Introduction
In recent decades, many natural molecules of
bacterial origin have been tested to evaluate their
anti-proliferative activity. Pigments produced by microbes, such as
melanin, flavin, phenazine, and violacein, are medically
interesting and are well known to show cytotoxic, antioxidant,
antimicrobial, and antimalarial activities (1). Violacein is a member of the class of
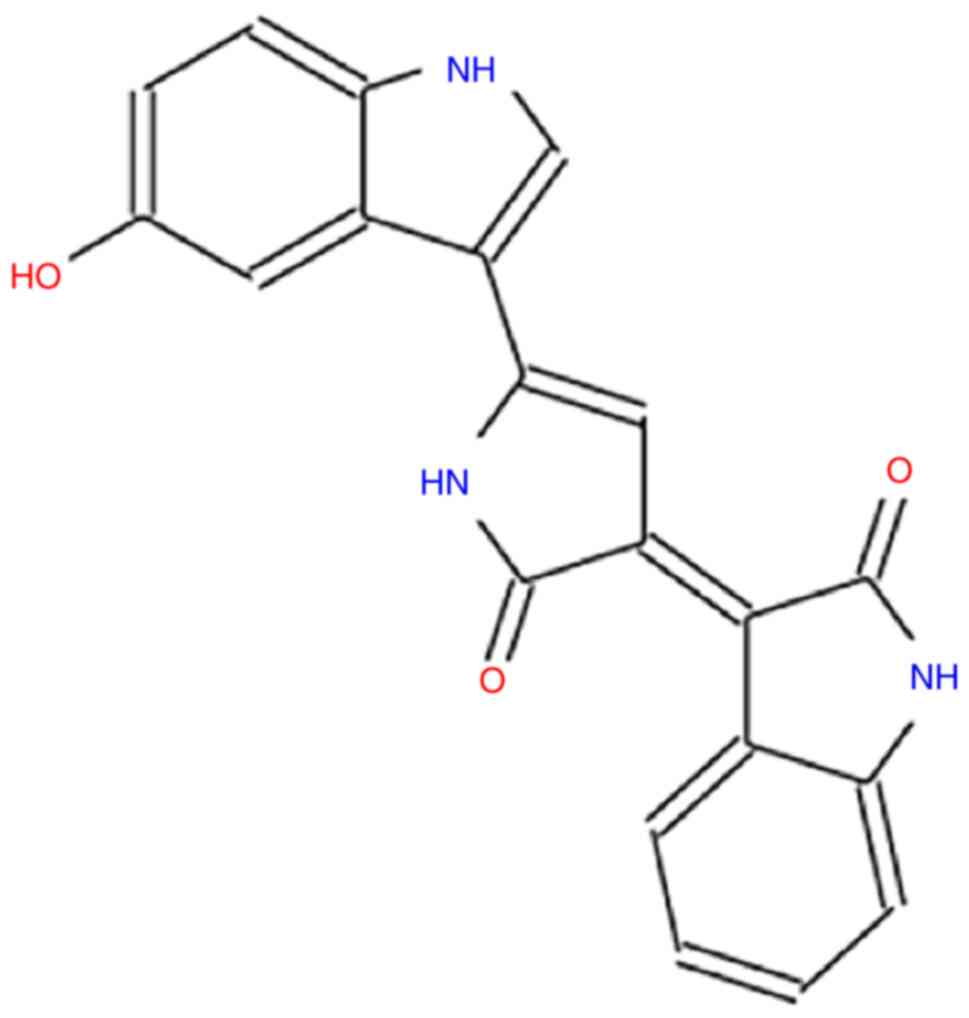
water-soluble hydroxyindoles, it is formed by the condensation of
two modified tryptophan molecules, with a molecular weight of
343.33 (C20H13N3O3)
(Fig. 1) (2). It is a natural pigment that confers a
blue violet color to bacteria that produce it, such as
Chromobacterium violaceum (C. violaceum), Janthinobacterium
lividum (J. lividum), Alteromonas luteoviolacea, Pseudoalteromonas
luteoviolacea, and Duganella sp. (3). These bacteria are all gram-negative,
facultative aerobes, and saprophytes belonging to Proteobacteria
phylum. They are mainly isolated from soil and water in tropical
and subtropical regions and are able to survive in hostile
environmental conditions (3). In
J. lividum and C. violaceum, the production of
violacein and biofilm appears to be regulated by a common metabolic
pathway (4). Violacein production
is a response to environmental stress and a key factor associated
with survival in hostile conditions (5).
Several studies have shown that this substance
possesses different activities: antibacterial (6), anti-fungal (7), trypanocidal (8), immunomodulating, analgesic,
antipyretic (9), and
anti-proliferative (9,10). Violacein is able to induce
apoptosis in HL60 leukemic cells through the activation of caspases
(11), which play an important
role in the induction of programmed cell death. The same study also
showed the anti-cancer activity of violacein versus leukemia cells
of the chronic myeloid line (K562 cell line). Furthermore,
violacein appears to be non-toxic in peripheral blood cells at a
concentration of 700 nM (11).
Programmed cell death is one of the biological events underlying
the regulation of tissue homeostasis, but it also plays a role in
the elimination of damaged, stressed, or infected cells. The
modalities of cell death can be traced back to two models: death
suffered, whose morphological aspect is necrosis, and cell death or
suicide, whose typical form (also physiological) is apoptosis
(12,13). Necrosis causes the involvement of
the structures surrounding inflammation. Apoptosis is a form of
programmed cell death that does not induce inflammation and helps
to keep the number of cells stable (14). Apoptosis is characterized by a
series of dramatic disturbances in the cellular architecture that
not only contribute to cell death, but also prepare cells for
phagocyte removal and prevent unwanted immune responses. Much of
what occurs during the breakdown phase of apoptosis is orchestrated
by members of the cysteine protease caspase family (12). Alterations of these mechanisms are
responsible for the onset of severe diseases (12). Corroborating data on the
anti-cancer activity of violacein can be found in studies carried
out on colon (15), breast
(16), and head and neck (17) cancer cell lines. Kodach et
al demonstrated that, in the HCT116 cell line, violacein
enhances the cytotoxic effect of 5-fluorouracil, a chemotherapeutic
agent used in the treatment of colorectal cancer (CRC) (18). The literature data show that in
Caco-2 cells, violacein causes tumor cell apoptosis, stimulating
the production of ROS, the release of cytochrome c and calcium into
the cytosol, and the activation of caspase-3 (15,19).
Bladder cancer is the second most frequent tumor of
the urinary tract, it is more common in men than in women (at a
ratio of 4:1) (20), representing
the seventh and seventeenth most diagnosed cancer types in the male
and female population worldwide, respectively (21). It mainly affects individuals aged
50–70 years (20) and the
incidence and mortality of this cancer are influenced by different
risk factors, such as tobacco smoking and prolonged exposure to
aromatic polyamines (22). At
diagnosis, bladder cancer is superficial in 75% of cases with a
disease limited to the mucosa (stage Ta, CIS) or submucosa (stage
T1). The main therapeutic strategies for urinary bladder tumor is
surgery followed by adjuvant and neoadjuvant therapy, such as
single immediate, post-operative intravesical instillation of
chemotherapy or repeated instillations, based on prognosis
(21).
Novel drugs represent an innovative pharmaceutical
tool for implementation of the current therapies for this type of
cancer (23). Owing to its
antibacterial and antitumoral properties, through bladder
instillations, violacein may represent a favorable therapeutic
option as an anticancer agent in both pre- and post-surgery. To
this purpose, the aim of the present study was to investigate, for
the first time, the anti-proliferative activity of violacein
against human bladder carcinoma cell lines T24 and 5637,
respectively, transitional cell carcinoma (low grade) and grade II
carcinoma (high grade), and to explore the mode of action of this
substance on these cell lines. The present preliminary in
vitro study on bladder carcinoma cell lines may represent the
first step in the design of violacein as a potential
antineoplastics drug.
Materials and methods
General
To evaluate the anti-proliferative activity of
violacein against urinary bladder cancer, the proliferation and
cell viability of two bladder cell lines, T24 and 5637, were
initially assessed following treatment with violacein. Cell
proliferation and viability were measured using the MTT assay, and
the viable cell count was conducted using the trypan blue assay.
Furthermore, the cytotoxic effect induced by violacein in the two
cell lines was examined through observation of the cell nuclei,
which were stained with DAPI fluorophore, using fluorescence
microscopy. Subsequently, using colorimetric assays and flow
cytometry, the cell cycle of T24 and 5637 following violacein
treatment, which were labelled with propidium iodide (PI) was
examined. In addition, the activation of the caspase-3 protease was
assessed by detecting the antibody directed against caspase-3
cleaved.
Materials
Violacein, dimethylsulfoxide (DMSO), methanol, and
acetic acid were purchased from Sigma-Aldrich. The PI was obtained
from Biotium Corporate Headquarters. The MTT assay and DAPI were
purchased from SERVA Electrophoresis GmbH. The T24 and 5637 cell
lines were purchased from the American Type Culture Collection
(ATCC). Media, RPMI-1640, Trypsin + EDTA, phosphate-buffered saline
(PBS), penicillin-streptomycin, and sera for the cell culture were
obtained from Corning and were endotoxin-free. For the
immunofluorescence experiments, anti-cleaved caspase-3 primary
antibody (1:20 dilution; ab2302) and goat anti-rabbit IgG-H&L
polyclonal secondary antibody (1:5 dilution; ab150077; Alexa
Fluor® 488) were purchased from Abcam plc. RNase A and
Lab-Tek™ II Chamber Slide™ were purchased from Thermo Fisher
Scientifics Baltics UAB. Tween-20 solution and paraformaldehyde
solution 4% in PBS were obtained from Bio-Rad Laboratories Srl.
Cell cultures
The human urinary bladder carcinoma T24 and 5637
cells were cultured in RPMI-1640, without phenol red, supplemented
with 10% fetal bovine serum (FBS), 2 mM glutamine, 100 U/ml
penicillin, and 100 µg/ml streptomycin. The T24 and 5637 cells were
obtained from ATCC and were grown in RPMI-1640, supplemented with
10% FBS, according to international guidelines. Mycoplasma testing
was performed for the cell lines. The cells were incubated at 37°C
in a humidified atmosphere of 5% CO2 for two weeks prior
to experimental use. To subculture the T24 and 5637 cells, they
were washed with PBS and incubated with 0.25% trypsin and 1 mM EDTA
at 37°C for 3–5 min (T24) and 5–7 min (5637). Then, the detached
cells were resuspended in a fresh serum-containing medium to
inactivate the trypsin and transferred to new flasks.
Cell proliferation assay (MTT)
The T24 and 5637 cells were seeded in parallel in
96-well plates at a density of 5.000 cells/well. The next day, the
cells were exposed to different concentrations of violacein (0.1,
0.2, 0.3, 0.5, and 1 µM) for 24 h. Two cellular controls were set
up in parallel, one containing only the cell culture medium
(RPMI-1640 supplemented with 10% FBS) and the other containing the
cell culture medium with an added percentage of DMSO used to
resuspend violacein (0.1% v/v). After the treatment, the cells were
treated with 20 µl of 5 mg/ml MTT in PBS, and the plates were
incubated for 4 h in the dark at 37°C and 5% CO2. Then,
the medium was removed, and the formed formazan salt was dissolved
by adding 100 µl of DSMO to each well. The optical density was
measured at 570 nm, with the reference set at 620 nm, using an
automatic microplate reader, Tecan Sunrise (24). The assay was carried out in
triplicate. To understand whether the cytotoxic effect was
permanent or reversible, the same test was performed 48 h after the
end of the violacein treatment. Briefly, after the treatment with
different concentrations of violacein, the medium (RPMI-1640
supplemented with 10% FBS) was discarded, and fresh RPMI-1640
medium with 10% of FBS was added to the cells, which were then
incubated at 37°C and 5% CO2 for 48 h. Then, the MTT
test was carried out as described above. The assay was performed in
triplicate. No absorbance differences were found between the two
cell controls, indicating that the percentage of DMSO used was
nontoxic for the cells (Fig. S1).
For this reason, only fresh medium with added DMSO was used as a
negative control in the following tests.
Vital staining with trypan blue
The cell viability was assessed using the dye,
trypan blue. T24 and 5637 cells seeded in 6-well plates at a
density of 1×106 cell/well were exposed to different
concentrations of violacein (0.1, 0.2, 0.3, 0.5, and 1 µM) and to
the cell medium alone as a control. At the end of the treatment,
the medium was collected and centrifuged at 1,200 × g for 5 min at
4°C to recover the dead cells present in the suspension. The cells
still adhering to the wells were detached by trypsinization and
centrifuged at 1,200 × g for 5 min at 4°C, and the pellet was
recovered and resuspended in 1 ml of a complete medium (RPMI-1640
supplemented with 10% FBS) also containing the dead cells that were
previously recovered. An aliquot was then taken and added to an
equal volume of trypan blue (0.4%). The cells were then counted
using an optical microscope via a Burker's chamber (25). The assay was carried out in
triplicate.
DAPI images
To evaluate the presence of morphological changes
characteristic of the cytotoxic effect, the T24 and 5637 cells were
seeded at a density of 2×105 cells/well in 8-well
Lab-Tek chamber slides and exposed to 1 µM of violacein or a fresh
medium (as a control) for 24 h at 37°C. Briefly, after the
violacein treatment, the medium was removed, and the remaining
adhering cells were washed with PBS and fixed with fixative (3:1
methanol/acetic acid) for 10 min at room temperature (rt).
Subsequently, the cells' nuclei were stained with a solution of 1
µg/ml DAPI in PBS at 37°C for 15 min. Excess solution dye was
removed, the polystyrene chambers were detached, and the remaining
slides were directly examined under a fluorescence microscope
(330–380 nm), Leica DM IRBE.
Cell cycle analysis
Flow cytometric analysis of the cells was performed
using a MACSQuant Analyzer 10-flow cytometer (Miltenyi Biotec
Inc.). To analyze the cell cycle distribution, the T24 and 5637
cells were treated with 1 µM of violacein and a fresh medium (as
control) for 24 h. After the treatment, the medium was removed and
centrifuged at 1,200 × g for 5 min at 4°C to recover the cellular
pellet, while the adhering cells were collected by trypsinization.
After fixation with 75% cold ethanol, the cells were washed in PBS
and resuspended in 1 ml of PBS containing 0.5 mg/ml of RNase A and
0.01 mg/ml of PI and incubated at room temperature in the dark for
30 min. The percentage of cells in the sub-G1, G0/G1, S, and G2/M
phases of the cell cycle was analyzed using the FlowJo software
(Becton, Dickinson and Company).
Caspase-3 activation
An indirect staining assay by flow cytometry was
used to test the caspase-3 activation. Briefly, the two cell lines
were seeded at a concentration of 5×105 cells/well in
12-well plates. The following day, the cells were exposed to 1 µM
of violacein or a fresh medium only (as a control) for 1, 3, 6, or
24 h. After the treatment, the cells were resuspended in a 4%
paraformaldehyde solution in PBS and kept on ice for 30 min. The
cells were then centrifuged at 300 × g for 5 min at rt, and the
pellet was resuspended in 0.5 ml of 0.2% Tween solution in PBS.
After three washes, the cell pellets were resuspended in a staining
solution containing the primary antibody (ab2302; anti-cleaved
caspase-3 antibody) and incubated for 10 min at rt. At the end of
the incubation, the cells were washed with PBS and then incubated
for 30 min at room temperature in a staining solution containing
the secondary antibody (ab150077). Subsequently, the obtained
solutions were transferred into appropriate tubes for flow
cytometric analysis.
Statistical analysis
Each assay was replicated at least three times, and
statistical significance was determined using GraphPad Prism 9
statistical software package (GraphPad Software, Inc.). Data are
expressed as means ± standard deviation (SD). Multiple comparisons
among group mean differences were analysed with one-way analysis of
variance (ANOVA) followed by Bonferroni's post hoc test. The
Student's t-test was used to compare paired and un-paired samples.
The means of the same group under two separate scenarios, were
compared with paired t-test (e.g., Fig. 2). The means of two independent or
unrelated groups (e.g., Fig. 3),
were compared with the unpaired t-test. Differences were considered
significant when P<0.05.
Results
Cell proliferation activity and
viability
After 24 h of exposure to different violacein
concentrations (0.1, 0.2, 0.3, 0.5, and 1 µM), the cytotoxic effect
of violacein on T24 and 5637 cells was evaluated through the
measurement of cell viability by MTT assay, and a vital count was
assessed with trypan blue dye. The assay was based on the principle
that proliferating cells are capable of transforming tetrazolium
salts (MTT) into formazan. No significant changes were observed in
the absorbance values after the restoration of the fresh medium for
48 h, compared to the values obtained by the MTT assay, which was
performed immediately after the treatment (Fig. S2), indicating that violacein
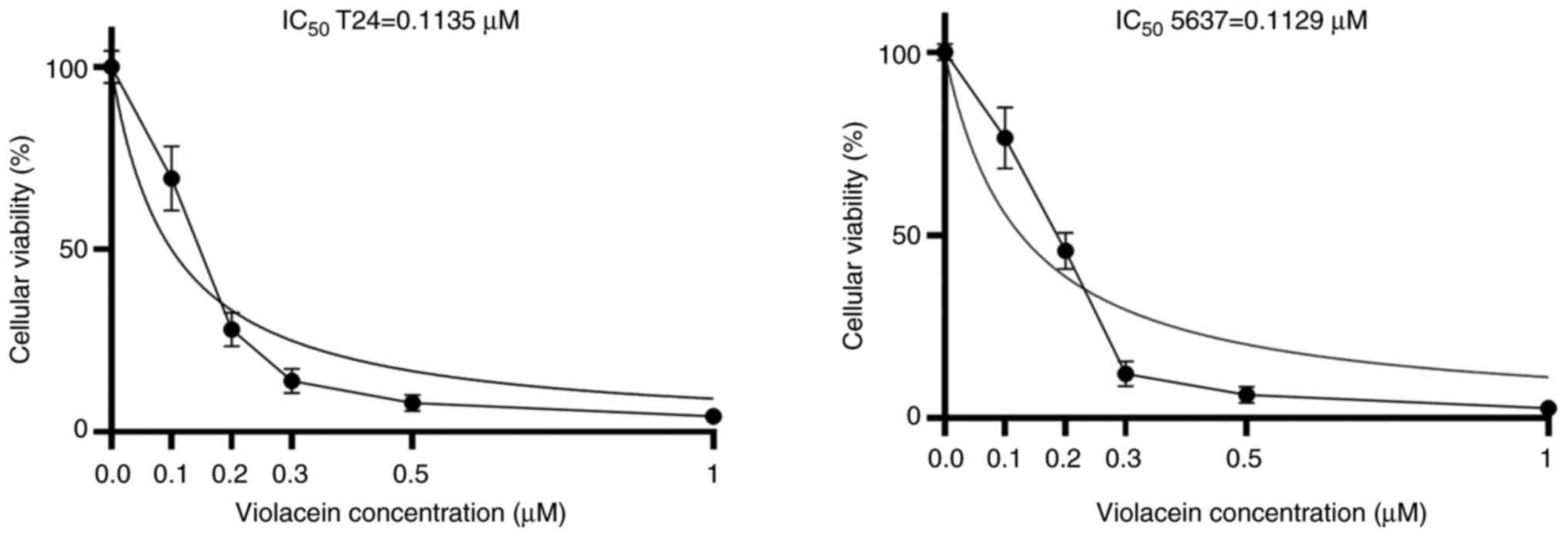
induces a permanent cytotoxic effect. As shown in Fig. 2, cell viability was evident in both
cell lines (T24 and 5637), but inhibited by the violacein treatment
in a dose-dependent manner, with an IC50 value of
0.1135±0.05 µM for the T24 cell line and an IC50 value
of 0.1129±0.1 µM for the 5637 cells. To determine whether a
reduction in cell proliferation also corresponded to an increase in
cell mortality, the vital count with trypan blue dye was added to
the MTT assay. Although inhibition of the cell proliferation had
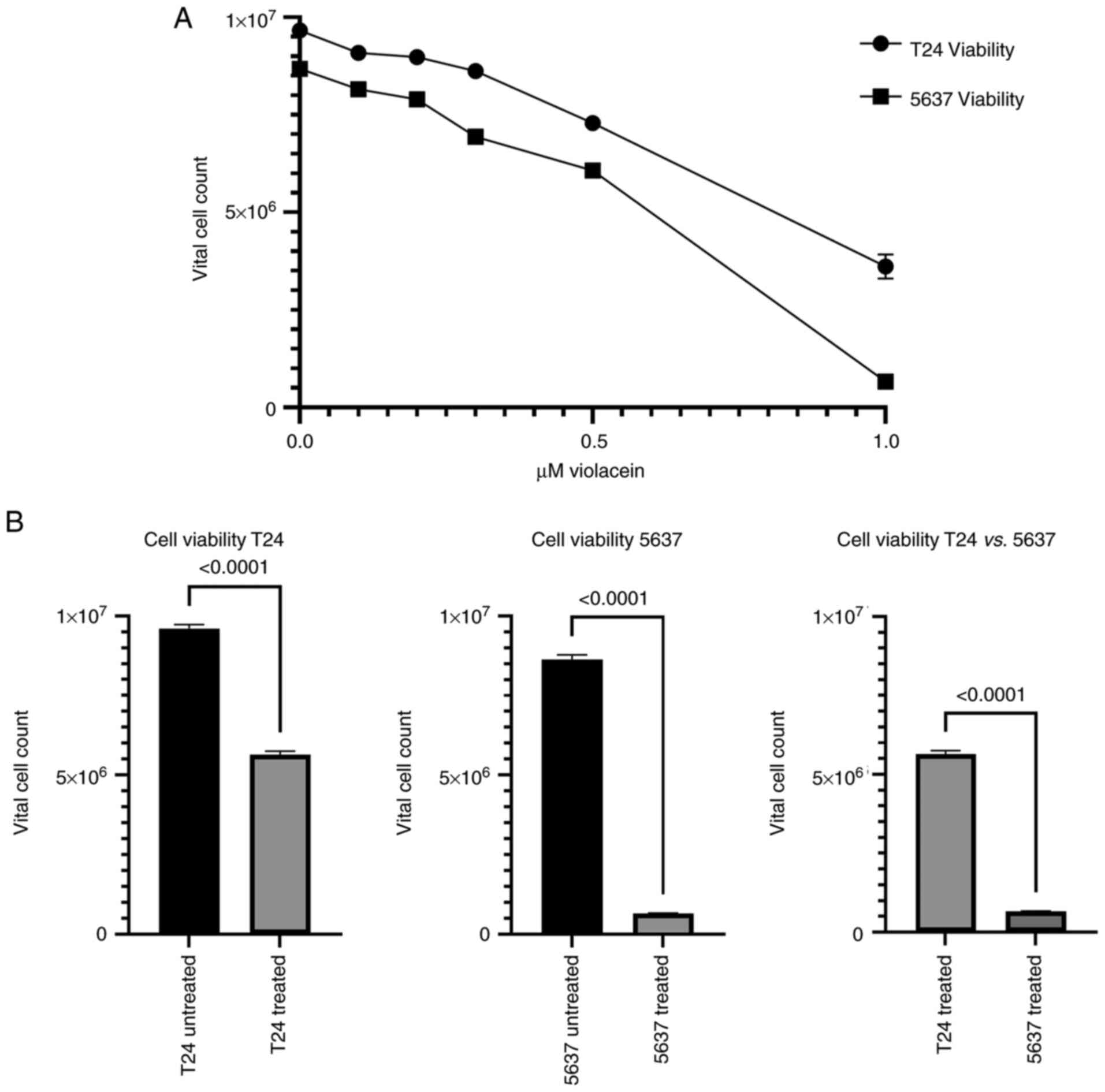
already started at a violacein concentration of 0.1 µM (Fig. 2), only at the 1 µM dose was there a
significant parallel increase in cell mortality. In fact, compared
to untreated cells, treatment with 1 µM of violacein caused a
significant decrease in the cellular viability in both cell lines
(P<0.0001) (Fig. 3A and B). In
order to confirm the difference between the cell lines used with
respect to violacein sensitivity, the viability level in T24 and
5637 cells treated for 24 h with 1 µM of violacein was examined
(Fig. 3B). A 2% viability
reduction for T24 and 10% for 5637 (P<0.0001), when compared
with their respective untreated cells, was observed (Fig. 3B). The 5637 cell line, a model of
second-grade bladder cancer, showed a greater sensitivity to
violacein, compared to the T24 cell line.
Morphological evaluation of cell
nuclei with DAPI
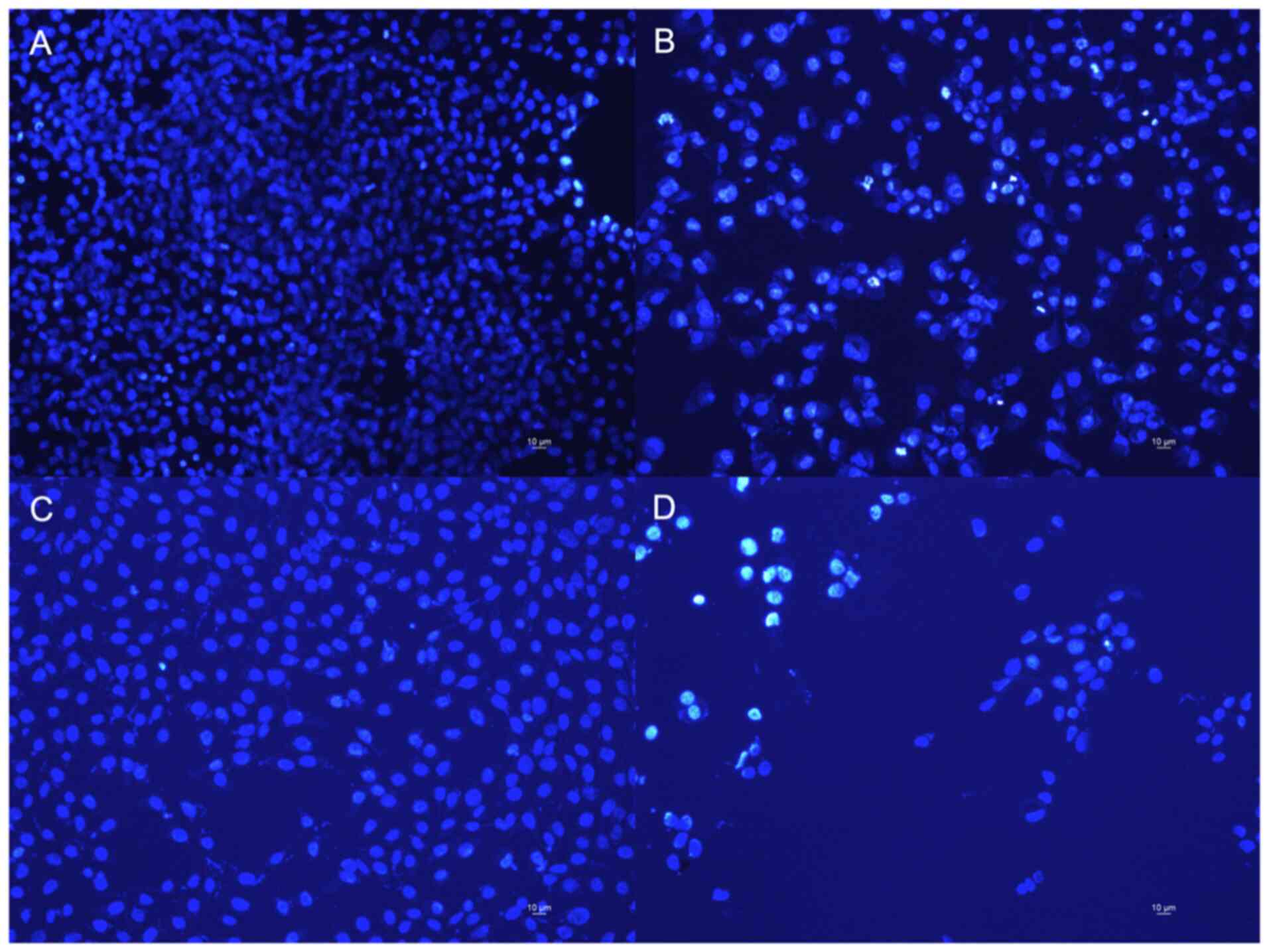
In order to evaluate the cytotoxic effect induced by
violacein, the nuclei of the two cell lines were stained with DAPI,
a fluorescent DNA stain, and visualized under a widefield optical
fluorescence microscopy (Leica DM 5000B). From the images detected
(Fig. 4), a decrease of the number
of nuclei per field is evident in both cell lines after the
treatment, compared to the untreated cells. On the other hand, the
nuclei show a normal phenotype and homogeneous light emission
signal in the untreated cells, while the nuclei of the
violacein-treated cells show a greater fluorescence intensity,
indicative of the damage. These results corroborate our
cytotoxicity data.
Cell cycle
To investigate the mechanism underlying the action
of violacein in the two cell lines, analysis of the progression of
the cell cycle after the violacein treatment was performed. The
cell cycle distribution analysis of both T24 and 5637 cell lines
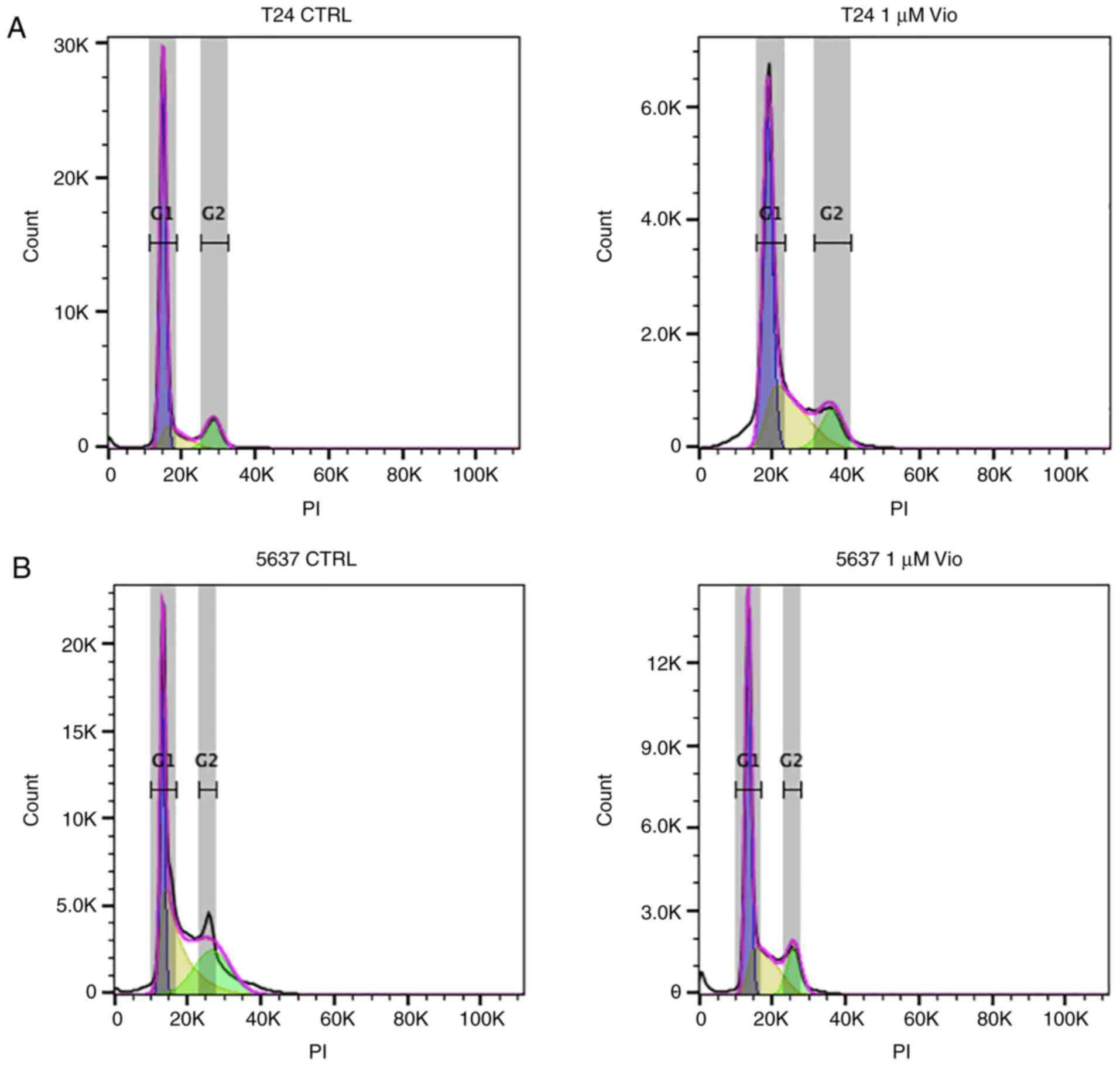
was performed after 24 h of 1 µM violacein exposure (Fig. 5). As shown in Fig. 5 and reported in Table I, the T24 G0/G1 population treated
with violacein decreased by 20%, compared to the untreated cells,
while the treatment caused an increase of 18% in the progression of
S-phase blocking cells in G2/M phase. The cell diminution in the
G0/G1 phase caused an increase in sub-G1 particles, with 2.5% more
apoptotic cells after the treatment, compared to the untreated
cells. The cell cycle analysis of 5637 cells revealed a possible
different mode of action of violacein, with respect to what was
observed in the T24 cells, with an increase in the G0/G1
population, compared to the untreated cells (25%), indicating a
block in this phase, with a decrease of the cell population in the
S phase (11.5%). A significant 1.2% increase in the sub-G1 cell
fraction, probably representing apoptotic cells, compared to the
untreated cells, was observed. This fraction would seem to be
greater in the T24 cell line, compared to 5637.
 | Table I.Value for the cell percentage in each
phase of the cell cycle. |
Table I.
Value for the cell percentage in each
phase of the cell cycle.
|
| T24 cells | 5637 cells |
|---|
|
|
|
|
|---|
| Cell cycle phase | Ctrl | Treated with 1 µM
of Violacein | Ctrl | Treated with 1 µM
of Violacein |
|---|
| Sub-G1 | 6.9±0.5 |
9.4±0.5b | 7.9±0.4 |
8.7±0.5a |
| G0/G1 | 65.6±3 | 46.6±2b | 24.8±1 | 48.6±2b |
| S | 13.5±1 | 31.4±2b | 39.2±2 | 27.7±1b |
| G2/M | 14.0±2 |
12.6±0.5a | 28.1±3 | 15.0±2b |
Active caspase-3 production
A distinctive feature of the early stages of
apoptosis is the activation of caspase enzymes, which participate
in the cleavage of protein substrates and the subsequent
disassembly of the cell. Caspase-3, a fundamental mediator of
apoptosis, also termed the death protease, is an effector caspase
that cuts precise protein substrates, thus initiating the apoptotic
process (26). To highlight the
onset of the apoptotic process, after the violacein treatment,
caspase-3 activation was evaluated by caspase-3 fluorometric
assay.
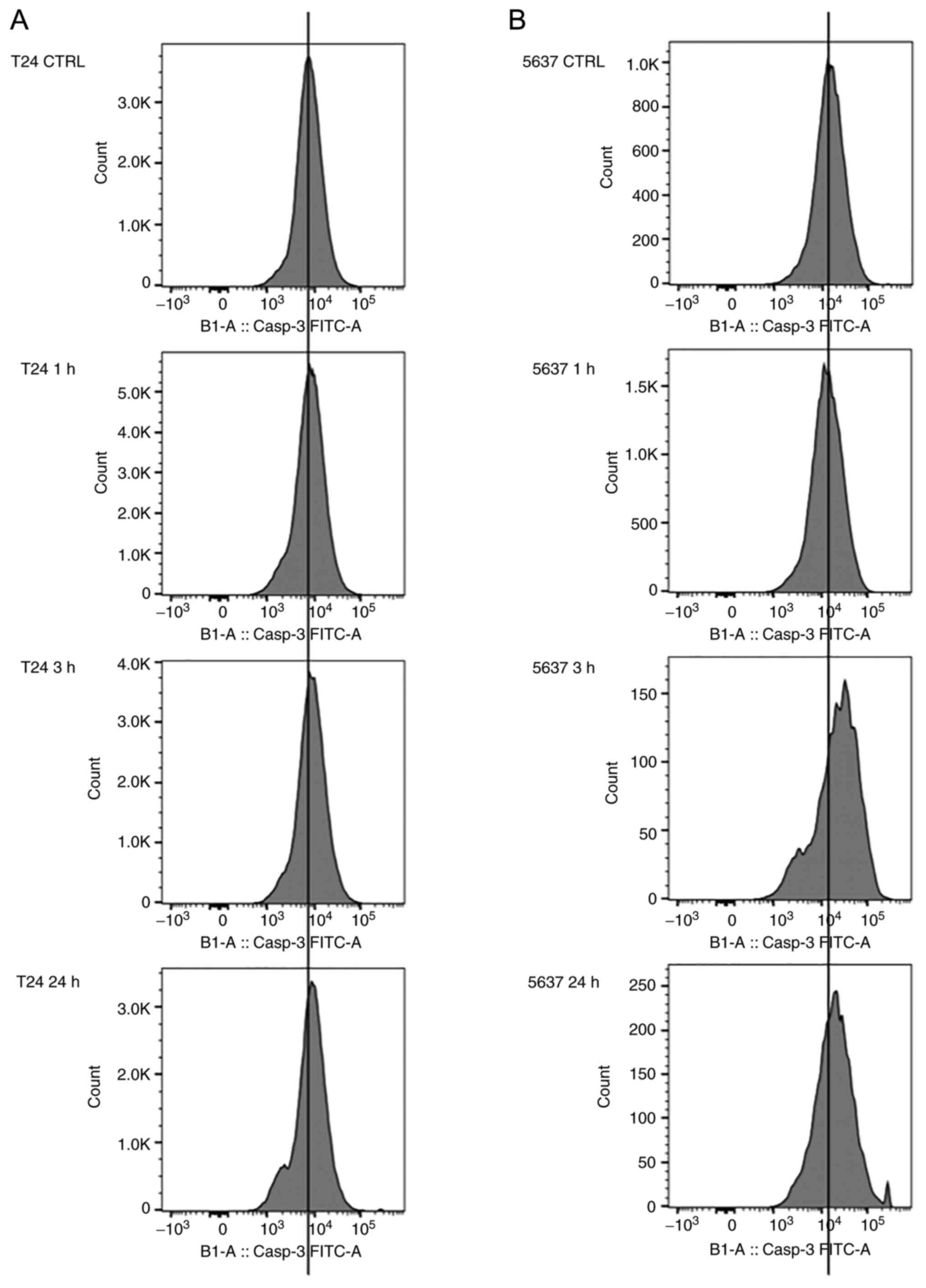
The two cell lines were subjected to a treatment of
1 µM violacein for 1, 3, or 24 h. The activation index of caspase-3
induced by the violacein treatment was determined as the ratio
between the fluorescence values of both the treated and the
untreated cells. In the two cell lines, the maximum expression of
active caspase-3 was obtained after 3 h of the treatment (Fig. 6). After 24 h of violacein exposure,
the expression level of caspase-3 returned to the untreated cell
values (Fig. S3). The expression
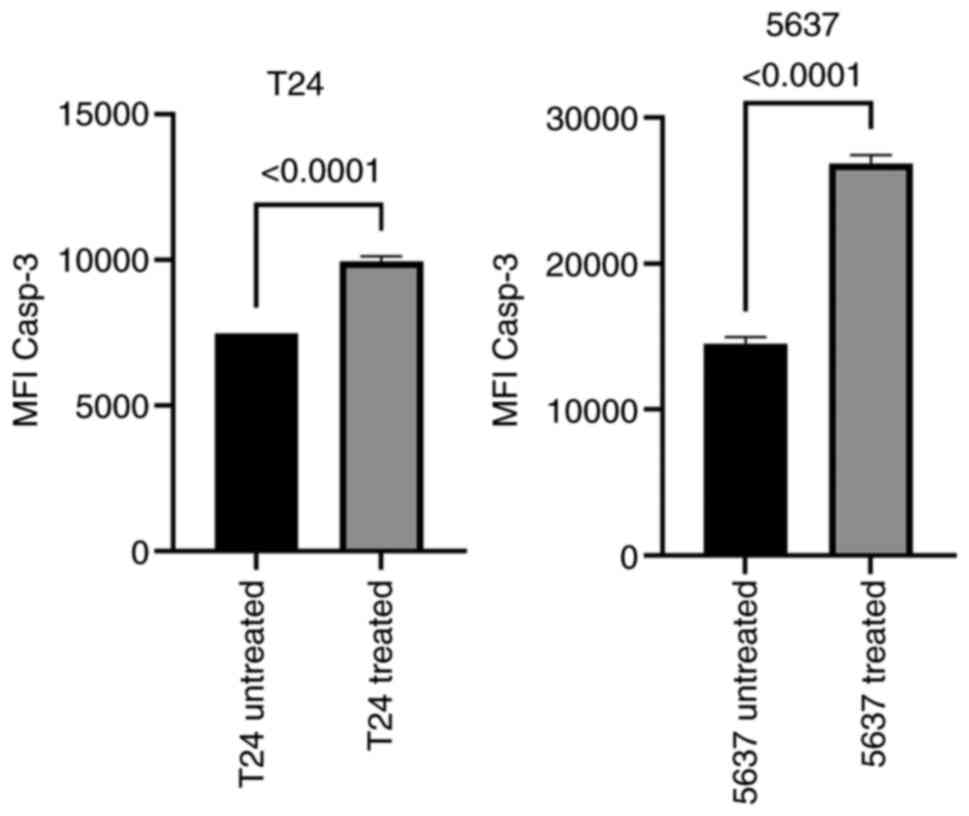
of the active caspase-3 was higher in the 5637 cell line, with
double the value for Median Fluorescence Intensity, compared to the
untreated cells (Figs. 6 and
7).
Discussion
The therapeutic approach for bladder cancer involves
interventions frequently used in combinations: surgery,
chemotherapy, or immunotherapy (21). Several natural compounds with the
ability to induce programmed cell death (apoptosis), causing
minimal damage to the surrounding cells and tissues, are currently
under evaluation. Among these compounds, the pigment, violacein,
has been highlighted for its anti-proliferative activity against
specific cell lines (17). The
violacein anti-proliferative activity appears to be due to the
induction of the apoptotic process, as already shown in several
cancer models (15–17,27).
In the present study, the anti-proliferative activity of this
substance was evaluated against two cell lines, T24 and 5637, which
are models of human transitional cell carcinoma, low-grade, and
high-grade, respectively. The effect of violacein on these cells of
the same derivation tissue, but at different tumor stages, has, to
the best of our knowledge, never been previously tested.
The DAPI staining assay confirmed the cytotoxicity
data of the present study showing, after 24 h of treatment with
violacein, a decrease in nuclei per field and an increased
fluorescence intensity for the nuclei of violacein-treated cells,
indicative of damage, thereby corroborating the cytotoxicity data
obtained in the present study. In order to better understand the
type of death activated by violacein further studies are necessary
including semi-quantitative analysis with DAPI, and assay with
annexin V-FITC and PI, as in the current study. Exploring violacein
activity, a proliferation inhibitory effect and an activation of
the caspase-3 protease were identified in the two cell lines, with
a greater antiproliferative activity against the second-grade tumor
model cell line, i.e., 5637. Moreover, from the cell cycle
analysis, a different violacein mode of action on these cell lines
emerged. In particular, violacein appears to block T24 cells in the
S phase of the cell cycle, thus preventing progression in the G2/M
division phase, while the 5637 cell line appeared to be blocked in
phase G1. Furthermore, a significant increase in sub-G1 particles
(probably representing apoptotic cells) after the violacein
treatment in both cell lines was observed. The distribution of T24
and 5637 cells in the different cell cycle phases showed a higher
apoptotic cell percentage in the T24 cell line, compared to 5637
(2.5 versus 1.2%, respectively). The violacein apoptotic induction
was evaluated by the expression of active caspase-3 in the lines
employed, after treatment with violacein. Caspase-3 is a protease
synthesized as an inactive zymogen in the cytosol of mammalian
cells. It is rapidly activated through a specific cascade of events
in the executive phase of apoptosis (28). A significant increase in active
caspase-3 expression was observed in the two cell lines after 3 h
of treatment with violacein. The 5637 cell line showed both a basal
expression of caspase-3 and a higher cellular proliferation
activity, than the T24 cells. The results of the present study
support the hypothesis that violacein may have different modes of
action on these cell lines possibly due to molecular differences
among cells. However, further investigations are required to gain a
better understanding of the violacein-induced cell death mechanism
in bladder cancer cells, with particular attention to the
differences observed between the two cell lines. Further studies
are also needed to determine how violacein affects caspase-3,
directly or indirectly. Moreover, for the 5637 cell line, the
apparent discrepancy in the percentage of death and apoptotic cells
obtained through the vital count with trypan blue (Fig. 3B) and the cell cycle analysis
(Fig. 4; Table I), respectively, could be supported
by the co-existence of different mechanisms of cell death. In
addition, the present preliminary in vitro study has shown,
through classical assays based on biochemical parameters and
morphological changes, the cytotoxic power of violacein against
bladder cancer cells assuming activation of the apoptotic pathway.
In order to identify molecular changes characteristic of apoptosis
and to attest the anti-proliferation activity of violacein, further
experiments, such as western blot analysis of caspase-3, Bcl-2,
Bax, and proliferating cell nuclear antigen (PCNA), are needed.
Furthermore, to investigate the death activated by violacein, to
allow the distinction between necrotic and apoptotic cells, a valid
approach was represented by flow cytometry analysis of annexin V/PI
assay. Future studies should examine the effect of violacein in
more advanced bladder cancer cell lines. In fact, in the case of
advanced and metastatic disease the tumor extends through the
bladder wall until it invades the pelvic and abdominal wall with
the ability to invade distant organs. In this case, the total
removal of the tumor was technically impossible and treatment with
chemotherapy to eradicate the tumor cells appears to be the main
therapeutic option. In this context, violacein may represent a
potential valid therapeutic strategy or an alternative/adjuvant to
existing ones focused on killing cancer cells, especially against
those at a more advanced stage. This preliminary in vitro
study on bladder carcinoma cell lines may be the first step in the
design of violacein as a potential antineoplastics drug.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This research was partly funded by Sapienza Fondi di Ateneo:
Avvio alla ricerca ‘2019 grant number AR11916B46803EC4’, PI Bruna
Neroni.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
BN conceptualized and designed the study, performed
the experimental part of the study, analyzed all the results, wrote
the manuscript and acquired the funding necessary for the study.
MAZ analyzed and interpreted the data regarding the cell cycle
analysis. GR analyzed results obtained from the MTT assay and
revised the manuscript. MRC analyzed the data regarding the cell
cycle analysis, and visualized and critically revised the
manuscript. LM supervised the experiments, performed the MTT assay
and wrote and critically revised the manuscript. FP conceptualized
the study, supervised experiments, analyzed the results and wrote
and revised the manuscript. SS conceptualized and designed the
study, supervised the experiments, analyzed results, wrote the
manuscript and critically revised it. SS and FP confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
References
|
1
|
Ramesh C, Vinithkumar NV, Kirubagaran R,
Venil CK and Dufossé L: Multifaceted applications of microbial
pigments: Current knowledge, challenges and future directions for
public health implications. Microorganisms. 7:1862019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Choi SY, Lim S, Cho G, Kwon J, Mun W, Im H
and Mitchell RJ: Chromobacterium violaceum delivers
violacein, a hydrophobic antibiotic, to other microbes in membrane
vesicles. Environ Microbiol. 22:705–713. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Durán N, Justo GZ, Durán M, Brocchi M,
Cordi L, Tasic L, Castro GR and Nakazato G: Advances in
Chromobacterium violaceum and properties of violacein-Its
main secondary metabolite: A review. Biotechnol Adv. 34:1030–1045.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Choi SY, Yoon K, Lee JI and Mitchell RJ:
Violacein: Properties and production of a versatile bacterial
pigment. Biomed Res Int. 2015:4650562015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pantanella F, Berlutti F, Passariello C,
Sarli S, Morea C and Schippa S: Violacein and biofilm production in
Janthinobacterium lividum. J Appl Microbiol. 102:992–999.
2007.PubMed/NCBI
|
|
6
|
Cazoto LL, Martins D, Ribeiro MG, Durán N
and Nakazato G: Antibacterial activity of violacein against
Staphylococcus aureus isolated from bovine mastitis. J
Antibiot (Tokyo). 64:395–397. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sasidharan A, Sasidharan NK, Amma DB, Vasu
RK, Nataraja AV and Bhaskaran K: Antifungal activity of violacein
purified from a novel strain of Chromobacterium sp. NIIST
(MTCC 5522). J Microbiol. 53:694–701. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bilsland E, Tavella TA, Krogh R, Stokes
JE, Roberts A, Ajioka J, Spring DR, Andricopulo AD, Costa FT and
Oliver SG: Antiplasmodial and trypanocidal activity of violacein
and deoxyviolacein produced from synthetic operons. BMC Biotechnol.
18:222018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rodrigues AL, Trachtmann N, Becker J,
Lohanatha AF, Blotenberg J, Bolten CJ, Korneli C, de Souza Lima AO,
Porto LM, Sprenger GA and Wittmann C: Systems metabolic engineering
of Escherichia coli for production of the antitumor drugs violacein
and deoxyviolacein. Metab Eng. 20:29–41. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hashimi SM, Xu T and Wei MQ: Violacein
anticancer activity is enhanced under hypoxia. Oncol Rep.
33:1731–1736. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ferreira CV, Bos CL, Versteeg HH, Justo
GZ, Durán N and Peppelenbosch MP: Molecular mechanism of
violacein-mediated human leukemia cell death. Blood. 104:1459–1464.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zeiss CJ: The apoptosis-necrosis
continuum: Insights from genetically altered mice. Vet Pathol.
40:481–495. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kerr JF, Winterford CM and Harmon BV:
Apoptosis. Its significance in cancer and cancer therapy. Cancer.
73:2013–2026. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
de Carvalho DD, Costa FTM, Duran N and
Haun M: Cytotoxic activity of violacein in human colon cancer
cells. Toxicol In Vitro. 20:1514–1521. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Alshatwi AA, Subash-Babu P and Antonisamy
P: Violacein induces apoptosis in human breast cancer cells through
up regulation of BAX, p53 and down regulation of MDM2. Exp Toxicol
Pathol. 68:89–97. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Masuelli L, Pantanella F, La Regina G,
Benvenuto M, Fantini M, Mattera R, Stefano ED, Mattei M, Silvestri
R, Schippa S, et al: Violacein, an indole-derived purple-colored
natural pigment produced by Janthinobacterium lividum,
inhibits the growth of head and neck carcinoma cell lines both in
vitro and in vivo. Tumour Biol. 37:3705–3717. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kodach LL, Bos CL, Durán N, Peppelenbosch
MP, Ferreira CV and Hardwick JCH: Violacein synergistically
increases 5-fluorouracil cytotoxicity, induces apoptosis and
inhibits Akt-mediated signal transduction in human colorectal
cancer cells. Carcinogenesis. 27:508–516. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
de Sousa Leal AM, de Queiroz JDF, de
Medeiros SRB, de Souza Lima TK and Agnez-Lima LF: Violacein induces
cell death by triggering mitochondrial membrane hyperpolarization
in vitro. BMC Microbiol. 15:1152015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
American Cancer Society: Key Statistics
for Bladder Cancer. https://www.cancer.org/cancer/bladder-cancer/about/key-statistics.htmlNovember
10–2021PubMed/NCBI
|
|
21
|
Professionals S-O: EAU Guidelines:
Non-muscle-invasive Bladder Cancer. Uroweb. https://uroweb.org/guideline/non-muscle-invasive-bladder-cancer/November
10–2021
|
|
22
|
Burger M, Catto JWF, Dalbagni G, Grossman
HB, Herr H, Karakiewicz P, Kassouf W, Kiemeney LA, Vecchia CL,
Shariat S and Lotan Y: Epidemiology and risk factors of urothelial
bladder cancer. Eur Urol. 63:234–241. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
DeGeorge KC, Holt HR and Hodges SC:
Bladder cancer: Diagnosis and treatment. Am Fam Physician.
96:507–514. 2017.PubMed/NCBI
|
|
24
|
Kumar P, Nagarajan A and Uchil PD:
Analysis of cell viability by the MTT assay. Cold Spring Harb
Protoc. Jun 1–2018.doi: 10.1101/pdb.prot095505, 2018. View Article : Google Scholar
|
|
25
|
Davis JD: The evolution of the
progressive-era hemocytometer. Caduceus. 11:164–183.
1995.PubMed/NCBI
|
|
26
|
Porter AG and Jänicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bromberg N, Dreyfuss JL, Regatieri CV,
Palladino MV, Durán N, Nader HB, Haun M and Justo GZ: Growth
inhibition and pro-apoptotic activity of violacein in Ehrlich
ascites tumor. Chem Biol Interact. 186:43–52. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cohen GM: Caspases: The executioners of
apoptosis. Biochem J. 326:1–16. 1997. View Article : Google Scholar : PubMed/NCBI
|