Introduction
Warthin's tumor is a type of benign tumor that
develops with a membrane, which is frequently composed of cystic
adenoid and papillary structures with lymphoid stroma (1). Malignant transformation may occur
occasionally and the epithelial components may transform into
squamous cell carcinoma, adenocarcinoma or mucoepidermoid carcinoma
(2,3), of which transformation into squamous
cell carcinoma is rarest (4,5). The
main morphological features of the malignant transformation of
Warthin's tumor into squamous cell carcinoma are a scaly
eosinophilic columnar epithelium, atypical hyperplasia and
cancerization. Immunohistochemistry frequently indicates
cytokeratin (CK)5/6, P40, CK7, CK18 and CK8 positivity (5). The present study reports on a case of
malignant transformation of Warthin's tumor to squamous cell
carcinoma and in addition, the relevant literature was reviewed to
explore the clinicopathological features, modes of diagnosis (and
differential diagnoses), biological behavior and prognosis of this
type of tumor.
Case report
Case presentation
A 67-year-old male patient was admitted to Xiaoshan
Affiliated Hospital of Wenzhou Medical University (Hangzhou, China)
in December 2021 due to a parotid gland mass that appeared 15 days
previously. The left and right sides of the patient's face were
asymmetrical and there was a lump below the right ear, with the
absence of pain and numbness. On physical examination, a lump in
the right parotid gland area with a clear boundary, medium
hardness, slight mobility, no tenderness and no deviation of the
mouth and other facial nerve injuries were noted. There was no
redness or swelling at the parotid duct orifice and a clear fluid
oozed out upon squeezing. B-ultrasound of the parotid gland
indicated a low-echo light mass with a size of ~4.4×2.2
cm2 in the right parotid gland, with a clear boundary
and irregular shape; the internal echo was grid-shaped. Cystic dark
areas and blood flow signals were observed in the light mass.
Initially, these findings were assumed to resemble a Warthin's
tumor. After two days, the mass of the right parotid gland and the
superficial and deep lobes of the right parotid gland were excised
under general anesthesia. During the operation, it was observed
that the tumor was wrapped in the parotid gland tissue and the mass
was removed together with the surrounding parotid gland tissue. The
patient did not receive any postoperative treatment. At the last
follow-up in February 2022, the patient was in a good postoperative
condition, with good wound healing and no postoperative
complications.
Pathological findings
Macro-examination
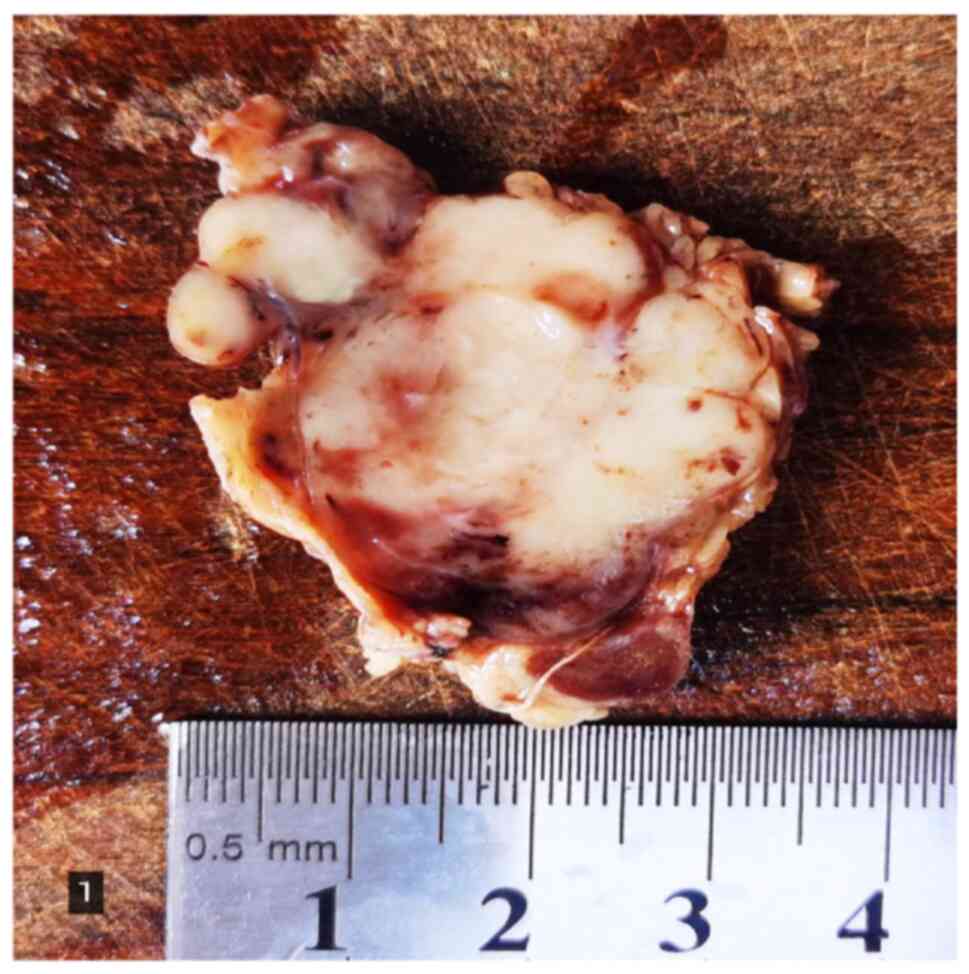
A piece of grayish-yellow parotid gland tissue,
measuring 5×3×3.5 cm; a grayish-white multi-nodular mass with a
size of 4.8×2.5×3.0 cm was observed on the cut surface and the
boundary with the surrounding parotid gland tissue was not clear.
There was a local capsule and the mass was solid and hard,
exhibiting local bleeding (Fig.
1). The tissue was fixed with 4% neutral formalin and embedded
in paraffin, and 4-µm serial sections were prepared that were
subjected to H&E staining and envision immunohistochemical
staining.
Microscopic observation
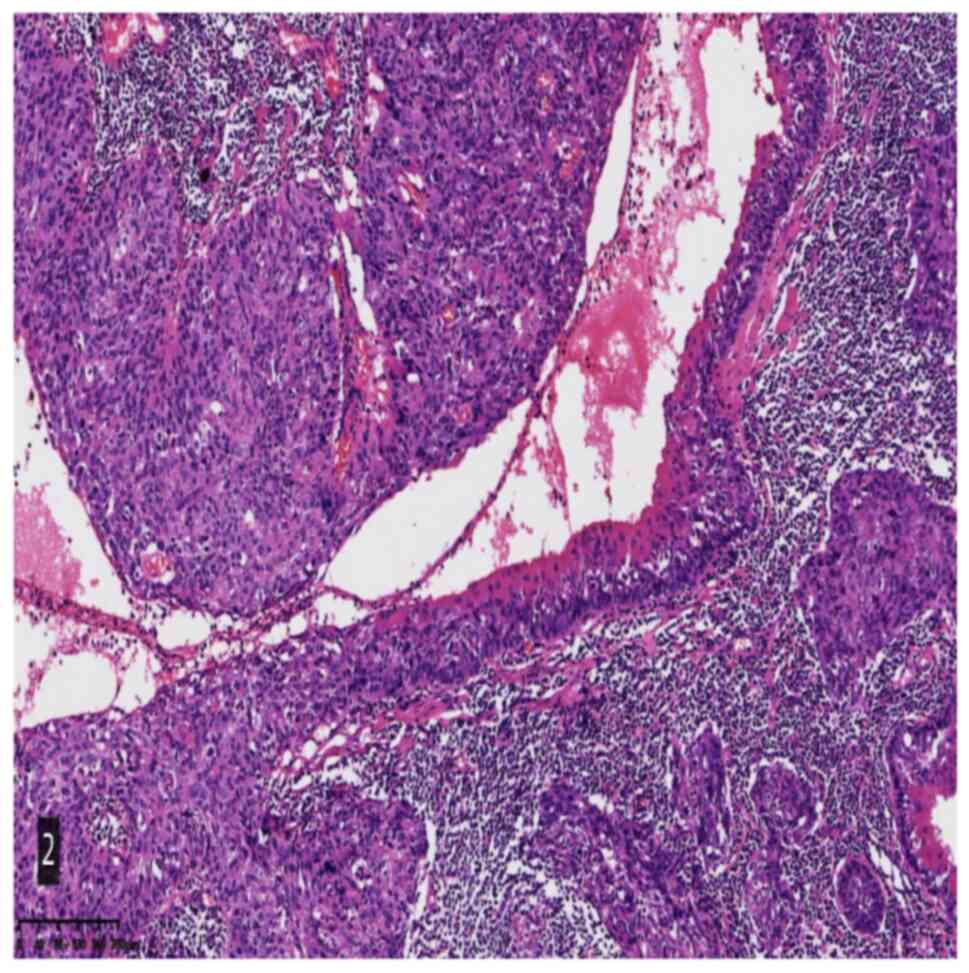
Histological analysis indicated that the tumor was
mainly composed of squamous cell carcinoma and lymphoid stroma. The
squamous cell carcinoma cells were arranged in a solid flake-like,
papillary and cystic shape. The squamous cell carcinoma cells were
clearly deemed to be heteromorphic and the mitotic images were easy
to observe. The focal tumor cells were transparent and vacuolar. A
large number of lymphoid stroma-associated centers were observed
between the cancer cells. Germinal centers were also present and
the remnants of benign Warthin's tumors were observed in certain
areas. It was also noted that the eosinophilic columnar epithelium
gradually underwent a dynamic process of scaling, atypical
hyperplasia and carcinogenesis (Fig.
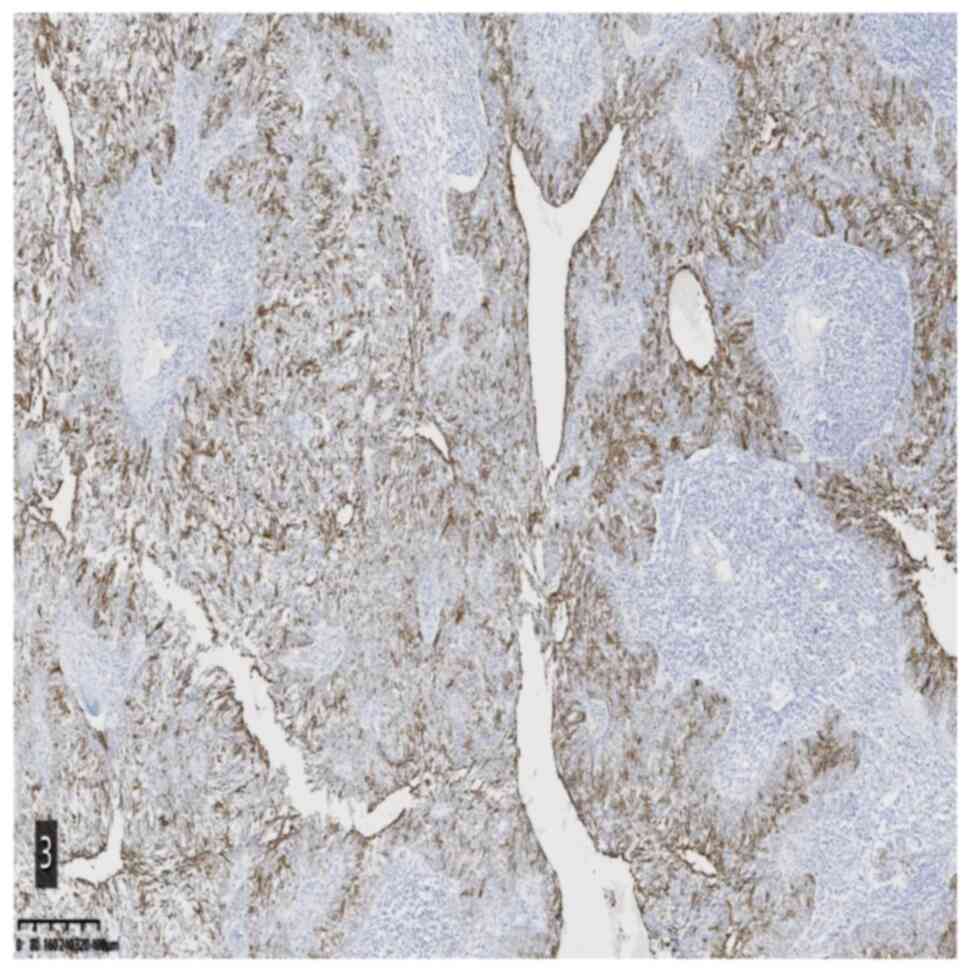
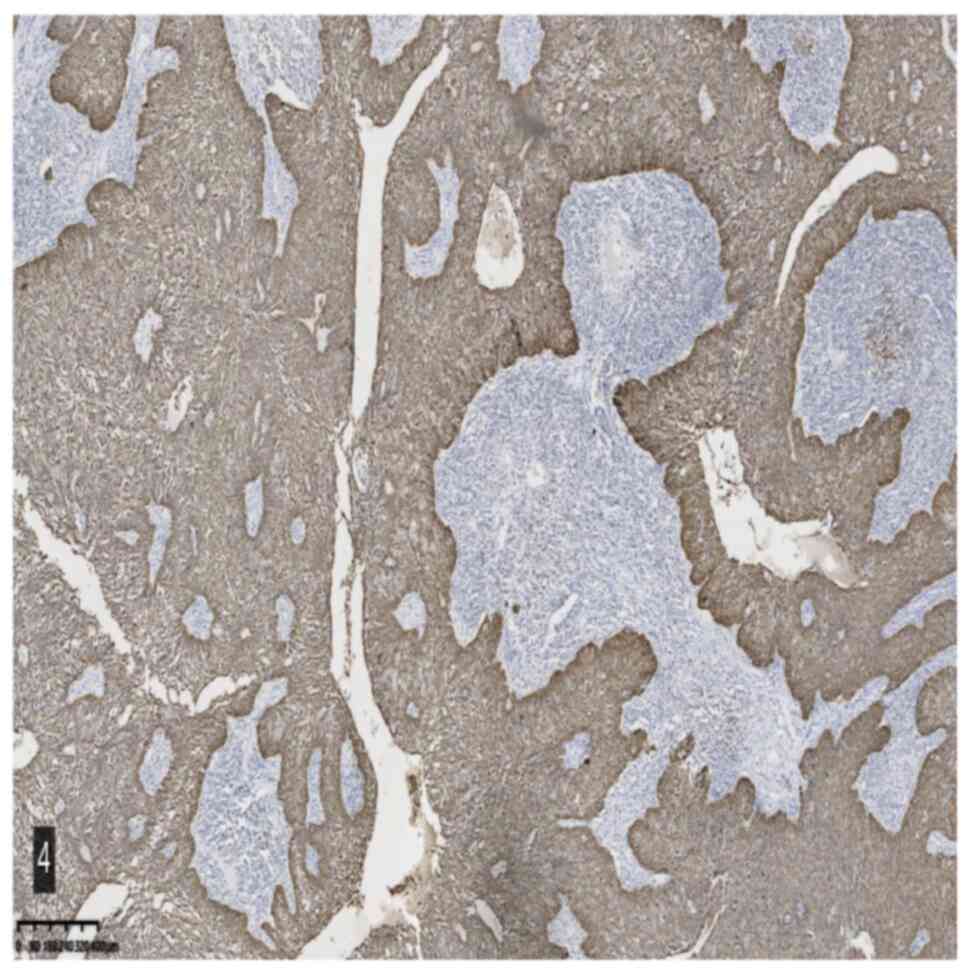
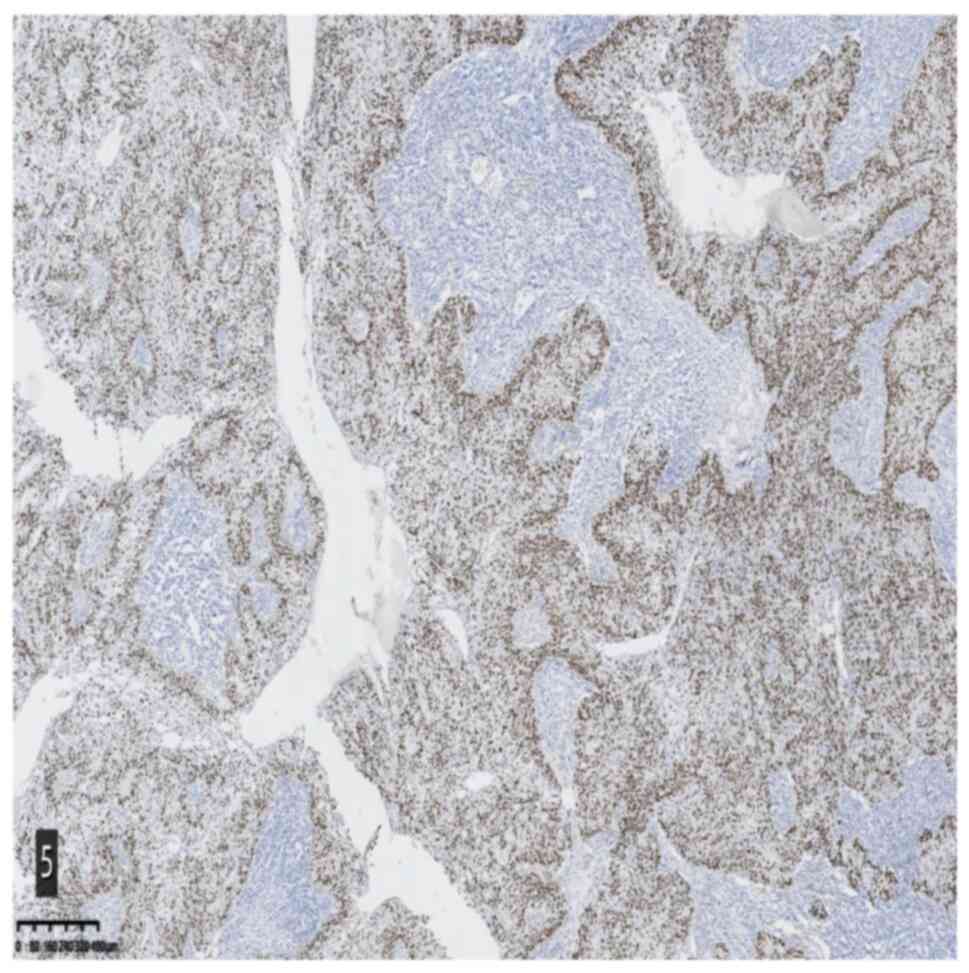
2). Immunohistochemical staining and specific staining provided
the following results: CK5/6 (epithelial +), Ki-67 (epithelial 25%
+), p53 (wild-type), P40 (epithelial +), CK7 (epithelial +), CK18
(epithelial +), CK8 (epithelial +), Epstein-Barr encoding region
(EBER) in situ hybridization (−), MutS protein homolog 2
(MSH2) (+) and Alcian blue/periodic acid-Schiff staining (focal +)
(Fig. 3,Fig. 4,Fig.
5).
Pathological diagnosis
The diagnosis was poorly differentiated squamous
cell carcinoma (malignant transformation from Warthin's tumor).
Discussion
Warthin's tumor, also known as ‘adenolymphoma’ or
‘papillary cystadenoma lymphomatosum’, is the second most common
type of salivary gland tumor. It frequently occurs in middle-aged
and elderly individuals (age, 40–70 years) and the condition is
also more prevalent among males. Patients frequently present with
painless tumors of the parotid gland, which may occur frequently or
symmetrically on both sides. The average size of the tumor is ~2–4
cm. Approximately 8% of Warthin's tumors occur in the cervical
lymph nodes outside the parotid gland (6). Warthin's tumor frequently presents
with a characteristic image through imaging techniques that are
used for diagnosis (7). When it
becomes malignant, imaging still retains the characteristics of
Warthin's tumor. Therefore, it may be easily distinguished from
other malignant tumor types of the parotid gland. In the present
case, initial B-ultrasound of the parotid gland also indicated
Warthin's tumor and malignant transformation was detected only
through subsequent pathological examination.
Warthin's tumor is generally a well-defined round or
oval mass with a smooth surface and capsule. The slices are
grayish-brown, cystic and lobulated, and are also characterized by
a sulfur granule paste. Its nuclei are oval and deeply stained,
close to the cavity surface, with inverted polarity and palisade
arrangement. The outer layers of bilateral structures are small
flat or cubic basal cells, which express immunohistochemical
markers such as CK5/6 and P40. The inner and outer cells are
usually staggered and arranged in a pseudo-stratified structure
(8). Mucinous metaplasia cells and
sebaceous metaplasia cells may also be observed in the lesions
(9). According to the number of
tumor cells and lymphatic stroma, the tumors may be divided into
four subtypes: Classic type, less stromal component type, more
stromal component type and degenerative variant type.
Most scholars have reached a consensus on the
pathogenesis of Warthin's tumor into squamous cell carcinoma and it
is thought that squamous metaplasia occurs in the inner layer
epithelium of eosinophilic hypercolumns. It occurs under the action
of multiple factors such as ischemia, hyperplasia and
cancerization, eventually developing into invasive squamous cell
carcinoma (4,5,10).
This dynamic process distinguishes this tumor from malignant tumors
that metastasize from other sites (11,12).
The lymphoid stroma frequently contains germinal centers, which may
be due to the immune response of the immune system to tumor
epithelial cells (13). Since the
superficial lymph nodes of the parotid gland are located on the
surface of the parotid gland, they drain the lymph nodes of the
forehead, skull top, temporal area, auricle, external auditory
canal, cheek and parotid gland; therefore, malignant tumors in the
parotid gland should be excluded from the above-mentioned malignant
tumors. In addition, malignant tumors with hematogenous metastasis
from the lungs, breasts, kidneys, gastrointestinal tract and colon
should also be excluded. In such circumstances, it is particularly
important to combine information from the patient's clinical
history.
Malignant transformation of Warthin's tumor to
squamous cell carcinoma should be differentiated from the following
diseases. First, lymphoepithelial carcinoma: It is a malignant
tumor prone to occurring at the parotid gland, with a wide age
distribution of 10–90 years and obvious ethnic and regional
distribution characteristics. The occurrence of certain
lymphoepithelial carcinomas is related to EB virus infection.
Microscopically, the tumor usually has no capsule and invades the
surrounding normal parotid gland tissue. Heterotypic neoplastic
epithelial cells are arranged in flakes or nests or have a single
scattered, infiltrating growth. The stroma is rich in lymphocytes
and the formation of lymphoid follicles may be observed. Tumor
cells are polygonal, with unclear cell borders; they are fused with
each other; have a lightly stained cytoplasm, round vacuoles in the
nucleus and obvious nucleoli; they also feature easy-to-observe
pathological mitosis and necrosis. Tumor cells are frequently
positive for expression of CKpan, CK5/6, P40 and Ki-67, and the
positive index is usually high; EBER is positive in most tumors
detected by in situ hybridization (14).
Second, the cyst wall epithelium in the parotid
cleft cyst turning malignant into squamous cell carcinoma: The
parotid cleft cyst is a developmental cyst, once known as the
‘lymphoepithelial cyst’, which primarily occurs before puberty. The
mass is slow-growing, well-defined, mobile and cystic. The cyst
contains an egg white-like liquid. The lining epithelium of the
cyst wall contains stratified squamous epithelium without obvious
keratosis, which may be mixed with stratified columnar epithelium
and may be accompanied by incomplete keratosis. Furthermore, the
epithelium is thin without the nail process. The fibrous capsule
wall contains a large number of lymphoid tissues, which may form
lymphoid follicles. The stratified squamous epithelium of the
capsule wall may produce atypical hyperplasia and cancerization
under the stimulation of numerous factors. It differs from the
malignant transformation of Warthin's tumor in that it does not
have a double-layered cellular structure (15).
Third, sebaceous adenocarcinoma: It usually occurs
in the elderly and is frequently located in areas rich in sebaceous
glands, i.e., in the eyelids, ears, head and neck. Microscopically,
it presents as irregular diffuse or solid nodular infiltrative
growth and is composed of basal-like cells and sebaceous gland
cells. It is frequently dominated by basal-like cells. Cells with
less cytoplasm, obvious atypia and clear mitotic images may be
observed without difficulty. Sebaceous gland cells are rich in
cytoplasm, are bright or vacuolar and have common scaly and ductal
structures; immunohistochemical characteristics are expression of
CKpan and epithelial membrane antigen (16).
Fourth, benign lymphoepithelial lesion: It is an
autoimmune disease that usually occurs in middle-aged and elderly
females. The clinical features are unilateral or bilateral parotid
or submandibular gland enlargement and diffusely enlarged salivary
glands with unclear borders, and certain tumors may form tumor-like
nodules. Patients occasionally experience symptoms of pain and dry
mouth. The disease is closely related to Shegren's syndrome.
Clinically, almost all patients with Shegren's syndrome suffer from
lymphoepithelial sialadenitis; however, only 50% of patients with
lymphoepithelial sialadenitis have manifestations of Shegren's
syndrome. The typical pathological changes are benign
lymphoepithelial lesions formed by dense multifocal, progressive
lymphocyte infiltration, acinar atrophy and residual ductal
hyperplasia. Lymphoid follicles containing germinal centers may
appear in dense lymphocyte infiltration areas (17).
The treatment of malignant transformation of
Warthin's tumor to squamous cell carcinoma is surgical resection
with negative margins. Postoperative radiotherapy or chemotherapy
usually have no effect on prognosis (5). Malignant transformation of Warthin's
tumor into squamous cell carcinoma may lead to local lymph node
metastasis, but distant metastasis is rare. Small size, negative
margins, no distant metastasis, low histological grade and early
clinical stage are indicators of good prognosis. Patients with
positive tumor margin and lymph node metastasis typically have poor
prognosis (9). The patient of the
present study was followed up for 6 months after complete resection
of the mass and there was no recurrence or metastasis.
In summary, the present study reported a case of
malignant transformation of Warthin's tumor into squamous cell
carcinoma. Squamous cell carcinoma is the rarest type. The present
case not only showcased that the squamous cell carcinoma cells are
arranged in a solid flake-like, papillary and cystic shape but also
emphasized the expression of CK5/6, P40, CK7, CK18, CK8 and MSH2 in
the diagnosis of malignant transformation of Warthin's tumor into
squamous cell carcinoma. The present study also explored the
clinicopathological features, diagnosis and differential diagnosis,
biological behavior and prognosis of this tumor type.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LS and JY drafted the manuscript and conceived the
study. XC was in charge of the case data collection. YJ revised the
manuscript and interpreted the data. LS and YJ confirm the
authenticity of all the raw data. All authors agreed on the journal
to which the article has been submitted and agreed to be
accountable for all aspects of the work. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided written informed consent for
the case study to be published.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kim JE and Kim TG: Squamous cell carcinoma
arising from Warthin's tumor in the parotid gland. BJR Case Rep.
5:201900322019.PubMed/NCBI
|
|
2
|
Sayar H, Öztarakçi H, Sayar Ç and Ağirbaş
Ş: Adenocarcinoma arising in warthin tumor of the parotid gland.
Turk Patoloji Derg. 28:278–281. 2012.PubMed/NCBI
|
|
3
|
Deodhar KK, Shah M and Chaturvedi P:
High-grade adenocarcinoma, (ductal type) arising in unilateral
Warthin tumor of the parotid gland. Indian J Pathol Microbiol.
54:574–577. 2011. View Article : Google Scholar
|
|
4
|
Yaranal PJ and T U: Squamous cell
carcinoma arising in Warthin's tumour: A case report. J Clin Diagn
Res. 7:163–165. 2013.PubMed/NCBI
|
|
5
|
Allevi F and Biglioli F: Squamous
carcinoma arising in a parotid Warthin's tumour. BMJ Case Rep.
2014:bcr20142078702014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brown NA and Elenitoba-Johnson KS: Update
from the 4th Edition of the World health organization
classification of head and neck tumours: Hematolymphoid tumours.
Head Neck Pathol. 11:96–109. 2017. View Article : Google Scholar
|
|
7
|
Yuan WH, Hsu HC, Chou YH, Hsueh HC, Tseng
TK and Tiu CM: Gray-scale and color Doppler ultrasonographic
features of pleomorphic adenoma and Warthin's tumor in major
salivary glands. Clin Imaging. 33:348–353. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Seethala RR, Thompson LD, Gnepp DR, Barnes
EL, Skalova A, Montone K, Kane S, Lewis JS Jr, Solomon LW, Simpson
RH, et al: Lymphadenoma of the salivary gland: Clinicopathological
and immunohistochemical analysis of 33 tumors. Mod Pathol.
25:26–35. 2012. View Article : Google Scholar
|
|
9
|
Sentani K, Ogawa I, Ozasa K, Sadakane A,
Utada M, Tsuya T, Kajihara H, Yonehara S, Takeshima Y and Yasui W:
Characteristics of 5015 salivary gland neoplasms registered in the
hiroshima tumor tissue registry over a period of 39 years. J Clin
Med. 8:5662019. View Article : Google Scholar
|
|
10
|
Flezar M and Pogacnik A: Warthin's tumour:
Unusual vs. common morphological findings in fine needle aspiration
biopsies. Cytopathology. 13:232–241. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kuzenko YV, Romanuk AM, Dyachenko OO and
Hudymenko O: Pathogenesis of Warthin's tumors. Interv Med Appl Sci.
8:41–48. 2016.
|
|
12
|
Moore FO, Abdel-Misih RZ, Berne JD, Zieske
AW, Rana NR and Ryckman JG: Poorly differentiated carcinoma arising
in a Warthin's tumor of the parotid gland: Pathogenesis,
histopathology, and surgical management of malignant Warthin's
tumors. Am Surg. 73:397–399. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Park CK, Manning JT Jr, Battifora H and
Medeiros LJ: Follicle center lymphoma and Warthin tumor involving
the same anatomic site. Report of two cases and review of the
literature. Am J Clin Pathol. 113:113–119. 2000. View Article : Google Scholar
|
|
14
|
Zheng HH, Weng SX and Gan MF:
Clinicopathological features of the lymphoepithelioma-like
carcinoma. Zhonghua Bing Li Xue Za Zhi. 49:74–76. 2020.(In
Chinese).
|
|
15
|
Thong HK, Athar PPSH and Mustaffa WMW:
Benign lymphoepithelial cyst: An unusual cause of parotid swelling
in two immunocompetent patients. Open Access Maced J Med Sci.
7:2142–2145. 2019. View Article : Google Scholar
|
|
16
|
Ihrler S, Weiler C, Eckert F and
Mollenhauer M: Cutaneous adnexal and salivary gland tumours.
Similarities and differences. Pathologe. 35:476–486. 2014.(In
German). View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jeong HS, Lee HK, Ha YJ, Kim DH and Suh
IS: Benign lymphoepithelial lesion of parotid gland and secondary
amyloidosis as concurrent manifestations in Sjögren's Syndrome.
Arch Plast Surg. 42:380–383. 2015. View Article : Google Scholar : PubMed/NCBI
|