Introduction
Ganglioneuroblastoma (GNB) is an embryonal tumor
originating from primitive neural crest cells in the neuroectoderm
(1). It is a transitional tumor
consisting of a mixture of mature ganglioneuromas (GNs) and
malignant neuroblastomas (NBs), hence its biological behavior is
intermediate between that of GN and NB. Furthermore, GNB is a
malignant or potentially malignant tumor (1,2). It
usually occurs in pediatric patients aged <10 years,
particularly between the ages of 1 and 2 years (3). By contrast, GNB is rare in
adolescents and adults, with previous studies reporting <50
cases in adults (4). The most
common site of origin for GNB is the adrenal medulla (~35%),
followed by the retroperitoneum, brain and mediastinum, with
tissues such as the orbit and parotid gland also reportedly
involved (5–13). Although cases of GNB in adolescents
or adults have been previously reported, GNB occurring in the
retroperitoneum is rare. The present study reported on a case of
retroperitoneal GNB in a 17-year-old female who had an abdominal
computed tomography (CT) finding of an occupying lesion in the head
of the pancreas and underwent retroperitoneal tumor resection,
which was confirmed by pathology to be GNB. On the third day after
surgery, the patient developed a stress duodenal ulcer perforation
and underwent an emergency major distal gastrectomy and duodenal
ulcer resection. The present study reviewed the clinical course of
this case together with an analysis and discussion of relevant
literature.
Case report
A 17-year-old woman was admitted to the Affiliated
Hospital of Guizhou Medical University (Guiyang, China) on
September 7, 2021, for a whole abdomen CT scan at 9 days
post-trauma that revealed a pancreatic head mass. However, the
patient had no symptoms of nausea, vomiting, acid reflux or
abdominal pain. Furthermore, no mass was palpated on examination
and no positive signs such as abdominal tenderness, rebound pain or
abdominal muscle tension were present. The patient had a previous
gastroscopy diagnosis of a peptic ulcer (no gastroscopy report was
available) and a history of prolonged and intermittent
non-steroidal drug (NSAID) use for menstrual pain. The patient had
a previous artificial abortion but no medical history of
hypertension, diabetes mellitus, hepatitis or tuberculosis.
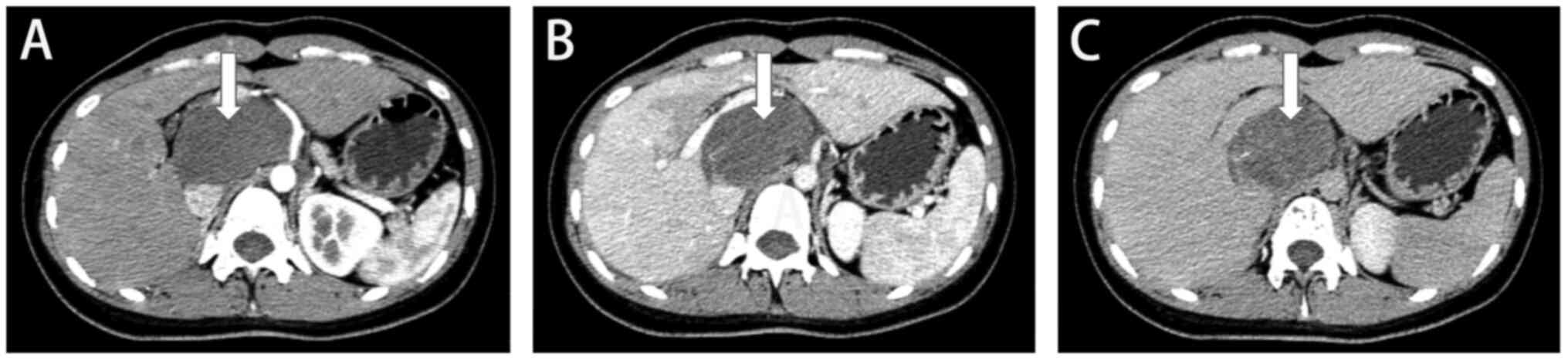
However, the patient had a cystic solid occupancy in the posterior
pancreatic head with clear borders ~120×64 mm in size as per the
color Doppler ultrasound and enhanced CT of the abdomen;
furthermore, the nature and origin of the tumor required further
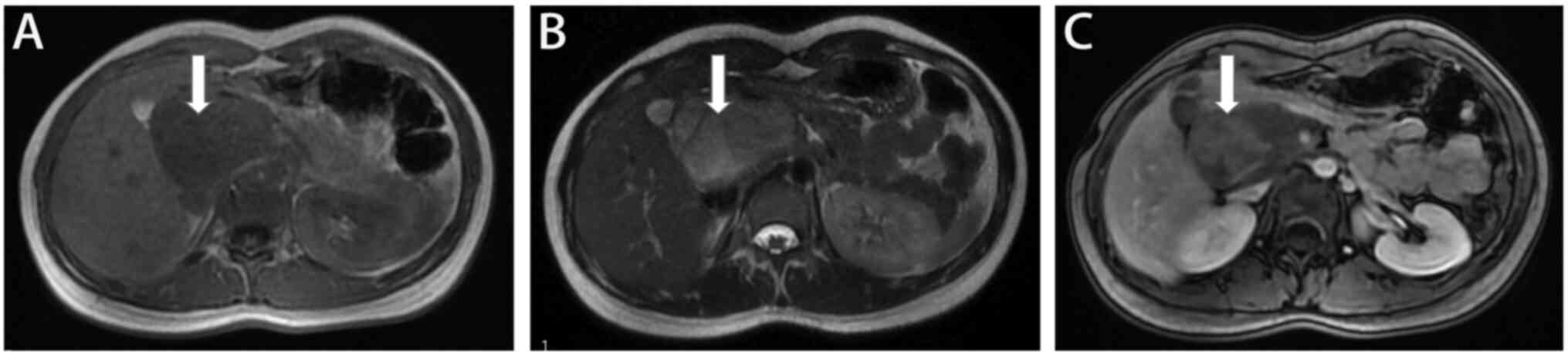
examination (Fig. 1). Magnetic
resonance cholangiopancreatography (MRCP) revealed a
morphologically irregular abnormal mass shadow behind the right
posterior pancreatic head in the peritoneal area (~62×52×125 mm)
with a non-uniform signal and well-defined borders; the enhancement
scan indicated that the mass was not significantly strengthened
(Fig. 2). After admission,
laboratory tests for routine blood, liver and renal function, as
well as coagulation and tumor markers were all unremarkable. The
preliminary diagnosis was a retroperitoneal occupancy neurogenic
tumor. The patient then underwent exploratory laparotomy and
retroperitoneal mass resection under general anesthesia on the 8th
day post-admission. The operation went well and the patient
returned safely to the ward after surgery and was given basic
treatment, such as pantoprazole sodium for acid suppression and
gastric protection and fluid supplementation. Based on the
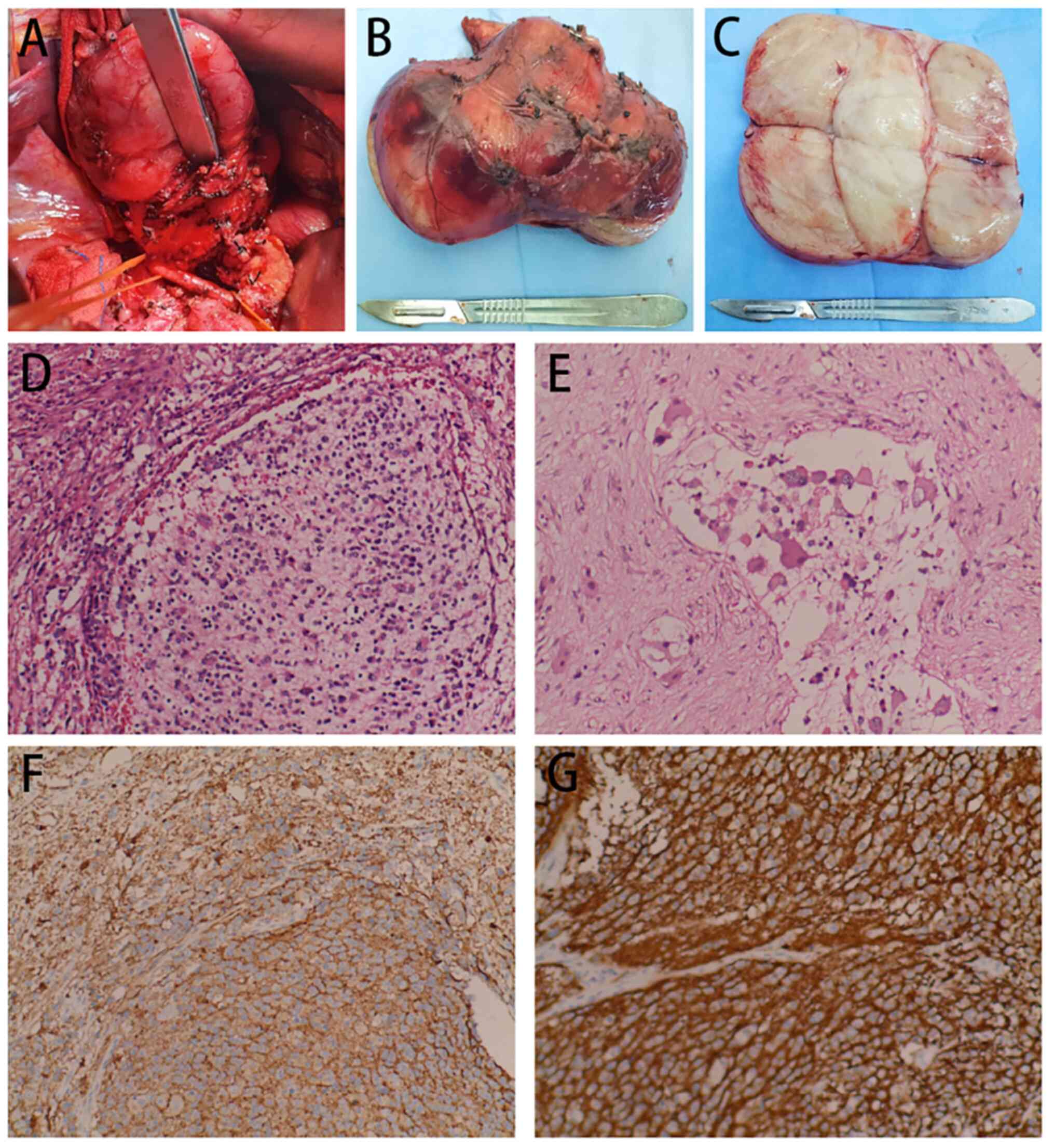
intraoperative findings (Fig.
3A-C), the mass was located in the right retroperitoneum of the
hepatoduodenal ligament, was ~12 cm in diameter, soft with a
complete capsule, movable and well defined. The mass was closely
associated with the surrounding nodal tissue, posterior aspect of
the head of the pancreas, portal veins, superior mesenteric veins
and pancreatic hooks, but no distant metastases or enlarged lymph
nodes were observed, as no images other than those of the tumor
were acquired. During the surgery, the tumor was completely removed
and the abdominal cavity was closed after careful examination and
confirmation that no lesions remained and no adjacent organs or
blood vessels were damaged. The tumor was irregular in shape,
measuring ~13×7×5 cm, was solid, grey-white, soft, and had
yellowish-white streaks on the cut surface. Microscopic observation
revealed scattered, thread-like and nested tumor cells with sheets
of necrosis. Certain cells had abundant cytoplasm and were
eosinophilic, various cells had displaced nuclei with clear,
vesicular nucleoli; certain cells had a nodular distribution with
nests of immature small cells within the nodules and deeply stained
heterogeneous nucleoli, and multinucleated cells and nuclear
divisions were also observed (Fig. 3D
and E). The immunohistochemistry results were as follows:
Vimentin(+), S100(+), Sox10(+), CD56(+), CD57(+), specific esterase
(SE)(+), neurofilament (NF)(+), synaptophysin (Syn)(+), friend
leukemia integration-1(+), glial fibrillary acidic protein (less
+), CgA (less +), myogenin (partial +), myogenic differentiation 1
(partial +), CD99 (partial +), smooth muscle actin(−), desmin (−),
caldesmon (−) and Ki-67 (average of ~2%; hotspots of ~10% +)
(Figs. 3F and G and S1). The pathological results were
consistent with the diagnosis of retroperitoneal GNB.
On the third day after surgery, the patient
presented with abdominal distension, abdominal pain and
palpitations. Physical examination revealed significant whole
abdominal pressure, rebound pain and abdominal muscle tension.
Emergency blood cell analysis revealed a white blood cell count of
22.41×109/l and neutrophil percentage of 90.50%.
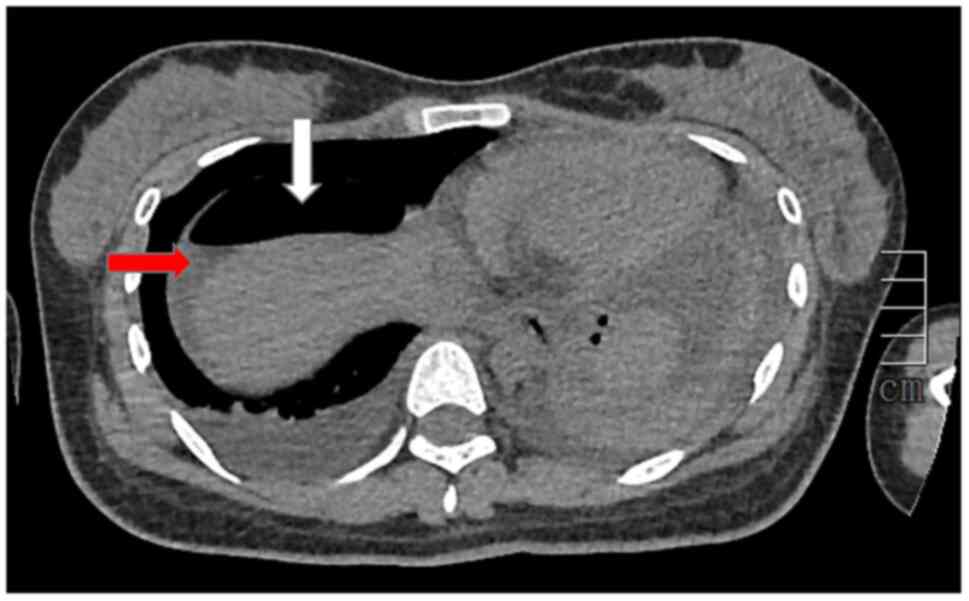
Abdominal CT indicated pneumoperitoneum, exudate and effusion in
the abdominal cavity and pelvis, as well as edema in the gastric
sinus and duodenal wall (Fig. 4).
Stress ulcers with peptic perforation were considered in the
context of the patient's physical signs, laboratory tests and
abdominal CT results. An emergency laparotomy was performed on the
4th day after the first surgery, which revealed a thick
accumulation of pus in the original surgical area and a perforation
in the posterior wall of the duodenal bulb ~3.0×2.0 cm, with
intestinal fluid spillage. After obtaining informed consent from
the patient's family, the patient underwent major distal
gastrectomy and duodenal ulcer resection. The pathological results
indicated old duodenal ulcers with inflammatory exudation,
granulation and fibrous tissue hyperplasia, scar formation and
ulcerative changes in the plasma membrane of the intestinal canal
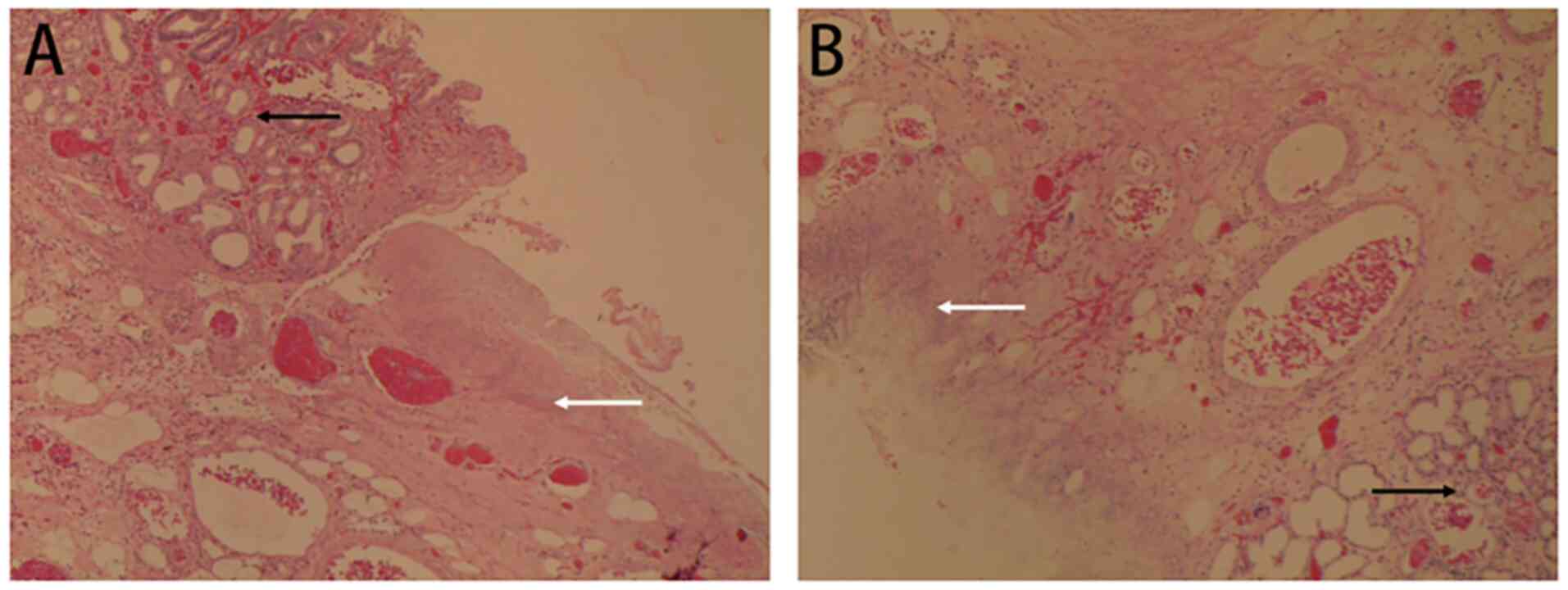
(Fig. 5).
The patient recovered well from the second surgery
and was discharged in good condition, with no recurrence or
metastatic tendencies noted at the follow-up examination.
Discussion
In 1947, Stout reported the first case of a tumor
consisting of a malignant NB nodule and a mature GN, which was
termed a mixed peripheral NB (14). In 1984, Shimada et al
(15) summarized 295 cases of NB
tumors and first introduced the concept of GNB; GNBs were then
classified as nodular or mixed. In 1999, the International
Classification of Neuroblastoma Pathology adopted Shimada's
classification and revised it in 2003, redefining neuroblastoma and
classifying it into NB, mixed GNB, GN and nodular GNB according to
histological features (16,17).
As part of the present study, the PubMed database
was searched using the subject key terms ‘ganglioneuroblastoma’,
‘GNB’, ‘retroperitoneum’ and ‘retroperitoneal’ (January 27, 2022).
A total of 330 papers with titles containing GNB were found, of
which only 14 (4.24%) were related to the retroperitoneum (12,13,18–29).
Among these papers, there were 59 cases involving retroperitoneal
GNB, and of these, 53 (89.8%) cases were in children (<12
years), one (1.7%) was in an adolescent (12–20 years) and five
(8.5%) were in adults (>20 years). Thus, retroperitoneal GNB in
adults or adolescents is rare. The present study reported the
clinical course of another case of retroperitoneal GNB in a
17-year-old female and summarized the data on non-pediatric cases
(age, >12 years) of GNB as follows (Table I).
 | Table I.Information on previously reported
non-pediatric cases of ganglioneuroblastoma and the present
case. |
Table I.
Information on previously reported
non-pediatric cases of ganglioneuroblastoma and the present
case.
| First author/s,
year | Country | Patient age,
years | Sex | Symptoms | Therapy | Diagnostic basis | Comorbidity | Outcome | (Refs.) |
|---|
| Marya and Gupta,
1979 | India | 14 | Male | Abdominal distension
and pain | Surgery | H&E | - | Improvement | (25) |
| Yamanaka et
al, 2001 | Japan | 60 | Male | Back pain | Surgery | H&E+IHC (NSE,
S100) | - | Died | (13) |
| Hayama et al,
2012 | Japan | 55 | Female | Abdominal pain | Surgery | H&E+IHC (S100,
NF, Syn) | Mature cystic
teratoma | Improvement | (21) |
| Jrebi et al,
2014 | USA | 33 | Male | Back pain | Surgery +
chemotherapy | H&E | - | Died | (19) |
| Chen et al,
2014 | UK | 21 | Female | Back pain | Surgery | H&E | Pregnancy | Improvement | (20) |
| Present study | China | 17 | Female | - | Surgery | H&E+IHC (Vim,
S100, CD56, CD57, SE, NF, Syn) | Stress ulcers | Improvement |
|
The clinical symptoms and signs of GNB and NB are
similar; however, the degree of malignancy and prognosis exhibit a
great variation. CT and MRI are the most commonly used imaging
methods to differentiate GNB from NB. A previous study has also
indicated that on CT, NB is mostly located in the adrenal region,
with vascular inclusions, local infiltration, organ and lymph node
metastases, and clustered or linear dilated vascular shadows around
and within the tumor. However, GNB tumors frequently display a
regular shape with well-defined margins and are accompanied by
pressure displacement of surrounding large vessels (18). Due to the diversity of GNB
components and the heterogeneous distribution of the fibrovascular
network (which in turn may undergo bleeding and necrosis), the
majority of CT enhancements are heterogeneous (18,30).
GNB exhibits equal or slightly low signals on MRI T1WI, whereas
T2WI is dominated by heterogeneous slightly high signals and the
necrotic area exhibits longer T2 signals. The signal level of T2WI
reflects the proportion of parenchymal cells and stroma in the
tumor to a certain extent and the slightly low signal is dominated
by parenchymal cells (31). In the
present case, no significant changes were observed on the enhanced
CT in the arterial phase, with mild enhancement in the venous and
delayed phases, whereas the pancreatic ducts and portal veins were
displaced by compression. The MRCP exhibited a predominantly
slightly low signal on the T1W1 image and a predominantly slightly
high signal on the T2W1 and T2-FS images, with a small amount of
plaque-like slightly low signals and separated shadows. Although CT
and MRI are useful in the diagnosis of GNB, the diagnosis of the
disease is still dependent on pathological examination of the tumor
tissue. The diagnosis is usually confirmed by microscopic findings
of NB components and mature ganglion cells, frequently with nuclear
schwannoma, hemorrhage, necrosis and calcification. In addition,
Syn, CD56, SE, NF, CgA and S100 may be considered as GNB-specific
markers (32).
Early radical surgery is the best treatment for
retroperitoneal GNB and its prognosis is related to the ratio of
neuroblasts to ganglion cells. If there are fewer neuroblasts, the
patient's prognosis is better; conversely, if there are more
neuroblasts, the patient's prognosis is worse as well. In addition,
patient age and tumor size are also associated with GNB prognosis.
Previous studies have indicated that patient age is inversely
related to prognosis of GNB, with older pediatric patients having
worse prognoses than younger pediatric patients (33–35).
Furthermore, adults with GNBs >8 cm in diameter are frequently
at risk of distant metastases (36). Compared to other reports of
retroperitoneal GNB lesions, the present case had a larger lesion
size (13 cm), hence the risk of recurrence may be increased.
Therefore, GNB lesions in adolescents or adults should be carefully
followed up, even after complete resection.
Stress ulcers are acute mucosal erosions and ulcers
of the gastrointestinal tract that occur in stressful conditions
such as shock, trauma, critical illness or surgery (37). The incidence of stress ulcers has
significantly decreased with the widespread use of proton pump
inhibitors (38–40). In the case of the present study,
the patient developed abdominal pain and elevated body temperature
on the third postoperative day, and laboratory tests and imaging
data confirmed a peptic ulcer with perforation. Review of the
surgical procedure did not indicate any injury to adjacent organs
or blood vessels. Combined with the patient's previous history of
peptic ulcer and history of NSAID use, the possibility of stress
ulcer with perforation was considered. As the patient's
pathological examination results indicated GNB with malignant
tendency and the ulcer perforation area was large (3.0×2.0 cm), it
was difficult to achieve the ideal treatment result with the
conventional ulcer repair surgery and the final decision was made
to perform ‘major distal gastrectomy and duodenal ulcer resection’.
Therefore, active and effective prevention of stress ulcers has an
important role in saving the patient's life as well as improving
the patient's quality of life.
Controversy still exists as to whether patients with
GNB should be treated with chemotherapy after surgery. The main
chemotherapeutic agents used in pediatric patients include
cyclophosphamide, vincristine, adriamycin and etoposide. Raina
et al (41) reported a case
wherein complete remission was achieved with a combination of
cyclophosphamide, vincristine, adriamycin, cisplatin, etoposide and
ifosfamide. In addition, certain studies have demonstrated that
neoadjuvant chemotherapy is effective in patients with GNB
(33,41–44),
but its prognostic impact remains uncertain due to the low
incidence of GNB. The pathological results of the patient of the
present study suggested a high proportion of NB and a poorly
located tumor with a close relationship to the surrounding tissues.
After multi-disciplinary treatment discussion, chemotherapy or
classical meta-iodobenzylguanidine treatment was recommended, but
the patient and her family decided not to proceed with chemotherapy
for the time being.
Recurrence of the disease mainly occurs 2 years
after surgery. Patients should therefore be followed up for a long
period and reviewed every 6 months, which should include a full
blood count and imaging of the primary tumor site (45).
In conclusion, GNB is a transitional tumor
frequently occurring in children. It consists of a mature GN and
malignant NB and is therefore malignant or potentially malignant.
to date, <50 adult or adolescent cases have been previously
reported and there are only five cases of retroperitoneal GNB in
adults or adolescents in the PubMed database.
Although the diagnosis of GNB depends on
histopathological analysis, imaging with CT and MRI may still aid
in GNB diagnosis. When an abdominal mass is found in an adult or
adolescent, clinicians should consider the possibility of GNB and
its malignancy and proactively prevent the development of
complications.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Special Fund for
Outstanding Young Scientific and Technological Talents of Guizhou
Province [grant no. (2019)5647] and the Science and Technology Fund
Project of Guizhou Health and Family Planning Commission (grant no.
gzwjkj2020-1-101).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
SLZ and BLX examined the patient, analyzed the
clinical, radiological and laboratory results, and wrote the
manuscript. YWZ assisted with data analysis. XYF and ZHZ were
involved in the surgical treatment. SZ designed the study,
including proofreading of the manuscript and revising it
critically. SLZ, BLX, YWZ, XYF, ZHZ and SZ confirm the authenticity
of all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient and their family for the publication of this case.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Badiu Tișa I, Samașca G, Aldea C, Lupan I,
Farcau D and Makovicky P: Ganglioneuroblastoma in children. Neurol
Sci. 40:1985–1989. 2019. View Article : Google Scholar
|
|
2
|
He WG, Yan Y, Tang W, Cai R and Ren G:
Clinical and biological features of neuroblastic tumors: A
comparison of neuroblastoma and ganglioneuroblastoma. Oncotarget.
8:37730–37739. 2017. View Article : Google Scholar
|
|
3
|
Akin M, Ergen SA, Oz B, Atkovar G and
Sahinler I: Ventricular ganglioneuroblastoma in an adult and
successful treatment with radiotherapy. Balkan Med J. 31:173–176.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vassallo L, Fasciano M, Baralis I,
Pellegrino L, Fortunato M, Orcioni GF and Sorrentino S: A rare case
of adrenal ganglioneuroblastoma-intermixed in an adult and a review
of literature. Radiol Case Rep. 16:2351–2356. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sekiguchi N, Noguchi T, Fukushima T,
Kobayashi T, Ozawa T, Sato Y, Takeda T, Yoshida K and Koizumi T:
Posterior mediastinal ganglioneuroblastoma in an adolescent: A case
report and review. Thorac Cancer. 11:451–455. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mousa AM, Shokouh-Amiri MH, Shah LM,
Garzon S and Xie KL: Adult-onset ganglioneuroblastoma of the
posterior mediastinum with osseous metastasis. Radiol Case Rep.
15:1676–1682. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Heidari Z, Kaykhaei MA, Jahantigh M and
Sheikhi V: Adrenal ganglioneuroblastoma in an adult: A rare case
report. Int J Endocrinol Metab. 16:e630552018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yao PS, Chen GR, Shang-Guan HC, Lin QS,
Wang XF, Zheng SF and Kang DZ: Adult hippocampal
ganglioneuroblastoma: Case report and literature review. Medicine
(Baltimore). 96:e88942017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ramaswamy B, Bhandarkar AM, Menon SS,
Agarwal AC and Nair SS: Ganglioneuroblastoma of skull base. J Clin
Diagn Res. 9:MD01–MD03. 2015.PubMed/NCBI
|
|
10
|
Bolzacchini E, Martinelli B and Pinotti G:
Adult onset of ganglioneuroblastoma of the adrenal gland: Case
report and review of the literature. Surg Case Rep. 1:792015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Patnaik A, Mishra SS, Mishra S, Das S and
Deo RC: Primary extradural spinal ganglioneuroblastoma: A case
report. Turk Neurosurg. 24:253–255. 2014.PubMed/NCBI
|
|
12
|
Nakaoka T, Uemura S, Nakagawa Y, Yano T
and Oda M: Retroperitoneal ganglioneuroblastoma resected 8 years
after mass screening: A case report. J Pediatr Surg. 42:E29–E32.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamanaka M, Saitoh F, Saitoh H, Nisimura
S, Sawada Y, Tsukui A, Kaimori M and Takahashi N: Primary
retroperitoneal ganglioneuroblastoma in an adult. Int J Urol.
8:130–132. 2001. View Article : Google Scholar
|
|
14
|
Stout AP: Ganglioneuroma of the
sympathetic nervous system. Surg Gynecol Obstet. 84:101–110.
1947.
|
|
15
|
Shimada H, Chatten J, Newton WA Jr, Sachs
N, Hamoudi AB, Chiba T, Marsden HB and Misugi K: Histopathologic
prognostic factors in neuroblastic tumors: Definition of subtypes
of ganglioneuroblastoma and an age-linked classification of
neuroblastomas. J Natl Cancer Inst. 73:405–416. 1984. View Article : Google Scholar
|
|
16
|
Munchar MJ, Sharifah NA, Jamal R and Looi
LM: CD44s expression correlated with the international
neuroblastoma pathology classification (Shimada system) for
neuroblastic tumours. Pathology. 35:125–129. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shimada H, Ambros IM, Dehner LP, Hata J,
Joshi VV, Roald B, Stram DO, Gerbing RB, Lukens JN, Matthay KK and
Castleberry RP: The international neuroblastoma pathology
classification (the Shimada system). Cancer. 86:364–372. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang H, Chen X, Liu H, Yu C and He L:
Computed tomography-based radiomics for differential of
retroperitoneal neuroblastoma and ganglioneuroblastoma in children.
Nan Fang Yi Ke Da Xue Xue Bao. 41:1569–1576. 2021.(In Chinese).
PubMed/NCBI
|
|
19
|
Jrebi NY, Iqbal CW, Joliat GR, Sebo TJ and
Farley DR: Review of our experience with neuroblastoma and
ganglioneuroblastoma in adults. World J Surg. 38:2871–2874. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen BF, Rathi M, Al-Samarrai S and
Rajeswary J: First reported case of ganglioneuroblastoma in
pregnancy and a review of the literature. Obstet Med. 7:128–130.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hayama S, Ohmi M, Yonemori A, Yamabuki T,
Inomata H, Nihei K and Hirano S: Ganglioneuroblastoma arising
within a retroperitoneal mature cystic teratoma. World J Clin
Oncol. 3:155–158. 2012. View Article : Google Scholar
|
|
22
|
Perego P, Bovo G, Cappellini A and Bratina
G: Adenocarcinoma of the large intestine associated with
retroperitoneal ganglioneuroblastoma. Report of a case.
Pathologica. 86:316–318. 1994.(In Italian).
|
|
23
|
Otal-Salaverri C, González-Cámpora R,
Hevia-Vazquez A, Lerma-Puertas E and Galera-Davidson H:
Retroperitoneal ganglioneuroblastoma. Report of a case diagnosed by
fine needle aspiration cytology and electron microscopy. Acta
Cytol. 33:80–84. 1989.
|
|
24
|
Ochoa Urdangarain O, Chávez Olivera R and
Molero Denes G: Retroperitoneal ganglioneuroblastoma. Presentation
of a case. Arch Esp Urol. 42:703–704. 1989.(In Spanish).
|
|
25
|
Marya SK and Gupta SP:
Ganglioneuroblastoma: A case report. Indian J Pediatr. 46:143–144.
1979. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Powers JM, Balentine JD, Wisniewski HM and
Terry RD: Retroperitoneal ganglioneuroblastoma: A kaleidoscope of
neuronal degeneration. A light and electron microscopic study. J
Neuropathol Exp Neurol. 35:14–25. 1976. View Article : Google Scholar
|
|
27
|
Nyãrãdi A, Kelle L and Klabuzai Z: High
catecholamine producing retroperitoneal ganglioneuroblastoma in an
adult. Orv Hetil. 112:2162–2164. 1971.(In Hungarian).
|
|
28
|
Likhachev AA: Retroperitoneal
ganglioneuroblastoma in a 14-month-old girl. Vopr Okhr Materin Det.
16:84–85. 1971.(In Russian).
|
|
29
|
Paulino F and Torres E: Retroperitoneal
ganglioneuroblastoma. Hospital (Rio J). 45:139–142. 1954.(In
Portuguese). PubMed/NCBI
|
|
30
|
Takeda Y, Sano H, Kawano A, Mochizuki K,
Takahashi N, Kobayashi S, Ohara Y, Tasaki K, Hosoya M and Kikuta A:
Usefulness of fluorodeoxyglucose positron emission
tomography/computed tomography for detection of a neuroblastic
nodule in a ganglioneuroblastoma: A case report. J Med Case Rep.
12:1192018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gahr N, Darge K, Hahn G, Kreher BW, von
Buiren M and Uhl M: Diffusion-weighted MRI for differentiation of
neuroblastoma and ganglioneuroblastoma/ganglioneuroma. Eur J
Radiol. 79:443–446. 2011. View Article : Google Scholar
|
|
32
|
Koike K, Iihara M, Kanbe M, Omi Y, Aiba M
and Obara T: Adult-type ganglioneuroblastoma in the adrenal gland
treated by a laparoscopic resection: Report of a case. Surg Today.
33:785–790. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Decarolis B, Simon T, Krug B, Leuschner I,
Vokuhl C, Kaatsch P, von Schweinitz D, Klingebiel T, Mueller I,
Schweigerer L, et al: Treatment and outcome of ganglioneuroma and
ganglioneuroblastoma intermixed. BMC Cancer. 16:5422016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mossé YP, Deyell RJ, Berthold F,
Nagakawara A, Ambros PF, Monclair T, Cohn SL, Pearson AD, London WB
and Matthay KK: Neuroblastoma in older children, adolescents and
young adults: A report from the international neuroblastoma risk
group project. Pediatr Blood Cancer. 61:627–635. 2014. View Article : Google Scholar
|
|
35
|
Brossard J, Bernstein ML and Lemieux B:
Neuroblastoma: An enigmatic disease. Br Med Bull. 52:787–801. 1996.
View Article : Google Scholar
|
|
36
|
Mizuno S, Iida T and Fujita S: Adult-onset
adrenal ganglioneuroblastoma-bone metastasis two years after
surgery: Report of a case. Surg Today. 40:482–486. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kasper P, Janssens U and Michels G: How
important are proton pump inhibitors in the prevention of stress
ulcers and stress-associated gastrointestinal bleeding in ICU
patients? Med Klin Intensivmed Notfmed. 114:350–354. 2019.(In
German). View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Eun CS: Does proton pump inhibitor
increase the clostridium difficile infection risk in the treatment
and prophylaxis of stress ulcers than histanime-2 receptor
antagonist? Gut Liver. 11:739–740. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cohen ME, Hathway JM, Salmasian H, Liu J,
Terry M, Abrams JA and Freedberg DE: Prophylaxis for stress ulcers
with proton pump inhibitors is not associated with increased risk
of bloodstream infections in the intensive care unit. Clin
Gastroenterol Hepatol. 15:1030–1036.e1. 2017. View Article : Google Scholar
|
|
40
|
Azab M, Doo L, Doo DH, Elmofti Y, Ahmed M,
Cadavona JJ, Liu XB, Shafi A, Joo MK and Yoo JW: Comparison of the
hospital-acquired clostridium difficile infection risk of using
proton pump inhibitors versus histamine-2 receptor antagonists for
prophylaxis and treatment of stress ulcers: A systematic review and
meta-analysis. Gut Liver. 11:781–788. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Raina V, Kamble R, Tanwar R, Singh SP and
Sharma S: Spinal ganglioneuroblastoma-complete response to
chemotherapy alone. Postgrad Med J. 69:746–748. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Irtan S, Brisse HJ, Minard-Colin V,
Schleiermacher G, Galmiche-Rolland L, Le Cossec C, Elie C, Canale
S, Michon J, Valteau-Couanet D and Sarnacki S: Image-defined risk
factor assessment of neurogenic tumors after neoadjuvant
chemotherapy is useful for predicting intra-operative risk factors
and the completeness of resection. Pediatr Blood Cancer.
62:1543–1549. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sawaguchi S, Kaneko M, Uchino J, Takeda T,
Iwafuchi M, Matsuyama S, Takahashi H, Nakajo T, Hoshi Y, Okabe I,
et al: Treatment of advanced neuroblastoma with emphasis on
intensive induction chemotherapy. A report from the study group of
Japan. Cancer. 66:1879–1887. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kilton LJ, Aschenbrener C and Burns CP:
Ganglioneuroblastoma in adults. Cancer. 37:974–983. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Murphy JM and La Quaglia MP: Advances in
the surgical treatment of neuroblastoma: A review. Eur J Pediatr
Surg. 24:450–456. 2014. View Article : Google Scholar : PubMed/NCBI
|