Introduction
Primary cilia are single, immotile organelles that
are present in all mammalian cells. These structures protruding
from the surface of the cell membrane function as extracellular
signal receptors for mechanical and chemical stimuli (1). The primary cilium is an organelle
that exists in the G0/G1 phase of the cell
cycle (1). It has been reported
that ciliogenesis, consisting of the assembly, degradation and
disappearance of primary cilia, is regulated by intracellular and
extracellular signaling and is involved in cell proliferation
(2). The endometrium is a tissue
that undergoes repeated proliferation and breakdown in response to
ovarian steroid hormones during the menstrual cycle, and the state
of primary cilia in this tissue undergoes change on a monthly basis
(3).
Disorder in this ciliary cycle, leading to
dysregulation of cell proliferation in various malignancies, has
been thought to result in a loss or reduction of ciliated cells. To
date, the changes associated with primary cilia have been reported
in tumor tissues or cultured cells that originated from such tumors
as glioblastoma (4), basal cell
carcinoma (5), colorectal cancer
(6), ovarian cancer (7), breast cancer (8), prostate cancer (9), renal cell carcinoma (10), pancreatic cancer (11) and cholangiocarcinoma (12). However, it has also been reported
that inhibition of ciliogenesis suppressed tumor growth in
medulloblastoma (13). Endometrial
cancer is one of the most common gynecological cancers, and its
incidence and mortality rates vary from country to country, perhaps
due to environmental factors (14,15).
Overall, ~20% of endometrial cancers are diagnosed as advanced
cancers, which are difficult to treat with local therapy alone and
are often resistant to systemic therapy, resulting in a poor
prognosis (16). Histological
classification of endometrial carcinoma includes endometrioid
carcinoma, serous carcinoma, clear cell carcinoma, undifferentiated
carcinoma, and so on (17). In
Japan, endometrioid carcinoma accounts for 90% of all endometrial
cancer (16). Endometrioid
carcinoma is defined as Grade 1, 2 or 3 according to the degree of
differentiation (16). Poorly
differentiated Grade 3 is known clinically to be highly malignant.
Although the state of motile cilia (18,19)
in endometrial cancer has been observed previously, the state of
primary cilia has not been reported as yet.
Autophagy is a self-cleaning pathway by which cells
break down intracellular proteins and organelles. In previous
years, autophagy has attracted attention as an important regulator
of ciliogenesis and cilia length. Tang et al showed that
autophagy promotes ciliogenesis through selective degradation of
Oral-facial-digital syndrome 1 protein (OFD1) (20). Lee et al reported that
genetic and pharmacological inhibition of autophagy in Hurthle cell
carcinoma results in the formation and the elongation of primary
cilia (21). In addition, Inami
et al reported that p62/Sequestosome 1 (p62), which
accumulates in hepatocellular carcinoma due to inhibition of
autophagy, is involved in tumor growth by activating the Nrf2
signaling system (22). Therefore,
the state of primary cilia and autophagy in cancers may act as
determinants of the tumor grade and prognosis (23).
In this study, we examined primary cilia in tissues
of normal endometrium, endometrioid carcinoma, and a typical
histological type of endometrial cancer. We also analyzed the
expression of OFD1, a ciliary protein, and p62, an autophagic
protein, in tissues of endometrioid carcinoma Grade 1 and Grade 3
to elucidate the relationship between the state of primary cilia
and autophagy in endometrioid carcinoma.
Materials and methods
Patients, study design and ethical
approval
All patients underwent a total hysterectomy at
Nagoya City University Hospital from January 2014 to December 2018.
Normal endometrium of 4 patients in the proliferative phase, 5
patients in the secretory phase and 4 patients in the menopausal
phase was used to examine differences in primary cilia at each
phase of the menstrual cycle. Normal endometrium was obtained from
patients who underwent a hysterectomy for cervical disease or
ovarian cancer (Table SI).
Obstetricians/gynecologists previously determined the menstrual
cycle stage by histologically examining the specimens (24). We have postmenopausal specimens
based on the medical records. For endometrial cancer, 17 patients
with Grade 1 and 8 patients with Grade 3 endometrioid carcinoma
were compared (Table SII).
Pathologists in Nagoya City University Hospital were responsible
for grading the endometrioid carcinomas. Medical information was
collected regarding the patient's age, menstrual cycle, and
previous medical history. For endometrial cancer patients, we
obtained information on the stage of surgery, as well as recurrence
or death following surgery to November 2021.
We examined the expression of primary cilia in
normal endometrium and compared their number and length in
proliferative, secretory, and menopausal endometrium as well as
between endometrioid carcinoma Grade 1 and Grade 3 by fluorescence
immunostaining.
Next, we examined the expression of p62 and OFD1 in
endometrioid carcinoma Grade 1 and Grade 3. The fluorescence
intensity of OFD1 was quantified and compared.
This study was approved by the Medical Research
Ethics Review Committee of the Nagoya City University Graduate
School of Medicine. Written consent was obtained from each patient
after being given an explanation of the purpose of the
research.
Antibodies
The following primary antibodies were used:
anti-acetylated-tubulin antibody (mouse monoclonal
IgG2B, Sigma, Cat# T7451, clone 6-11-B1; IF 1:500),
anti-γ-tubulin antibody (mouse monoclonal IgG1, Sigma,
Cat#5326, clone GTU-88; IF 1:500), anti-Ki67 antibody (rabbit
monoclonal IgG, Abcam, Cat#16667, IF 1:200), anti-SQSTM1/p62
antibody (mouse monoclonal IgG1, Fitzgerald,
Cat#10R5910, IF 1:200) and anti-OFD1 antibody (rabbit polyclonal
IgG, NovusCat#32843, 1:200). The secondary antibodies used were:
tetramethylrhodamine isothiocyanate (TRITC)-labeled goat anti-mouse
IgG2B (SouthernBiotech, Ca #1090-03), Alexa Fluor
633-labeled goat anti-mouse IgG1 (Invitrogen, Ca
#A21126) and Alexa Fluor 488-labeled goat anti-rabbit IgG(H+L).
Rabbit IgG (Cell Signaling Technology, Ca#2729S) was used for a
negative control of the primary antibody. Hoechst33342 (Invitrogen,
Ca #H3570) was used for nuclear staining.
Immunostaining
After formalin fixation, paraffin-embedded tissue
blocks were thinly sliced into 8 µm pieces for immunostaining. For
deparaffinization, paraffin-embedded tissue slides were heated in a
dry incubator at 50°C for 10 min and sequentially washed with 100%
xylene (4 times), 100% ethanol (2 times), 90% ethanol, 70% ethanol
and pure water. All washing was performed at room temperature.
Permeabilization was performed by heating in a microwave oven at
500W for 5 min with TrisEDTA (2 times). Tissue slides were blocked
with phosphate buffered saline (PBS) with goat serum (5%) (Thermo
Fisher, Cat#16210064) for 60 min at room temperature. Primary
antibodies were incubated on the tissue slides overnight at 4°C.
The slides were then washed 3 times with PBS. Secondary antibodies
with Hoechst were incubated on the slides for 60 min at room
temperature. Each slide was mounted with a cover glass (Matsunami,
Cat# No1-s, 0.16-0.19 mm thickness) using Aqueous Permanent
Mounting Medium (Diagnostic BioSystems, Cat#K002-xx).
Confocal microscopy
Images were acquired using a confocal laser scanning
microscope (Olympus, FV3000). To reduce variation, we examined one
slide per sample and at three locations on each slide. Images were
taken every 0.63 µm over a thickness of 5 µm for Z stacks. Z images
were projected using FV3000 software.
Image analysis
All image analyses were performed using ImageJ Fiji.
We counted numbers of ciliated cells and Ki67-positive cells, and
measured the length of primary cilia. OFD1 expression was
quantified by fluorescence intensity. Primary cilia were counted
only in cells with both axonemes and basal bodies. The total number
of stromal cells and the number of ciliated cells per field of view
were counted. Each count was performed manually using an ImageJ
Fiji cell counter. The lengths of the cilia were determined using a
tool only for measuring the length of the axoneme, and the basal
body was not included. The total number of cells per field of view
and the number of Ki67-positive cells were counted. For
quantification of the intensity, images obtained under the same
staining and imaging conditions were used. We quantified the
fluorescence intensity of OFD1 by setting the threshold of the
intensity to 100–255 and binarizing it.
Protein extraction from FFPE
blocks
Protein extraction from FFPE blocks was performed
using a Qproteome FFPE Tissue kit (Qiagen, Ca#37623). We cut each
block into 10 µm sections. For deparaffinization, we washed the
sections with 100% xylene for 10 min (2 times), 100% ethanol for 10
min (2 times), 96% ethanol for 10 min (2 times), 70% ethanol for 10
min (2 times) and pure water for 30 sec. We transferred the tumor
area only, as confirmed by HE staining, into a 1.5 ml collection
tube using a needle. Samples and 100 µl of Extraction Buffer EXB
Plus supplemented with beta-mercaptoethanol were incubated on ice
for 5 min and mixed using a vortex mixer. These tubes were heated
at 100°C for 20 min and then at 80°C for 2 h with a Thermomixer at
750 rpm. Concentrations of the extracted proteins were determined
using a Pierce BCA protein assay kit (ThermoFisher, Ca#23227).
Western blotting
Proteins were separated on 12.5% SDS-PAGE and then
transferred to a PVDF membrane. The membrane was blocked with 5%
milk in PBST for 1 h at room temperature and then incubated with
the following primary antibodies: anti-SQSTM1/p62antibody (rabbit
polyclonal IgG, Proteintech, Ca#18420, 1:1,000), anti-OFD1 antibody
(rabbit polyclonal IgG, Proteintech, Ca#22851, 1:1,000) and
anti-β-actin antibody (rabbit monoclonal IgG, Cell Signaling,
Ca#4970, 1:1,000). Goat anti-rabbit IgG(H+L) (BIORAD Ca#1708241,
1:5,000) was used as the secondary antibody. The results were
analyzed with an Amersham Imager 600 (GE Healthcare). We used
β-actin as the index of the loaded protein content in each
sample.
Statistical analysis
BellCurve for Excel (Social Survey Research
Information Co., Ltd.) was used for statistical analysis. All data
were expressed as mean values ± Standard Error (SE). One-way ANOVA
followed by Bonferroni's multiple comparison test was used to
compare three groups, and an unpaired t-test was used to compare
two groups. Dot plots and Kaplan-Meier curves were also plotted
using BellCurve for Excel. The Kaplan-Meier plotter (www.kmplot.com), an online database including gene
expression data and clinical data, was used to examine OFD1
expression.
Results
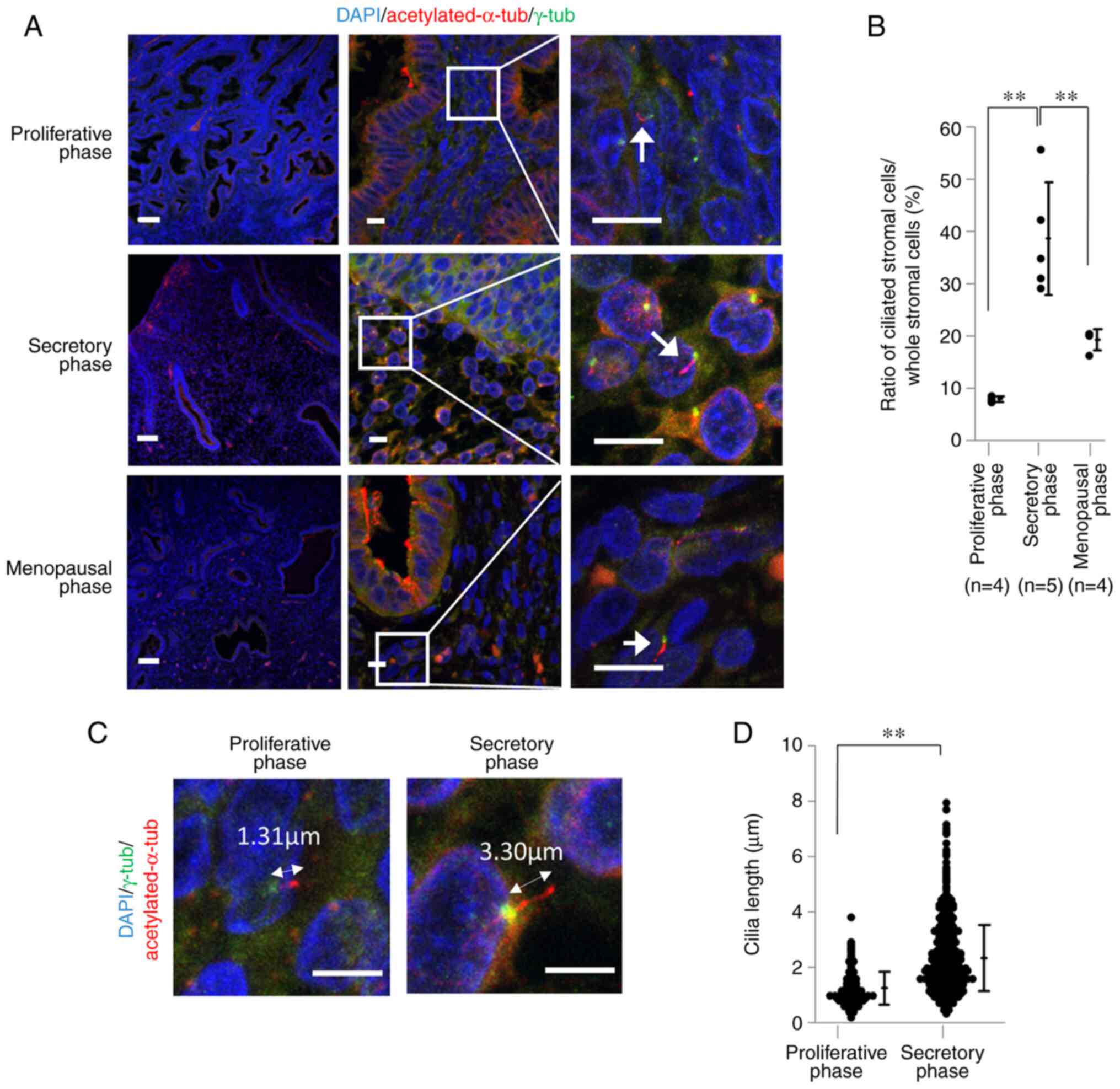
The state of ciliated endometrial
stromal cells during the menstrual cycle
Endometrial stromal cells with primary cilia were
counted at proliferative and secretory phases of the menstrual
cycle as well as at the menopausal phase. Median ages (range) of
patients with proliferative, secretory, and menopausal endometrium
were 49 (45–50), 46 (40–47), and 60.5 (52–69) years (Table SI), respectively. Primary cilia
were visualized by fluorescence staining using acetylated α-tubulin
antibody for the axonemes of primary cilia and γ-tubulin antibody
for the basal bodies (Fig. 1A;
arrows). The numbers of nuclei of proliferating, secretory and
menopausal endometrial stromal cells were 6,387 (n=4; 782–2074),
5,612 (n=5; 545–1936) and 13,651 (n=5; 1127–4595), respectively.
The median rate of endometrial stromal cells with primary cilia was
7.22% (7.47–8.66), 32.70% (29.22–55.78), and 20.56% (16.36–32.13)
(Fig. 1B). Bonferroni test results
showed statistically significant differences between each group
(Table I). The same images were
used to measure the length of primary cilia on endometrial stromal
cells at proliferative and secretory phases (Fig. 1C) and the average length was 1.24
µm (n=191) and 2.34 µm (n=812), respectively. An unpaired t-test
showed a statistically significant difference between these two
groups (95% confidence interval (CI)=0.92–1.27, P<0.001)
(Fig. 1D).
 | Table I.Differences in percentages of primary
cilia of endometrial stromal cells in proliferative, secretory and
menopausal phases. |
Table I.
Differences in percentages of primary
cilia of endometrial stromal cells in proliferative, secretory and
menopausal phases.
|
|
|
|
|
| 95% CI |
|---|
|
|
|
|
|
|
|
|---|
| Group A | Group B | Mean
difference | Standard Error | P-value | Lower limit | Upper limit |
|---|
| PP | SP | 30.70 | 4.64 | <0.01 | 21.61 | 39.79 |
| PP | MP | 11.37 | 4.89 |
0.12 | 1.79 | 20.95 |
| SP | MP | 19.33 | 4.64 | <0.01 | 10.24 | 28.42 |
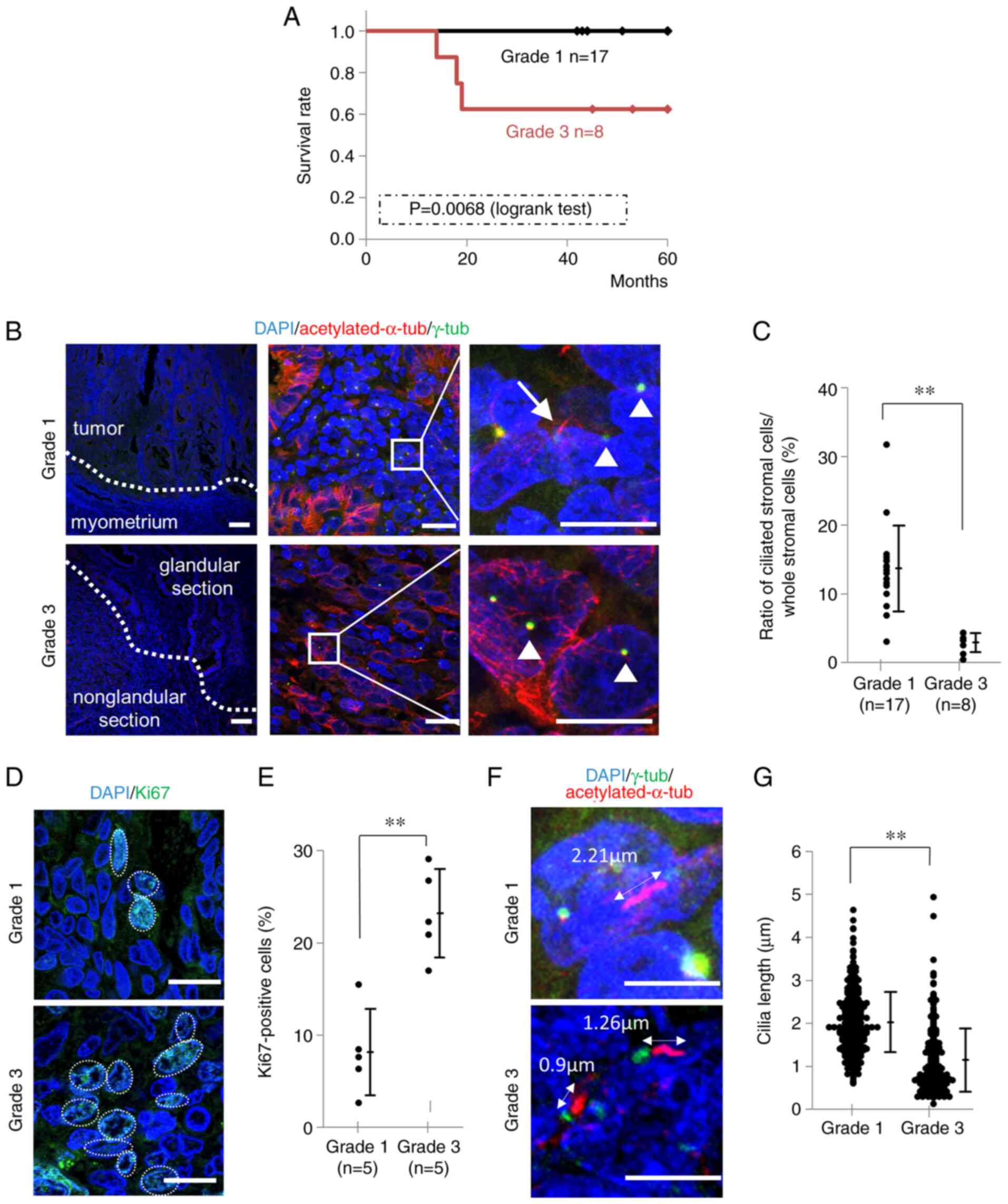
The state of primary cilia on Grade 1
and Grade 3 endometrioid carcinoma cells
The median ages (range) of patients with Grade 1 and
Grade 3 endometrioid carcinoma was 51 (44–71) and 60 (47–76),
respectively (Table II). All
Grade 1 patients had Stage I or II early-stage cancer, while 5 out
of 8 Grade 3 patients had Stage III or IV advanced cancer. During
the observation period, there were no recurrences or deaths in any
of the Grade 1 patients, whereas 3 patients died after recurrence
in the Grade 3 group. Kaplan-Meier curves for the survival rates of
patients of both groups are shown in Fig. 2A. Primary cilia of endometrioid
carcinoma Grade 1 and Grade 3 were visualized with fluorescence
immunostaining as shown in Fig.
2B. In Grade 3, the number of primary cilia was counted in the
less-differentiated, solid portion. Numbers of nuclei of
endometrioid carcinoma cells of Grade 1 and Grade 3 were 9,554
(n=17; 193–1190) and 5,615 (n=8; 340–985), respectively, and the
median rates of ciliated cells were 13.50% (3.11–31.79) and 2.91%
(0.43–4.37). An unpaired t-test showed that the differences were
statistically significant (95% CI=7.89–15.05, P<0.001) (Fig. 2C). Fluorescence immunostaining was
performed using Ki67 antibody as a proliferative marker for human
tumor cells (Fig. 2D).
Ki67-positive rates of Grade 1 and Grade 3 were shown with a dot
plot (Fig. 2E). Nuclei of Grade 1
and Grade 3 numbered 7,245 (n=5; 419–569) and 5,147 (n=5; 340–985).
An unpaired t-test showed that the differences were statistically
significant (95% CI=11.17–19.35, P<0.001). On measuring the
lengths of primary cilia on Grade 1 and Grade 3 cells (Fig. 2F), the mean length of Grade 1 was
2.02 µm (n=372) and that of Grade 3 was 1.14 µm (n=225) (Fig. 2G). An unpaired t-test showed that
this was a statistically significant difference (95% CI=0.76-0.99,
P<0.001) (Fig. 2G).
 | Table II.Characteristics of patients with
endometrioid carcinoma. |
Table II.
Characteristics of patients with
endometrioid carcinoma.
| Endometrioid
carcinoma histology | Grade
1a (n=17) | Grade
3b (n=8) |
|---|
| Mean age, (age
range) | 51 (44–71) | 58.5 (47–76) |
| Surgical Stage,
n |
|
|
| I,
II | 17 | 3 |
| III,
IV | 0 | 5 |
| Recurrence, n | 0 | 3 |
| Death, n | 0 | 3 |
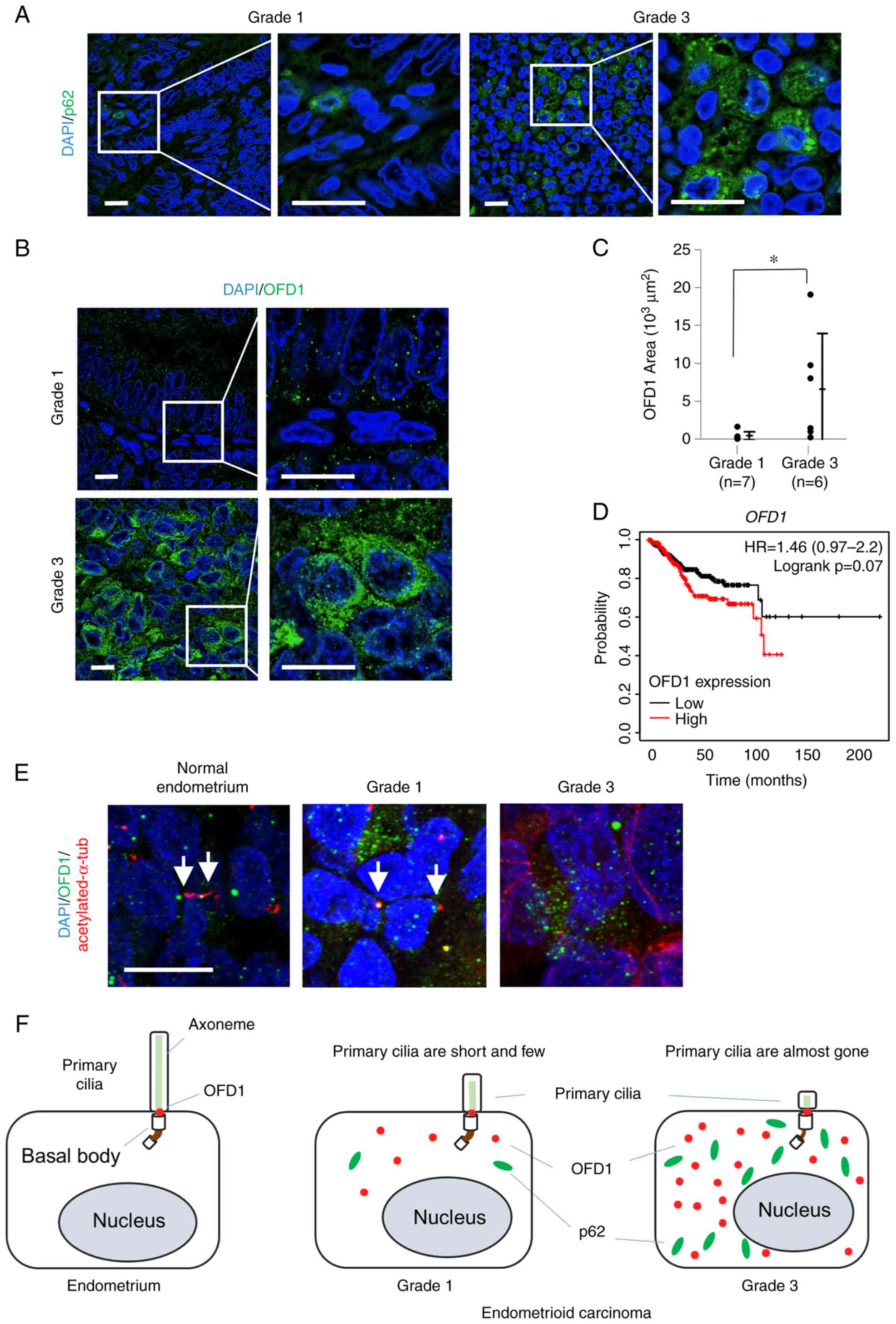
The expression of OFD1 in endometrioid
carcinoma Grade 1 and Grade 3
The inhibition of autophagy induces the accumulation
of p62, which is an autophagy receptor. As previously reported,
fluorescence immunostaining with p62 antibody showed that the
accumulation of p62 in Grade 3 endometrioid carcinoma cells was
more than that of Grade 1 (Fig.
3A). In addition, the expression of p62 was confirmed by
western blotting with proteins extracted from the same FFPE samples
as the ones used for immunostaining (Fig. S1). p62 was highly expressed in
Grade 3 carcinoma compared to Grade 1 or normal tissue. Both
overexpression and a defect in OFD1, a ciliary protein, induced
abnormal primary cilia or a loss of primary cilia (20,25).
The inhibition of autophagy led to the accumulation of OFD1 in the
cytoplasm (20). OFD1 accumulated
in Grade 3 cells more than in Grade 1 (Fig. 3B). The Kaplan-Meier plotter showed
that the patients with high OFD1 expression tended to have a
poor prognosis (Fig. 3D). For each
image, the mean of the values of OFD1 intensity per three areas was
dot plotted (Fig. 3C,
95%CI=133-12248, P=0.046), and the relationship between the
overexpression of OFD1 and primary cilia was examined. Fluorescence
immunostaining for OFD1 with acetylated-α-tubulin antibodies was
performed using tissues of normal endometrium, and Grade 1 and 3
endometrioid carcinoma (Fig. 3E).
In normal endometrial stromal cells, OFD1 was localized at the
basal bodies of primary cilia. In Grade 1, abnormal overexpression
of OFD1 was found in cells with short axonemes, while in Grade 3,
the accumulation of OFD1 was even greater and fewer primary cilia
were observed.
Discussion
In the present study, we found that the state of
primary cilia on endometrial stromal cells changes during the
normal menstrual cycle and the number of cells with longer primary
cilia is highest in the secretory phase. We also observed more
endometrioid carcinoma cells without primary cilia or with shorter
primary cilia in Grade 3 compared to Grade 1. In addition, we found
excessive accumulation of p62 and OFD1, which possibly inhibit
primary ciliogenesis, in endometrioid carcinoma cells that were
highly malignant (Fig. 3F).
Primary cilia are organelles that appear during the
quiescent phase of the cell cycle (26). Disassembly of primary cilia is
thought to trigger re-entry of quiescent cells into the mitotic
cycle and induce proliferation (27). Indeed, the excess expression of
aurora A kinase (AURA), which is known as a ciliolysis-associated
protein, has been reported in ovarian cancer (7). However, Ki67-negative cells were
found in endometrioid cancer cells without primary cilia,
indicating that ciliogenesis in the stromal cells of endometrial
carcinoma may be regulated in a manner independent of the cell
cycle. Therefore, this study focused on the failure of autophagy in
order to elucidate a mechanism for abnormal primary ciliogenesis in
endometrial carcinoma. The relationship between malignant tumors
and autophagy has recently received attention. The excessive
expression of p62, which represents the failure of autophagy, has
been reported in various malignant tumors such in liver (22,28),
breast (29), kidney (30), colon (31), ovary (32), as well as uterus (33). In this study, we also found the
excessive expression of p62 in these types of endometrioid
carcinoma. Since we found an abnormal accumulation of OFD1, whose
proper degradation by autophagy around the centrosome is necessary
for the formation of primary cilia (13), the abnormal expression of OFD1
induced by dysfunction of autophagy in Grade 3 seems to cause a
loss of primary cilia or shorter primary cilia in Ki67-negative
endometrioid carcinoma cells in the quiescent phase of the cell
cycle. The data showing the poor prognosis of endometrial cancer
patients with a high expression of OFD1 also support our model.
Interestingly, OFD1 expression was also increased in human
papilloma virus (HPV)-positive oropharyngeal carcinoma compared
with HPV-negative carcinoma and was associated with tumor
proliferation and invasiveness (25). Our model may explain a mechanism
for abnormal primary ciliogenesis not only in endometrial carcinoma
but also in other malignant tumors.
One advantage of the approaches used in this study
was the availability of patient specimens with clinical information
rather than having to rely on experimental systems such as the use
of cultured cells. A variety of endometrioid carcinoma specimens
was employed in this study. Some specimens of Grade 3 endometrioid
carcinoma were taken from advanced cancers at the time of
diagnosis, and some were recurrent cancers which led to patient
death. In other words, the distribution of cilia and the expression
of p62/OFD1 could be examined in tissues of cancers that are
considered to be highly metastatic and have proliferative
potential. If we had used cell lines for this study, ciliogenesis
and autophagy could not have been examined in the different states
of endometrial carcinoma cells. One of the weaknesses was the
limited use of paraffine-fixed specimens and our inability to
perform additional studies such as functional analysis. Another
limitation was the relatively small sample size of endometrioid
carcinoma Grade 3. Considering the above two limitations, our
observations in this study must be confirmed in future by using a
large number of patient samples.
From the findings of this study, we hypothesized
that autophagy is severely blocked in highly malignant endometrial
cancer, leading to the abnormal accumulation of OFD1 and the
inhibition of ciliogenesis. Therefore, the restoration of autophagy
may induce proper expression of OFD1 around the ciliary basal body
and generate primary cilia on endometrioid cancer cells. We were
not able to discover the triggers of autophagy inhibition nor any
correlation between OFD1 and ciliogenesis in this study. These
issues must be addressed in the future. In addition, we intend to
further examine whether the restoration of primary cilia on
endometrioid carcinoma cells might lead to the suppression of tumor
growth.
In conclusion, the present study found an increase
in the number of primary cilia from the proliferative to the
secretory phase of the normal menstrual cycle. We also found a
decrease in the number of primary cilia as well as an excessive
accumulation of the ciliary protein OFD1 induced by the impairment
of autophagy in highly malignant endometrial cancers. Since the
prognosis of endometrial carcinoma patients with high expression of
OFD1 is poor, the extent of OFD1 expression may be used as an index
to determine prognosis in endometrial carcinoma patients.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
The authors would like to thank the clinical
laboratory technician Mr. T. Sakakibara (Pathology and Molecular
Diagnostics, Nagoya City University Graduate School of Medical
Sciences, Nagoya City, Japan) for slicing the paraffin-embedded
tissues.
Funding
This work was supported by the Japanese Society for the
Promotion of Science, KAKENHI, Grants-in-Aid for Scientific
Research (grant no. 20K18226).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding authors on reasonable
request.
Authors' contributions
RK was involved in sample collection, investigation,
methodology, analysis, writing of the original draft and funding
acquisition. EH, FO, CYN and SG performed the experiments. SO, SM
and RN performed experiments and writing. HI provided analysis and
interpretation of data. YK was involved in the conceptualization,
writing and review of the manuscript and supervision of the
research. MSO was involved in the conceptualization, writing,
review and editing of the manuscript as well as supervision. All
authors approved the final version of the manuscript. RK and YK
confirmed the authenticity of all the raw data.
Ethics approval and consent to
participate
This study was approved by the Medical Research
Ethics Review Committee of the Nagoya City University Graduate
School of Medicine. Written consent was obtained from each patient
after being given an explanation of the purpose of the
research.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Breslow DK and Holland AJ: Mechanism and
regulation of centriole and cilium biogenesis. Annu Rev Biochem.
88:691–724. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goto H, Inoko A and Inagaki M: Cell cycle
progression by the repression of primary cilia formation in
proliferating cells. Cell Mol Life Sci. 70:3893–3905. 2013.
View Article : Google Scholar
|
|
3
|
Goranova V and Chaldakov GN: Ciliated
fibroblasts and smooth cells in the rat uterus. Experientia.
46:488–489. 1990. View Article : Google Scholar
|
|
4
|
Sarkisian MR, Siebzehnrubl D, Hoang-Minh
L, Deleyrolle L, Silver DJ, Siebzehnrubl FA, Guadiana SM,
Srivinasan G, Semple-Rowland S, Harrison JK, et al: Detection of
primary cilia in human glioblastoma. J Neurooncol. 117:15–24. 2014.
View Article : Google Scholar
|
|
5
|
Wong SY, Seol AD, So PL, Ermilov AN,
Bichakjian CK, Epstein EH Jr, Dlugosz AA and Reiter JF: Primary
cilia can both mediate and suppress Hedgehog pathway-dependent
tumorigenesis. Nat Med. 15:1055–1061. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rocha C, Papon L, Cacheux W, Marques Sousa
P, Lascano V, Tort O, Giordano T, Vacher S, Lemmers B, Mariani P,
et al: Tubulin glycylases are required for primary cilia, control
of cell proliferation and tumor development in colon. EMBO J.
33:2247–2260. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Egeberg DL, Lethan M, Manguso R, Schneider
L, Awan A, Jørgensen TS, Byskov AG, Pedersen LB and Christensen ST:
Primary cilia and aberrant cell signaling in epithelial ovarian
cancer. Cilia. 1:152012. View Article : Google Scholar
|
|
8
|
Hassounah NB, Nunez M, Nunez M, Fordyce C,
Roe D, Nagle R, Bunch T and McDermott KM: Inhibition of
ciliogenesis promotes Hedgehog signaling, tumorigenesis, and
metastasis in breast cancer. Mol Cancer Res. 15:1421–1430. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hassounah NB, Nagle R, Saboda K, Roe DJ,
Dalkin BL and McDermott KM: Primary cilia are lost in preinvasive
and invasive prostate cancer. PLoS One. 8:e685212013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin CJ, Dang A, Hernandez E and Hsieh JT:
DAB2IP modulates primary cilia formation associated with renal
tumorigenesis. Neoplasia. 23:169–180. 2021. View Article : Google Scholar
|
|
11
|
Kobayashi T, Nakazono K, Tokuda M, Mashima
Y, Dynlacht BD and Itoh H: HDAC2 promotes loss of primary cilia in
pancreatic ductal adenocarcinoma. EMBO Rep. 18:334–343. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gradilone SA, Radtke BN, Bogert PS, Huang
BQ, Gajdos GB and LaRusso NF: HDAC6 inhibition restores ciliary
expression and decreases tumor growth. Cancer Res. 73:2259–2270.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Barakat MT, Humke EW and Scott MP: Kif3a
is necessary for initiation and maintenance of medulloblastoma.
Carcinogenesis. 34:1382–1392. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Concin N, Matias-Guiu X, Vergote I, Cibula
D, Mizra MR, Marnitz S, Ledermann J, Bosse T, Chargari C, Fagotti
A, et al: ESGO/ESTRO/ESP guidelines for the management of patients
with endometrial carcinoma. Int J Gynecol Cancer. 31:12–39. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamagami W, Mikami M, Nagase S, Tabata T,
Kobayashi Y, Kaneuchi M, Kobayashi H, Yamada H, Hasegawa K,
Fujiwara H, Katabuchi H and Aoki D: Initial treatment for
endometrial cancer. In: Japan Society of Gynecologic Oncology 2018
guidelines for treatment of uterine body neoplasms. J Gynecol
Oncol. 31:e182020. View Article : Google Scholar
|
|
16
|
Nagase S, Ohta T, Takahashi F and Yaegashi
N; Board Members of the 2020 Committee on Gynecologic Oncology of
the Japan Society of Obstetrics and Gynecology, . Annual Report of
the Committee on Gynecologic Oncology, the Japan Society of
Obstetrics and Gynecology: Annual Patient Report for 2017 and
Annual Treatment Report for 2012. J Obstet Gynaecol Res.
47:1631–1642. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
WHO Classification of Tumors Editorial
Board, . WHO classification of tumours. 5th edition. Vol. 4. Female
Genital Tumors. World Health Organization; 2020
|
|
18
|
Gould PR, Li L, Henderson DW, Barter RA
and Papadimitriou JM: Cilia and ciliogenesis in endometrial
adenocarcinomas. An ultrastructural analysis. Ach Patho Lab Med.
110:326–330. 1986.
|
|
19
|
Haibach H, Oxenhandler RW and Luger AM:
Ciliated adenocarcinoma of the endometrium. Acta Obstet Gynecol
Scand. 64:457–462. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tang Z, Lin MG, Stowe TR, Chen S, Zhu M,
Stearns T, Franco B and Zhong Q: Autophagy promotes primary
ciliogenesis by removing OFD1 from centriolar satellites. Nature.
502:254–257. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee J, Yi S, Kang YE, Chang JY, Kim JT,
Sul HJ, Kim JO, Kim JM, Kim J, Porcelli AM, et al: Defective
ciliogenesis in thyroid hürthle cell tumors is associated with
increased autophagy. Oncotarget. 7:79117–79130. 2016. View Article : Google Scholar
|
|
22
|
Inami Y, Waguri S, Sakamoto A, Kouno T,
Nakada K, Hino O, Watanabe S, Ando J, Iwadate M, Yamamoto M, et al:
Persistent activation of Nrf2 through p62 in hepatocellular
carcinoma cells. J Cell Biol. 193:275–284. 2011. View Article : Google Scholar
|
|
23
|
Ko JY, Lee EJ and Park JH: Interplay
between primary cilia and autophagy and its controversial roles in
cancer. Biomol Ther (Seoul). 27:337–341. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nucci MR: Gynecologic pathology. A volume
in foundations in diagnostic pathology series. 2nd edition.
Elsevier; 2019
|
|
25
|
Meng HX, Yang XX, Liu RQ, Bao JJ, Hou YJ,
Sun J, Miao SS and Qu GF: The Relationship between human
papillomavirus, OFD1 and primary ciliogenesis in the progression of
oropharyngeal cancer: A retrospective cohort study. Pharmgenomics
Pers Med. 13:633–644. 2020.PubMed/NCBI
|
|
26
|
Ford MJ, Yeyati PL, Mali GR, Keighren MA,
Waddell SH, Mjoseng HK, Douglas AT, Hall EA, Sakaue-Sawano A,
Miyawaki A, et al: A cell/cilia biosensor for single-cell kinetics
reveals persistence of cilia after G1/S transition is a general
property in cells and mice. Dev Cell. 47:509–523.e5. 2018.
View Article : Google Scholar
|
|
27
|
Gabriel E, Wason A, Ramani A, Gooi LM,
Keller P, Pozniakovsky A, Poser I, Noack F, Telugu NS, Calegari F,
et al: CPAP promotes timely cilium disassembly to maintain neural
progenitor pool. EMBO J. 35:803–819. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu L, Sheng JQ, Wang MR, Gan Y, Wu XL,
Liao JZ, Tian DA, He XX and Li PY: Primary cilia blockage promotes
the malignant behaviors of hepatocellular carcinoma via induction
of autophagy. Biomed Res Int. 2019:52027502019.
|
|
29
|
Cai-McRae X and Karantza V: p62: A hub of
multiple signaling pathways in HER2-induced mammary tumorigenesis.
Mol Cell Oncol. 2:e9750352015. View Article : Google Scholar
|
|
30
|
Mathew R, Karp CM, Beaudoin B, Vuong N,
Chen G, Chen HY, Bray K, Reddy A, Bhanot G, Gelinas C, et al:
Autophagy suppresses tumorigenesis through elimination of p62.
Cell. 137:1062–1075. 2009. View Article : Google Scholar
|
|
31
|
Lei C, Zhao B, Liu L, Zeng X, Yu Z and
Wang X: Expression and clinical significance of p62 protein in
colon cancer. Medicine (Baltimore). 99:e187912020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Iwadate R, Inoue J, Tsuda H, Takano M,
Furuya K, Hirasawa A, Aoki D and Inazawa J: High expression of
SQSTM1/p62 protein is associated with poor prognosis in epithelial
ovarian cancer. Acta Histochem Cytochem. 47:295–301. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Iwadate R, Inoue J, Tsuda H, Takano M,
Furuya K, Hirasawa A, Aoki D and Inazawa J: High expression of p62
protein is associated with poor prognosis and aggressive phenotypes
in endometrial cancer. Am J Pathol. 185:2523–2533. 2015. View Article : Google Scholar
|