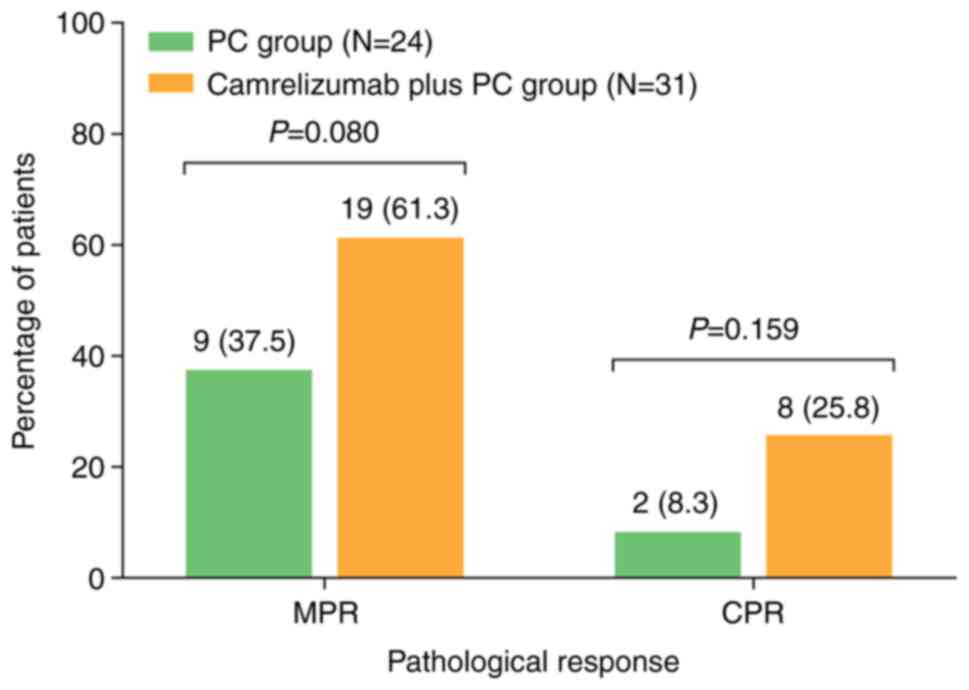
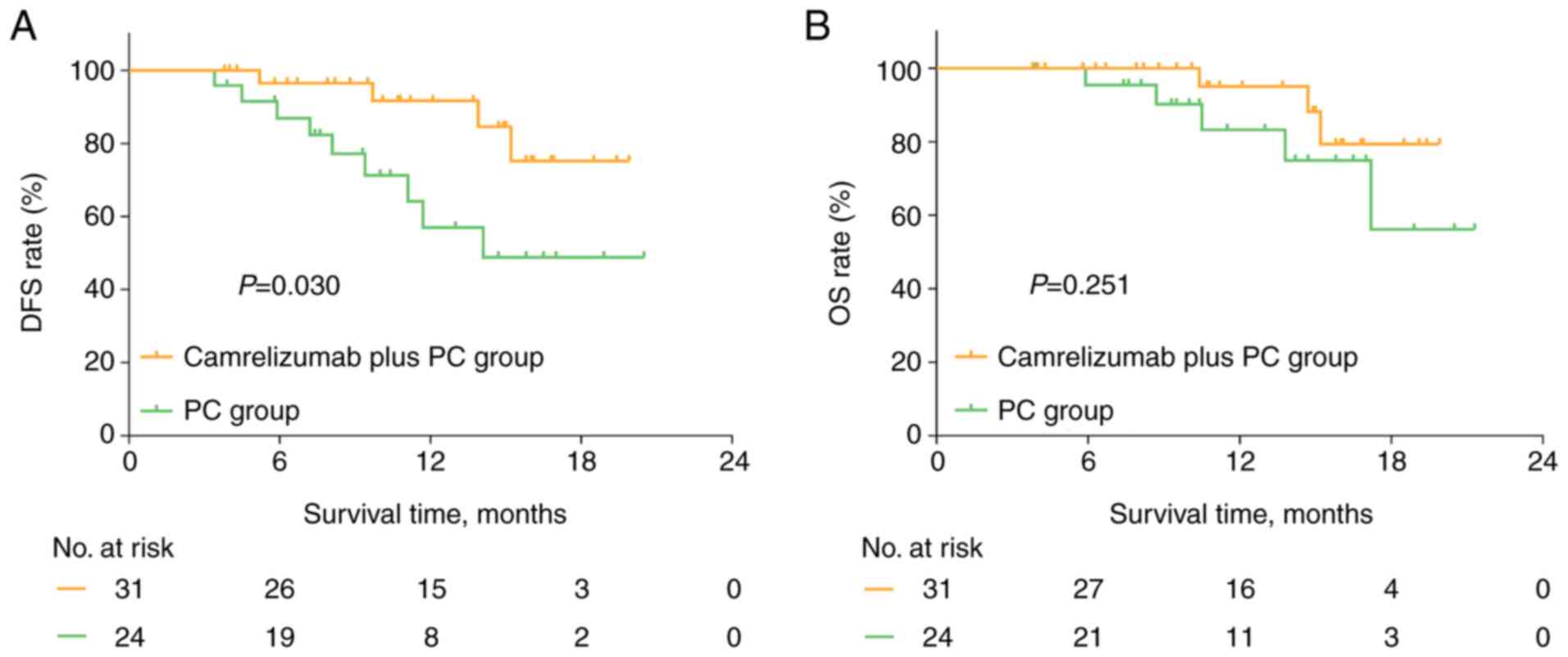
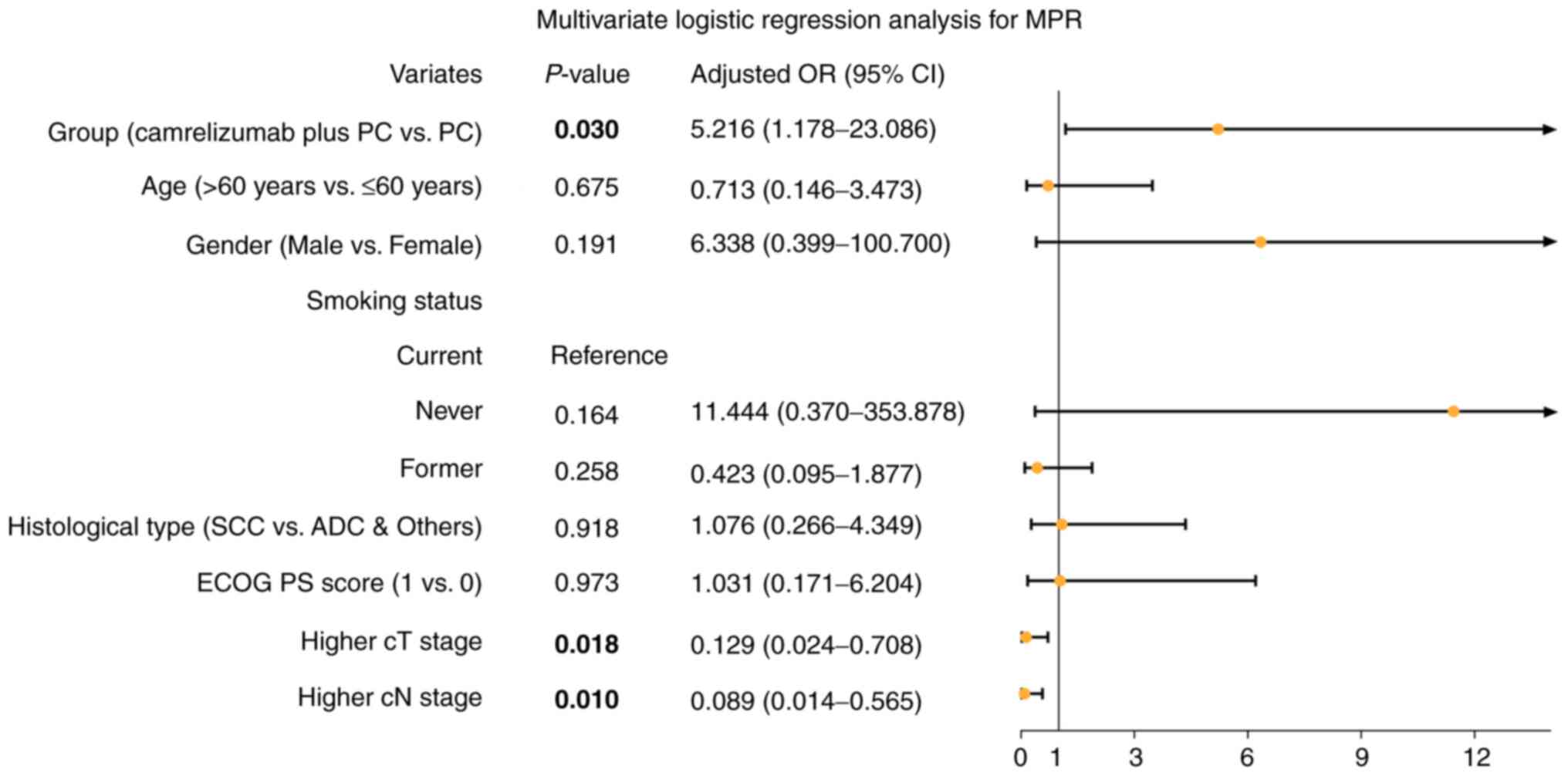
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Clark SB and Alsubait S: Non Small Cell
Lung Cancer. StatPearls. Treasure Island; FL: 2021
|
|
3
|
Ikeda N: Updates on minimally invasive
surgery in non-small cell lung cancer. Curr Treat Options Oncol.
20:162019. View Article : Google Scholar
|
|
4
|
Alexander M, Kim SY and Cheng H: Update
2020: Management of non-small cell lung cancer. Lung. 198:897–907.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jazieh AR, Zeitouni M, Alghamdi M,
Alrujaib M, Lotfi S, Daff SA, Alomair A, Alshehri S, Alhusaini H,
Allehebi A, et al: Management guidelines for stage III non-small
cell lung cancer. Crit Rev Oncol Hematol. 157:1031442021.
View Article : Google Scholar
|
|
6
|
Patane AK: Minimal invasive surgery in
locally advanced N2 non-small cell lung cancer. Transl Lung Cancer
Res. 10:519–528. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Passiglia F, Bertolaccini L, Del Re M,
Facchinetti F, Ferrara R, Franchina T, Malapelle U, Menis J,
Passaro A, Pilotto S, et al: Diagnosis and treatment of early and
locally advanced non-small-cell lung cancer: The 2019 AIOM (Italian
Association of Medical Oncology) clinical practice guidelines. Crit
Rev Oncol Hematol. 148:1028622020. View Article : Google Scholar
|
|
8
|
Ren S, Xu A, Lin Y, Camidge DR, Maio MD,
Califano R, Hida T, Rossi A, Guibert N, Zhu C and Shen J: A
narrative review of primary research endpoints of neoadjuvant
therapy for lung cancer: Past, present and future. Transl Lung
Cancer Res. 10:3264–3275. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xiong L, Lou Y, Bai H, Li R, Xia J, Fang
W, Zhang J, Zhang HH, Lizaso A, Li B, et al: Efficacy of erlotinib
as neoadjuvant regimen in EGFR-mutant locally advanced non-small
cell lung cancer patients. J Int Med Res. 48:3000605198872752020.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang C, Li SL, Nie Q, Dong S, Shao Y,
Yang XN, Wu YL, Yang Y and Zhong WZ: Neoadjuvant crizotinib in
resectable locally advanced non-small cell lung cancer with ALK
rearrangement. J Thorac Oncol. 14:726–731. 2019. View Article : Google Scholar
|
|
11
|
Zhao S, Zhu S, Lei X, Xu D, Shi T, Chen Q,
Ren F, Chen G, Huang D and Xu S: Use of crizotinib as neoadjuvant
therapy for non-small cell lung cancers patient with ROS1
rearrangement: A case report. Thorac Cancer. 12:2815–2818. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Markham A and Keam SJ: Camrelizumab: First
global approval. Drugs. 79:1355–1361. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Luo H, Lu J, Bai Y, Mao T, Wang J, Fan Q,
Zhang Y, Zhao K, Chen Z, Gao S, et al: Effect of camrelizumab vs
placebo added to chemotherapy on survival and progression-free
survival in patients with advanced or metastatic esophageal
squamous cell carcinoma: The ESCORT-1st randomized clinical trial.
JAMA. 326:916–925. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang Y, Zhang Z, Liao W, Hu K and Wang Z:
Combination of sorafenib, camrelizumab, transcatheter arterial
chemoembolization, and stereotactic body radiation therapy as a
novel downstaging strategy in advanced hepatocellular carcinoma
with portal vein tumor thrombus: A case series study. Front Oncol.
11:6503942021. View Article : Google Scholar
|
|
15
|
Qu YY, Zhang HL, Guo H, Luo H, Zou Q, Xing
N, Xia S, Sun Z, Zhang X, He C, et al: Camrelizumab plus famitinib
in patients with advanced or metastatic renal cell carcinoma: Data
from an open-label, multicenter phase II basket study. Clin Cancer
Res. 27:5838–5846. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou C, Chen G, Huang Y, Zhou J, Lin L,
Feng J, Wang Z, Shu Y, Shi J, Hu Y, et al: Camrelizumab plus
carboplatin and pemetrexed versus chemotherapy alone in
chemotherapy-naive patients with advanced non-squamous
non-small-cell lung cancer (CameL): A randomised, open-label,
multicentre, phase 3 trial. Lancet Respir Med. 9:305–314. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou N, Jiang M, Li T, Zhu J, Liu K, Hou H
and Zhang X: Anlotinib combined with anti-PD-1 antibody,
camrelizumab for advanced NSCLCs after multiple lines treatment: An
open-label, dose escalation and expansion study. Lung Cancer.
160:111–117. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang P, Fang X, Yin T, Tian H, Yu J and
Teng F: Efficacy and safety of anti-pd-1 plus anlotinib in patients
with advanced non-small-cell lung cancer after previous systemic
treatment failure-a retrospective study. Front Oncol.
11:6281242021. View Article : Google Scholar
|
|
19
|
Chansky K, Detterbeck FC, Nicholson AG,
Rusch VW, Vallières E, Groome P, Kennedy C, Krasnik M, Peake M,
Shemanski L, et al: The IASLC lung cancer staging project: External
validation of the revision of the TNM stage groupings in the eighth
edition of the TNM classification of lung cancer. J Thorac Oncol.
12:1109–1121. 2017. View Article : Google Scholar
|
|
20
|
Mischel AM and Rosielle DA: Eastern
cooperative oncology group performance status #434. J Palliat Med.
25:508–510. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pataer A, Kalhor N, Correa AM, Raso MG,
Erasmus JJ, Kim ES, Behrens C, Lee JJ, Roth JA, Stewart DJ, et al:
Histopathologic response criteria predict survival of patients with
resected lung cancer after neoadjuvant chemotherapy. J Thorac
Oncol. 7:825–832. 2012. View Article : Google Scholar
|
|
23
|
Travis WD, Dacic S, Wistuba I, Sholl L,
Adusumilli P, Bubendorf L, Bunn P, Cascone T, Chaft J, Chen G, et
al: IASLC multidisciplinary recommendations for pathologic
assessment of lung cancer resection specimens after neoadjuvant
therapy. J Thorac Oncol. 15:709–740. 2020. View Article : Google Scholar
|
|
24
|
Chen AP, Setser A, Anadkat MJ, Cotliar J,
Olsen EA, Garden BC and Lacouture ME: Grading dermatologic adverse
events of cancer treatments: The common terminology criteria for
adverse events version 4.0. J Am Acad Dermatol. 67:1025–1039. 2012.
View Article : Google Scholar
|
|
25
|
Shu CA, Gainor JF, Awad MM, Chiuzan C,
Grigg CM, Pabani A, Garofano RF, Stoopler MB, Cheng SK, White A, et
al: Neoadjuvant atezolizumab and chemotherapy in patients with
resectable non-small-cell lung cancer: An open-label, multicentre,
single-arm, phase 2 trial. Lancet Oncol. 21:786–795. 2020.
View Article : Google Scholar
|
|
26
|
Aguilar EJ, Ricciuti B, Gainor JF, Kehl
KL, Kravets S, Dahlberg S, Nishino M, Sholl LM, Adeni A, Subegdjo
S, et al: Outcomes to first-line pembrolizumab in patients with
non-small-cell lung cancer and very high PD-L1 expression. Ann
Oncol. 30:1653–1659. 2019. View Article : Google Scholar
|
|
27
|
Wei F, Huang Q, He J, Luo L and Zeng Y:
Lenvatinib plus camrelizumab versus lenvatinib monotherapy as
post-progression treatment for advanced hepatocellular carcinoma: A
short-term prognostic study. Cancer Manag Res. 13:4233–4240. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang G, Su X, Yang H, Luo G, Gao C, Zheng
Y, Xie W, Huang M, Bei T, Bai Y, et al: Neoadjuvant programmed
death-1 blockade plus chemotherapy in locally advanced esophageal
squamous cell carcinoma. Ann Transl Med. 9:12542021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu Z, Zheng Q, Chen H, Xiang J, Hu H, Li
H, Pan Y, Peng Y, Yao X, Liu P, et al: Efficacy and safety of
neoadjuvant chemotherapy and immunotherapy in locally resectable
advanced esophageal squamous cell carcinoma. J Thorac Dis.
13:3518–3528. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Qiao ZY, Zhang ZJ, Lv ZC, Tong H, Xi ZF,
Wu HX, Chen XS, Xia L, Feng H, Zhang JJ and Xia Q: Neoadjuvant
programmed cell death 1 (PD-1) inhibitor treatment in patients with
hepatocellular carcinoma before liver transplant: A cohort study
and literature review. Front Immunol. 12:6534372021. View Article : Google Scholar
|
|
31
|
Provencio M, Nadal E, Insa A,
García-Campelo MR, Casal-Rubio J, Dómine M, Majem M,
Rodríguez-Abreu D, Martínez-Martí A, Carpeño JD, et al: Neoadjuvant
chemotherapy and nivolumab in resectable non-small-cell lung cancer
(NADIM): An open-label, multicentre, single-arm, phase 2 trial.
Lancet Oncol. 21:1413–1422. 2020. View Article : Google Scholar
|
|
32
|
Chen T, Ning J, Campisi A, Dell'Amore A,
Ciarrocchi AP, Li Z, Song L, Huang J, Yang Y, Stella F and Luo Q:
Neoadjuvant PD-1 inhibitors and chemotherapy for locally advanced
NSCLC: A retrospective study. Ann Thorac Surg. 113:993–999. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang J, Li J, Cai L, Chen S and Jiang Y:
The safety and efficacy of neoadjuvant programmed death 1 inhibitor
therapy with surgical resection in stage IIIA non-small cell lung
cancer. Ann Transl Med. 9:4862021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen Z, Lu X and Koral K: The clinical
application of camrelizumab on advanced hepatocellular carcinoma.
Expert Rev Gastroenterol Hepatol. 14:1017–1024. 2020. View Article : Google Scholar
|
|
35
|
Yang C, Xu C, Li X and Zhang Y, Zhang S,
Zhang T and Zhang Y: Could camrelizumab plus chemotherapy improve
clinical outcomes in advanced malignancy? A systematic review and
network meta-analysis. Front Oncol. 11:7001652021. View Article : Google Scholar
|