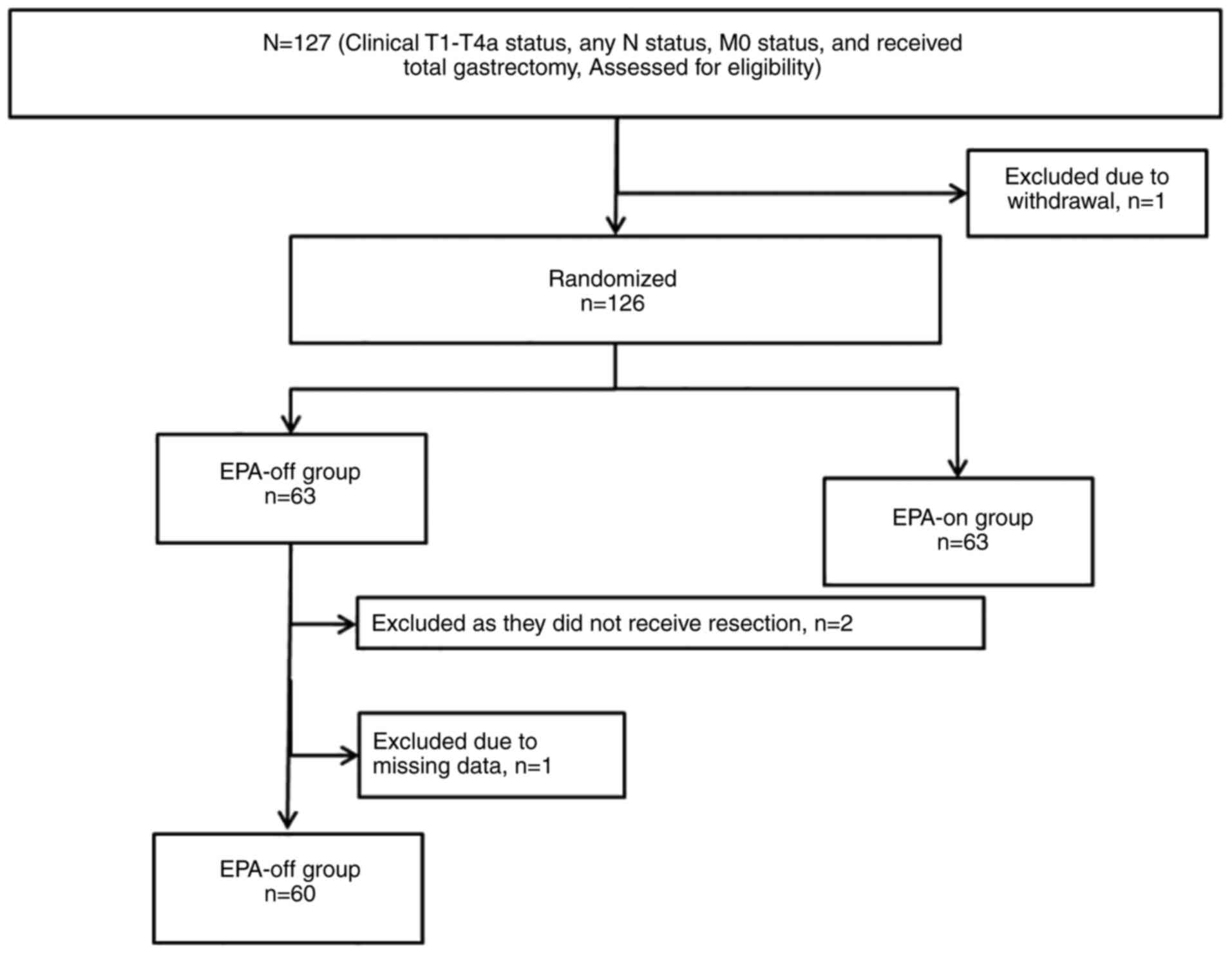
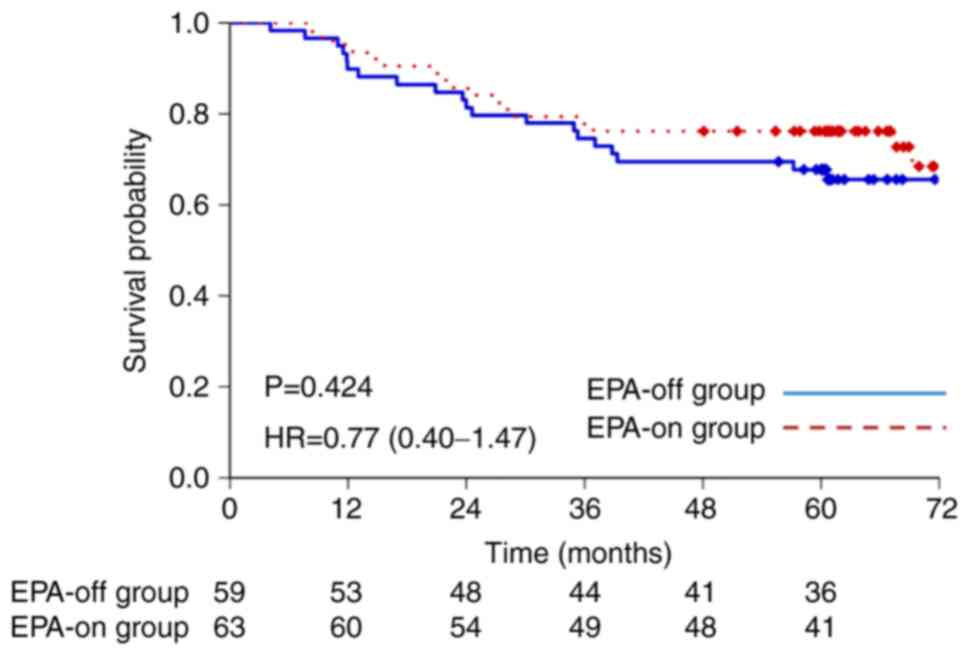
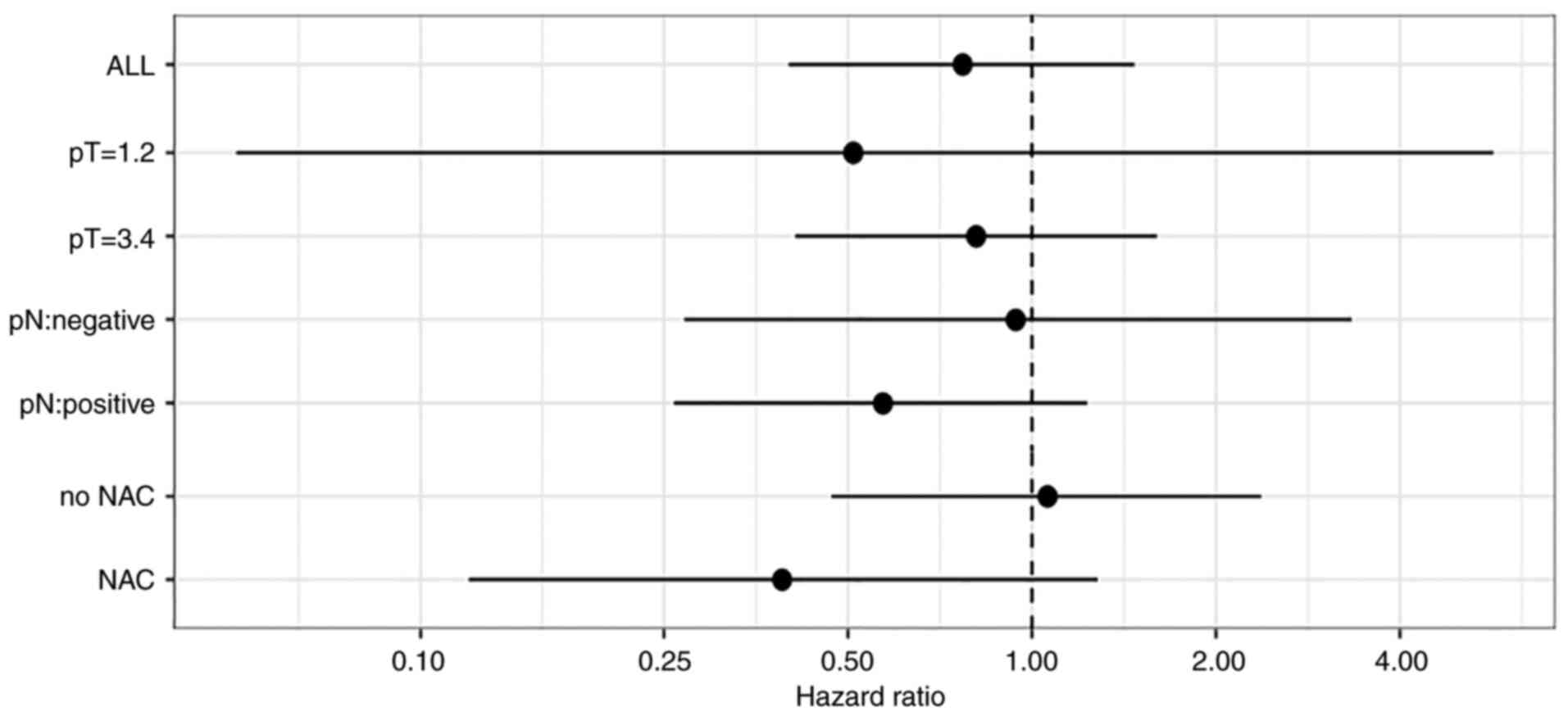
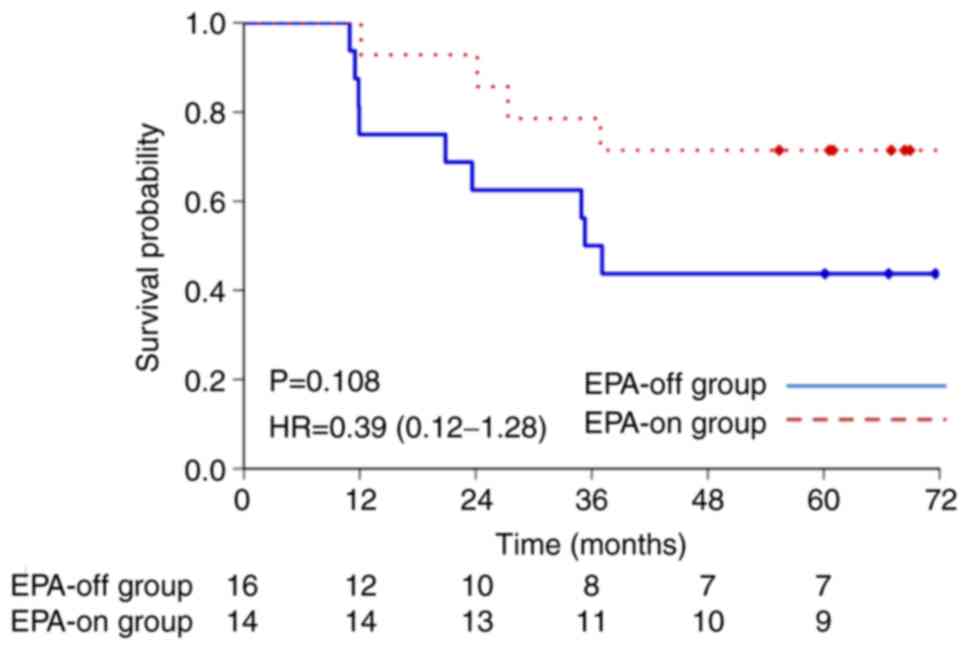
|
1
|
Ferlay J, Colombet M, Soerjomataram I,
Mathers C, Parkin DM, Piñeros M, Znaor A and Bray F: Estimating the
global cancer incidence and mortality in 2018: GLOBOCAN sources and
methods. Int J Cancer. 144:1941–1953. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Smyth EC, Verheij M, Allum W, Cunningham
D, Cervantes A and Arnold D; ESMO Guidelines Committee, : Gastric
cancer: ESMO clinical practice guidelines for diagnosis, treatment
and follow-up. Ann Oncol. 27 (Suppl 5):v38–v49. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Japanese Gastric Cancer Association, .
Japanese gastric cancer treatment guidelines 2018. ((5th edition)).
Gastric Cancer. 24:1–21. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
NCCN, . NCCN clinical practice guidelines
in oncology. 2018.http://www.nccn.org
|
|
6
|
Fearon KC, Von Meyenfeldt MF, Moses AG,
Van Geenen R, Roy A, Gouma DJ, Giacosa A, Van Gossum A, Bauer J,
Barber MD, et al: Effect of a protein and energy dense N-3 fatty
acid enriched oral supplement on loss of weight and lean tissue in
cancer cachexia: A randomised double blind trial. Gut.
52:1479–1486. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moses AW, Slater C, Preston T, Barber MD
and Fearon KC: Reduced total energy expenditure and physical
activity in cachectic patients with pancreatic cancer can be
modulated by an energy and protein dense oral supplement enriched
with n-3 fatty acids. Br J Cancer. 90:996–1002. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kubota H, Matsumoto H, Higashida M,
Murakami H, Nakashima H, Oka Y, Okumura H, Yamamura M, Nakamura M
and Hirai T: Eicosapentaenoic acid modifies cytokine activity and
inhibits cell proliferation in an oesophageal cancer cell line.
Anticancer Res. 33:4319–4324. 2013.PubMed/NCBI
|
|
9
|
Wigmore SJ, Fearon KC, Maingay JP and Ross
JA: Down-regulation of the acute-phase response in patients with
pancreatic cancer cachexia receiving oral eicosapentaenoic acid is
mediated via suppression of interleukin-6. Clin Sci (Lond).
92:215–221. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Beck SA, Smith KL and Tisdale MJ:
Anticachectic and antitumor effect of eicosapentaenoic acid and its
effect on protein turnover. Cancer Res. 51:6089–6093.
1991.PubMed/NCBI
|
|
11
|
Pahl HL: Activators and target genes of
Rel/NF-kappaB transcription factors. Oncogene. 18:6853–6866. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zubair A and Frieri M: Role of nuclear
factor-ĸB in breast and colorectal cancer. Curr Allergy Asthma Rep.
13:44–49. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Falconer JS, Fearon KC, Ross JA, Elton R,
Wigmore SJ, Garden OJ and Carter DC: Acute-phase protein response
and survival duration of patients with pancreatic cancer. Cancer.
75:2077–2082. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
McNamara MJ, Alexander HR and Norton JA:
Cytokines and their role in the pathophysiology of cancer cachexia.
JPEN J Parenter Enteral Nutr. 16 (Suppl 6):50S–55S. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cockbain AJ, Volpato M, Race AD, Munarini
A, Fazio C, Belluzzi A, Loadman PM, Toogood GJ and Hull MA:
Anticolorectal cancer activity of the omega-3 polyunsaturated fatty
acid eicosapentaenoic acid. Gut. 63:1760–1768. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
West NJ, Clark SK, Phillips RK, Hutchinson
JM, Leicester RJ, Belluzzi A and Hull MA: Eicosapentaenoic acid
reduces rectal polyp number and size in familial adenomatous
polyposis. Gut. 59:918–925. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ueno M, Sugimori K, Taguri M, Ohkawa S,
Kobayashi S, Miwa H, Kaneko T, Morimoto M and Yamanaka T:
Randomized phase II study of gemcitabine monotherapy vs gemcitabine
with an EPA-enriched oral supplement in advanced pancreatic cancer.
Nutr Cancer. 74:122–130. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yoshikawa T, Hiki N, Taguri M, Sano T,
Nunobe S, Taniguchi H, Fukushima R, Cho H, Morita S and Tsuburaya
A: A Phase III trial to evaluate the effect of perioperative
nutrition enriched with eicosapentaenoic acid on body weight loss
after total gastrectomy for T2-T4a gastric cancer. Jpn J Clin
Oncol. 42:459–462. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Aoyama T, Yoshikawa T, Ida S, Cho H,
Sakamaki K, Ito Y, Fujitani K, Takiguchi N, Kawashima Y, Nishikawa
K, et al: Effects of perioperative eicosapentaenoic acid-enriched
oral nutritional supplement on lean body mass after total
gastrectomy for gastric cancer. J Cancer. 10:1070–1076. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Japanese Gastric Cancer Association, .
Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric
Cancer. 14:113–123. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Aoyama T, Nishikawa K, Fujitani K, Tanabe
K, Ito S, Matsui T, Miki A, Nemoto H, Sakamaki K, Fukunaga T, et
al: Early results of a randomized two-by-two factorial phase II
trial comparing neoadjuvant chemotherapy with two and four courses
of cisplatin/S-1 and docetaxel/cisplatin/S-1 as neoadjuvant
chemotherapy for locally advanced gastric cancer. Ann Oncol.
28:1876–1881. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Iwasaki Y, Terashima M, Mizusawa J,
Katayama H, Nakamura K, Katai H, Yoshikawa T, Ito S, Kaji M, Kimura
Y, et al: Gastrectomy with or without neoadjuvant S-1 plus
cisplatin for type 4 or large type 3 gastric cancer (JCOG0501): An
open-label, phase 3, randomized controlled trial. Gastric Cancer.
24:492–502. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sakuramoto S, Sasako M, Yamaguchi T,
Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi
Y, Imamura H, et al: Adjuvant chemotherapy for gastric cancer with
S-1, an oral fluoropyrimidine. N Engl J Med. 357:1810–1820. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hurwitz H, Fehrenbacher L, Novotny W,
Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S,
Holmgren E, et al: Bevacizumab plus irinotecan, fluorouracil, and
leucovorin for metastatic colorectal cancer. N Engl J Med.
350:2335–2342. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fuchs CS, Tomasek J, Yong CJ, Dumitru F,
Passalacqua R, Goswami C, Safran H, Dos Santos LV, Aprile G, Ferry
DR, et al: Ramucirumab monotherapy for previously treated advanced
gastric or gastro-oesophageal junction adenocarcinoma (REGARD): An
international, randomised, multicentre, placebo-controlled, phase 3
trial. Lancet. 383:31–39. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wilke H, Muro K, Van Cutsem E, Oh SC,
Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, et
al: Ramucirumab plus paclitaxel versus placebo plus paclitaxel in
patients with previously treated advanced gastric or
gastro-oesophageal junction adenocarcinoma (RAINBOW): A
double-blind, randomised phase 3 trial. Lancet Oncol. 15:1224–1235.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
de Gramont A, Van Cutsem E, Schmoll HJ,
Tabernero J, Clarke S, Moore MJ, Cunningham D, Cartwright TH, Hecht
JR, Rivera F, et al: Bevacizumab plus oxaliplatin-based
chemotherapy as adjuvant treatment for colon cancer (AVANT): A
phase 3 randomised controlled trial. Lancet Oncol. 13:1225–1233.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Van Cutsem E, de Haas S, Kang YK, Ohtsu A,
Tebbutt NC, Ming Xu J, Peng Yong W, Langer B, Delmar P, Scherer SJ
and Shah MA: Bevacizumab in combination with chemotherapy as
first-line therapy in advanced gastric cancer: A biomarker
evaluation from the AVAGAST randomized phase III trial. J Clin
Oncol. 30:2119–2127. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Clavien PA and Strasberg SM: Severity
grading of surgical complications. Ann Surg. 250:197–198. 2009.
View Article : Google Scholar : PubMed/NCBI
|