Introduction
Over the past 20 years, the incidence and mortality
of bladder cancer in Japan have increased approximately 1.8 and 2.1
times, respectively. However, given that the age-adjusted morbidity
and mortality rates are almost stable (around 7.2/100,000
population/year and 2.0/100,000 population/year in 2018,
respectively), the increase in the number of cases and deaths can
be attributed to population aging. In general, most patients with
bladder cancer are elderly, with more than 95% being over 45 years
old and 80% over 65 years old (1).
About 25% of diagnosed bladder cancers are
classified as muscle-invasive bladder cancer (MIBC) at the time of
initial diagnosis (2).
Non-muscle-invasive bladder cancer without intrinsic muscle layer
invasion is often curable with minimally invasive treatments such
as transurethral resection of bladder tumor (TURBT) and
intravesical chemotherapy. However, when bladder cancer progresses
to MIBC, the standard treatment is radical cystectomy (RC) with
urinary tract diversion, which may lead to treatment-related
serious complications and a decline in postoperative quality of
life (QOL). Elderly patients with poor performance status (PS) and
serious comorbidities are considered ineligible to undergo highly
invasive surgery, originating challenges in the current population
aging scenario. Furthermore, due to the growing interest in
health-related QOL, a considerable number of patients refuse to
undergo surgery, regardless of their favorable PS. Regarding
bladder-sparing therapy for muscle-invasive cancer, TURBT followed
by external beam radiation or chemotherapy has been used as an
alternative treatment to RC for patients who refused the latter.
However, previous studies have reported limited effectiveness of
TURBT or chemotherapy only, and its routine use is not recommended
(3–6). Currently, the most common
bladder-sparing therapy for MIBC is trimodal therapy (TMT), which
consists of maximum TURBT followed by external beam radiation and
chemotherapy for radiosensitization (3,4).
However, in clinical practice, some patients who refuse RC are
still treated with TURBT monotherapy or TURBT plus chemotherapy,
even though the risks of these non-definitive therapies have not
been extensively reported. Furthermore, to date, there have been
few reports on the risks and outcomes of inadequate treatment for
MIBC.
In this study, we analyzed the risks and survival
outcomes of non-definitive therapy for MIBC at our hospital, which
may provide valuable information for patient informed consent in
future treatment selection.
Materials and methods
Study cohort and design
In this study, we retrospectively analyzed 124
patients who were diagnosed and treated for MIBC (cT2-4aN1-2M0) at
Kurume University hospital (Kurume, Japan) from 2013 to 2020. The
criteria for MIBC diagnosis include the following for all cases:
TURBT with histopathological diagnosis of the invasion of the
muscularis propria, lamina propria or further, and the invasion of
the muscle layer or further were confirmed by imaging (computed
tomography/magnetic resonance). As for lymph node metastasis, only
cases with pelvis metastasis were included, while cases with
distant metastasis, such as common iliac lymph node metastasis and
visceral metastasis, were excluded. Clinical T (cT) stages were
uniformly adjusted according to the 2017 Tumor-Node-Metastasis
classification system (7).
Post-treatment surveillance was performed by physical examination,
urine cytology, and cystoscopy at 2 to 3-month intervals, and blood
examinations and computed tomography at 4 to 6-month intervals.
Metastasis and local recurrence were defined as urothelial tumor
recurrence outside the residual urinary tract. The choice of
treatment was at the discretion of each attending physician,
considering the patient's physical findings. The patients were
classified into RC (the standard treatment) and TMT (an alternative
treatment option for patients with MIBC) treatment groups, defined
as the definitive therapy (DT) group, according to the European
Association of Urology and the National Comprehensive Cancer
Network guidelines (3,4); and TURBT monotherapy or TURBT plus
chemotherapy, defined as the non-definitive therapy (nDT) group.
All pathological diagnoses were performed by expert pathologists at
our hospital. All clinical data were obtained from hospital medical
records.
Statistical analysis
Statistical analysis data were presented as the
median and interquartile range for continuous variables because,
Shapiro-Wilk test confirmed the non-normality distribution of
continuous variables, while categorical variables were presented as
percentages of events. Therefore, we used the Mann-Whitney U test,
the Chi-square test, and Fisher's exact test as appropriate, to
evaluate differences between the DT and nDT groups. Since this
study was conducted in elderly patients with advanced cancer, we
evaluated the overall survival (OS), cancer-specific survival (CSS)
excluding the effect of death from other causes, and
progression-free survival (PFS) reflecting disease progression.
Survival outcomes were estimated using the Kaplan-Meier method and
compared using the log-rank test. Survival data were collected on
April 30, 2021. For patients with whom the hospital had lost
contact during follow-up, we considered the data from the date of
the last contact. Survival outcomes were based on the date of
TURBT: OS was calculated from the date of TURBT to the date of
mortality from any cause, while CSS and PFS rates were calculated
from the date of TURBT to the date of cancer-associated mortality
or disease progression. Disease progression was determined by the
appearance of distant metastases and local relapse. The following
clinical variables were analyzed as survival-affecting: age at the
time of TURBT, sex, Eastern Cooperative Oncology Group PS, smoking
history, Charlson comorbidity index (CCI), cT stage, lymph node
metastasis, tumor size, presence of hydronephrosis,
neutrophil-lymphocyte ratio (NLR), and treatment method. A high NLR
(≥2.1) was defined according to receiver operating characteristic
(ROC) curves. ROC curves were plotted for the NLR for the
evaluation of OS and CSS rates. The NLR threshold was 2.1 for both
OS and CSS, and the area under the curve was 0.618 and 0.604,
respectively. The NLR sensitivity for OS and CSS was 46.6 and
44.1%, respectively, and the specificity was 78.4 and 80%,
respectively (data not shown). Cox proportional hazards regression
models were used for univariate and multivariate analyses. All
statistical analyses were performed using JMP version 16.0.0 (SAS
Institute Inc.). All tests were two-sided, and P<0.05 was
considered statistically significant. This study was approved by
the Research Ethics Committee of our hospital.
Results
Clinicopathological characteristics of
MIBC
The characteristics for the 124 patients with MIBC
are shown in Table I. Of the 124
patients, 56 (45%) were treated with nDT, of whom 28 out of 56
(50%) were treated with TURBT monotherapy and 28 (50%) with TURBT
plus chemotherapy. Meanwhile, RC, the most common treatment, was
performed in 47 out of 124 patients (38%) whereas TMT was performed
in the remaining 21 (17%) patients. For TURBT plus chemotherapy,
the median number of chemotherapy cycles was 4; 23 out of 28
patients (82%) were treated with a combination of gemcitabine and
cisplatin, whereas the remaining 5 patients (18%) were treated with
a gemcitabine and carboplatin combination due to renal dysfunction.
There were no statistically significant differences in outcomes
between the groups treated with TURBT monotherapy and TURBT plus
chemotherapy (median OS: 562 vs. 738 days, P=0.274; median PFS: 349
vs. 402 days, P=0.768, respectively; log-rank test). The TMT group
was treated with radiotherapy (median: 58 Gy) delivered to the
bladder with concurrent multiple-agent radiosensitizing
chemotherapy with cisplatin. The median age of patients treated
with nDT was 77 years (69–81 years), which was significantly higher
than that of patients treated with DT. In addition, the proportion
of patients with poor PS, high CCI, and high NLR values was
significantly higher in the nDT group compared to the DT group
(P=0.003, P=0.044, and P=0.001, respectively). However, there were
no significant differences in clinical stage or presence of lymph
node metastasis between the two groups (Table I).
 | Table I.Clinicopathological characteristics of
the 124 patients with muscle invasive bladder cancer. |
Table I.
Clinicopathological characteristics of
the 124 patients with muscle invasive bladder cancer.
| Variables | All patients
(n=124) | Definitive therapy
(n=68) | Non-Definitive
therapy (n=56) | P-value |
|---|
| Median age, years
(IQR) | 73 (68–80) | 71 (66–77) | 77 (69–81) | 0.002a,b |
| Sex, n (%) |
|
|
|
|
| Male | 92 (74.2) | 52 (76.5) | 40 (71.4) | 0.543c |
|
Female | 32 (25.8) | 16 (23.5) | 16 (28.6) |
|
| ECOG performance
status, n (%) |
|
|
|
|
| 0 or
1 | 108 (87.1) | 65 (95.6) | 43 (76.8) | 0.003b,d |
| ≥2 | 16 (12.9) | 3 (4.4) | 13 (23.2) |
|
| Smoking history, n
(%) |
|
|
|
|
| Yes | 78 (63.4) | 46 (67.7) | 32 (58.2) | 0.347c |
| No | 45 (36.6) | 22 (32.3) | 23 (41.8) |
|
| Charlson comorbidity
index, n (%) |
|
|
|
|
| ≤2 | 99 (79.8) | 59 (86.8) | 40 (71.4) | 0.044b,c |
| ≥3 | 25 (20.2) | 9 (13.2) | 16 (28.6) |
|
| Presence of
hydronephrosis, n (%) |
|
|
|
|
| Yes | 37 (29.8) | 21 (30.9) | 16 (28.6) | 0.845c |
| No | 87 (70.2) | 47 (69.1) | 40 (71.4) |
|
| Median tumor size, mm
(IQR) | 35.0 (22.0-49.8) | 33.5 (21.3-49.8) | 35.0 (23.0-49.5) | 0.833a |
| cT stage, n (%) |
|
|
|
|
| cT2 | 43 (34.7) | 23 (33.8) | 20 (35.7) | 0.950d |
| cT3 | 62 (50.0) | 34 (50.0) | 28 (50.0) |
|
| cT4a | 19 (15.3) | 11 (16.2) | 8 (14.3) |
|
| Lymph node
involvement, n (%) |
|
|
|
|
|
Positive | 104 (83.9) | 60 (88.2) | 44 (78.6) | 0.219d |
|
Negative | 20 (16.1) | 8 (11.8) | 12 (21.4) |
|
| Tumor grade, n
(%) |
|
|
|
|
| High | 115 (95.8) | 63 (94.0) | 52 (98.1) | 0.382d |
|
Low | 5 (4.2) | 4 (6.0) | 1 (1.9) |
|
| Median NLR
(IQR) | 2.54
(1.97-3.65) | 2.24
(1.73-2.9) | 3.19
(2.11-4.41) | 0.001a,b |
| Treatment, n
(%) |
|
|
|
|
| Radical
cystectomy | 47 (37.9) | 47 (69.1) | - |
|
|
Chemoradiotherapy | 21 (16.9) | 21 (30.9) | - |
|
|
Chemotherapy | 28 (22.6) | - | 28 (50.0) |
|
| TURBT
monotherapy | 28 (22.6) | - | 28 (50.0) |
|
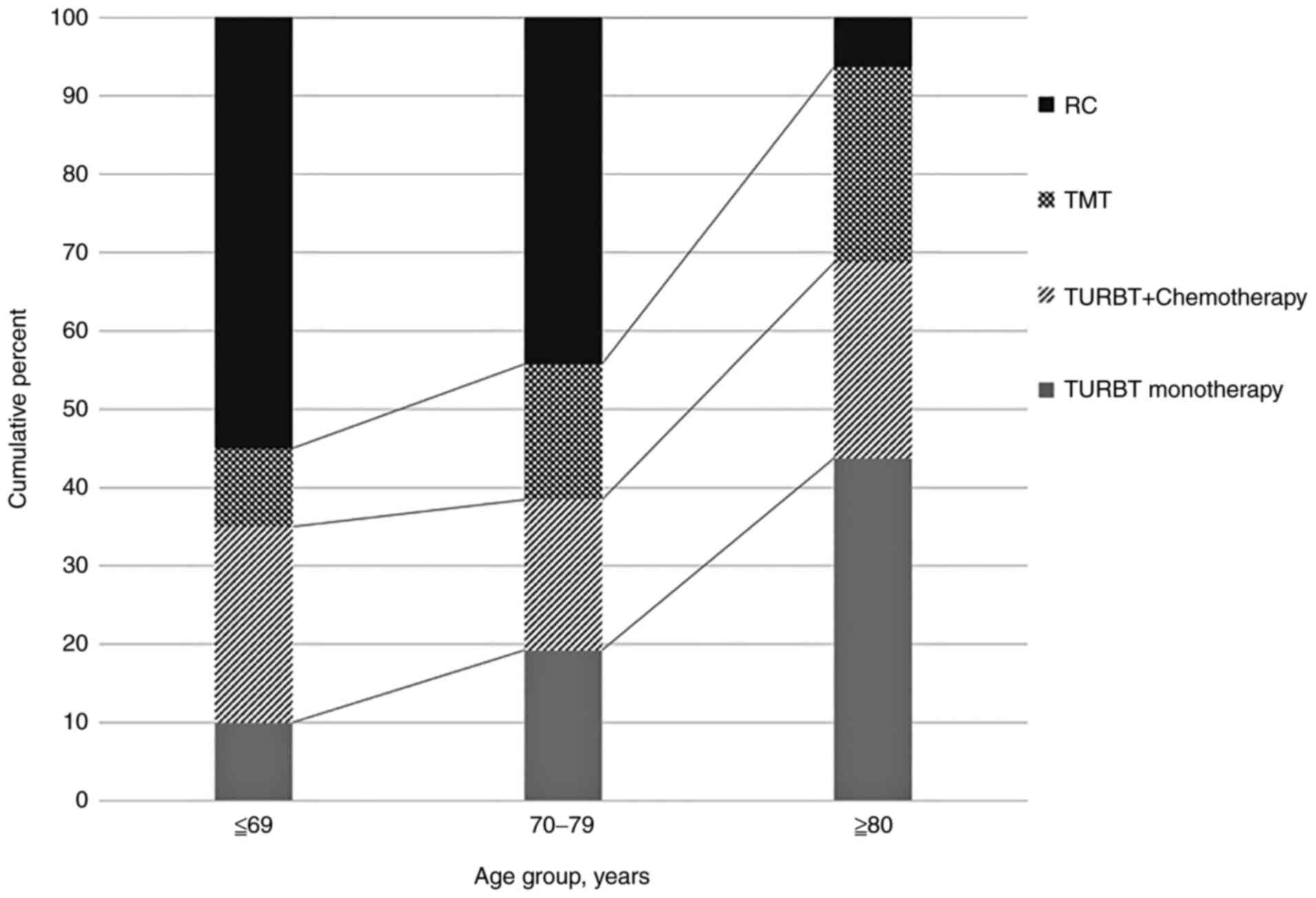
The choice of treatment varied significantly by age.
Younger patients had the highest rate of RC, which steadily
decreased with age. Conversely, the rate of bladder-sparing
therapies such as TURBT and TMT increased with age (Fig. 1).
Survival analysis
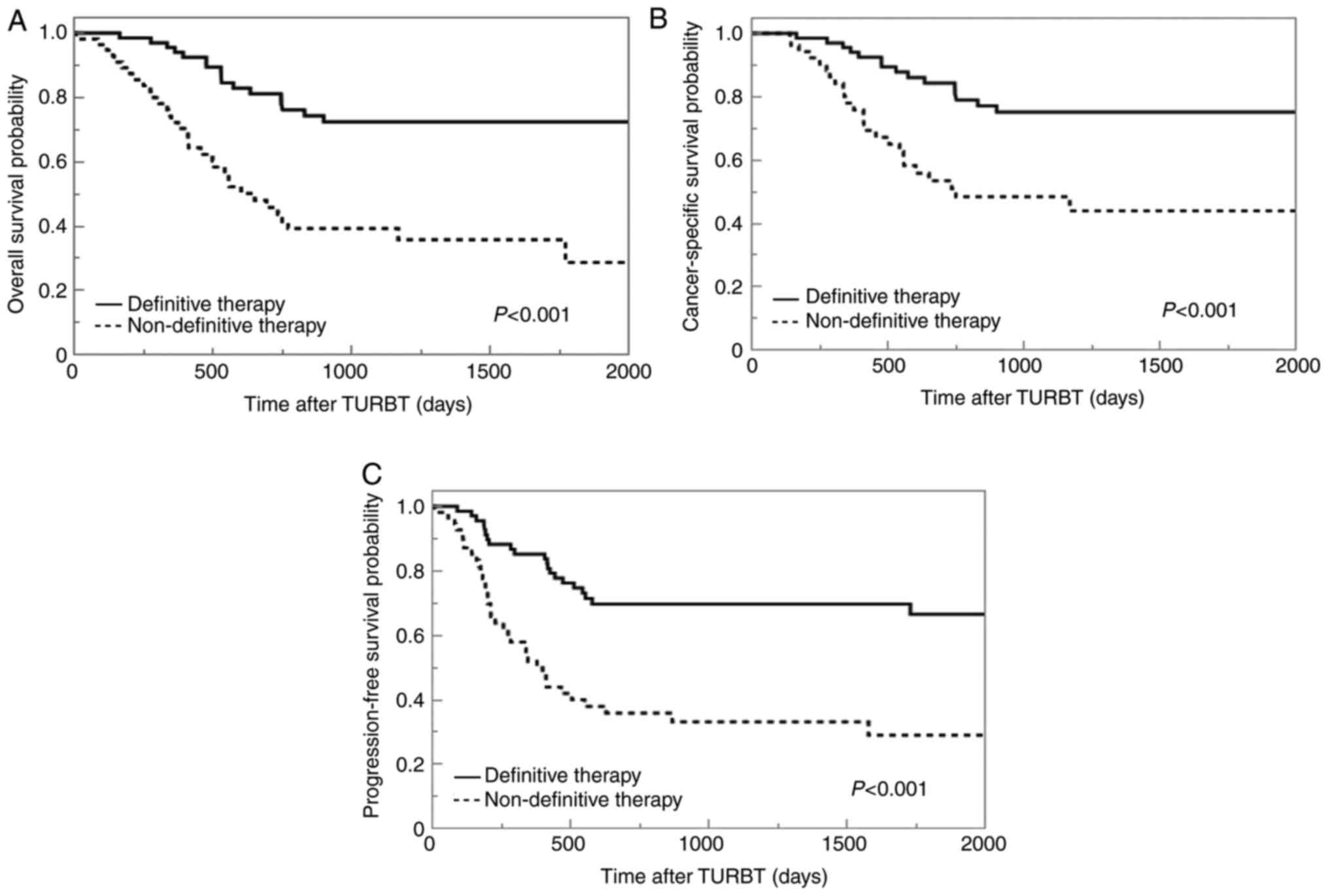
The median OS, CSS, and PFS times for patients
treated with nDT were 605, 753, and 382 days, respectively. For
those treated with DT, the median OS and CSS were not reached, and
for PFS it was 2492 days. The 5-year OS, CSS, and PFS rates were
23, 33, and 15%, respectively, for patients treated with nDT, and
69, 74, and 61%, respectively, for those treated with DT. The
Kaplan-Meier curves demonstrated that nDT is associated with
significantly reduced OS, CSS, and PFS rates compared with DT
(P<0.001) (Fig. 2).
Univariate and multivariate
analysis
Univariate analysis revealed that female sex, a high
PS (≥2), advanced cT stage (≥T3), high NLR (≥T2.1), and nDT were
significantly associated with OS, CSS, and PFS. According to
multivariate analysis, nDT was a significant poorer prognostic
factor for OS (HR 2.91, 95% CI 1.47-5.77, P=0.002), CSS (HR 3.28,
95% CI 1.58-6.81, P<0.001), and PFS (HR 3.54, 95% CI 1.88-6.66,
P<0.001). Additionally, an advanced cT stage (≥T3) was a
significant poorer prognostic factor for OS (HR 4.89, 95% CI
2.27-10.5, P<0.001), CSS (HR 8.34, 95% CI 2.84-24.5,
P<0.001), and PFS (HR 5.22, 95% CI 2.39-11.4, P<0.001)
(Table II).
 | Table II.Univariate and multivariate analyses
of clinical factors influencing PFS, CSS and OS in the 124 patients
with muscle invasive bladder cancer. |
Table II.
Univariate and multivariate analyses
of clinical factors influencing PFS, CSS and OS in the 124 patients
with muscle invasive bladder cancer.
|
| PFS | CSS | OS |
|---|
|
|
|
|
|
|---|
|
| Univariate
analysis | Multivariate
analysis | Univariate
analysis | Multivariate
analysis | Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|
|
|
|---|
| Variable | Hazard ratio (95%
CI) | P-value | Hazard ratio (95%
CI) | P-value | Hazard ratio (95%
CI) | P-value | Hazard ratio (95%
CI) | P-value | Hazard ratio (95%
CI) | P-value | Hazard ratio (95%
CI) | P-value |
|---|
| Age | 1.01 | 0.402 | - | - | 1.02 | 0.373 | - | - | 1.04 | 0.051 | - | - |
| (continuous) | (0.98-1.05) |
|
|
| (0.98-1.06) |
|
|
| (1.00-1.08) |
|
|
|
| Sex | 2.46 |
<0.001a | - | - | 2.14 | 0.022a | - | - | 1.79 | 0.055 | - | - |
| (female vs.
male) | (1.44-4.21) |
|
|
| (1.12-4.11) |
|
|
| (0.99-3.24) |
|
|
|
| ECOG PS | 2.29 | 0.019a | - | - | 2.55 | 0.025a | - | - | 4.14 |
<0.001a | 2.55 | 0.007a |
| (≥2 vs. ≤1) | (1.15-4.55) |
|
|
| (1.13-5.79) |
|
|
| (2.19-7.82) |
| (1.30-5.04) |
|
| CCI | 0.77 | 0.468 | - | - | 0.79 | 0.602 | - | - | 1.54 | 0.179 | - | - |
| (≥3 vs. ≤2) | (0.38-1.57) |
|
|
| (0.33-1.89) |
|
|
| (0.82-2.89) |
|
|
|
| Clinical stage | 4.48 |
<0.001a | 5.22 |
<0.001a | 7.08 |
<0.001a | 8.34 |
<0.001a | 3.62 |
<0.001a | 4.89 |
<0.001a |
| (≥cT3 vs. cT2) | (2.19-9.15) |
| (2.39-11.40) |
| (2.52-19.90) |
| (2.84-24.50) |
| (1.76-7.45) |
| (2.27-10.50) |
|
| LNI (positive | 2.45 | 0.004a | - | - | 2.05 | 0.051 | - | - | 1.74 | 0.105 | - | - |
| vs. negative) | (1.33-4.50) |
|
|
| (1.00-4.20) |
|
|
| (0.89-3.42) |
|
|
|
| NLR | 2.30 | 0.007a | - | - | 2.83 | 0.009a | - | - | 2.58 | 0.006a | - | - |
| (≥2.1 vs. 2.0) | (1.26-4.22) |
|
|
| (1.30-6.15) |
|
|
| (1.32-5.03) |
|
|
|
| Treatment | 3.06 |
<0.001a | 3.54 |
<0.001a | 3.04 |
<0.001a | 3.28 |
<0.001a | 3.46 |
<0.001a | 2.91 | 0.002a |
| (nDT vs. DT) | (1.79-5.24) |
| (1.88-6.66) |
| (1.60-5.79) |
| (1.58-6.81) |
| (1.93-6.19) |
| (1.47-5.77) |
|
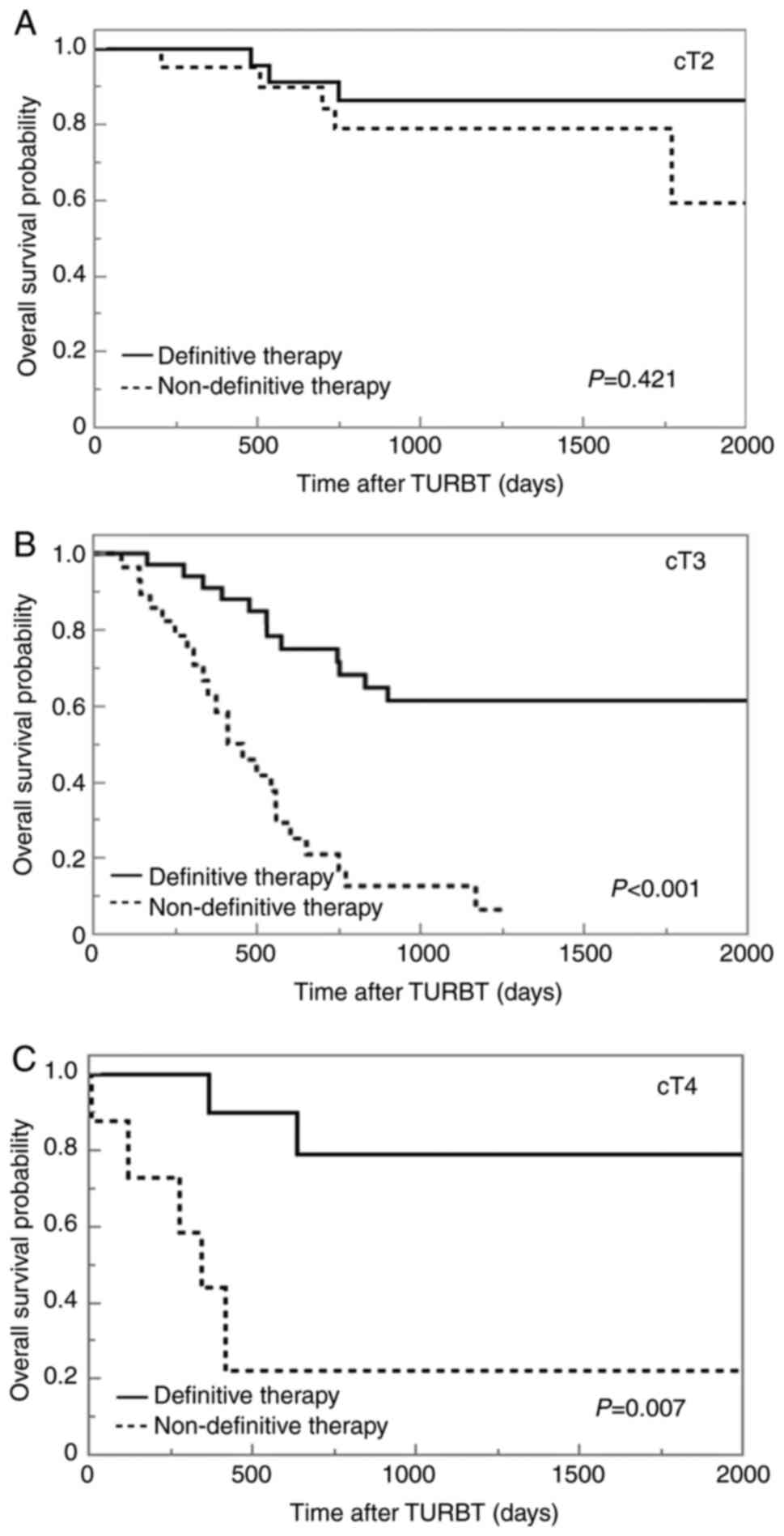
Overall survival analysis of cT
stage
Kaplan-Meier curves for survival outcomes stratified
according to cT stage are presented in Fig. 3. For cT2 cases, the median OS was
not significantly different between the two groups (not reached in
either group, P=0.421), and that for cT3 and cT4a cases was
significantly lower in the nDT group (not reached vs. 414 days,
P<0.001 and not reached vs. 343 days, P=0.007, respectively)
(Fig. 3).
Discussion
Generally, RC is the standard treatment for MIBC.
However, it remains a high-risk procedure associated with high
morbidity and high surgical mortality (8). Therefore, with population aging,
there is an increasing number of cases in which MIBC is not
adequately treated due to old age, poor PS, serious comorbidities
such as cardiac dysfunction, or refusal to undergo RC due to
health-related QOL concerns even in the absence of physical
problems.
The choice of treatment for patients with MIBC
varies widely. In a survey that included 28691 patients with MIBC
in the United States, 52.5% of patients received active treatment
such as RC or TMT. On the other hand, 25.9% of patients opted for
observation or TURBT only. It was also reported that the proportion
of patients opting for non-curative treatment increased with older
age (9). The results of the
present study were similar, with 54.8% of patients opting for
aggressive treatment and 25.8% treated with TURBT only. Moreover,
with increasing age, the proportion of patients who opted for
aggressive treatment such as RC decreased significantly.
Patients who do not undergo RC are generally treated
with TURBT, cisplatin-based chemotherapy, radiation therapy, or a
combination of these. Nevertheless, only a limited number of
patients respond to TURBT monotherapy or TURBT in combination with
chemotherapy. Previous reports suggest that the ideal patient for
TURBT monotherapy would be a cT2 patient with a tumor size of less
than 3 cm, no hydronephrosis, no evidence of metastasis or
adenocarcinoma, negative resection bed biopsies at initial TURBT or
negative repeat TURBT, and no evidence of upper urinary tract
cancer (5,10). Solsona et al (5) reported that, under these criteria,
the outcomes of TURBT monotherapy were CSS rates of 81.9, 79.5, and
76.7% at 5, 10, and 15 years, respectively, and PFS rates with
bladder preservation of 75.5, 64.9, and 57.8%, respectively.
Meanwhile, TURBT plus chemotherapy may have been chosen due to
concerns about the potential for short- and long-term adverse
events affecting the QOL associated with radiation therapy.
However, in a large retrospective study by Audenet et al
(6), the OS percentages for TURBT
plus chemotherapy at 2 and 5 years were only 49 and 32.9% for all
patients and 52.6 and 36.2% for cT2 patients, respectively. In
addition, they reported that patients who underwent TURBT plus
chemotherapy had a significantly shorter OS than those who
underwent RC (median: 23.9 months vs. 48.1 months) (6). In the current study, there was no
significant difference in the survival outcomes of cT2 patients
treated with TURBT monotherapy or TURBT plus chemotherapy and those
treated with DT, which is similar to previous reports. On the other
hand, these data indicate that there is a possibility of long-term
survival for some patients, but the routine use of these therapies
is not recommended.
Currently, TMT combining TURBT, chemotherapy with
radiosensitizers, and radiation therapy is recommended as an
effective alternative for patients who are unable or unwilling to
undergo RC (3,4). Seisen et al (11) reported a comparative study of RC
and TMT for MIBC in a large scale using the National Cancer Data
Base. The survey was conducted on 1257 patients (9.8%) treated with
TMT and 11586 patients (90.2%) treated with RC. TMT was associated
with a significantly lower long-term OS at 25 months of follow-up
or later (hazard ratio: 1.37, 95% CI 1.16-1.59) according to
inverse probability of treatment weighting-adjusted Cox regression
analysis with a time-varying covariate. However, the difference in
treatment effectiveness between TMT and RC decreases with age
(P=0.004). Therefore, the survival benefits of RC should be weighed
against the risks of surgery, especially in older patients
(11). On the other hand, a large
systematic review reported that the OS and disease-specific
survival (DSS) of RC and TMT were comparable. The mean 10-year OS
for the 1536 patients who received TMT was 30.9%, compared with
35.1% for the 5163 RC-treated patients (P=0.32). Meanwhile, the
average 10-year DSS was 50.9% for the 1,205 patients who received
TMT and 57.8% for the 4,856 RC-treated patients (P=0.26) (12). Overall, the outcomes of bladder
preservation with TMT are acceptable and can be a reasonable
treatment option in appropriately selected patients. Furthermore,
TMT was reported to have a better post-treatment QOL than RC and
may be a suitable treatment for patients who wish to receive
bladder-sparing therapy (13). In
this study and previous reports, the proportion of patients opting
for non-curative treatment increased with older age; therefore, it
may be necessary to recommend TMT for the elderly, which offers
both survival benefit and QOL (9).
Patients diagnosed with bladder cancer had a
significant decline in physical, mental, and social health-related
QOL compared to healthy individuals, regardless of the progression
degree. This decline was particularly pronounced in patients with
MIBC. Further, patients who underwent RC had a significant decline
in health-related QOL compared to patients who did not undergo RC
(14). However, in another recent
review, QOL after orthotopic urinary diversion (neobladder) was
significantly improved compared to QOL after ileal conduit
(15). Urinary incontinence is a
complication of neobladder; daytime continence improved by 92%
during more than 12 to 18 months post-operation, while nighttime
continence improved from 28% on the first 3 months after the
procedure to 51% during more than 18 to 36 months post-operation
(16). For patients who value
health-related QOL, RC with orthotopic urinary diversion or TMT may
provide both cure and maintenance of QOL.
This study showed that nDT had a significantly
poorer prognosis than DT not only in terms of OS but also in terms
of CSS (HR 3.28, 95% CI 1.58-6.81). Moreover, cT3 and cT4a patients
had an extremely poor prognosis, with a median survival of about
313–414 days due to inadequate treatment. On the other hand,
although a high CCI (≥3) and older age were not poor prognostic
factors in this study, Maffezzini et al (17) reported that RC worsened the
prognosis in patients older than 70 years and with a high CCI
(>3). In view of this, the survival benefits, and the
disadvantages of treatment-related complications associated with
DT, should be carefully considered when the patients with reduced
physical function choose DT.
The limitations of this study are that it was a
single-center, retrospective study with a small patient sample, and
the possibility of selection bias cannot be excluded. Therefore, in
future, further external validation and a large-scale multicenter
trial is necessary to confirm and reinforce this study result.
However, to the best of our knowledge, this is the first study on
the clinical outcomes of nDT for MIBC in Japan, and the results may
be meaningful to provide valuable data for adequate patient
informed consent in future treatment selection.
In conclusions, nDT for patients with MIBC is
associated with a high risk of cancer-related death, even in the
elderly with serious comorbidities. The most appropriate treatment
method should be discussed with the patient after providing
sufficient information on the risks and benefits of each one,
instead of simply choosing the easy route of nDT.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KN, HK, KoU, SS and TI designed the study and
revised the manuscript. NO, TH, KC, KE, KeU and MN contributed to
the collection, analysis and interpretation of the data. HK and SS
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Research
Ethics Committee of Kurume University (approval no. 21244; Fukuoka,
Japan). The requirement for informed consent was waived due to the
retrospective nature of the study using medical records only. The
research content was available publicly on the website of the
Research Ethics Committee of Kurume University, which ensured
opportunities for participants to opt out of the research without
any disadvantage.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
MIBC
|
muscle invasive bladder cancer
|
|
nDT
|
non-definitive therapy
|
|
DT
|
definitive therapy
|
|
RC
|
radical cystectomy
|
|
TURBT
|
transurethral resection of bladder
tumor
|
|
PS
|
performance status
|
|
QOL
|
quality of life
|
|
CCI
|
Charlson comorbidity index
|
|
TMT
|
trimodal therapy
|
|
OS
|
overall survival
|
|
CSS
|
cancer-specific survival
|
|
PFS
|
progression-free survival
|
References
|
1
|
No authors listed. Cancer Statistics in
Japan. Cancer information service, national cancer center; Japan:
(Vital Statistics of Japan, Ministry of Health, Labour and
Welfare). Available via DIALOG. https://ganjoho.jp/en/professional/statistics/table_download.htmlAugust
20–2021
|
|
2
|
Kamat AM, Hahn NM, Efstathiou JA, Lerner
SP, Malmström PU, Choi W, Guo CC, Lotan Y and Kassouf W: Bladder
cancer. Lancet. 388:2796–2810. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Witjes JA, Bruins HM, Cathomas R, Compérat
EM, Cowan NC, Gakis G, Hernández V, Linares Espinós E, Lorch A,
Neuzillet Y, et al: European association of urology guidelines on
muscle-invasive and metastatic bladder cancer: Summary of the 2020
guidelines. Eur Urol. 79:82–104. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
No authors listed. NCCN Guidelines.
Bladder cancer. Available via DIALOG. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1417August
20–2021
|
|
5
|
Solsona E, Iborra I, Collado A,
Rubio-Briones J, Casanova J and Calatrava A: Feasibility of radical
transurethral resection as monotherapy for selected patients with
muscle invasive bladder cancer. J Urol. 184:475–480. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Audenet F, Waingankar N, Ferket BS, Niglio
SA, Marqueen KE, Sfakianos JP and Galsky MD: Effectiveness of
transurethral resection plus systemic chemotherapy as definitive
treatment for muscle invasive bladder cancer in population level
data. J Urol. 200:996–1004. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brierley JD, Gospodarowicz MK and
Wittekind C: UICC TNM classification of malignant tumours. 8th
edition. Wiley Blackwell; New Jersey, NY: 2016
|
|
8
|
Koie T, Ohyama C, Fujimoto H, Nishiyama H,
Miyazaki J, Hinotsu S, Kikuchi E, Sakura M, Inokuchi J, Hara T, et
al: Diversity in treatment modalities of Stage II/III urothelial
cancer in Japan: Sub-analysis of the multi-institutional national
database of the Japanese urological association. Jpn J Clin Oncol.
46:468–474. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gray PJ, Fedewa SA, Shipley WU, Efstathiou
JA, Lin CC, Zietman AL and Virgo KS: Use of potentially curative
therapies for muscle-invasive bladder cancer in the United States:
results from the national cancer data base. Eur Urol. 63:823–829.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Herr HW: Transurethral resection of
muscle-invasive bladder cancer: 10-Year outcome. J Clin Oncol.
19:89–93. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Seisen T, Sun M, Lipsitz SR, Abdollah F,
Leow JJ, Menon M, Preston MA, Harshman LC, Kibel AS, Nguyen PL, et
al: Comparative effectiveness of trimodal therapy versus radical
cystectomy for localized muscle-invasive urothelial carcinoma of
the bladder. Eur Urol. 72:483–487. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fahmy O, Khairul-Asri MG, Schubert T,
Renninger M, Malek R, Kübler H, Stenzl A and Gakis G: A systematic
review and meta-analysis on the oncological long-term outcomes
after trimodality therapy and radical cystectomy with or without
neoadjuvant chemotherapy for muscle-invasive bladder cancer. Urol
Oncol. 36:43–53. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mak KS, Smith AB, Eidelman A, Clayman R,
Niemierko A, Cheng JS, Matthews J, Drumm MR, Nielsen ME, Feldman
AS, et al: Quality of life in long-term survivors of
muscle-invasive bladder cancer. Int J Radiat Oncol Biol Phys.
96:1028–1036. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Smith AB, Jaeger B, Pinheiro LC, Edwards
LJ, Tan HJ, Nielsen ME and Reeve BB: Impact of bladder cancer on
health-related quality of life. BJU Int. 121:549–557. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cerruto MA, D'Elia C, Siracusano S,
Gedeshi X, Mariotto A, Iafrate M, Niero M, Lonardi C, Bassi P,
Belgrano E, et al: Systematic review and meta-analysis of non RCT's
on health related quality of life after radical cystectomy using
validated questionnaires: Better results with orthotopic neobladder
versus ileal conduit. Eur J Surg Oncol. 42:343–360. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Clifford TG, Shah SH, Bazargani ST,
Miranda G, Cai J, Wayne K, Djaladat H, Schuckman AK and Daneshmand
S: Prospective evaluation of continence following radical
cystectomy and orthotopic urinary diversion using a validated
questionnaire. J Urol. 196:1685–1691. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Maffezzini M, Fontana V, Pacchetti A,
Dotta F, Cerasuolo M, Chiappori D, Guano G, Mantica G and Terrone
C: Age above 70 years and charlson comorbidity index higher than 3
are associated with reduced survival probabilities after radical
cystectomy for bladder cancer. Data from a contemporary series of
334 consecutive patients. Arch Ital Urol Androl. 93:15–20. 2021.
View Article : Google Scholar : PubMed/NCBI
|