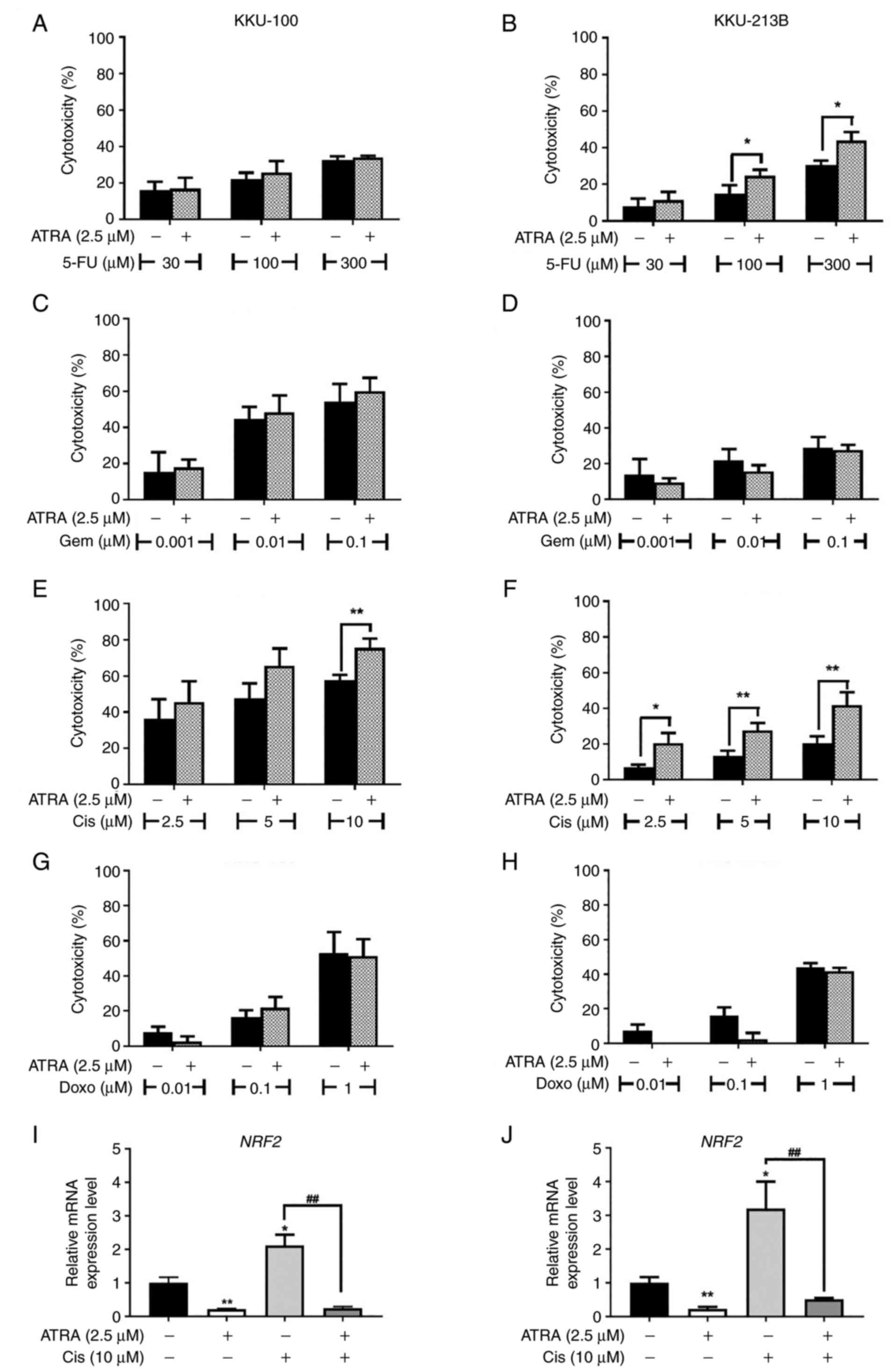
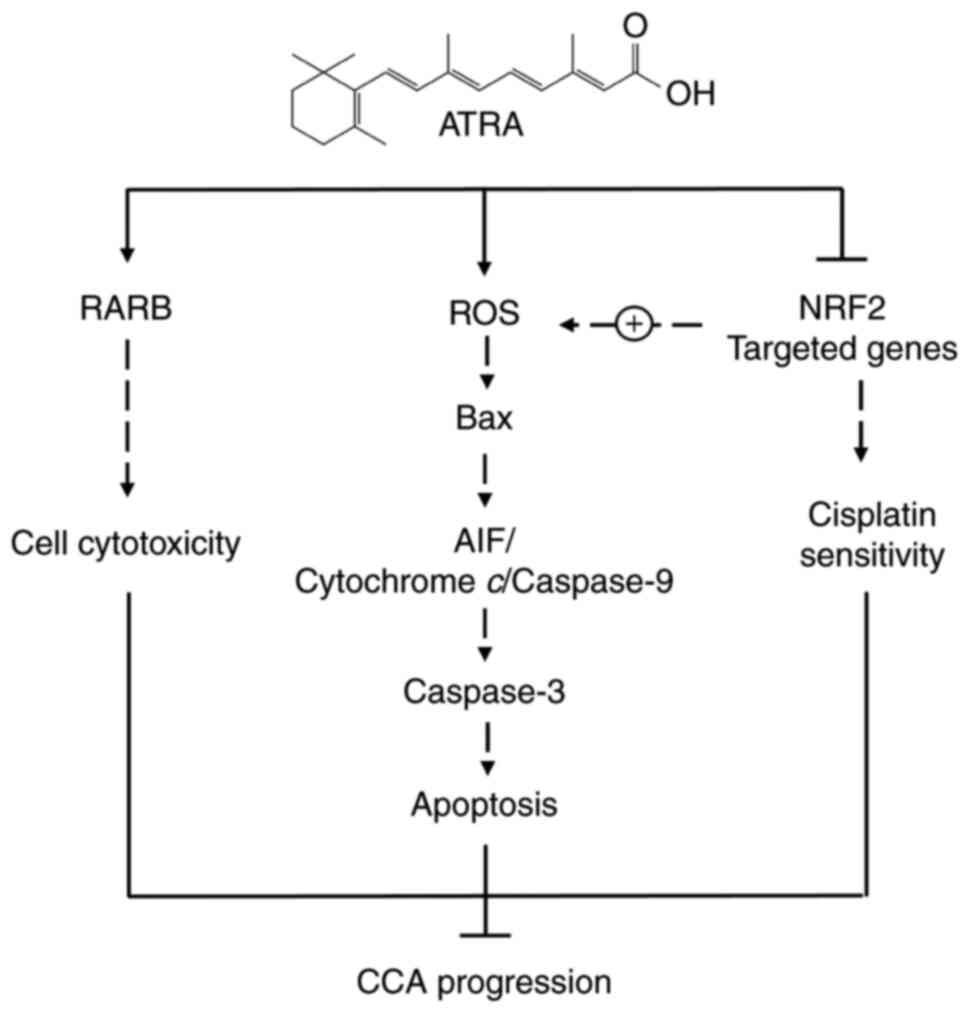
|
1
|
Anderson CD, Pinson CW, Berlin J and Chari
RS: Diagnosis and treatment of cholangiocarcinoma. Oncologist.
9:43–57. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Braconi C and Patel T: Cholangiocarcinoma:
New insights into disease pathogenesis and biology. Infect Dis Clin
North Am. 24871–884. (vii)2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Global Burden of Disease Cancer and
Collaboration, . Fitzmaurice C, Dicker D, Pain A, Hamavid H,
Moradi-Lakeh M, MacIntyre MF, Allen C, Hansen G, Woodbrook R, et
al: The global burden of cancer 2013. JAMA Oncol. 1:505–527. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Banales JM, Cardinale V, Carpino G,
Marzioni M, Andersen JB, Invernizzi P, Lind GE, Folseraas T, Forbes
SJ, Fouassier L, et al: Expert consensus document:
Cholangiocarcinoma: Current knowledge and future perspectives
consensus statement from the European Network for the Study of
Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol.
13:261–280. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Blechacz B: Cholangiocarcinoma: Current
knowledge and new developments. Gut Liver. 11:13–26. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Valle JW, Furuse J, Jitlal M, Beare S,
Mizuno N, Wasan H, Bridgewater J and Okusaka T: Cisplatin and
gemcitabine for advanced biliary tract cancer: A meta-analysis of
two randomised trials. Ann Oncol. 25:391–398. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rhinn M and Dolle P: Retinoic acid
signalling during development. Development. 139:843–858. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Blomhoff R and Blomhoff HK: Overview of
retinoid metabolism and function. J Neurobiol. 66:606–630. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barnard JH, Collings JC, Whiting A,
Przyborski SA and Marder TB: Synthetic retinoids:
Structure-activity relationships. Chemistry. 15:11430–11442. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Idres N, Marill J, Flexor MA and Chabot
GG: Activation of retinoic acid receptor-dependent transcription by
all-trans-retinoic acid metabolites and isomers. J Biol Chem.
277:31491–31498. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tallman MS, Andersen JW, Schiffer CA,
Appelbaum FR, Feusner JH, Ogden A, Shepherd L, Willman C,
Bloomfield CD, Rowe JM and Wiernik PH: All-trans-retinoic acid in
acute promyelocytic leukemia. N Engl J Med. 337:1021–1028. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tosi P, Visani G, Gibellini D, Zauli G,
Ottaviani E, Cenacchi A, Gamberi B, Manfroi S, Marchisio M and Tura
S: All-trans retinoic acid and induction of apoptosis in acute
promyelocytic leukemia cells. Leuk Lymphoma. 14:503–507. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fang J, Chen SJ, Tong JH, Wang ZG, Chen GQ
and Chen Z: Treatment of acute promyelocytic leukemia with ATRA and
As2O3: A model of molecular target-based cancer therapy. Cancer
Biol Ther. 1:614–620. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brigger D, Schlafli AM, Garattini E and
Tschan MP: Activation of RARα induces autophagy in SKBR3 breast
cancer cells and depletion of key autophagy genes enhances ATRA
toxicity. Cell Death Dis. 6:e18612015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cui J, Gong M, He Y, Li Q, He T and Bi Y:
All-trans retinoic acid inhibits proliferation, migration, invasion
and induces differentiation of hepa1-6 cells through reversing EMT
in vitro. Int J Oncol. 48:349–357. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li N, Lu Y, Li D, Zheng X, Lian J, Li S,
Cui H, Zhang L, Sang L, Wang Y, et al: All-trans retinoic acid
suppresses the angiopoietin-Tie2 pathway and inhibits angiogenesis
and metastasis in esophageal squamous cell carcinoma. PLoS One.
12:e01745552017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mei D, Lv B, Chen B, Xiao S, Jiang J, Xie
Y and Jiang L: All-trans retinoic acid suppresses malignant
characteristics of CD133-positive thyroid cancer stem cells and
induces apoptosis. PLoS One. 12:e01828352017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kuroda H, Tachikawa M, Uchida Y, Inoue K,
Ohtsuka H, Ohtsuki S, Unno M and Terasaki T: All-trans retinoic
acid enhances gemcitabine cytotoxicity in human pancreatic cancer
cell line AsPC-1 by up-regulating protein expression of
deoxycytidine kinase. Eur J Pharm Sci. 103:116–121. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Y, Guan DX, Shi J, Gao H, Li JJ,
Zhao JS, Qiu L, Liu J, Li N, Guo WX, et al: All-trans retinoic acid
potentiates the chemotherapeutic effect of cisplatin by inducing
differentiation of tumor initiating cells in liver cancer. J
Hepatol. 59:1255–1263. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yan Y, Li Z, Xu X, Chen C, Wei W, Fan M,
Chen X, Li JJ, Wang Y and Huang J: All-trans retinoic acids induce
differentiation and sensitize a radioresistant breast cancer cells
to chemotherapy. BMC Complement Altern Med. 16:1132016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Najafzadeh N, Mazani M, Abbasi A,
Farassati F and Amani M: Low-dose all-trans retinoic acid enhances
cytotoxicity of cisplatin and 5-fluorouracil on CD44(+) cancer stem
cells. Biomed Pharmacother. 74:243–251. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chung KD, Jeong YI, Chung CW, Kim DH and
Kang DH: Anti-tumor activity of all-trans retinoic
acid-incorporated glycol chitosan nanoparticles against HuCC-T1
human cholangiocarcinoma cells. Int J Pharm. 422:454–461. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ren HY, Chen B, Huang GL, Liu Y and Shen
DY: Upregulation of retinoic acid receptor-beta reverses drug
resistance in cholangiocarcinoma cells by enhancing susceptibility
to apoptosis. Mol Med Rep. 14:3602–3608. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
de Oliveira MR: Vitamin A and retinoids as
mitochondrial toxicants. Oxid Med Cell Longev. 2015:1402672015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Oliveira MR: The neurotoxic effects of
vitamin A and retinoids. An Acad Bras Cienc. 87 (2
Suppl):S1361–S1373. 2015. View Article : Google Scholar
|
|
26
|
Conte da Frota ML Jr, Gomes da Silva E,
Behr GA, Roberto de Oliveira M, Dal-Pizzol F, Klamt F and Moreira
JC: All-trans retinoic acid induces free radical generation and
modulate antioxidant enzyme activities in rat sertoli cells. Mol
Cell Biochem. 285:173–179. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Miyoshi T, Arai T, Yamashita K, Sasada M
and Uchiyama T: NB4 cells treated with all-trans retinoic acid
generate toxic reactive oxygen species that cause endothelial
hyperpermeability. Leuk Res. 34:373–378. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kittiratphatthana N, Kukongviriyapan V,
Prawan A and Senggunprai L: Luteolin induces cholangiocarcinoma
cell apoptosis through the mitochondrial-dependent pathway mediated
by reactive oxygen species. J Pharm Pharmacol. 68:1184–1192. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sompakdee V, Prawan A, Senggunprai L,
Kukongviriyapan U, Samathiwat P, Wandee J and Kukongviriyapan V:
Suppression of Nrf2 confers chemosensitizing effect through
enhanced oxidant-mediated mitochondrial dysfunction. Biomed
Pharmacother. 101:627–634. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Samatiwat P, Prawan A, Senggunprai L,
Kukongviriyapan U and Kukongviriyapan V: Nrf2 inhibition sensitizes
cholangiocarcinoma cells to cytotoxic and antiproliferative
activities of chemotherapeutic agents. Tumour Biol. 37:11495–11507.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang XJ, Hayes JD, Henderson CJ and Wolf
CR: Identification of retinoic acid as an inhibitor of
transcription factor Nrf2 through activation of retinoic acid
receptor alpha. Proc Natl Acad Sci USA. 104:19589–19594. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sripa B, Leungwattanawanit S, Nitta T,
Wongkham C, Bhudhisawasdi V, Puapairoj A, Sripa C and Miwa M:
Establishment and characterization of an opisthorchiasis-associated
cholangiocarcinoma cell line (KKU-100). World J Gastroenterol.
11:3392–3397. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sripa B, Seubwai W, Vaeteewoottacharn K,
Sawanyawisuth K, Silsirivanit A, Kaewkong W, Muisuk K, Dana P,
Phoomak C, Lert-Itthiporn W, et al: Functional and genetic
characterization of three cell lines derived from a single tumor of
an Opisthorchis viverrini-associated cholangiocarcinoma patient.
Hum Cell. 33:695–708. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liang W, Cui J, Zhang K, Xi H, Cai A, Li
J, Gao Y, Hu C, Liu Y, Lu Y, et al: Shikonin induces ROS-based
mitochondria-mediated apoptosis in colon cancer. Oncotarget.
8:109094–109106. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang R, Humphreys I, Sahu RP, Shi Y and
Srivastava SK: In vitro and in vivo induction of apoptosis by
capsaicin in pancreatic cancer cells is mediated through ROS
generation and mitochondrial death pathway. Apoptosis.
13:1465–1478. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang G, Wang Q, Zhou Q, Wang R, Xu M,
Wang H, Wang L, Wilcox CS, Liu R and Lai EY: Protective effect of
tempol on acute kidney injury through PI3K/Akt/Nrf2 signaling
pathway. Kidney Blood Press Res. 41:129–138. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang L, Zhu Z, Liu J, Zhu Z and Hu Z:
Protective effect of N-acetylcysteine (NAC) on renal
ischemia/reperfusion injury through Nrf2 signaling pathway. J
Recept Signal Transduct Res. 34:396–400. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sablina AA, Budanov AV, Ilyinskaya GV,
Agapova LS, Kravchenko JE and Chumakov PM: The antioxidant function
of the p53 tumor suppressor. Nat Med. 11:1306–1313. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Choi EJ, Whang YM, Kim SJ, Kim HJ and Kim
YH: Combinational treatment with retinoic acid derivatives in
non-small cell lung carcinoma in vitro. J Korean Med Sci. 22
(Suppl):S52–S60. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Flamini MI, Gauna GV, Sottile ML, Nadin
BS, Sanchez AM and Vargas-Roig LM: Retinoic acid reduces migration
of human breast cancer cells: Role of retinoic acid receptor beta.
J Cell Mol Med. 18:1113–1123. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Patrad E, Niapour A, Farassati F and Amani
M: Combination treatment of all-trans retinoic acid (ATRA) and
gamma-secretase inhibitor (DAPT) cause growth inhibition and
apoptosis induction in the human gastric cancer cell line.
Cytotechnology. 70:865–877. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hayashi K, Yokozaki H, Naka K, Yasui W,
Lotan R and Tahara E: Overexpression of retinoic acid receptor beta
induces growth arrest and apoptosis in oral cancer cell lines. Jpn
J Cancer Res. 92:42–50. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen H, Zhang H, Lee J, Liang X, Wu X, Zhu
T, Lo PK, Zhang X and Sukumar S: HOXA5 acts directly downstream of
retinoic acid receptor beta and contributes to retinoic
acid-induced apoptosis and growth inhibition. Cancer Res.
67:8007–8013. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
el-Metwally TH, Hussein MR, Pour PM,
Kuszynski CA and Adrian TE: Natural retinoids inhibit proliferation
and induce apoptosis in pancreatic cancer cells previously reported
to be retinoid resistant. Cancer Biol Ther. 4:474–483. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gumireddy K, Sutton LN, Phillips PC and
Reddy CD: All-trans-retinoic acid-induced apoptosis in human
medulloblastoma: Activation of caspase-3/poly(ADP-ribose)
polymerase 1 pathway. Clin Cancer Res. 9:4052–4059. 2003.PubMed/NCBI
|
|
46
|
Mangiarotti R, Danova M, Alberici R and
Pellicciari C: All-trans retinoic acid (ATRA)-induced apoptosis is
preceded by G1 arrest in human MCF-7 breast cancer cells. Br J
Cancer. 77:186–191. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Dhandapani L, Yue P, Ramalingam SS, Khuri
FR and Sun SY: Retinoic acid enhances TRAIL-induced apoptosis in
cancer cells by upregulating TRAIL receptor 1 expression. Cancer
Res. 71:5245–5254. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yang L, Luo C, Chen C, Wang X, Shi W and
Liu J: All-trans retinoic acid protects against doxorubicin-induced
cardiotoxicity by activating the ERK2 signalling pathway. Br J
Pharmacol. 173:357–371. 2016. View Article : Google Scholar : PubMed/NCBI
|