Introduction
Lung cancer was ranked as the second most prevalent
type of cancer in 2020 and as the cancer with the highest mortality
worldwide according to Global Cancer Statistics for that year; of
these lung cancer cases, ~85% correspond to non-small cell lung
cancer (NSCLC) (1,2). Surgical resection with or without
adjuvant therapy is currently the optimal choice for patients with
NSCLC and achieves relatively favorable outcomes; however, the
majority of patients with NSCLC are diagnosed at the advanced
stage, and therefore are not suitable for surgery (3,4). In
order to give patients the opportunity of undergoing surgery,
neoadjuvant chemotherapy has been proposed, particularly in
patients with locally advanced NSCLC (5–8).
Notably, in patients with locally advanced NSCLC exhibiting
sensitive gene mutations [such as epidermal growth factor receptor
(EGFR), anaplastic lymphoma kinase (ALK) and proto-oncogene
tyrosine-protein kinase ROS (ROS1) alterations], the addition of a
tyrosine kinase inhibitor to chemotherapy as the neoadjuvant
regimen could further improve the patient prognosis; however, in
patients without sensitive gene alterations, these novel drugs are
not applicable (9–11).
Camrelizumab, as a programmed cell death protein 1
(PD-1) inhibitor, affects antitumor activity by inhibiting the
binding of PD-1 to programmed cell death 1 ligand 1 (PD-L1) to
prevent the immune escape of tumor cells (12). Although camrelizumab has been only
been commercially available since 2019, it has been applied in
numerous cancer types, including esophageal, hepatocellular and
renal cell carcinoma, as well as NSCLC, and exhibits good efficacy
and an acceptable tolerance (12–16).
Regarding NSCLC treatment, camrelizumab plus chemotherapy or
anti-angiogenic drugs has improved the outcomes of patients with
unresectable advanced NSCLC (16–18).
However, to the best of our knowledge, the application of
camrelizumab as neoadjuvant treatment bridging to tumor resection
in patients with NSCLC has not been reported to date.
Therefore, the present cohort study aimed to
investigate the efficacy and safety of camrelizumab plus
chemotherapy versus chemotherapy alone as a neoadjuvant regimen in
patients with locally advanced NSCLC.
Materials and methods
Patients
Between July 2019 and February 2021, the present
prospective, cohort, observational study consecutively enrolled 31
patients with locally advanced NSCLC who received neoadjuvant
therapy consisting of camrelizumab plus a paclitaxel and
carboplatin (PC) regimen in Daqing Oil Field General Hospital
(Daqing, China). The inclusion criteria were: i) Pathologically
confirmed NSCLC; ii) age >18 years; iii) TNM stage IIIA-IIIB
(T1-T4N2M0, T3-T4N1M0 and T4N0M0) (19) and suitable for surgical resection;
iv) Eastern Cooperative Oncology Group performance status (ECOG PS)
score from 0 to 1 (20); v) no
EGFR, ALK or ROS1 sensitizing alterations; and vi) choice of
receiving neoadjuvant therapy of camrelizumab plus PC. The
exclusion criteria were as follows: i) Patients with
contraindications to the treatment or allergy to the drugs used in
the study; ii) patients with NSCLC accompanied by other
malignancies; iii) patients who had difficulty in attending regular
follow-ups; and iv) pregnant or lactating women. In addition to the
aforementioned 31 patients, the present study concurrently analyzed
25 patients with locally advanced NSCLC who only received a PC
regimen as a neoadjuvant therapy during the same period. These 25
patients also met the aforementioned criteria with the exception
that they chose to receive neoadjuvant therapy of PC, and therefore
served as controls in the present study.
The current study did not intervene in the treatment
of the patients. The patients received the corresponding treatment
regimen according to their willingness and disease conditions, but
such treatment regimens were not assigned by the researchers.
Patients who received camrelizumab combined with the PC regimen
were considered as the camrelizumab plus PC group (n=31), while
patients who only received the PC regimen were considered as the PC
group (n=25). The present study was approved by the Ethics
Committee of Daqing Oil Field General Hospital (Daqing, China;
approval no. KS1952). Written informed consent for participation
and data use was provided by all the patients included in the
study.
Treatment procedures
Patients in the camrelizumab plus PC group received
camrelizumab combined with the PC regimen for 3 cycles prior to
surgery. Camrelizumab was administered every 2 weeks by intravenous
drip at a dose of 200 mg for 30 min, while paclitaxel was provided
intravenously at a dose of 200 mg/m2 on day 1 and
carboplatin was administered with an area under the curve of 6 (6
mg/ml per min) on day 1. Both paclitaxel and carboplatin were
administered every 21 days (which was the duration of a treatment
cycle). Approximately 4 weeks after the completion of neoadjuvant
treatment, patients underwent surgical resection. Following
recovery from the surgery, patients received ≥2 cycles of adjuvant
therapy with camrelizumab monotherapy or camrelizumab plus the PC
regimen.
Patients in the PC group received neoadjuvant
treatment with 3 cycles of the PC regimen prior to surgery and
underwent surgery ~4 weeks after completion of the neoadjuvant
treatment. Upon recovery from surgery, the patients also received
≥2 cycles of PC regimen as adjuvant therapy. The administration of
the PC regimen was performed in the same manner in both groups.
Outcome evaluation
An enhanced chest computed tomography (CT) scan was
performed to evaluate the clinical response of the tumor ~4 weeks
after the last dose of neoadjuvant treatment, according to the
Response Evaluation Criteria In Solid Tumors (RECIST) (21). The clinical response outcomes were
classified as complete response (CR), partial response (PR), stable
disease (SD) and progressive disease (PD). The objective response
rate (ORR) was also calculated as the sum of the CR and PR rates.
At the time of surgery, the resected primary tumors of the patients
were examined by pathologists to assess their pathological response
according to a previously reported methodology (22). Major pathological response (MPR)
was defined as ≤10% residual viable tumor in the surgically removed
tumor and lymph node tissues (23), while complete pathological response
(CPR) was defined as lack of any viable tumor cells in the
surgically removed tumor and lymph node tissues (23). Adverse events (AEs) during the
administration of camrelizumab were documented and graded based on
the Common Terminology Criteria for Adverse Events (version 4.0)
(24). Since the AEs of patients
in the PC group were not recorded in detail, they were not included
in the analysis. Patients were followed up by outpatient visits or
telephone conversations. The last follow-up was completed on August
31, 2021. Disease-free survival (DFS) and overall survival (OS)
times were calculated from the date of surgery until disease
recurrence or mortality, respectively (25). Tumor PD-L1 expression was detected
by immunohistochemistry with a human anti-PD-L1 antibody (cat. no.
MAB1561; R&D Systems Europe, Ltd.; UK) and was calculated as
the percentage of tumor cells with positive membranous staining,
according to a previous study (26). In brief, tumor tissues were fixed
using formalin (10%) at room temperature for 24 h and embedded in
paraffin, and then cut into 4-µm slices. The slices were
deparaffinized using xylene, rehydrated using gradient ethanol, and
then antigen retrieval was performed. The slice was blocked with 5%
goat serum (Beyotime Institute of Biotechnology) at room
temperature for 1 h. Subsequently, the slices were incubated with
PD-L1 antibody (1:200 dilution; cat. no. MAB1561; R&D Systems
Europe, Ltd.) at 4°C overnight, and then incubated with anti-rabbit
IgG H&L (HRP) antibody (1:1,000 dilution; cat. no. ab6721;
Abcam). Finally, the staining image was obtained via a light
microscope (Nikon Corporation) and evaluated using ImageJ software
Version 1.8.0 (National Institutes of Health). The detection of
PD-L1 was not specifically performed for this study but was a
routine test at Daqing Oil Field General Hospital.
Statistical analysis
Comparison of clinical characteristics between two
groups was conducted with an unpaired Student's t-test, Wilcoxon
rank sum test or χ2 test. Comparison of the clinical and
pathological responses between two groups was performed with
Wilcoxon's rank sum test, the χ2 test or Fisher's exact
test as appropriate. Notably, in the PC group, there was a patient
with PD who exhibited contralateral lymph node metastasis (as
revealed by CT) after neoadjuvant treatment; based on a
comprehensive evaluation conducted by the physician, it was decided
that the patient was no longer suitable for surgery. Therefore,
this patient only had clinical response data documented and was not
included in the analysis of pathological response or survival. DFS
and OS were calculated using the Kaplan-Meier method, and the
comparison of DFS and OS between two groups was conducted using the
log-rank test. The factors associated with MPR were analyzed with a
multivariable logistic regression model, while the factors
associated with DFS or OS were analyzed with the multivariable
Cox's proportional hazards regression model. SPSS 22.0 (IBM Corp.)
and GraphPad Prism 7.02 (GraphPad Software, Inc.) were applied for
analysis and graphical representation of the data, respectively.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Patient characteristics
The mean age (± SD) of the 31 patients in the
camrelizumab plus PC group was 59.4±7.2 years, and 80.6% of them
were male, while 19.4% were female. The mean age (± SD) of the 25
patients in the PC group was 62.3±7.3 years, and 72.0% were male,
while 28.0% were female. No difference was observed in terms of
age, sex, smoking status, histological type, ECOG PS or TNM stage
between the camrelizumab plus PC group and the PC group (all
P>0.05; Table I). In addition,
20 (64.5%) patients had tumor PD-L1 expression >50%, while 11
(35.5%) patients had tumor PD-L1 expression ≤50% in the
camrelizumab plus PC group.
 | Table I.Clinical characteristics. |
Table I.
Clinical characteristics.
| Characteristic | PC group (n=25) | Camrelizumab plus PC
group (n=31) | P-value |
|---|
| Mean age ± SD,
years | 62.3±7.3 | 59.4±7.2 | 0.134 |
| Sex, n (%) |
|
| 0.446 |
|
Female | 7 (28.0) | 6 (19.4) |
|
| Male | 18 (72.0) | 25 (80.6) |
|
| Smoke status, n
(%) |
|
| 0.360 |
|
Never | 5 (20.0) | 5 (16.1) |
|
|
Former | 9 (36.0) | 17 (54.9) |
|
|
Current | 11 (44.0) | 9 (29.0) |
|
| Histological type, n
(%) |
|
| 0.359 |
| ADC | 10 (40.0) | 14 (45.2) |
|
| SCC | 15 (60.0) | 15 (48.3) |
|
|
Others | 0 (0.0) | 2 (6.5) |
|
| ECOG PS score, n
(%) |
|
| 0.514 |
| 0 | 19 (76.0) | 26 (83.9) |
|
| 1 | 6 (24.0) | 5 (16.1) |
|
| Tumor PD-L1
expression, n (%) |
|
| - |
|
≤50% | - | 11 (35.5) |
|
|
>50% | - | 20 (64.5) |
|
| cT stage, n
(%) |
|
| 0.978 |
|
cT1 | 0 (0.0) | 1 (3.2) |
|
|
cT2 | 9 (36.0) | 10 (32.3) |
|
|
cT3 | 14 (56.0) | 17 (54.8) |
|
|
cT4 | 2 (8.0) | 3 (9.7) |
|
| cN stage, n
(%) |
|
| 0.359 |
|
cN0 | 1 (4.0) | 1 (3.2) |
|
|
cN1 | 11 (44.0) | 10 (32.3) |
|
|
cN2 | 13 (52.0) | 20 (64.5) |
|
| cTNM stage, n
(%) |
|
| 0.593 |
|
cT1N2M0 | 0 (0.0) | 1 (3.2) |
|
|
cT2N2M0 | 9 (36.0) | 10 (32.3) |
|
|
cT3N1M0 | 10 (40.0) | 8 (25.8) |
|
|
cT3N2M0 | 4 (16.0) | 9 (29.0) |
|
|
cT4N0M0 | 1 (4.0) | 1 (3.2) |
|
|
cT4N1M0 | 1 (4.0) | 2 (6.5) |
|
Clinical response
Following neoadjuvant therapy, the clinical response
was assessed according to RECIST, which revealed that 0.0, 64.5,
35.5 and 0.0% of patients exhibited CR, PR, SD and PD,
respectively, in the camrelizumab plus PC group, while 0.0, 40.0,
52.0 and 8.0% of patients had CR, PR, SD and PD, respectively, in
the PC group. The camrelizumab plus PC group achieved a better
clinical response than the PC group (P=0.046). Furthermore, the
camrelizumab plus PC group exhibited a higher ORR than the PC
group, although this was not statistically significant (64.5 vs.
40.0%; P=0.067) (Table II).
 | Table II.Clinical response in the PC (n=25)
and camrelizumab plus PC (n=31) groups. |
Table II.
Clinical response in the PC (n=25)
and camrelizumab plus PC (n=31) groups.
| Response | PC group, n
(%) | Camrelizumab plus
PC group, n (%) | P-value |
|---|
| Clinical
response |
|
| 0.046a |
| CR | 0 (0.0) | 0 (0.0) |
|
| PR | 10 (40.0) | 20 (64.5) |
|
| SD | 13 (52.0) | 11 (35.5) |
|
| PD | 2 (8.0) | 0 (0.0) |
|
| ORR (CR+PR) | 10 (40.0) | 20 (64.5) | 0.067 |
Pathological response
The resected tumor was evaluated by pathological
examination to assess the pathological response, which revealed
that MPR exhibited a higher trend in the camrelizumab plus PC group
than in the PC group, although the difference was not statistically
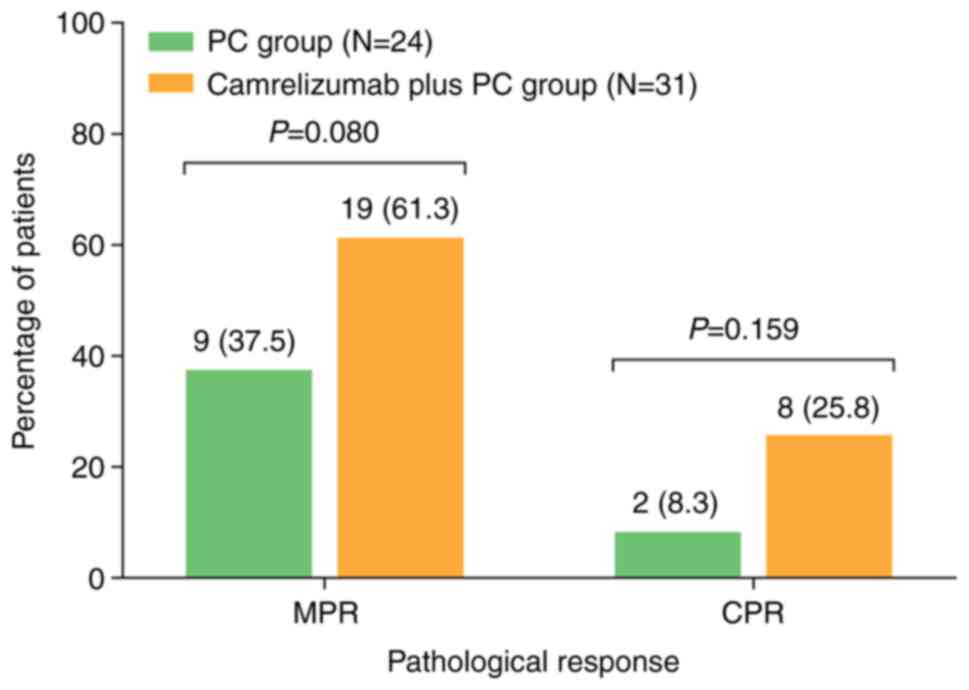
significant (61.3 vs. 37.5%; P=0.080). In addition, CPR also
exhibited an elevated trend in the camrelizumab plus PC group
compared with that of the PC group, but again the result was not
statistically significant (25.8 vs. 8.3%; P=0.159) (Fig. 1).
Survival outcome
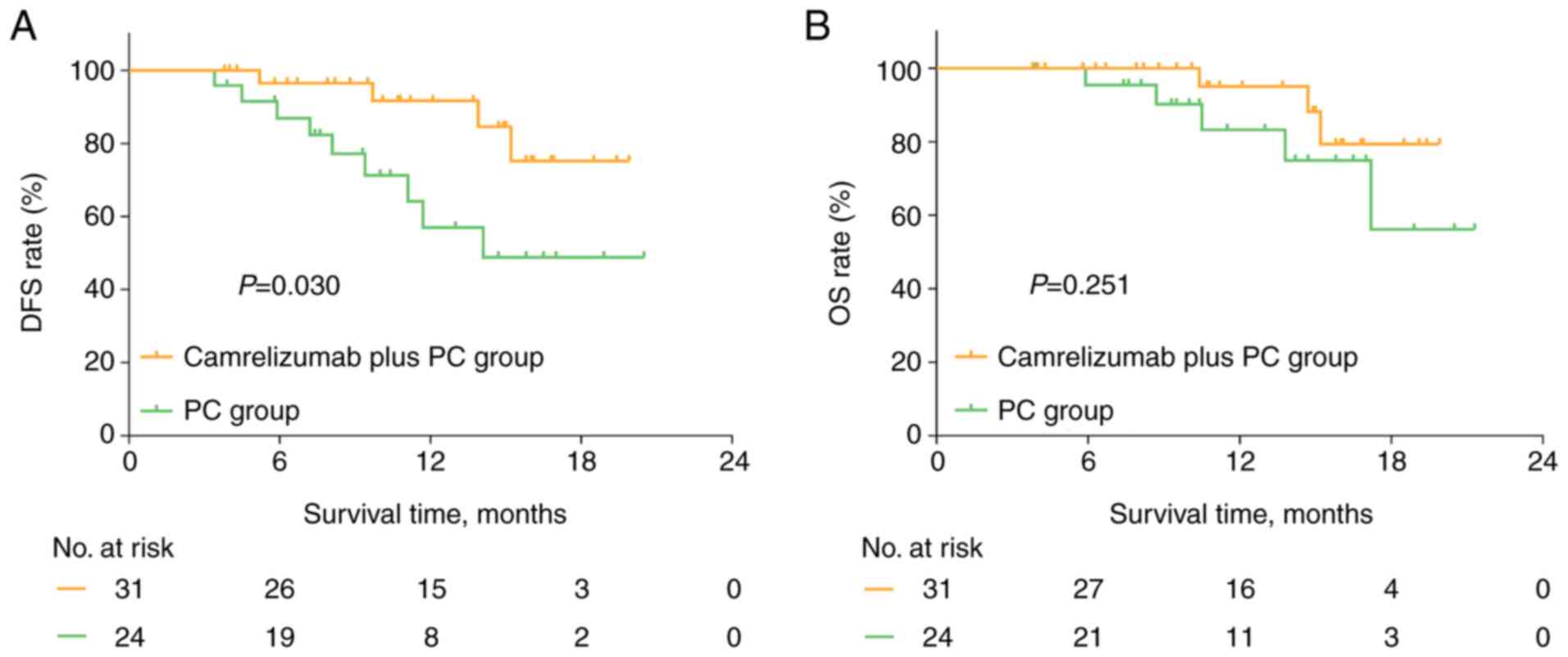
During a median follow-up time of 11.8 months, DFS
time was prolonged in the camrelizumab plus PC group compared with
that in the PC group (P=0.030; Fig.
2A). In addition, the 1-year DFS rate was 91.6% in the
camrelizumab plus PC group and 57.0% in the PC group. In terms of
OS, there was no significant difference between the camrelizumab
plus PC and PC groups (P=0.251; Fig.
2B). The 1-year OS was 95.0% in the camrelizumab plus PC group
and 83.2% in the PC group. In the camrelizumab plus PC group, it
was observed that patients with PD-L1 expression >50% tended to
have a longer DFS time compared with that of patients with PD-L1
expression ≤50%, although the difference was not statistically
significant (P=0.233; Fig. S1A).
The OS time also did not differ significantly between the two
groups (P=0.542; Fig. S1B).
Adjustment by multivariate
analyses
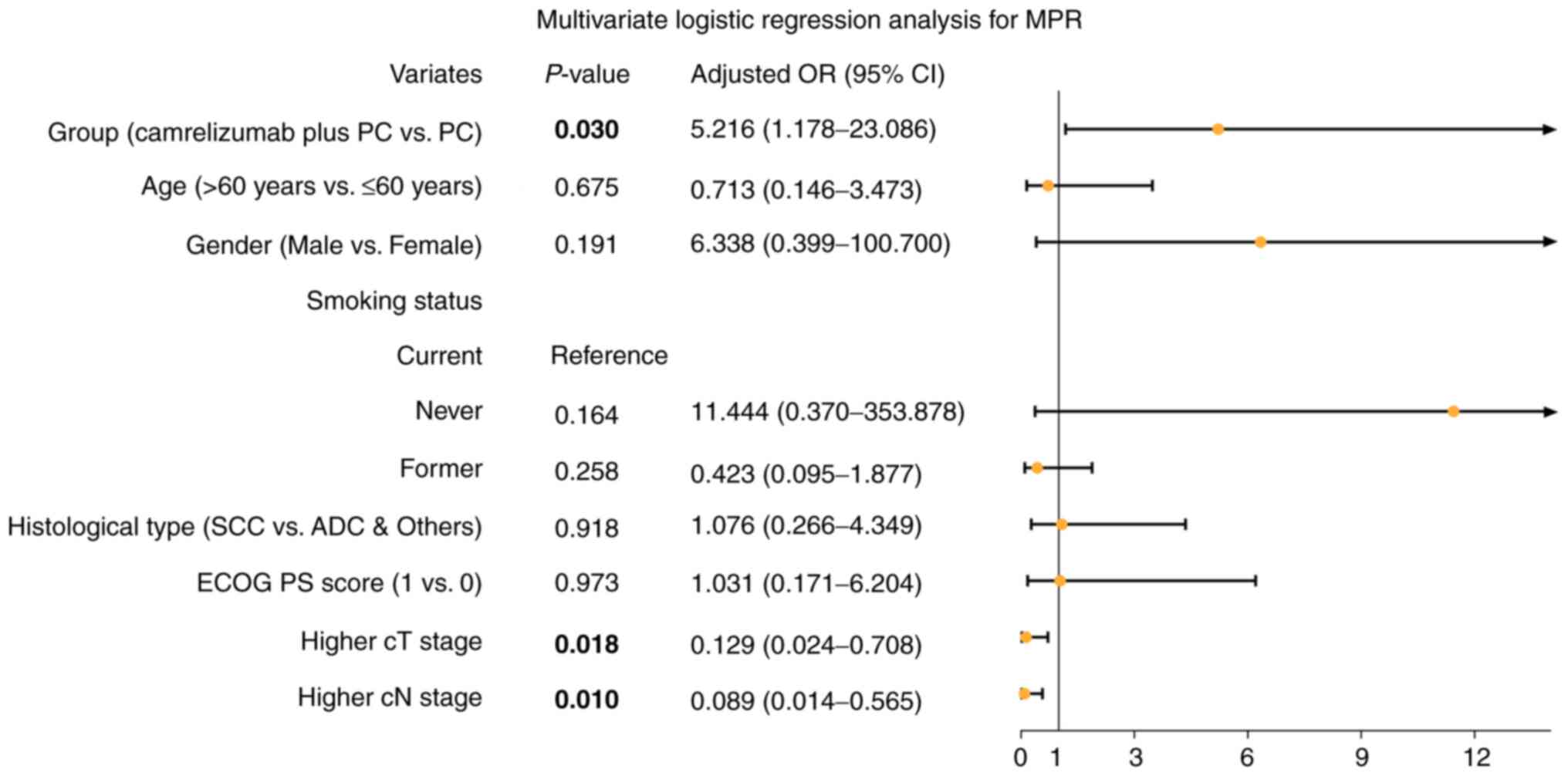
Upon adjustment by multivariate logistic regression
analysis, the camrelizumab plus PC regimen versus the PC regimen
were independently associated, with higher MPR [odds ratio (OR),
5.216; 95% confidence interval (CI), 1.178-23.086; P=0.030;
Fig. 3]. Furthermore, higher T and
N stages were independently associated with a lower MPR.
Following adjustment by multivariate Cox's
proportional hazards regression analysis, the camrelizumab plus PC
regimen was superior to the PC regimen regarding both DFS [hazard
ratio (HR), 0.055; 95% CI, 0.007-0.442; P=0.006] and OS (HR, 0.025;
95% CI, 0.002-0.416; P=0.010) (Table
III) times. Furthermore, a higher TNM stage was independently
associated with poor DFS and OS (both P=0.002).
 | Table III.Multivariable Cox's proportional
hazards regression analysis for DFS and OS. |
Table III.
Multivariable Cox's proportional
hazards regression analysis for DFS and OS.
|
| DFS | OS |
|---|
|
|
|
|
|---|
| Variable | P-value | HR (95% CI) | P-value | HR (95% CI) |
|---|
| Group (camrelizumab
plus PC vs. PC) | 0.006a | 0.055
(0.007-0.442) | 0.010a | 0.025
(0.002-0.416) |
| Age (>60 vs. ≤60
years) | 0.624 | 0.681
(0.146-3.166) | 0.116 | 0.096
(0.005-1.781) |
| Sex (male vs.
female) | 0.403 | 0.421
(0.055-3.200) | 0.456 | 48.644
(0.002-1336754.889) |
| Smoke status |
|
|
|
|
|
Current | Reference | - | Reference | - |
|
Never | 0.189 | 0.155
(0.010-2.506) | 0.660 | 9.603
(0.000-231266.344) |
|
Former | 0.991 | 1.008
(0.256-3.969) | 0.853 | 0.782
(0.058-10.557) |
| Histological type
(SCC vs. ADC & others) | 0.900 | 0.910
(0.207-3.999) | 0.746 | 1.462
(0.146-14.602) |
| ECOG PS score (1
vs. 0) | 0.901 | 1.114
(0.206-6.020) | 0.194 | 7.002
(0.372-131.960) |
| cTNM stage (IIIB
vs. IIIA) | 0.002a | 29.007
(3.488-241.219) | 0.002a | 110.594
(5.863-2085.989) |
AEs
The most common AEs of the neoadjuvant camrelizumab
plus PC regimen were alopecia (51.6%), nausea and vomiting (45.2%),
anemia (41.9%), fatigue (41.9%), neutropenia (38.7%), reactive
cutaneous capillary endothelial proliferation (RCCEP) (35.5%),
leukopenia (29.0%), peripheral neuropathy (25.8%), thrombopenia
(22.6%) and anorexia (22.6%) (Table
IV). The majority of AEs associated with the camrelizumab plus
PC regimen were at grade 1–2, and only a few AEs were observed at
grade 3–4, including neutropenia (9.7%), nausea and vomiting
(6.5%), fatigue (6.5%), anemia (3.2%), leukopenia (3.2%), anorexia
(3.2%) and elevated transaminase (3.2%).
 | Table IV.Adverse events in the camrelizumab
plus paclitaxel and carboplatin group. |
Table IV.
Adverse events in the camrelizumab
plus paclitaxel and carboplatin group.
| Adverse event | Total, n (%) | Grade 1-2, n
(%) | Grade 3-4, n
(%) |
|---|
| Alopecia | 16 (51.6) | 16 (51.6) | 0 (0.0) |
| Nausea and
vomiting | 14 (45.2) | 12 (38.7) | 2 (6.5) |
| Anemia | 13 (41.9) | 12 (38.7) | 1 (3.2) |
| Fatigue | 13 (41.9) | 11 (35.5) | 2 (6.5) |
| Neutropenia | 12 (38.7) | 9 (29.0) | 3 (9.7) |
| RCCEP | 11 (35.5) | 11 (35.5) | 0 (0.0) |
| Leukopenia | 9 (29.0) | 8 (25.8) | 1 (3.2) |
| Peripheral
neuropathy | 8 (25.8) | 8 (25.8) | 0 (0.0) |
| Thrombopenia | 7 (22.6) | 7 (22.6) | 0 (0.0) |
| Anorexia | 7 (22.6) | 6 (19.4) | 1 (3.2) |
| Constipation | 6 (19.4) | 6 (19.4) | 0 (0.0) |
| Elevated
bilirubin | 6 (19.4) | 6 (19.4) | 0 (0.0) |
| Elevated
transaminase | 6 (19.4) | 5 (16.1) | 1 (3.2) |
| Diarrhea | 5 (16.1) | 5 (16.1) | 0 (0.0) |
| Rash | 4 (12.9) | 4 (12.9) | 0 (0.0) |
| Hypothyroidism | 3 (9.7) | 3 (9.7) | 0 (0.0) |
Discussion
Camrelizumab is a PD-1 inhibitor, which was recently
developed in China and is currently widely used for the treatment
of advanced tumors (12–18,27).
For instance, in one study, camrelizumab plus lenvatinib increased
the ORR and disease control rate, and also prolonged the
progression-free survival (PFS) time, compared with lenvatinib
alone when treating advanced hepatocellular carcinoma (27). In terms of NSCLC, treatment with
camrelizumab plus carboplatin and pemetrexed led to a favorable PFS
compared with treatment with carboplatin and pemetrexed
chemotherapy alone in patients with advanced non-squamous NSCLC
without EGFR or ALK mutations (16). Furthermore, camrelizumab plus
anlotinib achieved a median PFS time of 8.2 months and a median OS
time of 12.7 months in patients with advanced NSCLC who had been
subjected to multiple failed lines of treatment (17). However, the use of camrelizumab as
a neoadjuvant therapy in cancer lacks sufficient evidence, and only
a limited number of reports have been published, including two
studies on neoadjuvant camrelizumab plus chemotherapy with or
without apatinib in locally advanced esophageal squamous cell
carcinoma and gastroesophageal junction adenocarcinoma (28,29),
and one study on neoadjuvant camrelizumab plus lenvatinib in
patients with hepatocellular carcinoma (HCC) who underwent a liver
transplant (30). In detail,
neoadjuvant camrelizumab plus nab-paclitaxel and S1 achieved a 33%
CPR and 75% MPR in locally advanced esophageal squamous cell
carcinoma (28), while another
study reported the use of neoadjuvant immunotherapy involving
camrelizumab plus chemotherapy, realizing a 34% CPR and 76% MPR in
locally advanced esophageal squamous cell carcinoma and
gastroesophageal junction adenocarcinoma (29). For HCC, neoadjuvant camrelizumab
plus lenvatinib achieved a 71% ORR and 85% DCR, and the patients
successfully underwent liver transplantation (30).
In terms of locally advanced NSCLC, various studies
have reported the advantages of PD-1 inhibitors as neoadjuvant
therapy (31–33). For instance, the neoadjuvant
chemotherapy and nivolumab in resectable non-small-cell lung cancer
(NADIM) trial indicated that neoadjuvant nivolumab plus PC
chemotherapy achieved a clinical response (according to RECIST
criteria) of a 4% CR and 76% ORR, and a pathological response
(according to pathological examination) of an 83% MPR and 63% CPR
in patients with TNM stage IIIA NSCLC (31). A recent retrospective cohort study
revealed that nivolumab or pembrolizumab plus PC chemotherapy led
to a 41.7% CPR and 75.0% MPR in patients with TNM stage IIIA/IIIB
NSCLC (32). In addition, a
prospective cohort study indicated that PD-1 inhibitors (including
multiple products) plus albumin paclitaxel and carboplatin produced
a CPR of 29.1% in patients with TNM stage IIIA NSCLC (33). However, the previous studies lacked
a control group or cohort, and the sample size was relatively
small. Furthermore, to the bets of our knowledge, in patients with
locally advanced NSCLC, no reports have been published to date on
the application of neoadjuvant camrelizumab for the treatment of
these patients.
The present study revealed that neoadjuvant
camrelizumab plus PC led to a 0.0% CR, 64.5% PR, 35.5% SD, 0.0% PD
and 64.5% ORR according to the RECIST criteria, and achieved a
25.8% CPR and 61.3% MPR according to the pathological examination
in patients with locally advanced NSCLC. These results were
partially in line with those from previous studies on other PD-1
inhibitors (31–33), although the CPR seemed relatively
low compared with that of the aforementioned studies, which may be
due to the following reasons: i) Patients with TNM stage IIIB were
also enrolled in the present study; ii) the duration of adjuvant
therapy differed among studies; and iii) different drugs were used.
Notably, the present study revealed that neoadjuvant camrelizumab
plus PC chemotherapy achieved a better clinical response than
neoadjuvant PC chemotherapy, and exhibited a higher ORR, CPR and
MRP compared with those of neoadjuvant PC chemotherapy in patients
with locally advanced NSCLC. A possible explanation could be that
camrelizumab synergized with PC chemotherapy by blocking immune
escape and enhancing chemosensitivity, therefore improving the
neoadjuvant treatment response (34,35).
The NADIM trial found that neoadjuvant nivolumab
plus PC chemotherapy achieved a 1-year PFS rate of 95.7%, a 2-year
PFS rate of 77.1%, a 1-year OS rate of 97.8% and a 2-year OS rate
of 89.9% in patients with TNM stage IIIA NSCLC (31). However, no relevant data on
neoadjuvant camrelizumab therapy in patients with locally advanced
NSCLC has been published to date. Although the follow-up duration
was relatively short, the present study revealed that neoadjuvant
camrelizumab plus PC chemotherapy led to a 1-year DFS rate of 91.6%
and a 1-year OS rate of 95.0% in patients with locally advanced
NSCLC, which was in accordance with previous studies on other PD-1
inhibitors (31,32). Importantly, a control cohort was
included in the current study, and it was observed that neoadjuvant
camrelizumab plus PC chemotherapy led to a prolonged DFS time
compared with that of neoadjuvant PC chemotherapy in patients with
locally advanced NSCLC, with a benefit from the synergy between
camrelizumab and chemotherapy (34,35).
In addition, since compounding factors may exist due
to the cohort study design, the present study further performed
multivariate logistic regression analysis and multivariate Cox's
proportional hazards regression analysis for adjustment, which
revealed that neoadjuvant camrelizumab plus PC chemotherapy versus
PC chemotherapy were independently associated with a higher MPR, as
well as prolonged DFS and OS times. This provided evidence of the
advantages of neoadjuvant camrelizumab plus PC chemotherapy for the
treatment of patients with locally advanced NSCLC.
Regarding safety, a previous study reported that the
most common AEs were hypertension, fatigue, transaminitis,
diarrhea, headache/dizziness and neutropenia in patients with
advanced NSCLC who underwent camrelizumab plus anlotinib treatment
(18). Another study revealed that
the most prevalent AEs were RECCP, decreased neutrophil, platelet
and white blood cell counts, anemia, and increased aspartate and
alanine aminotransferases in patients with advanced NSCLC who
underwent camrelizumab plus carboplatin and pemetrexed chemotherapy
(16). However, the safety profile
of neoadjuvant camrelizumab in locally advanced NSCLC remains
unclear. The current study revealed that the most common AEs were
alopecia, nausea, vomiting, anemia, fatigue, neutropenia, RCCEP,
leukopenia, peripheral neuropathy, thrombopenia and anorexia in
patients with locally advanced NSCLC who underwent neoadjuvant
camrelizumab plus PC chemotherapy. In addition, the majority of AEs
of neoadjuvant camrelizumab plus PC chemotherapy were at grade 1–2,
while only a few AEs were at grade 3–4. This suggested an
acceptable tolerance to neoadjuvant camrelizumab plus PC
chemotherapy in these patients. However, since PD-1 inhibitors
directly affect T cells, which can increase the risk of
hematological AEs, this issue needs to be monitored during its
application (16).
The present study has several limitations: i) Due to
the present cohort study design, a further randomized, controlled
study to validate the findings would be useful; ii) the current
study was a single-center-based study, and therefore patient
selection bias and physician assessment bias may exist; thus, a
multiple center-based study should be conducted in the future; and
iii) the follow-up duration was relatively short in the present
study due to the limited time that camrelizumab had been available
on the market (the camrelizumab was on the market for ~2.2 years at
the last follow-up date; therefore, the follow-up duration for each
patient was within 2.2 years), and therefore the follow-up time
should be prolonged in future studies.
In conclusion, the present study revealed that
neoadjuvant camrelizumab plus PC chemotherapy exhibited a superior
pathological response and survival profile over neoadjuvant PC
chemotherapy, and was tolerable in patients with locally advanced
NSCLC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL conceived and designed the study. XH and XS
collected and analyzed the data. JL prepared the figures and
tables. XH and XS wrote the manuscript. XH and XS confirm the
authenticity of all the raw data. JL revised the manuscript. All
authors read and approved the submitted version.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Daqing Oil Field General Hospital (Daqing, China;
approval no. KS1952). Written informed consent for participation
and data use was provided by all the patients included in the
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Clark SB and Alsubait S: Non Small Cell
Lung Cancer. StatPearls. Treasure Island; FL: 2021
|
|
3
|
Ikeda N: Updates on minimally invasive
surgery in non-small cell lung cancer. Curr Treat Options Oncol.
20:162019. View Article : Google Scholar
|
|
4
|
Alexander M, Kim SY and Cheng H: Update
2020: Management of non-small cell lung cancer. Lung. 198:897–907.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jazieh AR, Zeitouni M, Alghamdi M,
Alrujaib M, Lotfi S, Daff SA, Alomair A, Alshehri S, Alhusaini H,
Allehebi A, et al: Management guidelines for stage III non-small
cell lung cancer. Crit Rev Oncol Hematol. 157:1031442021.
View Article : Google Scholar
|
|
6
|
Patane AK: Minimal invasive surgery in
locally advanced N2 non-small cell lung cancer. Transl Lung Cancer
Res. 10:519–528. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Passiglia F, Bertolaccini L, Del Re M,
Facchinetti F, Ferrara R, Franchina T, Malapelle U, Menis J,
Passaro A, Pilotto S, et al: Diagnosis and treatment of early and
locally advanced non-small-cell lung cancer: The 2019 AIOM (Italian
Association of Medical Oncology) clinical practice guidelines. Crit
Rev Oncol Hematol. 148:1028622020. View Article : Google Scholar
|
|
8
|
Ren S, Xu A, Lin Y, Camidge DR, Maio MD,
Califano R, Hida T, Rossi A, Guibert N, Zhu C and Shen J: A
narrative review of primary research endpoints of neoadjuvant
therapy for lung cancer: Past, present and future. Transl Lung
Cancer Res. 10:3264–3275. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xiong L, Lou Y, Bai H, Li R, Xia J, Fang
W, Zhang J, Zhang HH, Lizaso A, Li B, et al: Efficacy of erlotinib
as neoadjuvant regimen in EGFR-mutant locally advanced non-small
cell lung cancer patients. J Int Med Res. 48:3000605198872752020.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang C, Li SL, Nie Q, Dong S, Shao Y,
Yang XN, Wu YL, Yang Y and Zhong WZ: Neoadjuvant crizotinib in
resectable locally advanced non-small cell lung cancer with ALK
rearrangement. J Thorac Oncol. 14:726–731. 2019. View Article : Google Scholar
|
|
11
|
Zhao S, Zhu S, Lei X, Xu D, Shi T, Chen Q,
Ren F, Chen G, Huang D and Xu S: Use of crizotinib as neoadjuvant
therapy for non-small cell lung cancers patient with ROS1
rearrangement: A case report. Thorac Cancer. 12:2815–2818. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Markham A and Keam SJ: Camrelizumab: First
global approval. Drugs. 79:1355–1361. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Luo H, Lu J, Bai Y, Mao T, Wang J, Fan Q,
Zhang Y, Zhao K, Chen Z, Gao S, et al: Effect of camrelizumab vs
placebo added to chemotherapy on survival and progression-free
survival in patients with advanced or metastatic esophageal
squamous cell carcinoma: The ESCORT-1st randomized clinical trial.
JAMA. 326:916–925. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang Y, Zhang Z, Liao W, Hu K and Wang Z:
Combination of sorafenib, camrelizumab, transcatheter arterial
chemoembolization, and stereotactic body radiation therapy as a
novel downstaging strategy in advanced hepatocellular carcinoma
with portal vein tumor thrombus: A case series study. Front Oncol.
11:6503942021. View Article : Google Scholar
|
|
15
|
Qu YY, Zhang HL, Guo H, Luo H, Zou Q, Xing
N, Xia S, Sun Z, Zhang X, He C, et al: Camrelizumab plus famitinib
in patients with advanced or metastatic renal cell carcinoma: Data
from an open-label, multicenter phase II basket study. Clin Cancer
Res. 27:5838–5846. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou C, Chen G, Huang Y, Zhou J, Lin L,
Feng J, Wang Z, Shu Y, Shi J, Hu Y, et al: Camrelizumab plus
carboplatin and pemetrexed versus chemotherapy alone in
chemotherapy-naive patients with advanced non-squamous
non-small-cell lung cancer (CameL): A randomised, open-label,
multicentre, phase 3 trial. Lancet Respir Med. 9:305–314. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou N, Jiang M, Li T, Zhu J, Liu K, Hou H
and Zhang X: Anlotinib combined with anti-PD-1 antibody,
camrelizumab for advanced NSCLCs after multiple lines treatment: An
open-label, dose escalation and expansion study. Lung Cancer.
160:111–117. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang P, Fang X, Yin T, Tian H, Yu J and
Teng F: Efficacy and safety of anti-pd-1 plus anlotinib in patients
with advanced non-small-cell lung cancer after previous systemic
treatment failure-a retrospective study. Front Oncol.
11:6281242021. View Article : Google Scholar
|
|
19
|
Chansky K, Detterbeck FC, Nicholson AG,
Rusch VW, Vallières E, Groome P, Kennedy C, Krasnik M, Peake M,
Shemanski L, et al: The IASLC lung cancer staging project: External
validation of the revision of the TNM stage groupings in the eighth
edition of the TNM classification of lung cancer. J Thorac Oncol.
12:1109–1121. 2017. View Article : Google Scholar
|
|
20
|
Mischel AM and Rosielle DA: Eastern
cooperative oncology group performance status #434. J Palliat Med.
25:508–510. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pataer A, Kalhor N, Correa AM, Raso MG,
Erasmus JJ, Kim ES, Behrens C, Lee JJ, Roth JA, Stewart DJ, et al:
Histopathologic response criteria predict survival of patients with
resected lung cancer after neoadjuvant chemotherapy. J Thorac
Oncol. 7:825–832. 2012. View Article : Google Scholar
|
|
23
|
Travis WD, Dacic S, Wistuba I, Sholl L,
Adusumilli P, Bubendorf L, Bunn P, Cascone T, Chaft J, Chen G, et
al: IASLC multidisciplinary recommendations for pathologic
assessment of lung cancer resection specimens after neoadjuvant
therapy. J Thorac Oncol. 15:709–740. 2020. View Article : Google Scholar
|
|
24
|
Chen AP, Setser A, Anadkat MJ, Cotliar J,
Olsen EA, Garden BC and Lacouture ME: Grading dermatologic adverse
events of cancer treatments: The common terminology criteria for
adverse events version 4.0. J Am Acad Dermatol. 67:1025–1039. 2012.
View Article : Google Scholar
|
|
25
|
Shu CA, Gainor JF, Awad MM, Chiuzan C,
Grigg CM, Pabani A, Garofano RF, Stoopler MB, Cheng SK, White A, et
al: Neoadjuvant atezolizumab and chemotherapy in patients with
resectable non-small-cell lung cancer: An open-label, multicentre,
single-arm, phase 2 trial. Lancet Oncol. 21:786–795. 2020.
View Article : Google Scholar
|
|
26
|
Aguilar EJ, Ricciuti B, Gainor JF, Kehl
KL, Kravets S, Dahlberg S, Nishino M, Sholl LM, Adeni A, Subegdjo
S, et al: Outcomes to first-line pembrolizumab in patients with
non-small-cell lung cancer and very high PD-L1 expression. Ann
Oncol. 30:1653–1659. 2019. View Article : Google Scholar
|
|
27
|
Wei F, Huang Q, He J, Luo L and Zeng Y:
Lenvatinib plus camrelizumab versus lenvatinib monotherapy as
post-progression treatment for advanced hepatocellular carcinoma: A
short-term prognostic study. Cancer Manag Res. 13:4233–4240. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang G, Su X, Yang H, Luo G, Gao C, Zheng
Y, Xie W, Huang M, Bei T, Bai Y, et al: Neoadjuvant programmed
death-1 blockade plus chemotherapy in locally advanced esophageal
squamous cell carcinoma. Ann Transl Med. 9:12542021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu Z, Zheng Q, Chen H, Xiang J, Hu H, Li
H, Pan Y, Peng Y, Yao X, Liu P, et al: Efficacy and safety of
neoadjuvant chemotherapy and immunotherapy in locally resectable
advanced esophageal squamous cell carcinoma. J Thorac Dis.
13:3518–3528. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Qiao ZY, Zhang ZJ, Lv ZC, Tong H, Xi ZF,
Wu HX, Chen XS, Xia L, Feng H, Zhang JJ and Xia Q: Neoadjuvant
programmed cell death 1 (PD-1) inhibitor treatment in patients with
hepatocellular carcinoma before liver transplant: A cohort study
and literature review. Front Immunol. 12:6534372021. View Article : Google Scholar
|
|
31
|
Provencio M, Nadal E, Insa A,
García-Campelo MR, Casal-Rubio J, Dómine M, Majem M,
Rodríguez-Abreu D, Martínez-Martí A, Carpeño JD, et al: Neoadjuvant
chemotherapy and nivolumab in resectable non-small-cell lung cancer
(NADIM): An open-label, multicentre, single-arm, phase 2 trial.
Lancet Oncol. 21:1413–1422. 2020. View Article : Google Scholar
|
|
32
|
Chen T, Ning J, Campisi A, Dell'Amore A,
Ciarrocchi AP, Li Z, Song L, Huang J, Yang Y, Stella F and Luo Q:
Neoadjuvant PD-1 inhibitors and chemotherapy for locally advanced
NSCLC: A retrospective study. Ann Thorac Surg. 113:993–999. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang J, Li J, Cai L, Chen S and Jiang Y:
The safety and efficacy of neoadjuvant programmed death 1 inhibitor
therapy with surgical resection in stage IIIA non-small cell lung
cancer. Ann Transl Med. 9:4862021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen Z, Lu X and Koral K: The clinical
application of camrelizumab on advanced hepatocellular carcinoma.
Expert Rev Gastroenterol Hepatol. 14:1017–1024. 2020. View Article : Google Scholar
|
|
35
|
Yang C, Xu C, Li X and Zhang Y, Zhang S,
Zhang T and Zhang Y: Could camrelizumab plus chemotherapy improve
clinical outcomes in advanced malignancy? A systematic review and
network meta-analysis. Front Oncol. 11:7001652021. View Article : Google Scholar
|