Introduction
Epithelial ovarian carcinoma (EOC) has the highest
worldwide mortality rate among malignant tumors of the female
genital tract (1). Owing to the
increase in life expectancy, the incidence and mortality rates of
ovarian carcinoma are increasing (2). Approximately 70% of cases are
diagnosed at advanced stages, half of which demonstrate tumor
progression above the pelvic brim (3). Cytoreductive surgery followed by
platinum-based combination chemotherapy is a critical element of
the standard initial therapy (4),
and optimal cytoreduction/debulking surgery involving the pelvis,
lower abdominal cavity and upper abdominal cavity can achieve a
considerable survival advantage (5,6). A
meta-analysis showed that a 10% increase in the optimal
cytoreduction rate prolonged the median survival time by 5.5%
(7). However, complete
cytoreduction depends on three factors: i)
physician/surgeon-related factors, including surgical training and
multidisciplinary team (MDT) collaboration offered at the center,
experience and risk tolerance; ii) patient-related factors, such as
performance status and comorbidity; and iii) disease-related
factors, such as tumor biological aggressive behaviour and
extension of disease. In different centers, the proportion of
patients who undergo optimal cytoreduction for advanced disease
varies between 15 and 85% (7).
Thus, gynecological oncologists could make substantial progress
with the help of teamwork and surgical training. Teamwork is also
involved in the use of enhanced recovery after surgery (ERAS)
protocols, including procedures such as preoperative counseling,
tailored anesthesia and analgesia, and early postoperative feeding
and mobilization, to improve perioperative management.
The evaluation of the radicality of surgery is
mainly based on the surgeon's visual estimation and experience.
Extensive upper abdominal surgery (EUAS), including diaphragmatic
peritonectomy, splenectomy, partial liver resection and distal
pancreatectomy can decrease the residual disease rate in patients
with advanced EOC; however, it can increase the incidence of
postoperative complications (8,9).
Therefore, the final decision of the surgeon on complete tumor
resection in primary debulking surgery (PDS) and interval debulking
surgery (IDS) remains controversial. Hynninen et al
(10) found that IDS had
significantly worse sensitivity and accuracy than PDS, leading to
incomplete tumor resection in potentially resectable areas. The
debates still remain regarding the technique for the best
progression-free survival (PFS) and overall survival (OS), the
incidence rates of postsurgical death and major infective
complications, and economic cost of the two types of surgical
strategies, considering the competing perspectives of the biology
of ovarian carcinoma in relation to the value of aggressive
debulking resection (11–14).
Most gynecological oncologists face the dilemma of
how to complete complex EUAS procedures, as >50% of cases with
optimal cytoreduction require EUAS to attain complete cytoreduction
(15,16). Recently, the Department of
Obstetrics and Gynecology (The First Affiliated Hospital of
University of Science and Technology of China, Hefei, China) has
made great progress in ovarian carcinoma surgical procedures with
the help of MDT members and ERAS protocols. To date, very few
studies have focused on the complications of extraradical debulking
surgery in patients with EOC (9,17–20).
The present study assessed and analyzed the risk factors of the
complications of primary surgery (including 76 cases of
extraradical cytoreduction) and their management in patients with
EOC at the Department of Obstetrics and Gynecology.
Materials and methods
Patients
After obtaining approval from the Institutional
Review Board of the First Affiliated Hospital of University of
Science and Technology of China (approval no. 2021-KY120), the
institutional database was retrospectively used to identify all
patients with stage I–IV EOC and fallopian tube carcinoma who
underwent primary cytoreduction at The First Affiliated Hospital of
USTC between December 2017 and 2019. Patients who underwent primary
surgery, regardless of whether optimal cytoreduction was attained,
were eligible for inclusion into the present study. Individual
records of all patients were reviewed, and demographic, clinical,
surgical, pathological and follow-up data were extracted. Patients
with non-epithelial carcinomas or borderline tumors, or those who
received neoadjuvant chemotherapy, were excluded. All tumors were
staged according to the Federation International of Gynecology and
Obstetrics (FIGO) staging system (21). The body mass index (BMI) of the
participants was measured to assess whether they were overweight or
obese. BMI (kg/m2) was calculated by dividing the body
weight (kg) by the square of the height (m2). A BMI of
<18.5 kg/m2 was classified as underweight, 18.5-23.9
kg/m2 as normal weight, 24.0-27.9 kg/m2 as
overweight and ≥28 kg/m2 as obese (22). The patients demographical and
clinicopathological characteristics are presented in Table I.
 | Table I.Clinical characteristics of patients
(n=161). |
Table I.
Clinical characteristics of patients
(n=161).
| Variable | Value |
|---|
| Mean age ± SD
(range), years | 54.23±9.70
(24–77) |
| Age, n
(%) |
|
| <50
years | 47 (29.19) |
| 50-59
years | 69 (42.86) |
| 60-69
years | 33 (20.50) |
| 70-79
years | 12 (7.45) |
| Mean body mass
index ± SD (range), kg/m2 | 22.90±2.85
(16.41-34.55) |
| Body mass index, n
(%) |
|
|
<18.5 | 8 (4.97) |
|
18.5-23.9 | 102 (63.35) |
|
24-27.9 | 44 (27.33) |
|
≥28 | 7 (4.35) |
| Comorbid illnesses,
n (%) | 52 (32.30) |
| Multiple comorbid
illnesses, n (%) | 18 (11.18) |
|
Diabetes | 4 (2.48) |
|
Hypertension | 18 (11.18) |
| Breast
cancer | 4 (2.48) |
| Other
cancer | 5 (3.11) |
| Other
diseases | 32 (19.88) |
| American Society of
Anesthesiologists score, n (%) |
|
| I | 6 (3.73) |
| II | 72 (44.72) |
|
III | 78 (48.45) |
| IV | 5 (3.11) |
| Primary site of
disease, n (%) |
|
|
Ovary | 149 (92.55) |
|
Fallopian tube | 10 (6.21) |
| Ovary +
fallopian tube | 2 (1.24) |
| FIGO stage, n
(%) |
|
| I | 24 (14.91) |
| II | 14 (8.70) |
|
III | 91 (56.52) |
| IV | 32 (19.88) |
| Histology, n
(%) |
|
|
Serous | 128 (79.50) |
|
Low grade | 4 (2.48) |
|
Middle grade | 0 (0.00) |
|
High grade | 121 (75.16) |
|
NA | 3 (1.86) |
| Mucinous | 4 (2.48) |
| Endometrial | 4 (2.48) |
|
Low grade | 0 (0.00) |
|
Middle grade | 1 (0.62) |
|
High-middle
grade | 1 (0.62) |
|
High grade | 1 (0.62) |
|
NA | 1 (0.62) |
| Clear cell | 12 (7.45) |
| Mixed | 8 (4.97) |
| Othera | 5 (3.11) |
| Mean preoperative
serum CA125 ± SD (range), units/ml |
1,525.04±2,479.24 |
|
|
(9.50-18,848.00) |
| Preoperative serum
CA125, n (%) |
|
| <500
U/ml | 76 (47.20) |
|
500-1,000 U/ml | 17 (10.56) |
|
1,000-2,000 U/ml | 30 (18.63) |
| ≥2,000
U/ml | 37 (22.98) |
| NA | 1 (0.62) |
| Mean preoperative
serum CA199 ± SD, U/ml | 958.99±8,199.25
(0.60-96,649.00) |
| Preoperative serum
CA199, n (%) |
|
| Normal
(<37 U/ml) | 118 (73.29) |
| 37-500
U/ml | 38 (23.60) |
| ≥500
U/ml | 7 (4.35) |
| NA | 8 (4.97) |
| Mean preoperative
serum HE4 ± SD (range), pM | 478.84±453.70
(4.00-2,148.00) |
| Preoperative serum
HE4, n (%) |
|
| <500
pM | 102 (63.35) |
|
500-1,000 pM | 37 (22.98) |
|
1,000-1,500 pM | 12 (7.45) |
| ≥1,500
pM | 8 (4.97) |
| NA | 2 (1.24) |
| Mean preoperative
albumin ± SD (range), g/l | 41.04±5.03
(24.90-51.20) |
| Preoperative
albumin, n (%) |
|
| <30
g/l | 6 (3.73) |
| 30-35
g/l | 12 (7.45) |
| 35-40
g/l | 43 (26.71) |
| ≥40
g/l | 100 (62.11) |
| Mean postoperative
albumin ± SD (range), g/l | 29.58±5.85
(10.00-41.40) |
| Postoperative
albumin, n (%) |
|
| <25
g/l | 29 (18.01) |
| 25-30
g/l | 42 (26.09) |
| 30-35
g/l | 67 (41.61) |
| ≥35
g/l | 23 (14.29) |
| Mean ascites + SD
(range), ml | 1,521.38±1,825.15
(0.00-10,000.00) |
| Ascites, n (%) |
|
| <500
ml | 64 (39.75) |
|
500-2,000 ml | 40 (24.84) |
|
2,000-5,000 ml | 45 (27.95) |
| ≥5,000
ml | 12 (7.45) |
| Mean estimated
blood loss ± SD (range), ml | 1,224.10±1,286.39
(0–10,000) |
| Estimated blood
loss, n (%) |
|
| <500
ml | 39 (24.22) |
|
500-2,000 ml | 91 (56.52) |
|
2,000-5,000 ml | 27 (16.77) |
| ≥5,000
ml | 4 (2.48) |
| Transfusion within
72 h of surgery, n (%) | 111 (68.94) |
| Mean operative time
± SD (range), min | 297.28±118.66
(48.00-680.00) |
| Operation type, n
(%) |
|
| Staging
surgery | 46 (28.57) |
|
Standard cytoreduction | 27 (16.77) |
| En bloc
debulking | 12 (7.45) |
|
Extra-radical debulking | 76 (47.20) |
| Residual disease, n
(%) |
|
| No
gross residual | 148 (91.93) |
| 0.1-1.0
cm | 9 (5.59) |
| >1
cm | 4 (2.48) |
| Surgical complexity
score, n (%) |
|
|
1-6 | 99 (61.49) |
|
7-9 | 27 (16.77) |
|
≥10 | 35 (21.74) |
| Mean intensive Care
Unit stay after surgery ± SD (range), h | 66.73±33.51
(24–144) |
| Intensive Care Unit
use after surgery, n (%) |
|
| Staging
surgery | 2 (1.24) |
|
Standard cytoreduction | 3 (1.86) |
| Radical
cytoreduction | 4 (2.48) |
|
Extra-radical
cytoreduction | 17 (10.56) |
| Mean postoperative
hospital stay ± SD (range), days | 17.33±11.29
(6–89) |
| Postoperative
hospital stays, n (%) |
|
| 0-14
days | 85 (52.80) |
| 15-28
days | 58 (36.02) |
| ≥29
days | 17 (10.56) |
| Died
before discharge | 1 (0.62) |
| Mean interval of
initial postoperative chemotherapy ± SD (range), days | 16.22±10.09
(6–63) |
| Interval of initial
postoperative chemotherapy, n (%) |
|
| 0-14
days | 91 (56.52) |
| 15-28
days | 46 (28.57) |
| ≥29
days | 17 (10.56) |
| NA | 7 (4.35) |
| Discharge status, n
(%) |
|
|
Home | 160 (99.38) |
|
Died | 1 (0.62) |
EUAS included diaphragmatic peritoneal stripping
and/or diaphragmatic resection, splenectomy, distal pancreatectomy,
partial liver resection, cardiophrenic angle lymph node resection,
portal lymph node resection, partial renal resection and
cholecystectomy.
The patients were treated with neoadjuvant
chemotherapy based on the adjusted inoperability criteria for
primary debulking according to the European Society of
Gynecological Oncology ovarian carcinoma surgery guidelines
(23) as follows: i) ≥80 years of
age; ii) stage IV carcinoma based on the findings of biopsy or
cytological pathology of neck lymph nodes and pleural fluid; iii)
American Society of Anesthesiologists (ASA) score of >4; and iv)
diffuse deep infiltration of the root of the small bowel mesentery,
diffuse carcinomatosis of the small bowel involving large parts
such that resection would lead to short bowel syndrome (remaining
bowel, <1.5 m), or diffuse involvement/deep infiltration of the
stomach or duodenum and head or middle part of the pancreas
observed during laparotomy or laparoscopy. The evaluation was
performed under the supervision of two gynecological oncologists
and one surgical oncologist.
The ERAS protocol presented in Table II was used in the Department of
Obstetrics and Gynecology (The First Affiliated Hospital of
University of Science and Technology of China) according to the
ERAS® Society recommendations for gynecological oncology
surgery (24,25). The exclusion criteria were as
follows: i) Metastatic disease; ii) simultaneous or metachronous
multiple carcinomas with disease-free survival ≤5 years; iii)
simultaneous surgery for other diseases; iv) emergency operation;
v) neoadjuvant chemoradiotherapy; vi) age ≥80 years; and vii) ASA
score of 5. Preoperative counseling was recommended to the
patients, as well as an opioid-sparing multimodal approach for pain
management, goal-directed fluid management, and early mobilization
and feeding. This required a multidisciplinary team effort and the
patient complying with the whole process with the help of their
family, nurses and doctors.
 | Table II.Overview of the enhanced recovery
after surgery pathway utilized in this study. |
Table II.
Overview of the enhanced recovery
after surgery pathway utilized in this study.
| Operative
stage | Intervention | Comment |
|---|
| Preoperative | Nutrition
evaluation with NRS 2002 and PG-SGA | NRS 2002 >5 and
PG-SGA ≥9 nutrition intervention for 1–2 weeks before
operation |
|
| Bowel prep | Select
circumstances with oral antibiotics and mechanical prep |
|
| Venous ultrasound
to examine VTE for D-Dimer >3.5 µg/ml | VTE, subcutaneous
heparin; pulmonary embolus |
|
| Respiratory
training with a ‘Triflow’ breathing apparatus | Alternative devices
with blowing a balloon every day 20 times |
| Intraoperative | Goal-directed fluid
therapy, temperature control and VTE prophylaxis management | Injected into the
fascial and subdermal layers at the end of the case (transversus
abdominis plane blocks) |
| Postoperative | Early feeding
without bowel surgical procedure | General diet
immediately |
|
| Early
ambulation | Physical and
occupational therapy services automatically consulted |
|
| Early
discontinuation of intravenous fluids | When patient
tolerating >400 ml of postoperative fluid per shift |
|
| Multimodal pain
medication | Scheduled
acetaminophen and NSAIDs |
|
| Chinese traditional
medicine wormwood patch or wormwood incense | No recommendation
for patients who had a bowel anastomosis. Continued until first
bowel movement or time of hospital discharge |
|
| Minimum urine
output tolerated 0.3-0.5 ml/kg/h |
|
|
| Respiratory
training with a ‘Triflow’ breathing apparatus | Alternative devices
with blowing a balloon every day 20 times |
Surgical procedure
All patients were evaluated by the MDT using
computed tomography (CT) before surgery, with staging surgery,
standard cytoreduction (total hysterectomy, bilateral
salpingo-oophorectomy, omentectomy, pelvic and para-aortic
lymphadenectomy), en bloc debulking and extraradical debulking, as
previously described (20,26,27).
En bloc debulking and extraradical debulking were performed by a
gynecological oncologist, surgical oncologist or
hepatopancreatobiliary/liver transplantation surgeon.
Preoperatively, the MDT discussed each case based on the CT scan
and history, and prepared the surgical equipment and surgeons.
Optimal cytoreduction was defined as the absence of a residual
tumor nodule measuring >1 cm in maximal dimension at the end of
the surgical procedure. The adjusted Aletti surgical complexity
score (SCS) (20,28) was revised, and the USTC-SCS was
used to evaluate the complexity of the surgical procedures
(Table SI). Complications were
graded on a 1–5-point scale according to the Clavien-Dindo
classification of surgical complications (Table SII) (29). Grade 3–5 complications lead to
invasive radiological intervention, reoperation, unplanned
intensive care unit (ICU) admission, chronic disability or death.
Perioperative complications were defined as complications occurring
within 60 days after surgery. As the present analysis focused on
the rate of complications after primary cytoreduction, a test was
performed on potential variables that could be associated with
preoperative and postoperative complications.
The pelvic and abdominal peritonea with carcinoma
were completely debulked when complete cytoreduction was deemed
suitable. The procedure was revised following report by Soleymani
et al (30). En bloc
resection of the pelvis (EnBRP) was performed using the 10-step
standardised technique described by Tozzi et al (27). A zero polydioxanone running suture
was used to reconstruct the pleura and a patch (knitted type) was
applied only when the diaphragmatic ends were under tension during
suturing. To decrease the risk of pneumothorax, the anesthetist
manually ventilated the patients, and a 10-Ch Foley catheter was
placed in the pleura. The Valsalva manoeuvre, together with suction
and catheter removal, was performed at the last stitch. Closed
chest drainage was placed in the patients who underwent mesh
repair. All patients underwent chest radiography to verify the
absence of a pneumothorax after surgery with diaphragmatic
resection. Drains were placed in all patients at the locations of
diaphragmatic stripping or resection, splenectomy, pancreatic
resection and bowel anastomosis.
Although there is no universally accepted grading
system for bowel leakage, the definition proposed by Rahbari et
al (31) is frequently used
for rectal carcinoma and comprises a three-grade scale. Grade A
requires no therapeutic intervention, grade B requires active
intervention without laparotomy and grade C requires laparotomy
(31,32).
The presence of a postoperative pancreatic fistula
(POPF) was evaluated according to the International Study Group for
Pancreatic Surgery definition: A drain output of any measurable
volume of fluid with an amylase level greater than three times the
upper institutional normal serum amylase level associated with a
clinically relevant development/condition directly related to the
POPF. POPF was graded as follows: grade A, biochemical leak; grade
B, postoperative pancreatic fistula requires a change in the
postoperative management; drains are either left in place >3
weeks or repositioned through endoscopic or percutaneous
procedures; grade C, postoperative pancreatic fistula refers to
those postoperative pancreatic fistula that require reoperation or
lead to single or multiple organ failure and/or mortality
attributable to the pancreatic fistula. (33).
All patients received the ERAS protocol before
surgery, according to the recommended guidelines (24,25,34,35).
The main strategies were analgesia, early oral feeding, early
ambulation, anesthesia management and goal-directed fluid therapy
(Table II).
Statistical analysis
Statistical analysis was performed using SPSS v.20.0
(IBM Corp.). The mean ± standard deviation, median (interquartile
range), range and count (percentages) values were computed to
describe the continuous and categorical variables, respectively.
For the categorical variables, differences among the patients with
complications were assessed using the χ2 test or
Fisher's exact test, as appropriate. For the continuous variables,
differences were evaluated using unpaired Student's t-test or the
Mann-Whitney test, as appropriate. Receiver operating
characteristic (ROC) curve analysis was performed to evaluate the
risk factors associated with the complications. Odds ratios (ORs)
and 95% confidence intervals (CIs) were calculated using
conventional univariate analysis or multivariate logistic
regression analysis to determine the association between the
different risk factors and occurrence of grade 3–5 complications.
We also conducted a multivariate logistic regression analysis with
bootstrapping using SPSS. Statistical significance was set at
P-values of <0.05 and all P-values were two-sided. Bootstrapping
with 5,000 permutated samples, including 161 patients, was
performed.
Results
Characteristics of the patients with
EOC according to primary surgery
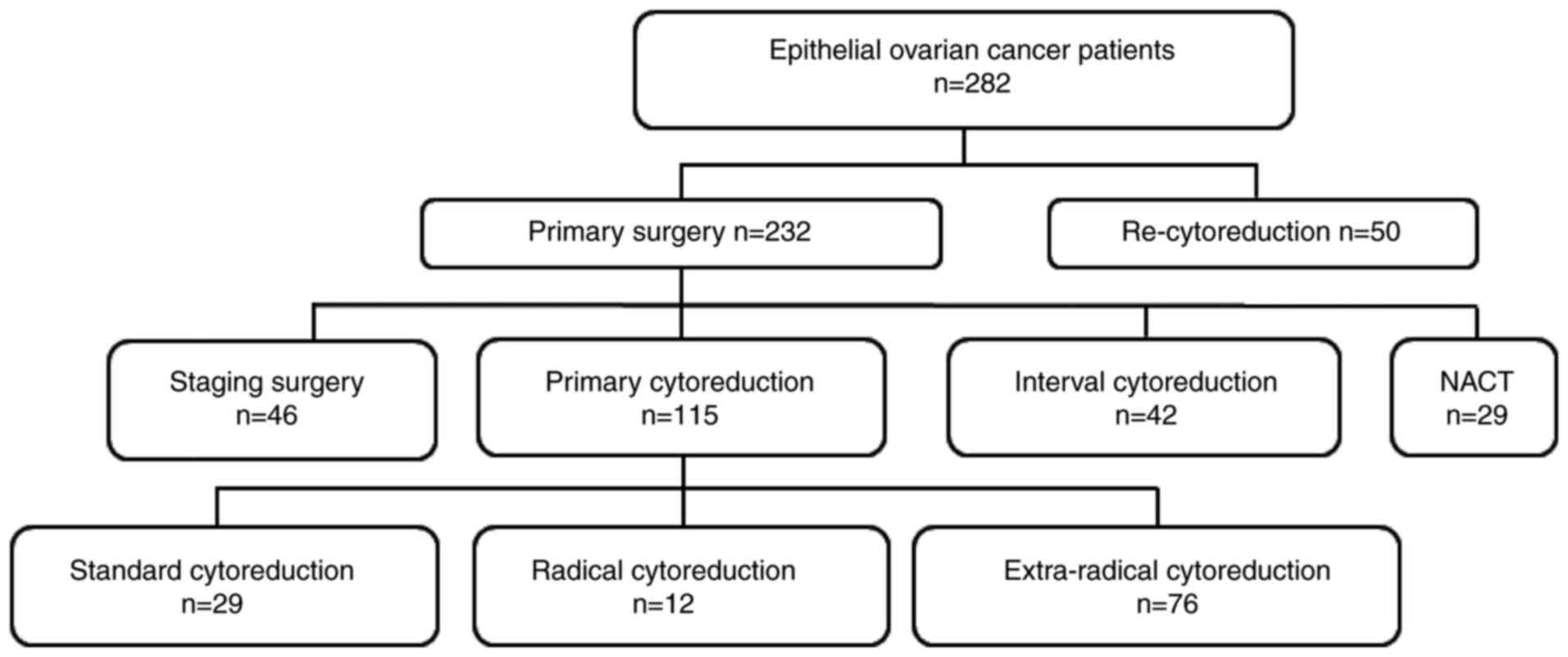
Between December 2017 and 2019, 282 patients were
hospitalised, including 50 patients who underwent recytoreduction
owing to a relapse of EOC, 29 who underwent neoadjuvant
chemotherapy after evaluation according to the inoperability
criteria for primary debulking, 42 who underwent interval
cytoreduction, 46 who underwent primary staging surgery and 115 who
underwent primary cytoreduction (Fig.
1). The demographic and clinicopathological characteristics of
161 patients who underwent primary surgery are presented in
Table I. A disseminated pelvic
abscess was detected in 1 patient with ovarian carcinoma where the
involved ureter was retained without resection in case of
anastomosis failure. The other 3 patients in whom optimal debulking
was not performed owing to enlarged mesenteric and celiac lymph
nodes were technically unsuitable for removal surgery.
Postoperative complications of the
patients with EOC according to the primary surgery
As shown in Table
III, 33 patients (20.50%) had grade 1–2 complications, such as
anemia and venous thrombosis, while 15 patients (9.32%) had grade
3–5 complications. A total of 39 patients had postoperative
complications, of which 24 patients had only grade 1–2
complications, 6 patients had only grade 3–5 complications and 9
patients had both grade 1–2 and grade 3–5 complications Table SIII. The distribution of numbers
of complications for each patient is shown in Table SIV. A dehiscent non-infected wound
was the most common grade 3–5 complication. A rectal fistula
developed in 1 patient, most likely caused by the burning of tumors
on the surface of the rectum and a stoma was created 1 week after
cytoreduction. Notably, this patient had a history of cervical
squamous carcinoma that was successfully treated by radiotherapy 3
years previously with no recurrence.
 | Table III.Postoperative complications (n=39,
24.22%). |
Table III.
Postoperative complications (n=39,
24.22%).
| Complications | n (%) |
|---|
| Grade 1–2 | 33 (20.50) |
| Renal
Insufficiency | 1 (0.62) |
|
Postoperative cognitive
dysfunction | 1 (0.62) |
|
Atelectasis | 2 (1.24) |
| Wound
infection | 1 (0.62) |
|
Pulmonary infection | 5 (3.11) |
|
Abdominal/pelvic
infection | 7 (4.35) |
| Urinary
system infection | 1 (0.62) |
| Venous
thrombosis | 6 (3.73) |
|
Pulmonary embolism | 1 (0.62) |
| Primary
intestinal obstruction | 3 (1.86) |
|
Anemia | 15 (9.32) |
| Urinary
incontinence | 1 (0.62) |
|
Arrhythmia | 2 (1.24) |
|
Septicemia | 1 (0.62) |
|
Biochemical pancreatic
fistula | 5 (3.11) |
| Bowel
fistula | 2 (1.24) |
| Grade 3–5 | 15 (9.32) |
| Closure
of dehiscent non-infected wound under anesthesia | 9 (5.59) |
|
Pancreatic leakage requiring
drainage | 2 (1.24) |
| Gastric
fistula requiring drainage | 1 (0.62) |
| Rectum
fistula requiring surgery | 1 (0.62) |
| Bladder
fistula | 1 (0.62) |
|
Respiratory failure | 2 (1.24) |
|
Cardiopulmonary failure | 1 (0.62) |
| Septic
shock | 1 (0.62) |
|
Death | 1 (0.62) |
Tables IV and
SI show the extensive radical
procedures that have been partly performed in the patients.
Extensive radical cytoreductions were performed in 76 patients. In
total, 2 patients who underwent diaphragm surgical procedures
developed pulmonary infection and were admitted to the ICU again, 1
patient died from septic shock due to multidrug resistant (MDR)
Klebsiella pneumoniae infections, and 1 patient underwent a
tracheotomy and recovered after respiratory rehabilitation every
day for 18 days as instructed by respiratory therapists. Overall, 2
patients underwent complete diaphragmatic resection and repair,
together with splenectomy, hysterectomy, oophorectomy, omentectomy,
appendectomy and lymphadenectomy. The other most common surgical
procedure was large bowel resection, which was performed in 45
patients (27.95%). Another 8 patients underwent a total colon
resection; 2 patients who underwent total colon resection developed
an anastomosis fistula identified on radiography and recovered
after drainage and rinsing without laparotomy. A single patient who
underwent partial rectal resection and colectomy developed a rectal
fistula and was treated with stoma creation 1 week after
cytoreduction. Another patient developed a bladder fistula after
partial bladder metastatic tumor resection and repair during
surgery; bladder repair was performed after six cycles of
chemotherapy and the patient then fully recovered. A further
patient developed pancreatic leakage and gastric fistula, and fully
recovered following drainage treatment, rinsing and feeding through
a gastrointestinal (GI) tube without laparotomy. The detailed data
of the 15 patients with grade 3–5 complications are shown in
Tables V and SIII.
 | Table IV.Extensive radical surgical
procedures. |
Table IV.
Extensive radical surgical
procedures.
| Procedure | n (%) |
|---|
| Diaphragm
peritonectomy | 32 (19.88) |
| Splenectomy | 25 (15.53) |
| Full-thickness
diaphragm resection | 16 (9.94) |
| Partial
hepatectomy | 6 (3.73) |
| Distal
pancreatectomy | 3 (1.86) |
|
Cholecystectomy | 3 (1.86) |
| Cardiophrenic angle
lymph nodes resection | 2 (1.24) |
| Portal lymph
node | 5 (3.11) |
| Small bowel
resection | 2 (1.24) |
| Partial renal
resection | 1 (0.62) |
| Ureter resection
and anastomosis | 1 (0.62) |
| Total colon
resection and anastomosis | 8 (4.97) |
| Partial large bowel
resection anastomosisa | 34 (21.12) |
| Partial large bowel
resection and stoma | 11 (6.83) |
| Inguinal lymph node
dissection | 2 (1.24) |
| Part of bladder
resection and repair | 1 (0.62) |
 | Table V.Univariate and multivariate analyses
of preoperative risk factors associated with grade 3–5
postoperative complications. |
Table V.
Univariate and multivariate analyses
of preoperative risk factors associated with grade 3–5
postoperative complications.
|
| Univariate
analysis | Multivariate
analysis |
|
|---|
|
|
|
|
|
|---|
| Factor | OR (95% CI) | P-value | OR (95% CI) | P-value | P-value
(bootstrap) |
|---|
| Age, years |
|
|
|
|
|
|
<50 | 1 | - | - | - | - |
|
50-59 | 1.260
(0.394-4.028) | 0.696 | - | - | - |
|
60-69 | NA | 0.071 | - | - | - |
|
70-79 | 0.700
(0.074-6.581) | 1.000 | - | - | - |
| BMI,
kg/m2 |
|
|
|
|
|
|
<18.5 | 1 | - | - | - | - |
|
18.5-23.9 | 0.051
(0.055-4.762) | 1.000 | - | - | - |
|
24-27.9 | 1.105
(0.115-10.648) | 1.000 | - | - | - |
|
≥28 | 1.167
(0.059-22.937) | 1.000 | - | - | - |
| ASA (III + IV vs. I
+ II) | 1.082
(0.373-3.135) | 0.884 | - | - | - |
| CA125 (≥1,012
U/ml) | 6.691
(1.808-24.761) | 0.001 | 3.866
(0.901-16.583) | 0.069 | 0.034 |
| HE4 (≥717 pM) | 9.680
(3.045-30.775) | <0.001 | 4.923
(1.368-17.715) | 0.015 | 0.007 |
| FIGO stage (IV vs.
IIIC) | 9.446
(2.747-32.478) | <0.001 | 7.070
(1.888-26.477) | 0.004 | 0.002 |
| Ascites (≥2,000
ml) | 2.240
(0.768-6.531) | 0.132 |
|
| - |
| Comorbidities | 1.042
(0.337-3.216) | 1.000 | - | - | - |
| Multiple
comorbidities | 0.550
(0.068-4.453) | 0.892 | - | - | - |
| Preoperative ALB
(<35 vs. ≥35 g/l) | 1.926
(0.493-7.523) | 0.586 | - | - | - |
Risk factor analysis for grade 3–5
complications
The potential risk factors significantly associated
with the preoperative risk factors for grade 3–5 complications were
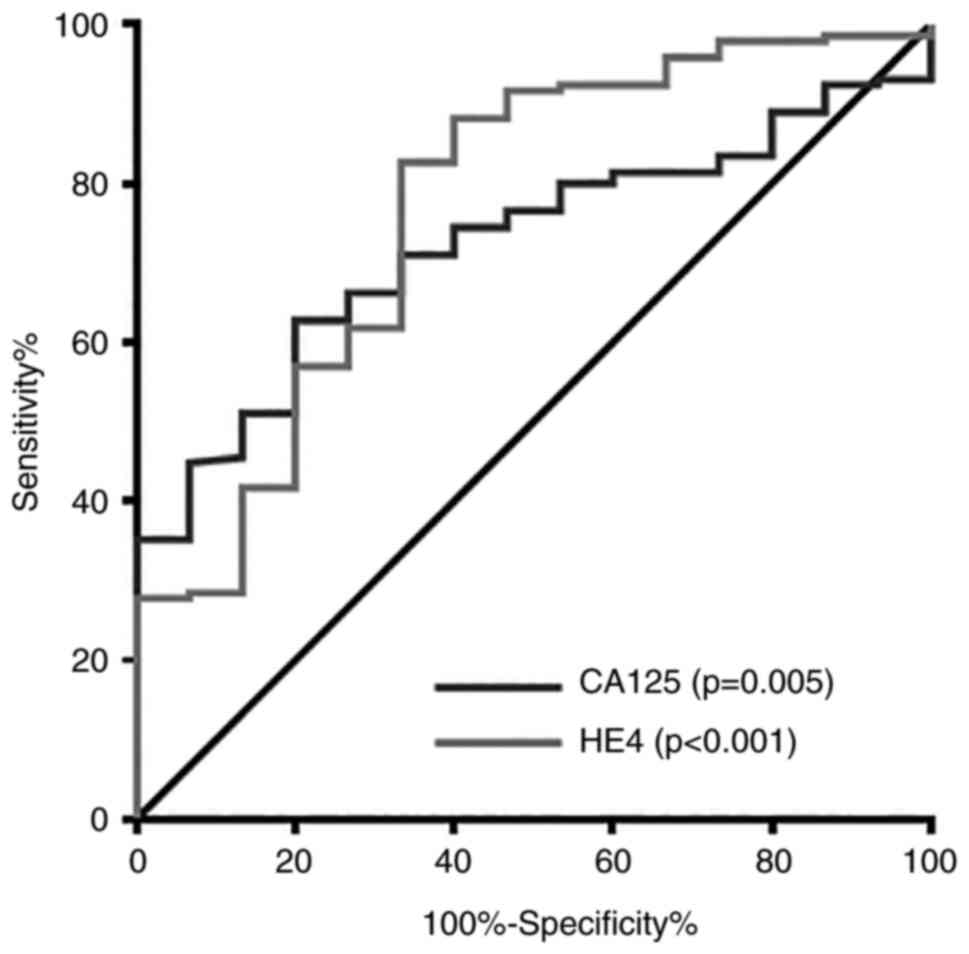
first analysed. The variables analyzed are listed in Table V. The univariate analysis
demonstrated that a CA125 level of ≥1,012 U/ml (P=0.001), an HE4
level of ≥717 pM (P<0.001) and FIGO stage IV (P<0.001;
compared with stage IIIC) were associated with grade 3–5
complications. The multivariate analysis revealed that an HE4 level
of ≥717 pM (P=0.015) and FIGO stage IV (P=0.004; compared with
stage IIIC) were associated with grade 3–5 complications. The
bootstrap analysis revealed that a CA125 level of ≥1,012 U/ml
(P=0.034), an HE4 level of ≥717 pM (P=0.007) and FIGO stage IV
(P=0.002, compared with stage IIIC) were significantly associated
with grade 3–5 complications. There were no significant
associations between age, BMI, ASA score, ascites (≥2,000 ml),
comorbidities, multiple comorbidities, preoperative hypoalbuminemia
(<3.5 g/l) and grade 3–5 complications. The cut off values and
ROC curves of the CA125 and HE4 levels related to grade 3–5
complications are presented in Fig.
2.
The potential factors that were significantly
associated with the operative risk factors associated with grade
3–5 complications were then assessed. The variables analyzed are
presented in Table VI. The
univariate analysis demonstrated that an SCS score of ≥10
(P=0.020), postoperative hypoalbuminemia (<25 g/l) (P=0.005),
operative blood loss of ≥1,100 ml (P=0.001) and postoperative
transfer to ICU (P=0.003) were associated with grade 3–5
complications. The multivariate analysis did not reveal any risk
factors associated with grade 3–5 complications, while the
bootstrap analysis revealed that transfer to the ICU after surgery
(P=0.026) might be associated with such complications.
 | Table VI.Univariate and multivariate analyses
of the operative factors associated with grade 3–5 postoperative
complications. |
Table VI.
Univariate and multivariate analyses
of the operative factors associated with grade 3–5 postoperative
complications.
|
| Univariate
analysis | Multivariate
analysis |
|
|---|
|
|
|
|
|
|---|
| Factor | OR (95% CI) | P-value | OR (95% CI) | P-value | P-value
(bootstrap) |
|---|
| SCS scores |
|
|
|
|
|
|
1-6 | 1 | - | 1 | - | - |
|
7-9 | 2.350
(0.524-10.531) | 0.484 | - | - | - |
|
≥10 | 4.700
(1.384-15.964) | 0.020 | 2.526
(0.441-14.480) | 0.298 | 0.352 |
| Postoperative ALB,
g/l |
|
|
|
|
|
|
≥30 | 1 | - | 1 | - | - |
|
25-30 | 2.263
(0.538-9.529) | 0.455 | - | - | - |
|
<25 | 6.841
(1.837-25.473) | 0.005 | 1.686
(0.287-9.907) | 0.563 | 0.446 |
| Estimated blood
loss, ml |
|
|
|
|
|
|
<1,100 | 1 | - | 1 | - | - |
|
≥1,100 | 6.587
(1.987-21.836) | 0.001 | 1.877
(0.308-11.434) | 0.494 | 0.542 |
| Operative time,
min |
|
|
|
|
|
|
≤120 | 1 |
| 1 | - | - |
|
121-360 | 0.180
(0.017-1.923) | 0.228 | - | - | - |
|
>360 | 1.200
(0.119-12.143) | 1.000 | - | - | - |
| ICU |
|
|
|
|
|
| No | 1 | - | 1 | - | - |
|
Yes | 5.849
(1.902-17.981) | 0.003 | 4.931
(0.805-30.205) | 0.084 | 0.026 |
Length of hospital stay (LOS) and
postoperative chemotherapy and prognosis
The mean LOS was 17.33±11.29 days after completion
of the initial postoperative chemotherapy, and the mean initial
postoperative chemotherapy interval was 16.22±10.09 days (range,
6–63 days).
The mean LOS was 11.29±3.66 and 19.71±12.35 days
among the patients who underwent staging surgery and cytoreductive
surgery after completion of the initial postoperative chemotherapy,
respectively.
A single patient died on postoperative day 19 and
did not undertake adjuvant chemotherapy, while another 154 patients
(95.65%) underwent adjuvant chemotherapy with carboplatin or
carboplatin and paclitaxel after surgery. A further 6 patients were
discharged and went to a local hospital for adjuvant chemotherapy
and were lost to follow-up. A total of 137 patients (85.09%)
completed the initial chemotherapy within 4 weeks of surgery, 91
(56.52%) of whom completed the initial chemotherapy within 2 weeks
of surgery. Overall, 15 patients did not complete the initial
chemotherapy within 4 weeks of surgery for reasons such as closure
of the dehiscent non-infected wound, intestinal obstruction,
infection and pancreatic or bowel anastomotic leakage. A further 2
patients refused to receive adjuvant chemotherapy immediately after
surgery but returned to the hospital to receive chemotherapy >4
weeks after surgery.
Discussion
The benefit of aggressive surgery increasing overall
survival must be balanced against the substantial risk of
perioperative severe complications. Nowadays, gynecological
oncologists emphasize the risk factors associated with
perioperative complications such as malnutrition, hypoalbuminemia,
low transferrin levels, obesity, insulin resistance, high levels of
C-reactive protein, interleukin-6 and increased platelet count
(36–38). Moreover, a peritoneal cancer index
≥21 and preoperative albumin concentration ≤33 g/l were also
independent predictors of high-grade complications (37,39).
Although the ERAS protocol can enhance the rehabilitation of a
patient, a recent study showed that 5.8% of 7,029 patients (76.5%
from Caucasian ethnicity) in the national surgical quality
improvement program database had experienced Clavien-Dindo IV
complications, and that 0.9% of patients died within 30 days. This
study also confirmed that increased age, emergency surgery,
ascites, bleeding disorder, low albumin, higher ASA score and a
higher extended procedure score are associated with serious
perioperative morbidity or mortality (40). The diverse demographics and
ethnicities in different countries also create a challenge for
clinicians caring for the patients in the perioperative period.
In the present study, cases were not selected from
patients with emergency surgery or those >80 years of age
(referring to ERAS exclusion criteria), and only preoperative tumor
loads, such as CA125 and HE4 levels, and FIGO stage IV were
associated with grade 3–5 perioperative complications. The National
Institute for Health and Care Excellence has reported that SCS can
define a higher risk operation (20), while preoperative higher tumor
loading can contribute to much more complex operations and elevated
SCS scores. In the present postoperative complication results, it
was found that SCS scores, postoperative ALB, blood loss and ICU
transfer were associated with grade 3–5 perioperative complications
in univariate analysis, but only ICU transfer was associated with
complications in multivariate bootstrapping analysis.
A decreased serum ALB level of ≥10 g/l on
postoperative day 1 has been reported to be associated with a
three-fold increased risk of complications after abdominal surgery
(36). In the present analysis,
there was no significant relationship between grade 3–5
complications and the albumin level (Table I). Some centers have reported that
a decreased preoperative serum albumin (≤35 g/l) level is
associated with postoperative complications and OS in patients with
EOC (41–44). Cham et al (40) also reported the use of preoperative
serum albumin levels in the validation of a risk calculator for
adverse perioperative outcomes in women with ovarian carcinoma. In
the present study, the patients with a preoperative albumin level
of ≥35 g/l accounted for 88.82% of all patients, and there was no
association between the preoperative serum albumin level (≤35 g/l)
and grade 3–5 complications. Although a postoperative albumin level
of ≤25 g/l was associated with grade 3–5 complications in the
univariate analysis, there was no significant difference found in
the multivariate and bootstrap analyses. Notably, the patients with
ovarian carcinoma at the advanced stage were transfused with
albumin 20–40 g during surgery and then 10–20 g every day after
surgery until a serum albumin level of >30 g/l was achieved.
Thus, although the albumin level may be related to the development
of postoperative complications, it was assumed that this was
prevented by albumin supplementation.
Cham et al (40) also used preoperative ascites for
the validation of the risk calculator for adverse perioperative
outcomes in women with ovarian carcinoma, and the calculator only
used negative or positive ascites for scoring, without the exact
quantity of ascites. A cohort study in the United States
demonstrated that >2,000 ml of ascites was associated with worse
PFS and OS, which might be associated with increased
immunosuppression (45,46). Günakan et al (47) reported that >500 ml of ascites
was associated with operative complications upon univariate
analysis and that there was no significant difference found upon
multivariate analysis. In the present study, 19.25% of the patients
had >2,000 ml of ascites; however, there was no association
observed between ascites and complications.
In the present study, a total of 13 cases of grade 3
complications, 1 case of grade 4 complications and 1 case of grade
5 complications were found after primary surgery. The independent
preoperative risk factors associated with grade 3–5 complications
included the HE4 level and FIGO stage. It is worth noting that
there was no comparison for the patients with ovarian cancer of
stage I and stage III/IV in this study. Firstly, the patients at
early stages achieved staging surgery, while the patients at
advanced stages received complete cytoreduction. The different
surgery strategies might not be suitable for making comparisons.
Secondly, the patients at early stages exhibited a better
performance status and nutrition status than those at advanced
stages. Therefore, for the complication risk factors, the advanced
stages between stage IIIc and stage IV were analyzed.
The CA125 level can also be considered a risk factor
according to the bootstrap analysis. Although no independent risk
factors associated with the postoperative complications were
observed, transfer to the ICU after surgery could be regarded as a
risk factor according to the bootstrap analysis. In the univariate
analysis, an SCS score of ≥10, a postoperative ALB level of <25
g/l and an estimated blood loss amount of ≥1,100 ml were also
associated with grade 3–5 complications.
Patients with advanced ovarian carcinoma usually
have metastases to multiple organs, such as the omentum, paracolic
sulcus, diaphragm, liver surface and spleen. A discussion among
MDTs should be conducted preoperatively to improve outcomes. The
final decision regarding resectability and the goal of achieving
<0.5 mm of residual tumor tissue are based on exploration to
offer the opportunity to investigate hidden spaces (e.g. posterior
aspect of the spleen, lesser sac and posterior margin of the liver
and diaphragm) (48–51). Postoperative management requires a
large amount of clinical experience to ensure that patients safely
overcome postoperative complications and begin adjuvant
chemotherapy within 4 weeks of surgery (52–54).
In the Department of Obstetrics and Gynecology (The First
Affiliated Hospital of University of Science and Technology of
China), 85.09% of the patients were treated with adjuvant
chemotherapy within 4 weeks of surgery.
ERAS protocols have been used in gynecological
oncology (24,25,55–57),
GI surgery, hepatopancreatic surgery and transplantation, and have
improved surgical outcomes and reduced hospitalisation times
(35,58,59).
Evaluation of postoperative complications and identification of
risk factors could further improve the outcomes of patients with
advanced ovarian carcinoma. In the Department of Obstetrics and
Gynecology, ERAS protocols have been used for ~2 years. In China,
patients do not have a family physician and the majority of the
patients in the present study were from the countryside; thus, they
were not discharged until they had completed chemotherapy following
surgery.
Tozzi et al (27) reported that the mean LOS among
patients who underwent EnBRP was 10.3 days (range, 6–91 days),
while in the present study, the mean LOS was 17.33±11.29 days
(range, 6–89 days). The LOS was longer than that in other national
centers despite the use of the ERAS protocol. The government is
attempting to use daytime wards to resolve such problems and
discharge patients without bowel procedures or other complications
on day 4 after surgery. With the help of GI, hepatopancreatic and
transplantation surgeries, EUAS was performed and the adjusted SCS
scores were revised (20) by
naming them USTC-SCS scores, as shown in Table SI. Preoperative and postoperative
respiratory training with a ‘Triflow’ breathing apparatus was
recommended for patients with metastases involving the diaphragm as
demonstrated on CT scans. This ERAS strategy was particularly used
for EUAS to prevent pulmonary infections.
Using ERAS protocols in gynecological surgery has
the following advantages: Reduced opioid use, enhanced patient
satisfaction, reduced LOS and complications, improved cost
effectiveness and decreased readmission rates. However, patients
with EOC are distinctly different from those with gynecological
carcinoma and other surgical patients. At the time of diagnosis,
patients often have an advanced stage with a high symptom burden,
including abdominal distension, dyspnea, nausea, GI dysfunction,
anemia, cachexia and malnutrition. Further, surgical procedures
often include multivisceral resection with a high postoperative
morbidity rate (60–64). Thus, ERAS protocols can be used in
patients with EOC to help prevent complications, hasten recover,
initiate adjuvant chemotherapy on schedule and achieve better
patient satisfaction scores. However, the ERAS protocol standard
cannot be evaluated using the LOS as a single criterion in patients
with EOC.
The problems with ERAS protocols that have not been
resolved in China and have resulted in a longer LOS compared with
that in other countries are as follows: i) The ideas among medical
staff and patients are profoundly traditional; ii) medical
administrations do not consider the application of ERAS protocols
as the status quo in the hospital, including every involved
department; iii) the medical treatment costs are very low in China;
iv) patients from the countryside would prefer not to travel home
after surgery and then return for chemotherapy a few days later; v)
the ward beds are cheaper than stays in a hotel and safer for the
observation of their conditions until the completion of the initial
chemotherapy; vi) patients do not have family physicians in China
and if they go home, local hospitals would deny their admission in
the presence of any complications; and vii) insurance companies
only pay for hospitalisation but not for the costs related to
regular visits in clinic.
Although no guidelines on the optimal interval
between debulking surgery and the initiation of adjuvant
chemotherapy are available, it is recommended that adjuvant
chemotherapy be initiated as soon as possible, as this treatment
may avoid early tumor growth within the interval. Recent reports
showed that delayed initiation of adjuvant chemotherapy was an
independent prognostic factor for worse OS after surgery and
concluded that a time-to-chemotherapy (TTC) delayed beyond 4 weeks
should be avoided (53,65). With the ERAS programme for surgery,
the patients in the present study recovered rapidly; 137 patients
(85.09%) completed the initial chemotherapy within 4 weeks of
surgery, with a mean TTC of 16.22±10.09 days (range, 6–63
days).
Recent studies have shown that 40% of patients
diagnosed with ovarian carcinoma have diaphragmatic metastases,
with the right diaphragm as the most common metastatic site
(63.3-80%) and with fewer cases involving both sides (5–36%) or the
left side only (0.7-15%) (66,67).
Intraoperative diaphragmatic evaluation has been suggested for all
patients undergoing cytoreductive surgery for advanced ovarian
carcinoma (30,66). In the present study, 40.99%
(66/161) of the patients were diagnosed with diaphragmatic
metastases, including 2 cases of metastases (1.24%) on the left
side, 32 cases (19.88%) on the right side and 32 cases (19.88%) on
both sides. Among the affected patients, 16 underwent
full-thickness diaphragmatic resection and repair, and 5 underwent
complete diaphragmatic resection and reconstruction of the
diaphragm via patch application (Table SV). The decision to perform
full-thickness resection of the diaphragm was based on tumor
infiltration of the muscle. For patients undergoing diaphragmatic
repair, who would be more likely to develop pleural effusion within
3 days of surgery, closed chest drainage tubes should be placed
with continuous low suction (18,67).
It is recommended that closed thoracic drainage should be performed
routinely after surgery and that chest radiography or CT should be
performed 3 days after surgery to assess the condition of the
peritoneal effusion and pulmonary atelectasis, and particularly to
check for infections. Once infection symptoms appear with closed
thoracic drainage, pleural fluid culture and drug sensitivity
testing should be performed in a timely manner, as well as
bronchioalveolar lavage as part of the ERAS protocol. When tumors
from the right diaphragm and spleen have to be removed, attention
should be paid to drainage and infection prevention in both
abdominal cavities. If both sides of the diaphragmatic abscess
develop and patients cannot breathe normally, they should be sent
to the ICU. Notably, 4% of patients admitted to the ICU are found
to be colonised or infected with strains of K. pneumoniae
with multi-resistance to ceftazidime, ciprofloxacin and tobramycin
(68). Infections with MDR strains
are common in patients with chronic diseases, advanced tumor
stages, exposure to chemotherapy or immunosuppression. Early
identification of K. pneumoniae with multi-resistance in
pulmonary infections is critical for patients after diaphragmatic
resection, which is the main cause of postoperative death.
In the present study, 8 patients (4.97%) underwent a
total colectomy. One of the side effects of a total colectomy is
the lack of adequate stool concentration and frequent postoperative
stool passage (69). To improve
the passage frequency, loperamide hydrochloride capsules with a
maximum daily dose of 12 mg as permanent medication were
administered in the present study. Patients could take this drug
after defecation, with an initial dose of 2 mg/day to relieve the
symptoms of watery diarrhoea; iron and multivitamins should also be
taken to alleviate anemia due to absorption dysfunction (70). The C-reactive protein level after
anastomosis should also be routinely assessed to monitor early
fistula formation or pelvic infection, in which case the level will
increase when an intestinal fistula occurs (71). Type A and B bowel fistulas can
resolve through continuous rinsing through a drainage tube and
anti-infective treatment (32).
Few centers have performed total colon resection, particularly deep
anterior resection with ileorectostomies. Son et al
(72) reported that the risk of
anastomotic leakage was highest in patients who underwent a
subtotal colectomy.
Although adjuvant chemotherapy can cause dehiscent
wounds (73), only 9/161 patients
(5.59%) developed dehiscent wounds and underwent retention suturing
under general anesthesia in the present study. The literature
recommends the use of a retention suture for patients with a high
risk of intra-abdominal hypertension to ensure the initiation of
adjuvant chemotherapy within 4 weeks of surgery, which may increase
bladder pressure and postoperative pain. Delayed wound healing is
typical in patients with advanced oncological diseases or
immunosuppression or in those receiving chemotherapy (74). Thus, careful wound monitoring and
cummerbund use for 3 months postoperatively are recommended.
Patients with ovarian carcinoma are distinctly
different from other gynecological surgical patients. Although ERAS
has been used for colorectal malignancies and other surgical
tumors, only a few relevant published studies are available on the
ERAS protocols due to the heterogeneity of ovarian cancer (75–80).
At the time of diagnosis, the patients often have an advanced stage
disease with complex symptoms, including abdominal distension,
dyspnea, impairment of gastrointestinal function and malnutrition,
which require aggressive cytoreductive procedures such as
multivisceral resections; patients also are prone to a high risk of
postoperative morbidity. Thus, the ERAS protocols achieved from
other surgical disciplines may be inappropriate to directly apply
to patients with ovarian malignancies (81,82).
The main dilemmas encountered in the present study were the strong
beliefs in traditional surgical paradigms held by the gynecological
surgeons and how to manage the extensive surgical procedures with
the ERAS protocol. Pain management and goal-directed fluid
management were the most important ERAS protocols. Firstly, pain
management is an important part of ERAS, especially for the long
incisions for the ovarian cytoreduction; freedom from pain can
facilitate the early mobility of the patient and an early return of
bowel function. Secondly, goal-directed fluid management also plays
an important role in the anesthetization process during
perioperative fluid management (81,83).
Appropriate volume status assessment can decrease postoperative
morbidities such as pulmonary and intestinal edema. Table II shows other ERAS protocols used
in the present study. It may be difficult to perform randomized
controlled trials to make comparisons between the patients with and
without ERAS in individuals with ovarian cancer. Firstly, the
patients have heterogeneity in tumor burden and nutrition status.
Secondly, it may not be ethically feasible to try randomized trials
of ERAS protocols given the positive evidence for ERAS elements
reported to date. Thirdly, the unproportionate medical insurance
and government management may challenge the multicenter and
international approach to perform a clinical trial. Efforts should
be undertaken to facilitate the work of those institutions willing
to revise their perioperative protocols, and cooperative trial
groups could be an appropriate platform to establish precise
evaluations with regard to the implantation of ERAS protocols in
the treatment of patients with ovarian cancer.
In summary, the present study demonstrated that
extraradical debulking is feasible, with a low mortality rate
(1/76, 1.3%) during hospitalisation, even among patients with an
advanced ASA score (≤4). ERAS protocols are useful and support the
early initiation of chemotherapy. Wound healing requires careful
supervision and management until adjuvant chemotherapy is
completed. Extraradical debulking in combination with ERAS
protocols and the early initiation of chemotherapy is feasible and
should be performed in select patient populations.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science
Foundation of China (grant nos. 82172773 and 81872110), the
National Key Research and Development Program (grant no.
2018YFC1003900), and the 2020 USTC-Affiliated Hospital Introduction
Project to Medical Leading Technology (grant no. 2020LXJS-05).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article (and its supplementary
information files).
Authors' contributions
ML, TZ, JZ, YL, WC, YX, WZ, RC, WW, GW, JQ, WZ, DW,
BN, ZS, YZ were responsible for the conception and design of the
study, and critical revisions of the manuscript. YZ and BN drafted
the manuscript. ML, TZ, JZ, YL, YX, WZ, RC, WW, GW, JQ, ZS and YZ
acquired, analysed and interpreted the data. ML, TZ, JZ, YX, WZ,
JQ, RC, WW, GW, WZ, DW, BN, ZS and YZ performed the statistical
analysis. BN and YZ confirm the authenticity of all the raw data.
All the authors have read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
The First Affiliated Hospital of University of Science and
Technology of China (approval no. 2021-KY120).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
IPC
|
initial postoperative chemotherapy
|
|
EOC
|
epithelial ovarian carcinoma
|
|
ICU
|
intensive care unit
|
|
FIGO
|
Federation International of Gynecology
and Obstetrics
|
|
MDT
|
multidisciplinary team
|
|
ERAS
|
enhanced recovery after surgery
|
|
EUAS
|
extensive upper abdominal surgery
|
|
PDS
|
primary debulking surgery
|
|
IDS
|
interval debulking surgery
|
|
PFS
|
progression-free survival
|
|
OS
|
overall survival
|
|
BMI
|
body mass index
|
|
ASA
|
American Society of
Anesthesiologists
|
|
CT
|
computed tomography
|
|
SCS
|
surgical complexity score
|
|
EnBRP
|
en bloc resection of the pelvis
|
|
POPF
|
postoperative pancreatic fistula
|
|
ROC
|
receiver operating characteristic
|
|
OR
|
odds ratio
|
|
CI
|
confidence interval
|
|
MDR
|
multidrug resistant
|
|
GI
|
gastrointestinal
|
|
ALB
|
albumin
|
|
LOS
|
length of hospital stay
|
|
TTC
|
time-to-chemotherapy
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huang Z, Zheng Y, Wen W, Wu C, Bao P, Wang
C, Zhong W, Gao YT, Jin F, Xiang YB, et al: Incidence and mortality
of gynaecological cancers: Secular trends in urban Shanghai, China
over 40 years. Eur J Cancer. 63:1–10. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Høgdall E: Approaches to the detection of
ovarian cancer. Scand J Clin Lab Invest Suppl. 245:S49–S53. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Winter WE III, Maxwell GL, Tian C, Carlson
JW, Ozols RF, Rose PG, Markman M, Armstrong DK, Muggia F and
McGuire WP; Gynecologic Oncology Group Study, : Prognostic factors
for stage III epithelial ovarian cancer: A gynecologic oncology
group study. J Clin Oncol. 25:3621–3627. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chi DS, Eisenhauer EL, Lang J, Huh J,
Haddad L, Abu-Rustum NR, Sonoda Y, Levine DA, Hensley M and Barakat
RR: What is the optimal goal of primary cytoreductive surgery for
bulky stage IIIC epithelial ovarian carcinoma (EOC)? Gynecol Oncol.
103:559–564. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bristow RE, Tomacruz RS, Armstrong DK,
Trimble EL and Montz FJ: Survival effect of maximal cytoreductive
surgery for advanced ovarian carcinoma during the platinum era: A
meta-analysis. J Clin Oncol. 20:1248–1259. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kehoe SM, Eisenhauer EL and Chi DS: Upper
abdominal surgical procedures: Liver mobilization and diaphragm
peritonectomy/resection, splenectomy, and distal pancreatectomy.
Gynecol Oncol. 111 (Suppl 2):S51–S55. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eisenhauer EL, Abu-Rustum NR, Sonoda Y,
Levine DA, Poynor EA, Aghajanian C, Jarnagin WR, DeMatteo RP,
D'Angelica MI, Barakat RR and Chi DS: The addition of extensive
upper abdominal surgery to achieve optimal cytoreduction improves
survival in patients with stages IIIC-IV epithelial ovarian cancer.
Gynecol Oncol. 103:1083–1090. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hynninen J, Lavonius M, Oksa S, Grénman S,
Carpén O and Auranen A: Is perioperative visual estimation of
intra-abdominal tumor spread reliable in ovarian cancer surgery
after neoadjuvant chemotherapy? Gynecol Oncol. 128:229–232. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lv X, Cui S, Zhang X and Ren C: Efficacy
and safety of neoadjuvant chemotherapy versus primary debulking
surgery in patients with ovarian cancer: A meta-analysis. J Gynecol
Oncol. 31:e122020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Siesto G, Cavina R, Romano F and Vitobello
D: Primary debulking surgery versus neoadjuvant chemotherapy in
advanced epithelial ovarian cancer: A propensity-matched analysis.
Am J Clin Oncol. 41:280–285. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen M, Chen Z, Xu M, Liu D, Liu T, He M
and Yao S: Impact of the time interval from neoadjuvant
chemotherapy to surgery in primary ovarian, tubal, and peritoneal
cancer patients. J Cancer. 9:4087–4091. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cole AL, Barber EL, Gogate A, Tran AQ and
Wheeler SB: Economic analysis of neoadjuvant chemotherapy versus
primary debulking surgery for advanced epithelial ovarian cancer
using an aggressive surgical paradigm. Int J Gynecol Cancer.
28:1077–1084. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eisenkop SM, Spirtos NM, Friedman RL, Lin
W-CM, Pisani AL and Perticucci S: Relative influences of tumor
volume before surgery and the cytoreductive outcome on survival for
patients with advanced ovarian cancer: A prospective study. Gynecol
Oncol. 90:390–396. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aletti GD, Dowdy SC, Gostout BS, Jones MB,
Stanhope CR, Wilson TO, Podratz KC and Cliby WA: Aggressive
surgical effort and improved survival in advanced-stage ovarian
cancer. Obstet Gynecol. 107:77–85. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chi DS, Zivanovic O, Levinson KL, Kolev V,
Huh J, Dottino J, Gardner GJ, Leitao MM Jr, Levine DA, Sonoda Y, et
al: The incidence of major complications after the performance of
extensive upper abdominal surgical procedures during primary
cytoreduction of advanced ovarian, tubal, and peritoneal
carcinomas. Gynecol Oncol. 119:38–42. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Benedetti Panici P, Di Donato V, Fischetti
M, Casorelli A, Perniola G, Musella A, Marchetti C, Palaia I,
Berloco P and Muzii L: Predictors of postoperative morbidity after
cytoreduction for advanced ovarian cancer: Analysis and management
of complications in upper abdominal surgery. Gynecol Oncol.
137:406–411. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tozzi R, Casarin J, Garruto-Campanile R,
Majd HS and Morotti M: Morbidity and reversal rate of ileostomy
after bowel resection during visceral-peritoneal debulking (VPD) in
patients with stage IIIC-IV ovarian cancer. Gynecol Oncol.
148:74–78. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Phillips A, Sundar S, Singh K, Pounds R,
Nevin J, Kehoe S, Balega J and Elattar A: The NICE classification
for ‘Ultra-radical (extensive) surgery for advanced ovarian cancer’
guidance does not meaningfully predict postoperative complications:
A cohort study. BJOG. 126:96–104. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Prat J; FIGO Committee on Gynecologic
Oncology, : FIGO's staging classification for cancer of the ovary,
fallopian tube, and peritoneum: Abridged republication. J Gynecol
Oncol. 26:87–89. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu Y: Overweight and obesity in China.
BMJ. 333:362–363. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Querleu D, Planchamp F, Chiva L,
Fotopoulou C, Barton D, Cibula D, Aletti G, Carinelli S, Creutzberg
C, Davidson B, et al: European society of gynaecological oncology
(ESGO) guidelines for ovarian cancer surgery. Int J Gynecol Cancer.
27:1534–1542. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nelson G, Altman AD, Nick A, Meyer LA,
Ramirez PT, Achtari C, Antrobus J, Huang J, Scott M, Wijk L, et al:
Guidelines for pre- and intra-operative care in
gynecologic/oncology surgery: Enhanced recovery after surgery
(ERAS®) society recommendations-part I. Gynecol Oncol.
140:313–322. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nelson G, Altman AD, Nick A, Meyer LA,
Ramirez PT, Achtari C, Antrobus J, Huang J, Scott M, Wijk L, et al:
Guidelines for postoperative care in gynecologic/oncology surgery:
Enhanced recovery after surgery (ERAS®) society
recommendations-part II. Gynecol Oncol. 140:323–332. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tozzi R, Gubbala K, Majd HS and Campanile
RG: Interval laparoscopic en-bloc resection of the pelvis (L-EnBRP)
in patients with stage IIIC-IV ovarian cancer: Description of the
technique and surgical outcomes. Gynecol Oncol. 142:477–483. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tozzi R, Hardern K, Gubbala K, Garruto
Campanile R and Soleymani Majd H: En-bloc resection of the pelvis
(EnBRP) in patients with stage IIIC-IV ovarian cancer: A 10 steps
standardised technique. Surgical and survival outcomes of primary
vs interval surgery. Gynecol Oncol. 144:564–570. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Aletti GD, Dowdy SC, Podratz KC and Cliby
WA: Relationship among surgical complexity, short-term morbidity,
and overall survival in primary surgery for advanced ovarian
cancer. Am J Obstet Gynecol. 197:676.e1–e7. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Strasberg SM, Linehan DC and Hawkins WG:
The accordion severity grading system of surgical complications.
Ann Surg. 250:177–186. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Soleymani Majd H, Ferrari F, Manek S,
Gubbala K, Campanile RG, Hardern K and Tozzi R: Diaphragmatic
peritonectomy vs full thickness resection with pleurectomy during
visceral-peritoneal debulking (VPD) in 100 consecutive patients
with stage IIIC-IV ovarian cancer: A surgical-histological
analysis. Gynecol Oncol. 140:430–435. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rahbari NN, Weitz J, Hohenberger W, Heald
RJ, Moran B, Ulrich A, Holm T, Wong WD, Tiret E, Moriya Y, et al:
Definition and grading of anastomotic leakage following anterior
resection of the rectum: A proposal by the international study
group of rectal cancer. Surgery. 147:339–351. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gessler B, Eriksson O and Angenete E:
Diagnosis, treatment, and consequences of anastomotic leakage in
colorectal surgery. Int J Colorectal Dis. 32:549–556. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bassi C, Marchegiani G, Dervenis C, Sarr
M, Abu Hilal M, Adham M, Allen P, Andersson R, Asbun HJ, Besselink
MG, et al: The 2016 update of the international study group (ISGPS)
definition and grading of postoperative pancreatic fistula: 11
Years after. Surgery. 161:584–591. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Feldheiser A, Aziz O, Baldini G, Cox BP,
Fearon KC, Feldman LS, Gan TJ, Kennedy RH, Ljungqvist O, Lobo DN,
et al: Enhanced recovery after surgery (ERAS) for gastrointestinal
surgery, part 2: Consensus statement for anaesthesia practice. Acta
Anaesthesiol Scand. 60:289–334. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Scott MJ, Baldini G, Fearon KC, Feldheiser
A, Feldman LS, Gan TJ, Ljungqvist O, Lobo DN, Rockall TA, Schricker
T and Carli F: Enhanced recovery after surgery (ERAS) for
gastrointestinal surgery, part 1: Pathophysiological
considerations. Acta Anaesthesiol Scand. 59:1212–1231. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Labgaa I, Joliat GR, Kefleyesus A,
Mantziari S, Schäfer M, Demartines N and Hübner M: Is postoperative
decrease of serum albumin an early predictor of complications after
major abdominal surgery? A prospective cohort study in a European
centre. BMJ Open. 7:e0139662017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bisch S, Nelson G and Altman A: Impact of
nutrition on enhanced recovery after surgery (ERAS) in gynecologic
oncology. Nutrients. 11:10882019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Barber EL, Boggess JF, Van Le L, Kim KH,
Bae-Jump VL, Brewster WR, Soper JT and Gehrig PA: Association of
preoperative thrombocytosis and leukocytosis with postoperative
morbidity and mortality among patients with ovarian cancer. Obstet
Gynecol. 126:1191–1197. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lomnytska M, Karlsson E, Jonsdottir B,
Lejon AM, Stålberg K, Poromaa IS, Silins I and Graf W: Peritoneal
cancer index predicts severe complications after ovarian cancer
surgery. Eur J Surg Oncol. 47:2915–2924. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cham S, Chen L, St Clair CM, Hou JY,
Tergas AI, Melamed A, Ananth CV, Neugut AI, Hershman DL and Wright
JD: Development and validation of a risk-calculator for adverse
perioperative outcomes for women with ovarian cancer. Am J Obstet
Gynecol. 220:571.e1–571.e8. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ataseven B, du Bois A, Reinthaller A,
Traut A, Heitz F, Aust S, Prader S, Polterauer S, Harter P and
Grimm C: Pre-operative serum albumin is associated with
post-operative complication rate and overall survival in patients
with epithelial ovarian cancer undergoing cytoreductive surgery.
Gynecol Oncol. 138:560–565. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ge LN and Wang F: Prognostic significance
of preoperative serum albumin in epithelial ovarian cancer
patients: A systematic review and dose-response meta-analysis of
observational studies. Cancer Manag Res. 10:815–825. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ayhan A, Günakan E, Alyazıcı İ, Haberal N,
Altundağ Ö and Dursun P: The preoperative albumin level is an
independent prognostic factor for optimally debulked epithelial
ovarian cancer. Arch Gynecol Obstet. 296:989–995. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Asher V, Lee J and Bali A: Preoperative
serum albumin is an independent prognostic predictor of survival in
ovarian cancer. Med Oncol. 29:2005–2009. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Coosemans AN, Baert T, D'Heygere V,
Wouters R, DE Laet L, VAN Hoylandt A, Thirion G, Ceusters J, Laenen
A, Vandecaveye V and Vergote I: Increased immunosuppression is
related to increased amounts of ascites and inferior prognosis in
ovarian cancer. Anticancer Res. 39:5953–5962. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Szender JB, Emmons T, Belliotti S, Dickson
D, Khan A, Morrell K, Khan ANMN, Singel KL, Mayor PC, Moysich KB,
et al: Impact of ascites volume on clinical outcomes in ovarian
cancer: A cohort study. Gynecol Oncol. 146:491–497. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Günakan E, Tohma YA, Tunç M, Akıllı H,
Şahin H and Ayhan A: Factors associated with surgical morbidity of
primary debulking in epithelial ovarian cancer. Obstet Gynecol Sci.
63:64–71. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bartl T, Schwameis R, Stift A,
Bachleitner-Hofmann T, Reinthaller A, Grimm C and Polterauer S:
Predictive and prognostic implication of bowel resections during
primary cytoreductive surgery in advanced epithelial ovarian
cancer. Int J Gynecol Cancer. 28:1664–1671. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Charnley RM: Effect of specialist
decision-making on treatment strategies for colorectal liver
metastases. Br J Surg. 2012.99:1263–1269. Br J Surg. 99:1269–1270.
2012. View Article : Google Scholar
|
|
50
|
Eng OS, Raoof M, Blakely AM, Yu X, Lee SJ,
Han ES, Wakabayashi MT, Yuh B, Lee B and Dellinger TH: A
collaborative surgical approach to upper and lower abdominal
cytoreductive surgery in ovarian cancer. J Surg Oncol. 118:121–126.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Jones RP, Vauthey JN, Adam R, Rees M,
Berry D, Jackson R, Grimes N, Fenwick SW, Poston GJ and Malik HZ:
Effect of specialist decision-making on treatment strategies for
colorectal liver metastases. Br J Surg. 99:1263–1269. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Aletti GD, Long HJ, Podratz KC and Cliby
WA: Is time to chemotherapy a determinant of prognosis in
advanced-stage ovarian cancer? Gynecol Oncol. 104:212–216. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Timmermans M, van der Aa MA, Lalisang RI,
Witteveen PO, Van de Vijver KK, Kruitwagen RF and Sonke GS:
Interval between debulking surgery and adjuvant chemotherapy is
associated with overall survival in patients with advanced ovarian
cancer. Gynecol Oncol. 150:446–450. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Alexander M, Beattie-Manning R, Blum R,
Byrne J, Hornby C, Kearny C, Love N, McGlashan J, McKiernan S,
Milar JL, et al: Guidelines for timely initiation of chemotherapy:
A proposed framework for access to medical oncology and haematology
cancer clinics and chemotherapy services. Intern Med J. 46:964–969.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wijk L, Udumyan R, Pache B, Altman AD,
Williams LL, Elias KM, McGee J, Wells T, Gramlich L, Holcomb K, et
al: International validation of enhanced recovery after surgery
society guidelines on enhanced recovery for gynecologic surgery. Am
J Obstet Gynecol. 221:237.e1–237.e11. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kay AH, Venn M, Urban R, Gray HJ and Goff
B: Postoperative narcotic use in patients with ovarian cancer on an
enhanced recovery after surgery (ERAS) pathway. Gynecol Oncol.
156:624–628. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Dowdy SC: Enhanced recovery after surgery
for ovarian cancer. Clin Adv Hematol Oncol. 17:217–219.
2019.PubMed/NCBI
|
|
58
|
Damania R and Cocieru A: Impact of
enhanced recovery after surgery protocols on postoperative
morbidity and mortality in patients undergoing routine hepatectomy:
Review of the current evidence. Ann Transl Med. 5:3412017.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Nelson G, Bakkum-Gamez J, Kalogera E,
Glaser G, Altman A, Meyer LA, Taylor JS, Iniesta M, Lasala J, Mena
G, et al: Guidelines for perioperative care in
gynecologic/oncology: Enhanced recovery after surgery (ERAS)
society recommendations-2019 update. Int J Gynecol Cancer.
29:651–668. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lindemann K, Kok PS, Stockler M, Jaaback K
and Brand A: Enhanced recovery after surgery for advanced ovarian
cancer: A systematic review of interventions trialed. Int J Gynecol
Cancer. 27:1274–1282. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Goulet D, Danilack V and Matteson KA:
Enhanced recovery pathways for improving outcomes after minimally
invasive gynecologic oncology surgery. Obstet Gynecol. 129:2072017.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Chapman JS, Roddy E, Ueda S, Brooks R,
Chen LL and Chen LM: Enhanced recovery pathways for improving
outcomes after minimally invasive gynecologic oncology surgery.
Obstet Gynecol. 128:138–144. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Carter J: Enhanced recovery in gynecologic
surgery. Obstet Gynecol. 122:13052013. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kalogera E, Bakkum-Gamez JN, Jankowski CJ,
Trabuco E, Lovely JK, Dhanorker S, Grubbs PL, Weaver AL, Haas LR,
Borah BJ, et al: Enhanced recovery in gynecologic surgery. Obstet
Gynecol. 122:319–328. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Rosa DD, Clamp A, Mullamitha S, Ton NC,
Lau S, Byrd L, Clayton R, Slade RJ, Kitchener HC, Shanks JH, et al:
The interval from surgery to chemotherapy in the treatment of
advanced epithelial ovarian carcinoma. Eur J Surg Oncol.
32:588–591. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Cliby W, Dowdy S, Feitoza SS, Gostout BS
and Podratz KC: Diaphragm resection for ovarian cancer: Technique
and short-term complications. Gynecol Oncol. 94:655–660. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Ye S, He T, Liang S, Chen X, Wu X, Yang H
and Xiang L: Diaphragmatic surgery and related complications in
primary cytoreduction for advanced ovarian, tubal, and peritoneal
carcinoma. BMC Cancer. 17:3172017. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Filippa N, Carricajo A, Grattard F, Fascia
P, El Sayed F, Defilippis JP, Berthelot P and Aubert G: Outbreak of
multidrug-resistant Klebsiella pneumoniae carrying qnrB1 and
blaCTX-M15 in a French intensive care unit. Ann Intensive Care.
3:182013. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Rao SSC, Tan G, Abdulla H, Yu S, Larion S
and Leelasinjaroen P: Does colectomy predispose to small intestinal
bacterial (SIBO) and fungal overgrowth (SIFO)? Clin Transl
Gastroenterol. 9:1462018. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Shichinohe T, Sasaki T, Kitashiro S,
Morita T, Ono K, Senmaru N, Ikeda J, Kojima T, Kyogoku N, Yamada H,
et al: Impact of elemental diet on early recovery after
laparoscopic colectomy: Findings of a randomized controlled trial.
Surg Today. 47:166–173. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Garcia-Granero A, Frasson M, Flor-Lorente
B, Blanco F, Puga R, Carratalá A and Garcia-Granero E:
Procalcitonin and C-reactive protein as early predictors of
anastomotic leak in colorectal surgery: A prospective observational
study. Dis Colon Rectum. 56:475–483. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Son JH, Kong TW, Paek J, Chang SJ and Ryu
HS: Perioperative outcomes of extensive bowel resection during
cytoreductive surgery in patients with advanced ovarian cancer. J
Surg Oncol. 119:1011–1015. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Spetzler VN, Schepers M, Pinnschmidt HO,
Fischer L, Nashan B and Li J: The incidence and severity of
post-hepatectomy bile leaks is affected by surgical indications,
preoperative chemotherapy, and surgical procedures. Hepatobiliary
Surg Nutr. 8:101–110. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Nashan B and Citterio F: Wound healing
complications and the use of mammalian target of rapamycin
inhibitors in kidney transplantation: A critical review of the
literature. Transplantation. 94:547–561. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Sánchez-Iglesias JL, Gómez-Hidalgo NR,
Pérez-Benavente A, Carbonell-Socias M, Manrique-Muñoz S, Serrano
MP, Gutiérrez-Barceló P, Bradbury M, Nelson G and Gil-Moreno A:
Importance of enhanced recovery after surgery (ERAS) protocol
compliance for length of stay in ovarian cancer surgery. Ann Surg
Oncol. 28:8979–8986. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Reuter S, Woelber L, Trepte CC, Perez D,
Zapf A, Cevirme S, Mueller V, Schmalfeldt B and Jaeger A: The
impact of enhanced recovery after surgery (ERAS) pathways with
regard to perioperative outcome in patients with ovarian cancer.
Arch Gynecol Obstet. Dec 27–2021.(Epub ahead of print). View Article : Google Scholar
|
|
77
|
Agarwal R, Rajanbabu A, Nitu PV, Goel G,
Madhusudanan L and Unnikrishnan UG: A prospective study evaluating
the impact of implementing the ERAS protocol on patients undergoing
surgery for advanced ovarian cancer. Int J Gynecol Cancer.
29:605–612. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Meyer LA, Shi Q, Lasala J, Iniesta MD, Lin
HK, Nick AM, Williams L, Sun C, Wang XS, Lu KH and Ramirez PT:
Comparison of patient reported symptom burden on an enhanced
recovery after surgery (ERAS) care pathway in patients with ovarian
cancer undergoing primary vs interval tumor reductive surgery.
Gynecol Oncol. 152:501–508. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Tankou JI, Foley O, Falzone M,
Kalyanaraman R and Elias KM: Enhanced recovery after surgery
protocols improve time to return to intended oncology treatment
following interval cytoreductive surgery for advanced gynecologic
cancers. Int J Gynecol Cancer. 31:1145–1153. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Duzgun O: Evaluation of enhanced recovery
after following a surgical protocol for cytoreductive surgery and
hyperthermic intraperitoneal chemotherapy for peritoneal
carcinomatosis. Med Arch. 73:331–337. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Noh JJ, Kim MS and Lee YY: The
implementation of enhanced recovery after surgery protocols in
ovarian malignancy surgery. Gland Surg. 10:1182–1194. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Sánchez-Iglesias JL, Carbonell-Socias M,
Pérez-Benavente MA, Monreal Clua S, Manrique-Muñoz S, García Gorriz
M, Burgos-Peláez R, Segurola Gurrutxaga H, Pamies Serrano M, Pilar
Gutiérrez-Barceló MD, et al: PROFAST: A randomised trial
implementing enhanced recovery after surgery for highcomplexity
advanced ovarian cancer surgery. Eur J Cancer. 136:149–158. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Bisch SP, Kooy J, Glaze S, Cameron A, Chu
P, Ghatage P, Nation J, Nelson G and Fitzmaurice GM: Impact of
transversus abdominis plane blocks versus non-steroidal
anti-inflammatory on post-operative opioid use in ERAS ovarian
cancer surgery. Int J Gynecol Cancer. 29:1372–1376. 2019.
View Article : Google Scholar : PubMed/NCBI
|