Introduction
Venous thromboembolism (VTE) in patients with cancer
(Ca) may exert a notable impact on both mortality and morbidity, as
VTE is the second leading cause of death in these patients
(1). Assessing the thrombotic
burden in patients with active Ca, referring to those who have been
diagnosed with a current or recent malignancy, those with
metastatic disease, or those who are receiving anticancer
treatment, remains a challenge, as patients with active Ca may
experience thromboembolic and bleeding complications (2). An individualized assessment of every
patient's profile is therefore required (3).
Many predisposing risk factors for VTE are comorbid
conditions that require active evaluation and management (4). Advanced age is recognized as a risk
factor (5), as the median age at
Ca diagnosis is 66 years (6), and
patients aged >40 years are at a higher risk of thrombosis,
while that risk doubles with each subsequent decade (7). Patients who are obese also experience
double the risk of VTE development, compared with normoweight
patients, and the results of a previous study demonstrated that the
higher the patient's weight, the higher the risk of VTE development
(7).
Patients with pancreatic, lung, gastric, uterine
corpus and cervical, kidney and brain primary tumors exhibit an
increased risk of VTE development (8,9).
Moreover, patients with metastatic disease at the time of diagnosis
present a 1.4–21.5X greater risk for thromboembolism, compared with
patients with non-metastatic disease. Additionally, previous
findings demonstrated that mucinous adenocarcinomas, such as those
of the pancreas or the lung, and cancers of the gastrointestinal
tract, exhibit the highest incidence rate of Ca-related VTE
(10–12).
Patients with active Ca who are undergoing systemic
treatment are among the highest-risk populations for thromboembolic
complications. High thrombotic burden agents (HTBAs) include
platinum compounds, 5-fluorouracil, capecitabine, gemcitabine,
hormonal therapy, anti-angiogenesis treatment, such as bevacizumab,
and supportive treatment, such as corticosteroids or erythropoietin
(13). In addition, VTE affects
patients who are being treated with immunotherapy, either as
monotherapy or in combination with other agents (14). The results of a previous study
demonstrated that ~1 in 3 patients may develop thrombosis, which
may exert a negative impact on survival (15).
Thrombotic events in patients with active Ca may
lead to complications in anticancer treatment, which may cause
delays in receiving treatment and affect treatment outcomes, thus
contributing to psychological and physical stress. The
aforementioned effects may also exacerbate the socioeconomic burden
of Ca, and they may exert a negative impact on the patients'
quality of life. Both progression-free survival (PFS) and overall
survival (OS) in patients who experience thromboembolic events
during systemic antineoplastic treatment are markedly affected,
compared with patients who do not experience VTE events (1,16,17).
In that context, the Hellenic Society of Medical
Oncology (HeSMO) conducted the Greek Management of Thrombosis
(GMaT) study (ClinicalTrials.gov identifier, NCT03292107) (18), a prospective multicenter
observational study aiming to record clinical practice in
Ca-associated thrombosis (CAT) management. The present post hoc
analysis aimed to obtain evidence for the justification of
prophylaxis administration in patients with active Ca, irrespective
of their assessment for VTE as this is evaluated via the Khorana
score. The present study also aimed to identify factors influencing
the decision making process of oncologists for the selection of
dose and duration of thromboprophylaxis, and to evaluate the
efficacy and safety of tinzaparin overall, as well as per dose
used.
Materials and methods
Study source and patient criteria
The present study is a post hoc analysis of primary
prophylaxis of CAT in patients with active Ca. The study source was
GMaT (18), a prospective
multicenter observational study (ClinicalTrials.gov code, NCT03292107), designed to
collect data associated with the management of CAT in routine
clinical practice and was conducted in a total of 18 oncology
departments across Greece. GMaT was conducted in Greece and a total
of 18 oncology departments participated. GMaT was conducted in
accordance to Helsinki declaration and was approved by the
Bioethics/Scientific Committees as St. Andrew Hospital, Patras,
Greece (approval number, 193-9/8/16, 9th August, 2016).
Participating patients signed an informed consent form. The
inclusion criteria were as follows: Age ≥18 years, histologically
confirmed solid tumors, use of anticoagulants for primary
prophylaxis or treatment, performance status of Eastern Cooperative
Oncology Group (ECOG) 0–2 and life expectancy beyond six
months.
The protocol used in the study source did not
provide specific guidance on the anticoagulant prophylaxis methods,
as oncologists followed their own individual or clinical practices.
The primary objective of the CAT prophylaxis was to record the
various approaches to thromboprophylaxis. Secondary aims were to
assess the incidence of VTE events, to record efficacy and bleeding
events and to assess the safety of current clinical practice. VTE
events were objectively confirmed by internationally recommended
imaging techniques (19,20).
For all patients, bleeding events were classified
using those recommended from the International Society on
Thrombosis and Haemostasis criteria (21–23).
The follow-up period was 12 months following patient enrolment. In
total, 546 patients with active Ca who were treated in an
ambulatory setting were included in the GMaT study. Out of these,
120 patients were diagnosed with objectively confirmed VTE, for
which they received treatment. Moreover, 426 patients were
considered to be at risk for VTE and received
thromboprophylaxis.
The study source reported the justification for the
administration of prophylaxis in patients with active Ca,
irrespective of Khorana score. In accordance with recent American
Society of Clinical Oncology guidelines, Khorana scores have
changed from ≥3 and <3, to ≥2 and <2 (24); thus, the present study reported
results based on the updated Khorana score values.
Moreover, the updated values were used to
investigate the low molecular weight heparin (LMWH) dose selection,
and to evaluate the safety and efficacy of the two different dose
schemes; namely, prophylactic dose (ProD) and intermediate dose
(InterD) used for primary prophylaxis of CAT.
As tinzaparin is used in the majority of cases and
different anticoagulation agents may exhibit differences in
efficacy and safety, the present study only focused on the use of
tinzaparin. The current analysis evaluated a total of 407 patients
who received thromboprophylaxis with tinzaparin.
Statistical analysis
The open source programme R (version 4.0.4) was used
for statistical analysis. Data were collected using the Excel 2007
spreadsheet (Microsoft Corporation), and were subsequently
conditioned and preprocessed, specifically: missing or erroneous
entries were easily identified and subsequently filled or corrected
and additional variables, such as patient age were calculated from
birth date and study inclusion date. The descriptive statistics for
the arithmetic data are presented as the mean value ± standard
deviation. Categorical data are presented as frequencies with
relative percentages. Comparisons among groups for the arithmetic
variables were performed using the Mann Whitney (MW) U test.
Non-parametric data were identified using the Kolmogorov-Smirnoff
test. Categorical variables were examined using the chi-square test
or Fisher's exact test, depending on the number of expected cases
within the groups under comparison. Moreover, in order to allow for
confounders that exhibited a role in the anticoagulation dose, the
odds ratios (ORs) were adjusted, and a forward selection logistic
regression model was used on all variables that were employed
during the univariate approach. P<0.05 was considered to
indicate a statistical significance, and all tests were two
sided.
Results
Patient characteristics
In total, 407 patients with active Ca receiving
thromboprophylaxis with tinzaparin were included in the current
cohort. Their baseline characteristics, the risk factors associated
with each patient, Ca type, treatment and biomarkers are displayed
in Table I, and are classified
according to the Khorana score (<2 or ≥2).
 | Table I.Baseline characteristics organized
into risk categories contributing to thrombotic burden related to
patients, cancer and treatment. |
Table I.
Baseline characteristics organized
into risk categories contributing to thrombotic burden related to
patients, cancer and treatment.
| Risk factor | All cases
(n=407) | Khorana score <2
(n=178; 43.7%) | Khorana score ≥2
(n=229; 56.3%) |
|---|
| Patient |
|
|
|
| Sex
(male), n (%) | 220 (54.1) | 81
(45.5) | 139 (60.7) |
| Age,
mean ± SD (% ≥65) | 65.2±11.3
(58.2) | 65.6±11.7
(43.9) | 64.9±10.9
(56.2) |
| BMI
>35 kg/m2, n (%) | 25 (6.1) | 3
(1.7) | 22 (9.6) |
| Smoking
(ex or current), n (%) | 244 (60.0) | 95
(53.4) | 149 (65.1) |
|
Previous surgical operation, n
(%) | 180 (44.3) | 91
(51.4) | 89
(38.9) |
|
Comorbidities, n (%) | 109 (26.9) | 43
(24.2) | 66
(28.8) |
| Severe
renal insufficiency, n (%) | 15 (3.7) | 8
(4.5) | 7
(3.1) |
| History
of trauma, (%) | 13 (3.2) | 12 (6.9) | 1
(0.5) |
| History
of immobility, n (%) | 61
(15.0) | 37
(20.8) | 24
(10.5) |
| History
of thrombosis, n (%) | 7
(1.7) | 6
(3.4) | 1
(0.5) |
| History
of bleeding, n (%) | 2
(0.5) | 1
(0.6) | 1
(0.4) |
| Ca, n (%) |
|
|
|
|
Lung | 102 (25.1) | 33
(32.4) | 69
(67.7) |
|
Pancreas | 58
(14.3) | 0
(0.0) | 58
(100.0) |
|
Breast | 37 (9.1) | 35
(94.6) | 2
(5.4) |
|
Stomach | 34 (8.4) | 0
(0.0) | 34
(100.0) |
|
Colorectal | 32 (7.9) | 26
(81.2) | 6
(18.8) |
|
Ovarian | 31 (7.6) | 15
(48.4) | 16
(51.6) |
|
Bladder | 22 (5.4) | 9
(40.9) | 13
(59.1) |
|
Prostate | 14 (3.4) | 13
(92.9) | 1
(7.1) |
|
Sarcomas | 11 (2.7) | 6
(54.5) | 5
(45.5) |
|
Liver | 5
(1.2) | 3
(60.0) | 2
(40.0) |
|
Testis | 5
(1.2) | 1
(20.0) | 4
(80.0) |
|
Cholangiocarcinoma | 4
(1.0) | 4
(100.0) | 0
(0.0) |
|
Larynx | 4
(1.0) | 3
(75.0) | 1
(25.0) |
|
Endometrial | 3
(0.7) | 0
(0.0) | 3
(100.0) |
|
Renal | 3
(0.7) | 3
(100.0) | 0
(0.0) |
|
Cervical | 2
(0.5) | 2
(100.0) | 0
(0.0) |
|
Oesophageal | 2
(0.5) | 2
(100.0) | 0
(0.0) |
|
Othera | 38 (9.3) | 23
(60.5) | 15
(39.5) |
|
Metastatic disease | 283 (69.5) | 121 (68.0) | 162 (70.7) |
| Treatment, n
(%) |
|
|
|
|
HTBAs | 270 (66.3) | 94
(52.8) | 176 (76.9) |
|
Platinum | 210 (51.6) | 87
(48.9) | 123 (53.7) |
|
Antimetabolites | 202 (49.6) | 73
(41.0) | 129 (56.3) |
|
Anti-angiogenesis | 32 (7.9) | 23
(12.9) | 9
(3.9) |
|
Immunotherapy | 20 (4.9) | 6
(3.4) | 14 (6.1) |
|
Erythropoietin | 71
(17.4) | 25
(14.0) | 46
(20.1) |
| Biomarker, n
(%) |
|
|
|
| Anemia
(Hg <10 g/l) | 85
(20.9) | 14 (7.9) | 71
(31.0) |
| PLT
count ≥350×109/liter | 153
(37.6) | 16 (9.0) | 137 (59.8) |
|
Leucocyte count
>11×109/liter | 95
(23.3) | 5
(2.8) | 90
(39.3) |
In the present cohort, the majority of patients who
received primary prophylaxis with tinzaparin had metastatic disease
(69.5%), and 270 (66.3%) patients were being treated with HTBAs.
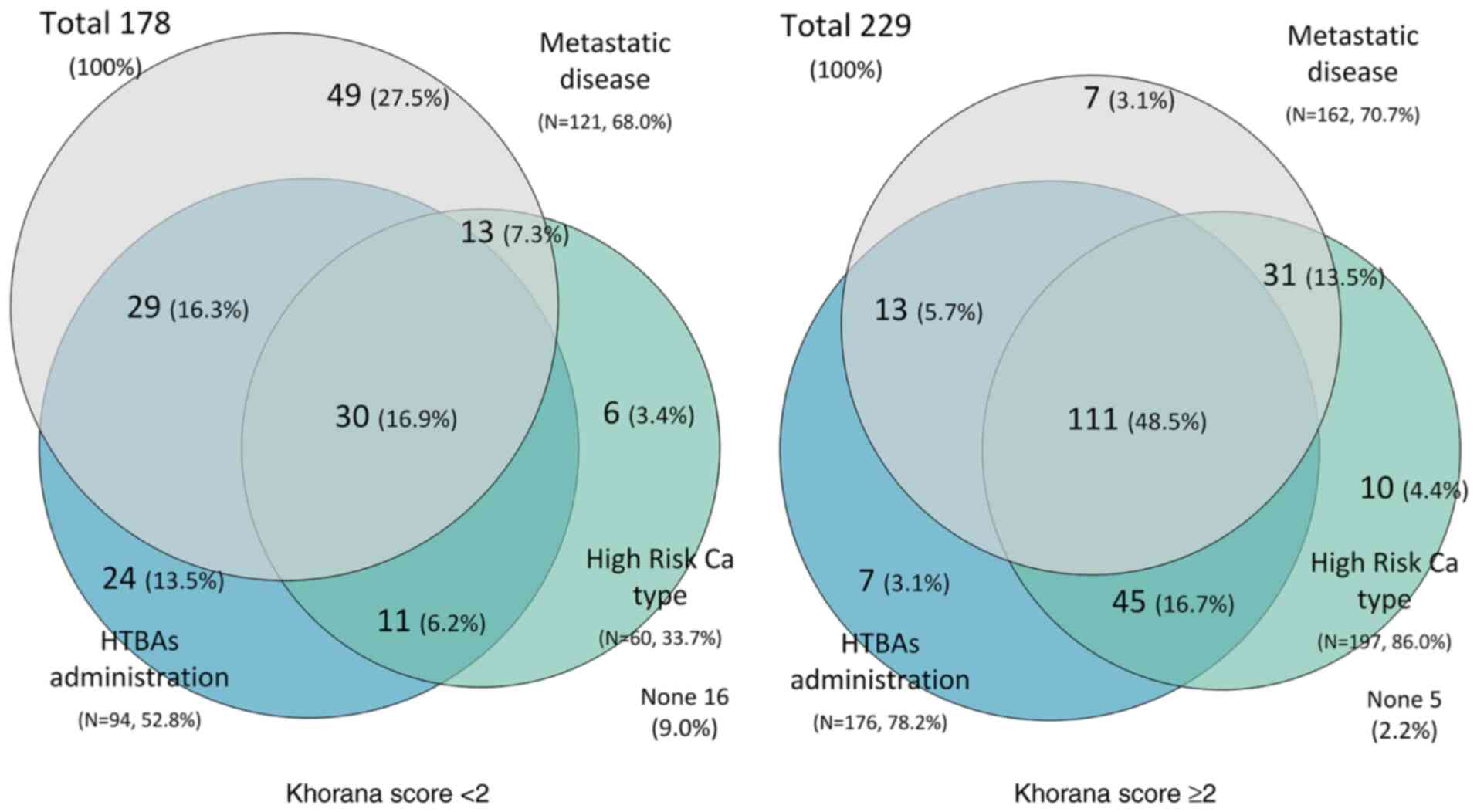
Out of all patients in the study, 178 (43.7%) exhibited a Khorana
score <2. Among them, 121 (68.0%) had metastatic disease, and 94
(52.8%) were being treated with HTBAs.
Notably, 30 of the patients in the group with a
Khorana score <2 (16.9%) had a high-risk for thrombosis Ca type
and metastatic disease, and were also being administered HTBAs. The
coexistence of metastatic disease together with HTBA administration
was observed in 29 (16.3%) patients. The number of patients with
metastatic disease who were receiving HTBAs and exhibited a Khorana
score <2 was 27.5%, compared with 3.1% in the Khorana ≥2 group
(P<0.0001). Similarly, the number of patients with
non-metastatic disease who were receiving HTBAs was 13.5% in the
Khorana score <2 group, compared with 3.1% in the Khorana score
≥2 group (P=0.0052). The coexistence of Ca and treatment-associated
risk factors are displayed in Fig.
1.
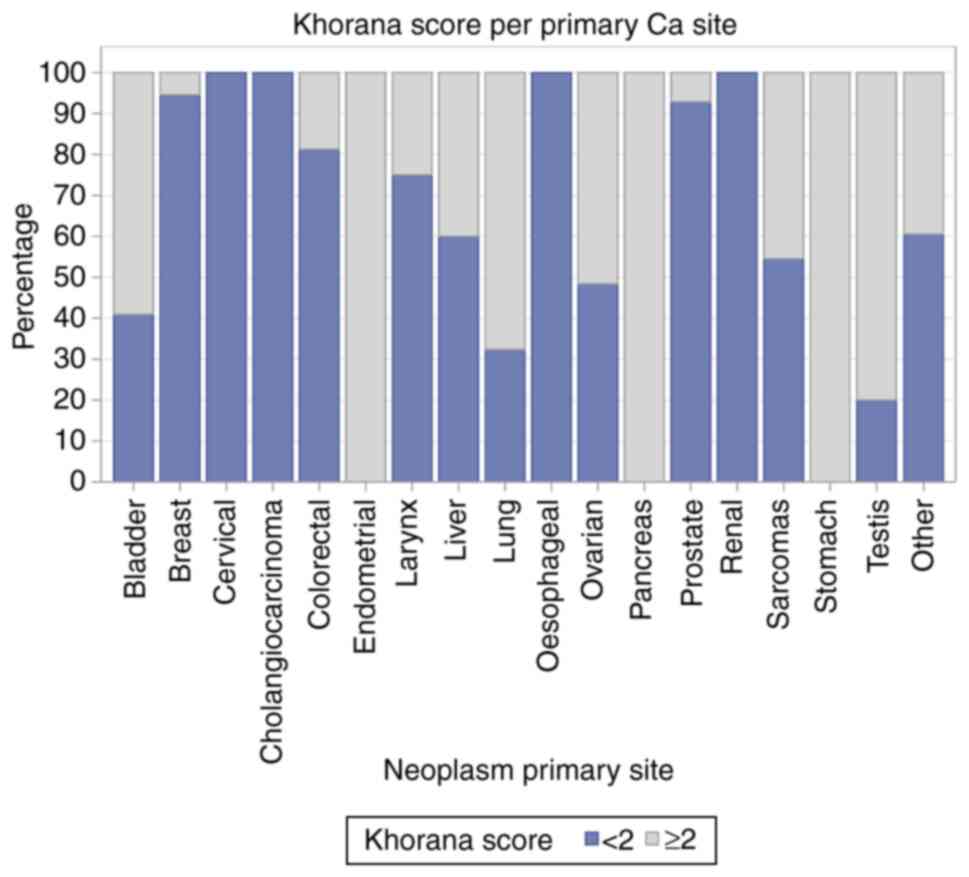
The distribution of patients with a Khorana score of
either <2 or ≥2, along with the Ca type, is displayed in
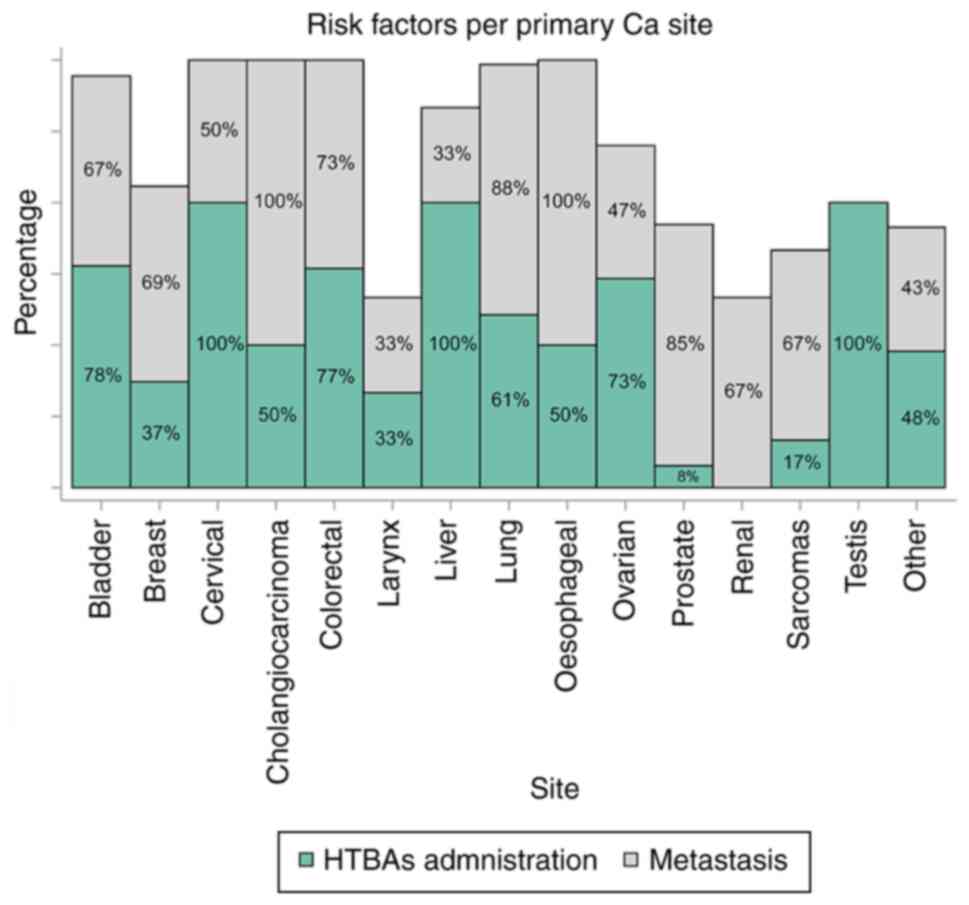
Fig. 2. The risk factors
associated with thrombosis and Ca, and the corresponding treatment
for patients with a Khorana score <2 are displayed in Fig. 3.
Thromboprophylaxis duration
On average, thromboprophylaxis with tinzaparin was
administered for 5.0±3.1 months. Patients with a Khorana score ≥2
received prophylaxis for a longer period of time (5.2±3.1 months),
compared to patients with a Khorana score <2. The latter
patients were receiving tinzaparin for 4.7±3.0 months; however, no
statistical significance was observed (MW test: P=0.0893). The
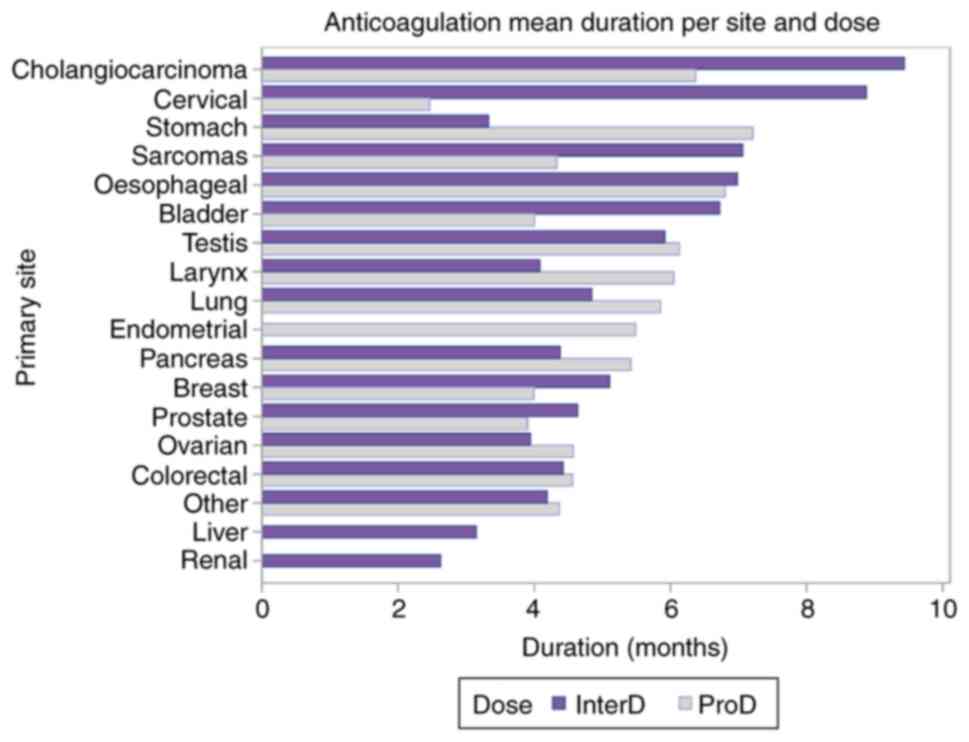
average duration of anticoagulation per Ca primary site is
demonstrated in Fig. 4.
Thromboprophylaxis dose
A total of 213 (52.4%) patients received an InterD
of 8,000-12,000 Anti-Xa IU, once daily. An InterD was administered
to 56.2% of the patients with a Khorana score <2, and 49.6% of
patients with a Khorana score ≥2 (OR, 0.76; 95% CI, 0.51–1.14;
P=0.1918).
A detailed univariate analysis between patients that
received a ProD of ≤4,500 IU compared with those who received an
InterD of tinzaparin was performed. A total of 21.6% of patients
who received an InterD had a body mass index (BMI) ≥30, compared
with 10.8% of those who received a ProD (x2 test:
P=0.0043). Moreover, a significant difference was observed between
the doses administered for patients with a BMI ≥35 (Table SI). Similarly, 69.1% of patients
who received an InterD were smokers or had a previous history of
smoking, compared with 53% of those who received a ProD
(χ2 test: P=0.0011). In the ProD group, 54.6% of
patients had a history of surgery, compared with 34% in the InterD
group. A total of 77% of patients who received an InterD were
suffering with metastatic disease, compared with 61.1% of those who
received a ProD (χ2 test: P=0.0007). A total of 26% of
those in the InterD group were administered erythropoietin,
compared with 9.2% in the ProD group (χ2 test:
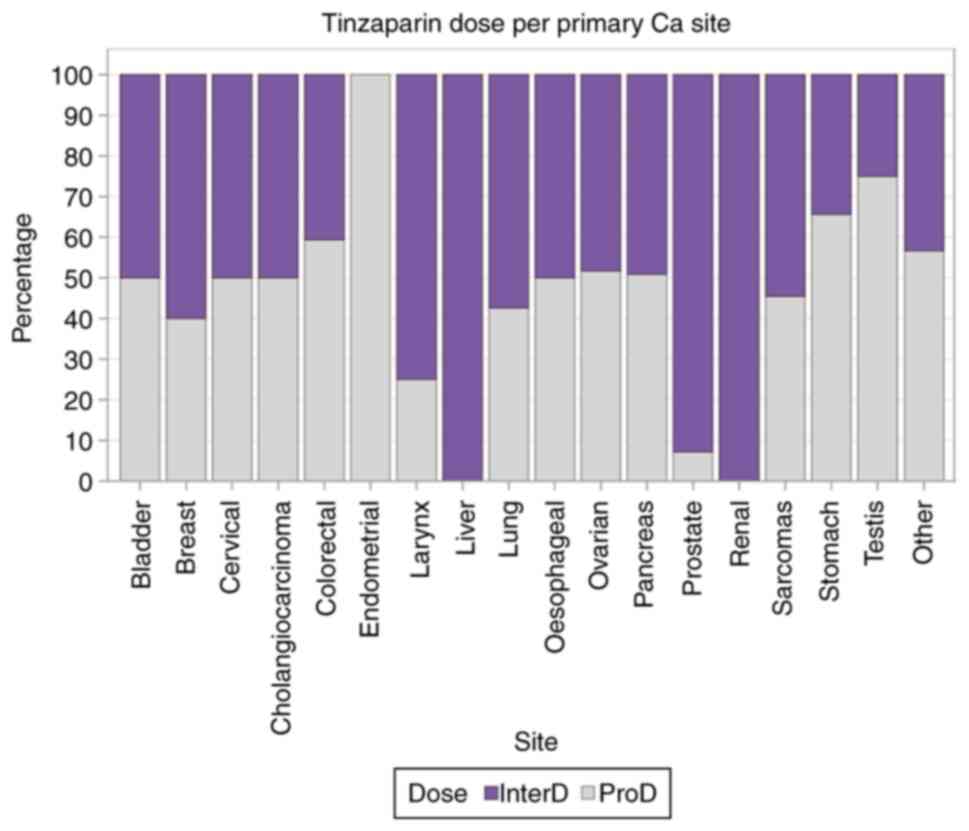
P<0.0001). The results are presented in Table SI. Finally, the percentages of
tinzaparin dose administered per primary Ca site is graphically
depicted in Fig. 5.
In addition to the aforementioned univariate
analysis, a multivariate analysis was performed using the logistic
regression method. The results obtained were similar to those
obtained using the univariate analysis. However, the results
demonstrated that a BMI cut-off value of 35 Kgr/m2
exerted no significant effects, and an InterD was more frequently
administered to patients who did not have low hemoglobin levels
(OR, 0.4; 95% CI, 0.2–0.8; P=0.013).
Thromboprophylaxis efficacy
Although arterial complications in patients with Ca
are less common than VTE, their incidence is continually observed
in a number of cases; therefore, both VTEs and arterial
complications were considered in the present study.
A total of 14 patients experienced thrombotic events
(3.4%; 95% CI, 2.1–5.7%); 8 experienced deep vein thrombosis, 4
experienced pulmonary embolism and 2 exhibited arterial thrombosis
events. Of these, a total of four events were incidental and 10
were symptomatic. In association with the primary Ca site, five
events were reported for patients with lung Ca, two were reported
for patients with breast Ca, while one event was reported in each
patient with either stomach, colorectal, cervical or bladder Ca.
Moreover, one case was reported in a patient with sarcoma and two
cases were reported for patients with Ca in other sites. Notably, a
high BMI was a significant risk factor. A total of 21% of patients
who experienced thrombotic events were grouped in class 2 (obese;
BMI ≥35 kg/m2), compared with 6% of patients who did not
experience thrombotic events, who were grouped in class 2 (obese;
OR, 4.6; 95% CI, 1.2–17.7; P=0.0476). Moreover, in association with
previous medical history, any type of trauma was associated with
thrombotic events (OR, 5.7; 95% CI, 1.1–28.8; P=0.0169).
Additionally, in terms of co-morbidities, 2 patients had
cardiological issues, 1 exhibited respiratory problems and 1 was
suffering from a metabolic disease. In total, 9 had metastatic
disease. Regarding those receiving treatment, 9 out of these 14
patients were being treated with HTBAs.
In total, 50% patients who experienced thrombosis
exhibited a Khorana score ≥2; 5 of them were administered with a
ProD, while 2 received an InterD of tinzaparin. Out of the
remaining 7 patients with a Khorana score <2, 5 received a ProD
and 2 received an InterD. Patients who received an InterD were less
likely to experience a thrombotic event, compared with those who
received a ProD (OR, 0.4; 95% CI, 0.1–1.1; P=0.0701). However, this
result was not statistically significant. When only VTE events are
considered, the OR becomes statistically significant (OR, 0.2; 95%
CI, 0.04–0.81; P=0.0126).
Thromboprophylaxis safety
Out of a total of 407 patients, 6 (1.5%; 95% CI,
0.7–3.1%) experienced a bleeding event, although all of these
events were categorized as minor. More specifically, 1 experienced
epistaxis, 1 experienced hematuria and 4 hemoptysis events were
observed. These events occurred in 0.5% of patients receiving ProD
and 2.3% of patients receiving InterD; however, no statistically
significant difference was observed. Table II demonstrates the various events
in association with Khorana score and the dose administered.
 | Table II.Observed events in relation to
tinzaparin dose and Khorana score. |
Table II.
Observed events in relation to
tinzaparin dose and Khorana score.
|
| ProD tinzaparin
dose ≤4,500 Anti-Xa IU, once daily (n=194) | InterD tinzaparin
dose 8,000-12,000 Anti-Xa IU, once daily (n=213) | Total tinzaparin
(n=407) |
|---|
|
|
|
|
|
|---|
| Adverse events | Number of events
(%) | KS | Number of events
(%) | KS | Number of events
(%) | KS |
|---|
| Thrombotic events
per KS | 5 (6.4) | <2 (n=78) | 2 (2.0) | <2 (n=100) | 7 (3.9) | <2 (n=178) |
|
| 5 (4.3) | ≥2 (n=116) | 2a (1.8) | ≥2 (n=113) | 7 (3.1) | ≥2 (n=229) |
| Total thrombotic
events, n (%) | 10 (5.2) |
| 4b (1.9) |
| 14 (3.4) |
|
| Bleeding events per
KS | 1 (1.3) | <2 (n=78) | 0 (0.0) | <2 (n=100) | 1 (0.6) | <2 (n=178) |
|
| 0 (0) | ≥2 (n=116) | 5 (4.4) | ≥2 (n=113) | 5 (2.2) | ≥2 (n=229) |
| Total bleeding
events, n (%) | 1 (0.5) |
| 5 (2.3) |
| 6 (1.5) |
|
Discussion
In the current cohort, 407 patients with various
types of active malignancies were administered tinzaparin as
primary prophylaxis. Almost 50% of the cohort population receiving
thromboprophylaxis exhibited a Khorana score <2. The
justification for using thromboprophylaxis was the presence of
metastatic disease, and whether HTBAs were being administered.
These factors were present in two thirds and >50% of the
patients, respectively. The average duration of prophylaxis was
5.0±3.1 months. In total, 213 (52.4%) received tinzaparin as an
InterD (8,000-12,000 Anti-Xa IU; once daily). Notably, the
following factors were found to be the main criteria for selection
of an InterD: A BMI >30 Kg/m2, previous history of
smoking, the presence of metastatic disease and previous
administration of erythropoietin. A total of 14 patients
experienced thrombotic events (3.4%), while 6 (1.5%) reported a
bleeding event; although all bleeding events were minor.
The risk for VTE was found to increase 6-fold in
outpatients receiving chemotherapy, as well as in those with
advanced stage of disease (25);
however, neither of these elements are included within the Khorana
score.
In the present cohort, factors influencing the
clinicians' decision to administer prophylaxis, apart from Khorana
score, were the presence of metastatic disease in 2 out of 3
patients, and the use of HTBAs in 50% of them.
Ca stage, rather than Ca type, is a dominant risk
factor for VTE. Specifically, metastasis is considered to be a key
risk factor for VTE (26).
Moreover, platinum compounds, 5-flurouracil and bevacizumab
(13) were received by ~50% of the
patients in the present study cohort, and these have been reported
to increase thrombotic risk by up to 18% as single agents (13). The coexistence of Ca and
treatment-associated factors further contributes to the risk of
thrombotic events.
With regards to patient-associated factors,
increased age and a history of smoking, which were found to be
present in 1 out of 2 patients with a Khorana score <2, have
previously been associated with an increased risk of VTE
development (27). Additionally, 1
out of 5 patients with a Khorana score <2 exhibited
comorbidities, including cardiovascular, endocrinological,
metabolic or respiratory disease, which could also contribute to
the overall risk of thrombotic events (4).
The average duration of prophylaxis with tinzaparin
lasted 5.0±3.1 months of anticancer treatment. A trend towards the
use of prophylaxis for longer periods was found in patients with a
Khorana score ≥2 (P=0.0893).
Regarding the dose administered, >50% patients
received tinzaparin as an InterD. No significant difference in the
selected dose (ProD or InterD) was observed between the groups of
patients with a Khorana score <2 or ≥2 (OR, 0.76; 95% CI,
0.51–1.14; P=0.1918). The main selection criteria for the use of an
InterD were as follows: a BMI >30 Kg/m2, previous
history of smoking, the presence of metastatic disease and previous
administration of erythropoietin. Notably, the administration of
InterD was consistent regardless of the aggressiveness of the
disease, and an increasing trend for the administration of InterD
was observed depending on the systemic anticancer treatment. The
percentages of patients receiving InterD were distributed as
follows: Preoperative, 23%; adjuvant, 34%; first-line, 50%;
second-line, 60%; and third-line, 65% (P=0.0364).
Two main RCTs (Randomized Control Trials) have
examined the impact of thromboprophylaxis on LMWHs in various Ca
types; namely, SAVE ONCO (28),
which looked at the use of semuloparin, and PROTECT (29), which examined the use of
nadroparin. The percentages of the different malignancies and those
of the different stages of the disease (advanced or metastatic)
which were included in the aforementioned studies were similar to
those included in the present post hoc analysis. In the PROTECT
study (29), the median
prophylaxis duration was <4 months, and in the SAVE ONCO study
(28) this was 3.5 months,
compared with the present cohort, in which the average duration was
longer (5±3.1 months). With regards to efficacy, thromboembolic
events were experienced by 2.0% of the patients treated with
nadroparin in the PROTECT study (29), and by 1.2% of the patients
receiving semuloparin in the SAVE ONCO study (28). In the present study, this was the
case with 5.2% of the patients who received a ProD, and 1.9% of the
patients who received an InterD of tinzaparin. In terms of safety,
minor bleeding events occurred in 7.4% of patients treated with
nadroparin in the PROTECT study, and major events in 0.7% of them.
The incidence rate of clinically relevant bleeding in the SAVE ONCO
study was 2.8%, and that of major bleeding was 1.2% in the
semuloparin group. In the present analysis, all bleeding events
reported were minor; specifically, the bleeding incidence was 0.5%
in the ProD group and 2.3% in the InterD group. In both the PROTECT
and SAVE ONCO trials, the dose used was the prophylactic dose.
A total of two DOAC (Direct Oral Anti-Coagulant)
trials examined the use of primary prophylaxis; the AVERT trial
(30), in which apixaban was
evaluated, and the CASSINI trial (31), in which patients were administered
with rivaroxaban. These studies only included patients with a
Khorana score ≥2. The comparable population in this cohort
(patients with a Khorana score ≥2) were 229 patients (56.3% of the
total population). In the AVERT trial (30), the median duration of the
prophylaxis period was 5.2 months in the apixaban group, and 4.3
months in the CASSINI trial, which is comparable with the median
duration in the present study (5±3.1 months). In the AVERT trial
(30), 4.2% cases of VTE were
reported, and in the CASSINI trial (31), 6.0% of the patients were diagnosed
with VTE. In the present analysis, VTE events were observed in 4.3%
of the patients receiving a ProD and in 1.8% of the patients
receiving an InterD of tinzaparin. Clinically relevant bleeding
events were observed in 9.0% of the patients treated with apixaban
in the AVERT study, and in 4.7% of the patients who received
rivaroxaban in the CASSINI trial. In the present study, bleeding
was not reported in patients who received a ProD, while minor
events were reported in 4.4% of the patients who received an
InterD.
Both the AVERT and CASSINI trials had excluded
patients with severe renal insufficiency [creatinine clearance
(CrCl) <30 ml/min]. Patients with chronic kidney disease are at
an increased risk of bleeding (32). In this cohort, severe renal
insufficiency was reported in 15 patients (3.7%), while one minor
bleeding event occurred in a patient with lung Ca. Tinzaparin
pharmacokinetics is of the first-order, as there is involvement of
both the cellular and renal elimination paths (33). In addition, tinzaparin does not
exhibit bioaccumulation, even in the presence of severe renal
impairment (34). Results of
previous studies demonstrated that tinzaparin does not accumulate
in patients with a CrCl as low as 20 ml/min (35). The aforementioned DOAC trial also
excluded patients with significant comorbidities, patients with a
predisposition for bleeding or a low-platelet count, and patients
undergoing chemotherapy, which may interact with DOACs. Notably, in
the present cohort, ~80% of the patients received anticancer
treatment with potential drug-drug interactions (DDIs) with DOACs.
Interactions with chemotherapy may reduce the efficacy of oral
anticoagulants or increase the risk of bleeding. Moreover,
chemotherapy may cause gastrointestinal disturbances, which may
affect oral anticoagulant bioavailability (36,37).
The present analysis demonstrated a potential
clinical benefit in the efficacy of thromboprophylaxis with the use
of ProD tinzaparin, consistent with the previously published data;
InterD of tinzaparin appeared to be more effective (4 thrombotic
events) compared with a ProD (10 thrombotic events) and this is
supported by marginally statistical significance (OR, 0.4; 95% CI,
0.1–1.1; P=0.0701). Considering only VTE events, patients who
received an InterD exhibited an 80% reduced risk of experiencing a
thrombotic event (OR, 0.2; 95% CI, 0.04–0.81; P=0.0126). Bleeding
is a frequent problem for patients with advanced Ca, with
approximately 10% of all patients having at least one episode
(38). Anticoagulation with
tinzaparin appeared to offer a balance between the competing risks
of clotting and bleeding in those patients, as only six minor
bleeding events were observed.
Patients included in this analysis had active Ca,
exhibited a high thrombotic burden, renal insufficiency and a type
of anticancer treatment. The present study was heavily reliant on
data obtained from day-to-day clinical practice and, as such, it is
not possible to make direct comparisons between the various groups.
An alternative approach is the use of DOACs for thromboprophylaxis
in patients with Ca, assuming the absence of significant risk
factors associated with bleeding and DDIs (24).
There were certain limitations to the present study,
as well as a number of advantages associated with observational
studies (39). Notably, the
present study involved a broad range of patients with no specific
focus on their characteristics. Thus, biases of an unknown nature
may have been present. For example, such heterogeneity between
patients may lead to a dilution of the beneficial effects of
prophylaxis against thrombosis. By contrast, such heterogeneity may
have allowed the impact of thrombosis-associated complications and
the potential benefits of anticoagulation intervention to be
highlighted in various different Ca types. Further limitations may
have been present; however, the present study summarized the
conditions of a common clinical oncology setting.
The present cohort includes practice-based evidence,
and its aim was to summarize the individualized stratification of
VTE risk in patients with active Ca.
Future randomized control studies, focusing on more
specific patient profiles with a high risk of thromboembolism
development, may be an attractive approach to assessing the
clinical benefits of thromboprophylaxis in terms of patient
outcomes.
In conclusion, personalized treatment is becoming an
increasingly attractive approach, and may allow oncologists to
consider the positive effects of antineoplastic agents without the
interference of challenges, such as thrombosis. The presence of
metastatic disease and the use of HTBAs appear to influence
oncologists' decisions for the use of thromboprophylaxis in
patients with active Ca, regardless of the Khorana score. The
results of the present study also demonstrated that an InterD of
tinzaparin was administered more frequently to patients with a BMI
≥30 kg/m2, a previous history of smoking and a history
of metastatic disease, along with a previous administration of
erythropoietin. Moreover, an InterD of tinzaparin was found to be
more efficacious for the prevention of VTE, without compromising
safety. Therefore, the administration of tinzaparin appears to
offer a promising solution for thromboprophylaxis in patients
undergoing a course of anticancer treatment.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Dr Abraham
Pouliakis, senior researcher at the 2nd Department of Pathology,
National and Kapodistrian University of Athens, Athens, Greece, for
statistical analysis.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AC, PPapakot, NT and IB conceived the study. AC, ET
and IB developed the methodology. AC and IB performed formal
analysis. AC, AAr, CP, GK, GPa, PPapakot, NT, CA, GS, PPapakos, GA,
NZ, MS, CK, ES, PM, GPe, AAt, HS, AB, AG, EST, IV, ET and IB
managed the subject patients included in the study and acquired the
data used in the analysis. AC wrote the original draft. IB reviewed
and edited the manuscript. IB supervised the study. NT and PPapakot
were responsible for project administration. AC, AAr, CP, GK, GPa,
PPapakot, NT, CA, GS, PPapakos, GA, NZ, MS, CK, ES, PM, GPe, AAt,
HS, AB, AG, EST, IV, ET and IB have critically revised the
manuscript for important intellectual content. AC, AAr, CP, GK,
GPa, PPapakot, NT, CA, GS, PPapakos, GA, NZ, MS, CK, ES, PM, GPe,
AAt, HS, AB, AG, EST, IV, ET and IB agreed to be accountable for
all aspects of this work in ensuring that questions related to the
accuracy or integrity of any part of the work are appropriately
investigated and resolved. AC, AAr and IB confirm the authenticity
of all the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was conformant with the Helsinki
Declaration and the relevant amendments and was approved by the
Bioethics Committee of ‘Saint Andrew’ General Hospital (Patras,
Greece). Written informed consent was obtained from all subjects
involved in the study for their participation.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Khorana AA, Francis CW, Culakova E,
Kuderer NM and Lyman GH: Thromboembolism is a leading cause of
death in cancer patients receiving outpatient chemotherapy. J
Thromb Haemost. 5:632–634. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
National Institute for Health and Care
Exellence, . Venous thromboembolic diseases: diagnosis, management
and thrombophilia testing. https://www.nice.org.uk/guidance/ng158/resources/venous-thromboembolic-diseases-diagnosis-management-and-thrombophilia-testing-pdf-66141847001797November
10–2021
|
|
3
|
Badescu MC, Ciocoiu M, Badulescu OV,
Vladeanu MC, Bojan IB, Vlad CE and Rezus C: Prediction of bleeding
events using the VTE-BLEED risk score in patients with venous
thromboembolism receiving anticoagulant therapy (Review). Exp Ther
Med. 22:13442021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Geerts WH, Pineo GF, Heit JA, Bergqvist D,
Lassen MR, Colwell CW and Ray JG: Prevention of venous
thromboembolism: The Seventh ACCP conference on antithrombotic and
thrombolytic therapy. Chest. 126 (Suppl 3):338S–400S. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ay C, Pabinger I and Cohen AT:
Cancer-associated venous thromboembolism: Burden, mechanisms, and
management. Thromb Haemost. 117:219–230. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
National Cancer Institute (NIH), . Age and
cancer risk. NIH; Bethesda, MD: 2021, https://www.cancer.gov/about-cancer/causes-prevention/risk/ageMarch
5–2021
|
|
7
|
American Heart Association, . Risk factors
for venous thromboembolism (VTE). American Heart Association;
Dallas, TX: 2021 https://www.heart.org/en/health-topics/venous-thromboembolism/risk-factors-for-venous-thromboembolism-vteLast
Reviewed. March 30–2017
|
|
8
|
Khorana AA and Connolly GC: Assessing risk
of venous thromboembolism in the patient with cancer. J Clin Oncol.
27:4839–4847. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Iorga RA, Bratu OG, Marcu RD, Constantin
T, Mischianu DLD, Socea B, Gaman MA and Diaconu CC: Venous
thromboembolism in cancer patients: Still looking for answers. Exp
Ther Med. 18:5026–5032. 2019.PubMed/NCBI
|
|
10
|
Haddad TC and Greeno EW:
Chemotherapy-induced thrombosis. Thromb Res. 118:555–568. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Blom JW, Osanto S and Rosendaal FR: The
risk of a venous thrombotic event in lung cancer patients: Higher
risk for adenocarcinoma than squamous cell carcinoma. J Thromb
Haemost. 2:1760–1765. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Khorana AA, Francis CW, Culakova E and
Lyman GH: Risk factors for chemotherapy-associated venous
thromboembolism in a prospective observational study. Cancer.
104:2822–2829. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Oppelt P, Betbadal A and Nayak L: Approach
to chemotherapy-associated thrombosis. Vasc Med. 20:153–161. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Roopkumar J, Swaidani S, Kim AS, Thapa B,
Gervaso L, Hobbs BP, Wei W, Alban TJ, Funchain P, Kundu S, et al:
Increased incidence of venous thromboembolism with cancer
immunotherapy. Med (NY). 2:423–434. 2021.PubMed/NCBI
|
|
15
|
Roopkumar J, Kim AS, Bicky T, Hobbs BP and
Khorana AA: Venous thromboembolism in cancer patients receiving
immunotherapy. Blood. 132 (Suppl 1):S25102018. View Article : Google Scholar
|
|
16
|
Sorensen HT, Mellemkjaer L, Olsen JH and
Baron JA: Prognosis of cancers associated with venous
thromboembolism. N Engl J Med. 343:1846–1850. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Khorana AA, Dalal MR, Lin J and Connolly
GC: Health care costs associated with venous thromboembolism in
selected high-risk ambulatory patients with solid tumors undergoing
chemotherapy in the United States. Clinicoecon Outcomes Res.
5:101–108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tsoukalas N, Papakotoulas P, Christopoulou
A, Ardavanis A, Koumakis G, Papandreou C, Papatsimpas G, Papakostas
P, Samelis G, Andreadis C, et al: Real-world data on
thromboprophylaxis in active cancer patients: Where are we? Are we
getting there? Cancers (Basel). 12:19072020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Estrada-Y-Martin RM and Oldham SA: CTPA as
the gold standard for the diagnosis of pulmonary embolism. Int J
Comput Assist Radiol Surg. 6:557–563. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zierler BK: Ultrasonography and diagnosis
of venous thromboembolism. Circulation. 109((12 Suppl 1)): SI9–S14.
2004.PubMed/NCBI
|
|
21
|
Elyamany G, Alzahrani AM and Bukhary E:
Cancer-associated thrombosis: An overview. Clin Med Insights Oncol.
8:129–137. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kaatz S, Ahmad D, Spyropoulos AC and
Schulman S; Subcommittee on Control of Anticoagulation, :
Definition of clinically relevant non-major bleeding in studies of
anticoagulants in atrial fibrillation and venous thromboembolic
disease in non-surgical patients: Communication from the SSC of the
ISTH. J Thromb Haemost. 13:2119–2126. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schulman S and Kearon C; Subcommittee on
Control of Anticoagulation of the Scientific and Standardization
Committee of the International Society on Thrombosis and
Haemostasis, : Definition of major bleeding in clinical
investigations of antihemostatic medicinal products in non-surgical
patients. J Thromb Haemost. 3:692–694. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Key NS, Khorana AA, Kuderer NM, Bohlke K,
Lee AYY, Arcelus JI, Wong SL, Balaban EP, Flowers CR, Francis CW,
et al: Venous thromboembolism prophylaxis and treatment in patients
with cancer: ASCO clinical practice guideline update. J Clin Oncol.
38:496–520. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Heit JA, O'Fallon WM, Petterson TM, Lohse
CM, Silverstein MD, Mohr DN and Melton LJ III: Relative impact of
risk factors for deep vein thrombosis and pulmonary embolism: A
population-based study. Arch Intern Med. 162:1245–1248. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ohashi Y, Ikeda M, Kunitoh H, Sasako M,
Okusaka T, Mukai H, Fujiwara K, Nakamura M, Oba MS, Kimura T, et
al: Venous thromboembolism in cancer patients: Report of baseline
data from the multicentre, prospective Cancer-VTE registry. Jpn J
Clin Oncol. 50:1246–1253. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gregson J, Kaptoge S, Bolton T, Pennells
L, Willeit P, Burgess S, Bell S, Sweeting M, Rimm EB, Kabrhel C, et
al: Cardiovascular risk factors associated with venous
thromboembolism. JAMA Cardiol. 4:163–173. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Agnelli G, George DJ, Kakkar AK, Fisher W,
Lassen MR, Mismetti P, Mouret P, Chaudhari U, Lawson F and Turpie
AG; SAVE-ONCO Investigators, : Semuloparin for thromboprophylaxis
in patients receiving chemotherapy for cancer. N Engl J Med.
366:601–609. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Agnelli G, Gussoni G, Bianchini C, Verso
M, Mandalà M, Cavanna L, Barni S, Labianca R, Buzzi F, Scambia G,
et al: Nadroparin for the prevention of thromboembolic events in
ambulatory patients with metastatic or locally advanced solid
cancer receiving chemotherapy: A randomised, placebo-controlled,
double-blind study. Lancet Oncol. 10:943–949. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Carrier M, Abou-Nassar K, Mallick R,
Tagalakis V, Shivakumar S, Schattner A, Kuruvilla P, Hill D,
Spadafora S, Marquis K, et al: Apixaban to prevent venous
thromboembolism in patients with cancer. N Engl J Med. 380:711–719.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Khorana AA, Soff GA, Kakkar AK, Vadhan-Raj
S, Riess H, Wun T, Streiff MB, Garcia DA, Liebman HA, Belani CP, et
al: Rivaroxaban for thromboprophylaxis in high-risk ambulatory
patients with cancer. N Engl J Med. 380:720–728. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Burlacu A, Genovesi S, Goldsmith D,
Rossignol P, Ortiz A, Kalra PA, Małyszko J, Banach M, Kanbay M and
Covic A: Bleeding in advanced CKD patients on antithrombotic
medication-a critical appraisal. Pharmacol Res. 129:535–543. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Johansen KB and Balchen T: Tinzaparin and
other low-molecular-weight heparins: What is the evidence for
differential dependence on renal clearance? Exp Hematol Oncol.
2:212013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Atiq F, van den Bemt PM, Leebeek FW, van
Gelder T and Versmissen J: A systematic review on the accumulation
of prophylactic dosages of low-molecular-weight heparins (LMWHs) in
patients with renal insufficiency. Eur J Clin Pharmacol.
71:921–929. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Siguret V, Pautas E, Février M, Wipff C,
Durand-Gasselin B, Laurent M, Andreux JP, d'Urso M and Gaussem P:
Elderly patients treated with tinzaparin (Innohep) administered
once daily (175 anti-Xa IU/kg): Anti-Xa and anti-IIa activities
over 10 days. Thromb Haemost. 84:800–804. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wu C and Lee AY: Novel or non-vitamin K
antagonist oral anticoagulants and the treatment of
cancer-associated thrombosis. Semin Thromb Hemost. 41:237–243.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Short NJ and Connors JM: New oral
anticoagulants and the cancer patient. Oncologist. 19:82–93. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Johnstone C and Rich SE: Bleeding in
cancer patients and its treatment: A review. Ann Palliat Med.
7:265–273. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Patsopoulos NA: A pragmatic view on
pragmatic trials. Dialogues Clin Neurosci. 13:217–224. 2011.
View Article : Google Scholar : PubMed/NCBI
|