Introduction
Endometrial cancer is the most common gynaecological
cancer and is diagnosed in one out of 35 women in developed
countries (1). When diagnosis of
endometrial cancer is made, the first-line treatment is surgery,
and then adjuvant treatment is recommended; however, adjuvant
treatment is determined according to the tumour invasion depth,
histological grade, patient age and lymph-vascular space invasion,
which have an important role in the staging of endometrial cancer
(2,3). While a high quality of life and
improved clinical outcomes have recently been achieved for patients
following use of chemotherapy and radiotherapy, the literature
shows that 8% of patients with endometrial cancer in the high-risk
group may develop distant metastases despite all treatment efforts
(4,5). The early diagnosis of endometrial
cancer will have a great impact on treatment management, the
prognostic process and cost. While a number of molecular profiling
studies indicate its value in the diagnosis and prognosis of
endometrial cancer and its place in the risk classification of
patients, the data available remain insufficient (6–9).
This situation has driven researchers to seek novel biomarkers and
target gene therapies.
Periostin (POSTN), a component of the extracellular
matrix (ECM) produced by fibroblasts, interacts with integrin
receptors and transmits signals to affect the cellular
differentiation, adhesion and migration regulated by cytokines
(10). The important role of POSTN
in physiological processes has been demonstrated in a number of
studies. For example, it has been shown that abnormal expression of
POSTN is associated with the pathophysiology of asthma, myocardial
damage and, most importantly, cancer (11–13).
In cancer, POSTN interacts with integrin αvβ3 in endothelial cells
and activates the focal adhesion kinase pathway, resulting in
tumour angiogenesis through vascular endothelial growth factor
(VEGF) regulating receptor-2 (VEGFR-2) (14).
Hiroi et al (15) first discovered that the expression
of POSTN in the human endometrium varies according to the menstrual
cycle, and is regulated by the oestrogen and progesterone released
from the ovary. The present study aimed to investigate the protein
and gene expression of POSTN and its utility as a biomarker in the
determination of higher histological grade in endometrial
cancer.
Materials and methods
Patients
A total of 15 patients diagnosed with endometrial
cancer and treated at the Department of Pathology Zeynep Kamil
Training and Research Hospital (Istanbul, Turkey) and 15 patients
who received surgery for non-tumour-related reasons between
December 2019 and May 2020 were included in the present study.
Three groups, International Federation of Gynaecology and
Obstetrics grades I, II and III, were collected from the cases
diagnosed with endometrial cancer via archive screening, with 5
patients in each group (16). All
patients underwent a hysterectomy and bilateral
salpingo-oophorectomy, as well as pelvic and/or paraaortic
lymphadenectomy operations according to the pathology results
obtained from frozen tissue samples. Tumour preparations of the
cases from the pathology archive were re-evaluated, and endometrial
cancer, non-tumour-related reason diagnosed blocks were selected
for ELISA and PCR studies.
The control group consisted of patients who
underwent a hysterectomy due to the indication of abnormal uterine
bleeding; no cancer cells were detected in the endometrial sampling
before the hysterectomy operation, and treatment-resistant bleeding
continued.
Patients diagnosed with leiomyosarcoma, ovarian
cancer and cervical cancer were excluded from the study.
Ethics
The present retrospective study was approved by the
Ethics Committee of Zeynep Kamil Training and Research Hospital
(Istanbul, Turkey; approval no. 116). All patients consented to
treatment in accordance with institutional guidelines, and provided
written informed consent at the time of the treatment. The present
study was performed in accordance with the Declaration of Helsinki
and the guidelines of the Ethics Committee of Zeynep Kamil Training
and Research Hospital (Istanbul, Turkey).
Gene expression of POSTN by reverse
transcription-quantitative (RT-q)PCR
From each paraffin block, five tissue sections (each
10-µm thick) were collected in 1.5-ml microfuge tubes. Extraction
of total RNA from paraffin-embedded tissues was performed in
duplicate using an FFPE RNA isolation kit (catalogue no. K156002;
Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. RNA (1 µg) was reverse transcribed to
cDNA with the High Capacity cDNA Reverse Transcription Kit (Applied
Biosystems; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. The following primers were used: POSTN
forward, 5′-TGCTCGAATCATCCATGGGAA-3′ and reverse,
5′-TGTGTAAGCACACGGTCAATG-3′; and GAPDH forward,
5′-AGGGCTGCTTTTAACTCTGGT-3′ and reverse 5′-CCCCACTTGATTTTGGAGGGA-3′
(Merck KGaA). Amplification was performed with an ABI StepOnePlus
detection system using SYBR Green PCR Master Mix (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The reaction
conditions were as follows: 95°C for 10 min, followed by 40 cycles
of 95°C for 15 sec and 60°C for 1 min. The results were analysed
using StepOne Software v2.3 (Applied Biosystems; Thermo Fisher
Scientific, Inc.) and using the 2−ΔΔCq method (17) and normalized to GAPDH mRNA. Data
are expressed as fold-change relative to the control.
Protein levels of POSTN as determined
by ELISA
A total of four formalin-fixed, paraffin-embedded
tissue sections (each 10- to 15-µm thick) were placed in a 1.5 ml
centrifuge tube. Samples were incubated with 250 µl buffer (pH 7.5;
0.05 M Tris, 1 mM EDTA and 0.5% Tween 20). The tube was placed at
100°C for 10 min and immediately placed into a dry ice ethanol
slurry until frozen. Protein extraction of all samples was
performed as previously described (18). Protein concentrations were measured
using the Bradford method (19).
POSTN levels were measured with the sandwich ELISA method following
the kit manufacturer's instructions (cat. no. EH0255; Wuhan Fine
Biotech Co., Ltd.) with an inter-assay coefficients of variability
(cv) of <12% and an intra-assay cv of <10%, respectively. The
mean minimum detectable quantity of human POSTN was 0.094 ng/ml.
POSTN values are presented as ng/µg protein. All ELISA measurements
were performed using a microplate reader (BioTek Instruments,
Inc.).
Histopathological evaluation
Histopathological evaluation was performed on the
samples of each of the 30 subjects. One sample per tumor centimeter
was taken from each tumor and the samples were embedded in paraffin
blocks and cut into 5-µm-thick sections using a Leica RM2125 RTS
microtome device (Leica Microsystems GmbH). The selected paraffin
sections were stained with haematoxylin and eosin (H/E) at room
temperature for morphological evaluation (Figs. 1 and 2). One sample from each endometrial tumor
was selected according to the WHO classification of tumors, 5th
edition (16). A single sample was
placed in each paraffin block. Proliferative endometrium samples
were taken from hysterectomy materials that were surgically
resected for reasons other than endometrial pathologies. All slides
were examined under a light microscope (Olympus BX51; Olympus
Corporation).
Statistical analysis
SPSS version 18.0 (SPSS, Inc.) was used for the
statistical analysis. Values are expressed as the mean ± standard
deviation for age, and as the mean ± standard error for the POSTN
gene expression and protein levels. One-way ANOVA was used to
analyse the differences among multiple groups, and an unpaired
Student's t-test was used to compare data between two groups.
Post-hoc tests analyses were performed using Tukey's test to
compare the groups after one-way ANOVA, as the variances were
homogeneous. Spearman's correlation analysis was used to analyse
the correlations between cancer grade and POSTN protein levels and
gene expression. P<0.05 was considered to indicate a
statistically significant difference.
Results
The mean age was 55.6±9.0 years for the total
patient cohort, with high similarity between the endometrial cancer
and non-pathological control groups (58.8±8.8 vs. 52.4±8.3 years,
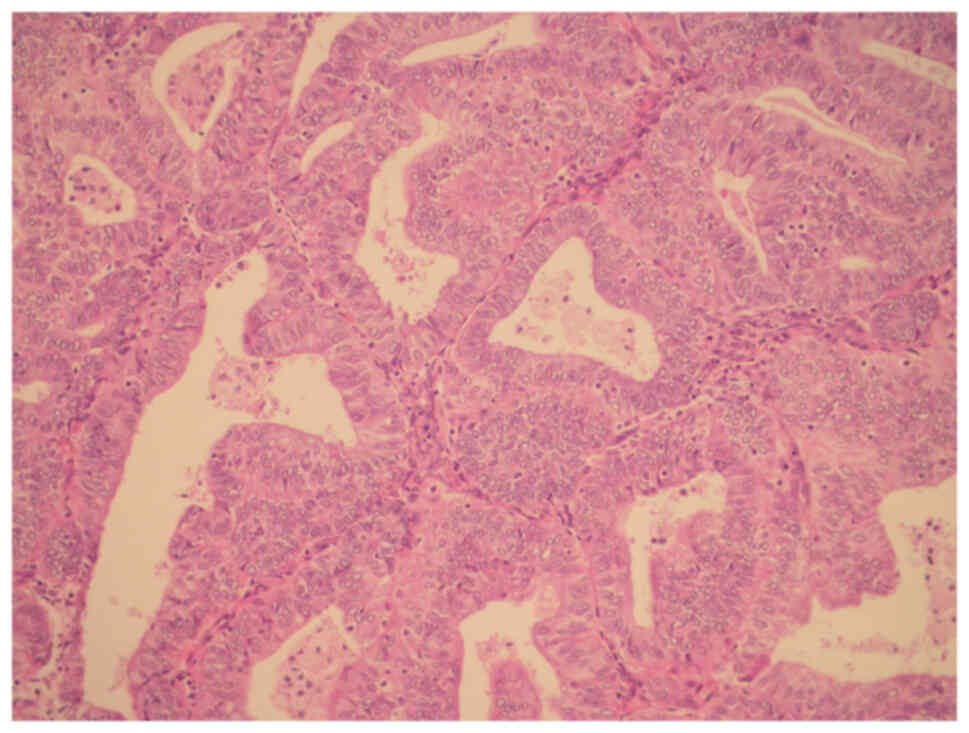
respectively; P=0.05). H/E staining for endometrial cancer in
Fig. 1; confluent glandular,
cribriform pattern and intervening loss of stroma were observed.
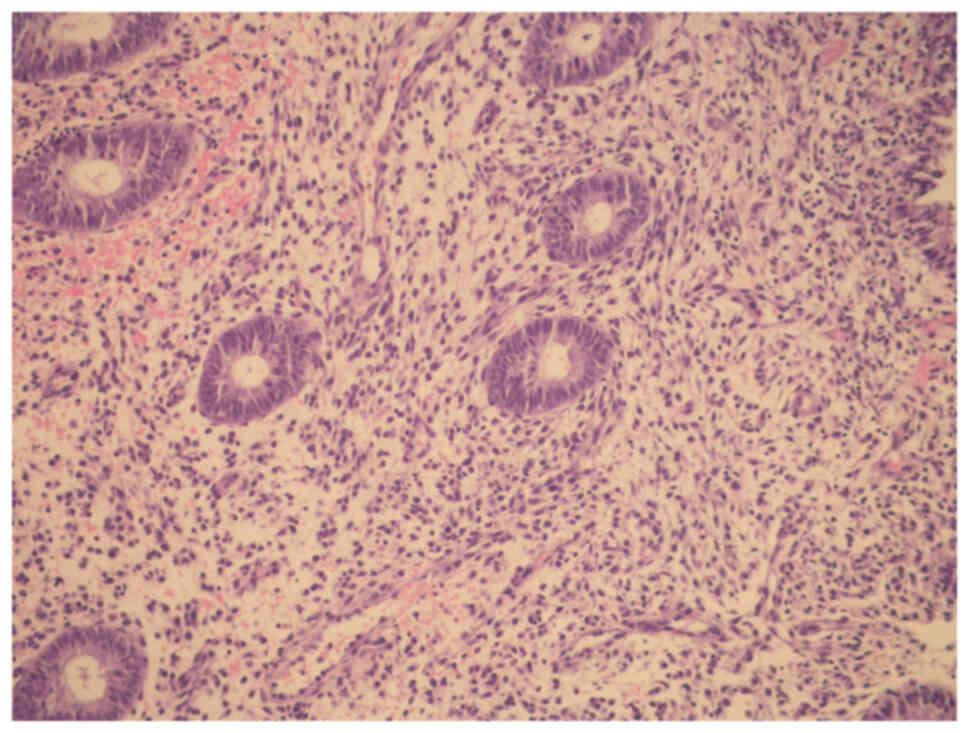
For proliferative endometrium (control) in Fig. 2; endometrial glands were observed
to be uniform and widely spaced, and glandular epithelium consisted
of low columnar cells.
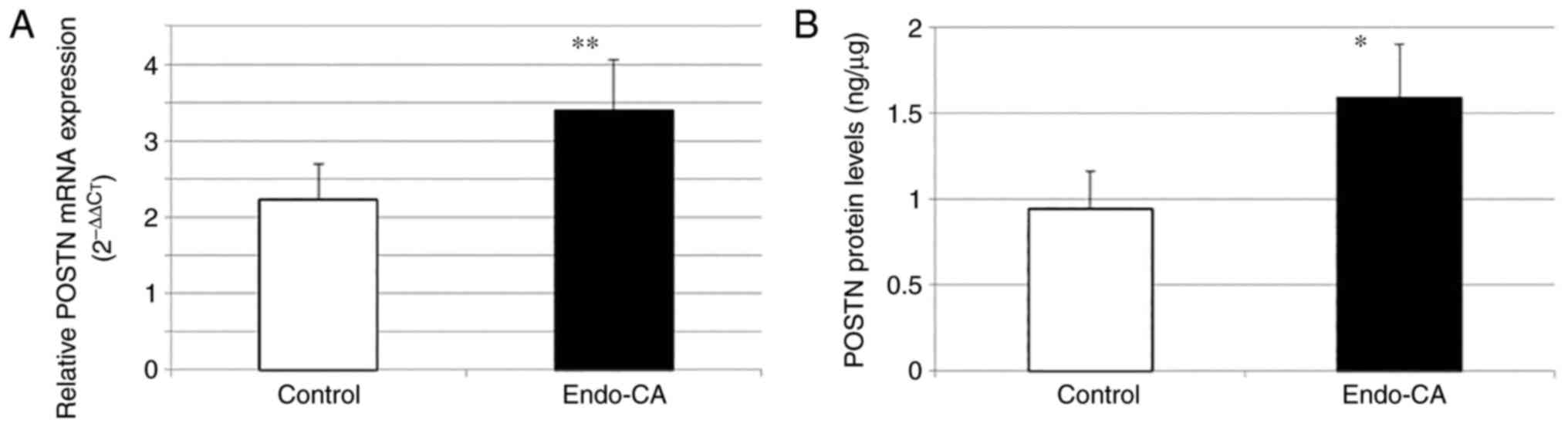
To explore the role of POSTN in endometrial cancer
tissues, the difference in expression was compared in the 15
endometrial cancer tissues and the 15 non-pathologically diagnosed
hysterectomy tissues (control). Significantly increased POSTN gene
expression was observed in cancer tissues compared with that in
tissues without a pathological diagnosis (3.40±0.66 vs. 2.23±0.47,
respectively) (Fig. 3A).
When the mean value for the protein level of POSTN
was examined, it was found to be higher in endometrial cancer
(1.59±0.31) compared with that in the control (0.94±0.22) (Fig. 3B).
Gene expression (P=0.005) and protein levels
(P=0.003) were found to be significantly higher in the endometrial
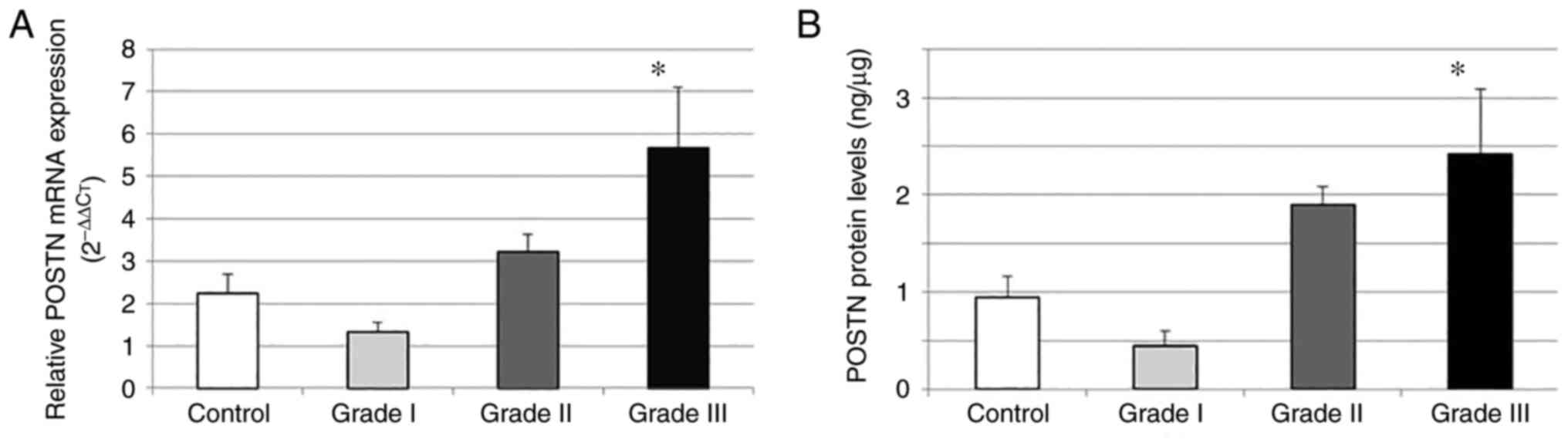
cancer group compared with those in the control group. Moreover,
gene expression (P=0.007; Fig. 4A)
and protein (P=0.015; Fig. 4B)
levels were found to be significantly higher in the grade III
patient group compared with those in the other grade groups and the
control group. Grade I and II and the control groups were similar
in terms of the mean gene expression and protein levels (P>0.05
for each).
In the results obtained in terms of demographic
data, the median age in the endometrial cancer group was 57 years
(range, 46–75 years) and the median age in the control group was 49
years (range, 43–67 years). The median age was 57 years (range,
53–75 years) in those with grade I disease, 52 years (range, 46–65
years) in those with grade II disease and 59 years (range, 50–73
years) in those with grade III disease.
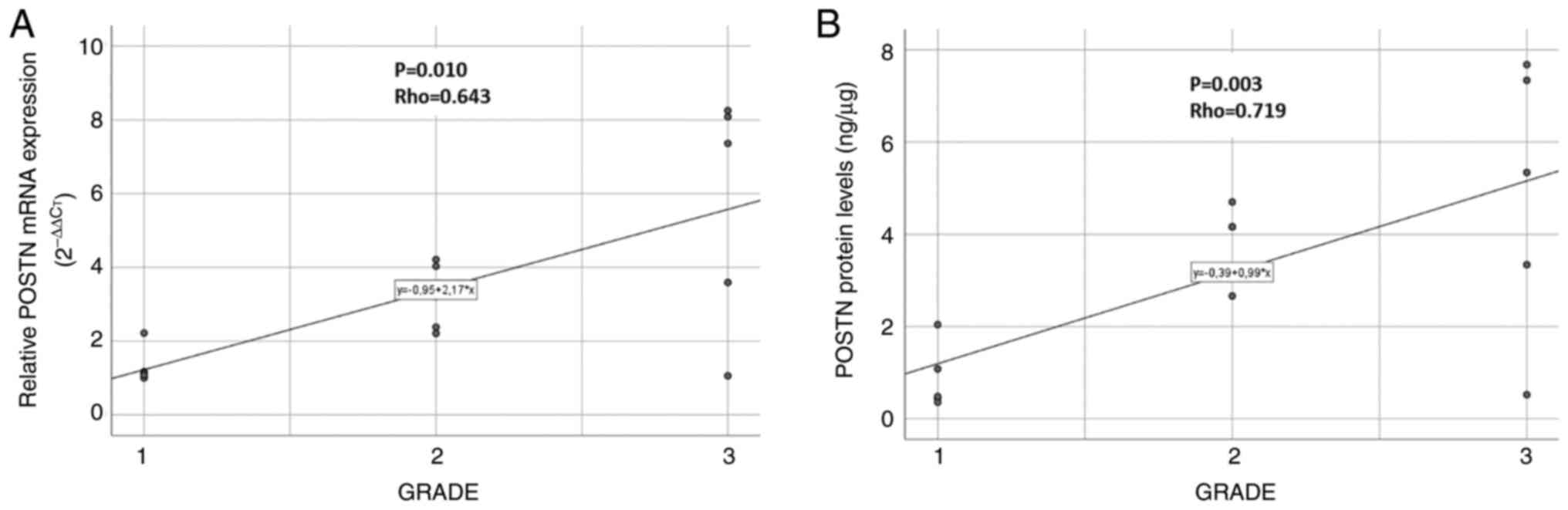
The endometrial cancer grade was found to be
significantly correlated with POSTN protein (P=0.003; Rho=0.719)
and gene expression (P=0.010; Rho=0.643) levels (Fig. 5A and B).
Discussion
Epithelial-mesenchymal transition (EMT) is known as
an important stage in cancer invasion and metastasis, and POSTN is
a demonstrated factor in cell migration and transformation via EMT
during cell differentiation and pathological progression (20). It has been shown that the effect of
POSTN on EMT is mediated by the PI3K/AKT signalling pathway, which
is regulated by cytokines, such as TGF-β1 (21,22).
While POSTN is a marker of EMT, it is also an inducer of EMT
(23). Previous studies have
indicated that POSTN is expressed in large quantities in the tumour
microenvironment and is one of the factors that mediate the
communication between tumour cells and the ECM (21–23).
The present study showed an increase in the protein level and gene
expression of POSTN in endometrial cancer, and to the best of our
knowledge, it is the first study reporting on this subject.
The endometrium consists of stromal and epithelial
cells with eutopic and ectopic localization. In a previous study on
the intrauterine physiological endometrium, POSTN expression was
reported to increase significantly in the mid-proliferative and
early secretory phases, while downregulated expression was observed
in the late-proliferative, mid-secretory and late-secretory phases
(15). The data obtained showed a
strong association between oestrogen and progesterone
supplementation and POSTN expression (15). POSTN expression is controlled by
ovarian steroid hormones, and this may have a strong effect on
physiological pregnancy and pathological processes (15). In other studies, POSTN was found to
be overexpressed in tumour metastases, similar to certain
mesenchymal cell markers, such as fibronectin and cadherin
(24,25). POSTN interacts with integrin
receptors to regulate the adhesion, differentiation and migration
of undifferentiated cells with their characteristic fasciclin 1
domains (26). Cancer-associated
fibroblasts are known as a major ECM component in the tumour
microenvironment, and POSTN is partially produced by fibroblasts in
metastatic lesions (27,28). In a previous study, it was
determined that active fibroblasts secrete POSTN and support the
differentiation, adhesion and migration of cholangiocarcinoma cells
(29). Moreover, increased
expression of POSTN has been shown to affect the tumour
microenvironment by remodelling the ECM and interacting with
integrins or other signalling molecules (10). The present study measured the
protein level and gene expression of POSTN with regard to all three
grades of endometrial cancer, and found that endometrial cancer
grade was significantly correlated with gene expression and protein
level, showing an increase in POSTN level as the grade increased.
The present data support the results of previous studies indicating
increased POSTN expression in malignant tissues such as colon,
esophageal, nasopharyngeal carcinoma and pancreatic cancer
(21–24).
In a large study conducted on patients diagnosed
with ovarian cancer, POSTN was associated with drug resistance in
epithelial ovarian cancer and gene expression profiling revealed a
correlation between POSTN expression and chemotherapy resistance
(30). In a previous study showing
increased resistance to chemotherapy in chemosensitive ovarian
cells under the influence of recombinant POSTN (31), it was reported that the
proliferation and spread of non-small cell lung cancer cells could
be inhibited, while sensitivity to chemotherapy was increased
(32). In conclusion, targeting
POSTN represents a novel therapeutic strategy for minimizing
chemoresistance. There have been attempts to demonstrate the
efficacy of targeting POSTN in the detection and treatment of
tumours in several preclinical models. One of them used
near-infrared fluorescence imaging with upper gastrointestinal
endoscopy to detect preneoplastic lesions via optical imaging of
POSTN (33). Kyutoku et al
(34) reported that treatment with
PN1-Ab, an anti-POSTN antibody in breast cancer, was able to
significantly inhibit the growth of primary tumours and the
formation of lung metastases in an experimental study.
The present study has certain limitations. For
example, patient serum samples were not assessed, as
paraffin-embedded tissues were used. The aim of the study was to
demonstrate the POSTN changes at the tissue level before proceeding
with measuring changes in the serum in larger groups in future
studies and the fact that the sample size was small was another
limitation of the study.
The data obtained in the present study suggest that
POSTN may be used as a biomarker in the early detection of
endometrial cancer and determination of higher histological grade.
Further comprehensive studies are required to fully elucidate the
clinical value and prognostic impact of POSTN.
Acknowledgements
The authors would like to thank Professor Canan
Kabaca (Zeynep Kamil Training and Research Hospital, Istanbul,
Turkey) for sharing her experience and knowledge and for her
contribution to this study by surgical techniques and literature
review.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
DH: Project development and manuscript writing, SG:
Data management, data analysis and manuscript writing, EK: Data
collection and management and data analysis, OC: Data analysis,
manuscript writing and editing. DH and OC confirm the authenticity
of all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the by the Ethics
Committee of Zeynep Kamil Training and Research Hospital (Istanbul,
Turkey; approval no. 116). All patients consented to treatment in
accordance with institutional guidelines, and provided written
informed consent at the time of the treatment.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Creutzberg CL, van Putten WL, Koper PC,
Lybeert ML, Jobsen JJ, Wárlám-Rodenhuis CC, De Winter KA, Lutgens
LC, van den Bergh AC, van de Steen-Banasik E, et al: Surgery and
postoperative radiotherapy versus surgery alone for patients with
stage-1 endometrial carcinoma: Multicentre randomised trial. PORTEC
study group. Post operative radiation therapy in endometrial
carcinoma. Lancet. 355:1404–1411. 2000. View Article : Google Scholar
|
|
3
|
Nout RA, Smit VT, Putter H,
Jürgenliemk-Schulz IM, Jobsen JJ, Lutgens LC, van der Steen-Banasik
EM, Mens JW, Slot A, Kroese MC, et al: Vaginal brachytherapy versus
pelvic external beam radiotherapy for patients with endometrial
cancer of high-intermediate risk (PORTEC-2): An open-label,
non-inferiority, randomised trial. Lancet. 375:816–823. 2010.
View Article : Google Scholar
|
|
4
|
Ørtoft G, Hansen ES and Bertelsen K:
Omitting adjuvant radiotherapy in endometrial cancer increases the
rate of locoregional recurrences but has no effect on long-term
survival: The danish endometrial cancer study. Int J Gynecol
Cancer. 23:1429–1437. 2013. View Article : Google Scholar
|
|
5
|
Thomas GM: A role for adjuvant radiation
in clinically early carcinoma of the endometrium? Int J Gynecol
Cancer. 20 (11 Suppl 2):S64–S66. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cancer Genome Atlas Research Network, .
Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H,
Robertson AG, Pashtan I, Shen R, et al: Integrated genomic
characterization of endometrial carcinoma. Nature. 497:67–73. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Talhouk A, McConechy MK, Leung S, Li-Chang
HH, Kwon JS, Melnyk N, Yang W, Senz J, Boyd N, Karnezis AN, et al:
A clinically applicable molecular-based classification for
endometrial cancers. Br J Cancer. 113:299–310. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Talhouk A and McAlpine JN: New
classification of endometrial cancers: The development and
potential applications of genomic-based classification in research
and clinical care. Gynecol Oncol Res Pract. 3:142016. View Article : Google Scholar
|
|
9
|
Talhouk A, McConechy MK, Leung S, Yang W,
Lum A, Senz J, Boyd N, Pike J, Anglesio M, Kwon JS, et al:
Confirmation of ProMisE: A simple, genomics-based clinical
classifier for endometrial cancer. Cancer. 123:802–813. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu AY, Zheng H and Ouyang G: Periostin, a
multifunctional matricellular protein in inflammatory and tumor
microenvironments. Matrix Biol. 37:150–156. 2014. View Article : Google Scholar
|
|
11
|
Woodruff PG, Boushey HA, Dolganov GM,
Barker CS, Yang YH, Donnelly S, Ellwanger A, Sidhu SS, Dao-Pick TP,
Pantoja C, et al: Genome-wide profiling identifies epithelial cell
genes associated with asthma and with treatment response to
corticosteroids. Proc Natl Acad Sci USA. 104:15858–15863. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dorn GW II: Periostin and myocardial
repair, regeneration, and recovery. N Engl J Med. 357:1552–1554.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sasaki H, Dai M, Auclair D, Kaji M, Fukai
I, Kiriyama M, Yamakawa Y, Fujii Y and Chen LB: Serum level of the
periostin, a homologue of an insect cell adhesion molecule, in
thymoma patients. Cancer Lett. 172:37–42. 2001. View Article : Google Scholar
|
|
14
|
Shao R, Bao S, Bai X, Blanchette C,
Anderson RM, Dang T, Gishizky ML, Marks JR and Wang XF: Acquired
expression of periostin by human breast cancers promotes tumor
angiogenesis through up-regulation of vascular endothelial growth
factor receptor 2 expression. Mol Cell Biol. 24:3992–4003. 2004.
View Article : Google Scholar
|
|
15
|
Hiroi H, Momoeda M, Nakazawa F, Koizumi M,
Tsutsumi R, Hosokawa Y, Osuga Y, Yano T, Tsutsumi O and Taketani Y:
Expression and regulation of periostin/OSF-2 gene in rat uterus and
human endometrium. Endocr J. 55:183–189. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Creasman WT, Odicino F, Maisonneuve P,
Quinn MA, Beller U, Benedet JL, Heintz AP, Ngan HY and Pecorelli S:
Carcinoma of the corpus uteri. FIGO 26th annual report on the
results of treatment in gynecological cancer. Int J Gynaecol
Obstet. 95 (Suppl 1):S105–S143. 2006. View Article : Google Scholar
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nicholson EM, Greenlee JJ and Hamir AN:
PrPSc detection in formalin-fixed paraffin-embedded tissue by
ELISA. BMC Res Notes. 4:4322011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lindsley A, Snider P, Zhou H, Rogers R,
Wang J, Olaopa M, Kruzynska-Frejtag A, Koushik SV, Lilly B, Burch
JB, et al: Identification and characterization of a novel Schwann
and outflow tract endocardial cushion lineage-restricted periostin
enhancer. Dev Biol. 307:340–355. 2007. View Article : Google Scholar
|
|
21
|
Bao S, Ouyang G, Bai X, Huang Z, Ma C, Liu
M, Anderson RM, Rich JN and Wang XF: Periostin potently promotes
metastatic growth of colon cancer by augmenting cell survival via
the Akt/PKB pathway. Cancer Cell. 5:329–339. 2004. View Article : Google Scholar
|
|
22
|
Baril P, Gangeswaran R, Mahon PC, Caulee
K, Kocher HM, Harada T, Zhu M, Kalthoff H, Crnogorac-Jurcevic T and
Lemoine NR: Periostin promotes invasiveness and resistance of
pancreatic cancer cells to hypoxia-induced cell death: Role of the
beta4 integrin and the PI3k pathway. Oncogene. 26:2082–2094. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Michaylira CZ, Wong GS, Miller CG,
Gutierrez CM, Nakagawa H, Hammond R, Klein-Szanto AJ, Lee JS, Kim
SB, Herlyn M, et al: Periostin, a cell adhesion molecule,
facilitates invasion in the tumor microenvironment and annotates a
novel tumor-invasive signature in esophageal cancer. Cancer Res.
70:5281–5292. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Luo W and Yao K: Molecular
characterization and clinical implications of spindle cells in
nasopharyngeal carcinoma: A novel molecule-morphology model of
tumor progression proposed. PLoS One. 8:e831352013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Soltermann A, Tischler V, Arbogast S,
Braun J, Probst-Hensch N, Weder W, Moch H and Kristiansen G:
Prognostic significance of epithelial-mesenchymal and
mesenchymal-epithelial transition protein expression in non-small
cell lung cancer. Clin Cancer Res. 14:7430–7437. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Seifert GJ: Fascinating fasciclins: A
surprisingly widespread family of proteins that mediate
ınteractions between the cell exterior and the cell surface. Int J
Mol Sci. 19:16282018. View Article : Google Scholar
|
|
27
|
Malanchi I, Santamaria-Martínez A, Susanto
E, Peng H, Lehr HA, Delaloye JF and Huelsken J: Interactions
between cancer stem cells and their niche govern metastatic
colonization. Nature. 481:85–89. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Qin X, Yan M, Zhang J, Wang X, Shen Z, Lv
Z, Li Z, Wei W and Chen W: TGFβ3-mediated induction of periostin
facilitates head and neck cancer growth and is associated with
metastasis. Sci Rep. 6:205872016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Utispan K, Thuwajit P, Abiko Y, Charngkaew
K, Paupairoj A, Chau-in S and Thuwajit C: Gene expression profiling
of cholangiocarcinoma-derived fibroblast reveals alterations
related to tumor progression and indicates periostin as a poor
prognostic marker. Mol Cancer. 9:132010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ryner L, Guan Y, Firestein R, Xiao Y, Choi
Y, Rabe C, Lu S, Fuentes E, Huw LY, Lackner MR, et al: Upregulation
of periostin and reactive stroma ıs associated with primary
chemoresistance and predicts clinical outcomes in epithelial
ovarian cancer. Clin Cancer Res. 21:2941–2951. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sung PL, Jan YH, Lin SC, Huang CC, Lin H,
Wen KC, Chao KC, Lai CR, Wang PH, Chuang CM, et al: Periostin in
tumor microenvironment is associated with poor prognosis and
platinum resistance in epithelial ovarian carcinoma. Oncotarget.
7:4036–4047. 2016. View Article : Google Scholar
|
|
32
|
Hu W, Jin P and Liu W: Periostin
contributes to cisplatin resistance in human non-small cell lung
cancer A549 cells via activation of Stat3 and Akt and upregulation
of survivin. Cell Physiol Biochem. 38:1199–1208. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wong GS, Habibollahi P, Heidari P, Lee JS,
Klein-Szanto AJ, Waldron TJ, Gimotty P, Nakagawa H, Taylor PR, Wang
TC, et al: Optical imaging of periostin enables early endoscopic
detection and characterization of esophageal cancer in mice.
Gastroenterology. 144:294–297. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kyutoku M, Taniyama Y, Katsuragi N,
Shimizu H, Kunugiza Y, Iekushi K, Koibuchi N, Sanada F, Oshita Y
and Morishita R: Role of periostin in cancer progression and
metastasis: Inhibition of breast cancer progression and metastasis
by anti-periostin antibody in a murine. Int J Mol Med. 28:181–186.
2011.PubMed/NCBI
|