Introduction
Lung cancer is prevalent worldwide, accounting for
nearly 20% of all cancer-related deaths (1). In 2015, Non-small cell lung cancer
(NSCLC) accounted for ~80% of all lung cancers worldwide (2). Radiotherapy has developed into an
important treatment modality for inoperable and postoperative
patients with NSCLC (3).
Radiotherapy can induce cell death via damaging the DNA of tumor
cells and subsequently impede the progress of tumors (4). The sensitivity of tumor cells to
radiation defines the efficacy of radiotherapy. Nevertheless, a
growing number of studies have indicated that patients with NSCLC
can acquire resistance to radiotherapy, which limits the effects of
radiotherapy (5–7). Therefore, it is crucial to explore
the underlying mechanism of radioresistance in NSCLC.
MicroRNAs (miRNAs or miRs) are a class of
short-chain non-coding RNAs (19–25 nucleotides), which are involved
in various biological processes and tumorigenesis (8). miRNAs can function as either a tumor
suppressor or oncogene via directly binding the 3′-untranslated
region (UTR) of the target mRNA (9). Notably, several miRNAs, including
miR-219a-5p (10), miRNA-218-5p
(11) and miR-129-5p (12), are related to the radiosensitivity
of NSCLC cells. At present, miR-148a has been identified as a tumor
suppressor in several cancer types, including cervical (13), pancreatic (14), and gastric cancer (15). Furthermore, several studies have
revealed the tumor suppressive role of miR-148a in the initiation
and progression of NSCLC (16–18).
However, no studies have been conducted on the role of miRNA-148a
in the radioresistance of NSCLC.
In the present study, miR-148a expression was
significantly decreased in radiotherapy-resistant patients with
NSCLC. In addition, miR-148a overexpression could inhibit the
progression of radiation-resistant NSCLC cells via silencing Son of
Sevenless 2 (SOS2) expression.
Materials and methods
Clinical samples
The present study was approved (approval no.
2019-KY-008) by the Ethics Committee of Xingtai People's Hospital
(Xingtai, China) and conformed to the Declaration of Helsinki. All
86 enrolled individuals provided signed informed consents. A total
of 86 patients with NSCLC (median age, 54 years; age range, 34–72
years; 61 males and 25 females) who underwent radiotherapy at
Xingtai People's Hospital (Xingtai, China) between January 2016 and
December 2018 were enrolled in the present study. The inclusion
criteria were as follows: i) All patients were pathologically
confirmed with NSCLC; ii) all patients had adequate organ function
to complete radiotherapy; iii) patients had full documentation of
their treatment response to radiotherapy. The patients received
60-Gy radiotherapy with 6 weeks treatment duration (2 Gy per
fraction per day, 5 days per week). In addition, a group of 47 age-
and sex-matched healthy participants were included in a control
cohort during the same period. The peripheral blood samples were
collected one day before radiotherapy and also after the patients
completed the course of treatment in the NSCLC cohort. Blood
samples were collected from the control cohort during physical
check. The peripheral blood samples were collected from all the
participants in serum gel separator tubes. Each sample was
centrifuged at 3,000 × g for 10 min to separate serum and then
stored at −80°C until tested. According to the RECIST 1.1 criterion
(19), patients with NSCLC were
included into the radiosensitive group (n=35, including complete
response, and partial response) and the radioresistant group (n=51,
including stable disease, and progressive disease).
Cell culture and irradiation
A549, H358, HCC827 and BEAS-2B cell lines were
obtained from the Central Culture Collection of the Chinese Academy
of Science (Shanghai, China). The cells were cultured in DMEM
(Thermo Fisher Scientific, Inc.) supplemented with 10% FBS (Gibco;
Thermo Fisher Scientific, Inc.) and maintained at 5% CO2
and 37°C. The A549 and H358 cell lines were selected to establish
radioresistant cells. When cell confluence reached 50%, A549 and
H358 cells were subjected to X-ray irradiation (2 Gy), cultured,
digested with trypsin at a 90% convergence rate and then
re-irradiated at a 50% convergence rate, with a 2 Gy daily fraction
size being administered 30 times for a total dose of 60 Gy. The
established radioresistant cell lines were defined as A549R
(radioresistant) and H358R cells.
Cell transfection
miR-148a mimics, miR-148a inhibitors and negative
controls (NC mimics, NC inhibitors), or small interfering RNA
against SOS2 (si-SOS2) and its negative control (si-NC) were
synthesized by Shanghai GenePharma Co., Ltd. NSCLC cells were
seeded into six-well plates at a density of 1×105 cells
per well and grown at 37°C for 24 h before transfection. Once they
reached 70% confluence, NSCLC cells were transfected with miR-148a
mimics, miR-148a inhibitors and respective controls, or si-SOS2 and
si-NC at a final concentration of 50 nM using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) at 37°C for 4 h. Subsequent experimentation was
performed 48 h after transfection. The primer sequences used were
as follows: miR-148a mimics, 5′-UCAGUGCACUACAGAACUUUGU-3′; NC
mimics, 5′-TTCTCCGAACGTGTCACGT-3′; miR-148a inhibitors,
5′-CACCGGCCAGACCCUCAGCUCCCGA-3′; NC inhibitors,
5′-CGGUACUUUGAUGUAGUACCA-3′; si-SOS2, 5′-GATAGAGTACAUGUAGAGATT-3′;
and si-NC, 5′-GTGGCUCAUGUGUCGUTT-3′.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA from clinical samples and cell lines was
extracted using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.). The cDNA was synthesized using a
PrimeScript RT reagent kit (Takara Bio, Inc.) according to the
manufacturer's protocol. RT-qPCR was carried out with a SYBR Green
I Master Mix kit (Invitrogen; Thermo Fisher Scientific, Inc.) on a
7300 Real-Time PCR System (Applied Biosystems; Thermo Fisher
Scientific, Inc.) following the manufacturer's protocol. The
thermocycling conditions were as follows: Initial denaturation at
95°C for 4 min, followed by 40 cycles of 95°C for 15 sec, 60°C for
30 sec and 72°C for 30 sec, with a final extension step at 72°C for
2 min. Samples were then held at 4°C. The relative expression of
SOS2 and miR-148a was calculated using 2−∆∆Cq method
with GAPDH and U6 as the endogenous control (20). The primer sequences are listed in
Table I.
 | Table I.RT-qPCR primer sequences. |
Table I.
RT-qPCR primer sequences.
| Gene name | Primer sequences
(5′→3′) |
|---|
| SOS2 | F:
CCGCAGCCTTACGAGTTCTTC |
|
| R:
GGATGCACTTGTTCCTGAACC |
| microRNA-148a | F:
TGCGCTCAGTGCACTACAGAAC |
|
| R:
CCAGTGCAGGGTCCGAGGTATT |
| GAPDH | F:
CCTTCATTGACCTCAACTACA |
|
| R:
GCTCCTGGAAGATGGTGAT |
| U6 | F:
CTCGCTTCGGCAGCACATATACT |
|
| R:
ACGCTTCACGAATTTGCGTGTC |
Western blot analysis
Proteins were extracted from A549R and H358R cells
using RIPA lysis buffer (Thermo Fisher Scientific, Inc.). Protein
concentration was determined by BCA assay (Thermo Fisher
Scientific, Inc.). Then, the protein lysates (30 µg) were separated
by using 10% SDS/PAGE and transferred onto a PVDF membrane
(MilliporeSigma). The membranes were blocked using 5% non-fat dry
milk for 1 h at room temperature. Membranes were incubated
overnight at 4°C with primary antibodies against SOS2 (1:1,000;
cat. no. ab154999) and GAPDH (1:1,000; cat. no. ab9485; both from
Abcam). After washing with TBST (0.05% Tween 20), the membranes
were incubated with corresponding HRP-conjugated goat anti-mouse
IgG secondary antibodies (1:5,000; cat. no. ab205719; Abcam) at
37°C for 1 h. Protein bands were visualized with the enhanced
chemiluminescence detection kit (Pierce™ Fast Western Blot Kit;
cat. no. 35050; Thermo Fisher Scientific, Inc.).
Cell Counting Kit (CCK)-8 assay
NSCLC cells were seeded into 96-well plates at a
density of 5×103 cells per well. A total of 10 µl of
CCK-8 solution (Dojindo Molecular Technologies, Inc.) was added at
24, 48 and 72 h, following which the plates were placed in the
incubator for 3 h. Finally, the absorbance at 450 nm was measured
by a microplate reader (Bio-Rad Laboratories, Inc.).
Transwell assay
Serum-free medium (200 µl) containing
5×104 cells was added to the upper compartments of
Transwell inserts (8.0 µm pore size; Costar; Corning, Inc.) for the
cell migration assay. As for the cell invasion assay, the chamber
was coated with Matrigel (50 µl; BD Biosciences) at 37°C for 3 h.
Then, 600 µl of basal medium containing 10% FBS was added to the
lower compartments. After 48 h of culture in a humidified
atmosphere with 5% CO2 at 37°C, migratory and invasive
cells were fixed with 4% paraformaldehyde for 15 min at room
temperature and stained with 0.5% crystal violet for 10 min at room
temperature. Five random fields were chosen and counted under an
inverted light microscope (Olympus Corporation; magnification,
×200).
Bioinformatics analysis
miRDB (http://mirdb.org/download.html), starBase v2.0
(http://starbase.sysu.edu.cn/index.php), and TargetScan
(http://www.targetscan.org/vert_70/)
databases were used to predict the target genes of miR-148a.
Luciferase reporter assay
The SOS2 fragments harboring the predicted wild-type
(WT) or mutant (MUT) binding site (Shanghai GenePharma Co., Ltd.)
were cloned into the pmirGLO luciferase plasmid (Promega
Corporation) to create the reporter plasmids WT-SOS2 or MUT-SOS2.
NSCLC cells were co-transfected with WT-SOS2 or MUT-SOS2, together
with miR-148a mimics or NC-mimics, miR-148a inhibitors or
NC-inhibitors using Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.). Relative reporter gene activity
was evaluated with normalization to Renilla luciferase
activity at 48 h post-transfection with a dual-luciferase reporter
assay system (Promega Corporation).
Statistical analysis
All data are expressed as the mean ± standard
deviation. Statistical evaluations were performed using SPSS 20.0
(IBM Corp.) and GraphPad Prism 8.01 (GraphPad Software, Inc.). All
experiments were repeated at least three times. Differences between
two groups were analyzed using the unpaired Student's t-test, with
the exception of miR-148a expression in the serum of patients with
NSCLC before or after radiotherapy which was compared using paired
Student's t-test. Differences between multiple groups were analyzed
using one-way ANOVA followed by Tukey's post hoc test. Correlation
between miR-148a and SOS2 was performed using Pearson's correlation
coefficient. Differences were considered to be statistically
significant when P<0.05.
Results
miR-148a is positively associated with
radiosensitivity in NSCLC tissues
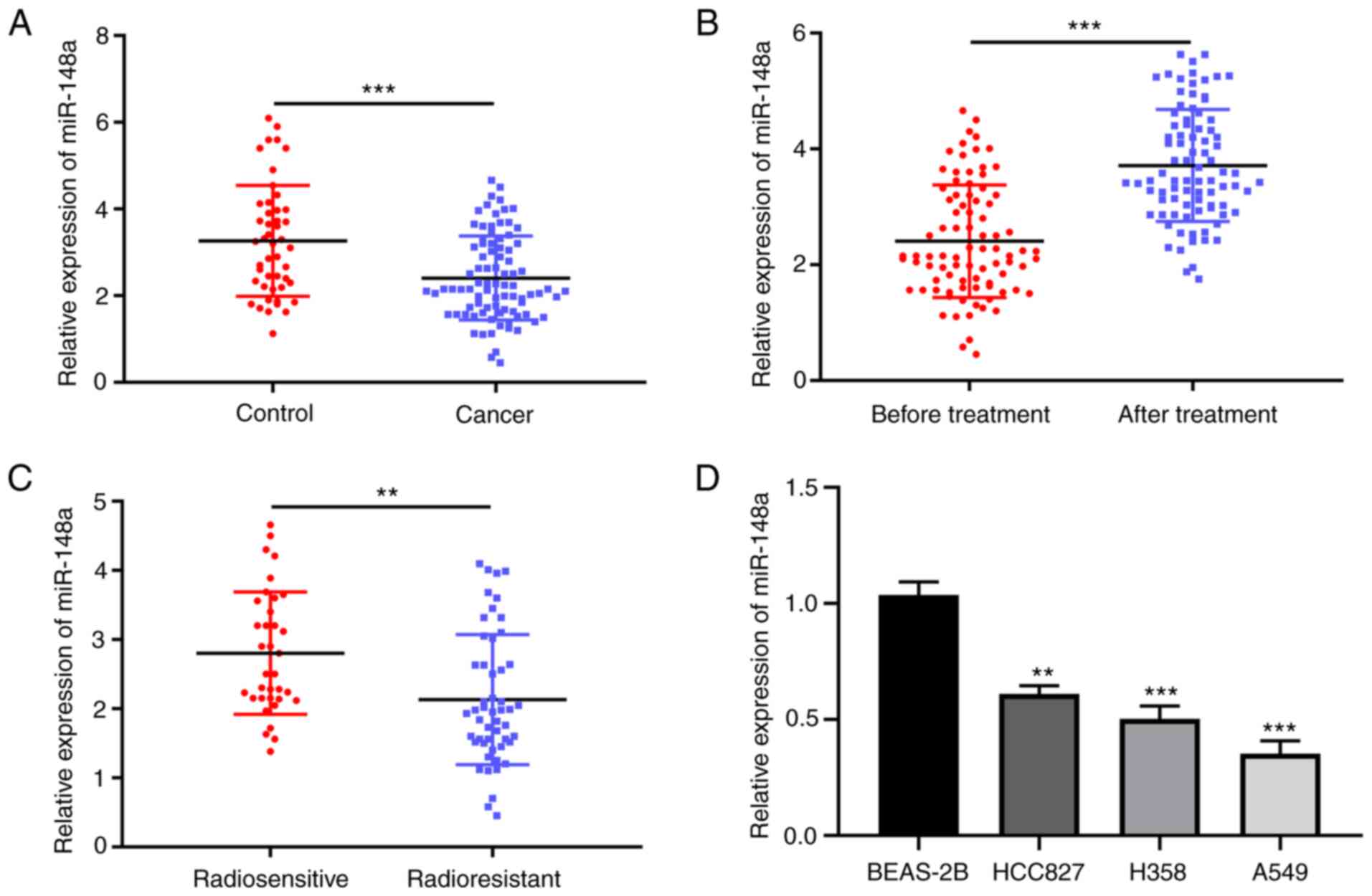
The expression of miR-148a in the serum of patients
with NSCLC and healthy controls was firstly compared, and the
RT-qPCR results showed that miR-148a expression was significantly
lower in NSCLC than controls (Fig.
1A). A significantly higher miR-148a expression was observed in
the serum of patients with NSCLC after radiotherapy compared with
that before intervention (Fig.
1B). Furthermore, the serum expression level of miR-148a was
significantly lower in radioresistant patients than that in
radiosensitive patients (Fig. 1C).
In addition, the miRNA-148a expression level was determined in a
series of NSCLC cell lines after 5 Gy of γ-ray irradiation and the
results revealed that the miRNA-148a expression was decreased in
NSCLC cells when compared with BEAS-2B cells (Fig. 1D). This suggested that miRNA-148a
may be a potential biomarker for radioresistance in NSCLC.
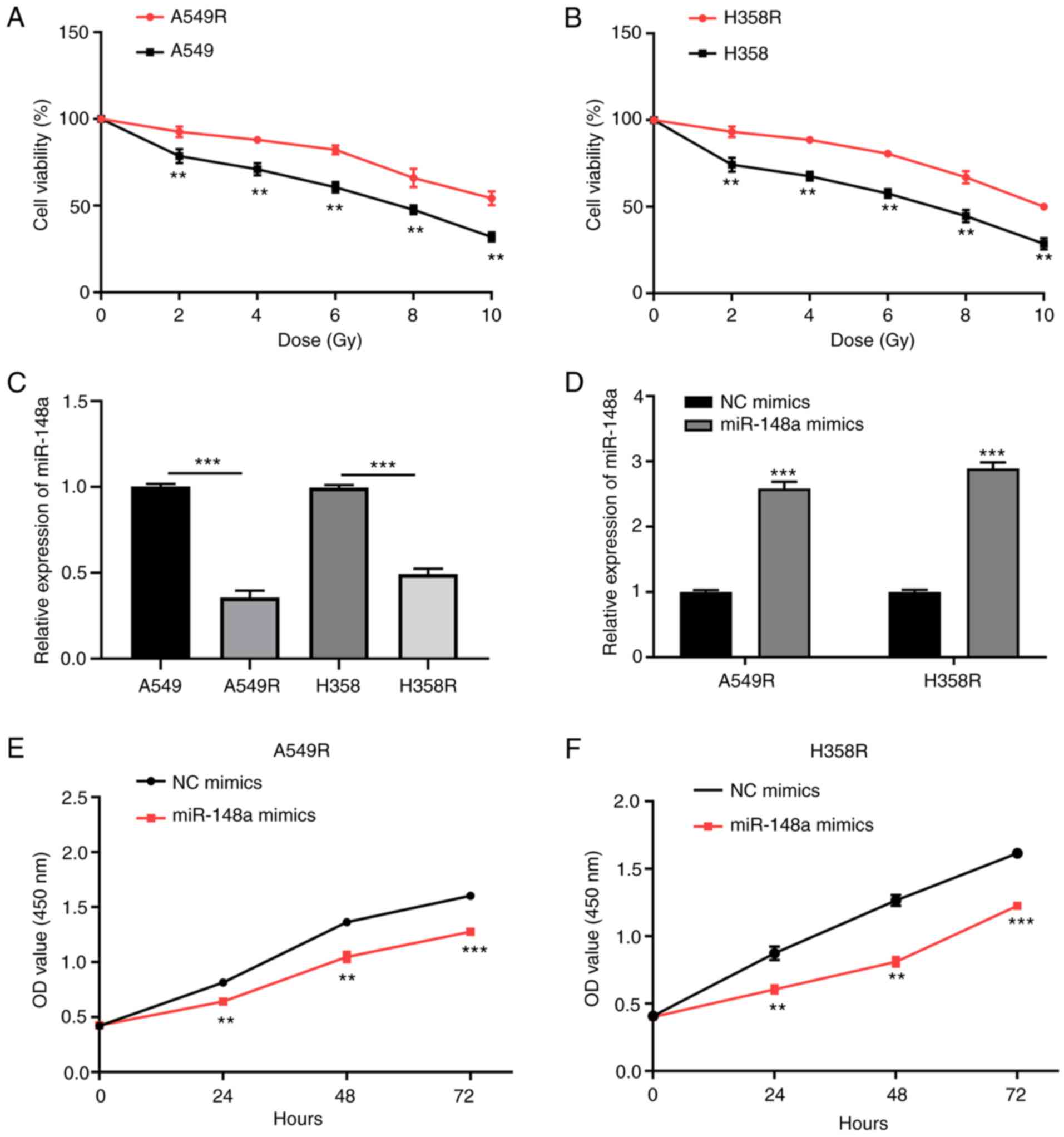
miR-148a inhibits cell proliferation,
migration and invasion of radiation-resistant NSCLC cells
As revealed in Fig. 2A
and B, the radiation-resistant cell lines (A549R and H358R) had
enhanced resistance to X-rays compared to their parental cells. As
revealed by RT-qPCR, the expression level of miRNA-148a was
significantly decreased in A549R and H358R cells compared with the
parental cells (Fig. 2C).
Subsequently, to evaluate the effect of miR-148a on the development
of radioresistance in A549 and H358 cells, A549R and H358R cells
were transfected with miR-148a or NC mimics and the transfection
efficiency was confirmed by RT-qPCR (Fig. 2D). In addition, miR-148a or NC
inhibitors were applied to silence miR-148a expression in A549R and
H358R cells and the transfection efficiency was confirmed by
RT-qPCR (Fig. S1A). Liu et
al (21) reported that
fractionated irradiation could promote epithelial-mesenchymal
transition and enhance the migration, invasion and stemness-like
properties in A549 cells, which elucidates the possible
radioresistance mechanisms of the cancer cells. Thus, the effects
of miR-148a on NSCLC cell progression, including proliferation,
migration and invasion, were detected. CCK-8 assays demonstrated
that the enhanced expression of miR-148a led to a significant
decrease in cell proliferation (Fig.
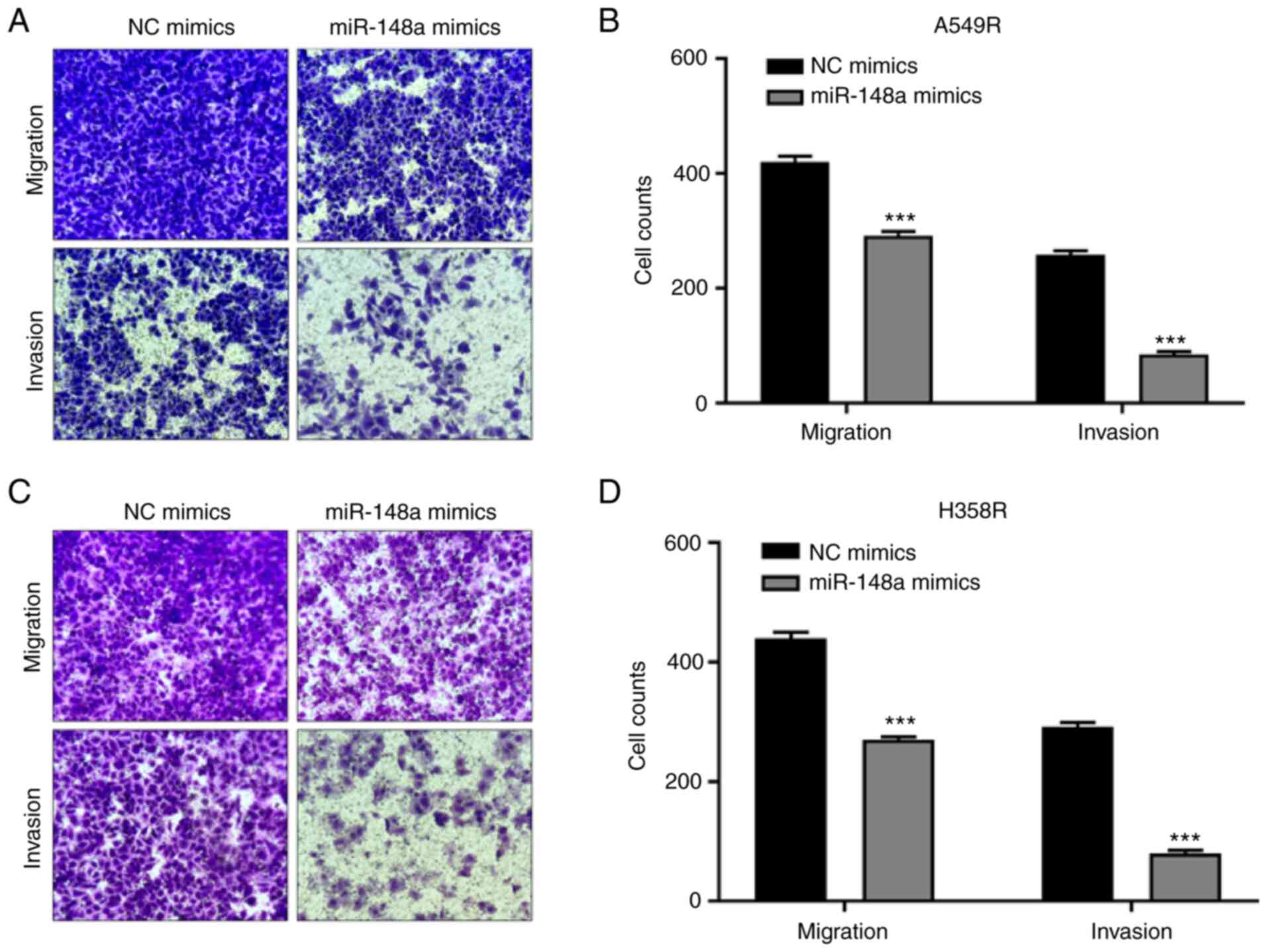
2E and F). Transwell migration and Matrigel invasion assays
demonstrated that the overexpression of miR-148a led to a
profoundly weaker cell migration ability in A549R cells (Fig. 3A and B). Similar results were
yielded for H358R cells (Fig. 3C and
D). The aforementioned results proved that miR-148a exerted a
tumor suppressive role in the development of radioresistance in
NSCLC cells.
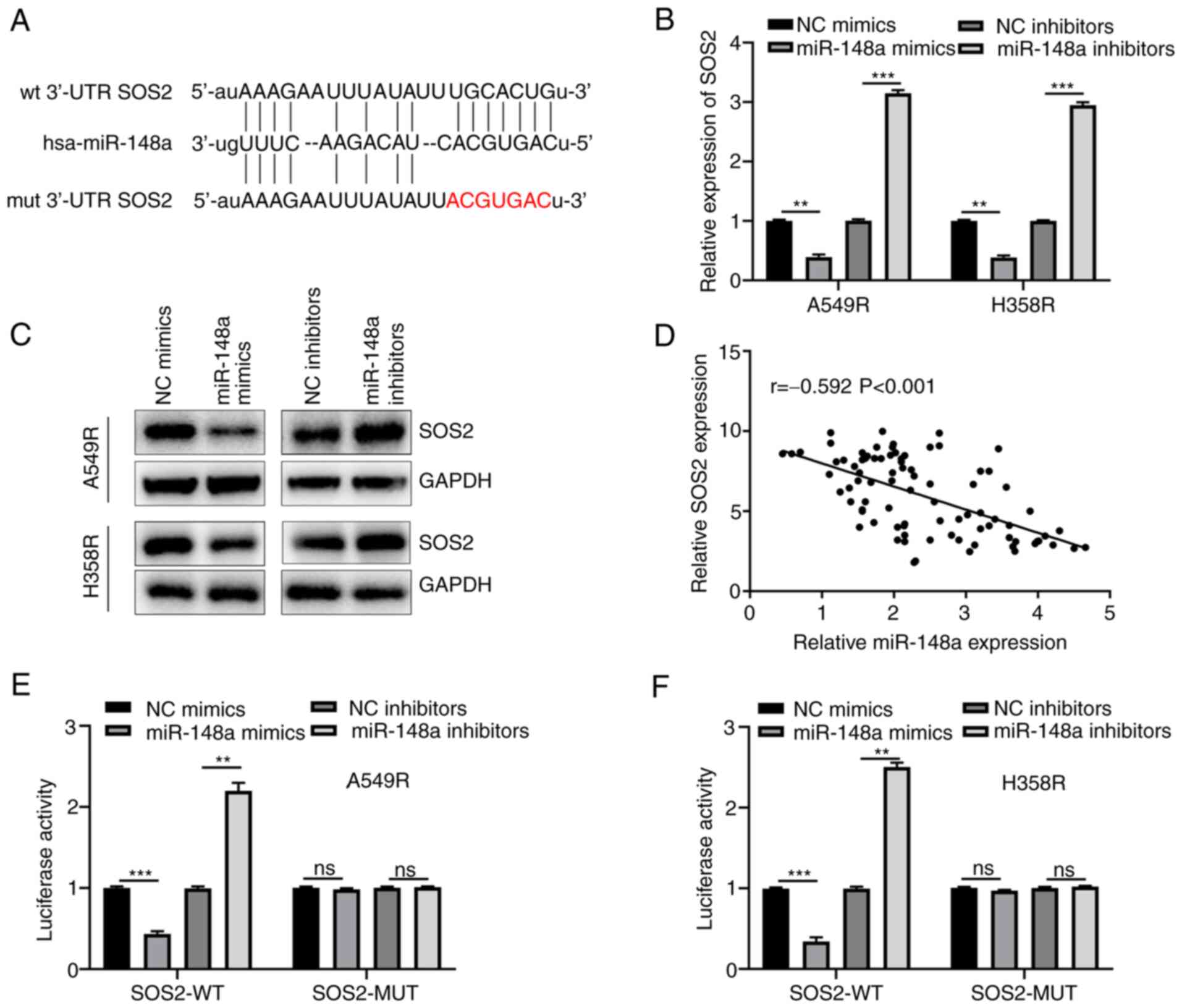
miR-148a directly targets SOS2 in
radiation-resistant NSCLC cells
To investigate the underlying mechanism of
miR-148a-induced regulation of radiosensitivity of NSCLC cells,
certain bioinformatics analysis was performed to identify the
targeted genes, which determined the putative binding sites between
miR-148a and SOS2 (Fig. 4A).
Subsequently, the correlations between miR-148a and SOS2 expression
both at mRNA and protein levels were analyzed using RT-qPCR and
western blotting, and the results revealed their prominent inverse
association (Fig. 4B and C). In
addition, the Pearson's correlation analysis between miR-148a and
SOS2 mRNA expression in the serum of NSCLC showed a consist trend
(Fig. 4D). As demonstrated in
Fig. 4E, miR-148a overexpression
could decrease SOS2-WT luciferase activity and miR-148a knockdown
could increase SOS2-WT luciferase activity, while no effect was
exerted on the SOS2-MUT A549R cells. Similar results were also
observed in H358R cells (Fig. 4F).
All the aforementioned data indicated that SOS2 was a direct target
of miR-148a.
SOS2 knockdown restores the miR-148a
inhibitor-induced promotion of proliferation, migration and
invasion
To further confirm whether the promotion of cell
proliferation, migration and invasion effects of miR-148a
inhibitors was mediated by SOS2, a ‘rescue’ strategy was adopted.
The si-SOS2 vector was co-transfected with miR-148a inhibitors into
A549 or A549R cells. The transfection efficiency of si-SOS2 in
A549R cells was confirmed by RT-qPCR and western blotting (Fig. S1B and C). As indicated by the cell
survival curve, knockdown of SOS2 overtly reversed miR-148a
inhibitor-mediated increase in survival fraction at different doses
of X-ray radiation (Fig. 5A). As
indicated by CCK-8 assays, SOS2 knockdown significantly rescued the
miR-148a inhibitor-induced promotion of cell proliferation
(Fig. 5B). As revealed by
Transwell and Matrigel assays, restoration of SOS2 significantly
reversed the promotion of cell migration and invasion induced by
miR-148a inhibitors (Fig. 5C and
D). These findings demonstrated that the downregulation of
miR-148a promoted proliferation, migration and invasion by
targeting SOS2 in A549R cells.
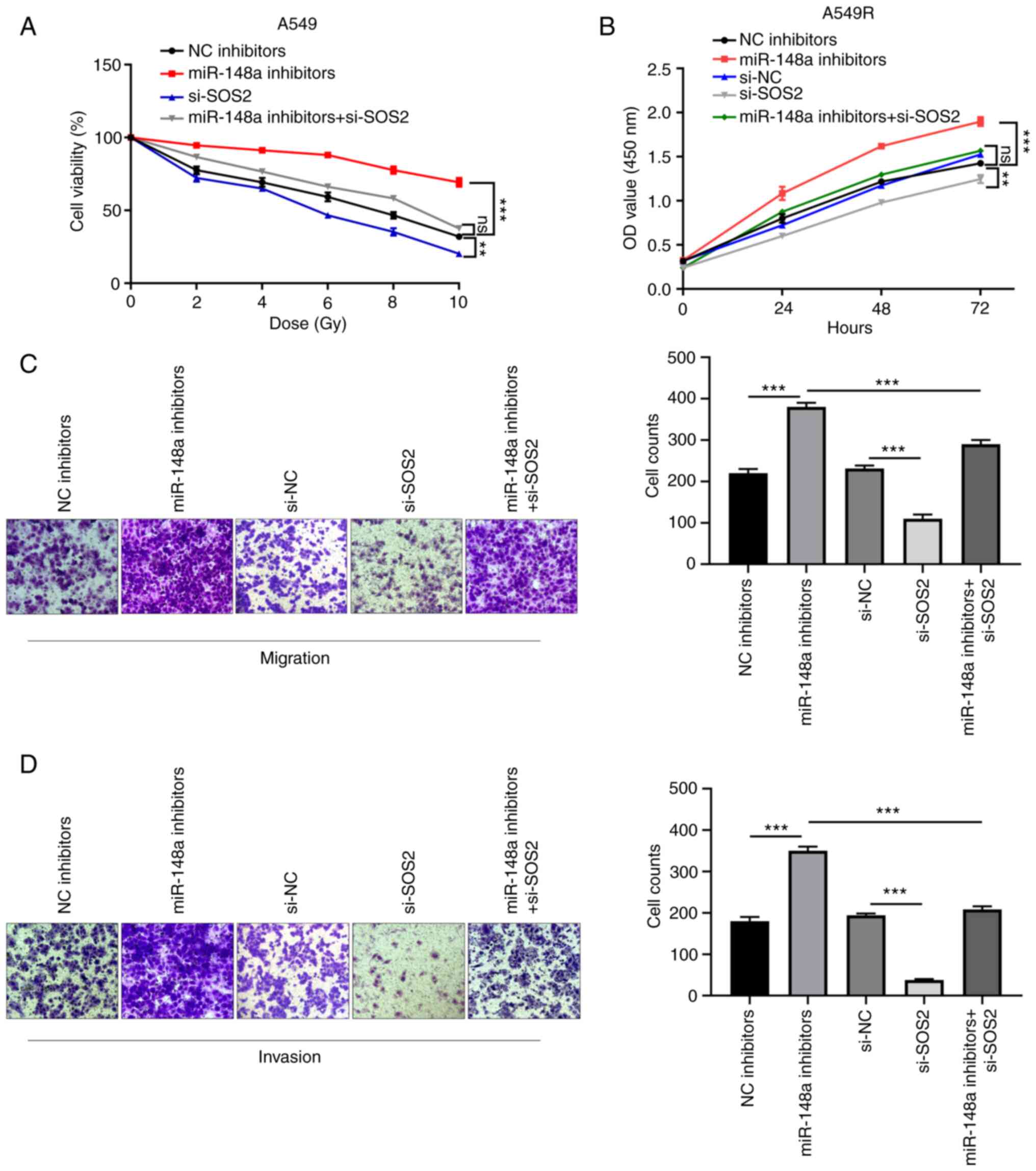 | Figure 5.Silencing SOS2 reverses the
tumor-promoting effects of miR-148a inhibitors in A549R cells. (A)
Cell survival curve was generated in A549 cells transfected with NC
inhibitors, miR-148a inhibitors, si-SOS2 and miR-148a inhibitors +
si-SOS2 at different doses of X-ray radiation. (B-D) CCK-8,
Transwell and Matrigel assays were performed to analyze the (B)
cell proliferation, (C) migration and (D) invasion capacity in
A549R cells after transfection with NC inhibitors, miR-148a
inhibitors, si-NC, si-SOS2 and miR-148a inhibitors + si-SOS2.
**P<0.01 and ***P<0.001. miR, microRNA; si-, small
interfering; NC, negative control. |
Discussion
Radioresistance has recently become a critical
barrier in the treatment of NSCLC (22). To date, numerous studies have
proposed the role of miRNAs in the occurrence of radioresistance in
NSCLC. For example, miR-147a upregulation increased the
radiosensitivity in NSCLC (23).
Yuan et al (24)
demonstrated that miR-410 overexpression could promote both
epithelial-mesenchymal transition and radioresistance in NSCLC.
Furthermore, it has been reported that miR-125a-5p promotes
apoptosis to increase the radiosensitivity of NSCLC cells (25). Thus, it is of great value to
identify more miRNAs during radioresistance in NSCLC.
At present, certain studies have implicated miR-148a
as a tumor suppressor in certain types of cancer. As reported,
miR-148a-3p suppressed cell growth and invasion in colon
adenocarcinoma (26). Elhelbawy
et al (27) revealed that
miRNA-148a expression was downregulated in patients with breast
cancer. In addition, the tumor suppressive role of miR-148a has
also been indicated in NSCLC. For example, Joshi et al
(17) reported that miR-148a
overexpression hindered lung tumorigenesis in vitro and
in vivo. He and Yue et al (16) demonstrated that miR-148a expression
was markedly downregulated in NSCLC tissues and cell lines and
could suppress proliferation and invasion. In the present study, it
was demonstrated that miR-148a was significantly downregulated in
NSCLC tissues and cell lines. Notably, the serum miR-148a
expression was decreased in the radioresistant patients compared
with the radiosensitivity patients. In addition, miR-148a
overexpression inhibited the cell proliferation, migration and
invasion of radiation-resistant NSCLC cells. These results
suggested that miR-148a may play a role in promoting the
radiosensitivity of NSCLC.
Furthermore, it is well acknowledged that miRNAs
function to regulate protein expression by binding to the 3′-UTR of
the target mRNAs (28). In the
present study, miR-148a had putative binding site with SOS2 and a
negative correlation was observed between the expression levels of
miR-148a and SOS2 in serum of patients with NSCLC. Further
experiments demonstrated that miR-148a overexpression inhibited the
mRNA and protein expression of SOS2 in radiation-resistant NSCLC
cells. The luciferase reporter assay confirmed the interaction
between miR-148a and SOS2. Silencing SOS2 expression significantly
inhibited miR-148a inhibitor-induced increase in radiosensitivity
in NSCLC. Overall, the present study indicated that miR-148a could
enhance radiosensitivity in NSCLC via directly regulating SOS2
expression.
In conclusion, the present study demonstrated that
miR-148a expression was decreased in radiotherapy-resistant
patients with NSCLC. Furthermore, miR-148a could enhance the
radiosensitivity of NSCLC cells through targeting SOS2, thus
providing potential therapeutic targets to improve the radiotherapy
in NSCLC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ and XH guided the study design, conducted
experiments, analyzed and interpreted the data, and drafted the
manuscript. Each author revised the article critically for
important intellectual content. The final manuscript was read and
approved by all authors. YZ and XH confirmed the authenticity of
all the raw data.
Ethics approval and consent to
participate
The present study was approved (approval no.
2019-KY-008) by the Ethics Committee of Xingtai People's Hospital
(Xingtai, China) and conformed to the Declaration of Helsinki. All
enrolled individuals provided signed informed consents.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Remark R, Becker C, Gomez JE, Damotte D,
Dieu-Nosjean MC, Sautès-Fridman C, Fridman WH, Powell CA, Altorki
NK, Merad M and Gnjatic S: The non-small cell lung cancer immune
contexture. A major determinant of tumor characteristics and
patient outcome. Am J Respir Crit Care Med. 191:377–390. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sundahl N and Lievens Y: Radiotherapy for
oligometastatic non-small cell lung cancer: A narrative review.
Transl Lung Cancer Res. 10:3420–3431. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Changizi V, Bahrami M, Esfahani M and
Shetab-Boushehri SV: Prevention of γ-radiation-induced DNA damage
in human lymphocytes using a serine-magnesium sulfate mixture.
Sultan Qaboos Univ Med J. 17:e162–e167. 2017.PubMed/NCBI
|
|
5
|
Wang Y, Huang J, Wu Q, Zhang J, Ma Z, Zhu
L, Xia B, Ma S and Zhang S: Decitabine sensitizes the
radioresistant lung adenocarcinoma to pemetrexed through
upregulation of folate receptor alpha. Front Oncol. 11:6687982021.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang Y, Chong Y, Chen M, Dai W, Zhou X, Ji
Y, Qiu G and Du X: Targeting lactate dehydrogenase a improves
radiotherapy efficacy in non-small cell lung cancer: From bedside
to bench. J Transl Med. 19:1702021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu LM, Yu H, Yuan YJ, Zhang J, Ma Y, Cao
XC, Wang J, Zhao LJ and Wang P: Overcoming of radioresistance in
non-small cell lung cancer by microRNA-320a through
HIF1α-suppression mediated methylation of PTEN. Front Cell Dev
Biol. 8:5537332020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li B, Cao Y, Sun M and Feng H: Expression,
regulation, and function of exosome-derived miRNAs in cancer
progression and therapy. FASEB J. 35:e219162021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Winter J, Jung S, Keller S, Gregory RI and
Diederichs S: Many roads to maturity: MicroRNA biogenesis pathways
and their regulation. Nat Cell Biol. 11:228–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wei T, Cheng S, Fu XN and Feng LJ:
MiR-219a-5p enhances the radiosensitivity of non-small cell lung
cancer cells through targeting CD164. Biosci Rep.
40:BSR201927952020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen X, Xu Y, Jiang L and Tan Q:
MiRNA-218-5p increases cell sensitivity by inhibiting PRKDC
activity in radiation-resistant lung carcinoma cells. Thorac
Cancer. 12:1549–1557. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xue T, Yin G, Yang W, Chen X, Liu C, Yang
W and Zhu J: MiR-129-5p promotes radio-sensitivity of NSCLC cells
by targeting SOX4 and RUNX1. Curr Cancer Drug Targets. 21:702–712.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen Q, Wang Y, Dang H and Wu X:
MicroRNA-148a-3p inhibits the proliferation of cervical cancer
cells by regulating the expression levels of DNMT1 and UTF1. Oncol
Lett. 22:6172021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fu X, Hong L, Yang Z, Tu Y, Xin W, Zha M,
Tu S, Sun G, Li Y and Xiao W: MicroRNA-148a-3p suppresses
epithelial-to-mesenchymal transition and stemness properties via
Wnt1-mediated Wnt/β-catenin pathway in pancreatic cancer. J Cell
Mol Med. 24:13020–13035. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bao C and Guo L: MicroRNA-148a-3p inhibits
cancer progression and is a novel screening biomarker for gastric
cancer. J Clin Lab Anal. 34:e234542020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
He M and Xue Y: MicroRNA-148a suppresses
proliferation and invasion potential of non-small cell lung
carcinomas via regulation of STAT3. Onco Targets Ther.
10:1353–1361. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Joshi P, Jeon YJ, Laganà A, Middleton J,
Secchiero P, Garofalo M and Croce CM: MicroRNA-148a reduces
tumorigenesis and increases TRAIL-induced apoptosis in NSCLC. Proc
Natl Acad Sci USA. 112:8650–8655. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xie Q, Yu Z, Lu Y, Fan J, Ni Y and Ma L:
MicroRNA-148a-3p inhibited the proliferation and
epithelial-mesenchymal transition progression of non-small-cell
lung cancer via modulating Ras/MAPK/Erk signaling. J Cell Physiol.
234:12786–12799. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TDP: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu L, Shang M, Song X, Zhang C and Guo W:
Fractionated irradiation enhances invasion and migration by
inducing epithelial-mesenchymal transition and stemness-like
properties in A549 cells. Ann Clin Lab Sci. 51:521–528.
2021.PubMed/NCBI
|
|
22
|
Xiong W and Hu XHW: AKR1C3 and β-catenin
expression in non-small cell lung cancer and relationship with
radiation resistance. J BUON. 26:802–811. 2021.PubMed/NCBI
|
|
23
|
Dai K, Chen L, Liu J, Ding Y, Gu C and Lu
X: MiR-147a mediated by sodium new houttuyfonate could enhance
radiosensitivity of non-small cell lung cancer cells via
suppressing STAT3. Adv Clin Exp Med. 30:173–181. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yuan Y, Liao H, Pu Q, Ke X, Hu X, Ma Y,
Luo X, Jiang Q, Gong Y, Wu M, et al: MiR-410 induces both
epithelial-mesenchymal transition and radioresistance through
activation of the PI3K/mTOR pathway in non-small cell lung cancer.
Signal Transduct Target Ther. 5:852020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun C, Zeng X, Guo H, Wang T, Wei L, Zhang
Y, Zhao J, Ma X and Zhang N: MicroRNA-125a-5p modulates
radioresistance in LTEP-a2 non-small cell lung cancer cells by
targeting SIRT7. Cancer Biomark. 27:39–49. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hu B, Chen Z, Wang X, Chen F, Song Z and
Cao C: MicroRNA-148a-3p directly targets SERPINE1 to suppress
EMT-mediated colon adenocarcinoma progression. Cancer Manag Res.
13:6349–6362. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Elhelbawy NG, Zaid IF, Khalifa AA, Gohar
SF and Fouda EA: MiRNA-148a and miRNA-30c expressions as potential
biomarkers in breast cancer patients. Biochem Biophys Rep.
27:1010602021.PubMed/NCBI
|
|
28
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|