Introduction
Lung cancer is the first and second most common
cause of cancer morbidity among males and females in China,
respectively, and is also a leading cause of cancer-related
mortality worldwide (1,2). Lung adenocarcinoma (LUAD) is the most
frequent subtype of lung cancer, and its incidence has been
increasing in recent years (3).
Current treatments for LUAD include surgical resection,
radiotherapy, chemotherapy, targeted therapy and immunotherapy
(4). Although multimodal therapies
have been used to treat LUAD, survival outcomes remain
unsatisfactory, ranging from 15 to 20% (2). Surgery and drugs used for
chemotherapy can lead to complications, such as infections
(Staphylococcus aureus, Escherichia coli, Streptococcus spp
and Fusarium spp), which can become severe, and even fatal
infections of the surgical site while in the hospital environment
(5–7). Therefore, there is an urgent need to
explore potential molecular biomarkers that can help determine
patient prognosis and be used to prescribe effective treatments for
LUAD.
Holliday junction-recognizing protein (HJURP) is a
protein that has recently been shown to be required for centromere
protein-A (CENP-A) loading in the centromeric chromatin and for the
assembly of functional kinetochores (8–10).
In humans, HJURP has been demonstrated to be a critical regulator
of DNA binding and phosphorylation, and is involved in the
regulation of chromosomal segregation and cell division (11,12).
Emerging evidence has revealed that HJURP expression is
significantly upregulated following DNA damage, that it
collaborates with components of the DNA repair machinery and that
it plays a role in homologous recombination (10,13).
In addition, the upregulation of HJURP, which has now been reported
in hepatocellular carcinoma, glioblastoma, breast cancer and
ovarian carcinoma, has been correlated with a poor prognosis
(14–17). However, there remains limited
understanding as to whether HJURP expression can act as a
prognostic biomarker for LUAD, despite continuing reports of the
role HJURP plays in carcinogenesis.
Thus, the objective of the current study was to
evaluate the prognostic value of HJURP expression in cases of human
LUAD, based on data obtained from The Cancer Genome Atlas (TCGA).
To gain further insights into the biological pathways involved in
LUAD pathogenesis related to the HJURP regulatory network, gene set
enrichment analysis (GSEA) was also performed.
Materials and methods
Cell lines
The human LUAD cell line, H1299, and the normal
bronchial epithelial BEAS-2B cell line, were purchased from the
Cell Bank of the Chinese Academy of Sciences. H1299 cells were
maintained in RPMI-1640 medium supplemented with 10% fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and maintained
at 37°C in an incubator containing 5% CO2, with 100 U/ml
penicillin and 100 mg/ml streptomycin. BEAS-2B cells were
maintained in Dulbecco's modified Eagle's medium (DMEM) (Gibco;
Thermo Fisher Scientific, Inc.) supplemented with 10% FBS and
maintained at 37°C in an incubator containing 5% CO2,
with 100 U/ml penicillin and 100 mg/ml streptomycin.
Western blotting
H1299 and BEAS-2B cells were lysed in RIPA buffer
(Beyotime Institute of Biotechnology). Protein concentrations were
then determined using a BCA Protein assay kit (Beyotime Institute
of Biotechnology). The protein samples (25 µg/sample) were
subjected to SDS-polyacrylamide gel electrophoresis (SDS-PAGE) on a
12% gel before being transferred to polyvinylidene difluoride
membranes. To block non-specific protein binding, the membranes
were incubated in 5% BSA for 1.5 h at room temperature with gentle
agitation. Next, the membranes were incubated with GAPDH (1:1,000;
cat. no. ab8245; Abcam) and HJURP (1:2,000; cat. no. ab233541;
Abcam) antibodies at 4°C for 15–18 h, washed three times with TBST
(Tris-buffered saline containing 0.1% Tween-20), and incubated with
HRP-conjugated secondary antibodies [1:1,000; cat. nos. A0208 (goat
anti-rabbit IgG) and A0216 (goat anti-mouse IgG); Beyotime
Institute of Biotechnology] for 1 h at room temperature. Finally,
the membranes were washed again with TBST and BeyoECL Plus [Ultra
Sensitive ECL Chemiluminescence kit (cat. no. P0018S; Beyotime
Institute of Biotechnolog]) was used to visualize the protein bands
on a ChemiDoc Touch (Bio-Rad Laboratories, Inc.). The bands were
quantified using Quantity One 1-D analysis software version 4.6.8
(Bio-Rad Laboratories, Inc.) (18). GAPDH immunoreactivity was used as
the loading control for each protein.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from cells using
TRIzol® (Thermo Fisher Scientific, Inc.) according to
the manufacturer's instructions. Using the PrimeScript RT reagent
kit (Takara Bio, Inc.), RNA was reverse-transcribed to synthesize
first-strand cDNA, which was then quantified using an SYBR Premix
Ex Taq kit (Takara Bio, Inc.), according to the manufacturer's
instructions. Primers used in this study were as follows: HJURP
forward, 5′-AGTGCCTTTATGTATTGGAG-3′ and reverse,
5′-AAGTGAGGGTCTGGATTTA-3′; and GAPDH forward,
5′-GAACATCATCCCTGCCTCTACT-3′ and reverse,
5′-ATTTGGCAGGTTTTTCTAGACG-3′. qPCR was performed with the following
thermocycling conditions: 95°C for 15 min, followed by 40 cycles of
95°C for 10 sec, 60°C for 20 sec and 72°C for 10 sec. Fluorescence
was detected using a Corbett Research RG-6000 Real-Time PCR Machine
(Corbett Life Science, Sydney, NSW, Australia). Each sample was run
in triplicate and was compared with GAPDH as the internal control.
Results were obtained using the 2−ΔΔCq method (19).
Collection of publicly available data
from TCGA and Gene Expression Omnibus (GEO) databases
Gene expression (HJURP) profiling data of 519 LUAD
samples and 54 normal tissue samples were downloaded from the
publicly available TCGA database (https://gdc.cancer.gov/). Another transcriptome
profiling dataset, GSE116959 (20), of 57 LUAD samples and 11
peritumoral normal lung tissue samples was obtained from the NCBI
GEO database (https://www.ncbi.nlm.nih.gov/geo/) to verify the
expression level of HJURP in LUAD cases. Log2FC>2
indicates that gene expression in tumor samples is upregulated 4
times compared with that of adjacent samples,
log2FC<-2 indicates that gene expression in tumor
samples is downregulated 4 times compared with that of adjacent
samples. HJURP protein expression profiling data were obtained from
the UALCAN database (http://ualcan.path.uab.edu/index.html). Relevant
clinicopathological information, including age, sex, T stage, N
status, M grade, stage (21) and
overall survival (OS) time were also extracted from the TCGA
database. A total of 480 patients with LUAD with complete follow-up
data were included, whose details were recorded prior to November
1, 2019. The clinical end point was OS, defined as the time from
surgery to death. In addition, patients who were alive at the last
follow-up were considered to be censored observations.
GSEA
GSEA is a computational method that determines
whether an a priori defined set of genes shows statistically
significant, concordant differences between two biological states
(22). In the present study, the
GSEA first generated an ordered list of all genes according to
their correlation with HJURP expression; tumor samples were divided
into high expression group and low expression group according to
the median value (4.3) of HJURP expression level. GSEA was then
performed to elucidate the significant survival differences
observed between the high and low expression HJURP groups. Gene set
permutations were performed 1,000 times for each analysis. The
level of HJURP expression was used as a phenotype label. The
nominal P-value and normalized enrichment score (NES) were used to
sort the pathways enriched in each phenotype.
Immune infiltration analysis
It is well known that interactions between a tumor
and the immune system play a crucial role in cancer initiation,
progression and response to treatment. The integrated repository
portal for tumor-immune system interactions (http://cis.hku.hk/TISIDB/index.php) (22) was used to examine tumor and immune
system interactions in 28 types of tumor-infiltrating lymphocytes
(TILs) seen across different human cancer types. The relative
abundance of TILs was inferred by using gene set variation analysis
based on the HJURP expression profile. Spearman's test was applied
to measure correlations between HJURP and TILs; P<0.05 was
considered to indicate a significant difference for all tests.
Statistical analyses
Scatter plots and paired plots were used to show
differences in HJURP expression between normal and tumor samples.
The cut-off value of HJURP expression was determined by the optimal
cutoff values determined by X-tile software (https://medicine.yale.edu/lab/rimm/research/software/).
The Wilcoxon rank-sum test or Kruskal-Wallis test was used to
assess the association between expression levels and
clinicopathological characteristics. The Kaplan-Meier method and
log-rank test were used to estimate associations between HJURP
expression and OS. Univariate and multivariable Cox proportional
hazards regression models were used to evaluate the impact of HJURP
expression on OS in the presence of other known risk factors.
Two-sided P<0.05 was considered to indicate a
statistically significant difference. All analyses were performed
using R (v.3.5.2; http://cran.r-project.org/bin/windows/base/old/3.5.2/).
Results
Patient characteristics
As shown in Table
1, 480 primary tumors with both clinical and gene expression
data were downloaded from the TCGA database during November 2019.
The study cohort included 221 (46.04%) males, with average patient
age being 66 years. In the cohort, the T-stage distribution of LUAD
was as follows: T1, 164 patients (34.17%); T2, 254 patients
(52.92%); T3, 40 patients (8.33%); and T4, 19 patients (3.96%). The
N status distribution of LUAD was as follows: N0, 310 patients
(64.58%); N1, 87 patients (18.13%); N2, 69 patients (14.38%); and
N3, 2 patients (0.42%). The cancer type distribution was as
follows: M0, 316 patients (65.83%) and M1, 25 patients (5.21%).
Stage I disease was found in 260 patients (54.16%), stage II in 107
patients (22.29%); stage III in 79 patients (16.46%); and stage IV
in 26 patients (5.42%). The median follow-up time for patients
alive at their last contact was 18.42 months (range, 0–227.07
months).
 | Table I.Characteristics of lung
adenocarcinoma patients from The Cancer Genome Atlas. |
Table I.
Characteristics of lung
adenocarcinoma patients from The Cancer Genome Atlas.
| Clinical
characteristic | Value |
|---|
| Median age at
diagnosis (range), years, | 66 (33–88) |
| Sex, n (%) |
|
|
Male | 221 (46.04) |
|
Female | 259 (53.96) |
| Clinical stage, n
(%) |
|
| I | 260 (54.17) |
| II | 107 (22.29) |
|
III | 79 (16.46) |
| IV | 26 (5.42) |
| NA | 8 (1.67) |
| Clinical T grade, n
(%) |
|
| T1 | 164 (34.17) |
| T2 | 254 (52.92) |
| T3 | 40 (8.33) |
| T4 | 19 (3.96) |
| NA | 3 (0.63) |
| Clinical N grade, n
(%) |
|
| N0 | 310 (64.58) |
| N1 | 87 (18.13) |
| N2 | 69 (14.38) |
| N3 | 2 (0.42) |
| NA | 12 (2.5) |
| Clinical M grade, n
(%) |
|
| M0 | 316 (65.83) |
| M1 | 25 (5.21) |
| NA | 139 (28.96) |
| Median follow-up
time | 18.42
(0-227.07) |
HJURP expression and its association
with clinicopathological variables
Compared with HJURP expression in normal lung
tissues (n=54), HJURP expression was significantly higher in LUAD
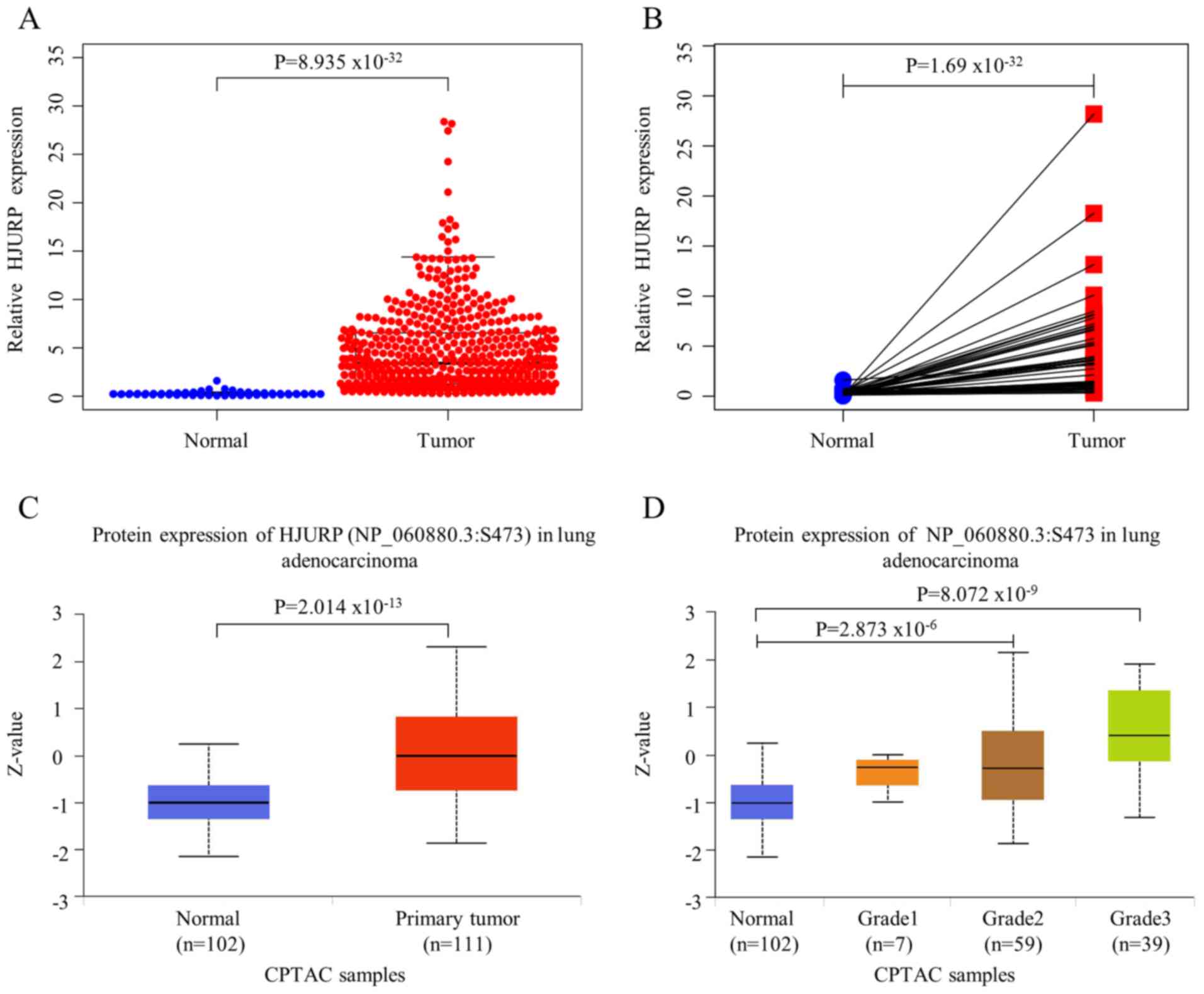
tissues (n=519) (P<0.05). The scatter plot (Fig. 1A) and paired plot (Fig. 1B) show the differences in HJURP
expression between normal and tumor samples. Expression profiling
data were also obtained from the GSE116959 dataset (including 57
LUAD samples and 11 normal lung tissue samples), after data
preprocessing and quality assessment using R software. According to
the cut-off criteria set (P<0.05 and |log2FC|>2.0), a total
of 329 differentially expressed genes were obtained, including 85
upregulated genes and 244 downregulated genes. To further
investigate HJURP protein expression in patients with LUAD, the
levels of HJURP proteomic expression were quantified in normal lung
tissues (n=102) and primary LUAD tissues (n=111). The results
showed that the expression of HJURP was significantly higher in
primary LUAD (median, 0) than in normal lung tissues (median,
−1.016) (P<0.01; Fig. 1C).
Significant differences were also found in HJURP protein expression
with regard to LUAD grades compared with the normal group: Grade 2
(median, −0.28; P<0.01) and grade 3 (median, 0.421; P<0.01).
However, the protein expression of HJURP may not be associated with
grade 1 LUAD (P=0.202) (Fig. 1D).
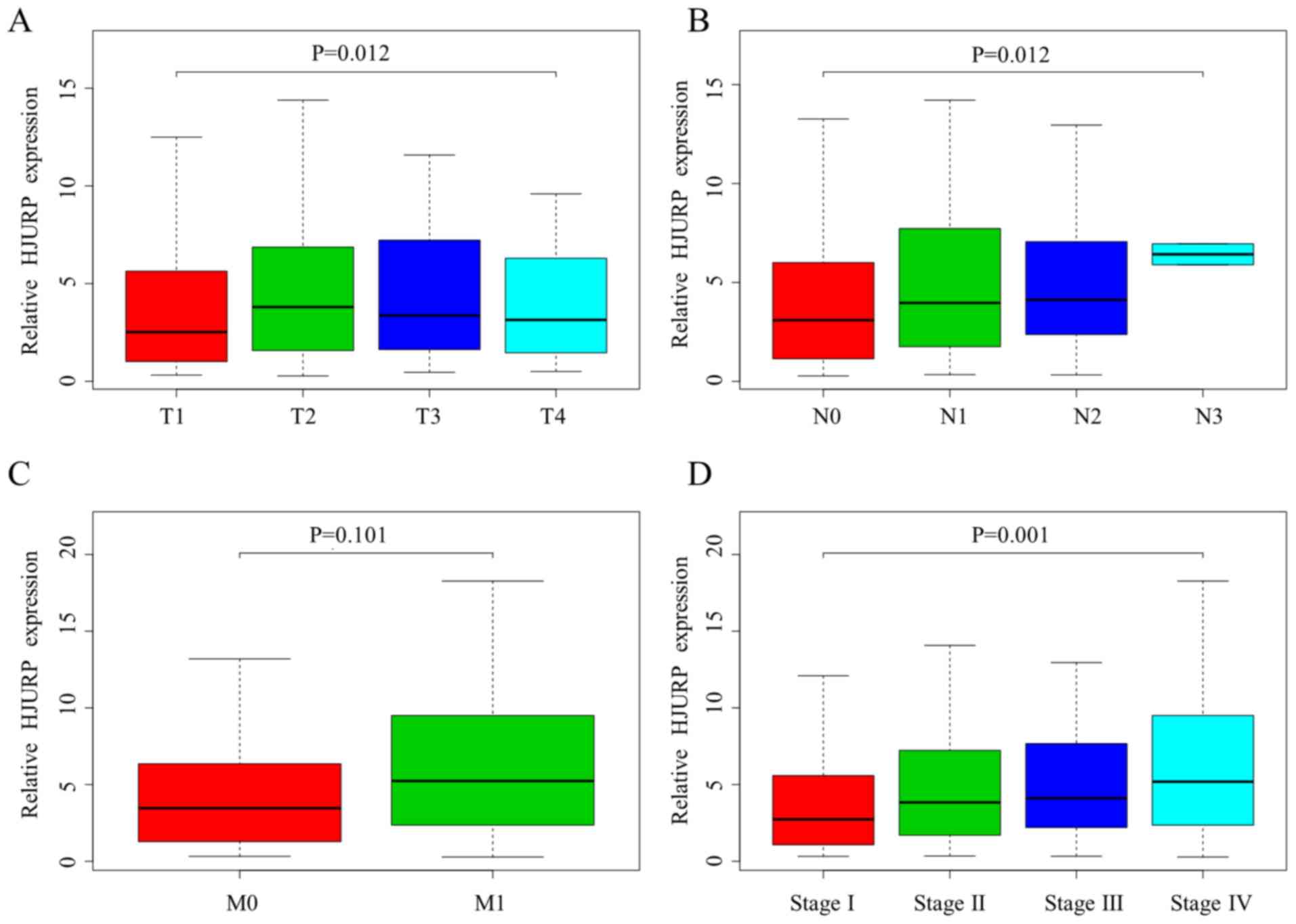
Significant differences in HJURP expression were observed with
regard to the T grade (P=0.012), N grade (P=0.012) and stage
(P=0.001) of LUAD (Fig. 2A, B and
D). However, the HJURP expression level was not associated with
M grade (P=0.101; Fig. 2C).
HJURP expression is associated with
the survival rate of patients with LUAD
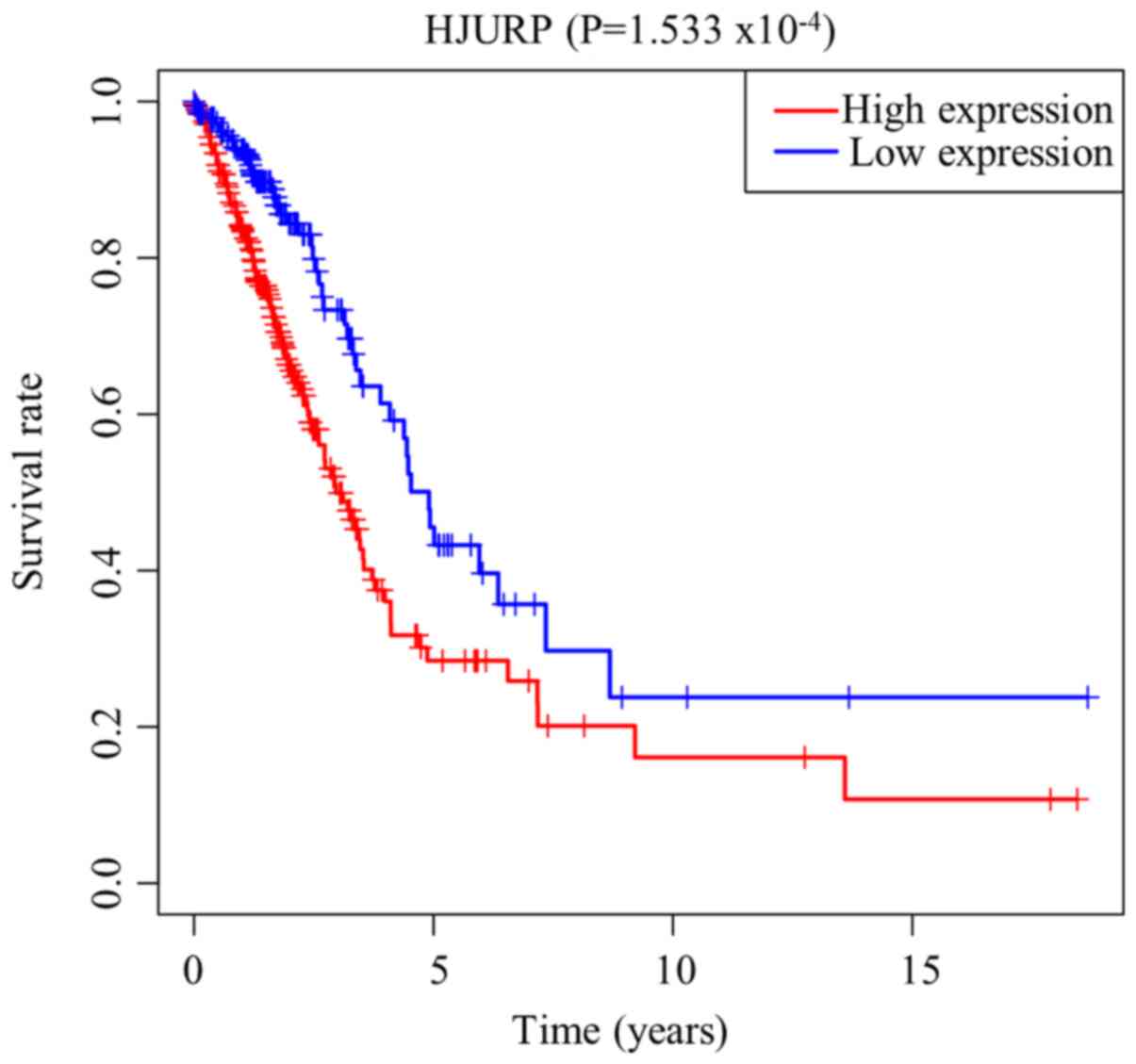
As presented in Fig.
3, Kaplan-Meier survival analysis showed that LUAD with high
expression of HJURP was associated with a worse prognosis compared
with LUAD with low expression of HJURP (P<0.001). The univariate
analysis revealed that high expression of HJURP was significantly
associated with a poor OS [hazard ratio (HR), 1.06; 95% confidence
interval (CI), 1.03-1.09; P<0.001]. Other clinicopathological
variables associated with poor survival included T grade, N grade
and stage (Table II). The
multivariate analysis showed that HJURP remained independently
associated with OS, with an HR of 1.32 (95% CI, 1.09-1.60;
P=0.004), along with stage (HR, 1.90; 95% CI, 1.19-3.03;
P=0.007).
 | Table II.Associations between overall survival
time and clinicopathological characteristics in patients from The
Cancer Genome Atlas according to univariate and multivariate Cox
regression analysis. |
Table II.
Associations between overall survival
time and clinicopathological characteristics in patients from The
Cancer Genome Atlas according to univariate and multivariate Cox
regression analysis.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Parameter | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age (years) | 1.00 | 0.99-1.02 | 0.686 | 1.02 | 0.10-1.04 | 0.128 |
| Sex (male vs.
female) | 1.03 | 0.72-1.48 | 0.866 | 0.89 | 0.62-1.29 | 0.554 |
| Stage (III–IV vs.
I–II) | 1.64 | 1.40-1.94 | ≤0.001 | 1.90 | 1.19-3.03 | 0.007 |
| T grade (T3-T4 vs.
T1-T2) | 1.65 | 1.33-2.04 | ≤0.001 | 1.22 | 0.96-1.55 | 0.100 |
| M grade (M1 vs.
M0) | 1.67 | 0.92-3.05 | 0.092 | 0.41 | 0.12-1.39 | 0.152 |
| N grade (N1+N2+N3
vs. N0) | 1.79 | 1.47-2.20 | ≤0.001 | 0.97 | 0.65-1.44 | 0.864 |
| HJURP expression
(high vs. low) | 1.06 | 1.03-1.09 | ≤0.001 | 1.32 | 1.09-1.60 | 0.004 |
Molecular mechanisms of HJURP in
LUAD
To identify signaling pathways that are
differentially activated in LUAD, GSEA was conducted between low
and high HJURP expression data sets. GSEA was used to identify
significant differences in signaling pathways from the MSigDB
Collection (c2.cp.kegg.v7.4.symbols.gmt). False discovery rate
<0.05 and nominal P<0.05 were used as thresholds to determine
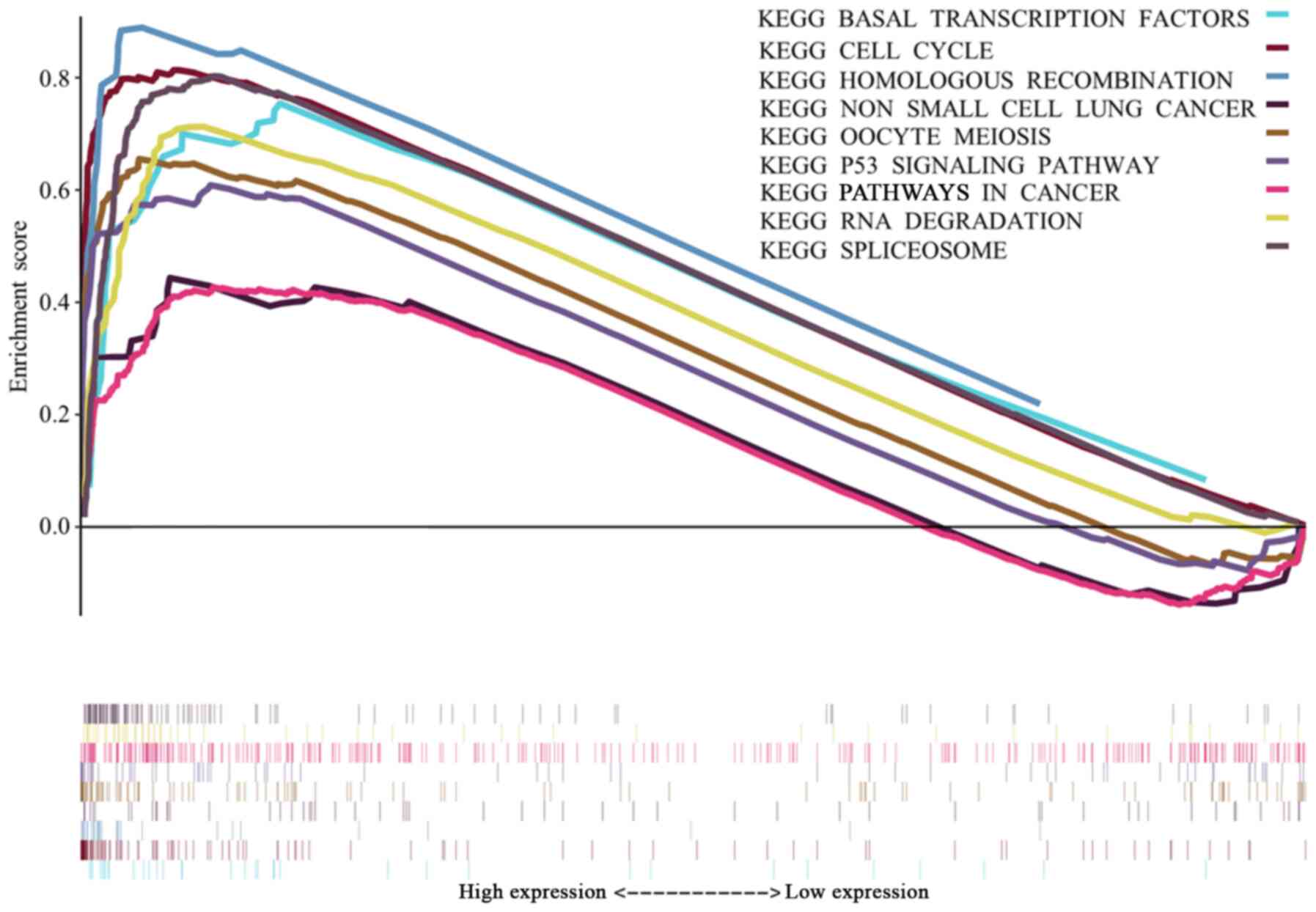
significantly enriched signaling pathways. The most significantly
enriched signaling pathways were selected based on their NES
(Fig. 4). Fig. 4 shows that ‘basal transcription
factors’, the ‘cell cycle’, ‘homologous recombination’, ‘non-small
cell lung cancer’ (NSCLC), ‘oocyte meiosis’, the ‘p53 signaling
pathway’, ‘pathways in cancer’, ‘RNA degradation’ and ‘spliceosome’
are differentially enriched in the high HJURP expression
phenotype.
Correlation of HJURP expression with
immune infiltration level
After identifying the HJURP-related signaling
pathways, a correlation analysis was performed to explore the
relationship between HJURP expression and immune infiltration level
in patients with LUAD. Significant correlations were found between
HJURP expression and 28 types of TILs across different human cancer
types (Fig. 5A). Significant
results were found for HJURP expression with the abundance of
activated CD4 T cells (ρ=0.591; P<0.001) were notably
correlated.
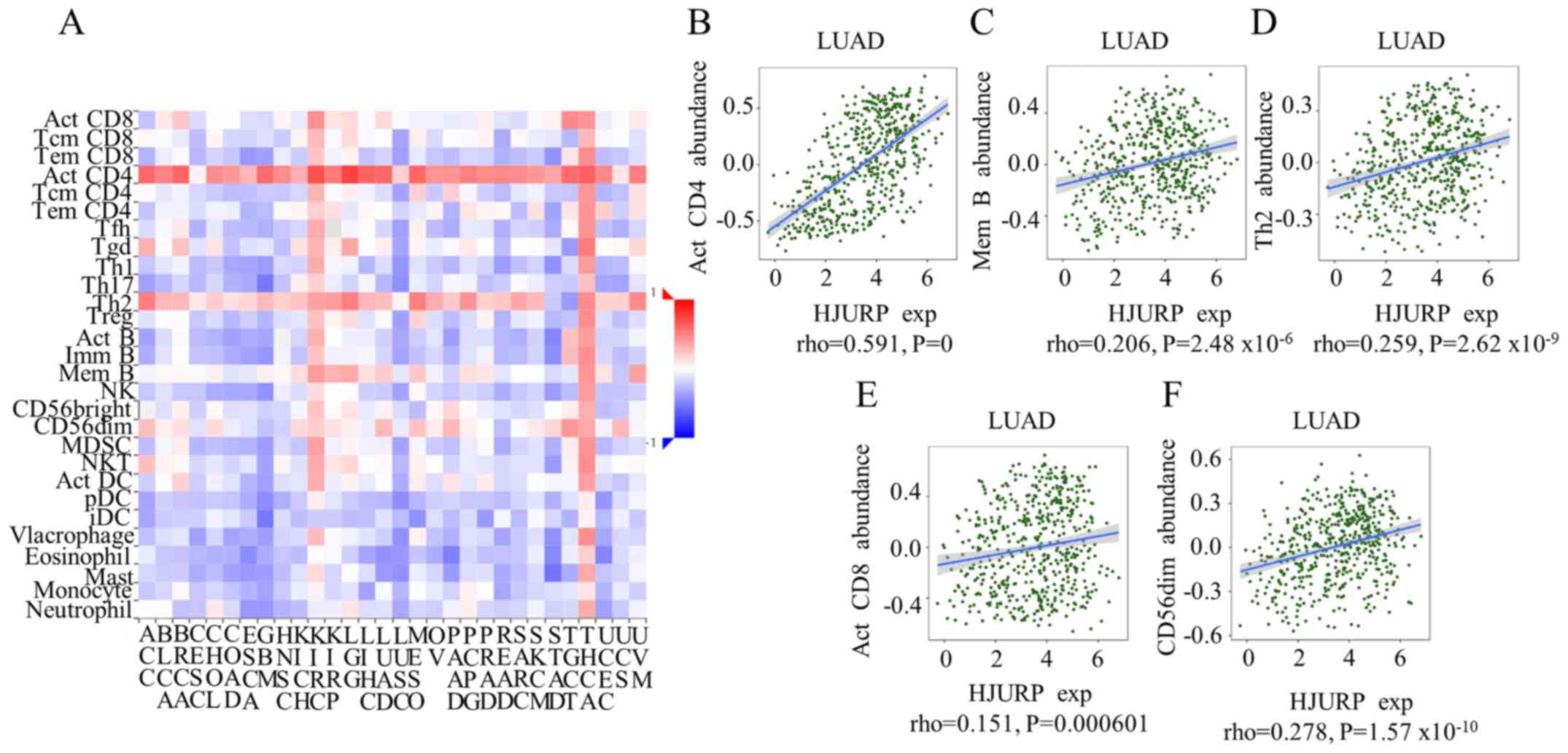 | Figure 5.Spearman correlations between the
expression of HJURP and TILs across different human cancer types.
(A) Relationships between the expression of HJURP and 28 types of
TILs across different human cancer types. (B-F) Significant results
were found for HJURP expression with regard to the abundance of
memory B cells, type 2 T-helper cells, activated CD8 T cells, and
CD56(dim) natural killer cells; however, only the abundance of
activated CD4 T cells was notably correlated. HJURP, Holliday
junction-recognizing protein; TILs, tumor-infiltrating lymphocytes;
LUAD, lung adenocarcinoma; Act, activated; Mem B, memory B cells;
Th2, type 2 T-helper cells; exp, expression. |
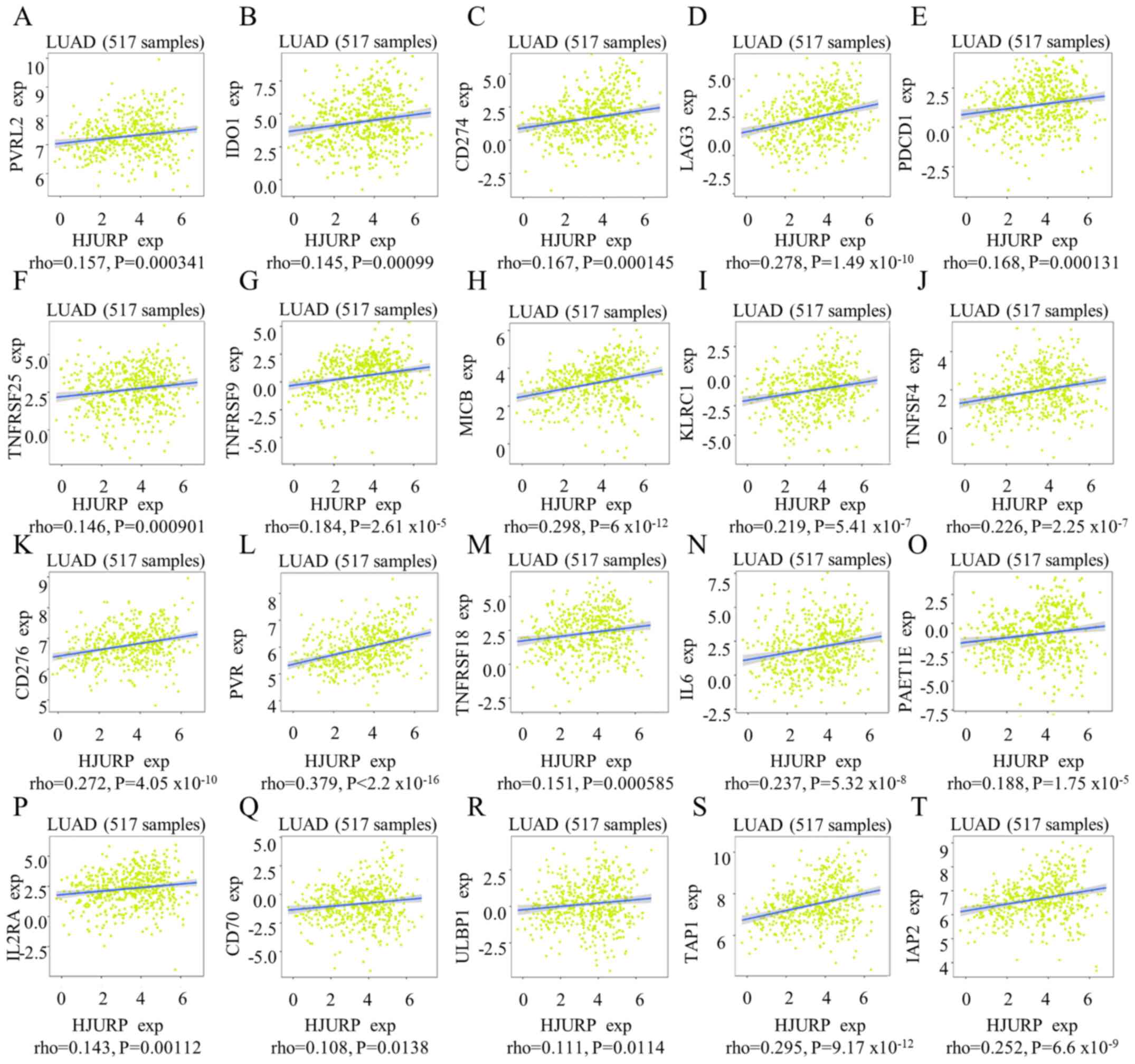
The relationships between three types of
immunomodulators, immune-inhibitors (Fig. 6A-E), immunostimulators (Fig. 6F-R) and major histocompatibility
complex molecules (Fig. 6S and T),
and the expression of HJURP were examined. Significant results were
observed using Spearman's correlation test (Fig. 6); however, only HJURP expression
and poliovirus receptor (an immunostimulator) exhibited a
coefficient indicating that the variables were notably
correlated.
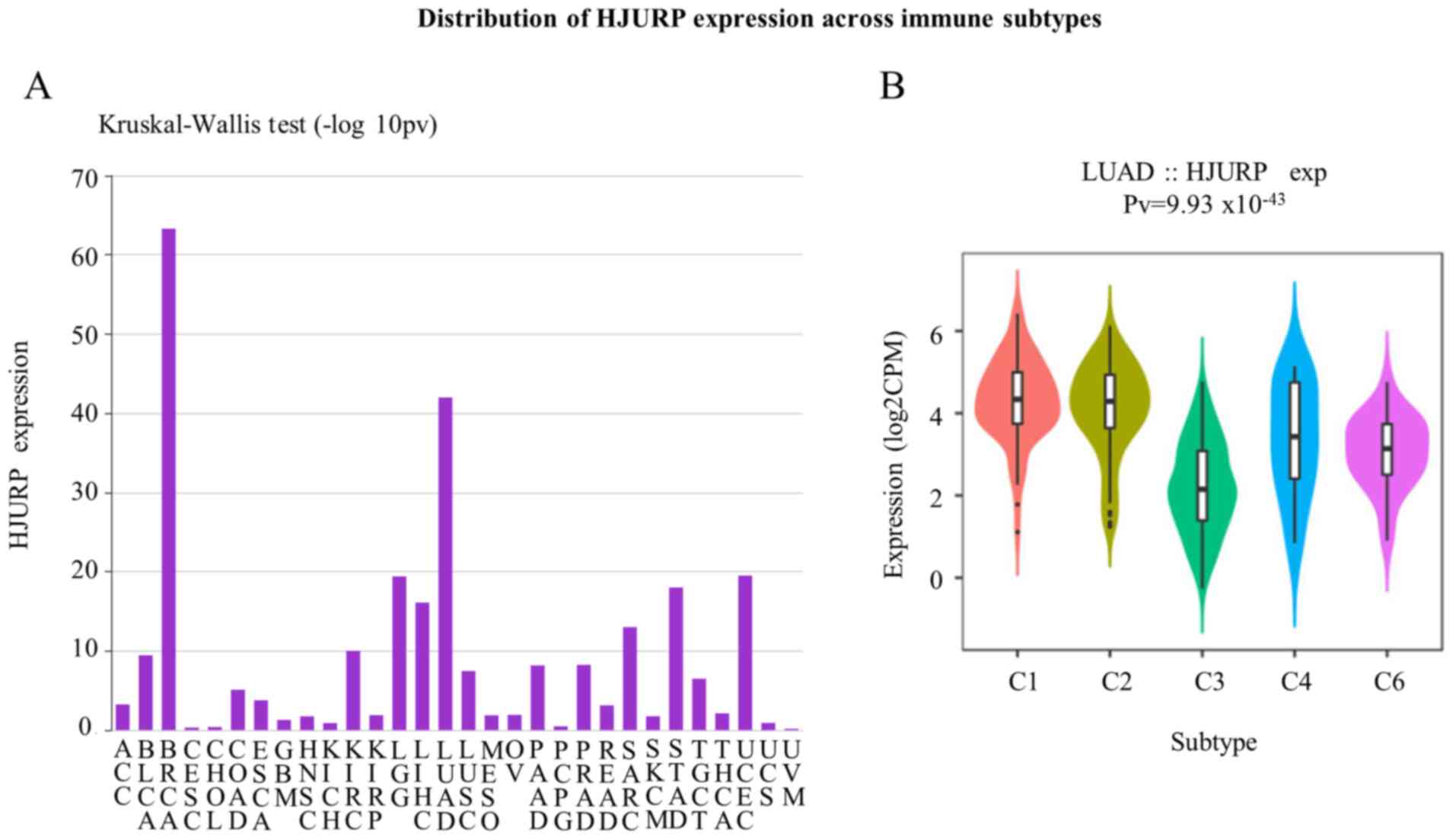
The distribution of HJURP expression across immune
and molecular subtypes was also explored. Fig. 7A shows the associations between
HJURP expression and immune subtypes across different human cancer
types. The violin plot shows the LUAD distribution across the
following subtypes: C1, wound healing; C2, IFN-γ dominant; C3,
inflammatory; C4, lymphocyte-depleted; C5, immunologically quiet;
and C6, TGF-β dominant (Fig. 7B).
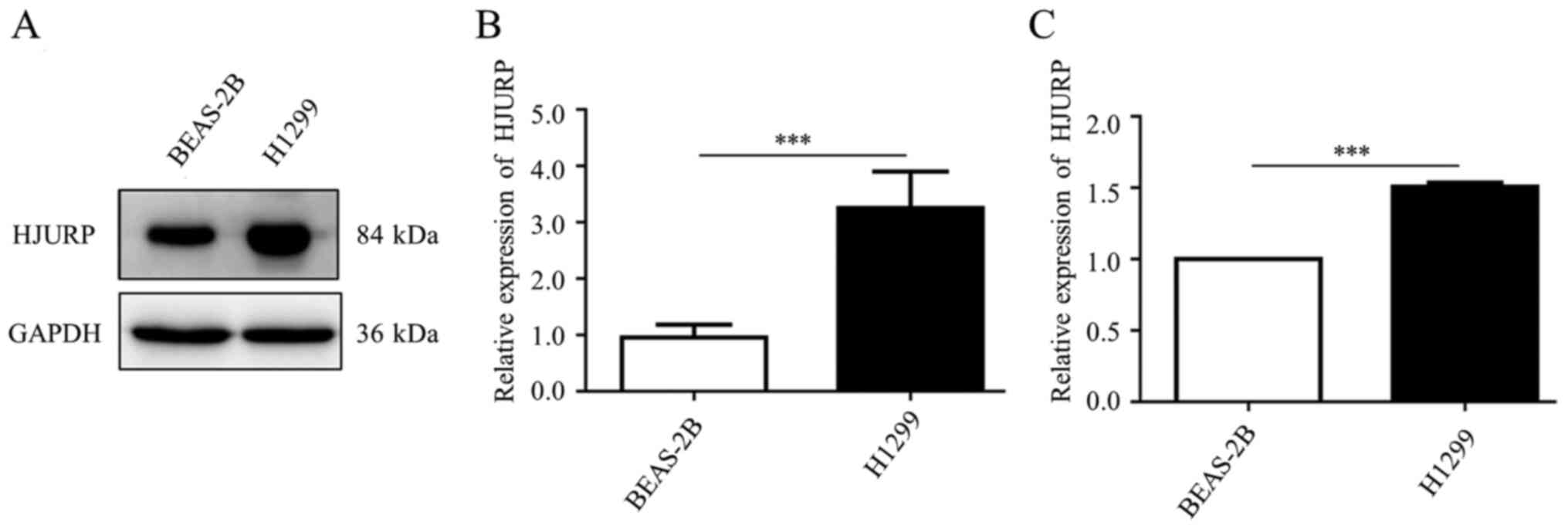
In addition, western blotting and qPCR results from a LUAD cell
line (H1299) and a normal bronchial epithelial cell line (BEAS-2B)
confirmed that HJURP was significantly increased in NSCLC (Fig. 8A-C). The research design of the
study is shown in Fig. 9.
Discussion
In the present study, an RNA sequencing dataset of
HJURP and relevant clinical parameters of 480 patients with LUAD
from the TCGA database were analyzed. The study found that the high
expression of HJURP could be considered to be an independent
prognostic factor in patients with LUAD, regardless of other
clinicopathological variables. HJURP may be a potentially useful
prognostic molecular biomarker of poor survival in LUAD cases.
Further experiments should be performed to elucidate the biological
effects of HJURP.
An increasing number of studies have found that
HJURP may be exploited as a potentially effective biomarker in the
diagnosis of and determination of progression and prognosis of
cancer (14–17). Hu et al (14) identified that high levels of HJURP
expression could predict a poorer prognosis in hepatocellular
carcinoma (HCC) and may promote HCC progression by accelerating HCC
cell proliferation. A study by Valente et al (15) found that HJURP plays an important
role in the maintenance of extremely proliferative cells of
high-grade gliomas and pointed to HJURP as a potential therapeutic
target for the development of novel treatments for patients with
glioma. Montes de Oca et al (16) identified HJURP as the first
biomarker that can be used to differentiate good and poor prognoses
in patients with luminal A breast cancer; the study also noted that
HJURP can support the integration of selected chromatin regulators
in the clinical setting to help guide treatment plans and improve
the overall management of patients with breast cancer. Recently, Li
et al (17) revealed that
increased expression of HJURP could act as an independent negative
prognostic biomarker for patients with advanced serous ovarian
cancer. These studies suggested that HJURP has potentially useful
clinical implications in improving prognostic predictions for
cancer. However, there remains a limited understanding on whether
HJURP expression is a prognostic biomarker in LUAD.
In the present study, bioinformatics analysis using
high-throughput RNA-sequencing data from TCGA demonstrated that the
upregulation of HJURP in LUAD was associated with advanced
clinicopathological characteristics (T grade, N grade and stage),
survival time and a poor prognosis. To further investigate the
functions of HJURP in LUAD, GSEA was performed using TCGA data.
This GSEA showed that ‘basal transcription factors’, the ‘cell
cycle’, ‘homologous recombination’, ‘non-small cell lung cancer’,
‘oocyte meiosis’, the ‘p53 signaling pathway’, ‘pathways in
cancer’, ‘RNA degradation’ and ‘spliceosome’ are enriched in the
high HJURP expression phenotype. This suggests that HJURP may serve
as a potential biomarker of prognosis and a therapeutic target in
LUAD.
The present findings are in agreement with those
previously reported for HJURP upregulation in lung tumors compared
with HJURP expression in normal lung tissue samples (23), as well as with the increased HJURP
levels seen in plasma sediments from patients with lung cancer
(24). Zhou et al (25) focused on plasma mRNA as a novel
non-invasive biomarker for diagnosing lung cancer. Blood specimens
were collected from 47 patients with primary lung cancer and 14
healthy individuals. Circulating HJURP and ADAMTS8 mRNAs with
superior sensitivity and specificity were revealed, and these
molecules were proposed as promising non-invasive biomarkers for
the diagnosis of lung cancer. Recently, Wei et al (24) confirmed that the increased
expression of HJURP was associated with advanced stage and a poor
prognosis, based on a small sample size of 74 patients with NSCLC.
Additionally, the study provided clues regarding the role of HJURP
as a tumor promoter in NSCLC via the activation of the
Wnt/β-catenin pathway.
In the present study, using GSEA, it was observed
that the HJURP high expression phenotype was associated with ‘basal
transcription factors’, the ‘cell cycle’, ‘homologous
recombination’, ‘non-small cell lung cancer’, ‘oocyte meiosis’, the
‘p53 signaling pathway’, ‘pathways in cancer’, ‘RNA degradation’
and ‘spliceosome’. Significant correlations were also found between
HJURP expression and immunomodulators, immune subtype and several
tumor-infiltrating immune cells, such as activated CD4 T cells.
There have been many reports on the molecular genetic alterations
of p53 in lung cancer. Dhieb et al (26) found that abnormal immunostaining of
p53 was detected in 56.16% of patients with LUAD. Abnormal p53
expression was slightly increased in European compared with Asian
populations. It has been reported that lung cancer is strongly
influenced by mutations of p53 (27). The role played by immune
infiltration in LUAD has been highlighted by certain studies. Varn
et al (28) determined that
naive B-cell and CD8+ T-cell infiltration was associated
with prolonged prognosis, while myeloid cell infiltration was
associated with shorter survival times. Wang et al (29) found that increased TTC21A
expression was correlated with an increased proportion of immune
cells, such as B cells, neutrophils, mast cells and T cells, in
patients with LUAD. Previous studies have also found that the
expression levels of HJURP mRNA are linked with the regulation of
the cell cycle (11,14). Several proteins have been reported
to interact with HJURP, including proteins affecting HJURP function
and downstream proteins regulated by HJURP. The most well-known
molecule regulated by HJURP is the histone H3 variant,
centromere-specific protein (CENP)-A. The cooperation between
CENP-A and its chaperon HJURP mediates a normal cell cycle, whereas
ectopic activation of HJURP is involved in the chromosomal
stability and immortality of cancer cells (11). The associations between HJURP
expression and ‘basal transcription factors’, ‘homologous
recombination’, ‘oocyte meiosis’, ‘RNA degradation’ and
‘spliceosomes’ were the first noted in the present study, although
the regulatory mechanisms remain to be further elucidated.
The present study found that the expression of HJURP
was significantly increased in patients with LUAD and associated
with several clinical features and immune infiltrations. HJURP may
be a potentially useful prognostic molecular biomarker of poor
survival in LUAD cases. The bioinformatics results were confirmed
with RT-qPCR and western blotting analyses in the normal bronchial
epithelium (BEAS-2B) and human NSCLC (H1299) cell lines. HJURP mRNA
and protein levels were significantly increased in the H1299 cells
compared with the levels in the BEAS-2B cells. These findings were
consistent with those of a previous study (24) and also demonstrated that higher
expression of HJURP was associated with advanced stage, distant
metastasis and a poor prognosis in cases of NSCLC. Similarly,
higher HJURP levels may be associated with early stage lung cancer
(11,25), and HJURP activation seems to play a
pivotal role in the immortality of cancer cells (11). Therefore, higher HJURP levels
promote a poor prognosis in NSCLC; a precise mechanism for this
showing that HJURP promotes tumor cell proliferation, migration and
invasion through the Wnt/β-catenin signaling pathway has been
reported (24), and the present
study has provided further support for this mechanism.
In conclusion, the present study demonstrated that
high levels of HJURP expression are correlated with a poor
prognosis in patients with LUAD. HJURP may be a promising
therapeutic target for the development of anticancer drugs and may
also act as a biomarker for LUAD diagnosis.
Acknowledgements
Not applicable.
Funding
This study was supported by Scientific Research Start Plan of
Shunde Hospital, Southern Medical University (grant no.
SRSP2019013) and The National Natural Scientific Foundation of
China (grant no. 81770148).
Availability of data and materials
The dataset analyzed during the present study can be
downloaded from TCGA (https://cancergenome.nih.gov/), GEO (https://www.ncbi.nlm.nih.gov/geo/), UALCAN
(http://ualcan.path.uab.edu/index.html) and TISIDB
(http://cis.hku.hk/TISIDB/index.php)
databases. The remaining data (including PCR and WB experiments)
are available from the corresponding author on reasonable
request.
Authors' contributions
LC and JY designed the experiments and wrote the
manuscript. LC and CZe performed the experiments. LY and WL
analyzed and interpreted the results. LC performed the statistical
analysis. LC, CZe and CZh assembled the data. JY contributed to
every process as a supervisor. LC, CZe, WL, LY, CZh and JY confirm
the authenticity of all the raw data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CENP-A
|
centromere protein-A
|
|
GSEA
|
gene set enrichment analysis
|
|
HCC
|
hepatocellular carcinoma
|
|
HJURP
|
Holliday junction-recognizing
protein
|
|
LUAD
|
lung adenocarcinoma
|
|
NES
|
normalized enrichment score
|
|
NSCLC
|
non-small cell lung cancer
|
|
OS
|
overall survival
|
|
TIL
|
tumor-infiltrating lymphocyte
|
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shi J, Hua X, Zhu B, Ravichandran S, Wang
M, Nguyen C, Brodie SA, Palleschi A, Alloisio M, Pariscenti G, et
al: Somatic genomics and clinical features of lung adenocarcinoma:
A retrospective study. PLoS Med. 13:e10021622016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shah DR and Masters GA: Precision medicine
in lung cancer treatment. Surg Oncol Clin N Am. 29:15–21. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ungureanu A, Zlatian O, Mitroi G, Drocaş
A, Ţîrcă T, Călina D, Dehelean C, Docea AO, Izotov BN, Rakitskii
VN, et al: Staphylococcus aureus colonisation in patients
from a primary regional hospital. Mol Med Rep. 16:8771–8780. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zlatian O, Balasoiu AT, Balasoiu M,
Cristea O, Docea AO, Mitrut R, Spandidos DA, Tsatsakis AM, Bancescu
G and Calina D: Antimicrobial resistance in bacterial pathogens
among hospitalised patients with severe invasive infections. Exp
Ther Med. 16:4499–4510. 2018.PubMed/NCBI
|
|
7
|
Tanase A, Colita A, Ianosi G, Neagoe D,
Branisteanu DE, Calina D, Docea AO, Tsatsakis A and Ianosi SL: Rare
case of disseminated fusariosis in a young patient with graft vs.
host disease following an allogeneic transplant. Exp Ther Med.
12:2078–2082. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Foltz DR, Jansen LE, Bailey AO, Yates JR
3rd, Bassett EA, Wood S, Black BE and Cleveland DW:
Centromere-specific assembly of CENP-a nucleosomes is mediated by
HJURP. Cell. 137:472–484. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dunleavy EM, Roche D, Tagami H, Lacoste N,
Ray-Gallet D, Nakamura Y, Daigo Y, Nakatani Y and
Almouzni-Pettinotti G: HJURP is a cell-cycle-dependent maintenance
and deposition factor of CENP-A at centromeres. Cell. 137:485–497.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Barnhart MC, Kuich PH, Stellfox ME, Ward
JA, Bassett EA, Black BE and Foltz DR: HJURP is a CENP-A chromatin
assembly factor sufficient to form a functional de novo
kinetochore. J Cell Biol. 194:229–243. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kato T, Sato N, Hayama S, Yamabuki T, Ito
T, Miyamoto M, Kondo S, Nakamura Y and Daigo Y: Activation of
Holliday junction recognizing protein involved in the chromosomal
stability and immortality of cancer cells. Cancer Res.
67:8544–8553. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mishra PK, Au WC, Choy JS, Kuich PH, Baker
RE, Foltz DR and Basrai MA: Misregulation of Scm3p/HJURP causes
chromosome instability in Saccharomyces cerevisiae and human cells.
PLoS Genet. 7:e10023032011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shuaib M, Ouararhni K, Dimitrov S and
Hamiche A: HJURP binds CENP-A via a highly conserved N-terminal
domain and mediates its deposition at centromeres. Proc Natl Acad
Sci U S A. 107:1349–1354. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hu B, Wang Q, Wang Y, Chen J, Li P and Han
M: Holliday junction-recognizing protein promotes cell
proliferation and correlates with unfavorable clinical outcome of
hepatocellular carcinoma. Onco Targets Ther. 10:2601–2607. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Valente V, Serafim RB, de Oliveira LC,
Adorni FS, Torrieri R, Tirapelli DP, Espreafico EM, Oba-Shinjo SM,
Marie SK, Paçó-Larson ML and Carlotti CG Jr: Modulation of HJURP
(Holliday junction-recognizing protein) levels is correlated with
glioblastoma cells survival. PLoS One. 8:e622002013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Montes de Oca R, Gurard-Levin ZA, Berger
F, Rehman H, Martel E, Corpet A, de Koning L, Vassias I, Wilson LO,
Meseure D, et al: The histone chaperone HJURP is a new independent
prognostic marker for luminal A breast carcinoma. Mol Oncol.
9:657–674. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li L, Li X, Meng Q, Khan AQ and Chen X:
Increased expression of Holliday junction-recognizing protein
(HJURP) as an independent prognostic biomarker in advanced-stage
serous ovarian carcinoma. Med Sci Monit. 24:3050–3055. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Askar N, Cirpan T, Toprak E, Karabulut B,
Selvi N, Terek MC, Uslu R, Sanli UA and Goker E: Arsenic trioxide
exposure to ovarian carcinoma cells leads to decreased level of
topoisomerase II and cytotoxicity. Int J Gynecol Cancer.
16:1552–1556. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zeng C, Shao Z, Wei Z, Yao J, Wang W, Yin
L, YangOu H and Xiong D: The NOTCH-HES-1 axis is involved in
promoting Th22 cell differentiation. Cell Mol Biol Lett. 26:72021.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Moreno Leon L, Gautier M, Allan R, Ilié M,
Nottet N, Pons N, Paquet A, Lebrigand K, Truchi M, Fassy J, et al:
The nuclear hypoxia-regulated NLUCAT1 long non-coding RNA
contributes to an aggressive phenotype in lung adenocarcinoma
through regulation of oxidative stress. Oncogene. 38:7146–7165.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ramos M, Franch P, Zaforteza M, Artero J
and Durán M: Completeness of T, N, M and stage grouping for all
cancers in the mallorca cancer registry. BMC Cancer. 15:8472015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Subramanian A, Tamayo P, Mootha VK,
Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub
TR, Lander ES, et al: Gene set enrichment analysis: A
knowledge-based approach for interpreting genome-wide expression
profiles. Proc Natl Acad Sci U S A. 102:15545–15550. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ru B, Wong CN, Tong Y, Zhong JY, Zhong SS,
Wu WC, Chu KC, Wong CY, Lau CY, Chen I, et al: TISIDB: An
integrated repository portal for tumor-immune system interactions.
Bioinformatics. 35:4200–4202. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wei Y, Ouyang GL, Yao WX, Zhu YJ, Li X,
Huang LX, Yang XW and Jiang WJ: Knockdown of HJURP inhibits
non-small cell lung cancer cell proliferation, migration, and
invasion by repressing Wnt/β-catenin signaling. Eur Rev Med
Pharmacol Sci. 23:3847–3856. 2019.PubMed/NCBI
|
|
25
|
Zhou D, Tang W, Liu X, An HX and Zhang Y:
Clinical verification of plasma messenger RNA as novel noninvasive
biomarker identified through bioinformatics analysis for lung
cancer. Oncotarget. 8:43978–43989. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dhieb D, Belguith I, Capelli L, Chiadini
E, Canale M, Bravaccini S, Yangui I, Boudawara O, Jlidi R,
Boudawara T, et al: Analysis of genetic alterations in tunisian
patients with lung adenocarcinoma. Cells. 8:5142019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zheng MM, Li YS, Jiang BY, Tu HY, Tang WF,
Yang JJ, Zhang XC, Ye JY, Yan HH, Su J, et al: Clinical utility of
cerebrospinal fluid cell-free DNA as liquid biopsy for
leptomeningeal metastases in ALK-rearranged NSCLC. J Thorac Oncol.
14:924–932. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Varn FS, Tafe LJ, Amos CI and Cheng C:
Computational immune profiling in lung adenocarcinoma reveals
reproducible prognostic associations with implications for
immunotherapy. Oncoimmunology. 7:e14310842018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang W, Ren S, Wang Z, Zhang C and Huang
J: Increased expression of TTC21A in lung adenocarcinoma infers
favorable prognosis and high immune infiltrating level. Int
Immunopharmacol. 78:1060772020. View Article : Google Scholar : PubMed/NCBI
|