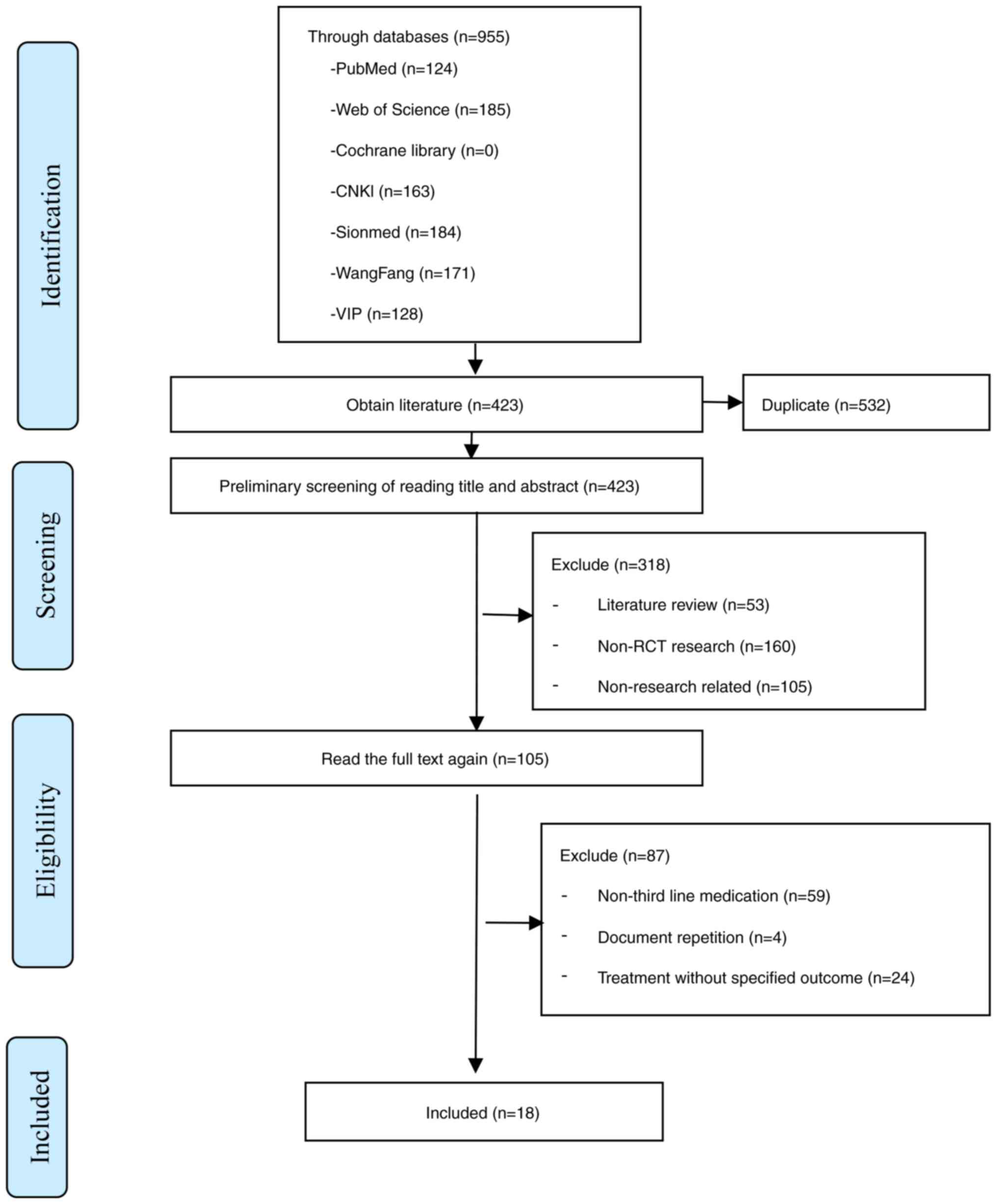
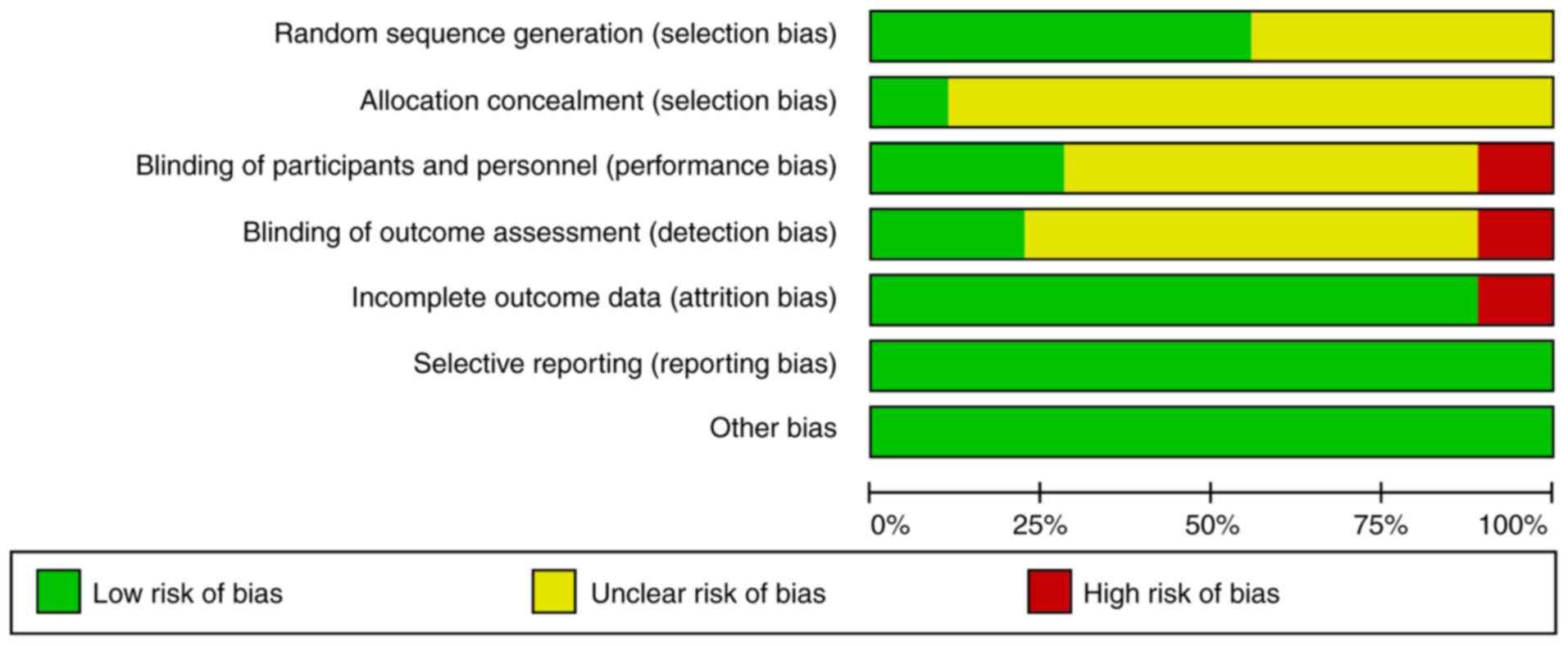
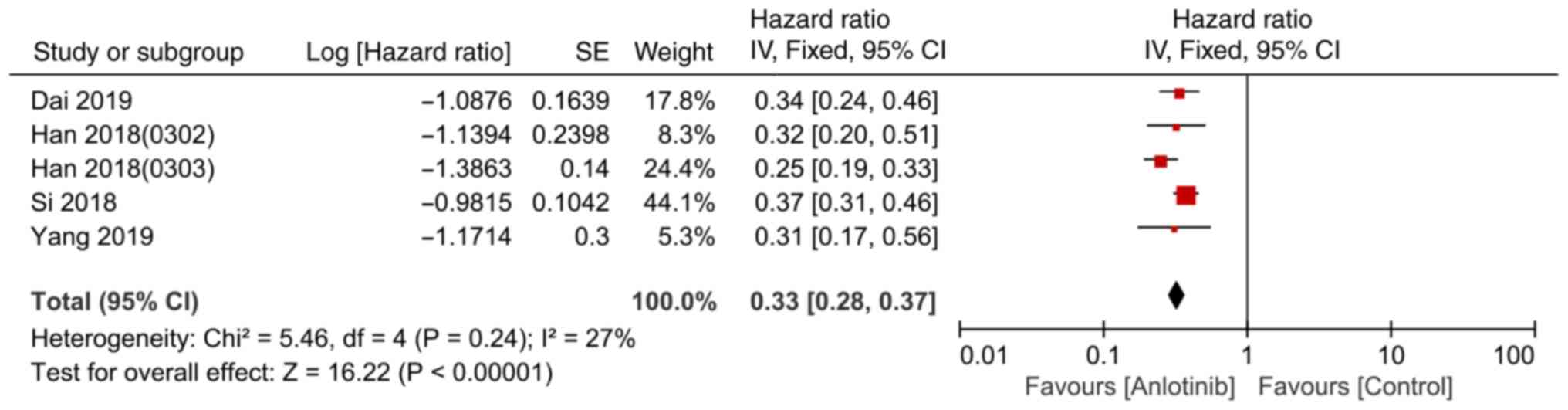
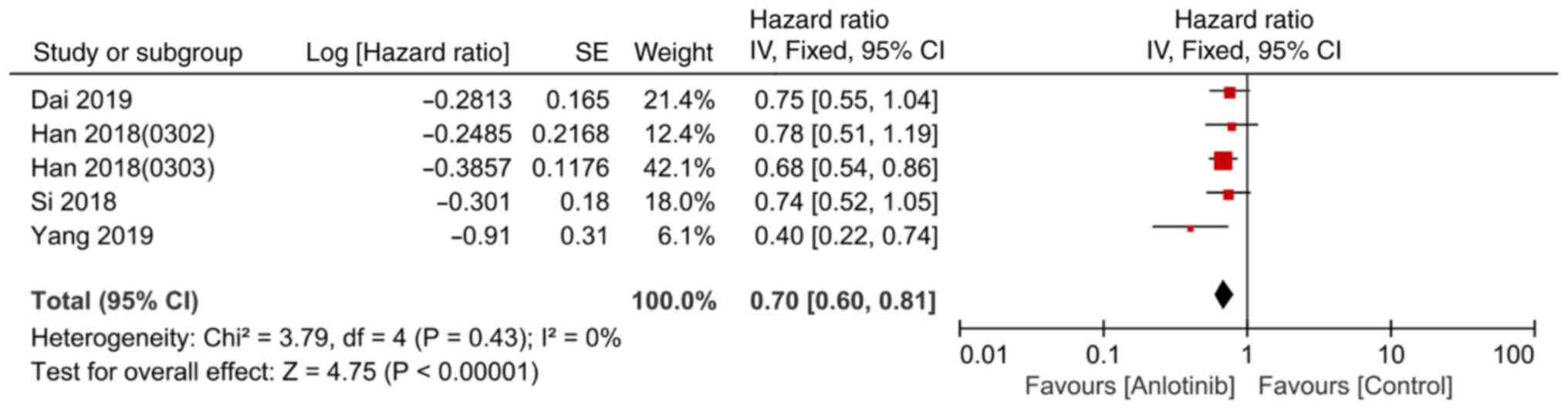
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global Cancer Statistics 2020:
GLOBOCAN estimates of incidence and mortality Worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Herbst RS, Morgensztern D and Boshoff C:
The biology and management of nonsmall cell lung cancer. Nature.
553:446–454. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Molina JR, Yang P, Cassivi SD, Schild SE
and Adjei AA: Non-small cell lung cancer: Epidemiology, risk
factors, treatment, and survivorship. Mayo Clin Proc. 83:584–594.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aokage K, Yoshida J, Hishida T, Tsuboi M,
Saji H, Okada M, Suzuki K, Watanabe S and Asamura H: Limited
resection for early-stage non-small cell lung cancer as
function-preserving radical surgery: A review. Jpn J Clin Oncol.
47:7–11. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Duma N, Santana-Davila R and Molina JR:
Non-small cell lung cancer: Epidemiology, Screening, diagnosis, and
treatment. Mayo Clin Proc. 94:1623–1640. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Broderick SR: Adjuvant and Neoadjuvant
immunotherapy in non-small cell lung cancer. Thorac Surg Clin.
30:215–220. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ruiz-Cordero R and Devine WP: Targeted
therapy and checkpoint immunotherapy in lung cancer. Surg Pathol
Clin. 13:17–33. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou C, Wu YL, Chen G, Feng J, Liu XQ,
Wang C, Zhang S, Wang J, Zhou S, Ren S, et al: Erlotinib versus
chemotherapy as first-line treatment for patients with advanced
EGFR mutation-positive non-small-cell lung cancer (OPTIMAL,
CTONG-0802): A multicentre, open-label, randomised, phase 3 study.
Lancet Oncol. 12:735–742. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mitsudomi T, Morita S, Yatabe Y, Negoro S,
Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, et
al: Gefitinib versus cisplatin plus docetaxel in patients with
non-small-cell lung cancer harbouring mutations of the epidermal
growth factor receptor (WJTOG3405): An open label, randomised phase
3 trial. Lancet Oncol. 11:121–128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liang W, Zhong R and He J: Osimertinib in
EGFR-mutated lung cancer. N Engl J Med. 384:6752021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Belani CP and Eckardt J: Development of
docetaxel in advanced non-small-cell lung cancer. Lung Cancer. 46
(Suppl 2):S3–S11. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cortot AB, Audigier-Valette C, Molinier O,
Le Moulec S, Barlesi F, Zalcman G, Dumont P, Pouessel D, Poulet C,
Fontaine-Delaruelle C, et al: Weekly paclitaxel plus bevacizumab
versus docetaxel as second- or third-line treatment in advanced
non-squamous non-small-cell lung cancer: Results of the IFCT-1103
ULTIMATE study. Eur J Cancer. 131:27–36. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shen G, Zheng F, Ren D, Du F, Dong Q, Wang
Z, Zhao F, Ahmad R and Zhao J: Anlotinib: A novel multi-targeting
tyrosine kinase inhibitor in clinical development. J Hematol Oncol.
11:1202018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Han B, Li K, Wang Q, Zhang L, Shi J, Wang
Z, Cheng Y, He J, Shi Y, Zhao Y, et al: Effect of anlotinib as a
third-line or further treatment on overall survival of patients
with advanced non-small cell lung cancer: The ALTER 0303 phase 3
Randomized clinical trial. JAMA Oncol. 4:1569–1575. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372:n712021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Higgins JP, Altman DG, Gøtzsche PC, Jüni
P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, et
al: The cochrane collaboration's tool for assessing risk of bias in
randomised trials. BMJ. 343:d59282011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tierney JF, Stewart LA, Ghersi D, Burdett
S and Sydes MR: Practical methods for incorporating summary
time-to-event data into meta-analysis. Trials. 8:162007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Borenstein M, Hedges LV, Higgins JP and
Rothstein HR: A basic introduction to fixed-effect and
random-effects models for meta-analysis. Res Synth Methods.
1:97–111. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen B, Sun J, Cai W and Chen J: Efficacy
of anlotinib as a third-line therapy for non-small cell lung
cancer. Chin J Clin Oncol Rehabil. 28:558–560. 2021.
|
|
20
|
Dai X, Wang Y, Han L, Liu Y and Li R:
Clinical emcacy of AIllotinib in the treatment of advanced
non-small cell lung cancer. Chin Med Herald. 16:95–98. 2019.
|
|
21
|
Feng Y, Yao F, Wang W, Guo J and Zhu Z:
Short-term curative effect of anlotinib on non-small cell lung
cancer and its influences on CTC and VGEF levels in peripheral
blood and quality of life. J Clin Exp Med. 19:2399–2403. 2020.
|
|
22
|
Gou F, Yu D, Qiao Q and Zhou X: Clinical
observation of anlotinib in the treatment of advanced lung
adenocarcinoma with targeted therapy. Anti-Tumor Pharm. 10:343–348.
2020.
|
|
23
|
Han B, Kan N and Wang S: Explore the
clinical efficacy and short-term prognosis of Anlotinib in the
third-line treatment of advanced non-small cell lung cancer
(NSCLC). Health Guide. 89–90. 2021.(In Chinese).
|
|
24
|
Han B, Li K, Zhao Y, Li B, Cheng Y, Zhou
J, Lu Y, Shi Y, Wang Z, Jiang L, et al: Anlotinib as a third-line
therapy in patients with refractory advanced non-small-cell lung
cancer: A multicentre, randomised phase II trial (ALTER0302). Br J
Cancer. 118:654–661. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang P, Li W and Zhang H: Effect of
anlotinib capsule on the level of VEGF and survival period in
advanced non-small cell lung cancer. J Pract Cancer. 35:360–362.
2020.
|
|
26
|
Kong Q: Clinical effect of antinib in the
thirdline treatment of nonsmall cell lung cancer and its clinical
significance security analysis. Chin J Mod Drug Appl. 14:164–166.
2020.
|
|
27
|
Liu Q, Li J, Wang K and Zhang Y: Clinical
efficacy of antinib in the treatment of patients with advanced lung
cancer after operation. Mod Diagn Treat. 31:2602–2604. 2020.
|
|
28
|
Luo K and Yu H: Clinical observation on
the treatment of advanced non-small cell lung cancer with Anrotinib
III. Healthmust-Readmagazine. 7:452021.(In Chinese).
|
|
29
|
Si XY, Wang HP, Zhang XT, Wang MZ and
Zhang L: Efficacy and safety of anlotinib in 16 patients with
advanced non-small cell lung cancer. Zhonghua Nei Ke Za Zhi.
57:830–834. 2018.(In Chinese). PubMed/NCBI
|
|
30
|
Sun X: Clinical efficacy and safety
analysis of third-line treatment with enrotinib for advanced
non-small cell lung cancer. Oriental Med Diet. 28:1172021.
|
|
31
|
Tian T, He M, Wu F, Lu Y and Liu Y:
Short-term efficacy of anlotinib in the treatment of non-small cell
lung cancer and its influence on serum tumor markers CTC VGEF and
side effects. J Hebei Nat Sci. 27:1908–1912. 2021.
|
|
32
|
Wang C: The efficacy of Anlotinib capsule
in the treatment of advanced non-small cell lung cancer. Chin Heal
Standard Management. 11:75762020.(In Chinese).
|
|
33
|
Yang Y: The Efficacy of Anlotinib as a
thirdor furtherline treatment in Patients with Advanced NSCLC.
Master's Thesis. Zhengzhou University; 2018, (In Chinese).
|
|
34
|
Yu C and Liu L: Observation on the effect
of third-line Anrotinib on advanced non-small cell lung cancer.
Medical Innovation of China. 18:69–72. 2021.(In Chinese).
|
|
35
|
Zhu Y: Short-term efficacy and life of
anlotinib thirdline and above in the treatment of advanced nonsmall
cell lung cancer. Healthmust-Readmagazine. 24:982021.(In
Chinese).
|
|
36
|
Nagano T, Tachihara M and Nishimura Y:
Molecular mechanisms and targeted therapies including immunotherapy
for non-small cell lung cancer. Current Cancer Drug Targets.
19:595–630. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhao T, Wang X, Xu T, Xu X and Liu Z:
Bevacizumab significantly increases the risks of hypertension and
proteinuria in cancer patients: A systematic review and
comprehensive meta-analysis. Oncotarget. 8:51492–51506. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Syed YY: Anlotinib: First global approval.
Drugs. 78:1057–1062. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xie C, Wan X, Quan H, Zheng M, Fu L, Li Y
and Lou L: Preclinical characterization of anlotinib, a highly
potent and selective vascular endothelial growth factor receptor-2
inhibitor. Cancer Sci. 109:1207–1219. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
He C, Wu T and Hao Y: Anlotinib induces
hepatocellular carcinoma apoptosis and inhibits proliferation via
Erk and Akt pathway. Biochem Biophys Res Commun. 503:3093–3099.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yu G, Shen Y, Xu X and Zhong F: Anlotinib
for refractory advanced non-small-cell lung cancer: A systematic
review and meta-analysis. PLoS One. 15:e02429822020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Abdel-Qadir H, Ethier JL, Lee DS,
Thavendiranathan P and Amir E: Cardiovascular toxicity of
angiogenesis inhibitors in treatment of malignancy: A systematic
review and meta-analysis. Cancer Treat Rev. 53:120–127. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ogawa C, Morita M, Omura A, Noda T, Kubo
A, Matsunaka T, Tamaki H, Shibatoge M, Tsutsui A, Senoh T, et al:
Hand-foot syndrome and post-progression treatment are the good
predictors of better survival in advanced hepatocellular carcinoma
treated with sorafenib: A multicenter study. Oncology. 93 (Suppl
1):S113–S119. 2017. View Article : Google Scholar
|