Introduction
Head and neck squamous cell carcinoma (HNSCC) is the
sixth most common cancer worldwide and by far the most common
malignancy of the upper aerodigestive tract (1). The main risk factors are alcohol and
tobacco abuse which act synergistically (2,3).
Despite the development of new treatment strategies in the last
decades, the median five-year survival rate for HNSCC remains
around 50%. Since, in about 90% of cases, the tumour overexpresses
the epidermal growth factor receptor (EGFR), it has become a region
of interest for novel therapy approaches (4).
EGFR, also known as HER1, together with HER2, HER3
and HER4, belongs to the group of receptor tyrosine kinases (RTK).
Receptor activation is initiated by ligand binding. This leads to
an autophosphorylation of the RTK via homodimerization with a
similar receptor or heterodimerization with other RTKs. HER3 is
unique in that it does not have sufficient autophosphorylation
ability. Therefore, it must be activated by heterodimerization with
other RTKs. In downstream signal transduction, the PI3K/AKT/mTOR
and the RAS/RAF/MEK/ERK pathways are of particular relevance. Each
RTK can be inhibited in its phosphorylation by tyrosine kinase
inhibitors (TKI) (5). In this
study, we used lapatinib and afatinib. Lapatinib only inhibits HER1
and HER2, while afatinib inhibits each of the four receptors HER
1–4.
A well-known example of successful TKI treatment is
chronic myeloid leukaemia (CML). Imatinib was the first TKI against
BCR-ABL fusion protein, and it became known as a breakthrough in
targeted therapy (6). TKIs were
then tried in many tumour entities with varying degrees of success.
Afatinib is approved for the treatment of locally advanced or
metastatic NSLC and lapatinib for the treatment of HER2-positive
breast cancer (7,8).
RTK-directed therapies have hardly any clinical
relevance in the therapy of HNSCC, except for the monoclonal EGFR
antibody cetuximab (9). Presently,
there is insufficient evidence supporting the use of TKI, such as
lapatinib or afatinib, in the therapy of HNSCC. Therefore, they are
in most cases used for clinical trials (10,11).
Preclinical studies have already produced promising
results for some TKIs, including lapatinib and afatinib; however,
in clinical studies, lapatinib has shown no additional benefit
compared to established therapy concepts (12,13).
Neither have mono kinase inhibitors such as Erlotinib or Gefitinib
been able to confirm the preclinical results (14), while more promising results were
obtained for afatinib. A phase 3 trial identified a subpopulation
of patients (p16-negative, EGFR-amplified, HER3-low, PTEN-high) who
could benefit from afatinib treatment (15). The identification of such
subpopulations and the investigation of resistance mechanisms is
essential for the application of targeted therapies. Various
resistance mechanisms are discussed as reasons for the poor
response of TKIs in HNSCC. Low mutation rates of RTKs in HNSCC must
be considered as well as receptor-independent activation of
downstream signalling pathways (14,16).
3D cell culture is becoming increasingly popular in
examining resistance mechanisms. The idea is to create a better
imitation of the in vivo-conditions through more cell-cell
interactions and different growth behaviours. There are already
studies showing a correlation between treatment response under 3D
cell culture and clinical outcome (17). Furthermore, 3D cell culture can be
set up with hypoxic areas as well as coculture with stromal cells
(18).
The HNSCC is supposed to include the presence of
cancer stem cells (CSC), which have increased drug resistance and
are capable of regenerating tumour tissue after chemotherapy.
Prince et al were the first to detect CD44+ cells
as a population with stem cell-typical properties in HNSCC
(19). Since then, there has been
increasing interest in defining this subpopulation of cells. More
additional markers, such as ALDH1A1, have been found, but an exact
definition of CSC has yet to be determined (20,21),
and 3D cell culture as a link between conventional 2D culture and
xenograft studies could be a key factor here (22).
There is evidence that 3D cell culture can change
the response and signal transduction of tumour cell lines (23–25).
We therefore investigated the response behaviour of TKIs in HNSCC
using 3D cell culture. Our focus was to compare 2D and 3D cell
cultures in terms of their response behaviour to lapatinib and
afatinib. Additionally, we examined the underlying signal
transduction.
Materials and methods
Cell culture and drug treatment
The cell lines UM-SCC-11B (11B), UM-SCC-22B (22B)
and UM-SCC-74A (74A) were obtained from Dr T.E. Carey (University
of Michigan, Ann Arbor, MI, USA). The cell line UD-SCC-1 was
obtained from Professor Wagenmann (University of Düsseldorf,
Germany). These lines originated from squamous cell carcinomas
(SCC) of the larynx (11B), the hypopharynx (22B), the tongue (74)
and the oropharynx (UD01). STR profiling was performed to test the
authenticity of the cell lines. The cells were cultivated in
Dulbecco's modified Eagle's medium (DMEM) (Thermo Fisher
Scientific, Inc.) supplemented with 10% foetal calf serum (FCS) and
antibiotics (Gibco, Life Technologies). 11B and 22B were chosen for
3D cell culture for their ability to form stable and viable
spheroids when cultivated in low attachment plates.
For 3D cell culture, the cells were suspended at a
density of 2.5×105 cells/ml and transferred into 96-well
Nunclon Sphera-Treated U-shaped-bottom Microplates (Thermo Fisher
Scientific, Inc.), so every spheroid contained 50.000 cells at the
beginning of cultivation. Spheroids were incubated for seven days
prior to treatment, with fresh mediums replaced every 48 h.
For drug treatment, we used afatinib and lapatinib
(Selleck Chemicals) diluted in dimethyl sulfoxide (DMSO) and
corresponding DSMO concentrations as the negative control. These
were added to the cells in 2D or 3D cultures with concentrations
ranging from 1 to 50 µM, depending on the experiment. The cells
were incubated with the TKIs for 48 h.
Alamar blue viability assay
Viability testing on 2D cell culture was performed
via Alamar Blue viability assay (Thermo Fisher Scientific, Inc.).
After treatment with TKI, 10 µl Alamar Blue solution was added to
each well, according to the manufacturer's instructions. Absorption
was measured after 3 h using an Infinite M Plex Microplate Reader
(Tecan Trading AG).
3D viability assay
The spheroids were transferred in 96 well white
clear bottom plates (Thermo Fisher Scientific, Inc.) and lysed with
100 µl CellTiter-Glo viability assay solution, according to the
manufacturer's instructions. Luminescence was measured after 30 min
using an Infinite M Plex Microplate Reader (Tecan Trading AG).
Western blotting
Cells were washed with PBS and lysed with RIPA
Buffer (Sigma-Aldrich; Merck KGaA). Protein concentration of the
cell lysates was calculated by DC Protein assay (Bio-Rad
Laboratories Inc.) according to the manufacturer's instructions,
and each probe was diluted to samples of 10 µg of protein. Samples
were mixed with 3.75 µl loading dye and separated by SDS-page. The
separated proteins were transferred to a polyvinylidene fluoride
membrane and then blocked with a TBST buffer and 5% non-fat dry
milk. Incubation with primary antibodies took place overnight. The
primary antibodies used were EGFR, pEGFR, HER3, pHER3, AKT, pAKT,
ERK, pERK and GAPDH. After three washing steps with the TBST
buffer, the membrane was incubated with a secondary antibody
followed by three additional washing steps. For luminescence
detection, the membrane was coated with 1 ml luminol and 1 ml
peroxide solution and then analysed with an IBright FL 1000
(Invitrogen; Thermo Fisher Scientific, Inc.).
Microscopy
For visual analysis of viability in 3D culture,
cells were dual-stained with ATP-Red dye (Sigma-Aldrich; Merck
KGaA) as a marker for viable cells and Sytox-Green dye (Thermo
Fisher Scientific, Inc.) as a marker for apoptotic/necrotic cells.
For the staining of cells under 2D culture, we used Calcein AM as a
marker for viable cells and Ethidiumhomodimer-1 for
apoptotic/necrotic cells (Thermo Fisher Scientific, Inc.). After
staining, the cells were cultured in 2D or 3D conditions and
treated with lapatinib or afatinib, as previously described.
Pictures were taken via light microscopy (Axiophot, Fa. Zeiss).
Statistical analysis
Each experiment was repeated three times (n=3) with
duplicates, and mean values as well as the standard deviations were
calculated. Viability was calculated in comparison to the
corresponding control group. IC50-doses were calculated
from the resulting dose-response curves using Graph Pad Prism.
Resistance was considered as an IC50-dose >50 µM.
Differences between multiple groups were analysed using one-way
ANOVA followed by Tukey's post hoc test. A probability value of
P≤0.05 was considered significant.
Results
HNSCC cell lines have a high response
to TKI therapy in 2D culture
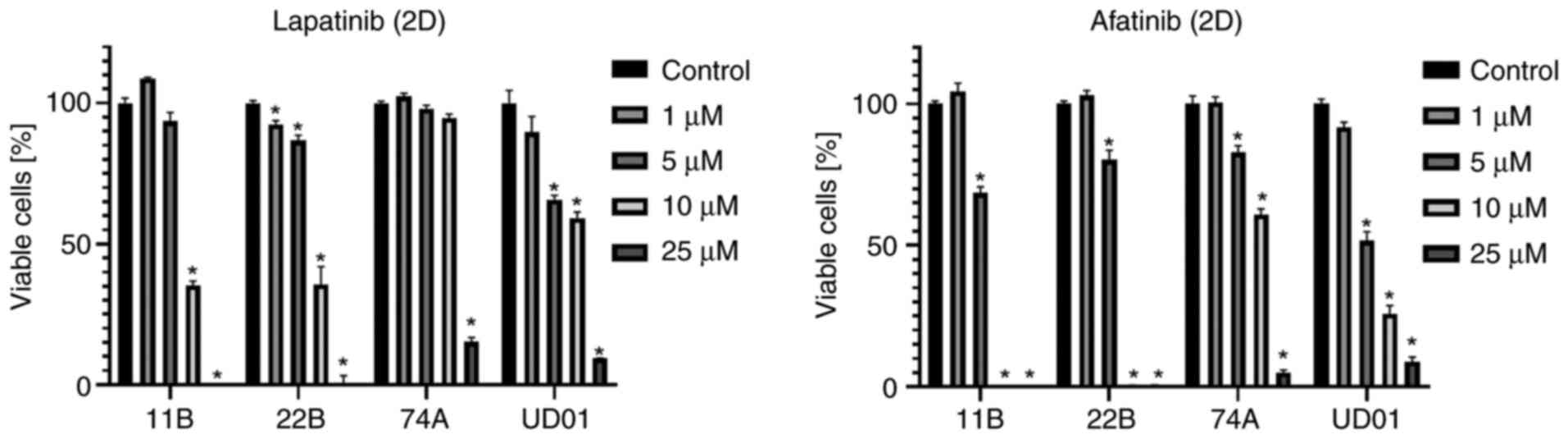
All the used cell lines responded very well to TKI
therapy in 2D cell cultures in terms of viability. Treatment with 5
µM afatinib resulted in a significant decrease of viability in each
of the four cell lines. Under treatment with 25 µM lapatinib or
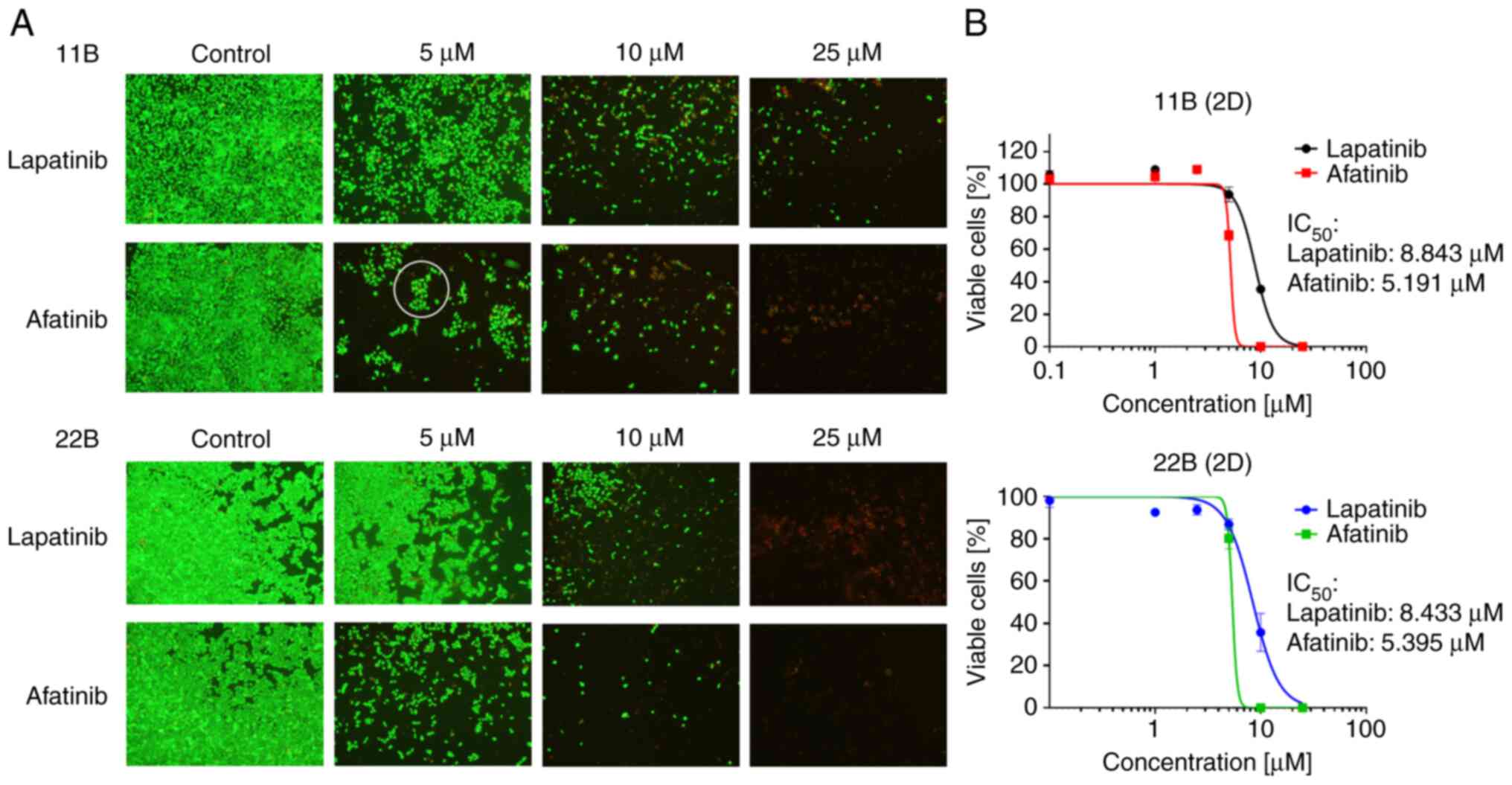
afatinib, little-to-no viable cells could be detected (Fig. 1). The evaluation of IC50
values of 11B and 22B resulted in 8.843 µM (11B) and 8.433 µM (22B)
for lapatinib. The IC50 values for afatinib were 5.191
µM (11B) and 5.395 µM (22B). In addition, the cells were
photographed under viability staining and treatment with TKI.
Concentrations were chosen close to their IC50 values
(Fig. 2). With increasing
concentration, the cell density and viability decreased. Under
treatment with 5 µM afatinib, 11B cells formed up clusters. In
areas with viable cell colonies, those cells seemed to be attached
to each other.
3D cell culture alters treatment
response to lapatinib in 11B cells
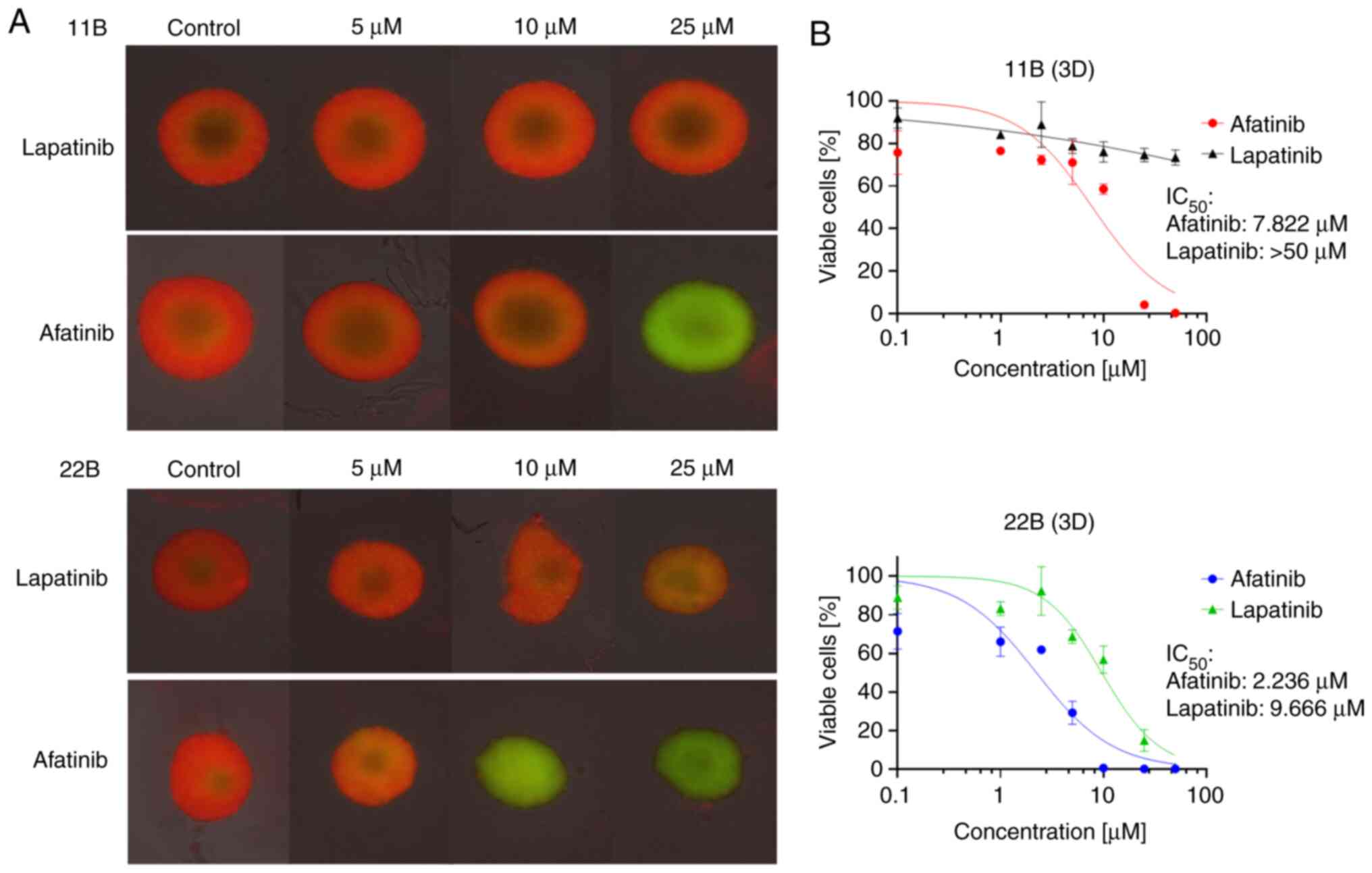
In 3D cell culture, we were able to determine a
strong resistance of 11B to lapatinib (IC50 >50 µM).
However, there was a strong response to afatinib
(IC50=7.822 µM) comparable to the IC50 seen
in 2D culture. Cell line 22B showed no resistance to lapatinib
(IC50=9.666 µM) or afatinib (IC50=2.2362 µM)
in 3D cell culture either. These results were re-evaluated using
microscopy (Fig. 3). The
lapatinib-resistant cell line 11B showed a homogeneous distribution
pattern of viable cells in the spheroid. Even with 25 µM lapatinib,
there was hardly any difference compared to the control. A very
homogeneous distribution pattern of apoptotic/necrotic cells with
hardly viable cells was found in spheroids of both cell lines when
treated with 25 µM afatinib.
11B shows higher phosphorylation in 3D
cell culture
Due to the limited response of 11B to lapatinib that
was explicitly present in the 3D culture, both cultures were
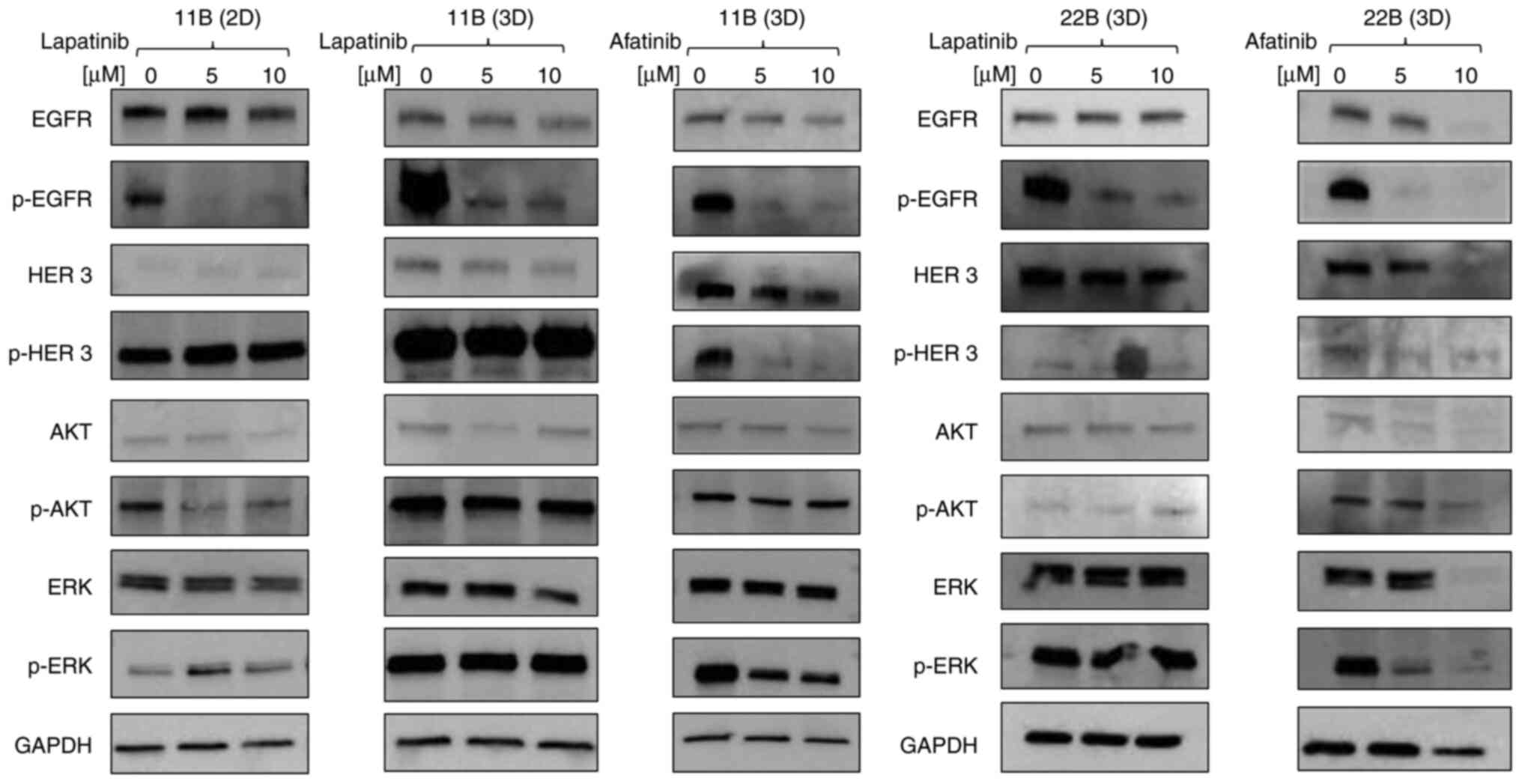
analysed via western blot after treatment with lapatinib (Fig. 4). A comparison of the two culture
models in the western blot showed a constantly high EGFR expression
in both 2D and 3D cultures. In 3D cell culture, the phosphorylation
of EGFR was stronger. This was also observed with HER3 and the
downstream signalling proteins AKT and ERK. The phosphorylation of
EGFR decreased after treatment with lapatinib, whereas the
phosphorylation of HER3 remained consistently high independent of
the TKI concentration.
Resistant cell line 11B has higher
expression of HER3
To compare the two cell lines regarding their
different behaviour in 3D culture, a western blot analysis of cell
lines 11B and 22B treated with afatinib and lapatinib was carried
out in 3D culture (Fig. 4). Both
lines showed similar expressions of EGFR and a reduction in EGFR
phosphorylation under treatment with afatinib or lapatinib. As
previously mentioned, a constantly high HER3 phosphorylation could
be detected in line 11B in 3D culture. This phosphorylation was
suppressed under treatment with afatinib. In line 22B, hardly any
pHER3 expression was detectable even under control conditions.
A similar result was observed with the downstream
signalling protein AKT. Neither lapatinib nor afatinib influenced
phosphorylation in line 11B. In contrast, pAKT was hardly
detectable for line 22B. A strong ERK expression was detected in
both cell lines. Lapatinib treatment had no effect on either the
expression of panERK nor of pERK. On the other hand, afatinib
treatment showed a slight decrease in ERK phosphorylation in 11B
and a complete blockage of phosphorylation in line 22B.
Discussion
We aim to use 3D cell culture for generating a
deeper understanding of cell-cell interactions and resistance
behaviour of tumour cells. The 3D cell culture appears to be more
suitable for this issue than the conventional 2D cell culture for
several reasons: a changed growth rate, better imitation of hypoxic
areas, and a changed drug response (26–28).
There are several publications on comparisons of drug response as
well as protein and gene expression between 2D and 3D cell cultures
(29,30). In the case of HNSCC, hardly any
studies on the response to TKI in 3D have been performed. We
therefore addressed the question of why, despite promising
preclinical results, there has so far been little clinical success
with TKI in the treatment of HNSCC. In the present work, we were
able to observe an altered treatment response to TKI using a 3D
cell culture model and to provide explanatory approaches for this
phenomenon.
Cell-cell interaction is essential for
survival
Preliminary tests have shown that all our HNSCC cell
lines show a very similar and strong response to TKI in 2D cell
culture. We therefore selected two cell lines that were reliably
capable of spheroid formation to examine them more closely in 3D.
In the microscopic examination of the 2D cells, we observed a
colony formation of the 11B cells, especially under treatment with
5 µM afatinib. This seems to represent a survival benefit for the
cell when it is in contact with other cells. The reason for this
could be altered signal transduction via growth factors, which
would also be in line with the higher IC50 doses that
tend to be observed in 3D cell cultures. In our experiments, we
observed some sort of resistance in cell line 11B, with an
IC50 dose >50 µM for lapatinib. It seems that the
cells have also changed their characteristics because of the
changed growth conditions. Even if a wide variety of mechanisms are
possible for this, it was likely that changes in the EGFR cascade
have occurred. This presumption was confirmed in the western blot
analysis. Both EGFR and HER3, as well as their downstream signal
molecules AKT and ERK, had higher phosphorylation under 3D cell
culture than under conventional 2D cell culture.
HER3 as a possible resistance inductor
The observed strong phosphorylation of the HER3
receptor in cell line 11B offers a plausible explanation for the
observed resistance to lapatinib, while maintaining its sensitivity
to afatinib. HER3 is already a discussed option for overcoming
resistance to EGFR-directed therapy in other tumour entities
(31,32). For HNSCC, there are preclinical
approaches to circumvent cetuximab resistance using HER3 targeting
(33). A frequently observed
problem of cetuximab therapy is acquired resistance, which
describes a therapy failure that occurs only after some time under
medication. It is assumed that an upregulation of other tyrosine
kinases of the EGFR family contributes to this behaviour.
Therefore, it seems that a therapy strategy aimed at the entire
EGFR family, as is already being used in NSCLC, seems promising
(34). We therefore assume that
successful clinical usage of TKI depends on how many opportunities
there are left for the development of resistance. From a
preclinical point of view, this explains why afatinib could be
superior to therapy with lapatinib, erlotinib or gefitinib.
The data from clinical studies on TKIs do not yet
justify their use in HNSCC (35).
Afatinib is still the most promising substance among the TKIs. A
phase 3 study by Guo et al evaluated the progression-free
survival of patients undergoing second-line therapy for HNSCC. They
detected significantly longer progression-free survival for
afatinib in comparison to methotrexate (36). In this regard, the results of our
study are in line with the clinical data.
A limiting factor in our study is certainly the
small number of lines examined. Therefore, even more cell lines or,
ideally, patient-derived spheroids would have to be examined to
confirm that the increased activation of pHER3 is a potential
resistance mechanism in 3D cell culture. However, the increased
phosphorylation activity observed in our cell line is a good
example of the changed signal transduction processes under
different culture conditions.
Altered conditions result in altered
reactions
The present study aims to address the leading
question, why tumour cells tend to have a limited treatment
response under 3D cell culture. One possibility would be that the
increased contact between the cells and the overall higher cell
density would also lead to increased communication between the
cells. We were also able to observe that the concentration of
receptors and intracellular signalling molecules of the EGFR family
tends to be higher in 3D cell cultures than in normal 2D cell
cultures. Whether this occurs through increased cell-to-cell
communication or through intracellular mechanisms would have to be
investigated more closely.
Another explanation for a higher rate of surviving
cells in the spheroids is based on the life cycle of the cancer
cells. Since mitosis is a very energy-consuming process, dormant
cells can often be found in hypoxic and nutrient-undersupplied
areas. These cells are less susceptible to cytostatic treatment and
therefore show higher resistance behaviour (37). In the 3D cell culture model, there
are significantly more dormant cells due to the different nutrient
and oxygen content in the spheroid.
The model of the cancer stem cell niche assumes that
such resistant cells are arranged in sub-areas. This hypothesis is
particularly widespread in HNSCC research. Compared to regular
tumour cells, tumour stem cells have an increased potential for
regeneration and metastasis. However, this stem cell potential
seems to be dependent on the environment of the stem cell,
including supportive tumour cells and tumour stromal cells
(38). It remains to be seen
whether these stem cells occur more frequently in 3D cell
cultures.
The most likely cause of changed resistance
behaviour in 3D cell cultures is a combination of various effects.
Therefore, it is even more important to establish a 3D cell culture
that gives a good representation of the in-vivo conditions.
Our long-term goal is to develop a 3D cell culture using
bioprinting that includes patient-derived cells and supportive
cells (39). An optimized 3D cell
culture model would then provide the basis for further resistance
testing of various drug groups. Possible new resistances that arise
from the different culture techniques could be examined by means of
genetic analysis. One possibility would be the comparative RNA
sequencing analysis between 2D and 3D culture or between different
forms of 3D culture.
Even though 3D cell culture is associated with
longer cultivation times and higher material costs, we believe that
these costs are reasonable if the method is well established and
used efficiently.
In conclusion, we found a HNSCC cell line (11B) with
an altered treatment response to lapatinib under 3D cell culture.
In addition, we found an upregulation of HER3 phosphorylation,
which is a plausible explanation for a resistance mechanism.
Limited therapy response compared to conventional cell culture is a
frequently observed phenomenon in clinical trials. We see this as
an indication that the 3D cell culture could be superior to the
conventional 2D cell culture for the investigation of drug
resistance development in cancer therapy.
Acknowledgements
The authors would like to thank Ms. Petra Prohaska
(Department of Otorhinolaryngology Head and Neck Surgery,
University Medical Centre Mannheim, Mannheim, Germany) for their
technical support.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JH wrote the initial manuscript. AL and JK conceived
and designed the present study. Experiments and data collection
were completed by JH, YJ and ET. AA and NR interpreted the results
and helped with the writing of the manuscript. JH and JK confirm
the authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sturgis EM, Wei Q and Spitz MR:
Descriptive epidemiology and risk factors for head and neck cancer.
Semin Oncol. 31:726–733. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Blot WJ, McLaughlin JK, Winn DM, Austin
DF, Greenberg RS, Preston-Martin S, Bernstein L, Schoenberg JB,
Stemhagen A and Fraumeni JF Jr: Smoking and drinking in relation to
oral and pharyngeal cancer. Cancer Res. 48:3282–3287.
1988.PubMed/NCBI
|
|
4
|
Grandis JR and Tweardy DJ: Elevated levels
of transforming growth factor alpha and epidermal growth factor
receptor messenger RNA are early markers of carcinogenesis in head
and neck cancer. Cancer Res. 53:3579–3584. 1993.PubMed/NCBI
|
|
5
|
Scaltriti M and Baselga J: The epidermal
growth factor receptor pathway: A model for targeted therapy. Clin
Cancer Res. 12:5268–5272. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Iqbal N and Iqbal N: Imatinib: A
breakthrough of targeted therapy in cancer. Chemother Res Pract.
2014:3570272014.PubMed/NCBI
|
|
7
|
Harvey RD, Adams VR, Beardslee T and
Medina P: Afatinib for the treatment of EGFR mutation-positive
NSCLC: A review of clinical findings. J Oncol Pharm Pract.
26:1461–1474. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu ZQ, Zhang Y, Li N, Liu PJ, Gao L, Gao X
and Tie XJ: Efficacy and safety of lapatinib and trastuzumab for
HER2-positive breast cancer: A systematic review and meta-analysis
of randomised controlled trials. BMJ Open. 7:e0130532017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vermorken JB, Trigo J, Hitt R, Koralewski
P, Diaz-Rubio E, Rolland F, Knecht R, Amellal N, Schueler A and
Baselga J: Open-label, uncontrolled, multicenter phase II study to
evaluate the efficacy and toxicity of cetuximab as a single agent
in patients with recurrent and/or metastatic squamous cell
carcinoma of the head and neck who failed to respond to
platinum-based therapy. J Clin Oncol. 25:2171–2177. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ferrarotto R and Gold KA: Afatinib in the
treatment of head and neck squamous cell carcinoma. Expert Opin
Investig Drugs. 23:135–143. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cohen RB: Current challenges and clinical
investigations of epidermal growth factor receptor (EGFR)- and ErbB
family-targeted agents in the treatment of head and neck squamous
cell carcinoma (HNSCC). Cancer Treat Rev. 40:567–577. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kozakiewicz P and Grzybowska-Szatkowska L:
Application of molecular targeted therapies in the treatment of
head and neck squamous cell carcinoma. Oncol Lett. 15:7497–7505.
2018.PubMed/NCBI
|
|
13
|
Weiss JM, Bagley S, Hwang WT, Bauml J,
Olson JG, Cohen RB, Hayes DN and Langer C: Capecitabine and
lapatinib for the first-line treatment of metastatic/recurrent head
and neck squamous cell carcinoma. Cancer. 122:2350–2355. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Agarwal V, Subash A, Nayar RC and Rao V:
Is EGFR really a therapeutic target in head and neck cancers? J
Surg Oncol. 119:685–686. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cohen EE, Licitra LF, Burtness B, Fayette
J, Gauler T, Clement PM, Grau JJ, Del Campo JM, Mailliez A, Haddad
RI, et al: Biomarkers predict enhanced clinical outcomes with
afatinib versus methotrexate in patients with second-line recurrent
and/or metastatic head and neck cancer. Ann Oncol. 28:2526–2532.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Picon H and Guddati AK: Mechanisms of
resistance in head and neck cancer. Am J Cancer Res. 10:2742–2751.
2020.PubMed/NCBI
|
|
17
|
Shuford S, Lipinski L, Abad A, Smith AM,
Rayner M, O'Donnell L, Stuart J, Mechtler LL, Fabiano AJ, Edenfield
J, et al: Prospective prediction of clinical drug response in
high-grade gliomas using an ex vivo 3D cell culture assay.
Neurooncol Adv. 3:vdab0652021.PubMed/NCBI
|
|
18
|
Costa EC, Moreira AF, de Melo-Diogo D,
Gaspar VM, Carvalho MP and Correia IJ: 3D tumor spheroids: An
overview on the tools and techniques used for their analysis.
Biotechnol Adv. 34:1427–1441. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Prince ME, Sivanandan R, Kaczorowski A,
Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF and Ailles
LE: Identification of a subpopulation of cells with cancer stem
cell properties in head and neck squamous cell carcinoma. Proc Natl
Acad Sci USA. 104:973–978. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Clay MR, Tabor M, Owen JH, Carey TE,
Bradford CR, Wolf GT, Wicha MS and Prince ME: Single-marker
identification of head and neck squamous cell carcinoma cancer stem
cells with aldehyde dehydrogenase. Head Neck. 32:1195–1201. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tahmasebi E, Alikhani M, Yazdanian A,
Yazdanian M, Tebyanian H and Seifalian A: The current markers of
cancer stem cell in oral cancers. Life Sci. 249:1174832020.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yamada KM and Cukierman E: Modeling tissue
morphogenesis and cancer in 3D. Cell. 130:601–610. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ayuso JM, Vitek R, Swick AD, Skala MC,
Wisinski KB, Kimple RJ, Lambert PF and Beebe DJ: Effects of culture
method on response to EGFR therapy in head and neck squamous cell
carcinoma cells. Sci Rep. 9:124802019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chan YH, Lee YC, Hung CY, Yang PJ, Lai PC
and Feng SW: Three-dimensional spheroid culture enhances
multipotent differentiation and stemness capacities of human dental
pulp-derived mesenchymal stem cells by modulating MAPK and NF-kB
signaling pathways. Stem Cell Rev Rep. 17:1810–1826. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee HK, Noh MH, Hong SW, Kim SM, Kim SH,
Kim YS, Broaddus VC and Hur DY: Erlotinib activates different cell
death pathways in EGFR-mutant lung cancer cells grown in 3D versus
2D culture systems. Anticancer Res. 41:1261–1269. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
DelNero P, Lane M, Verbridge SS, Kwee B,
Kermani P, Hempstead B, Stroock A and Fischbach C: 3D culture
broadly regulates tumor cell hypoxia response and angiogenesis via
pro-inflammatory pathways. Biomaterials. 55:110–118. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fontoura JC, Viezzer C, Dos Santos FG,
Ligabue RA, Weinlich R, Puga RD, Antonow D, Severino P and Bonorino
C: Comparison of 2D and 3D cell culture models for cell growth,
gene expression and drug resistance. Mater Sci Eng C Mater Biol
Appl. 107:1102642020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schmidt M, Scholz CJ, Polednik C and
Roller J: Spheroid-based 3-dimensional culture models: Gene
expression and functionality in head and neck cancer. Oncol Rep.
35:2431–2440. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Imamura Y, Mukohara T, Shimono Y,
Funakoshi Y, Chayahara N, Toyoda M, Kiyota N, Takao S, Kono S,
Nakatsura T and Minami H: Comparison of 2D- and 3D-culture models
as drug-testing platforms in breast cancer. Oncol Rep.
33:1837–1843. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mosaad E, Chambers K, Futrega K, Clements
J and Doran MR: Using high throughput microtissue culture to study
the difference in prostate cancer cell behavior and drug response
in 2D and 3D co-cultures. BMC Cancer. 18:5922018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Watanabe S, Yonesaka K, Tanizaki J,
Nonagase Y, Takegawa N, Haratani K, Kawakami H, Hayashi H, Takeda
M, Tsurutani J and Nakagawa K: Targeting of the HER2/HER3 signaling
axis overcomes ligand-mediated resistance to trastuzumab in
HER2-positive breast cancer. Cancer Med. 8:1258–1268. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Temraz S, Mukherji D and Shamseddine A:
Dual targeting of HER3 and EGFR in colorectal tumors might overcome
anti-EGFR resistance. Crit Rev Oncol Hematol. 101:151–157. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang D, Qian G, Zhang H, Magliocca KR,
Nannapaneni S, Amin AR, Rossi M, Patel M, El-Deiry M, Wadsworth JT,
et al: HER3 targeting sensitizes HNSCC to cetuximab by reducing
HER3 activity and HER2/HER3 dimerization: Evidence from cell line
and patient-derived xenograft models. Clin Cancer Res. 23:677–686.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Iida M, Bahrar H, Brand TM, Pearson HE,
Coan JP, Orbuch RA, Flanigan BG, Swick AD, Prabakaran PJ, Lantto J,
et al: Targeting the HER family with Pan-HER effectively overcomes
resistance to cetuximab. Mol Cancer Ther. 15:2175–2186. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
von der Grün J, Rödel F, Brandts C, Fokas
E, Guckenberger M, Rödel C and Balermpas P: Targeted therapies and
immune-checkpoint inhibition in head and neck squamous cell
carcinoma: Where do we stand today and where to go? Cancers
(Basel). 11:4722019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Guo Y, Ahn MJ, Chan A, Wang CH, Kang JH,
Kim SB, Bello M, Arora RS, Zhang Q, He X, et al: Afatinib versus
methotrexate as second-line treatment in Asian patients with
recurrent or metastatic squamous cell carcinoma of the head and
neck progressing on or after platinum-based therapy (LUX-head &
neck 3): an open-label, randomised phase III trial. Ann Oncol.
30:1831–1839. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Phan TG and Croucher PI: The dormant
cancer cell life cycle. Nat Rev Cancer. 20:398–411. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Faber A, Aderhold C, Goessler UR, Hoermann
K, Schultz JD, Umbreit C, Walliczek U and Stern-Straeter J:
Interaction of a CD44+ head and neck squamous cell carcinoma cell
line with a stromal cell-derived factor-1-expressing supportive
niche: An in vitro model. Oncol Lett. 7:82–86. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lammert A, Affolter A, Gvaramia D, Heid J,
Jungbauer F, Scherl C, Tenschert E, Rotter N, Willett N and Kern J:
The tumor stem cell niche of head and neck-point of intersection
with therapeutic potential? Laryngorhinootologie. 100:23–29.
2021.(In German). PubMed/NCBI
|