underlying mechanisms of KIF18B in
mediating BC tumorigenesis, the factors closely associated with
KIF18B expression in BC were explored. TRIP13 was revealed to be
positively associated with KIF18B expression in BC and exhibited a
similar expression profile to that of KIF18B, in which it was
upregulated in BC, particularly in the TNBC subtype. Similar to
KIF18B, TRIP13 was also most highly expressed in the HCC-1937 cell
line. Moreover, the results of the present study validated that
KIF18B was able to bind to TRIP13 and positively regulate its
expression levels. These results strongly suggested the involvement
of TRIP13 in KIF18B-mediated BC tumorigenesis. Further results
revealed that TRIP13 overexpression markedly abolished the
inhibitory effect of KIF18B knockdown on HCC-1937 cell
proliferation, migration and invasion, further suggesting that
TRIP13 had an indispensable role in KIF18B-mediated BC
tumorigenesis.
KIF18B has previously been reported to activate the
Wnt/β-catenin signaling pathway, promoting the tumor progression of
hepatocellular carcinoma (23),
cervical cancer (24) and BC
(11). In the present study, it
was demonstrated that the decreased β-catenin, c-Myc and cyclin D1
protein expression levels in HCC-1937 cells were attributed to
KIF18B knockdown. The Wnt/β-catenin signaling pathway has been
reported to play a crucial role in cancer cell proliferation,
survival, migration and invasion (25). When Wnt ligands bind to
transmembrane receptors, Wnt signaling is initiated. Cyclin D1 is
the upstream signaling molecule of β-catenin and c-Myc is one of
the target genes of β-catenin (26). The results of the present study
demonstrated that KIF18B functioned as an oncogene in BC via the
activation of the Wnt/β-catenin signaling pathway, and therefore
promoted the migration and invasion of HCC-1937 cells. Furthermore,
it was demonstrated that TRIP13 overexpression reactivated the
Wnt/β-catenin signaling pathway, previously being inactivated due
to KIF18B knockdown. The TRIP13-mediated activation of the
Wnt/β-catenin signaling pathway has also been reported in lung,
colorectal and cervical cancer (27–29).
It was therefore suggested that the activation of the Wnt/β-catenin
signaling pathway in BC might be also caused by TRIP13. However,
further research is required, in order to validate this finding.
However, this study also had several limitations. Firstly, whether
KIF18B overexpression can alter the effects mediated by TRIP13
silencing on BC cell progression, as well as the underlying
mechanisms, may also be worthy of investigation. Moreover, the use
of other TNBC or BC cell lines and the application of in
vivo experiments, using animal models alongside the evaluation
of clinical patient samples are required to further validate the
conclusions of the present study. In addition, although the
inhibition on cancer cell proliferation, migration and invasion is
sufficient to illustrate the anti-cancer effect in vitro
(30,31), the involvement of cell death and
apoptosis has to be also assessed in vitro and in
vivo, to further confirm this effect in future research.
In conclusion, the present study indicated that
KIF18B/TRIP13 was upregulated in BC, particularly in the TNBC
subtype, in both cell lines and tissues. Furthermore, KIF18B
knockdown suppressed the malignant process in TNBC cells by
decreasing the TRIP13 expression levels, which may have inactivated
the Wnt/β-catenin signaling pathway. Overall, these findings
suggest that KIF18B/TRIP13 may play a crucial role in BC
tumorigenesis. Thus, this axis may prove to be a novel prognostic
biomarker for patients with BC in the future.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Construction of Women's Life
Cycle Health Management Service Model in Baoji Area, Key R&D
Projects in Shaanxi Province (grant no. 2021SF-211).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL and LL contributed to the conception and design
of the study. LL, ZZ and XX contributed to the acquisition of data.
LL and ZZ contributed to the analysis or interpretation of the
data. JL and LL drafted the manuscript and revised it critically
for important intellectual content. All authors have read and
approved the final manuscript. JL and LL confirm the authenticity
of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Coughlin SS: Epidemiology of breast cancer
in women. Adv Exp Med Biol. 1152:9–29. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cheung KL: Treatment strategies and
survival outcomes in breast cancer. Cancers (Basel). 12:7352020.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Falzone L, Grimaldi M, Celentano E,
Augustin LSA and Libra M: Identification of modulated MicroRNAs
associated with breast cancer, diet, and physical activity. Cancers
(Basel). 12:25552020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Arnedos M, Roulleaux Dugage M,
Perez-Garcia J and Cortes J: Window of opportunity trials for
biomarker discovery in breast cancer. Curr Opin Oncol. 31:486–492.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Emens LA: Breast cancer immunotherapy:
Facts and hopes. Clin Cancer Res. 24:511–520. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhong A, Tan FQ and Yang WX:
Chromokinesin: Kinesin superfamily regulating cell division through
chromosome and spindle. Gene. 589:43–48. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hirokawa N and Tanaka Y: Kinesin
superfamily proteins (KIFs): Various functions and their relevance
for important phenomena in life and diseases. Exp Cell Res.
334:16–25. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lucanus AJ and Yip GW: Kinesin
superfamily: Roles in breast cancer, patient prognosis and
therapeutics. Oncogene. 37:833–838. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fu Y, Zhou QZ, Zhang XL, Wang ZZ and Wang
P: Identification of Hub genes using co-expression network analysis
in breast cancer as a tool to predict different stages. Med Sci
Monit. 25:8873–8890. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li TF, Zeng HJ, Shan Z, Ye RY, Cheang TY,
Zhang YJ, Lu SH, Zhang Q, Shao N and Lin Y: Overexpression of
kinesin superfamily members as prognostic biomarkers of breast
cancer. Cancer Cell Int. 20:1232020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jiang J, Liu T, He X, Ma W, Wang J, Zhou
Q, Li M and Yu S: Silencing of KIF18B restricts proliferation and
invasion and enhances the chemosensitivity of breast cancer via
modulating Akt/GSK-3β/β-catenin pathway. Biofactors. 47:754–767.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miniowitz-Shemtov S, Eytan E, Kaisari S,
Sitry-Shevah D and Hershko A: Mode of interaction of TRIP13
AAA-ATPase with the Mad2-binding protein p31comet and with mitotic
checkpoint complexes. Proc Natl Acad Sci USA. 112:11536–11540.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lu S, Qian J, Guo M, Gu C and Yang Y:
Insights into a Crucial Role of TRIP13 in human cancer. Comput
Struct Biotechnol J. 17:854–861. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dazhi W, Mengxi Z, Fufeng C and Meixing Y:
Elevated expression of thyroid hormone receptor-interacting protein
13 drives tumorigenesis and affects clinical outcome. Biomark Med.
11:19–31. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang K, Sturt-Gillespie B, Hittle JC,
Macdonald D, Chan GK, Yen TJ and Liu ST: Thyroid hormone receptor
interacting protein 13 (TRIP13) AAA-ATPase is a novel mitotic
checkpoint-silencing protein. J Biol Chem. 289:23928–23937. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vedoya GM, López Nigro MM and Martín GA:
The secretome of non-tumorigenic mammary cells MCF-10A elicits DNA
damage in MCF-7 and MDA-MB-231 breast cancer cells. Toxicol In
Vitro. 70:1050182021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
van den Brand AD, Villevoye J, Nijmeijer
SM, van den Berg M and van Duursen MBM: Anti-tumor properties of
methoxylated analogues of resveratrol in malignant MCF-7 but not in
non-tumorigenic MCF-10A mammary epithelial cell lines. Toxicology.
422:35–43. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gobin E, Bagwell K, Wagner J, Mysona D,
Sandirasegarane S, Smith N, Bai S, Sharma A, Schleifer R and She
JX: A pan-cancer perspective of matrix metalloproteases (MMP) gene
expression profile and their diagnostic/prognostic potential. BMC
Cancer. 19:5812019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Barzaman K, Karami J, Zarei Z,
Hosseinzadeh A, Kazemi MH, Moradi-Kalbolandi S, Safari E and
Farahmand L: Breast cancer: Biology, biomarkers, and treatments.
Int Immunopharmacol. 84:1065352020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tsang JYS and Tse GM: Molecular
classification of breast cancer. Adv Anat Pathol. 27:27–35. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lyons TG: Targeted therapies for
triple-negative breast cancer. Curr Treat Options Oncol. 20:822019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang B, Wang S, Xie H, Wang C, Gao X, Rong
Y, Liu Z and Lu Y: KIF18B promotes hepatocellular carcinoma
progression through activating Wnt/β-catenin-signaling pathway. J
Cell Physiol. 235:6507–6514. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu Y, Wang A, Zhu B, Huang J, Lu E, Xu H,
Xia W, Dong G, Jiang F and Xu L: KIF18B promotes tumor progression
through activating the Wnt/β-catenin pathway in cervical cancer.
Onco Targets Ther. 11:1707–1720. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Krishnamurthy N and Kurzrock R: Targeting
the Wnt/beta-catenin pathway in cancer: Update on effectors and
inhibitors. Cancer Treat Rev. 62:50–60. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tian D, Tian M, Ma ZM, Zhang LL, Cui YF
and Li JL: Anesthetic propofol epigenetically regulates breast
cancer trastuzumab resistance through IL-6/miR-149-5p axis. Sci
Rep. 10:88582020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li ZH, Lei L, Fei LR, Huang WJ, Zheng YW,
Yang MQ, Wang Z, Liu CC and Xu HT: TRIP13 promotes the
proliferation and invasion of lung cancer cells via the Wnt
signaling pathway and epithelial-mesenchymal transition. J Mol
Histol. 52:11–20. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Agarwal S, Behring M, Kim HG,
Chandrashekar DS, Chakravarthi BVSK, Gupta N, Bajpai P, Elkholy A,
Al Diffalha S, Datta PK, et al: TRIP13 promotes metastasis of
colorectal cancer regardless of p53 and microsatellite instability
status. Mol Oncol. 14:3007–3029. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu X, Shen X and Zhang J: TRIP13 exerts a
cancer-promoting role in cervical cancer by enhancing Wnt/β-catenin
signaling via ACTN4. Environ Toxicol. 36:1829–1840. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhu J, Zhou L, Wei B, Qian Z, Wang J, Hui
H and Sun Y: MiR-142-5p inhibits pancreatic cancer cell migration
and invasion by targeting PIK3CA. Mol Med Rep. 22:2085–2092. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang S, Sun S, Xu W, Yu B, Wang G and Wang
H: Astragalus polysaccharide inhibits breast cancer cell migration
and invasion by regulating epithelial-mesenchymal transition via
the Wnt/β-catenin signaling pathway. Mol Med Rep. 21:1819–1832.
2020.PubMed/NCBI
|