Introduction
Multiple myeloma (MM) is a mature B cell malignancy
characterized by an excessive production of monoclonal antibodies
in the bloodstream, which leads to end organ damage, such as renal
failure and lytic bone lesions (1,2). It
is worth noting that, due to improvements in the pharmaceutical
industry, a number of novel therapies have recently been developed
for the treatment of MM (3).
Bortezomib (BTZ), one of the novel chemotherapy drugs, is widely
used to treat MM (4–6). Previous studies found that BTZ
inhibited proteasome degradation and lead to a favorable clinical
outcome in patients with MM (4–6).
However, some patients with MM develop BTZ resistance following BTZ
administration for a prolonged period of time, partly due to
dysregulated cellular metabolic activity and altered intracellular
signaling pathways (7,8). Furthermore, a poor prognosis has been
reported in MM cases with BTZ resistance, reflected by a shorter
overall survival time and disease recurrence (9). Thus, relevant studies on the
mechanism of BTZ resistance are required to reverse this
resistance.
β-catenin, a key protein of the Wnt/β-catenin
signaling pathway, plays a critical role in MM pathogenesis
(10,11). Furthermore, from the existing
evidence, β-catenin inhibitors have been shown to exert a positive
effect in the treatment of MM. As a commonly used β-catenin
inhibitor, ICG-001 induces MM cell apoptosis via the activation of
pro-apoptotic proteins, phorbol-12-myristate-13-acetate-induced
protein 1 and p53 upregulated modulator of apoptosis (12). Another β-catenin inhibitor,
pyrvinium (PP), promotes intracellular β-catenin degradation and
leads to enhanced cell apoptosis activity in MM cells (13). In addition, another in vitro
study showed that PP enabled the suppression of mitochondrial
respiratory complex I to inhibit cell proliferation in MM cells
(14). Apart from ICG-001 and PP,
other β-catenin inhibitors (such as C-82) are available; however,
it has not been used to treat hematology-related diseases (15). Previous studies have investigated
the direct therapeutic effect of ICG-001 and PP on MM; however, the
synergistic effect of β-catenin inhibitors plus BTZ requires
further investigation. Therefore, the aim of the present study was
to investigate the therapeutic effect of β-catenin inhibitors
administered with BTZ in BTZ-resistant MM cells.
Materials and methods
Cell culture and reagents
The RPMI-8226 (cat. no. JCRB00344), KMS-11 (cat. no.
JCRB1179) and BTZ-resistant KMS-11 (KMS-11BR; cat. no. JCRB1642)
human MM cell lines were purchased from the Japanese Collection of
Research Bioresources Cell Bank and cultured with RPMI-1640 medium
(cat. no. A3823) containing 10% FBS (cat. no. 26170035) (both from
Thermo Fisher Scientific, Inc.). The BTZ-resistant RPMI-8226
(RPMI-8226BR) cell line was constructed by increasing the
concentration of BTZ stepwise over a period of 3 months and was
adapted to a final concentration of 20 nM BTZ, and the detailed
procedure was adapted from a previous study (16). BTZ (cat. no. S1013), ICG-001 (cat.
no. S262) and PP (cat. no. 5816) were purchased from Selleck
Chemicals. The Cell Counting Kit (CCK)-8 (cat. no. C0037), Annexin
V-FITC Apoptosis Detection kit (cat. no. C1067S), RIPA Lysis Buffer
(cat. no. P0013C), SDS-PAGE sample loading buffer (cat. no. P0015),
BCA Protein Assay kit (cat. no. P0012S), 4-20% precast PAGE (cat.
no P0670) and BeyoECL Plus (cat. no. P0018FS) were purchased from
Beyotime Institute of Biotechnology. The nitrocellulose (NC) filter
membrane (cat. no. GSWP037000) was purchased from Merck KGaA.
BTZ treatment
The RPMI-8226, RPMI-8226BR, KMS-11 and KMS-11BR cell
lines were cultured with different concentrations (0, 1, 2, 3, 4, 8
and 16 nM) of BTZ for 24 h. The cell viability was then evaluated
using CCK-8, according to the manufacturer's instructions.
Western blot analysis
To evaluate the protein expression levels of
β-catenin, GSK-3β, phosphorylated (p)GSK-3β and c-Myc, western blot
analysis was performed. The RPMI-8226, RPMI-8226BR, KMS-11 and
KMS-11BR cell lines were lysed with RIPA Lysis Buffer. Following
quantification with the BCA Protein Assay kit, 20 µg total protein
was separated using 4-20% precast PAGE. The protein was then
transferred to a NC membrane. The membrane was blocked with 5% BSA
at 37°C for 1 h and incubated with the primary antibodies at 4°C
overnight, then incubated with a secondary antibody at 37°C for 1
h. Finally, the protein bands were visualized with BeyoECL Plus.
The antibodies used for western blot analysis are listed in
Table SI. For densitometry
analysis, ImageJ v1.8.0 (National Institutes of Health) was used.
The relative protein expression for β-catenin and c-Myc was
calculated as the absolute protein density/GAPDH density, while the
relative protein expression for pGSK-3β was calculated as the
pGSK-3β density/GSK-3β density.
ICG-001 and PP treatment
Different concentrations of ICG-001 (0, 1, 2, 4, 8,
16 and 32 µM) or PP (0, 1, 2, 4, 8, 16 and 32 nM) were added to the
RPMI-8226BR and KMS-11BR cell lines for 24 h. Next, CCK-8 was used
to determine cell viability.
Combination treatment
he RPMI-8226BR cell line was cultured with 8 nM BTZ
alone, 32 µM ICG-001 alone, 32 nM PP alone, 8 nM BTZ plus 32 µM
ICG-001 or 8 nM BTZ plus 32 nM PP for 24 h, while the KMS-11BR cell
line was cultured with 6 nM BTZ alone, 32 µM ICG-001 alone, 32 nM
PP alone, 6 nM BTZ plus 32 µM ICG-001 or 6 nM BTZ plus 32 nM PP for
24 h. Following incubation, the CCK-8 and Annexin V-FITC Apoptosis
Detection kits were used to assess cell viability and apoptosis,
respectively. The RPMI-8226BR and KMS-11BR cells cultured in normal
medium were used as a control. Furthermore, the Chou-Talalay method
was used to calculate the combination index of the β-catenin
inhibitors and BTZ, according to a previous study (17).
Cell viability
CCK-8 was used to determine relative cell viability.
In brief, the cells were washed with PBS, and then 10 µl CCK-8
regent and 100 µl serum-free RPMI-1640 medium were added to cells.
Next, the cells were incubated for 2 h, and their optical density
(OD) value was recorded at 450 nm. Finally, the relative cell
viability was calculated as the OD value in each tested group/OD
value in the control group ×100.
Cell apoptosis
The Annexin V-FITC Apoptosis Detection kit was used
to assess the cell apoptosis rate. In brief, the cells were
collected and suspended in PBS. Next, 5 µl Annexin V and 5 µl PI
were added to the cell solution for a 15-min incubation in the
dark, at room temperature. Finally, the apoptosis rates were
detected using a BD FACSCelesta™ Flow Cytometer (BD Biosciences)
and analyzed by FlowJo (v7.6.5; FlowJo LLC).
Statistical analysis
GraphPad Prism v7.02 (GraphPad Software Inc.) was
used for data analysis and presentation. The data are presented as
the mean ± standard deviation. Statistical significance was
determined using one-way ANOVA, followed by Dunnett's or Tukey's
multiple comparisons tests. Unpaired Student's t-test was used to
compare two groups. All the experiments were repeated three times.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Validation of BTZ-resistant MM
cells
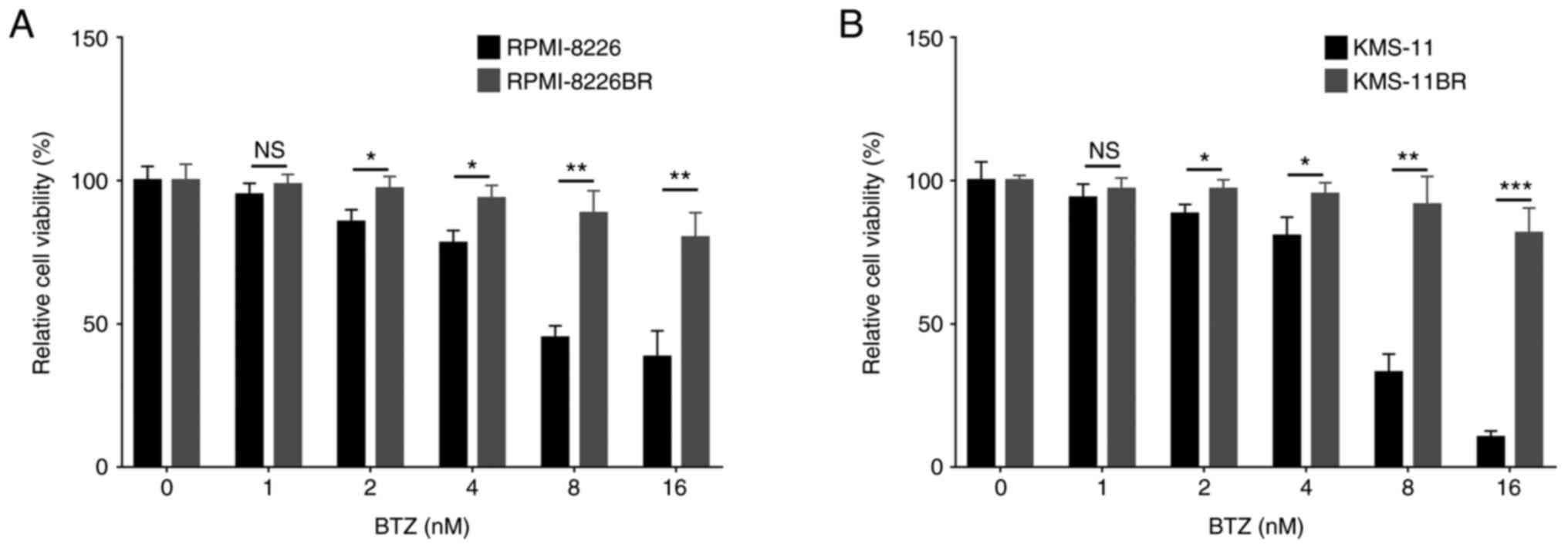
Compared with RPMI-8226 cell line, the relative cell
viability of the RPMI-8226BR cell line was increased under 2
(P<0.05), 4 (P<0.05), 8 (P<0.01) and 16 nM (P<0.01) BTZ
treatment (Fig. 1A). The relative
cell viability of the KMS-11BR cell line was increased following 2
(P<0.05), 4 (P<0.05), 8 (P<0.01) and 16 nM (P<0.001)
BTZ treatment compared with that in the KMS-11 cell line (Fig. 1B).
Activation of the Wnt/β-catenin
signaling pathway in BTZ-resistant MM cells
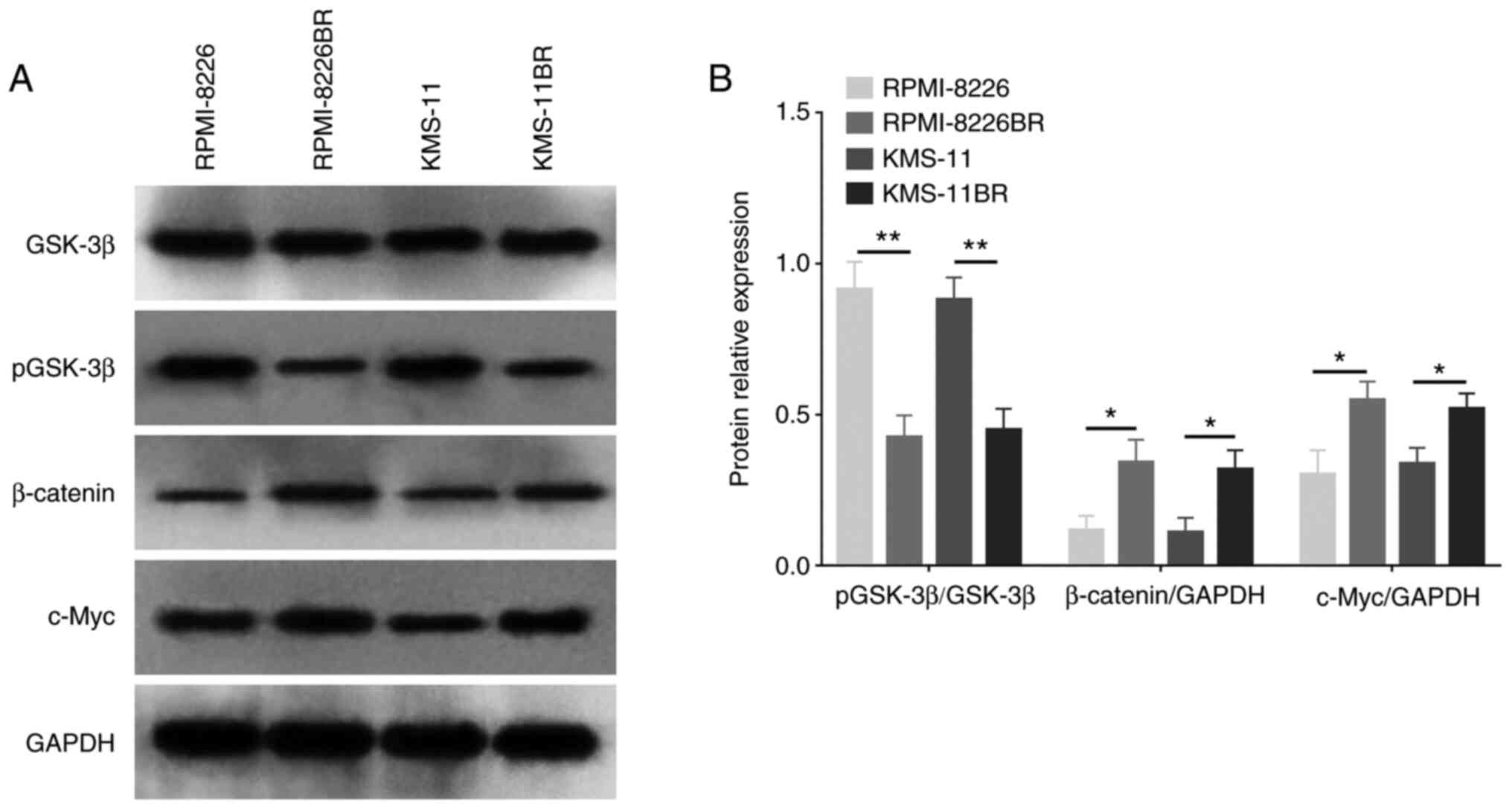
The protein expression levels of both β-catenin and
c-Myc were increased in the BTZ-resistant cell lines compared with
that in the normal MM cell lines (both P<0.05) (Fig. 2A and B). However, pGSK-3β
expression level was decreased in the RPMI-8226BR cell line
compared with that in the RPMI-8226 cell line (P<0.01) (Fig. 2A and B), as well as in the KMS-11BR
cell line compared with that in the KMS-11 cell line (P<0.05)
(Fig. 2A and B). These data
suggested that the activation of the Wnt/β-catenin signaling
pathway might be involved in the mechanism of BTZ resistance in
MM.
Effect of β-catenin inhibitors
(ICG-001 and PP) on BTZ-resistant MM cells
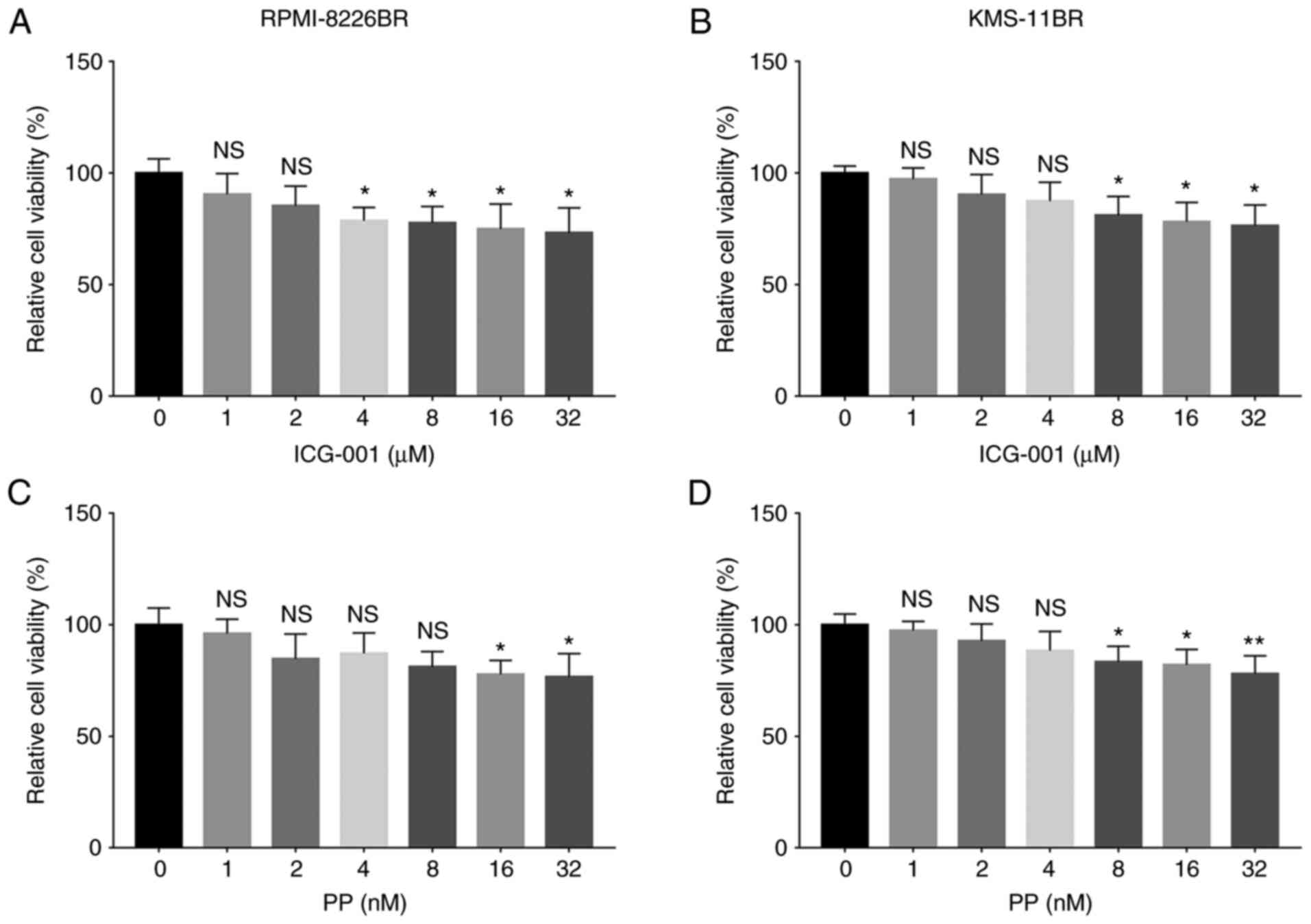
The relative cell viability was significantly
reduced following 4, 8, 16 and 32 µM ICG-001 treatment (all
P<0.05) compared with that in cells treated with 0 µM ICG-001 in
the RPMI-8226BR cell line (Fig.
3A). The relative cell viability was significantly decreased
following 8, 16 and 32 µM ICG-001 treatment (all P<0.05)
compared with that in cells treated with 0 µM ICG-001 in the
KMS-11BR cell line (Fig. 3B).
Furthermore, the relative cell viability was decreased following 16
and 32 nM PP treatment (both P<0.05) compared with that in cells
treated with 0 nM PP in the RPMI-8226BR cell line (Fig. 3C). The relative cell viability was
reduced following 8, 16 and 32 nM (all P<0.05) PP treatment
compared with that in cells treated with 0 nM PP in the KMS-11BR
cell line (Fig. 3D).
β-catenin inhibitors sensitize
BTZ-resistant MM cells to BTZ
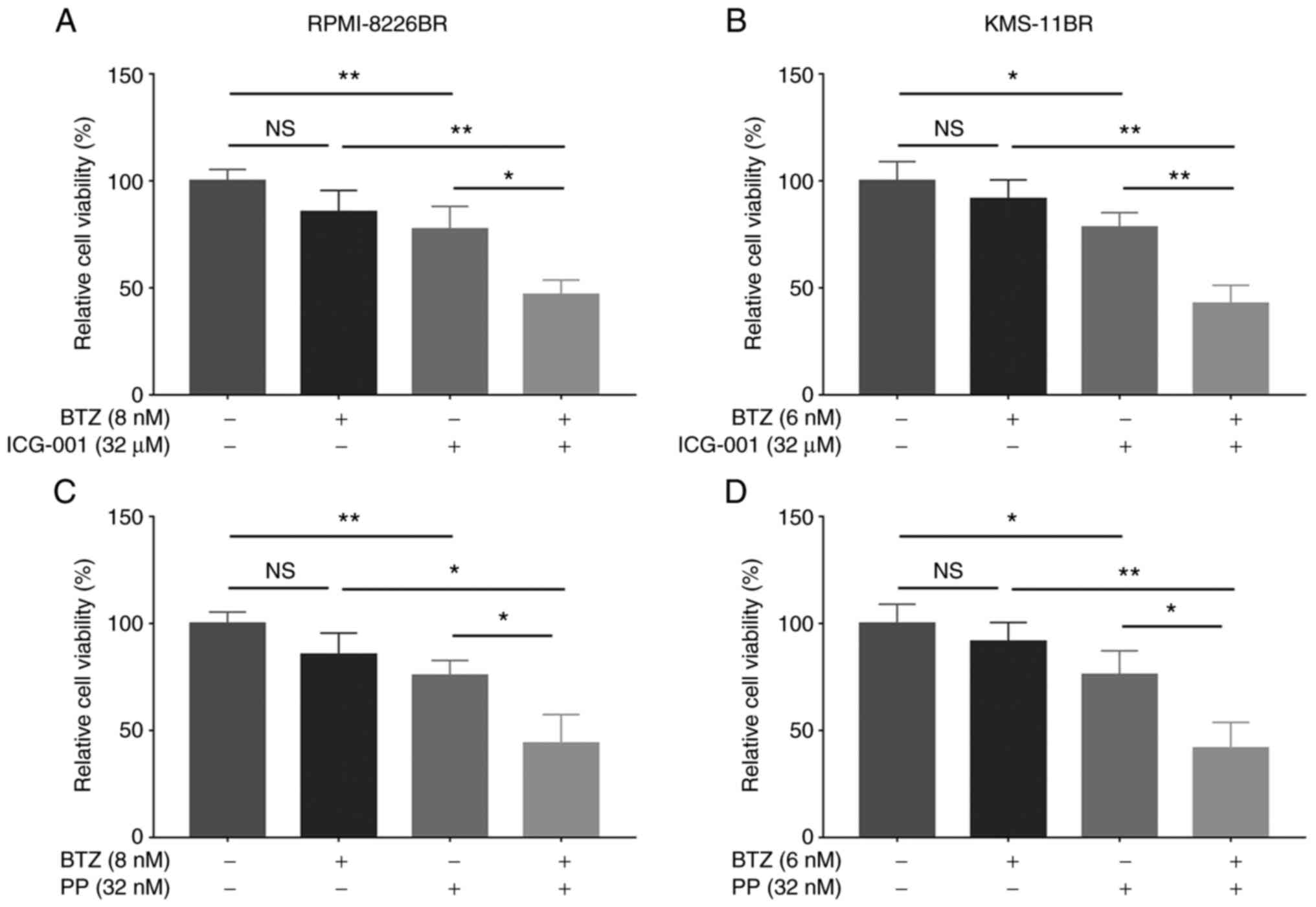
The combination of BTZ with ICG-001 significantly
decreased the relative cell viability compared with that in cells
treated with ICG-001 or BTZ alone in the RPMI-8226BR (all
P<0.05; Fig. 4A) and KMS-11BR
cell lines (all P<0.01; Fig.
4B). In addition, BTZ combined with PP significantly reduced
the relative cell viability compared with that in cells treated
with PP or BTZ alone, in the RPMI-8226BR (all P<0.05; Fig. 4C) and KMS-11BR cell lines (all
P<0.05; Fig. 4D).
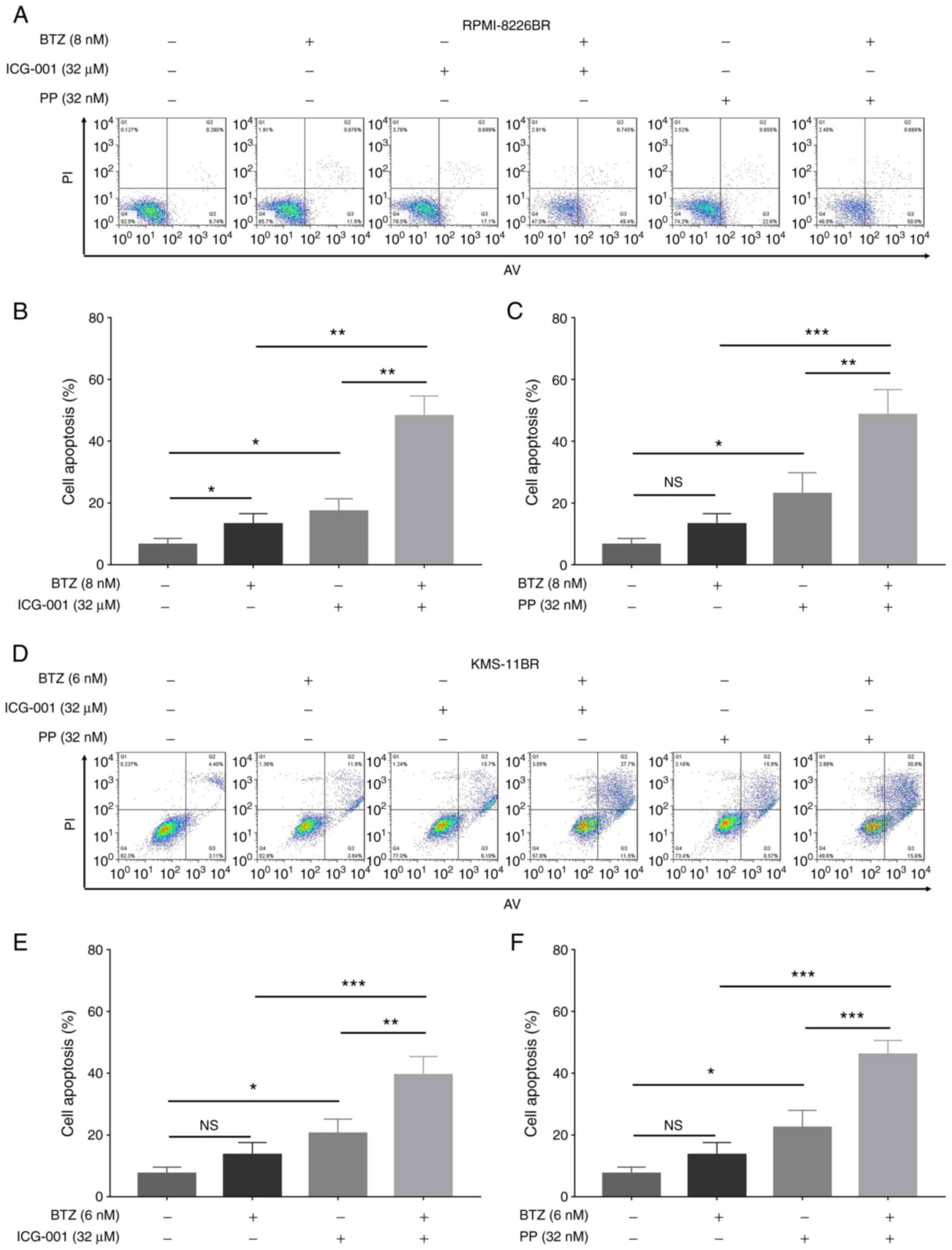
Furthermore, BTZ combined with ICG-001 (all
P<0.01) and BTZ combined with PP (all P<0.05) significantly
increased the cell apoptosis rate compared with that in cells
treated with ICG-001, PP or BTZ alone in the RPMI-8226BR cell line
(Fig. 5A-C). In addition, BTZ
combined with ICG-001 (all P<0.05) and BTZ combined with PP (all
P<0.01) also significantly increased the cell apoptosis rate,
compared with that in cells treated with ICG-001, PP or BTZ alone
in the KMS-11BR cell line (Fig.
5D-F).
Wnt/β-catenin pathway-related
molecular alteration under ICG-001 or PP treatment
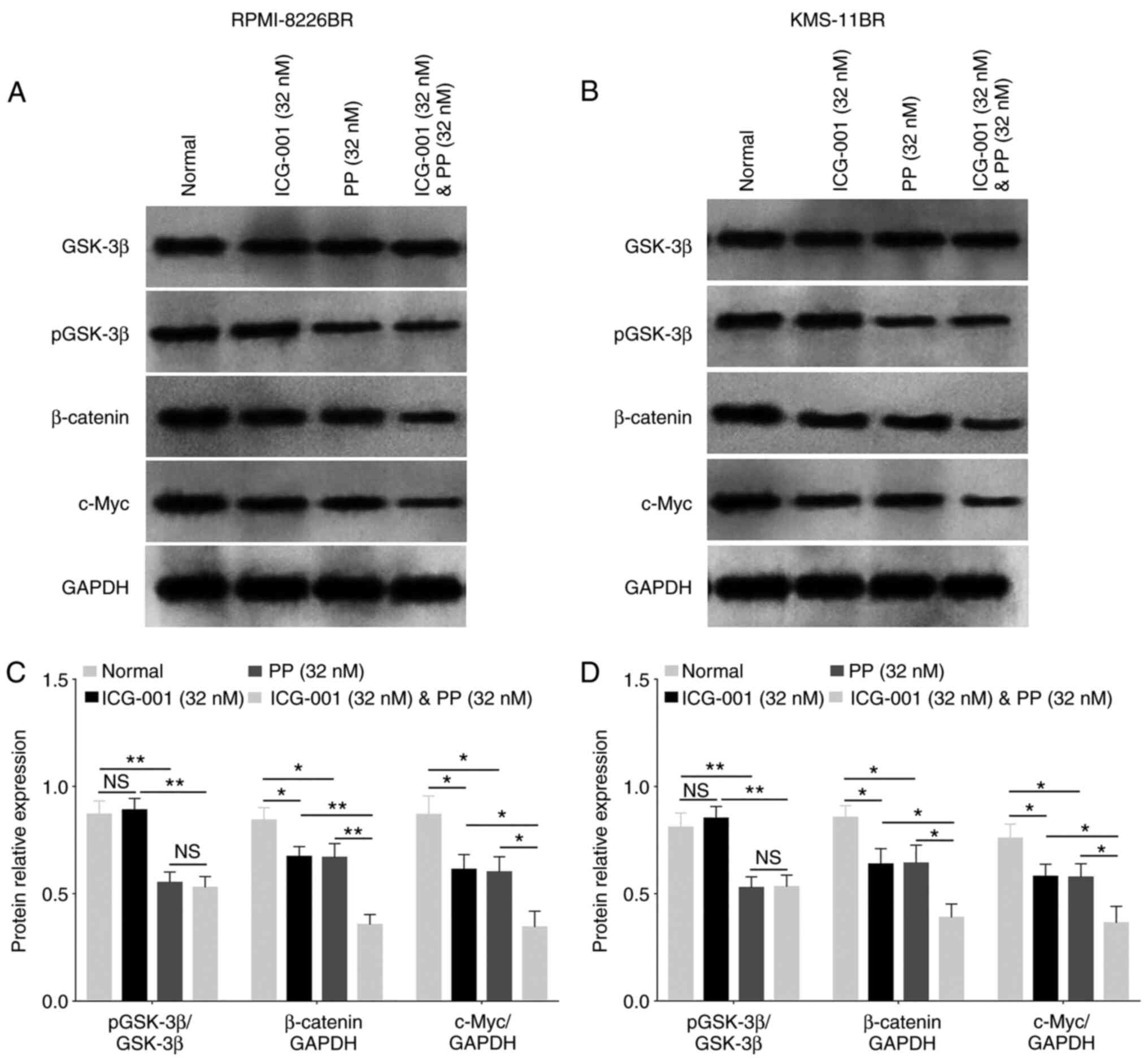
In the RPMI-8226BR cell line, ICG-001 decreased the
protein expression level of β-catenin and c-Myc compared with that
in cells receiving no treatment (both P<0.05; Fig. 6A and C). Furthermore, PP reduced
the pGSK-3β, β-catenin and c-Myc protein expression levels (all
P<0.05; Fig. 6A and C) compared
with those in cells receiving no treatment. Furthermore, ICG-001
plus PP also significantly decreased the pGSK-3β protein expression
level compared with that in cells treated with ICG-001 alone
(P<0.001; Fig. 6A and C). In
addition, ICG-001 plus PP also significantly reduced the β-catenin
and c-Myc protein expression level compared with that in the cells
treated with ICG-001 or PP alone (both P<0.05; Fig. 6A and C).
In the KMS-11BR cell line, ICG-001 decreased the
β-catenin and c-Myc protein expression level compared with that in
cells receiving no treatment (both P<0.01; Fig. 6B and D). In addition, PP reduced
pGSK-3β, β-catenin and c-Myc protein expression level (all
P<0.01; Fig. 6B and D) compared
with that in cells receiving no treatment. Furthermore, ICG-001
plus PP also significantly decreased pGSK-3β protein expression
level compared with that in cells treated with ICG-001 alone
(P<0.001; Fig. 6B and D).
Lastly, ICG-001 plus PP also reduced β-catenin and c-Myc protein
expression level compared with that in cells treated with ICG-001
or PP alone (all P<0.05; Fig. 6B
and D). The data suggested that ICG-001 and PP could
downregulate β-catenin and c-Myc protein expression level.
Combination index of β-catenin
inhibitors and BTZ in BTZ-resistant MM cells
The combination index of BTZ plus ICG-001 in the
RPMI-8226BR and KMS-11BR cell lines was 0.7081 and 0.5538,
respectively. Furthermore, the combination index of BTZ plus PP in
the RPMI-8226BR and KMS-11BR cell lines was 0.5950 and 0.6593,
respectively. These combination values were all <1, which
suggested a synergistic effect of the β-catenin inhibitors and BTZ
in the BTZ-resistant MM cell lines.
Discussion
BTZ has been classified as a peptide boronate, based
on its chemical properties (18);
it has also been shown to be more potent and stable than proteasome
inhibitor peptide aldehydes, which were invented first (18). With respect to its mode of action,
BTZ reversibly inhibits the autophagic-lysosomal pathway and
suppresses protein degradation in MM (6,19).
BTZ suppresses MM cell proliferation via multiple signaling
pathways, such as the NF-κβ and Wnt/β-catenin signaling pathways
(6,20); it also promotes reactive oxygen
species formation, which further leads to mitochondrial damage and
cell apoptosis (21). Notably, it
has been approved for MM treatment in patients with refractory MM
since 2003 and in newly diagnosed patients with MM since 2008 by
the US Food and Drug Administration (6). More importantly, BTZ combined with
dexamethasone can achieve improved clinical outcomes, including an
improved 3-year survival rate and lower hematological toxicity
incidence rate as compared with conventional therapy in patients
with MM, as the first phase of pharmacological therapy (22).
A previous study found that BTZ induced cytotoxicity
in MM cells via multiple signaling pathways (18,21).
For example, BTZ has been shown to decrease β-catenin and c-Myc
protein expression levels in MM cells, indicating the involvement
of the Wnt/β-catenin signaling pathway in its cytotoxicity
(20). The same study also
revealed that the activation of the Wnt/β-catenin signaling pathway
led to a decrease in cell proliferation and an increase in
apoptosis (20). However, limited
research has been performed on the role of the Wnt/β-catenin
signaling pathway in BTZ-resistant MM cells; therefore, the present
study aimed to investigate this area, and it was discovered that
the activation of the Wnt/β-catenin signaling pathway
(characterized by the upregulation of β-catenin and c-Myc and
downregulation of pGSK-3β) might participate in the mechanism of
BTZ resistance using western blot analysis. A possible reason for
this could be due to the binding of the Wnt ligand to its receptor
(Frizzled and LRP 5/6), which causes the phosphorylation of
disheveled, the disassociation of β-catenin from the destruction
complex and the phosphorylation of GSK-3β into its inactive form,
pGSK-3β (23). As a result of the
high intracellular level of β-catenin, the accumulated β-catenin is
translocated and binds to TCF/LEF, as a promotor for target gene
transcription (23,24). Meanwhile, c-Myc is one of the
target genes of β-catenin, which is responsible for cell
proliferation (23). Thus, the
intracellular elevation of β-catenin was a result of GSK-3β
phosphorylation, suggesting the activation of the Wnt/β-catenin
signaling pathway in BTZ-resistant MM cells.
Alternatively, the intrinsic increase in β-catenin
might have led to the overexpression of a deubiquitination enzyme,
such as ubiquitin-specific peptidase (USP)-47, resulting in the
accumulation of a reduced unfolded protein, which decreased
cellular stress and enhanced cell survival, thus leading to BTZ
resistance (25,26). However, the present study did not
detect USP-7 expression level in BTZ-resistant MM cells, which
could be performed in future studies. Overall, the Wnt/β-catenin
signaling pathway plays a critical role in both BTZ normal function
and its resistance acquisition in MM cells.
However, BTZ resistance has become a serious issue,
as it worsens the clinical outcome of patients with MM (6,19).
Therefore, the identification of alternative treatments is required
to improve prognosis. As aforementioned, it was discovered that the
activation of the Wnt/β-catenin signaling pathway might be involved
in BTZ resistance in MM cells. Thus, it was suggested that the
inhibition of β-catenin might reverse BTZ resistance and sensitize
BTZ-resistant MM cells to BTZ. Several previous studies have shown
that β-catenin suppression plays a role in reversing drug
resistance (27,28). For example, in one study, β-catenin
knockdown increased apoptosis and autophagy activity, which was
characterized by an increase in the expression level of apoptotic
proteins, including Bcl-2 and capase-3, and autophagy-related
proteins, including beclin-1 and microtube-associated protein 1
light chain 3, in BTZ-resistant MM cells (27). Another study found that the
suppression of β-catenin sensitized lenalidomide-resistant MM cells
to lenalidomide through the use of short hairpin RNAs (28). Apart from β-catenin inhibition
using molecular techniques, certain β-catenin inhibitors have been
used to reverse the established drug resistance to therapeutic
drugs.
ICG-001, a commonly used β-catenin inhibitor,
reduced cancer stem cell stemness, which is characterized by
decreased in vitro tumor sphere formation, to reverse
chemosensitivity in both gastric cancer and nasopharyngeal
carcinoma cells with acquired resistance (29,30).
In addition, ICG-001, combined with tamoxifen treatment, reversed
endocrine resistance in breast cancer cells (31). ICG-001, combined with cisplatin
therapy, also reserved cisplatin resistance in nasopharyngeal
carcinoma cells, manifesting as a suppression of proliferation both
in vitro and in vivo (30). However, few studies have reported
the role of ICG-001 in BTZ-resistant MM cells. In the present
study, ICG-001 was found to decrease cell viability and sensitize
BTZ-resistant MM cells to BTZ. The possible reasons for this are as
follows: i) ICG-001 might inhibit the interaction between CREB
binding protein and β-catenin, thereby leading to cell
differentiation in the BTZ-resistant MM cells and further resulting
in antibody production by mature B cells to eliminate MM cells
(32); and ii) ICG-001 might
downregulate several cancer stem cell markers, such as SRY-box 2,
octamer-binding transcription factor 4 and cluster of
differentiation 44, and inhibit tumor sphere formation to suppress
BTZ-resistant MM cell stemness, eventually resulting in enhanced
BTZ chemosensitivity (29,30). In combination, ICG-001 might induce
apoptosis and enhance BTZ cytotoxicity in BTZ-resistant MM.
PP, another β-catenin inhibitor, was first
identified as an anthelmintic drug and showed tumor suppression
activity in ovarian cancer and MM (10,33).
Preclinical experiments showed that PP targeted casein kinase 1α to
induce a paclitaxel chemotherapy response in renal carcinoma cells
(34). In addition, PP inhibited
signal transducer and activator of transcription 3 to selectively
target reactive oxygen species activation and suppress aerobic
glycolysis in Kirsten rat sarcoma viral oncogene homolog-mutant
lung cancer (35). However, few
studies report the effect of PP on MM. One previous study revealed
that PP inhibited MM cell proliferation through suppression of
mitochondrial respiratory complex I and STAT3 (14). In addition, another study
discovered that PP promoted MM cell apoptosis and destabilized
β-catenin by downregulating the Wnt/β-catenin pathway in MM cells
(13). In the present study,
several experiments were performed to discover the effect of PP
combined with BTZ in BTZ-resistant MM cell lines. It was found that
PP induced MM cell apoptosis and inhibited MM cell viability.
Furthermore, PP reversed sensitivity to BTZ in BTZ-resistant MM
cells. The possible reasons for this could be as follows: i) PP
might dysregulate the cellular lipid anabolism process in
triple-negative breast cancer stem cells, which could further
inhibit the lipid biosyncretic pathway in cancer stem cells and
reduce BTZ resistance in BTZ-resistant MM cells (36); and ii) PP might suppress the
transcriptional activity of glucose-regulated protein 78 to promote
unfolded protein response in the endoplasmic reticulum, thus
leading to an enhanced BTZ effect on, and chemosensitivity in,
BTZ-resistant MM cells (37). In
combination, these results suggested that PP might induce cell
apoptosis and enhance BTZ chemosensitivity in BTZ-resistant MM.
These findings could be important in the clinical setting and could
be used by clinicians to develop a treatment for patients with MM
and BTZ resistance in the future.
There are some limitations in the present study.
Firstly, the CalcuSyn software was not used to calculate the
combination effect of the β-catenin inhibitors with BTZ in MM
cells. Secondly, further molecular experiments are required to
reveal the detailed mechanism of ICG-001 on cancer stemness.
Thirdly, further investigation is required to detect cell
behaviours at multiple time points. Finally, normal cells, as
negative controls, were not used in the present study.
In conclusion, the β-catenin inhibitors, ICG-001 and
PP not only increased apoptosis, but also sensitized BTZ-resistant
MM cells to BTZ, indicating its potential therapeutic application
in MM.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
CW designed this study and completed the data
collection, interpretation and article writing. CW has read and
approved the final manuscript. CW confirms the authenticity of all
the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
MM
|
multiple myeloma
|
|
BTZ
|
bortezomib
|
|
PP
|
pyrvinium
|
|
KMS-11BR
|
BTZ-resistant KMS-11
|
|
RPMI-8226BR
|
BTZ-resistant RPMI-8226
|
|
CCK-8
|
Cell Counting Kit-8
|
|
NC
|
nitrocellulose
|
|
pGSK-3β
|
phosphorylated GSK-3β
|
|
OD
|
optical density
|
|
USP
|
ubiquitin-specific peptidase
|
References
|
1
|
Barwick BG, Gupta VA, Vertino PM and Boise
LH: Cell of origin and genetic alterations in the pathogenesis of
multiple myeloma. Front Immunol. 10:11212019. View Article : Google Scholar
|
|
2
|
Kyle RA and Rajkumar SV: Multiple myeloma.
Blood. 111:2962–2972. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shah N, Aiello J, Avigan DE, Berdeja JG,
Borrello IM, Chari A, Cohen AD, Ganapathi K, Gray L, Green D, et
al: The society for immunotherapy of cancer consensus statement on
immunotherapy for the treatment of multiple myeloma. J Immunother
Cancer. 8:e0007342020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rajkumar SV: Multiple myeloma: 2018 update
on diagnosis, risk-stratification, and management. Am J Hematol.
93:981–1114. 2018. View Article : Google Scholar
|
|
5
|
Dingli D, Ailawadhi S, Bergsagel PL, Buadi
FK, Dispenzieri A, Fonseca R, Gertz MA, Gonsalves WI, Hayman SR,
Kapoor P, et al: Therapy for relapsed multiple myeloma: Guidelines
from the mayo stratification for myeloma and risk-adapted therapy.
Mayo Clin Proc. 92:578–598. 2017. View Article : Google Scholar
|
|
6
|
Scott K, Hayden PJ, Will A, Wheatley K and
Coyne I: Bortezomib for the treatment of multiple myeloma. Cochrane
Database Syst Rev. 4:CD0108162016.PubMed/NCBI
|
|
7
|
Tibullo D, Giallongo C, Romano A, Vicario
N, Barbato A, Puglisi F, Parenti R, Amorini AM, Wissam Saab M,
Tavazzi B, et al: Mitochondrial functions, energy metabolism and
protein glycosylation are interconnected processes mediating
resistance to bortezomib in multiple myeloma cells. Biomolecules.
10:6962020. View Article : Google Scholar
|
|
8
|
Zaal EA, Wu W, Jansen G, Zweegman S, Cloos
J and Berkers CR: Bortezomib resistance in multiple myeloma is
associated with increased serine synthesis. Cancer Metab. 5:72017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Robak P, Drozdz I, Szemraj J and Robak T:
Drug resistance in multiple myeloma. Cancer Treat Rev. 70:199–208.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Spaan I, Raymakers RA, van de Stolpe A and
Peperzak V: Wnt signaling in multiple myeloma: A central player in
disease with therapeutic potential. J Hematol Oncol. 11:672018.
View Article : Google Scholar
|
|
11
|
Pai SG, Carneiro BA, Mota JM, Costa R,
Leite CA, Barroso-Sousa R, Kaplan JB, Chae YK and Giles FJ:
Wnt/beta-catenin pathway: Modulating anticancer immune response. J
Hematol Oncol. 10:1012017. View Article : Google Scholar
|
|
12
|
Grigson ER, Ozerova M, Pisklakova A, Liu
H, Sullivan DM and Nefedova Y: Canonical Wnt pathway inhibitor
ICG-001 induces cytotoxicity of multiple myeloma cells in
Wnt-independent manner. PLoS One. 10:e01176932015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu F, Zhu Y, Lu Y, Yu Z, Zhong J, Li Y and
Pan J: Anthelmintic pyrvinium pamoate blocks Wnt/β-catenin and
induces apoptosis in multiple myeloma cells. Oncol Lett.
15:5871–5878. 2018.
|
|
14
|
Harada Y, Ishii I, Hatake K and Kasahara
T: Pyrvinium pamoate inhibits proliferation of
myeloma/erythroleukemia cells by suppressing mitochondrial
respiratory complex I and STAT3. Cancer Lett. 319:83–88. 2012.
View Article : Google Scholar
|
|
15
|
Hirakawa T, Nasu K, Miyabe S, Kouji H,
Katoh A, Uemura N and Narahara H: β-catenin signaling inhibitors
ICG-001 and C-82 improve fibrosis in preclinical models of
endometriosis. Sci Rep. 9:200562019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Salem K, Brown CO, Schibler J and Goel A:
Combination chemotherapy increases cytotoxicity of multiple myeloma
cells by modification of nuclear factor (NF)-κB activity. Exp
Hematol. 41:209–218. 2013. View Article : Google Scholar
|
|
17
|
Chou TC: Drug combination studies and
their synergy quantification using the Chou-Talalay method. Cancer
Res. 70:440–446. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Thibaudeau TA and Smith DM: A practical
review of proteasome pharmacology. Pharmacol Rev. 71:170–197. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Terpos E, Kleber M, Engelhardt M, Zweegman
S, Gay F, Kastritis E, van de Donk NW, Bruno B, Sezer O, Broijl A,
et al: European myeloma network guidelines for the management of
multiple myeloma-related complications. Haematologica.
100:1254–1266. 2015. View Article : Google Scholar
|
|
20
|
Dai Y, Guo X and Yang C: Effect of
bortezomib on proliferation and apoptosis of myeloma cells by
activating Wnt/β-catenin signaling pathway. Oncol Lett.
20:1295–1299. 2020. View Article : Google Scholar
|
|
21
|
Guo N and Peng Z: MG132, a proteasome
inhibitor, induces apoptosis in tumor cells. Asia Pac J Clin Oncol.
9:6–11. 2013. View Article : Google Scholar
|
|
22
|
Harousseau JL, Attal M, Avet-Loiseau H,
Marit G, Caillot D, Mohty M, Lenain P, Hulin C, Facon T, Casassus
P, et al: Bortezomib plus dexamethasone is superior to vincristine
plus doxorubicin plus dexamethasone as induction treatment prior to
autologous stem-cell transplantation in newly diagnosed multiple
myeloma: Results of the IFM 2005-01 phase III trial. J Clin Oncol.
28:4621–4629. 2010. View Article : Google Scholar
|
|
23
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012. View Article : Google Scholar
|
|
24
|
Taciak B, Pruszynska I, Kiraga L, Bialasek
M and Krol M: Wnt signaling pathway in development and cancer. J
Physiol Pharmacol. 69:2018.
|
|
25
|
Shi J, Liu Y, Xu X, Zhang W, Yu T, Jia J
and Liu C: Deubiquitinase USP47/UBP64E regulates beta-Catenin
ubiquitination and degradation and plays a positive role in Wnt
signaling. Mol Cell Biol. 35:3301–3311. 2015. View Article : Google Scholar
|
|
26
|
Peterson LF, Sun H, Liu Y, Potu H,
Kandarpa M, Ermann M, Courtney SM, Young M, Showalter HD, Sun D, et
al: Targeting deubiquitinase activity with a novel small-molecule
inhibitor as therapy for B-cell malignancies. Blood. 125:3588–3597.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Su N, Wang P and Li Y: Role of
Wnt/beta-catenin pathway in inducing autophagy and apoptosis in
multiple myeloma cells. Oncol Lett. 12:4623–4629. 2016. View Article : Google Scholar
|
|
28
|
Bjorklund CC, Ma W, Wang ZQ, Davis RE,
Kuhn DJ, Kornblau SM, Wang M, Shah JJ and Orlowski RZ: Evidence of
a role for activation of Wnt/beta-catenin signaling in the
resistance of plasma cells to lenalidomide. J Biol Chem.
286:11009–11020. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu Y, Chen H, Zheng P, Zheng Y, Luo Q,
Xie G, Ma Y and Shen L: ICG-001 suppresses growth of gastric cancer
cells and reduces chemoresistance of cancer stem cell-like
population. J Exp Clin Cancer Res. 36:1252017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chan KC, Chan LS, Ip JC, Lo C, Yip TT,
Ngan RK, Wong RN, Lo KW, Ng WT, Lee AW, et al: Therapeutic
targeting of CBP/β-catenin signaling reduces cancer stem-like
population and synergistically suppresses growth of EBV-positive
nasopharyngeal carcinoma cells with cisplatin. Sci Rep. 5:99792015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Won HS, Lee KM, Oh JE, Nam EM and Lee KE:
Inhibition of β-Catenin to overcome endocrine resistance in
tamoxifen-resistant breast cancer cell line. PLoS One.
11:e01559832016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhao Y, Masiello D, McMillian M, Nguyen C,
Wu Y, Melendez E, Smbatyan G, Kida A, He Y, Teo JL and Kahn M:
CBP/catenin antagonist safely eliminates drug-resistant
leukemia-initiating cells. Oncogene. 35:3705–3717. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang C, Zhang Z, Zhang S, Wang W and Hu
P: Targeting of Wnt/β-Catenin by anthelmintic drug pyrvinium
enhances sensitivity of ovarian cancer cells to chemotherapy. Med
Sci Monit. 23:266–275. 2017. View Article : Google Scholar
|
|
34
|
Cui L, Zhao J and Liu J: Pyrvinium
sensitizes clear cell renal cell carcinoma response to chemotherapy
via Casein Kinase 1alpha-dependent inhibition of Wnt/β-Catenin. Am
J Med Sci. 355:274–280. 2018. View Article : Google Scholar
|
|
35
|
Feng J, Jiang W, Liu Y, Huang W, Hu K, Li
K, Chen J, Ma C, Sun Z and Pang X: Blocking STAT3 by pyrvinium
pamoate causes metabolic lethality in KRAS-mutant lung cancer.
Biochem Pharmacol. 177:1139602020. View Article : Google Scholar
|
|
36
|
Dattilo R, Mottini C, Camera E, Lamolinara
A, Auslander N, Doglioni G, Muscolini M, Tang W, Planque M,
Ercolani C, et al: Pyrvinium pamoate induces death of
triple-negative breast cancer stem-like cells and reduces
metastases through effects on lipid anabolism. Cancer Res.
80:4087–4102. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yu DH, Macdonald J, Liu G, Lee AS, Ly M,
Davis T, Ke N, Zhou D, Wong-Staal F and Li QX: Pyrvinium targets
the unfolded protein response to hypoglycemia and its anti-tumor
activity is enhanced by combination therapy. PLoS One. 3:e39512008.
View Article : Google Scholar : PubMed/NCBI
|