Introduction
Colorectal cancer (CRC) is the third most commonly
diagnosed cancer and the second leading cause of cancer-related
mortality worldwide (1). The
prediction of prognosis for cancer depends on the Tumor, Node,
Metastasis (TNM) classification system and the features of tumor
cell differentiation. This approach serves as a useful model for
selecting postoperative treatment options. However, this
classification does not provide sufficient information to predict
prognosis. Therefore, a better classification system is required to
achieve this purpose.
CRC is characterized by the infiltration of immune
cells comprising subpopulations of granulocytes, lymphocytes, and
macrophages. Accumulating evidence indicates the significant
potential of analyzing the presence of tumor-infiltrating immune
cells in the tumor microenvironment (TME) as a means for predicting
the prognosis of cancer (2–4). In
particular, tumor-infiltrating lymphocytes (TILs) and
tumor-associated neutrophils (TANs) are essential for the
progression of CRC (5–8). Moreover, CD8 is typically used as a
marker of cytotoxic T cells that exert antitumor effects on the TME
and our previous study indicates that CD8+TILs are
useful biomarkers for predicting early relapse of CRC further
revealing its potential in this field (9).
Human neutrophils with surface expression of markers
such as CD11b, CD16, and CD66b make the identification of mature
neutrophils possible (10).
Furthermore, CD66b, which is also known as carcinoembryonic
antigen-related cell adhesion molecule 8, NCA-95, and CD67, is also
a reliable surface marker to identify neutrophils in cancer tissues
(11). While, CD66b+
TANs are generally associated with worse prognosis for diverse
tumors (12,13). The significance of
CD66b+ TANs in CRC is rather controversial (14–16).
Moreover, the type of tumor-infiltrating immune
cells, their densities as well as their spatial distributions in
the TME, are important for clinical means, and their locations in
the different compartments of a tumor are associated with different
clinical outcomes. For example, TILs play antitumor roles in the
tumor nest and the invasive margin (5), however, the clinical significance of
TANs in the invasive margin and the interaction between TILs and
TANs in CRC largely remains unclear.
In this study, we hypothesized that TILs and TANs in
the invasive margin could provide independent prognostic
information for curatively resected patients with stages I–III CRC.
We based our study off the definition of the invasive margin which
was based on the recommendation by the International
Immuno-Oncology Biomarker Working Group (2). We evaluated the densities of TILs and
TANs in this area. Furthermore, we determined the clinical
importance of CD8+ TILs and CD66b+ TANs in
CRC and focused on the combined prognostic significance of these
immune-cell subsets in the invasive margin.
Materials and methods
Patients and sample collection
We retrospectively enrolled 131 consecutive patients
with stages I–III CRC who underwent curative resection at the Mie
University Hospital from 2013 to 2015. 28 patients were excluded,
of which, 15 patients received preoperative neoadjuvant therapy,
and 13 patients could not be evaluated for immunohistochemical
analysis. The patients' median age was 71 years and ranged from 38
to 94 years, and 38.8% of patients were women while 62.2% were
male. The clinicopathological characteristics of patients are
presented in Table I. The protocol
for this research project was approved by the institutional review
board of Mie University Hospital (approval no. H2019-187). Informed
consent was obtained in the form of opt-out on the web-site. Those
who rejected were excluded. The study was conducted in accordance
with the Declaration of Helsinki. All patients were classified
according to the International Union against Cancer TNM
Classification (7th Edition) and underwent resection of the primary
tumor.
 | Table I.Clinicopathological characteristics
of patients with CRC (n=103). |
Table I.
Clinicopathological characteristics
of patients with CRC (n=103).
| Variables | N (%) |
|---|
| Median age (range),
years | 71 (38–94) |
| Gender |
|
|
Male | 63 (61.2) |
|
Female | 40 (38.8) |
| Location |
|
|
Colon | 59 (57.3) |
|
Rectum | 44 (42.7) |
|
Differentiation |
|
|
Differentiated | 95 (92.2) |
|
Undifferentiated | 8 (7.8) |
| Pathological T
category |
|
| T1 | 19 (18.5) |
| T2 | 25 (24.3) |
| T3 | 47 (45.6) |
| T4 | 12 (11.7) |
| Lymph node
metastasis |
|
| N0 | 70 (67.9) |
| N1 | 33 (32.1) |
| UICC stage
classification |
|
| Stage
I | 38 (36.9) |
| Stage
II | 32 (31.1) |
| Stage
III | 33 (32.0) |
| MSI |
|
|
MSI-H | 8 (7.8) |
|
MSI-L/MSS | 95 (92.2) |
| KRAS |
|
|
Wild | 54 (52.4) |
|
Mutation | 49 (47.6) |
| BRAF |
|
|
Wild | 97 (94.2) |
|
Mutation | 6 (5.8) |
After surgery, all patients with stage III CRC
received 5-fluorouracil-based chemotherapy, whereas patients with
stage I or II CRC were not administered adjuvant chemotherapy.
Patients were observed in 3-month intervals for 24 months after the
completion of surgery, then every 6 months for the next 3 years,
and lastly yearly thereafter. During each annual hospital visit,
all patients underwent a chest X-ray, colonoscopy, and abdominal
computed tomography. Moreover, at each visit there was a physical
examination performed to further document their patient histories.
Retrospective clinical data were obtained from medical records and
pathological reports, including sex, age, anatomical site, tumor
differentiation, depth of invasion, vessel invasion, lymph node
metastasis, UICC TNM classification, KRAS status, BRAF status,
microsatellite instability, and survival data [disease-free
survival (DFS) and overall survival (OS)]. After treatment,
formalin-fixed and paraffin-embedded tissue sections were used for
further immunohistochemical analysis.
Immunohistochemical analysis
Formalin-fixed and paraffin-embedded specimens were
sliced into 5-µm sections and subjected to immunohistochemical
analysis to detect the expression of CD8+ TILs and
CD66b+ TANs in the invasive tumor margin. The primary
antibodies used were a monoclonal rabbit anti-human CD8 (clone:
EP1150, dilution 1:1,000; GeneTex, San Antonio, TX, USA) and a
monoclonal mouse anti-human CD66b (clone: G10F5, dilution 1:200;
Biolegend, San Diego, CA, USA).
Immunohistochemical evaluation of
CD8+ TILs and CD66b+ TANs
Two independent observers who were uninformed of
clinical outcomes evaluated the sections using an inverted research
microscope (BX-50, Olympus, Japan). With a magnification of 400×,
CD8+ TILs and CD66b+ TANs were photographed
in three representative high-power fields (HPFs) at the invasive
tumor margin. According to the recommendation by the International
Immuno-Oncology Biomarker Working Group, the ‘invasive margin’ was
defined as the region centered on the border separating the host
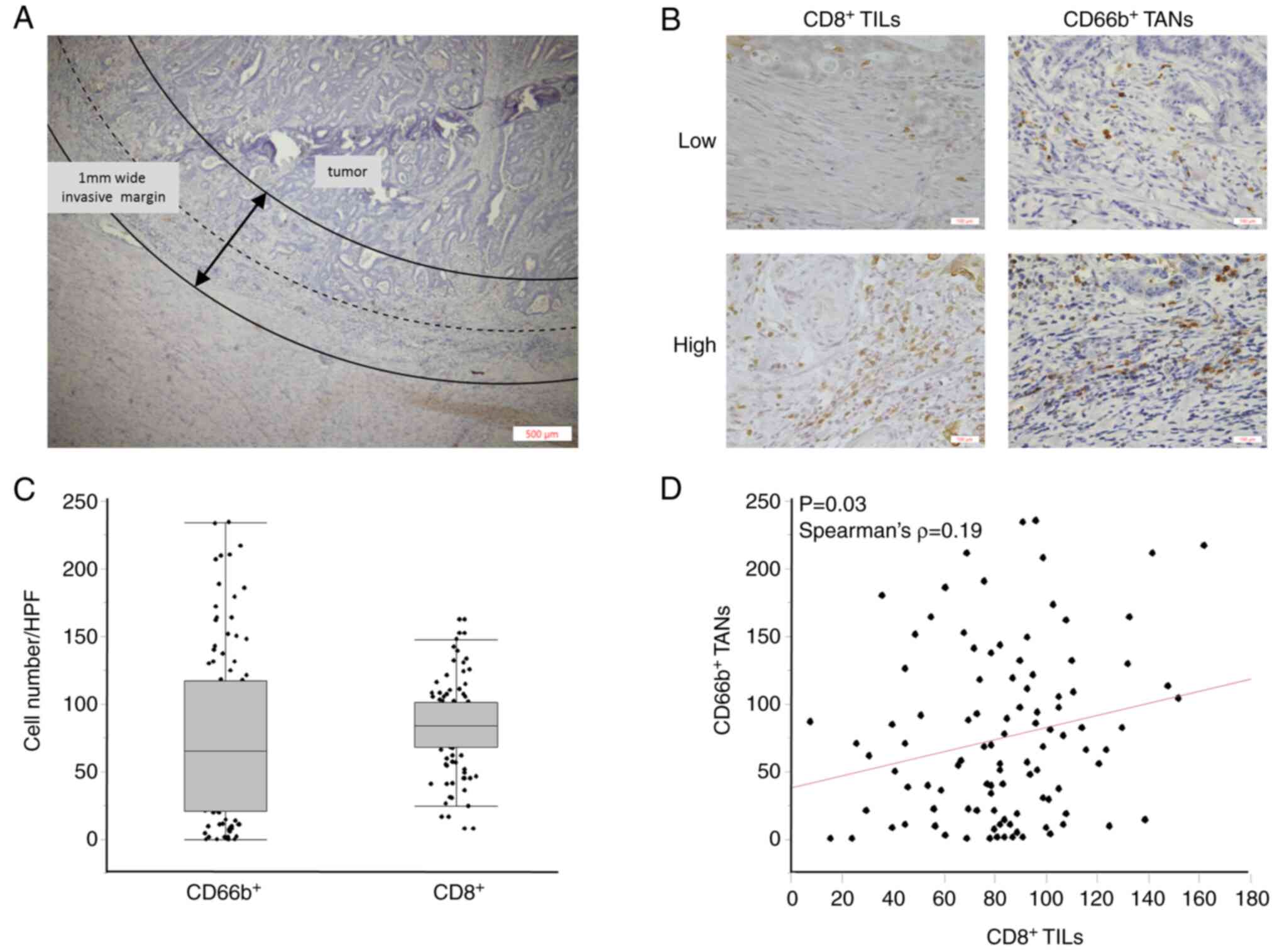
tissue from the malignant nets, with an extent of 1 mm (2) (Fig.
1A). Representative images are presented in Fig. 1B.
Statistical analysis
Statistical analysis was performed using JMP
software version 10 (SAS Institute, Cary, NC) and MedCalc
Statistical Software version 19.1.2 (MedCalc Software bv, Ostend,
Belgium). Spearman's rank correlation analysis was used to
determine the relation between non-normally distributed continuous
variables. Differences between groups were estimated using the Mann
Whitney U, Kruskal-Wallis followed by Steel-Dwass test or one-way
ANOVA followed by Tukey's Kramer when appropriate. Shapiro-Wilk
tests were performed to evaluate the normality of the data
distribution, and Levene's tests were conducted to assess the
equality of variance for comparable groups. For time-to-event
analyses, survival estimates were calculated using the Kaplan-Meier
method, and groups were compared using the log-rank test. Receiver
operating characteristic curves with Youden's index was generated
to determine the cut-off values for analyzing prognosis. Univariate
and multivariate analysis was performed using Cox proportional
hazards regression. For multivariate analysis, variables with
P-values <0.20 in univariate analysis were included in the
multivariate regression model. All P-values were 2-sided, and
P<0.05 was considered significant.
Results
Association between clinical
characteristics and tumor-infiltrating immune cells in CRC
We first performed histopathological analysis of
tissue sections to evaluate the densities of CD8+ TILs
and CD66+ TANs in tumor margins as well as evaluated the
associations between different clinicopathological factors and the
degree of TILs. The median densities of CD8+ TILs and
CD66b+ TANS in the tumor margin were 84/HPF (8-162/HPF)
and 65/HPF (0-234/HPF), respectively (Fig. 1C). There was a weak positive
correlation between the densities of CD8+ TILs and
CD66b+ TANs in the tumor margin (Spearman's correlation
coefficient: 0.19, P=0.03) (Fig.
1D). Interestingly, the decreased expression of CD8+
TILs in tumor margin was significantly associated with mutated KRAS
status (P=0.03), and the decreased expression of CD66+
TANs in the tumor margin was significantly associated with lymph
node metastasis (P=0.01) (Table
II).
 | Table II.Association between the infiltration
of immune cells and clinicopathological characteristics of patients
with CRC. |
Table II.
Association between the infiltration
of immune cells and clinicopathological characteristics of patients
with CRC.
| Variables | CD8+
number/HPF mean ± SD | P-value | CD66b+
number/HPF [median (IQR)] | P-value |
|---|
| Agea |
| 0.10 |
| 0.85 |
| Low
(<71 years) | 85.9±31.6 |
| 68.7
(17.9-115.2) |
|
| High
(≥71 years) | 81.1±27.9 |
| 60.8
(21.0-117.5) |
|
| Gender |
| 0.98 |
| 0.96 |
|
Male | 83.5±31.6 |
| 60.8
(20.7-117.0) |
|
|
Female | 83.4±26.9 |
| 70.0
(19.8-116.5) |
|
| Location |
| 0.54 |
| 0.59 |
|
Colon | 81.9±29.6 |
| 65.0
(20.7-108.3) |
|
|
Rectum | 85.6±30.1 |
| 67.5
(23.4-127.8) |
|
|
Differentiation |
| 0.15 |
| 0.22 |
|
Differentiated | 82.3±29.9 |
| 67.7
(20.7-121.0) |
|
|
Undifferentiated | 97.3±25.0 |
| 45.8
(15.8-74.1) |
|
| Pathological T
category |
| 0.78 |
| 0.52 |
|
pT1/2 | 84.4±31.3 |
| 67.7
(37.5-106.9) |
|
|
pT3/4 | 82.8±28.7 |
| 60.8
(11.3-129.2) |
|
| Vessel
invasion |
| 0.32 |
| 0.70 |
|
Absent | 87.0±34.2 |
| 65.0
(12.5-115.0) |
|
|
Present | 81.1±26.2 |
| 62.7
(27.5-118.0) |
|
| Lymph node
metastasis |
| 0.73 |
| 0.01b |
|
Absent | 82.9±29.6 |
| 34.0
(9.0-82.7) |
|
|
Present | 84.8±30.1 |
| 79.5
(36.9-126.2) |
|
| UICC stage
classification |
| 0.96 |
| 0.05b,c |
| Stage
I | 82.7±31.6 |
| 73.5
(39.0-109.3) |
|
| Stage
II | 83.1±27.4 |
| 84.5
(15.3-146.3) |
|
| Stage
III | 84.8±30.4 |
| 34.0
(9.0-82.7) |
|
| MSI |
| 0.40 |
| 0.75 |
|
MSI | 92.1±31.4 |
| 72.7
(55.8-101.7) |
|
|
MSS | 82.8±29.6 |
| 60.8
(19.6-118) |
|
| KRAS |
| 0.03b |
| 0.68 |
|
Mutation | 76.8±31.6 |
| 69.8
(22.0-110.2) |
|
|
Wild | 89.6±26.7 |
| 55.1
(13.3-120.8) |
|
| BRAF |
| 0.37 |
| 0.16 |
|
Mutation | 94.0±14.0 |
| 40.3
(7.9-79.1) |
|
|
Wild | 82.8±30.3 |
| 67.7
(20.8-119.5) |
|
Low densities of CD8+ TILs
and CD66+ TANs in the tumor margin correlates with poor
prognosis and disease recurrence of patients with stages I–III
CRC
Next, to evaluate the significance of the
association between tumor-infiltrating immune cells and survival,
we defined the cutoff values of CD8+ TILs and
CD66+ TANs, to be 91 and 77 cells per HPF, respectively,
according to the receiver operating characteristic analysis and
Youden's index. We then conducted Kaplan-Meier survival analysis
according to the densities of CD8+ TILs and
CD66+ TANs. Consistent with our previous research on
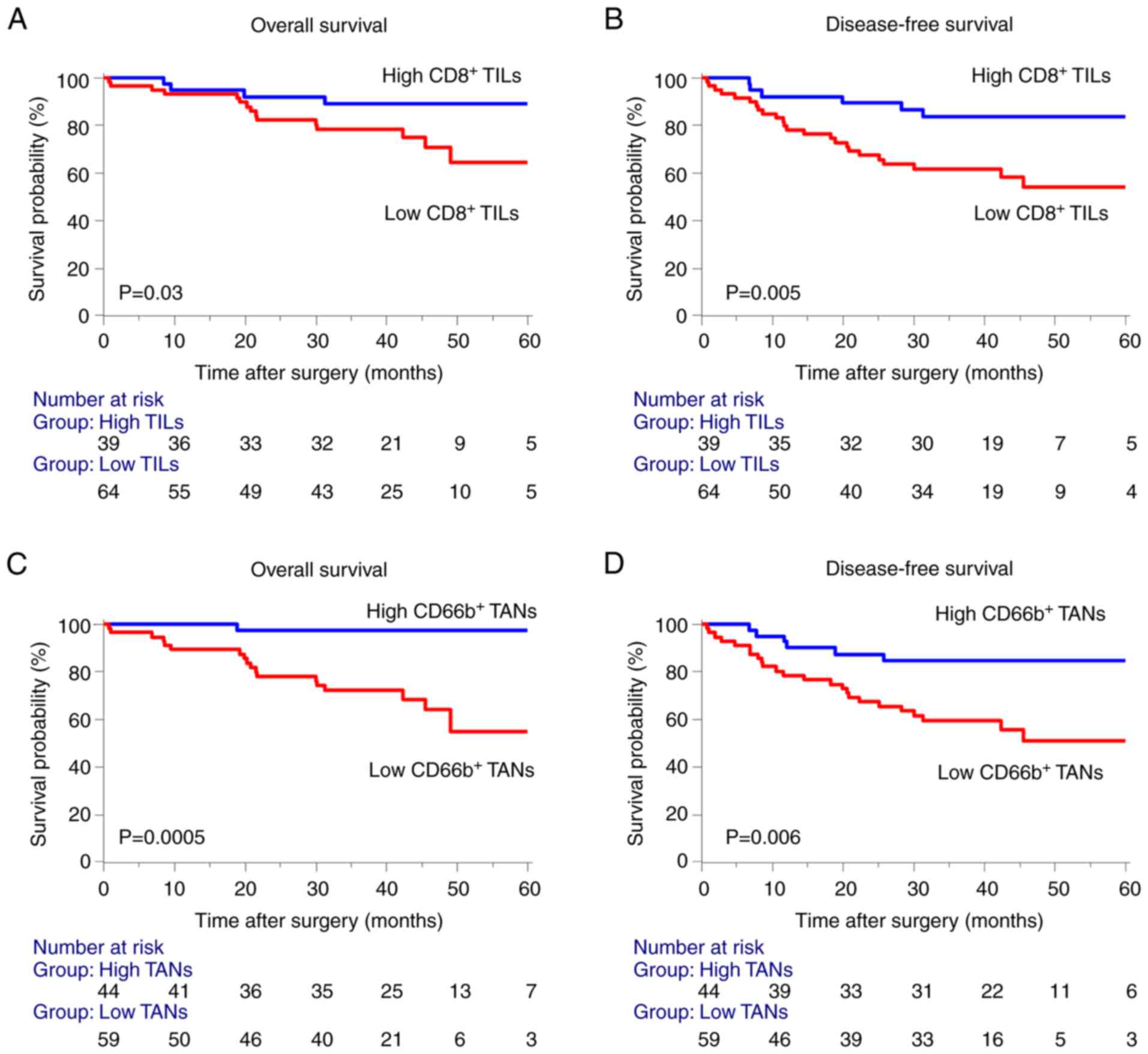
CD8+ TILs (9), we found
that a low density of CD8+ TILs in the invasive margin
significantly correlated with poor prognosis for OS and DFS of
patients with stages I–III CRC (Fig.
2A and B). Furthermore, low density of CD66+ TANs in
the invasive margin was unexpectedly associated with a poor
prognosis in OS and DFS (Fig. 2C and
D).
Combined assessment of CD8+
TILs and CD66b+ TANs densities (Model 1) in the invasive
margin
We hypothesized that the immune signature of the
invasive margin improves the prognostic impact of established
clinicopathological parameters and assumed that the different
densities of CD8+ TILs or CD66b+ TANs in the
invasive margin could represent different levels of antitumor
immunity. We therefore investigated whether the favorable
signatures, identified in the patient populations with CRC stages I
to III, have prognostic value for the clinical outcomes of
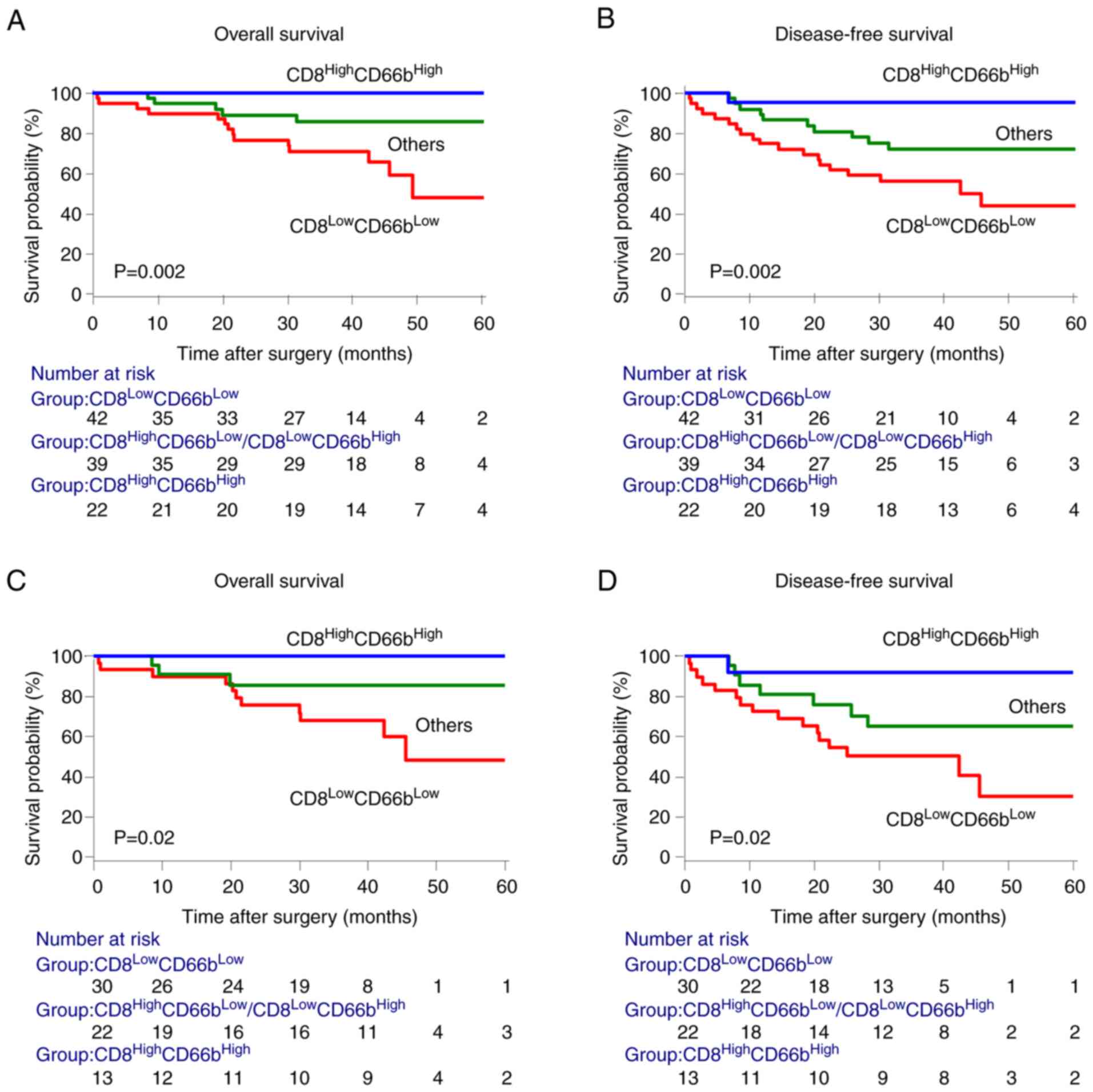
subgroups of patients. In the end, the data revealed that cancers
of patients infiltrated by CD8+ TILs and
CD66b+ TANs were characterized by a favorable prognosis,
whereas patients with low densities of CD8+ TILs and
CD66b+ TANs had the poorest prognosis (Fig. 3A and B). Similar results were
obtained for patients with stages II and III CRC, who may require
further post-surgical treatment (Fig.
3C and D).
To further determine whether the potential of
tumor-infiltrating immune cells could function as predictive
biomarkers for cancer recurrence and prognosis was influenced by
other variables, we incorporated an immune feature comprising
CD8+ TILs and CD66b+ TANs
(CD8LowCD66bLow) into a Cox regression
proportional hazard model. Univariate analysis revealed that the
pathological T category, lymph node metastasis, and the immune
signature were significantly associated with DFS, further revealing
that low densities of CD8+ TILs and CD66b+
TANs correlated with poor prognosis. Also, multivariate analysis
revealed that the signature of the invasive margin served as an
independent prognostic factor for OS (HR=3.80, 95% CI, 1.48-11.1,
P=0.005) of patients with CRC (Table
III). Furthermore, this signature served as an independent
prognostic factor for DFS (HR=2.52, 95% CI, 1.18-5.58, P=0.02)
(Table IV).
 | Table III.Multivariate analysis for OS of
patients with stages I–III CRC. |
Table III.
Multivariate analysis for OS of
patients with stages I–III CRC.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Gender (Male) | 1.39 | 0.55-3.95 | 0.49 |
|
|
|
| Age (≥71
years)a | 1.52 | 0.63-3.91 | 0.36 |
|
|
|
| Location
(Rectum) | 1.09 | 0.44-2.65 | 0.84 |
|
|
|
| Differentiation
(Undifferentiated) | 4.22 | 1.20-11.7 | 0.03c | 2.90 | 0.82-8.16 | 0.09 |
| Pathological T
category (T3/T4) | 1.84 | 0.74-5.20 | 0.19 | 1.56 | 0.61-4.49 | 0.36 |
| Vessel invasion
(Present) | 1.21 | 0.49-3.22 | 0.69 |
|
|
|
| Lymph node
metastasis (Present) | 1.58 | 0.62-3.85 | 0.32 |
|
|
|
| MSI
(MSI-L/MSS) | 1.49 | 0.31-26.8 | 0.68 |
|
|
|
| KRAS (Wild) | 1.73 | 0.71-4.63 | 0.23 |
|
|
|
| BRAF (Wild) | 1.11 | 0.23-19.9 | 0.92 |
|
|
|
| CD66b+
(Low) | 8.82 | 2.51-55.9 | 0.0002c |
|
|
|
| CD8+
(Low) | 3.15 | 1.14-11.1 | 0.04c |
|
|
|
|
CD8+CD66b+
(Low-Low)b | 4.41 | 1.75-12.6 | 0.002c | 3.80 | 1.48-11.0 | 0.005c |
 | Table IV.Multivariate analysis of DFS of
patients with stage I–III CRC. |
Table IV.
Multivariate analysis of DFS of
patients with stage I–III CRC.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95%CI | P-value |
|---|
| Gender (Male) | 1.54 | 0.73-3.53 | 0.26 |
|
|
|
| Age (≥71
years)a | 0.83 | 0.41-1.70 | 0.60 |
|
|
|
| Location
(Rectum) | 1.00 | 0.48-2.04 | 0.99 |
|
|
|
| Differentiation
(Undifferentiated) | 2.17 | 0.64-5.57 | 0.19 | 1.58 | 0.46-4.21 | 0.43 |
| Pathological T
category (T3/T4) | 2.48 | 1.15-5.92 | 0.02c | 1.87 | 0.82-4.71 | 0.14 |
| Vessel invasion
(Present) | 1.64 | 0.78-3.78 | 0.19 | 1.32 | 0.59-3.17 | 0.51 |
| Lymph node
metastasis (Present) | 2.18 | 1.06-4.44 | 0.04c | 1.22 | 0.54-2.79 | 0.62 |
| MSI
(MSI-L/MSS) | 2.51 | 0.54-44.6 | 0.29 |
|
|
|
| KRAS (Wild) | 1.19 | 0.59-2.46 | 0.63 |
|
|
|
| BRAF (Wild) | 2.02 | 0.43-36.0 | 0.44 |
|
|
|
| CD66b+
(Low) | 3.12 | 1.41-7.88 | 0.004 |
|
|
|
| CD8+
(Low) | 3.35 | 1.46-9.06 | 0.003 |
|
|
|
|
CD8+CD66b+
(Low-Low)b | 3.04 | 1.48-6.47 | 0.002c | 2.52 | 1.18-5.58 | 0.02c |
Low TANs-to-TILs ratio (Model 2) in
the invasive margin correlates with poor prognosis of patients with
stages I–III
From a statistical standpoint, a continuous variable
is more amenable to analysis. Therefore, we evaluated significance
of TANs-to-TILs ratio as a prognostic marker in patients with
stages I–III CRC. Kaplan-Meier survival analysis showed that
patients with low TANs-to-TILs ratio in the invasive margin had a
better prognosis (Fig. S1).
Multivariate Cox regression analysis indicated TANs-to-TILs ratio
was an independent prognostic factor both for OS (P=0.008) of
patients with stages I–III CRC, but not for DFS (P=0.07) (Tables SI and SII).
Discussion
To improve the poor prognosis of patients with CRC,
we require better stratification analyses to predict the disease
development. Accumulating evidence reveals that tumor-infiltrating
immune cells may serve as markers for the prognosis of various
types of cancer. However, the clinical significance of
CD66b+ TANs in CRC remains controversial due to
conflicting results as both tumor-promoting and tumor-suppressing
roles of CD66b+ TANs in CRC have been reported (14–16).
Inflammatory cells are essential components of the
immune microenvironment. More specifically, lymphocytes and
neutrophils play crucial roles in the pathogenesis of several
diverse diseases including cancer (17). In the TME, surface markers
expressed by TILs include CD3, CD8, or FoxP3. CD3+ TILs
and CD8+ TILs in the invasive margin play a central role
in immunity against CRC (5,18,19).
The ‘Immunoscore’ which assesses TILs in CRC can serve as a strong
predictive measure of DFS and OS which is superior to the
traditional tumor-node-metastasis staging system of the AJCC/UICC
(20). Therefore, fully comparing
the results from this study to the traditional system, we chose the
same definition of tumor margins to assess TANs. However, TANs
recruited in tumors exhibit different phenotypes compared with
those of circulating peripheral blood neutrophils (21).
Neutrophils recruited into tissues engage in complex
bidirectional interactions with macrophages, dendritic cells,
natural killer cells, as well as B and T cells (17). In the TME, TANs exhibit complex
phenotypic heterogeneity and functional versatility. Neutrophils
were classified as antitumorigenic ‘N1’ and protumorigenic ‘N2’
phenotypes (22–24). In humans, CD66b is traditionally
defined as a marker expressed on the surface of neutrophils, which
are myeloid cells with a short half-life and a specific nuclear
morphology (25,26).
Research on TANs generally employs
immunohistochemistry to evaluate their densities. However,
published studies give conflicting messages regarding the clinical
significance of a high density of CD66b+ TANs and are
difficult to compare and interpret due to the analyses of diverse
tumor types (4,12,13,27,28).
These mixed results can be partly explained by inconsistent
evaluation areas in the various studies. For example, Zhu et
al quantitatively assessed the association between the density
of CD66b+ TANs and prognosis using a training cohort of
337 patients and a validation cohort of 245 patients who had CRC.
They demonstrated that patients with a low density of
CD66b+ TANs experienced better clinical outcomes
compared with those with a high density of such cells (14). However, this study did not clarify
the areas (tumor center or invasive margin) used to evaluate the
densities of CD66b+ cells which is one of the reasons
why the results of the study differ from ours.
Moreover, Ye et al conducted tissue
microarray (TMA) and immunohistochemical analyses on patients with
CRC revealing that patients with CRC with a high density of
infiltrating CD66b+ TANs experienced better prognosis
(15). We also noticed that many
studies used TMAs to evaluate the expression of tumor-infiltrating
immune cells (15,16). However, most TMAs only assess the
tumor-infiltrating immune cells of the tumor nest and not in the
invasive margin which is difficult to evaluate.
To our knowledge, there have only been two studies
that have evaluated the role of CD66b+ TANs in the
invasive margin of CRC (11,29).
One of the two being, Galdiero et al who defined the
invasive margin as ‘50% of the entire microscopic field was
cancerous tissue’. In their study, all CD66b+ cells in
this microscopic area were counted, but the count may increase
compared with the true value (11). Moreover, Wikberg et al
assessed CD66b+ neutrophil infiltration in the tumor
front and center using a semi-quantitative score. However, this
study did not specifically define ‘the tumor front’ and chose a
semi-quantitative evaluation method because it does not require a
precise definition of the evaluation area. In the end, the results
revealed that CD66b+ TANs infiltration maintained an
independent significance in the multivariable analysis of stage
I–II colon cancers, but not of stage III–IV stage colon cancer. All
patients (23.8% were stage IV) were divided into 4 groups according
to expression of CDd66b+ TANs and CD8+ TILs.
The patients with high CD66b+ TANs and high
CD8+ TILs in tumor front had the best prognosis in OS.
However, in total there was no significant difference between the
other three groups (29). We also
believe that most stage IV CRC patients will die from metastatic
disease and should not be included in the survival analysis for OS
discounting some of the data and conclusions gained from this
study. More specifically, why the combined assessment of
CD8+ TILs and CD66b+ TANs did not show
greater potency. At last in our study we defined the invasive
margin as ‘the region centered on the border separating the host
tissue from the malignant nets with an extent of 1 mm’, which is
consistent with the ‘Immunoscore’ (5,20)
and the prognostic indicator has been well validated in stages
I–III of colon cancer.
In sum, we found in this study that low densities of
CD8+ TILs and CD66b+ TANs in the invasive
margin of tumor significantly correlated with shorter OS and DFS of
patients with stages I–III CRC, suggesting that these
tumor-infiltrating immune cells configured the complex
microenvironment that influences tumor development. Also, in
patients with lymph node metastasis (stage III), the density of
CD66b+ TANs decreased significantly, suggesting that
density of CD66b+ TANs in invasive margin may be linked
to immune escape (30). In
addition, low density of CD8+ TILs in the invasive
margin was correlated significantly with KRAS mutation. Some
evidence suggests that KRAS mutation may not only activate many
downstream signaling pathways (31), but can also mediate immune evasion
in various tumors (32). Smakman
et al reported that silencing KRAS(D12) significantly
reduced the tumorigenic potential of C26 cells in mice with intact
immune systems. The incidence of tumor formation by KRAS(D12)
knockdown cells remained at 100% in immune-deficient hosts
(33). Thus, this finding suggests
that KRAS-driven tumorigenicity is due to in-part to the
suppression of host immunity. Moreover, Zdanov et al
reported mutant KRAS conversed conventional T cells into regulatory
T cells (Tregs) and enhanced the function of Tregs. In this study,
the CD4+CD25− T cells were cocultured with
mutant KRAS tumor cells (SW620, SW480), and a high percentage of
CD4+CD25− T cells that were converted to
Tregs expressed FOXP3 and CTLA-4. In contrast, cocultures
established with WT KRAS tumor cells (colo320, widr) contained
significantly fewer Tregs (34).
Thus, this reveals that KRAS mutation may lead to inhibition of
CD8+ TILs activation and proliferation.
A major finding of our present study is that the
combined evaluation of CD8+ TILs and CD66b+
TANs in the invasive margin of CRC achieved improved stratification
and prognostic accuracy. Also, simultaneous low tumor infiltration
by CD8+ TILs and CD66b+ TANs was associated
significantly with poorer prognosis compared with that of
CD8+ TILs or CD66b+ TANs, suggesting that
interaction between CD8+ TILs and CD66b+ TANs
enhances the independent effect against CRC. Furthermore, we found
that an immune signature with low densities of CD8+ TILs
and CD66b+ TANs served as an independent prognostic
factor for OS and DFS. Moreover, patients with stages II and III
CRC with this signature experienced terrible survival outcomes,
suggesting that we should pay more attention to postoperative
treatment strategies.
Although there is increasing evidence suggesting
that neutrophils are involved in adaptive immunity (17,35),
the interplay between these TANs and other immune cells in TME is
poorly understood. Furthermore, there is much difficulty associated
with research on the mechanism of neutrophils which is due to the
effect of various cell separation procedures on assays of
neutrophil function. For example, in-vitro experiments that
work with isolated neutrophils do not behave normally because they
are primed or preactivated during isolation (36). CD8+ T cells play a
central role in anti-tumor effect. TGF-β induces neutrophils to
acquire N2 phenotype neutrophils (pro-tumor). Moreover, an in
vivo study revealed that the antitumor effect of TGF-β receptor
kinase inhibitor was lost in mice with CD8+ T cell
depletion (treated with anti-CD8 antibody). On the other hand, the
depletion of N2 phenotype neutrophils affects the activation of
CD8+ T cells (23).
Another in vitro study also found that CD66b+
TANs (anti-tumor) frequently co-localize with CD8+ T
cells and the co-culture with CD66b+ TANs enhances
CD8+ T cell activation and proliferation in CRC
(3). Furthermore, simultaneous low
tumor infiltration by CD8+ TILs and CD66b+
TANs is associated significantly with poorer prognosis compared
with that of CD8+ TILs or CD66b+ TANs,
suggesting that the interaction between CD8+ TILs and
CD66b+ TANs enhances the independent effect against
CRC.
We also tried another combined assessment method,
TANs-to-TILs ratio, which is more amenable to analysis as it is a
continuous variable. Model 1 and Model 2 yielded similar results
since low density of CD66b+ TANs showed the highest
hazard ratio for OS (HR: 8.82; 95% CI, 2.51-55.9) in univariate
analysis. However, compared to Model 2, Model 1 had a stronger risk
stratification ability to identify patients with worse
prognosis.
Immunotherapy has changed the treatment strategy for
many tumors. Previous studies have shown that PD-1/PD-L1 blockade
reinvigorated function of CD8+ cytotoxic T lymphocytes
(37), and CTLA-4 mAbs required
depletion of regulatory T cells (Tregs) (38). On the other hand, the role of TANs
remains unclear in immunotherapy as they can activate or inhibit
TILs (3,39). Tumor with the absence of TILs is
often referred to as ‘cold tumors’ and associate with initial
resistance to immunotherapy. Therefore, CRC patients with low
densities of CD8+ TILs and CD66b+ TANs may be
related to poor immunotherapy response, requiring a closer
follow-up and more aggressive adjuvant therapy. On the other hand,
patients with high densities of CD8+ TILs and
CD66b+ TANs may benefit from immunotherapy when local
recurrence or distant metastasis occurs.
We acknowledge several potential limitations to this
study as it is a single instructional and retrospective study with
a sample size somewhat smaller. Small number of end-point events
limited multivariate Cox regression analysis. Therefore, larger
prospective trials and validation cohorts are needed to further
confirm the potential of TILs and TANs in the invasive margins as a
prognostic marker for patients with CRC.
In conclusion, our present study provides evidence
for the clinical significance of CD66b+ TANs in the
invasive margin of CRC. Combined assessment of CD8+ TILs
and CD66b+ TANs in the invasive margin better stratified
high-risk CRC patients. These analyses may help surgeons and
oncologists to design more effective postoperative oncological
follow-up strategies for managing patients with advanced CRC.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
The authors would like to thank Mr. Arul Goel (La
Canada High School, La Canada Flintridge, CA, USA) for editing a
draft of this manuscript and English language correction.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CY, YOku and YT conceived and designed the study.
YOku, AY, TK, TS, MK, MT, YOki, MO and YT provided the samples and
acquired the clinical and prognostic information. CY, YOku and YT
analyzed and interpreted the data. CY, YOku and YT performed
statistical analysis. CY, YOku, AY, TK, TS, MK, MT, YOki, MO and YT
contributed to the writing of the manuscript, and agreed with the
manuscript's results and conclusions. YT and CY confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The protocol for this research project was approved
by the institutional review board of Mie University Hospital
(approval no. H2019-187). Informed consent was obtained in the form
of opt-out on the web-site. Those who rejected were excluded. The
study was conducted in accordance with the Declaration of
Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
TANs
|
tumor-associated neutrophils
|
|
CRC
|
colorectal cancer
|
|
TILs
|
tumor-infiltrating lymphocytes
|
|
TME
|
tumor microenvironment
|
|
DFS
|
disease-free survival
|
|
OS
|
overall survival
|
|
TMA
|
tissue microarray
|
|
TNM
|
Tumor, Node, Metastasis
|
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hendry S, Salgado R, Gevaert T, Russell
PA, John T, Thapa B, Christie M, van de Vijver K, Estrada MV,
Gonzalez-Ericsson PI, et al: Assessing tumor-infiltrating
lymphocytes in solid tumors: A practical review for pathologists
and proposal for a standardized method from the international
immunooncology biomarkers working group: Part 1: Assessing the host
immune response, TILs in invasive breast carcinoma and ductal
carcinoma in situ, metastatic tumor deposits and areas for further
research. Adv Anat Pathol. 24:235–251. 2017. View Article : Google Scholar
|
|
3
|
Governa V, Trella E, Mele V, Tornillo L,
Amicarella F, Cremonesi E, Muraro MG, Xu H, Droeser R, Däster SR,
et al: The interplay between neutrophils and CD8+ T
cells improves survival in human colorectal cancer. Clin Cancer
Res. 23:3847–3858. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jiang Y, Xie J, Huang W, Chen H, Xi S, Han
Z, Huang L, Lin T, Zhao LY, Hu YF, et al: Tumor immune
microenvironment and chemosensitivity signature for predicting
response to chemotherapy in gastric cancer. Cancer Immunol Res.
7:2065–2073. 2019.PubMed/NCBI
|
|
5
|
Galon J, Costes A, Sanchez-Cabo F,
Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M,
Berger A, Wind P, et al: Type, density, and location of immune
cells within human colorectal tumors predict clinical outcome.
Science. 313:1960–1964. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Swierczak A, Mouchemore KA, Hamilton JA
and Anderson RL: Neutrophils: Important contributors to tumor
progression and metastasis. Cancer Metastasis Rev. 34:735–751.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao Y, Ge X, He J, Cheng Y, Wang Z, Wang
J and Sun L: The prognostic value of tumor-infiltrating lymphocytes
in colorectal cancer differs by anatomical subsite: A systematic
review and meta-analysis. World J Surg Oncol. 17:852019. View Article : Google Scholar
|
|
8
|
Idos GE, Kwok J, Bonthala N, Kysh L,
Gruber SB and Qu C: The prognostic implications of tumor
infiltrating lymphocytes in colorectal cancer: A systematic review
and meta-analysis. Sci Rep. 10:33602020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mori K, Toiyama Y, Saigusa S, Fujikawa H,
Hiro J, Kobayashi M, Ohi M, Araki T, Inoue Y, Tanaka K, et al:
Systemic analysis of predictive biomarkers for recurrence in
colorectal cancer patients treated with curative surgery. Dig Dis
Sci. 60:2477–2487. 2015. View Article : Google Scholar
|
|
10
|
Lakschevitz FS, Hassanpour S, Rubin A,
Fine N, Sun C and Glogauer M: Identification of neutrophil surface
marker changes in health and inflammation using high-throughput
screening flow cytometry. Exp Cell Res. 342:200–209. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Galdiero MR, Bianchi P, Grizzi F, Di Caro
G, Basso G, Ponzetta A, Bonavita E, Barbagallo M, Tartari S,
Polentarutti N, et al: Occurrence and significance of
tumor-associated neutrophils in patients with colorectal cancer.
Int J Cancer. 139:446–456. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Carus A, Ladekarl M, Hager H, Nedergaard
BS and Donskov F: Tumour-associated CD66b+ neutrophil count is an
independent prognostic factor for recurrence in localised cervical
cancer. Br J Cancer. 108:2116–2122. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ilie M, Hofman V, Ortholan C, Bonnetaud C,
Coëlle C, Mouroux J and Hofman P: Predictive clinical outcome of
the intratumoral CD66b-positive neutrophil-to-CD8-positive T-cell
ratio in patients with resectable nonsmall cell lung cancer.
Cancer. 118:1726–1737. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu B, Luo J, Jiang Y, Yu L, Liu M and Fu
J: Prognostic significance of nomograms integrating IL-37
expression, neutrophil level, and MMR status in patients with
colorectal cancer. Cancer Med. 7:3682–3694. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ye L, Zhang T, Kang Z, Guo G, Sun Y, Lin
K, Huang Q, Shi X, Ni Z, Ding N, et al: Tumor-infiltrating immune
cells Act as a marker for prognosis in colorectal cancer. Front
Immunol. 10:23682019. View Article : Google Scholar
|
|
16
|
Rao HL, Chen JW, Li M, Xiao YB, Fu J, Zeng
YX, Cai MY and Xie D: Increased intratumoral neutrophil in
colorectal carcinomas correlates closely with malignant phenotype
and predicts patients' adverse prognosis. PLoS One. 7:e308062012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mantovani A, Cassatella MA, Costantini C
and Jaillon S: Neutrophils in the activation and regulation of
innate and adaptive immunity. Nat Rev Immunol. 11:519–531. 2011.
View Article : Google Scholar
|
|
18
|
Fridman WH, Pagès F, Sautès-Fridman C and
Galon J: The immune contexture in human tumours: Impact on clinical
outcome. Nat Rev Cancer. 12:298–306. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Laghi L, Bianchi P, Miranda E, Balladore
E, Pacetti V, Grizzi F, Allavena P, Torri V, Repici A, Santoro A,
et al: CD3+ cells at the invasive margin of deeply invading
(pT3-T4) colorectal cancer and risk of post-surgical metastasis: A
longitudinal study. Lancet Oncol. 10:877–884. 2009. View Article : Google Scholar
|
|
20
|
Pagès F, Kirilovsky A, Mlecnik B, Asslaber
M, Tosolini M, Bindea G, Lagorce C, Wind P, Marliot F, Bruneval P,
et al: In situ cytotoxic and memory T cells predict outcome in
patients with early-stage colorectal cancer. J Clin Oncol.
27:5944–5951. 2009. View Article : Google Scholar
|
|
21
|
Eruslanov EB: Phenotype and function of
tumor-associated neutrophils and their subsets in early-stage human
lung cancer. Cancer Immunol Immunother. 66:997–1006. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Silvestre-Roig C, Hidalgo A and Soehnlein
O: Neutrophil heterogeneity: Implications for homeostasis and
pathogenesis. Blood. 127:2173–2181. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fridlender ZG, Sun J, Kim S, Kapoor V,
Cheng G, Ling L, Worthen GS and Albelda SM: Polarization of
tumor-associated neutrophil phenotype by TGF-beta: ‘N1’ versus ‘N2’
TAN. Cancer Cell. 16:183–194. 2009. View Article : Google Scholar
|
|
24
|
Fridlender ZG and Albelda SM:
Tumor-associated neutrophils: Friend or foe? Carcinogenesis.
33:949–955. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ng LG, Ostuni R and Hidalgo A:
Heterogeneity of neutrophils. Nat Rev Immunol. 19:255–265. 2019.
View Article : Google Scholar
|
|
26
|
Pillay J, Tak T, Kamp VM and Koenderman L:
Immune suppression by neutrophils and granulocytic myeloid-derived
suppressor cells: Similarities and differences. Cell Mol Life Sci.
70:3813–3827. 2013. View Article : Google Scholar
|
|
27
|
Posabella A, Köhn P, Lalos A, Wilhelm A,
Mechera R, Soysal S, Muenst S, Güth U, Stadlmann S, Terracciano L,
et al: High density of CD66b in primary high-grade ovarian cancer
independently predicts response to chemotherapy. J Cancer Res Clin
Oncol. 146:127–136. 2020. View Article : Google Scholar
|
|
28
|
Huang X, Pan Y, Ma J, Kang Z, Xu X, Zhu Y,
Chen J, Zhang W, Chang W and Zhu J: Prognostic significance of the
infiltration of CD163+ macrophages combined with
CD66b+ neutrophils in gastric cancer. Cancer Med.
7:1731–1741. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wikberg ML, Ling A, Li X, Öberg Å, Edin S
and Palmqvist R: Neutrophil infiltration is a favorable prognostic
factor in early stages of colon cancer. Hum Pathol. 68:193–202.
2017. View Article : Google Scholar
|
|
30
|
Mlecnik B, Tosolini M, Kirilovsky A,
Berger A, Bindea G, Meatchi T, Bruneval P, Trajanoski Z, Fridman
WH, Pagès F and Galon J: Histopathologic-based prognostic factors
of colorectal cancers are associated with the state of the local
immune reaction. J Clin Oncol. 29:610–618. 2011. View Article : Google Scholar
|
|
31
|
Zhu G, Pei L, Xia H, Tang Q and Bi F: Role
of oncogenic KRAS in the prognosis, diagnosis and treatment of
colorectal cancer. Mol Cancer. 20:1432021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pylayeva-Gupta Y, Lee KE, Hajdu CH, Miller
G and Bar-Sagi D: Oncogenic Kras-induced GM-CSF production promotes
the development of pancreatic neoplasia. Cancer Cell. 21:836–847.
2012. View Article : Google Scholar
|
|
33
|
Smakman N, Veenendaal LM, van Diest P, Bos
R, Offringa R, Borel Rinkes IH and Kranenburg O: Dual effect of
Kras(D12) knockdown on tumorigenesis: Increased immune-mediated
tumor clearance and abrogation of tumor malignancy. Oncogene.
24:8338–8342. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zdanov S, Mandapathil M, Abu Eid R,
Adamson-Fadeyi S, Wilson W, Qian J, Carnie A, Tarasova N,
Mkrtichyan M, Berzofsky JA, et al: Mutant KRAS conversion of
conventional T cells into regulatory T cells. Cancer Immunol Res.
4:354–365. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vono M, Lin A, Norrby-Teglund A, Koup RA,
Liang F and Loré K: Neutrophils acquire the capacity for antigen
presentation to memory CD4+ T cells in vitro and ex
vivo. Blood. 129:1991–2001. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Glasser L and Fiederlein RL: The effect of
various cell separation procedures on assays of neutrophil
function. A critical appraisal. Am J Clin Pathol. 93:662–669. 1990.
View Article : Google Scholar
|
|
37
|
Topalian SL, Drake CG and Pardoll DM:
Immune checkpoint blockade: A common denominator approach to cancer
therapy. Cancer Cell. 27:450–461. 2015. View Article : Google Scholar
|
|
38
|
Du X, Liu M, Su J, Zhang P, Tang F, Ye P,
Devenport M, Wang X, Zhang Y, Liu Y and Zheng P: Uncoupling
therapeutic from immunotherapy-related adverse effects for safer
and effective anti-CTLA-4 antibodies in CTLA4 humanized mice. Cell
Res. 28:433–447. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Germann M, Zangger N, Sauvain MO, Sempoux
C, Bowler AD, Wirapati P, Kandalaft LE, Delorenzi M, Tejpar S,
Coukos G and Radtke F: Neutrophils suppress tumor-infiltrating T
cells in colon cancer via matrix metalloproteinase-mediated
activation of TGFβ. EMBO Mol Med. 12:e106812020. View Article : Google Scholar : PubMed/NCBI
|