Introduction
Lung cancer is the leading cause of
cancer-associated death and the second most diagnosed cancer
worldwide. Approximately 228,820 people are diagnosed with lung
cancer and at least 135,700 people succumb to the disease each year
(1). Non-small cell lung cancer
(NSCLC) is the most common histological type of lung cancer,
accounting for ~85% of lung cancer cases (2,3).
Although the prognosis of patients with NSCLC has improved over the
last decade with the development of target drugs and immune
checkpoint inhibitors, NSCLC is still a highly malignant, rapidly
progressing and incurable malignant tumor (4,5). The
5-year survival rate of patients with NSCLC is <20% (6). Therefore, elucidating the underlying
molecular mechanisms and identifying the therapeutic targets are
critical for developing efficient treatments of NSCLC.
Sine oculis homeobox homolog 1 (SIX1) is an
important transcription factor for the development of various
organs and is not expressed in most normal adult tissues. However,
SIX1 expression is frequently found in a number of malignant cells
and plays a significant role in tumorigenesis, including cell
proliferation, apoptosis, invasion, epithelial to mesenchymal
transition and stem cell maintenance (7–11).
More importantly, previous studies have confirmed that SIX1 is
mainly involved in aerobic glycolysis, a metabolic feature of
malignancy, by positively regulating the expression of key
glycolytic enzymes at the transcriptional level (12). SIX1 knockdown results in a decrease
in glucose uptake, ATP level and lactate production (13). Additionally, SIX1 levels are
increased in lung cancer and are correlated with a poor prognosis
(14). Hence, finding new
compounds to block SIX1 expression in NSLSC may contribute to the
development of successful cancer therapy.
Tanshinone IIA (Tan IIA) is an active constituent
extracted from the plant Salvia miltiorrhiza, the dried root
of which forms the traditional Chinese herb Danshen. Tan IIA is
found to exert antitumor effects in multiple human cancer types,
including ovarian cancer (15),
oral cancer (16), prostate cancer
(17), acute leukemia (18) and lung cancer (19). Multiple mechanisms are involved in
the antitumor action of Tan IIA in lung cancer. For example, Liao
et al (20) reported that
Tan IIA induced cell apoptosis through the phosphatidylinositol
3-kinase/protein kinase B pathway. Tan IIA also inhibits NSCLC cell
growth by regulating epidermal growth factor receptor signaling
(19) and prevents A549 cell
proliferation by targeting the kinase domain of vascular
endothelial growth factor receptor 2 (21). Additionally, the antitumor effects
of Tan IIA have been shown to deplete pyruvate kinase isozyme M2
(PKM2) levels, leading to the inhibition of glycolysis (22). Therefore, we hypothesize that the
antitumor activity of Tan IIA in NSCLC cells may involve its
antimetabolic role.
The present study investigated the antitumor effects
of Tan IIA on NSCLC via the repression of SIX1 expression and the
subsequent inhibition of aerobic glycolysis.
Material and methods
Clinical specimens
A total of 30 samples of NSCLC primary tumor tissues
and their adjacent noncancerous tissues were obtained from newly
diagnosed patients admitted to the Hebei Provincial Chest Hospital
(Shijiazhuang, Hebei, China) between September 2017 and August
2019. The tumor tissues were obtained via surgical resection, and
the distance between normal lung tissue and corresponding
non-cancerous lung tissues was >3 cm. The patient clinical
characteristics are shown in Table
I. The mean age of the patients was 63.9 years (range, 49-74
years). None of patients received chemotherapy or radiotherapy
before surgery. The inclusion criterion was histologically proven
non-small cell lung cancer. The exclusion criterion was any patient
undergoing chemotherapy or radiotherapy. TNM stage was defined
according to TNM Stage Groupings in the Forthcoming (Eighth)
Edition of the NM Classification for Lung Cancer (23). All the tissues were stored in the
liquid nitrogen immediately for the next experiments. All the
protocols of this study were approved by the Ethics Committee of
the Hebei Provincial Chest Hospital. Written informed consent was
obtained from all patients.
 | Table I.Clinicopathological characteristics
of the non-small cell lung caner patients. |
Table I.
Clinicopathological characteristics
of the non-small cell lung caner patients.
| Characteristic | Number of
cases |
|---|
| Age, years |
|
|
≤60 | 13 |
|
>60 | 17 |
| Sex |
|
|
Male | 16 |
|
Female | 14 |
| Tumor size, cm |
|
| ≤3 | 22 |
|
>3 | 8 |
| TNM stage |
|
|
I+II | 24 |
|
III+IV | 6 |
| Smoking |
|
|
Yes | 20 |
| No | 10 |
Cell culture and transfection
Human NSCLC A549 and H292 cell lines were obtained
from the Shanghai Cell Bank of the Chinese Academy of Science. A549
and H292 cells were cultured in Dulbecco's modified Eagle's medium
(Gibco; Thermo Fisher Scientific, Inc.) with 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin-streptomycin. Cells were cultured in an incubator with
5% CO2 at saturated humidity at 37°C.
The small interfering (si)RNAs specifically
targeting SIX1 were designed by Suzhou GenePharma Co., Ltd., and
had the following the sequences: siSIX1-1 forward,
5′-GGUGGACUUUCACAAAUAUUU-3′ and reverse,
5′-AUAUUUGUGAAAGUCCACCUU-3′; and siSIX1-2 forward,
5′-CAGGUCAGCAACUGGUUUAUU-3′ and reverse,
5′-UAAACCAGUUGCUGACCUGUU-3′. A scrambled sequence was used as the
negative control, which had the following sequences: Forward,
5′-GCUGCUUCUACUCGUAAGUTT-3′ and reverse,
5′-AUUCAGGAGUAGAGAGACCTT-3′. Cells were transfected with siRNA
using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
Briefly, 20 pmol siRNA oligomer and 1 µl Lipofectamine 2000 were
diluted in 50 µl Opti-MEM medium (Gibco; Thermo Fisher Scientific,
Inc.) without serum, respectively. After a 5-min incubation at room
temperature, the diluted oligomer with the diluted Lipofectamine
2000 were combined and incubated for 20 min at room temperature.
The oligomer-Lipofectamine 2000 complexes were then added to each
well containing cells and cultured at 37°C in a 5% CO2
incubator. After 6 h, cells were introduced to normal medium.
Transfected cells were incubated at 37°C in a 5% CO2
incubator for 24 h and then collected to be used in the subsequent
experiments.
Cell viability assay
Cell viability was assessed with the CCK-8 Kit
(Bestbio) following the manufacturer's instructions. Briefly,
1×106 cells were seeded in 100 µl culture medium on
96-well plates and then treated with different concentrations of
Tan IIA (0.25, 0.5, 1, 2, 4, 8, 16 and 32 µM). After 48 h, 10 µl
CCK-8 assay solution was added to each well and the cells were
further incubated at 37°C with 5% CO2 for 2 h. The
optical density values were measured using an ELx808 absorbance
reader (BioTek Instruments, Inc.) at 450 nm.
Protein extraction and western
blotting
Cultured cells or tissues were lysed with RIPA
buffer (Abcam). The BCA method was used for protein determination.
Then 10–30 µg total protein was separated by 10% SDS-PAGE and
electrotransferred to polyvinylidene fluoride membranes
(MilliporeSigma). Membranes were blocked with 5% non-fat milk for 2
h at 37°C and then incubated with the primary antibodies overnight
at 4°C with primary antibody recognizing SIX1 (catalog no.
10709-1-AP; 1:1,000), hexokinases 2 (HK2; catalog no. 66974-1-Ig;
1:1,000), PKM2 (catalog no. 15822-1-AP; 1:1,000), lactate
dehydrogenase A (LDHA; catalog no. 19987-1-AP; 1:1,000), hypoxia
inducible factor 1α (HIF1α; catalog no. 20960-1-AP 1:1,000) and
β-actin (catalog no. 66009-1-Ig; 1:2,000) (all Wuhan Sanying
Biotechnology). The next day, after washing with TBST (containing
0.05% Tween-20), the membranes were incubated with the horse radish
peroxidase-conjugated secondary antibodies (1:5,000; anti-mouse,
catalog no. ab205719; anti-rabbit, catalog no. ab205718; Abcam).
Finally, the blots were treated with the Immobilo™ Western
(MilliporeSigma) and imaged with ECL Fuazon Fx (Vilber Lourmat
Deutschland GmbH) on FusionCapt Advance Fx5 software (Vilber
Lourmat Deutschland GmbH).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from tissues and cells using
QIAzol Lysis Reagent (Qiagen GmbH) following the manufacturer's
protocol. The M-MLV First-Strand Kit (Thermo Fisher Scientific,
Inc.) was used for the reverse transcription of RNA (2 µg) to cDNA
according to the manufacturer's instructions. The Platinum SYBR
Green qPCR Super Mix UDG Kit (Invitrogen; Thermo Fisher Scientific,
Inc.) was used for the PCR on a CFX96TM Real-Time System (Bio-Rad
Laboratories, Inc.) with the following primers: SIX1 forward,
5′-CCAGGTCAGCAACTGGTTTAAG-3′ and reverse,
5′-ATAGTTTGAGCTCCTGGCGT-3′; PKM2 forward,
5′-GTGGCTCGTGGTGATCTAGG-3′ and reverse, 5′-GTGGAGTGACTTGAGGCTCG-3′;
HK2 forward, 5′-GCCATCCTGCAACACTTAGGGCTTGAG-3′ and reverse,
5′-GTGAGGATGTAGCTTGTAGAGGGTCCC-3′; LDHA forward,
5′-ATGGCAACTCTAAAGGATCA-3′ and reverse, 5′-GCAACTTGCAGTTCGGGC-3′;
HIF1α forward, 5′-TACTCAGCACTTTTAGATGCTGTT-3′ and reverse,
5′-ACGTTCAGAACTTATCCTACCAT-3′; and β-actin forward,
5′-GAGCTACGAGCTGCCTGAC-3′ and reverse,
5′-GGTAGTTTCGTGGATGCCACAG-3′. The qPCR conditions were as follows:
Initial denaturation at 95°C for 10 min, followed by 40 cycles of
95°C for 5 sec, 60°C for 30 sec and 72°C for 20 sec, with a final
extension at 72°C for 5 min. All the relative gene expression
levels were calculated using the 2−ΔΔCq formula
(24).
Glucose uptake, lactate production and
ATP assays
Glucose uptake and lactate production were assessed
using a Glucose Uptake Colorimetric Assay Kit (catalog no.
ab136955; Abcam) and a Lactate Assay Kit II (catalog no. K267-100;
BioVision, Inc.), respectively, following the manufacturers'
instructions. Briefly, 5×105 cells were seeded into
96-well plates and treated with Tan IIA (5 µM) for 48 h. After
washing with PBS, the cells were preincubated with 100 ml
Krebs-Ringer-Phosphate-HEPES buffer containing 2% BSA for 40 min at
room temperature. Next, 2-deoxy-D-glucose was added, and the cells
were incubated for 20 min at 37°C. Afterward, cells were lysed with
90 ml extraction buffer and then 10 ml neutralization buffer was
added. After centrifugation (1,500 × g, for 10 min at room
temperature), the supernatant was used to measure glucose uptake at
412 nm and lactate production at 450 nm in a microplate reader
(BioTek Instruments, Inc.).
For ATP level analysis, cells were lysed in the same
manner as for the glucose uptake and lactate production assays. The
cells were collected and extracted in 100 ml of the ATP Assay
Buffer from the ATP calorimetric assay kit (BioVision, Inc.). After
centrifugation (1,500 × g, for 10 min at room temperature), the
supernatants were incubated for 30 min at room temperature, and
then absorbance at 570 nm was measured using a microplate reader
(BioTek Instruments, Inc.).
Xenograft model
A total of 12 male BALB/c-nu/nu nude male mice (4–6
weeks old; weight, 22–25 g) were purchased from Vital River
Laboratory Animal Technology Co., Ltd. Mice were maintained in
specific-pathogen-free conditions under a constant humidity of
60–70% and room temperature of 18–20°C in the Laboratory Animal
Center of Hebei Medical University (Shijiazhuang, Hebei, China). A
total of 5×106 A549 cells were mixed with 50% Matrigel
matrix (BD Biosciences) and subcutaneously injected into the nude
mice. On the 8th day after injection, the mice were then randomly
divided into two groups (6 mice in each group), namely, the Tan
IIA-treated and control groups. In the Tan IIA-treated group, Tan
IIA (20 mg/kg) was administered to the mice intraperitoneally every
day for 2 weeks, as described previously (25). The control group was injected with
the same amount of saline as the negative control. After 28 days,
the mice were euthanized using carbon dioxide asphyxiation as
follows: The mice were placed into a euthanasia box (20×12×10 cm)
and then carbon dioxide (concentration of 99.9%) was injected into
the box at a flow rate of 1 l/min (~33% of the box volume/min). The
mice were exposed to the carbon dioxide until a complete cessation
of breathing was determined (usually within 4-8 min) and observed
for at least 2 min afterwards. The mice were then taken out of the
box and cervical dislocation was performed to ensure death. The
tumors were excised from the mice for further study. Tumor volume
was calculated as follows: Volume = (length × width2)/2.
All animal experiments were approved by the Institutional Animal
Care and Use Committee of Hebei Medical University (approval no.
HebMU 20,080,026).
TCGA analysis
Kaplan-Meier plot analysis of TCGA data was
performed by using OncoLnc (http://www.oncolnc.org/kaplan). A total of 489
patients with LUAD were contained in the database. The cut-off
value was 25%.
Statistical analysis
Data are presented as the mean ± standard deviation
of three independent experiments. Student's t-test was used to
determine the significance of differences between two groups.
One-way ANOVA followed by Bonferroni's post hoc test was used for
multigroup comparisons. Correlations between SIX1 expression and
LDH level were analyzed using Pearson's correlation test. P<0.05
was considered to indicate a statistically significant difference.
Statistical analyses were performed using GraphPad Prism (version
8; GraphPad Software, Inc.).
Results
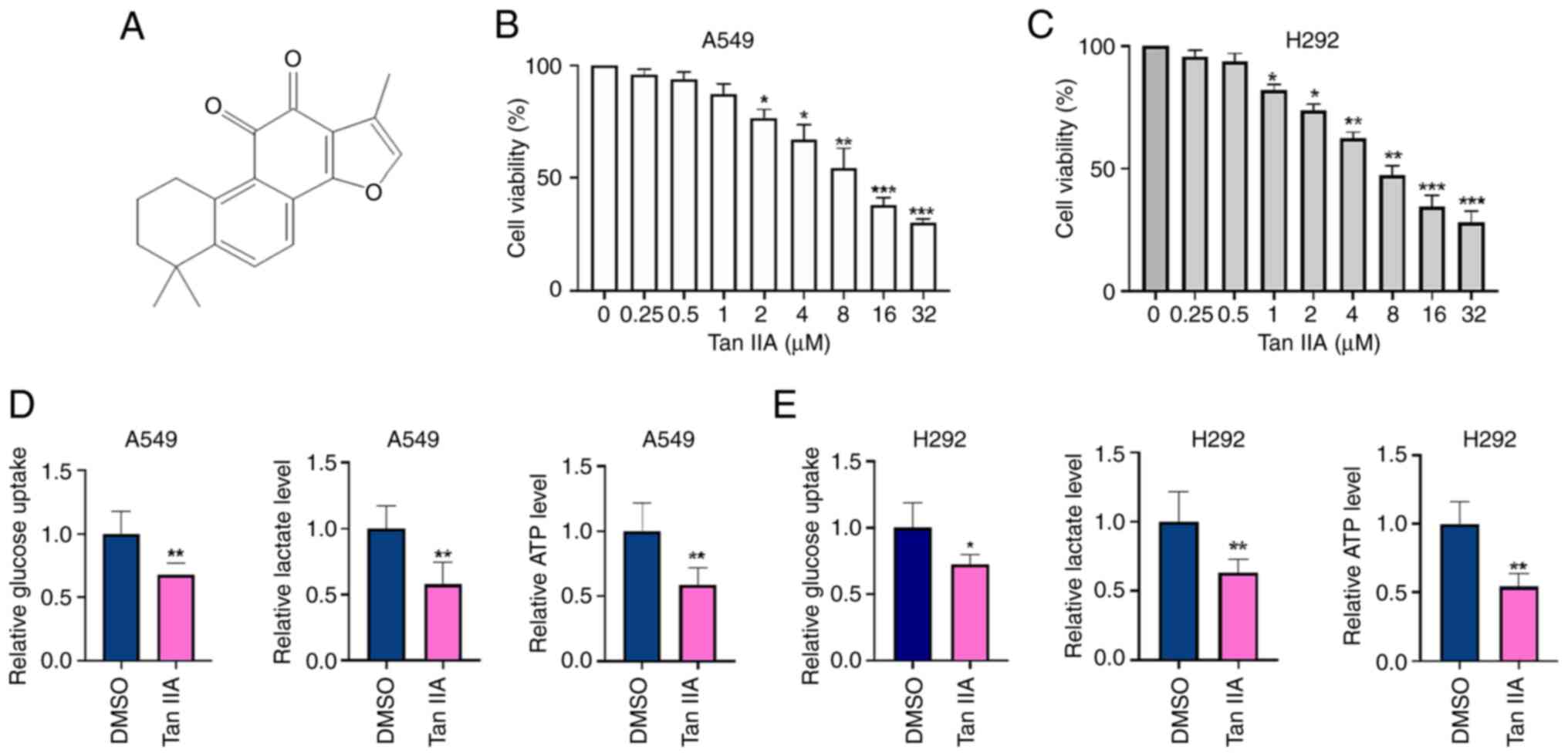
Tan IIA inhibits cell proliferation
and glycolysis in NSCLC cells
The structure of Tan IIA is shown in Fig. 1A. To investigate the antitumor
effect of Tan IIA in NSCLC cells, A549 and H292 cells were treated
with different concentrations of Tan IIA for 48 h and then cell
viability was detected by CCK-8 assay. As shown in Fig. 1B and 1C, Tan IIA significantly
inhibited the proliferation of NSCLC cells in a dose-dependent
manner. The half maximal inhibitory concentration of Tan IIA was
5.45 µM in A549 cells and 5.78 µM in H292 cells. To clarify the
effects of Tan IIA on the glycolysis of NSCLC cells, the levels of
glucose consumption, lactate production and ATP were detected. As
shown in Fig. 1D and E, glucose
uptake, lactate production and ATP levels were significantly
decreased by 30, 40 and 52% in A549 cells, and 25, 38 and 50% in
H292 cells, respectively, in the Tan IIA-treated group compared
with those of the DMSO group. These results indicate that Tan IIA
inhibits cell viability and suppresses cell glycolysis activity in
NSCLC cells in vitro.
Tan IIA downregulates SIX1 expression
in NSCLC cells
To detect whether Tan IIA inhibited the glycolysis
in NSCLC cells, the mRNA and protein levels of glycolysis
rate-limiting enzymes (PKM2, HK2 and LDHA) were measured in Tan
IIA-treated A549 cells using RT-qPCR and western blot analysis,
respectively. As shown in Fig. 2A,
Tan IIA treatment markedly decreased the mRNA levels of PKM2, HK2
and LDHA compared with those measured in DMSO-treated controls.
Consistent with the RT-qPCR results, the western blotting results
showed that Tan IIA-treated A549 cells contained lower protein
levels of PKM2, HK2 and LDHA compared with DMSO-treated controls
(Fig. 2B), suggesting that Tan IIA
inhibited glycolysis by repressing the activity of enzymes involved
in aerobic glycolysis. A previous study reported that HIF1α and
SIX1 contribute to regulate the levels of enzymes involved in
aerobic glycolysis level in multiple cancer types (13). Therefore, the present study
analyzed the mRNA and protein levels of SIX1 and HIF1α by RT-qPCR
and western blot analysis, respectively. As shown in Fig. 2C and D, SIX1 mRNA and protein
levels were markedly decreased by Tan IIA, whereas HIF1α expression
was not affected. Additionally, A549 cells were treated with Tan
IIA (2.5, 5 and 10 µM) and then RT-qPCR was used to detect the
expression levels of PKM2, LDHA, HK2 and SIX1. As shown in Fig. 2E, the treatment with Tan IIA
decreased the mRNA expression levels of PKM2, LDHA, HK2, and SIX1.
However, there was no significant different between Tan IIA used at
5 and 10 µM concentrations. Furthermore, it was found that SIX1
mRNA expression in the A549 cell line was higher than that in the
H292 cell line (Fig. 2F).
Therefore, the A549 cell line was chosen for the following
experiments. Together, these data suggest that Tan IIA may
influence glycolysis activity by regulating SIX1 expression.
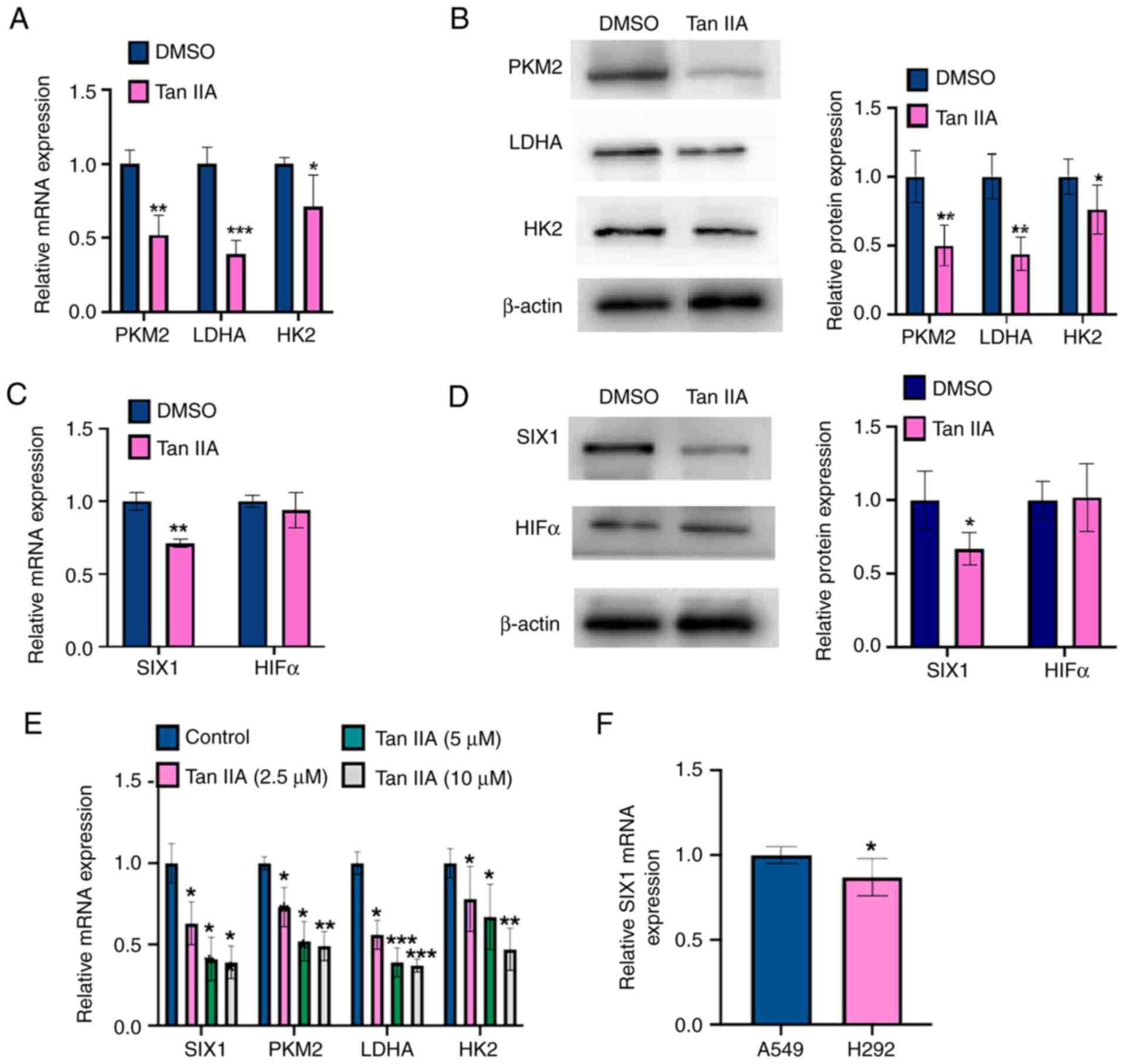 | Figure 2.Tan IIA downregulates SIX1 expression
in NSCLC cells. (A) A549 cells were treated with 5 µM Tan IIA for
48 h. The mRNA levels of PKM2, LDHA and HK2 were detected by
RT-qPCR. *P<0.05, **P<0.01 and ***P<0.001 vs. DMSO. (B)
A549 cells were treated as in (A), and the protein levels of PKM2,
LDHA and HK2 were detected by western blot analysis. The right
panel shows the densitometric analysis of three independent
experiments. *P<0.05 and **P<0.01 vs. DMSO. (C) A549 cells
were treated as in (A), and the mRNA levels of SIX1 and HIF1α were
detected by RT-qPCR. **P<0.01 vs. DMSO. (D) A549 cells were
treated as in (A), and the protein levels of SIX1 and HIF1α were
detected by western blot analysis. The right panel shows the
densitometric analysis of three independent experiments. *P<0.05
vs. DMSO. (E) A549 cells were treated with Tan IIA (2.5, 5 and 10
µM) and mRNA levels of PKM2, LDHA, HK2 and SIX1 were detected by
RT-qPCR. *P<0.05, **P<0.01 and ***P<0.001 vs. DMSO. (F)
RT-qPCR was used to detect the mRNA level of SIX1 in A549 and H292
cell lines. *P<0.05 vs. A549 cell line. Tan IIA, Tanshinone IIA;
SIX1, sine oculis homeobox homolog 1; PKM2, pyruvate kinase subtype
M2; HK2, hexokinases 2; LDHA, lactate dehydrogenase A; HIF1α,
hypoxia inducible factor 1α; RT-qPCR, reverse
transcription-quantitative PCR. |
Knockdown of SIX1 represses glycolysis
in NSCLC cells
To investigate the role of SIX1 in glycolysis in
NSCLC cells, loss-of-function experiments were performed. First,
SIX1 expression was knocked down in A549 cells using SIX1-specific
siRNA. The transfection of siRNAs against SIX1 (si-SIX1-1 and
si-SIX1-2) efficiently decreased SIX1 mRNA and protein levels of
SIX1 compared with those in the negative control (Fig. 3A and B), with si-SIX1-1 knocking
down 70% of SIX1 mRNA expression. Therefore, si-SIX1-1 was chosen
for all subsequent experiments. The knockdown of SIX1 decreased
glucose uptake level, lactate production level and ATP level in
A549 cells compared with those found in the negative control
(Fig. 3C). Western blotting and
RT-qPCR results showed that SIX1 depletion led to a significant
decrease in PKM2, HK2 and LDHA expression at the mRNA and protein
levels compared with those of the negative control (Fig. 3D and E). These results demonstrate
that depletion of SIX1 decreases glycolysis in an NSCLC cell
line.
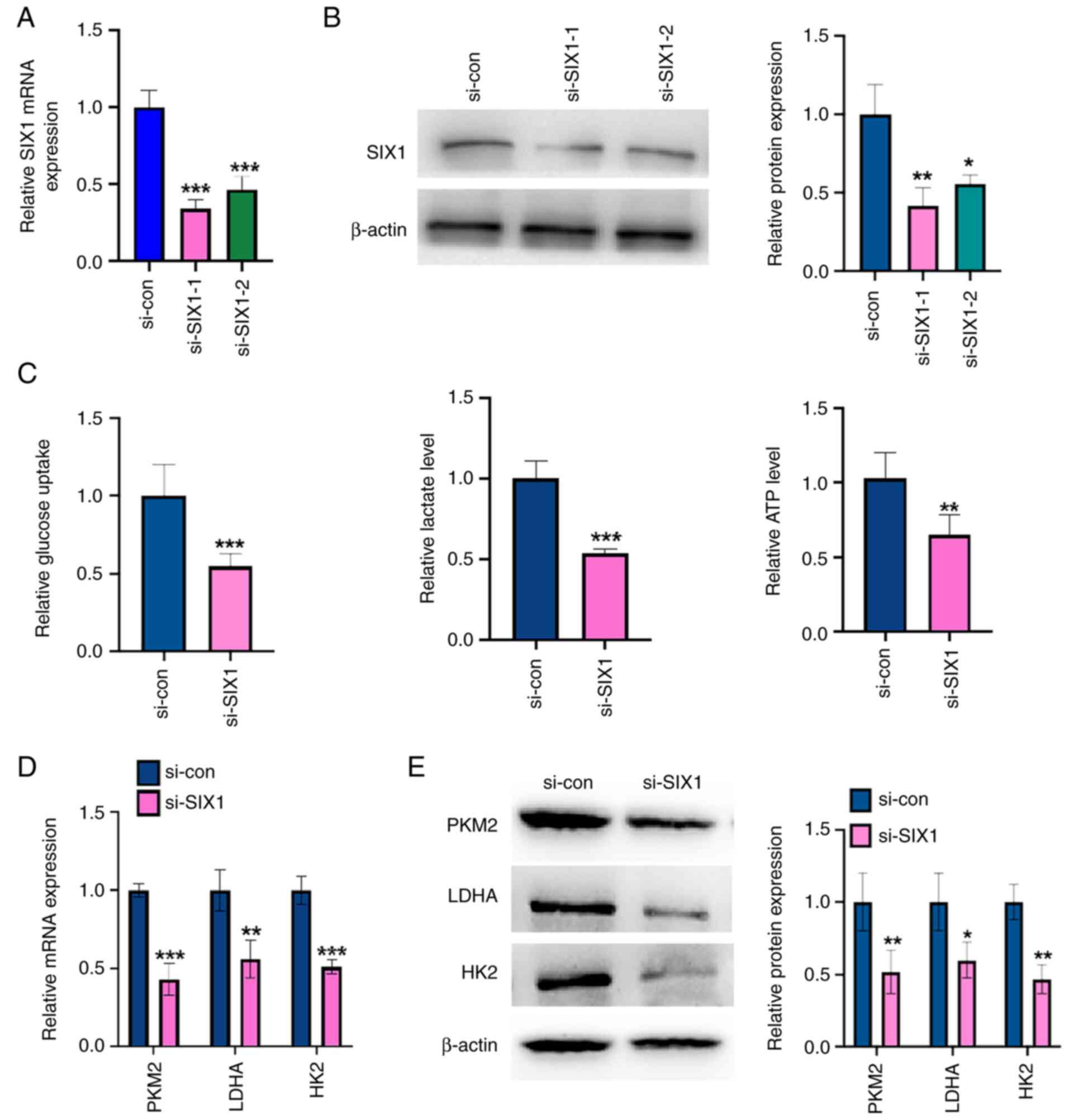 | Figure 3.Knockdown of SIX1 represses
glycolysis in non-small cell lung cancer cells. (A) A549 cells were
transfected with si-SIX1 (si-SIX1-1 and si-SIX1-2) or si-con,
respectively. The mRNA level of SIX1 was detected by RT-qPCR.
***P<0.001 vs. si-con. (B) A549 cells were treated as in (A),
and the protein level of SIX1 was measured by western blot
analysis. The right panel shows the densitometric analysis of three
independent experiments. *P<0.05 and **P<0.01 vs. si-con. (C)
A549 cells were transfected with si-SIX1 or si-con respectively.
Glucose uptake, lactate production and ATP level were measured by
corresponding assays. **P<0.01 and ***P<0.001 vs. si-con. (D)
A549 cells were treated as in (C), and the mRNA levels of PKM2,
LDHA and HK2 were detected by RT-qPCR. **P<0.01 and
***P<0.001 vs. si-con. (E) A549 cells were treated as in (C),
and the protein levels of PKM2, LDHA and HK2 were detected by
western blot analysis. The right panel shows the densitometric
analysis of three independent experiments. *P<0.05 and
**P<0.01 vs. si-con. Tan IIA, Tanshinone IIA; SIX1, sine oculis
homeobox homolog 1; PKM2, pyruvate kinase subtype M2; HK2,
hexokinases 2; LDHA, lactate dehydrogenase A; RT-qPCR, reverse
transcription-quantitative PCR; si-, small interfering; con,
control. |
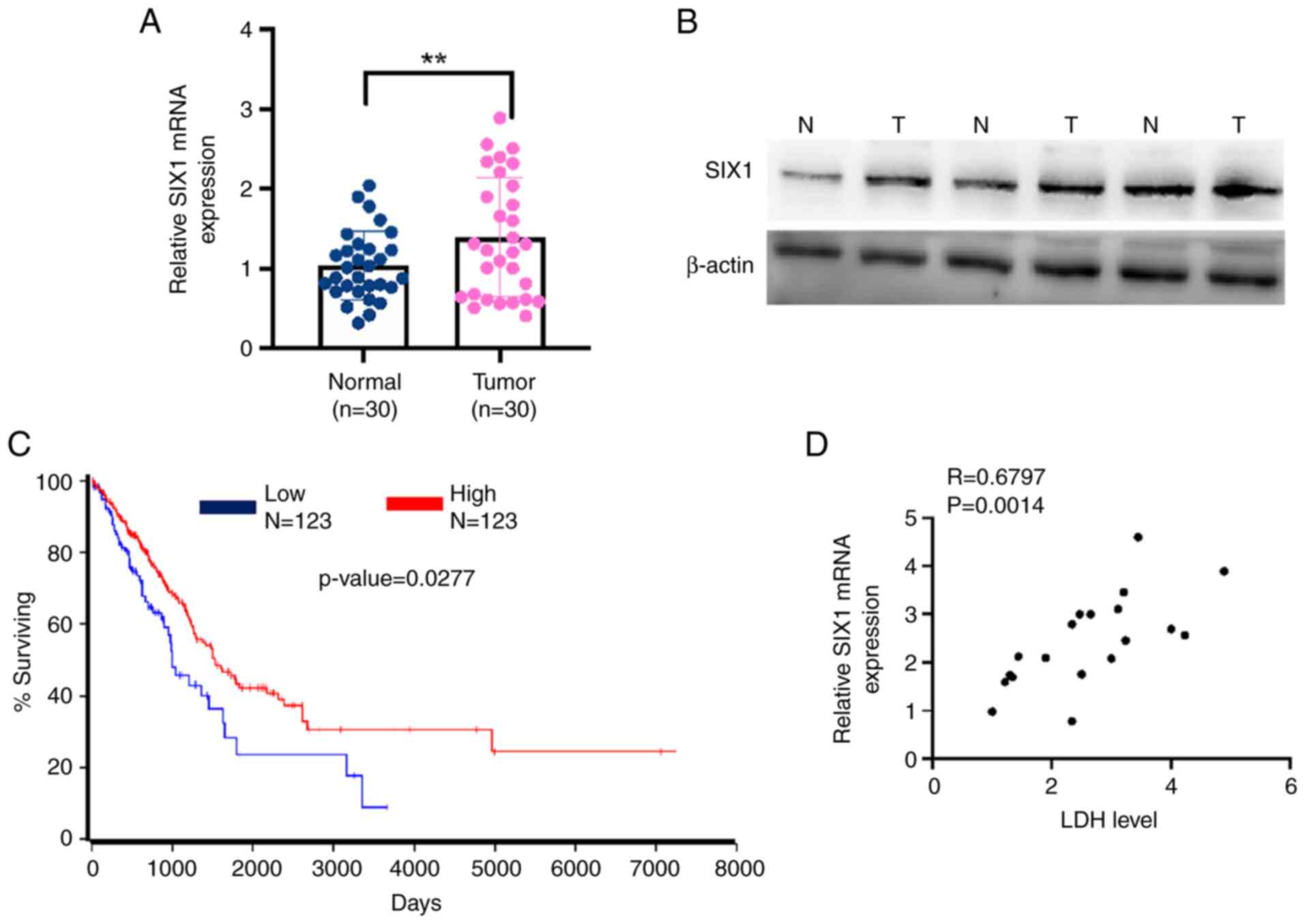
SIX1 expression is upregulated in
NSCLC tissues and correlates with LDH level in patients with
NSCLC
Previous studies have shown that SIX1 expression is
correlated with the prognosis of multiple types of cancer (26,27).
Therefore, the present study investigated SIX1 expression in NSCLC
tissues. As shown in Fig. 4A and
B, SIX1 expression was markedly upregulated in NSCLC tissues
compared with the levels found in adjacent noncancerous lung
tissues. More importantly, Kaplan-Meier survival analysis of TCGA
data using OncoLnc (http://www.oncolnc.org/) revealed that a low level of
SIX1 was associated with a lower survival rate in patients with
NSCLC (Fig. 4C). A positive
correlation was also found between SIX1 expression and LDH level
(R=0.6797). These data support the fact that lower expression of
SIX1 correlates with the increased LDH level and the poor prognosis
of patients with NSCLC.
Tan IIA decreases glycolysis in NSCLC
cells through SIX1
To investigate whether SIX1 plays a role in the Tan
IIA-induced decrease in glycolysis, rescue experiments were
performed. A549 cells were transfected with si-SIX1 or negative
control and then treated with Tan IIA or DMSO. As shown in Fig. 5A-C, glycolysis in NSCLC cells was
inhibited by Tan IIA treatment and this inhibitory effect was
enhanced by the simultaneous knockdown of SIX1. Western blotting
and RT-qPCR data revealed that Tan IIA treatment decreased the
protein and mRNA levels of SIX1, PKM2, HK2 and LDHA, and that the
knockdown of SIX1 combined with Tan IIA treatment significantly
enhanced this effect compared with Tan IIA treatment alone
(Fig. 5D). Collectively, these
findings demonstrate that SIX1 plays a crucial role in the decrease
of glycolysis induced by Tan IIA in NSCLC cells.
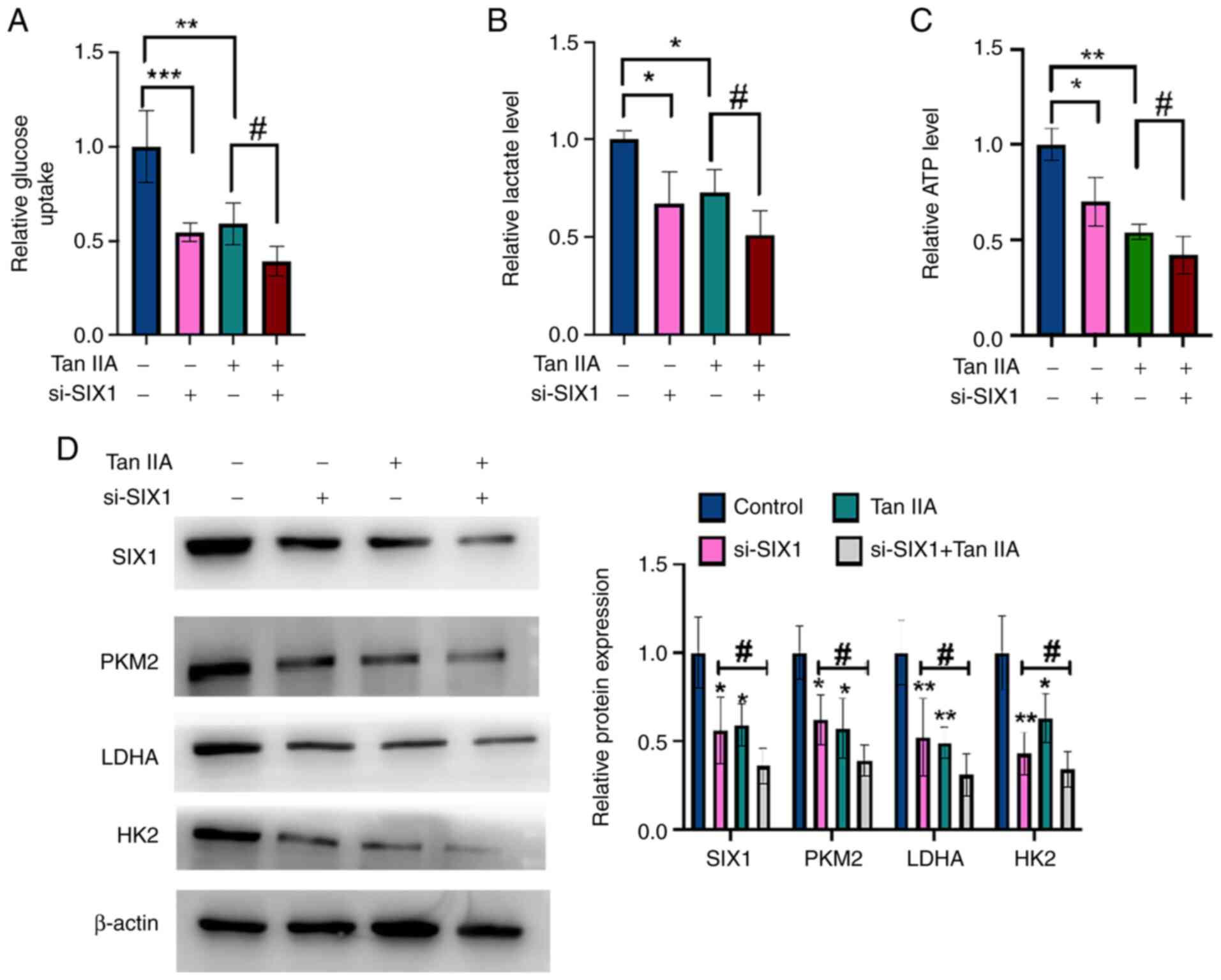 | Figure 5.Tan IIA decreases glycolysis in
non-small cell lung cancer cells through SIX1. (A) A549 cells were
transfected with si-SIX1 or si-con and then treated with Tan IIA or
DMSO. Glucose uptake was detected by glucose assay kit. (B) A549
cells were treated as in (A), and lactate level was detected by
lactate assay kit. (C) A549 cells were treated as in (A), and ATP
level was detected by ATP assay kit. (D) A549 cells were treated as
in (A), and the protein levels of SIX1, PKM2, LDHA and HK2 were
detected by western blot analysis. The right panel shows the
densitometric analysis of three independent experiments.
*P<0.05, ***P<0.001, **P<0.01 and ##P<0.05
vs. corresponding control. SIX1, sine oculis homeobox homolog 1;
Tan IIA, Tanshinone IIA; si-, small interfering; con, control;
PKM2, pyruvate kinase subtype M2; HK2, hexokinases 2; LDHA, lactate
dehydrogenase A. |
Tan IIA suppresses NSCLC cell growth
in vivo
To further confirm the antitumor role of Tan IIA in
NSCLC, a nude mouse xenograft model of NSCLC was established.
First, A549 cells were implanted into nude mice. After 8 days, the
mice were administered Tan IIA. As shown in Fig. 6A-C, the Tan IIA-treated group
presented with smaller tumor volumes and lower tumor weights
compared with those measured in the control group. In addition, the
expression levels of SIX1, PKM2, HK2 and LDHA were determined in
the tumor tissues by RT-qPCR and western blot analysis. As shown in
Fig. 6D and E, SIX1, PKM2, HK2 and
LDHA levels were significantly decreased in the tumors of the Tan
IIA-treated group compared with those of the controls. These data
reveal that Tan IIA treatment decreases tumor growth and suppresses
SIX1 expression in vivo.
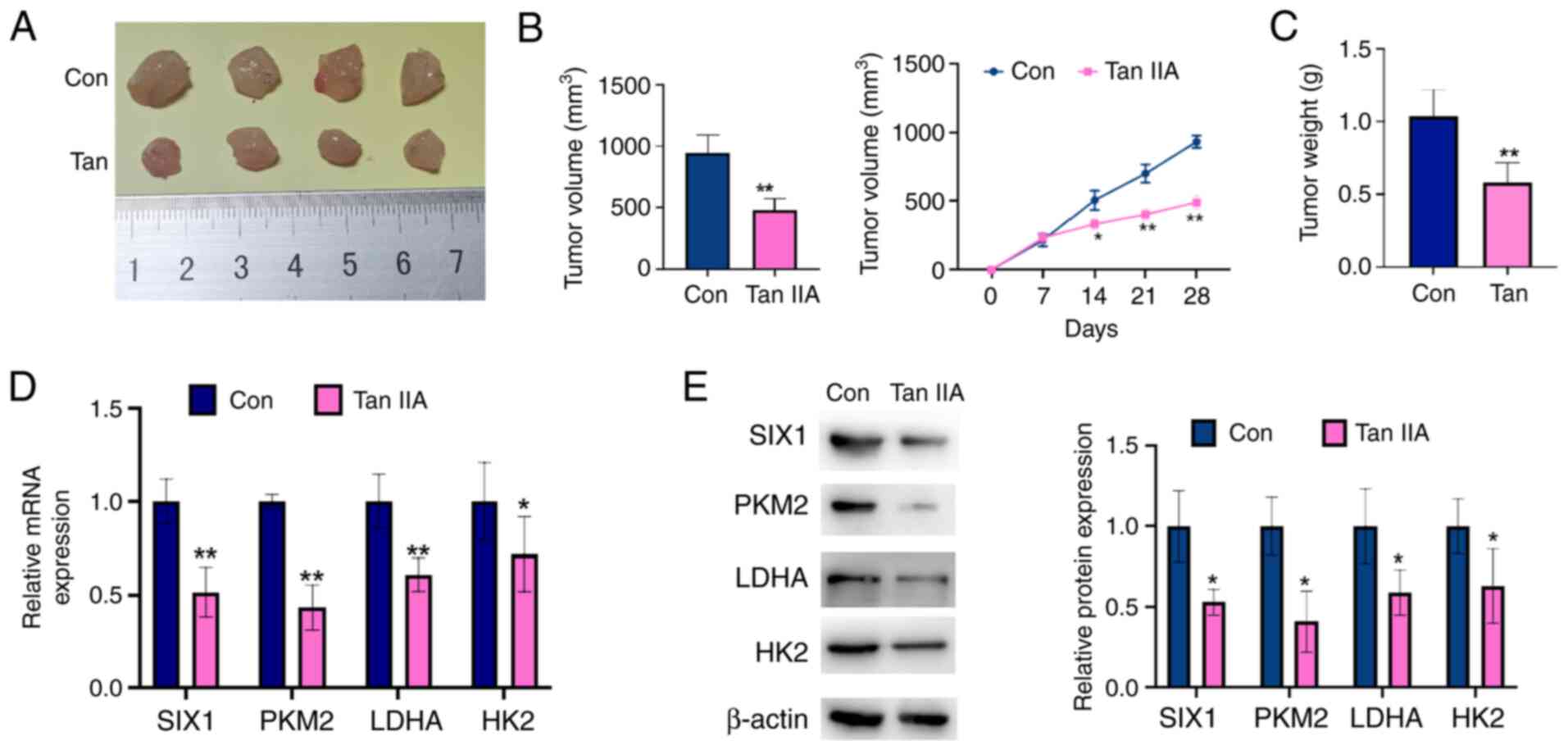 | Figure 6.Tan IIA suppresses non-small cell
lung cancer cell growth in vivo. A549 cells were injected
into the right posterior ankle of the nude mice to establish
xenograft tumors. From the eighth day, mice were intraperitoneally
injected with Tan IIA (20 mg/kg) for 2 weeks. (A) Representative
tumor sizes in each group of mice. (B) Tumor volumes were monitored
by direct measurement. *P<0.05 and **P<0.01 vs. control
group. (C) Xenograft tumor wet weight in each group of mice.
**P<0.01 vs. control group. (D) The mRNA levels of SIX1, PKM2,
LDHA and HK2 in xenograft tumor tissues were detected by reverse
transcription-quantitative PCR. *P<0.05 and **P<0.01 vs.
control group. (E) The protein levels of SIX1, PKM2, LDHA and HK2
in xenograft tumor tissues were detected by western blot analysis.
The right panel shows the densitometric analysis of three
independent experiments. *P<0.05 vs. control group. SIX1, sine
oculis homeobox homolog 1; Tan IIA, Tanshinone IIAcon, control;
PKM2, pyruvate kinase subtype M2; HK2, hexokinases 2; LDHA, lactate
dehydrogenase A. |
Discussion
It is well known that the uncontrolled proliferation
of tumor cells is mainly due to their monoclonality. However,
increasing evidence indicates that malignancy is not only a clonal
disease, but also a metabolic disease (28). Indeed, the rapid growth of tumor
cells depends on their unique metabolic pathways, especially in
glucose metabolism (29). The
Warburg effect allows tumor cells to undergo aerobic glycolysis to
provide energy, even in the presence of sufficient oxygen, leading
to increased glucose uptake and lactate production (30,31).
This characteristic of glucose metabolism renders tumor cells more
adaptable to hypoxia and contributes to cancer progression
(32,33). Therefore, inhibiting aerobic
glycolysis and then blocking the energy metabolism of neoplastic
cells may constitute an effective antitumor treatment.
In the present study, it was demonstrated that Tan
IIA, a constituent of Salvia miltiorrhiza, significantly
inhibited NSCLC cell growth in vivo and in vitro,
which was consistent with earlier studies (19,34).
Moreover, Tan IIA decreased the glucose uptake, ATP level and
lactate production of the NSCLC cells suggesting that Tan IIA
exerts an anti-Warburg effect in NSCLC. Previous studies showed
that Tan IIA inhibited the Warburg effect in various neoplasms. For
example, Liu et al (35)
reported that Tan IIA treatment led to decreased glycolysis in
cervical cancer and subsequently to cell apoptosis. Li et al
(36) revealed that Tan IIA
exerted its antitumor effect by suppressing HK2-mediated glycolysis
in oral squamous cell carcinoma. In the present study, it was
demonstrated that Tan IIA treatment effectively suppressed
glycolysis by interfering with glucose uptake, lactate production
and ATP level. Moreover, Zhang et al (37) reported that Tan IIA suppressed PKM2
expression in esophageal cancer cells by upregulating the microRNA
(miR)-122 level. Liu et al (35) also showed that treatment of Tan IIA
downregulated PKM2 and HK2 expression in cervical cancer cells,
thus leading to cell apoptosis. The present study confirmed that
Tan IIA treatment decreased PKM2, LDHA and LDHA mRNA and protein
levels. These are key enzymes in the glycolytic pathway in A549
cells. Although the anticancer effect of Tan IIA in NSCLC cells was
shown in vivo and in vitro, clinical trials to test
the safety and efficacy of Tan IIA in patients with cancer are
needed.
Since Li et al (13) first demonstrated that SIX1 was a
critical transcription factor involved in the Warburg effect and
contributing to tumorigenesis, research has focused on suppressing
SIX1 gene expression. For example, Nie et al (38) reported that miR-140-5p targeted
SIX1 directly in leukemia cells. Translation of miR-140-5p mimics
markedly downregulated SIX1 level and inhibited leukemia cell
growth. In addition, miR-23a-3p negatively regulated the
post-transcription level of SIX1 in head and neck squamous cell
carcinomas (HNSCC). SIX1 expression was decreased and HNSCC cell
growth was suppressed in vitro by transfection of miR-23a-3p
mimics (39). Furthermore, Zhou
et al revealed that SIX1 was highly modified by
O-GlcNAcylation in HCC cells, which could inhibit the
ubiquitination degradation of SIX1 (40). In addition, Chu et al
(41) reported that the compound
8430 decreased SIX1 transcriptional activity and MCF7 cell
proliferation in vitro, indicating that 8430 might serve as
a promising adjuvant in the future. In the present study, it was
demonstrated that Tan IIA treatment downregulated the mRNA and
protein levels of SIX1 in A549 cells, indicating that Tan IIA might
function as a SIX1 inhibitor. Knockdown of SIX1 expression
downregulated the mRNA and protein levels of PKM2, HK2 and LDHA.
Importantly, depletion of SIX1 expression led to a decrease in ATP
level, lactate production and glucose uptake. These results further
explained the mechanisms by which Tan IIA regulated PKM2, HK2 and
LDHA expression. Previous studies found that Tan IIA decreased the
HIF1α level in colorectal cancer and breast cancer (42,43).
However, in the present study, Tan IIA treatment (5 µM) did not
affect the HIF1α expression in A549 cells, possibly as a different
cell line was used, in which Tan IIA might trigger different
mechanisms. Therefore, the underlying mechanism involved in the
regulation of SIX1 expression by Tan IIA in NSCLC cells needs to be
further investigated.
The high expression of SIX1 is linked to the
prognosis of patients with cancer. For example, in a previous
study, SIX1 expression was increased in prostate cancer tissues
compared with that in normal tissues, The high level of SIX1 was an
independent prognostic indicator and correlated with a high
histological grade and clinical stage in prostate cancer (44). In glioma, the higher expression of
SIX1 is more frequent in high-grade glioma and is correlated with a
poor clinical outcome (22).
Consistent with these previous studies, the present study found
increased SIX1 expression levels in NSCLC tissues and cell lines.
TCGA database analysis revealed that lower expression of SIX1 was
closely associated with the poor prognosis of patients with NSCLC.
Notably, increased SIX1 expression was positively correlated with
the LDH serum levels of the patients with NSCLC. These results also
confirmed the important role of SIX1 in metabolism. The association
between SIX1 and LDH in NSCLC tissues provides a basis for the
future selection of treatment for clinical patients. Indeed,
patients with higher serum LDH levels might benefit from Tan IIA
treatment.
In conclusion, the present study revealed the
antitumor effect of Tan IIA in lung cancer in vitro and
in vivo. Tan IIA decreased SIX1 expression and inhibited
glycolysis in NSCLC cells. The results indicate that Tan IIA is an
anti-Warburg effect agent and may constitute a novel treatment for
patients with NSCLC.
Acknowledgements
Not applicable.
Funding
This study was supported by the Scientific Research Foundation
of Hebei Administration of Traditional Chinese Medicine (grant no.
2022383).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request. The data showing the association between SIX1 and the
prognosis of patients with lung cancer are available from OncoLnc
(http://www.oncolnc.org/kaplan).
Authors' contributions
YL was responsible for the conception and design of
the study. HQ, ZC and XW were responsible for study data
acquisition. HQ, ZZ and YQ was involved in the development of the
study methodology, analysis and interpretation of the data. HQ, ZC,
YQ and YL were involved in the writing, reviewing and revision of
the article, and analyzed the relevant literature. HQ and YL
confirm the authenticity of the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
All the protocols of this study were approved by the
Ethics Committee of Hebei Chest Hospital. All patients provided
written informed consent. All animal experiments were approved by
the Institutional Animal Care and Use Committee of Hebei Medical
University (approval no. HebMU 20,080,026). Hebei Provincial Chest
Hospital is an affiliated hospital of Hebei Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
TCGA
|
The Cancer Genome Atlas
|
|
NSCLC
|
non-small cell lung cancer
|
|
Tan IIA
|
Tanshinone IIA
|
|
SIX1
|
sine oculis homeobox homolog 1
|
|
PKM2
|
pyruvate kinase subtype M2
|
|
HK2
|
hexokinases 2
|
|
LDHA
|
lactate dehydrogenase A
|
|
RT-qPCR
|
reverse transcription-quantitative
PCR
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ettinger DS, Wood DE, Aggarwal C, Aisner
DL, Akerley W, Bauman JR, Bharat A, Bruno DS, Chang JY, Chirieac
LR, et al: NCCN guidelines insights: Non-small cell lung cancer,
version 1.2020. J Natl Compr Canc Netw. 17:1464–1472. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen Z, Fillmore CM, Hammerman PS, Kim CF
and Wong KK: Non-small-cell lung cancers: A heterogeneous set of
diseases. Nat Rev Cancer. 14:535–546. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Herbst RS, Morgensztern D and Boshoff C:
The biology and management of non-small cell lung cancer. Nature.
553:446–454. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rosner S, Reuss JE and Forde PM: PD-1
blockade in early-stage lung cancer. Annu Rev Med. 70:425–435.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pastorino U, Buyse M, Friedel G, Ginsberg
RJ, Girard P, Goldstraw P, Johnston M, McCormack P, Pass H and
Putnam JB Jr; International Registry of Lung Metastases, :
Long-term results of lung metastasectomy: Prognostic analyses based
on 5206 cases. J Thorac Cardiovasc Surg. 113:37–49. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu W, Ren Z, Li P, Yu D, Chen J, Huang R
and Liu H: Six1: A critical transcription factor in tumorigenesis.
Int J Cancer. 136:1245–1253. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chu Y, Chen Y, Li M, Shi D, Wang B, Lian
Y, Cheng X, Wang X, Xu M, Cheng T, et al: Six1 regulates leukemia
stem cell maintenance in acute myeloid leukemia. Cancer Sci.
110:2200–2210. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Coletta RD, Christensen KL, Micalizzi DS,
Jedlicka P, Varella-Garcia M and Ford HL: Six1 overexpression in
mammary cells induces genomic instability and is sufficient for
malignant transformation. Cancer Res. 68:2204–2213. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Coletta RD, McCoy EL, Burns V, Kawakami K,
McManaman JL, Wysolmerski JJ and Ford HL: Characterization of the
Six1 homeobox gene in normal mammary gland morphogenesis. BMC Dev
Biol. 10:42010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang Y, Wang S, Liu Z, Yang L, Liu J and
Xiu M: Increased Six1 expression in macrophages promotes
hepatocellular carcinoma growth and invasion by regulating MMP-9. J
Cell Mol Med. 23:4523–4533. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang C, Xu W, Gong J, Chai F, Cui D and
Liu Z: Six1 overexpression promotes glucose metabolism and invasion
through regulation of GLUT3, MMP2 and snail in thyroid cancer
cells. Onco Targets Ther. 13:4855–4863. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li L, Liang Y, Kang L, Liu Y, Gao S, Chen
S, Li Y, You W, Dong Q, Hong T, et al: Transcriptional regulation
of the Warburg effect in cancer by SIX1. Cancer Cell.
33:368–385.e7. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Q, Li A, Tian Y, Liu Y, Li T, Zhang C,
Wu JD, Han X and Wu K: The expression profile and clinic
significance of the SIX family in non-small cell lung cancer. J
Hematol Oncol. 9:1192016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li N, Yang L, Zhang B and Chen S:
Tanshinone IIA effects on ovarian cancer cell line. J Pharm
Pharmacol. 70:1369–1377. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
International BR: Retracted: Tanshinone
IIA induces apoptosis in human oral cancer KB cells through a
mitochondria-dependent pathway. Biomed Res Int.
2017:94964852017.PubMed/NCBI
|
|
17
|
Chiu SC, Huang SY, Chen SP, Su CC, Chiu TL
and Pang CY: Tanshinone IIA inhibits human prostate cancer cells
growth by induction of endoplasmic reticulum stress in vitro and in
vivo. Prostate Cancer Prostatic Dis. 16:315–322. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nie ZY, Zhao MH, Cheng BQ, Pan RF, Wang
TR, Qin Y and Zhang XJ: Tanshinone IIA regulates human AML cell
proliferation, cell cycle, and apoptosis through miR-497-5p/AKT3
axis. Cancer Cell Int. 20:3792020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gao F, Li M, Liu W and Li W: Inhibition of
EGFR signaling and activation of mitochondrial apoptosis contribute
to tanshinone IIA-mediated tumor suppression in non-small cell lung
cancer cells. Onco Targets Ther. 13:2757–2769. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liao XZ, Gao Y, Huang S, Chen ZZ, Sun LL,
Liu JH, Chen HR, Yu L, Zhang JX and Lin LZ: Tanshinone IIA combined
with cisplatin synergistically inhibits non-small-cell lung cancer
in vitro and in vivo via down-regulating the phosphatidylinositol
3-kinase/Akt signalling pathway. Phytother Res. 33:2298–2309. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xie J, Liu J, Liu H, Liang S, Lin M, Gu Y,
Liu T, Wang D, Ge H and Mo SL: The antitumor effect of tanshinone
IIA on anti-proliferation and decreasing VEGF/VEGFR2 expression on
the human non-small cell lung cancer A549 cell line. Acta Pharm Sin
B. 5:554–563. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang X and Xu R: Six1 expression is
associated with a poor prognosis in patients with glioma. Oncol
Lett. 13:1293–1298. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chassagnon G, Bennani S and Revel MP: New
TNM classification of non-small cell lung cancer. Rev Pneumol Clin.
73:34–39. 2017.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yen JH, Huang ST, Huang HS, Fong YC, Wu
YY, Chiang JH and Su YC: HGK-sestrin 2 signaling-mediated autophagy
contributes to antitumor efficacy of Tanshinone IIA in human
osteosarcoma cells. Cell Death Dis. 9:10032018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chao L, Liu J and Zhao D: Increased Six1
expression is associated with poor prognosis in patients with
osteosarcoma. Oncol Lett. 13:2891–2896. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li W, Qin Y, Zhou R, Liu Y and Zhang G:
High expression of SIX1 is an independent predictor of poor
prognosis in endometrial cancer. Am J Transl Res. 13:2840–2848.
2021.PubMed/NCBI
|
|
28
|
Bose S and Le A: Glucose metabolism in
cancer. Adv Exp Med Biol. 1063:3–12. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Luengo A, Gui DY and Vander Heiden MG:
Targeting metabolism for cancer therapy. Cell Chem Biol.
24:1161–1180. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pascale RM, Calvisi DF, Simile MM, Feo CF
and Feo F: The Warburg effect 97 years after its discovery. Cancers
(Basel). 12:28192020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Vaupel P, Schmidberger H and Mayer A: The
Warburg effect: Essential part of metabolic reprogramming and
central contributor to cancer progression. Int J Radiat Biol.
95:912–919. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ishibashi K, Egami R, Nakai K and Kon S:
An anti-tumorigenic role of the Warburg effect at emergence of
transformed cells. Cell Struct Funct. 43:171–176. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bar-Or D, Carrick M, Tanner A II, Lieser
MJ, Rael LT and Brody E: Overcoming the Warburg effect: Is it the
key to survival in sepsis? J Crit Care. 43:197–201. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li Z, Zhang Y, Zhou Y, Wang F, Yin C, Ding
L and Zhang S: Tanshinone IIA suppresses the progression of lung
adenocarcinoma through regulating CCNA2-CDK2 complex and AURKA/PLK1
pathway. Sci Rep. 11:236812021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu Z, Zhu W, Kong X, Chen X, Sun X, Zhang
W and Zhang R: Tanshinone IIA inhibits glucose metabolism leading
to apoptosis in cervical cancer. Oncol Rep. 42:1893–1903.
2019.PubMed/NCBI
|
|
36
|
Li M, Gao F, Zhao Q, Zuo H, Liu W and Li
W: Tanshinone IIA inhibits oral squamous cell carcinoma via
reducing Akt-c-Myc signaling-mediated aerobic glycolysis. Cell
Death Dis. 11:3812020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang HS, Zhang FJ, Li H, Liu Y, Du GY and
Huang YH: Tanshinone IIA inhibits human esophageal cancer cell
growth through miR-122-mediated PKM2 down-regulation. Arch Biochem
Biophys. 598:50–56. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nie ZY, Liu XJ, Zhan Y, Liu MH, Zhang XY,
Li ZY, Lu YQ, Luo JM and Yang L: miR-140-5p induces cell apoptosis
and decreases Warburg effect in chronic myeloid leukemia by
targeting SIX1. Biosci Rep. 39:BSR201901502019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang H, Xue W, Ouyang W and Jiang X and
Jiang X: miR-23a-3p/SIX1 regulates glucose uptake and proliferation
through GLUT3 in head and neck squamous cell carcinomas. J Cancer.
11:2529–2539. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhou H, Blevins MA, Hsu JY, Kong D,
Galbraith MD, Goodspeed A, Culp-Hill R, Oliphant MUJ, Ramirez D,
Zhang L, et al: Identification of a small-molecule inhibitor that
disrupts the SIX1/EYA2 complex, EMT, and metastasis. Cancer Res.
80:2689–2702. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chu Y, Jiang M, Wu N, Xu B, Li W, Liu H,
Su S, Shi Y, Liu H, Gao X, et al: O-GlcNAcylation of SIX1 enhances
its stability and promotes hepatocellular carcinoma proliferation.
Theranostics. 10:9830–9842. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sui H, Zhao J, Zhou L, Wen H, Deng W, Li
C, Ji Q, Liu X, Feng Y, Chai N, et al: Tanshinone IIA inhibits
β-catenin/VEGF-mediated angiogenesis by targeting TGF-β1 in
normoxic and HIF-1α in hypoxic microenvironments in human
colorectal cancer. Cancer Lett. 403:86–97. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li G, Shan C, Liu L, Zhou T, Zhou J, Hu X,
Chen Y, Cui H and Gao N: Tanshinone IIA inhibits HIF-1α and VEGF
expression in breast cancer cells via mTOR/p70S6K/RPS6/4E-BP1
signaling pathway. PLoS One. 10:e01174402015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zeng J, Shi R, Cai CX, Liu XR, Song YB,
Wei M and Ma WL: Increased expression of Six1 correlates with
progression and prognosis of prostate cancer. Cancer Cell Int.
15:632015. View Article : Google Scholar : PubMed/NCBI
|