Introduction
Although cytotoxic drugs, such as 5-fluorouracil
(5-FU), platinums, taxanes and mitomycin, as well as some of the
new targeted drugs, including the EGFR-antibody cetuximab, have
been successfully used together with radiotherapy, it is not always
apparent whether chemoradiotherapy provides a benefit over
radiotherapy alone (1–6). Thus, there is a requirement for drugs
that more selectively kill and/or radiosensitize tumour cells that
are resistant to chemotherapy and radiotherapy.
The dominant mode of action of photon radiation used
in radiotherapy for cancer is indirect radiolysis of water
molecules, which generates free oxygen radicals. This will induce
both DNA-DNA and DNA-protein cross-links and base lesions, as well
as cause damage to sugar moieties in the backbone of DNA, resulting
in single-strand breaks and double-strand breaks (DSBs). The number
of DSBs is considered to be the major determinant of
radiation-induced damage. Increased oxygen levels cause tumours to
be more sensitive to radiation and, although both normal and tumour
tissues vary in oxygenation, only tumours are considered to be
sufficiently hypoxic to impair cell killing induced by radiation
(7–11).
Nitazoxanide is a Food and Drug
Administration-approved antiprotozoal drug with several
characteristics theoretically suitable for the radiosensitization
of solid tumours (12). This drug
was recently identified to be selectively toxic to quiescent and
glucose-deprived tumour cells and was described as an oxidative
phosphorylation (OXPHOS) inhibitor (12). Nitazoxanide was also shown to
reduce hypoxia (12) in spheroids
and it is well established that hypoxic areas in tumours are
associated with resistance to radiotherapy (13). These findings suggest that
nitazoxanide, by increasing tumour oxygenation, could potentially
enhance the number of DSBs caused by the radiation-induced
formation of free oxygen radicals in hypoxic tumours.
Based on the aforementioned findings, the present
study aimed to investigate a potential radiosensitization effect of
nitazoxanide in clinically relevant models that have been recently
described (12). To evaluate the
effect of nitazoxanide and radiation, the colon cancer cell line,
HCT116 green fluorescent protein (GFP), was cultured as monolayers
or spheroids and evaluated using total cell kill or clonogenic
assays as read-outs. The expression level of γ-H2A histone family
member X (H2AX) was used to evaluate DSBs and the in vivo
antitumour effect was evaluated using HCT116 GFP cell xenograft
tumours in mice.
Materials and methods
Cells and cell culture
HCT116 GFP cells (human colon cancer cell line
HCT116 transfected with GFP; AntiCancer, Inc.) were cultured at
37°C in a humidified incubator containing 5% CO2 in
McCoy's 5A medium (Sigma-Aldrich; Merck KGaA) supplemented with 10%
heat-inactivated FBS, L-glutamine (2 mM), penicillin (100 U/ml) and
streptomycin (100 µg/ml). The morphology and proliferation of cells
were monitored on a weekly basis and the cell line was split twice
a week. The cell line was authenticated via short-term repeat
analysis performed by the cell bank (AntiCancer, Inc.). Cells were
passaged for <3 months after resuscitation.
Drugs, irradiation and cell culture
experiments
Drugs
Nitazoxanide was obtained from Selleck Chemicals.
For comparison and reference, the standard cytotoxic drug 5-FU
(Sigma-Aldrich; Merck KGaA) was included. 5-FU is the backbone
therapy for colorectal cancer chemotherapy regimens and is also in
clinical use as a radiosensitizer (1,14).
The oxygen and moisture free MiniPod™ system (Roylan
Developments Ltd.) was used to store source plates that were
prepared with drugs solubilized in DMSO. The liquid handling system
ECHO® 550 (Labcyte, Inc.) was used to add the drugs to
the experimental plates.
Monolayer experiments
The monolayer experiments were performed as
described previously, with minor modifications (15). Briefly, on day 0 of the experiment,
50 µl cell suspension (1,000 cells/well) was added into 384-well
plates and pre-incubated in 37°C overnight at standard cell culture
conditions. On day 1, the drug was added and experimental plates
were irradiated with an external low dose-rate γ radiation source
(GammaCell 40 Exactor; Best Theratronics, Ltd.) 4–6 h thereafter.
Cell viability was assessed using the fluorometric microculture
cytotoxicity assay (FMCA) and clonogenic assay as later
described.
Spheroid experiments
The formation of HCT116 GFP quiescent multicellular
tumour spheroids (Q-MCTS) was previously described in detail
(12). Briefly, 384-well
Corning® black clear bottom ultra-low attachment
microplates (Corning, Inc.) were used with 50 µl cell suspension,
with 10,000 cells seeded into each well on day 0. Q-MCTS formed
during the 7 day culture without a change of medium. The
ECHO® 550 liquid handler was used to add the drugs on
day 7. Spheroids were incubated with drugs for 4–6 h and then
irradiated as aforementioned. Following 7 days of drug incubation,
cell viability was assessed using the FMCA and the GFP assay for
total cell kill, while a clonogenic assay was used for the
assessment of the effect in clonogenic cells only (see below).
Measurement of cellular cytotoxicity
Total cell kill assay in monolayers
Following 3 or 7 days of drug incubation,
cytotoxicity in the total cell population was assessed using FMCA
as previously described (15). The
FMCA is based on fluorescein diacetate that is converted to
fluorescent fluorescein by viable cells with an intact plasma
membrane (15).
Total cell kill assay in spheroids
Following the aforementioned spheroid experiments,
cytotoxicity in the total cell population was assessed using FMCA
and GFP assays. The GFP assay is based on the fluorescent signal
generated from viable HCT116 GFP cells of the intact spheroids, as
described previously (12). In the
FMCA, 40 µl medium was aspirated from each well and 50 µl
Accumax/well was added. The plates were then incubated at 37°C for
30 min, followed by the dissociation of spheroids into single cells
using a multipipette. The FMCA procedure was then performed for the
monolayer cultured cells, as described previously (15).
Clonogenic assay on monolayer and
spheroid cultures
In the monolayer cultures, ~20 h after radiation, 40
µl medium was aspirated from each well and 50 µl Accumax/well was
added. After incubation at 37°C for 30 min, mixing, centrifugation,
removal of Accumax solution, the addition of 50 µl fresh medium and
subsequent mixing, 20 µl cell solution from each well was
transferred together with 3 ml fresh medium into each well in
6-well plates (Nalge Nunc International; Thermo Fisher Scientific,
Inc.).
Spheroids were dissociated into single cells as
aforementioned at ~20 h after radiation, followed by removal of
Accumax solution and then addition of 50 µl fresh medium. In total,
20 µl cell solution from each well was transferred together with 3
ml fresh medium to 6-well plates (Nalge Nunc International; Thermo
Fisher Scientific, Inc.) and the procedure was then performed
identical to that described for monolayer experiments.
The 6-well plates were incubated at 37°C for 10 days
and cells were then fixed and stained as previously described by
Franken et al (16). A
Canon iR-ADV C5235i printer (Canon, Inc.) was used to scan plates
and number of colonies were then counted on the computer screen.
Only colonies with >50 cells were counted.
Assessment of DSBs via
immunohistochemistry (IHC)
The formation of spheroids and exposure to drugs and
radiation were performed as previously described. Subsequently, 24
h after radiation, spheroids were harvested into Eppendorf tubes
and washed once in PBS. Spheroids were embedded in paraffin,
sectioned and evaluated for DSBs using the anti-γ H2AX
(phosphorylated S139) antibody [9F3] against the synthetic peptide
phosphorylated (Ser139) human H2AX (cat. no. ab26350; Abcam) and
for apoptosis using antibodies against Annexin V (cat. no.
ab108321; Abcam) and caspase-3 (cat. no. 9664; Cell Signaling),
according to the manufacturer's protocols. In the staining process,
the commercially available MACH1 Universal HRP-Polymer Detection
kit (Biocare Medical) with peroxide block for 5 min with Biocare's
Peroxidazed 1 (without protein block) was used. Antibodies were
used at the following dilutions: H2AX (1:1,000), Annexin V
(1:1,000) and caspase-3 (1:100). The incubation time was 20 min.
IHC staining was assessed using a light microscope at ×400
magnification.
Assessment of nitazoxanide and radiation
effects in tumour xenografts
In total, 40 female 8-week old NMRI nu/nu mice
(Crl:NMRI-Foxn1nu) from Charles River Laboratories were injected
subcutaneously at the right flank with a 50-µl cell suspension
containing 5×106 HCT116 GFP cells mixed with 50 µl
Matrigel. The animals were included into the study in groups of 10
animals/group when the majority of the tumours had reached a size
of ~0.1 cm3. The groups were as follows: Group 1,
vehicle; group 2, vehicle + irradiation; group 3, nitazoxanide; and
group 4, nitazoxanide + irradiation. Starting at day 0,
drug/vehicle administration occurred twice daily for 3 days (there
was only one administration on day 2). Vehicle control (1%
carboxymethylcellulose in PBS with 8% DMSO) and nitazoxanide (200
mg/kg) were administered via oral gavage. Temperature was kept at
21–22°C, humidity was 50–60% and a 12-h light, 12-h dark cycle was
applied. Autoclaved tap water was available ad libitum in
water bottles and the animals received R70, irradiated, diet
(Lantmännen AB).
At ~4 h after the last drug administration, the
tumours of the animals were irradiated with 6 Gy. The irradiated
area, with the tumour centred, measured 2×2 cm on the right rear
flank. With the setting of 320 kV and filter F2, the total
radiation dose of 6 Gy was delivered at 0.73 Gy/min. An X-RAD 340
(PXi; Precision X-ray, Inc.) was used for the radiation. The length
and width of each tumour was measured using a calliper twice
weekly, and the tumour volume (TV) was calculated using the
formula: Length (cm) × width (cm) × width (cm) × 0.44. Body weight
was recorded at the start of the study, along with tumour
measurements, and at the end of the study. The animals were checked
daily for any change in activity and appearance, as signs of a
change in general health status. The animals were euthanized via
cervical dislocation at the end of the study on day 28, and the
tumours were then dissected and placed into 4% buffered
formaldehyde solution for subsequent histopathological
examination.
Tumours were embedded in paraffin, sectioned,
evaluated for H&E staining and electronically scanned according
to standard protocols. The analysis algorithm ‘Positive Pixel Count
v9’ in Aperio ImageScope (v12.3.2.8013; Leica Microsystems GmbH)
was slightly modified (Table SI).
Using this algorithm, strong positive areas (SPA) corresponded well
to areas with cells visually classified as HCT116 GFP cells under
the microscope. Thus, SPA/(weak positive areas (WPA) + SPA) ×100
was used as a surrogate marker for the fraction of HCT116 GFP cells
in the tumour and was referred to as the tumour cell percentage
(TCP). The tumour cell volume (TCV) was calculated as TCP ×
TV/100.
Statistical analysis and
presentation
The calculations and graphical presentations were
performed in GraphPad Prism 7 (GraphPad Software, Inc.).
Dose-response curves are presented as the mean ± SEM for the number
of experiments indicated. Cell viability data in the FMCA for both
spheroid and monolayer experiments are presented as survival index
(SI), defined as the fluorescence in experimental wells in % of
that in unexposed control wells, with the fluorescence of the blank
wells subtracted. An AUTO SI was calculated in the GFP assay, and
defined as the spheroid fluorescence in experimental wells in % of
that in the same wells immediately before addition of
drug/radiation 7 days earlier.
In the clonogenic assays, a slightly modified
definition was used to calculate surviving fraction (SF), which was
defined as the number of colonies in % of that in unexposed control
wells, since plating efficiency was not assessed. The interaction
between drug and radiation was characterized using the mean SI (or
SF) for wells treated with drug only (SId or
SFd) and the mean SI (or SF) for wells irradiated only
(SIr or SFr). An expected combination SI or
SF (SIe or SFe) was calculated as follows:
SId × SIr=SIe (or SFd ×
SFr=SFe), in accordance with independent
Bliss interaction (17). To obtain
an interaction ratio for each individual experiment, the SI (or SF)
actually observed for the combination SIo (or
SFo) was divided by SIe (or SFe).
For each group in Tables I and
II, based on the number of
experiments indicated in the table legend, one-sample t-test was
used to calculate if the interaction ratio was different from the
value 1.
 | Table I.Interaction ratios of nitazoxanide
and radiation combinations in the FMCA assay. |
Table I.
Interaction ratios of nitazoxanide
and radiation combinations in the FMCA assay.
|
| Monolayer (3
days) | Monolayer (7
days) |
|
|---|
|
|
|
|
|
|---|
|
SIo/SIe (µM) | 2 Gy | 4 Gy | 6 Gy | 2 Gy | 4 Gy | 6 Gy | Spheroids 6 Gy |
|---|
| 100 | - | - | - | - | - | - | - |
| 50 | - | - | - | - | - | - | - |
| 25 | 1.030 | 1.154 | 1.390 | 1.221a | 1.156a | 1.263a | - |
| 12.5 | 1.096 | 1.123 | 1.269a | 2.186a | 3.742a | 4.435a | 1.043 |
| 6.5 | 1.064 | 1.091 | 1.169 | 1.301a | 2.544a | 3.254a | 0.996b |
| 3 | 1.060 | 1.063a | 1.152 | 1.201 | 1.552a | 1.819a | 1.037 |
| 1.5 | 1.105 | 1.096 | 1.174 | 1.051 | 1.267a | 1.379a | 1.020 |
| 1 | 1.047a | 1.042 | 1.144 | 1.057 | 1.195 | 1.381a | 1.054 |
| 0.5 | 1.032 | 1.035 | 1.094 | 1.023 | 1.139a | 1.292 | 1.064 |
 | Table II.Interaction ratios of drug and
radiation combinations in the clonogenic assay in cells cultured as
spheroids. |
Table II.
Interaction ratios of drug and
radiation combinations in the clonogenic assay in cells cultured as
spheroids.
|
SFo/SFe (µM) | 5-FU + 4 Gy | 5-FU + 6 Gy | Nitazoxanide + 4
Gy | Nitazoxanide + 6
Gy |
|---|
| 100 | 0.57b | 0.67 | - | - |
| 50 | 0.61a | 0.58 | - | - |
| 12.5 | 0.88 | 0.96 | - | - |
| 6.5 | 0.81 | 0.90 | 0.16b | 0.14b |
| 3 | - | - | 0.47c | 0.39a |
In the tumour xenograft experiments, TVs are
presented as the mean ± SEM. Differences in TV between the groups
were calculated using a repeated measures (from day 0 to 28)
two-way ANOVA followed by Tukey's multiple comparisons test in
GraphPad Prism. When animals were euthanized pre-term, the last
data point was carried forward and included in the calculations of
the means and in the statistical analysis. Differences in TCP and
TCV were calculated using one-way ANOVA followed by Tukey's
multiple comparisons test in GraphPad Prism. In total, three
animals that were euthanized pre-term were excluded from the
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
Total cell kill assay in monolayer-
and spheroid cultures
The selective activity of nitazoxanide against
nutrient-deprived cells grown as multicellular tumour spheroids
(IC50 1.44 and 2.41 µM in the GFP assay and FMCA,
respectively), compared with monolayer cultures (IC50
5.35 µM in the FMCA), following 7 days of drug exposure was
confirmed in the total cell kill assay (Fig. 1A upper panel and B). This effect
was in contrast to most other drugs, which are usually considerably
more active against monolayer cells compared with cells grown as
spheroids (12). Radiation alone
was modestly active in monolayer cultures, with a slightly greater
effect after 3 vs. 7 days of culture following radiation (Fig. 1A lower panel).
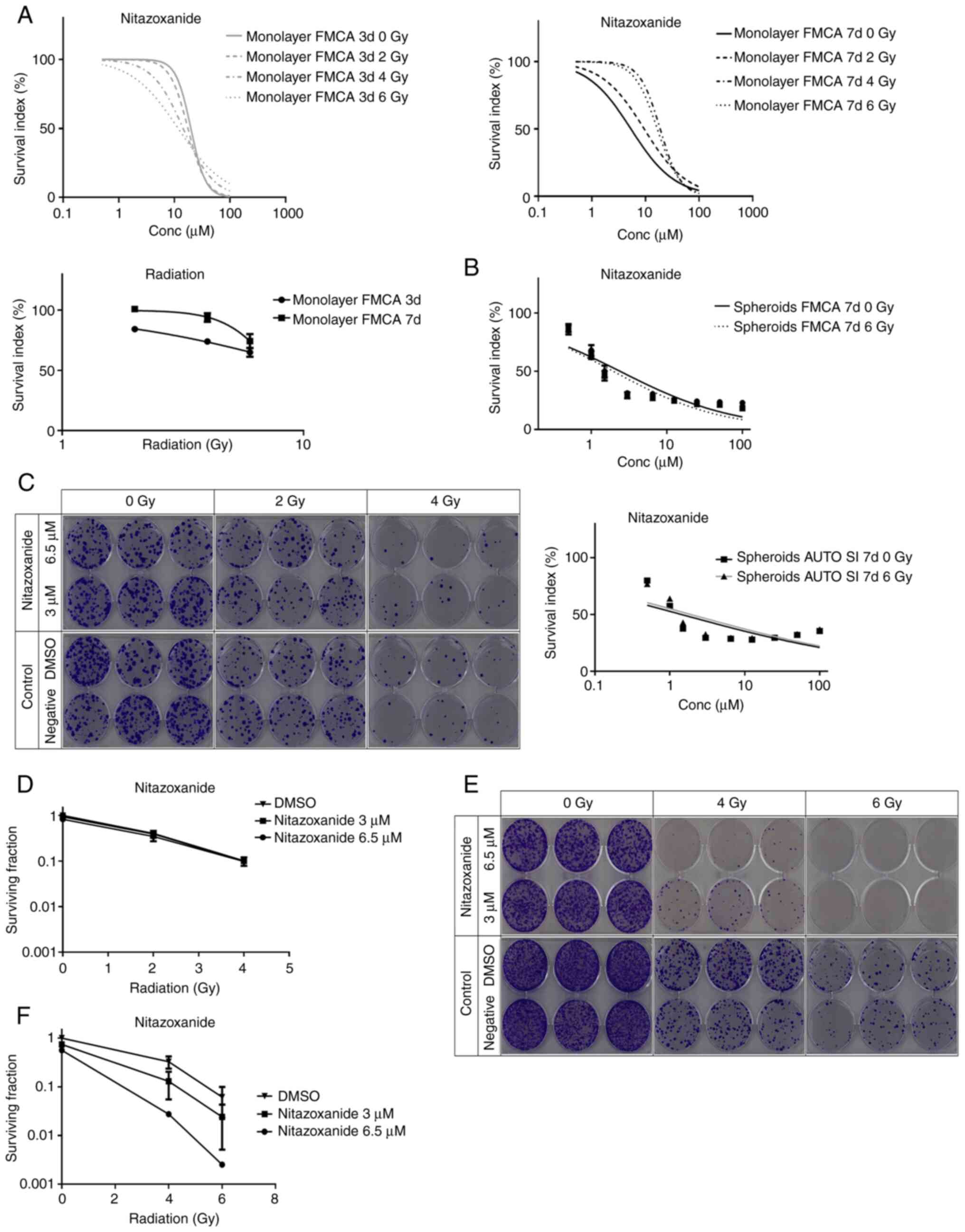 | Figure 1.Cell survival in the FMCA-, GFP- and
clonogenic assays. (A) Cell survival in the FMCA assay, expressed
as SI of HCT116 GFP cells cultured as monolayers, pre-incubated
overnight and then incubated with drugs for 3 or 7 days with
irradiation (2–6 Gy) at 4–6 h after addition of drug (upper panel).
Effect of 2, 4 and 6 Gy radiation in HCT116 GFP cells incubated
with DMSO for 3 and 7 days (lower panel). n=3 independent
experiments; SEM (generally <8) were omitted in upper panels.
(B) Cell survival in the total cell kill assay, expressed as SI
(FMCA, upper panel) or AUTO SI (GFP assay, lower panel) of HCT116
GFP cells cultured as spheroids for 7 days, then incubated with
drugs for 7 days with irradiation (6 Gy) 4–6 h after addition of
drug. n=7-8 independent experiments. Drug concentrations used were:
0.5, 1.0, 1.5, 3.0, 6.5, 12.5, 25, 50 and 100 µM. (C) Clonogenic
assay with nitazoxanide, shown as growth of HCT116 GFP cells
cultured as monolayers, pre-incubated overnight in 384-well plates,
with no irradiation (control) or irradiated (2 or 4 Gy) at 4–6 h
after drug addition and 20 h later dissociated into single cells,
transferred to six-well plates and incubated for 10 days.
Triplicate wells were used for each drug concentration. (D) Cell
survival in the clonogenic assay, expressed as surviving fraction
of HCT116 GFP cells cultured as monolayers, pre-incubated overnight
in 384-well plates, with no irradiation (DMSO control) or
irradiated (2 or 4 Gy) at 4–6 h after drug addition and 20 h later
dissociated into single cells, transferred to six-well plates and
incubated for 10 days. n=4 independent experiments. (E) Clonogenic
assay with nitazoxanide, shown as growth of HCT116 GFP cells
cultured as spheroids for 7 days, with no irradiation (control) or
irradiated (4 or 6 Gy) at 4–6 h after drug addition and 20 h later
dissociated into single cells, transferred to six-well plates and
incubated for 10 days. Triplicate wells were used for each drug
concentration. (F) Cell survival in the clonogenic assay, expressed
as surviving fraction of HCT116 GFP cells cultured as spheroids for
7, with no irradiation (DMSO control) or irradiated (4 or 6 Gy) at
4–6 h after drug addition, and 20 h later dissociated into single
cells, transferred to six-well plates and incubated for 10 days.
n=3 independent experiments, with triplicate wells for each drug
concentration. Data are presented as the mean ± SEM. SI, survival
index; FMCA, fluorometric microculture cytotoxicity assay; Conc,
concentration; GFP, green fluorescent protein. |
Interestingly, in the monolayer experiments,
radiation seemingly ‘protected’ the cells from the effect of
nitazoxanide after 7 days, but not after 3 days, of drug exposure
(Fig. 1A upper panel). Cell
survival was higher after 7 days compared with that at 3 days after
radiation only, indicating that the radiation doses used in this
experiment induced transient inhibition of cell proliferation
rather than cell death.
Radiation at 6 Gy had little effect on cell survival
in spheroids (SI 92 and 93% in the GFP assay and FMCA,
respectively). Thus, monolayer cells were more sensitive than
spheroids to radiation (Fig. 1A and
B). Moreover, nitazoxanide showed no statistically significant
synergistic effects with radiation in the total cell kill assays
for monolayer and spheroid cultures (Table I).
Clonogenic assay in monolayer and
spheroid cultures
The nitazoxanide-radiation interaction was further
investigated in the clonogenic assay (Fig. 1C-F; Table II). Nitazoxanide interacted
synergistically with radiation in a radiation dose-dependent manner
in cells grown as spheroids (Fig. 1E
and F; Table II). By
contrast, there was no synergy in monolayer cell cultures (Fig. 1C and D). The clonogenicity of cells
from spheroids was markedly affected by radiation only [SF
36.1±5.16 and 8.13±1.94% (mean ± SEM) at 4 and 6 Gy, respectively],
but less so than cells from monolayers [SF 40.3±6.05 and 10.1±2.57%
(mean ± SEM) at 2 and 4 Gy, respectively]. Although the clinically
most used radiosensitizer in colorectal cancer, 5-FU, at high
concentrations (50–100 µM) showed radiosensitizing effects in
spheroids, the effect was not as strong as that with nitazoxanide
(Table II). The lack of the
effect of nitazoxanide alone in the clonogenic assay in spheroids
was expected from the short incubation time in this assay.
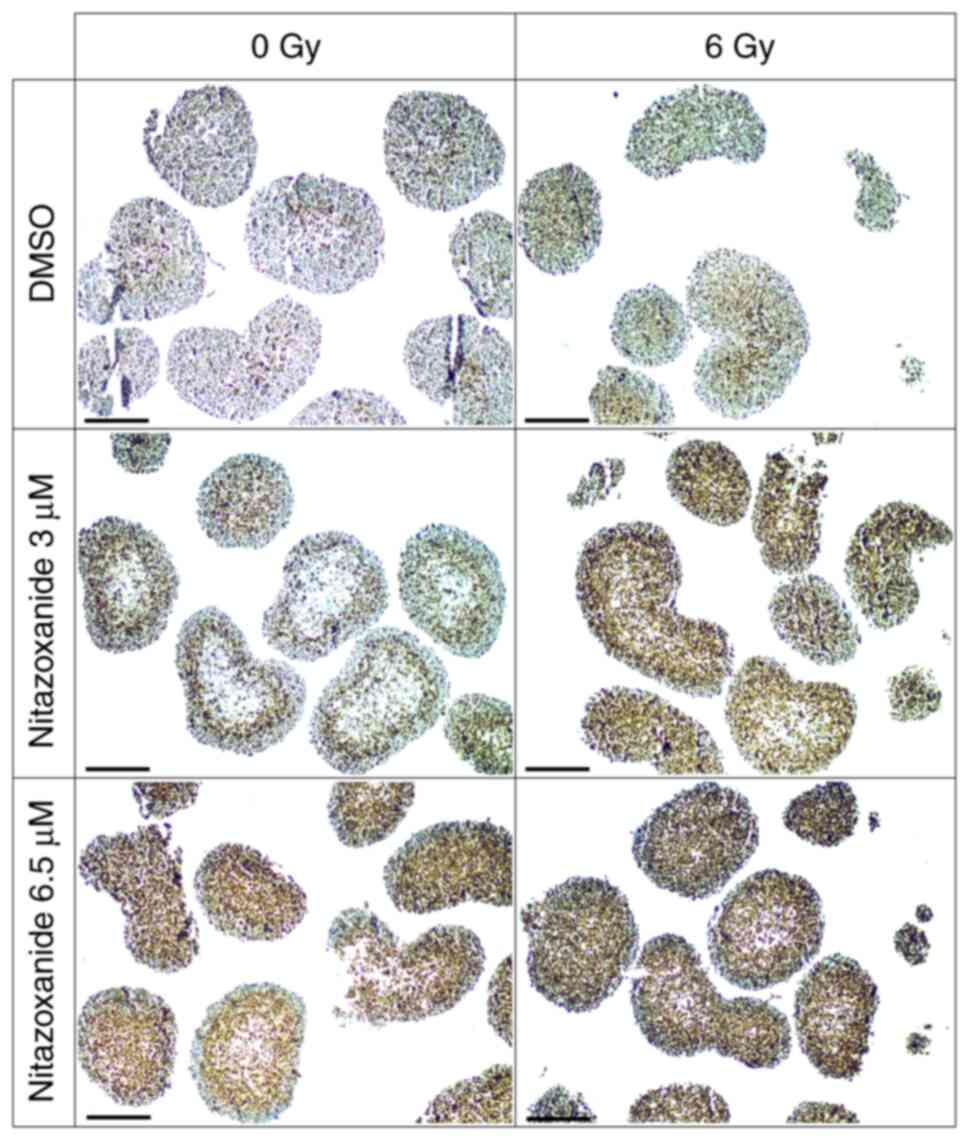
IHC for assessment of DSBs
A necrosis-like pattern was observed centrally in
spheroids exposed to nitazoxanide (Fig. 2). Since γ-H2AX expression may
reflect apoptosis, as well as DSBs, apoptosis was evaluated using
antibodies against both Annexin V and caspase-3 (Figs. S1 and S2) No notable difference
was observed between the treatment groups and, thus, a difference
in γ-H2AX expression, as determined via IHC, was considered due to
DSBs.
DSBs were induced in spheroids by 3 and 6.5 µM
nitazoxanide, and were determined using the IHC assessment of
γ-H2AX expression. Exposure to nitazoxanide and radiation resulted
in higher γ-H2AX expression compared with that after each treatment
alone (Fig. 2). The intensity of
the γ-H2AX staining was considerably stronger in central parts of
spheroids exposed to the combination compared with spheroids
exposed to nitazoxanide or radiation only, which was indicative of
a selective radiosensitizing effect towards more centrally located
cells. As shown in Fig. 2, DSBs
were markedly induced in spheroids by 6 Gy of radiation.
Nitazoxanide and radiation effects in
tumour xenografts
The antitumour activity and radiosensitizing
properties of nitazoxanide were investigated in HCT116 GFP cell
xenograft tumours in mice. It was found that, over time, ulcers
developed on some tumours that penetrated the skin, and one animal
in each group, but the nitazoxanide + radiation group, was
euthanized pre-term. There was a temporary decrease in body weight
after the treatments started in the two irradiated groups, but the
decrease was recaptured 4 days later, and the animals then gained
weight with no obvious differences between the groups. Animal
behaviour was seemingly normal and similar in all study groups. The
maximum size observed for a single tumor during the present study
was length 2.30 cm and width 1.60 cm, corresponding to a volume of
2.59 cm3 and was observed at day 28 in the control
vehicle group.
The TVs increased in all groups throughout the study
and the vehicle-treated animals developed the largest tumours
(Fig. 3A). Animals treated with
nitazoxanide and/or radiation developed smaller tumours compared
with those treated with the vehicle. Moreover, the combination
treatment of nitazoxanide + radiation resulted in no further
inhibition of tumour growth compared with radiation only.
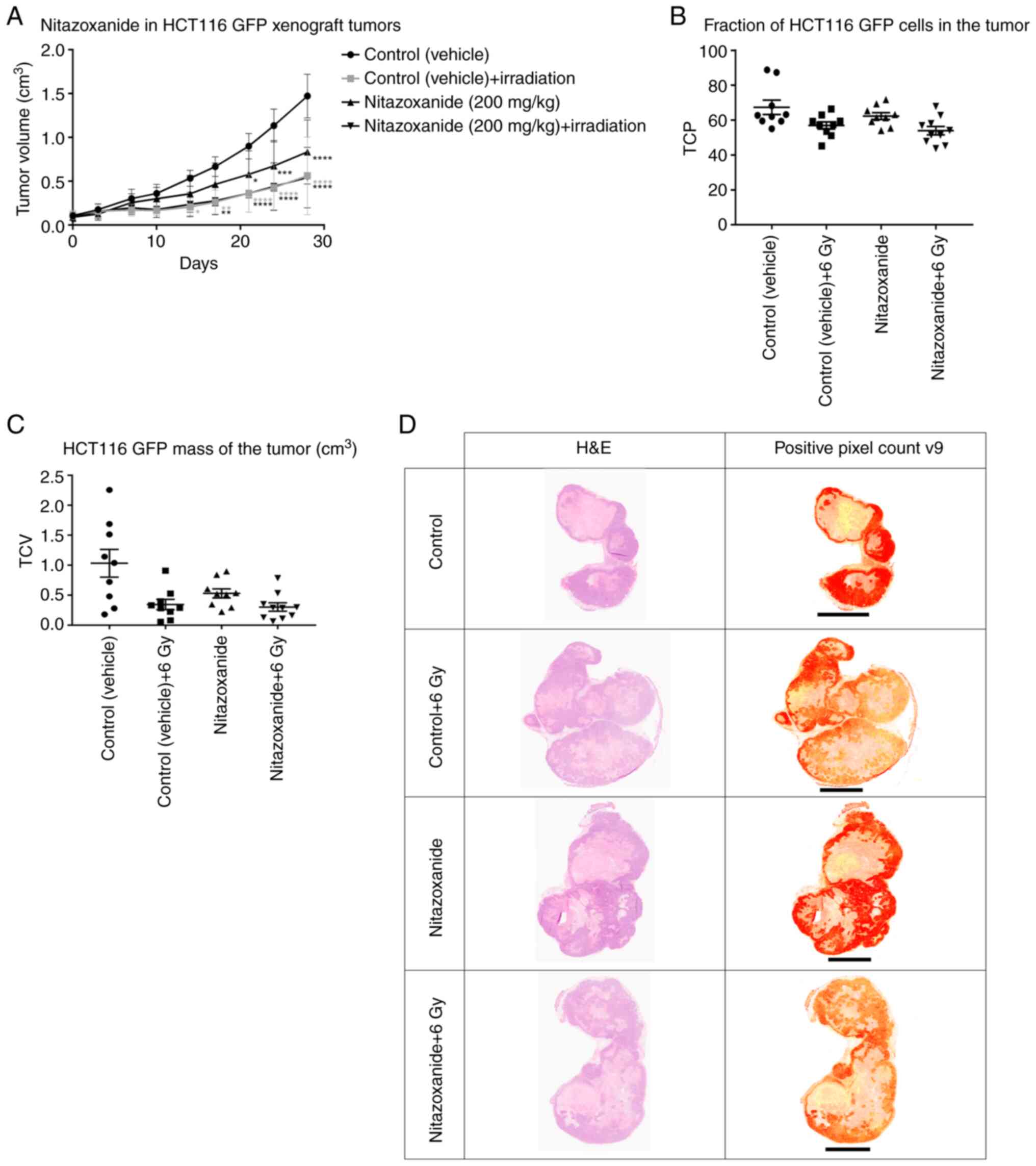 | Figure 3.HCT116 GFP cell xenograft tumours in
mice. (A) At day 0, drug/vehicle administration started twice daily
for 3 days (only one administration was given on day 2). Vehicle
control (1% CMC in PBS with 8% DMSO) and nitazoxanide (200 mg/kg)
were administered via oral gavage. At 3–4 h after the last
administration, the animal tumours were irradiated with 6 Gy.
*P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 vs. vehicle
control. HCT116 GFP cell xenograft tumours were embedded in
paraffin, sectioned, evaluated for H&E staining and scanned.
(B) TCP was calculated with the analysis algorithm ‘Positive Pixel
Count v9’ in Aperio ImageScope (v12.3.2.8013). (C) TCV was
calculated to evaluate the HCT116 GFP cell mass of the tumour. (D)
Typical examples from the four treatment groups (control, control +
6 Gy, nitazoxanide and nitazoxanide + 6 Gy) are shown. Please note
that the size of tumour in this figure is dependent on level of
sectioning and does not necessarily correlate to tumour volume.
Scale bar, 4 mm. CMC, carboxymethylcellulose; GFP, green
fluorescent protein; TCV, tumour cell volume; TCP, tumour cell
percentage. |
Histopathological examination of
tumour xenografts
The nitazoxanide + radiation group was the only
group with a TCP significantly lower than that of the control
(Fig. 3B; Table III); however, there was also a
strong trend towards lower TCP in the radiation only group,
compared with control (Fig. 3B;
Table III). Both groups treated
with radiation +/- nitazoxanide developed a TCV that was
significantly lower than that of the control (Fig. 3C; Table III). Furthermore, there was a
strong trend towards a lower TCV in the nitazoxanide only group
compared with the control group (Fig.
3C; Table III). Visual
inspection results indicated that the tumour xenografts typically
consisted of an outer rim of viable and more centrally located
pyknotic HCT116 GFP cells and necrosis, and a lesser degree of
mouse tissue (stroma, fat, blood vessels and immune cells)
(Fig. 3D).
 | Table III.TCP and TCV. |
Table III.
TCP and TCV.
| A, TCP |
|---|
|
|---|
| Treatment | P-value |
|---|
| Control (vehicle)
vs. control (vehicle) + 6 Gy | 0.0587 |
| Control (vehicle)
vs. nitazoxanide | 0.5880 |
| Control (vehicle)
vs. nitazoxanide + 6 Gy | 0.0074a |
| Control (vehicle) +
6 Gy vs. nitazoxanide | 0.5303 |
| Control (vehicle) +
6 Gy vs. nitazoxanide + 6 Gy | 0.8631 |
| Nitazoxanide vs.
nitazoxanide + 6 Gy | 0.1503 |
| B, TCV |
|
Treatment | P-value |
| Control (vehicle)
vs. control (vehicle) + 6 Gy | 0.0045a |
| Control (vehicle)
vs. nitazoxanide | 0.0529 |
| Control (vehicle)
vs. nitazoxanide + 6 Gy | 0.0019a |
| Control (vehicle) +
6 Gy vs. nitazoxanide | 0.7579 |
| Control (vehicle) +
6 Gy vs. nitazoxanide + 6 Gy | 0.9956 |
| Nitazoxanide vs.
nitazoxanide + 6 Gy | 0.6047 |
Discussion
Nitazoxanide, formally known as
2-(acetyloxy)-N-(5-nitro-2-thiazolyl) benz-amide NTZ, is a
thiazolide compound that was discovered in 1984 and originally
developed as a veterinary anthelminthic (18–20).
It is a non-toxic and well-tolerated compound, with a broad
anti-microbial activity, that was developed for human use in the
1990s and is the only anti-protozoal drug approved for use in
children (21). Drug
administration and pharmacokinetics of nitazoxanide are
advantageous for use in humans. Thus, plasma concentrations above 6
µmol/l can be reached in humans after a single oral dose of 500 mg
and Cmax in plasma is reached at 2–6 h after
administration (12,22).
The potential drug-repurposing of nitazoxanide into
an anticancer drug could bypass early stages of drug development,
and offers a faster and cheaper drug development process compared
to with de novo anticancer drug discovery (23). Moreover, oral administration of
nitazoxanide offers many numerous economic advantages compared with
standard intravenous administrated chemotherapy since it requires
less personnel and equipment.
Although initially designed as an anti-microbial
drug, the preclinical antitumour activities of nitazoxanide in
vitro and in vivo have been observed over the last
decade (12,18,19,21,24,25).
The reported preclinical anti-cancer properties are promising for
the development of nitazoxanide into a novel chemotherapeutic
compound. These properties involve crucial antitumoural metabolic
and pro-death signalling, such as drug detoxification, unfolded
protein response, autophagy, immunological responses and
interference in cell signalling pathways, such as the MAPK pathway
via interference with glutathione S-transferase π 1 or c-Myc
(18,19,21).
Our previous study described nitazoxanide as a
hit-compound in a screening process designed to identify drugs with
selective activity against quiescent and glucose-deprived tumour
cells, and exposure of colon cancer cells to nitazoxanide resulted
in OXPHOS-inhibition and reduced hypoxia in spheroids (12). Moreover, the nitro group in the
nitrothiazole moiety can be converted into a free radical (21,26).
These properties could potentially be of substantial value in the
development of a novel radiosensitizer, since the major effect of
radiation is via the formation of free oxygen radicals, leading to
DSBs.
In line with our previous observations, the present
results demonstrated that nitazoxanide showed selective activity
against spheroids compared with monolayers, as determined in the
total cell kill assay. The seemingly protective effect of radiation
to nitazoxanide in the monolayer experiments incubated for 7, but
not 3 days was unexpected, and is suggested to be explained by the
selective effect of nitazoxanide against the quiescent and
glucose-deprived cells in wells not irradiated (12), due to faster cell proliferation in
these wells secondary to lack of inhibitory radiation. The
non-irradiated cells exposed to nitazoxanide will be confluent and
glucose-deprived after, but not before, 3 days, compared with
irradiated cells. This would explain the higher effect of
nitazoxanide after 7 days, compared with 3 days, in non-irradiated
cells, and the transient radiation effect would explain the higher
cell survival after 7 days compared with 3 days in irradiated
wells. This was in agreement with nitazoxanide being more active
against cells in medium with low glucose and pH (12), and is theoretically advantageous
since lower toxicity toward normal cells, exposed to normal glucose
levels and pH, is expected.
No synergistic interaction between nitazoxanide and
radiation could be observed for monolayer or spheroid cultured
cells in the total cell kill assay. This assay is useful for
viability measurements in high-throughput screening experiments but
may miss effects secondary to cell cycle arrest and cell
senescence, as well as those in small subpopulations of cells.
Thus, the radiosensitizing property observed in the clonogenic
assay on spheroids was in accordance with the synergistic cell
inhibitory effect of nitazoxanide on clonogenic cells, rather than
synergistic cytotoxicity among all cells.
The possible mechanism underlying the induction in
DSBs in spheroids by nitazoxanide itself is beyond the scope of the
present study, but was not unexpected since nitazoxanide can induce
cell growth inhibition via the formation of free radicals (21,26).
Interestingly, the increase in DSBs in central parts of spheroids
exposed to the combination of nitazoxanide and radiation compared
with spheroids exposed to nitazoxanide or radiation only indicates
a selective radiosensitizing effect towards more centrally located
cells. This supports the hypothesis that reduced hypoxia and
formation of free radicals are important mechanisms underlying the
radiosensitization. However, a potential study limitation is the
absence of experiments analyzing reoxygenation in spheroids during
the present study. The radiosensitizing effect in spheroids could
be observed in the clonogenic assay, but not in the total cell kill
assay, and may be compatible with a low number of DSBs formed after
exposure to the combination treatment that are only sufficient to
inhibit the clonogenicity of tumour driving cells, but not enough
to further induce direct cytotoxicity. Since clonogenic
tumour-driving stem cells are considered to be located in quiescent
and glucose-deprived parts of tumours, a selective synergistic
interaction with radiation in this tumour compartment may be
beneficial.
Overall, the current data support the hypothesis
that nitazoxanide acts as a radiosensitizer in tumour-driving,
quiescent parts of tumours. Theoretically, the combination of a
radiosensitizer effective against high-proliferative tumour cells
(e.g. 5-FU) (1) with a
radiosensitizer that targets quiescent clonogenic tumour cells
(e.g. nitazoxanide) could enhance the anticancer effect of
radiation via both an increased bioequivalent radiation dose to the
tumour and a reduced radiation effect in surrounding normal
cells.
Enhancement of the radiation effect by nitazoxanide
was not observed in vivo as determined via tumour size.
However, given the synergistic interaction between nitazoxanide and
radiation only in quiescent clonogenic cells, as suggested from the
in vitro experiments, this finding was not surprising. In
addition, the current study recognized that a major problem with
examining the radiosensitization effects in the HCT116 GFP cell
murine xenograft model was that the tumours only partially consist
of tumour cells, alongside considerable parts with necrosis and
mouse tissue. Therefore, it was important to evaluate the TCP and
TCV in the xenografts. Notably, the only group that developed a
significantly lower TCP compared with the control was the
nitazoxanide + radiation group. This may indicate a selective
radiosensitization effect of nitazoxanide against the
tumour-driving HCT116 GFP cell mass of the tumour. However, no
difference was observed compared with the radiation only group. As
expected, although a strong trend towards a lower TCP compared with
the control was identified in the radiation only group, the in
vivo data suggests that this relatively high radiation dose
also affects normal cells to a considerable extent.
Single treatment with nitazoxanide for only 3 days
resulted in a statistically significant inhibition of tumour growth
in vivo. A strong trend towards a smaller TCV compared with
the control, in contrast to similar TCPs, is compatible with the
selective effect of nitazoxanide against the tumour-driving HCT116
GFP cell mass of the tumour, but is more suggestive of an
inhibitory effect on quiescent and glucose-deprived tumour
compartments consisting of both HCT116 GFP cells and transformed
normal cells. This was unexpected, since continuous treatment with
nitazoxanide for 28 days in a similar xenograft model did not
significantly inhibit tumour growth (12). However, the antitumour effect of
single treatment with nitazoxanide indicates that the mechanisms
important in vivo are complex. Drug doses, administration
schedules, the build-up and wash-out of an active compound in the
tumour, radiation dose and follow-up time are factors that may
impact on the possibility to observe radiosensitization and single
drug effects in vivo. Moreover, although spheroids are
considered to better reflect solid tumours in vivo compared
with monolayers, the situation in vivo is more complex and
several factors, such as pharmacokinetics, interaction with other
cell types (stroma, immune cells, fat) and elevated intratumor
pressure, will affect anticancer drug and radiation therapy, and
are difficult to mimic in vitro.
In conclusion, the current study demonstrated that
nitazoxanide presented several characteristics that make it highly
interesting for repurposing into an anticancer drug, as well as for
use in combination with radiation. However, other models are
required to reflect the potential of nitazoxanide as a
radiosensitizer in vivo. Since nitazoxanide shows selective
effects against quiescent and glucose-deprived tumour compartments
and radiosensitization of clonogenic cells in these regions, in
vivo experiments with serial orthotopic transplantation of
tumours exposed to nitazoxanide and radiation may be needed to
provide proof of principle evidence of the long term
radiosensitization effects of this combination.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
The laboratory work by Ms Lena Lenhammar, our late
Ms Christina Leek and Dr Sharmineh Mansoori at the Department of
Medical Sciences, Uppsala University Hospital (Uppsala, Sweden) is
gratefully acknowledged. Adlego Biomedical AB (Astrid Fagreaus
Laboratoriet, Solna, Sweden) carried out the tumour xenograft
experiments in mice.
Funding
This work was supported by grants from Swedish Cancer Society
(grant no. 462430020), Swedish Foundation for Strategic Research
(grant no. 2008-03000) and Lions Cancer Research Fund.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HK and PN designed the experiments, wrote the paper
and are responsible for confirming the authenticity of the raw
data. MF and RL helped design the experiments. WS helped design the
spheroid experiments. The laboratory work was performed by HK. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Mouse experiments were approved by the Regional
Animal Experimental Ethics Committee in Stockholm (North; approval
nos. N37/15 and N188/15).
Patient consent for publication
Not applicable.
Competing interests
Uppsala University has received an unconditional
grant from Romark LC for studies of nitazoxanide. This grant has
not supported any part of the experiments described in this
manuscript.
References
|
1
|
Shewach DS and Lawrence TS: Antimetabolite
radiosensitizers. J Clin Oncol. 25:4043–4050. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lawrence TS, Blackstock AW and McGinn C:
The mechanism of action of radiosensitization of conventional
chemotherapeutic agents. Semin Radiat Oncol. 13:13–21. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dai T and Shah MA: Chemoradiation in
oesophageal cancer. Best Pract Res Clin Gastroenterol. 29:193–209.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Spithoff K, Cummings B, Jonker D and Biagi
JJ; Gastrointestinal Cancer Disease Site Group, : Chemoradiotherapy
for squamous cell cancer of the anal canal: A systematic review.
Clin Oncol (R Coll Radiol). 26:473–487. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Morris ZS and Harari PM: Interaction of
radiation therapy with molecular targeted agents. J Clin Oncol.
32:2886–2893. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Koning CC, Wouterse SJ, Daams JG,
Uitterhoeve LL, van den Heuvel MM and Belderbos JS: Toxicity of
concurrent radiochemotherapy for locally advanced non-small-cell
lung cancer: A systematic review of the literature. Clin Lung
Cancer. 14:481–487. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
DeVita VT, Lawrence TS and Rosenberg SA:
Cancer Principles and Practice of Oncology. Wolters Kluwer;
Philadelphia, PH: pp. pp2802015
|
|
8
|
Ivashkevich A, Redon CE, Nakamura AJ,
Martin RF and Martin OA: Use of the γ-H2AX assay to monitor DNA
damage and repair in translational cancer research. Cancer Lett.
327:123–133. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Radford IR: The level of induced DNA
double-strand breakage correlates with cell killing after
X-irradiation. Int J Radiat Biol Relat Stud Phys Chem Med.
48:45–54. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brown JM: The hypoxic cell: A target for
selective cancer therapy-eighteenth Bruce F. Cain Memorial Award
lecture. Cancer Res. 59:5863–5870. 1999.PubMed/NCBI
|
|
11
|
Bernier J, Hall EJ and Giaccia A:
Radiation oncology: A century of achievements. Nat Rev Cancer.
4:737–747. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Senkowski W, Zhang X, Olofsson MH, Isacson
R, Höglund U, Gustafsson M, Nygren P, Linder S, Larsson R and
Fryknäs M: Three-Dimensional cell culture-based screening
identifies the anthelmintic drug nitazoxanide as a candidate for
treatment of colorectal cancer. Mol Cancer Ther. 14:1504–1516.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Overgaard J: Hypoxic radiosensitization:
Adored and ignored. J Clin Oncol. 25:4066–4074. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
DeVita VT Jr and Chu E: A history of
cancer chemotherapy. Cancer Res. 68:8643–8653. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lindhagen E, Nygren P and Larsson R: The
fluorometric microculture cytotoxicity assay. Nat Protoc.
3:1364–1369. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Franken NA, Rodermond HM, Stap J, Haveman
J and van Bree C: Clonogenic assay of cells in vitro. Nat Protoc.
1:2315–2319. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yeh PJ, Hegreness MJ, Aiden AP and Kishony
R: Drug interactions and the evolution of antibiotic resistance.
Nat Rev Microbiol. 7:460–466. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Di Santo N and Ehrisman J: A functional
perspective of nitazoxanide as a potential anticancer drug. Mutat
Res. 768:16–21. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Muller J, Sidler D, Nachbur U, Wastling J,
Brunner T and Hemphill A: Thiazolides inhibit growth and induce
glutathione-S-transferase Pi (GSTP1)-dependent cell death in human
colon cancer cells. Int J Cancer. 123:1797–1806. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rossignol JF and Maisonneuve H:
Nitazoxanide in the treatment of Taenia saginata and Hymenolepis
nana infections. Am J Trop Med Hyg. 33:511–512. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fan-Minogue H, Bodapati S, Solow-Cordero
D, Fan A, Paulmurugan R, Massoud TF, Felsher DW and Gambhir SS: A
c-Myc activation sensor-based high-throughput drug screening
identifies an antineoplastic effect of nitazoxanide. Mol Cancer
Ther. 12:1896–1905. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stockis A, Deroubaix X, Lins R,
Jeanbaptiste B, Calderon P and Rossignol JF: Pharmacokinetics of
nitazoxanide after single oral dose administration in 6 healthy
volunteers. Int J Clin Pharmacol Ther. 34:349–351. 1996.PubMed/NCBI
|
|
23
|
Wurth R, Thellung S, Bajetto A, Mazzanti
M, Florio T and Barbieri F: Drug-repositioning opportunities for
cancer therapy: Novel molecular targets for known compounds. Drug
Discov Today. 21:190–199. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Di Santo N and Ehrisman J: Research
perspective: Potential role of nitazoxanide in ovarian cancer
treatment. Old drug, new purpose? Cancers (Basel). 5:1163–1176.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brockmann A, Strittmatter T, May S,
Stemmer K, Marx A and Brunner T: Structure-function relationship of
thiazolide-induced apoptosis in colorectal tumor cells. ACS Chem
Biol. 9:1520–1527. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hemphill A, Mueller J and Esposito M:
Nitazoxanide, a broad-spectrum thiazolide anti-infective agent for
the treatment of gastrointestinal infections. Expert Opin
Pharmacother. 7:953–964. 2006. View Article : Google Scholar : PubMed/NCBI
|