Introduction
Current therapeutic strategies for cancer have
shifted from focusing on the tumor cells themselves to targeting
the tumor microenvironment (TME) (1). Multiple cellular components and
signaling pathway networks create a TME that surrounds the tumor
cells and supports tumor growth (2). Among these components,
cancer-associated fibroblasts (CAFs) are important for promoting
tumor progression through various mechanisms, including remodeling
of extracellular matrix, maintenance of stemness and angiogenesis
(3,4). Previous reports confirmed that CAFs
underwent metabolic reprogramming to enhance glycolysis and form
metabolic couplings with colon cancer cells, thereby promoting
malignant tumor behaviors in vitro (5). The tumor-promoting effects of CAFs
could be attenuated by hindering glycolysis (6).
Sudachitin is a polymethoxylated flavone derived
from the peel of the citrus fruit Citrus sudachi, a
specialty food in Tokushima Prefecture, Japan. Sudachitin has been
reported to have anti-inflammatory activities (7) and induce mitochondrial biogenesis,
which protects against metabolic disorders (8). Liu N et al have reviewed that
flavonoids derived from citrus peels, which are normally wasted,
may prevent cancer through various mechanisms and may be
health-promoting food components (9). Therefore, in our study, we
investigated the effects of sudachitin on tumor cells and the TME,
with a specific focus on CAFs. The aim of this study was to update
knowledge regarding the biological activities of sudachitin and
determine its usefulness as a safe anticancer adjuvant.
Materials and methods
Cell culture and reagents
Human colorectal cancer cell lines HCT-116 (ECACC
91091005) and HT-29 (ECACC 91072201) were purchased from The
European Collection of Authenticated Cell Cultures (ECACC) and
cultured in McCoy's 5A medium (Thermo Fisher Scientific, Inc.)
supplemented with 10% fetal bovine serum (FBS, Thermo Fisher
Scientific, Inc.) and 1% penicillin-streptomycin (Thermo Fisher
Scientific, Inc.). Human cholangiocarcinoma cell lines HuCCT1
(RRID: CVCL_0324) and RBE (RRID: CVCL_4896) were purchased from
Cell Bank, RIKEN BioResource Research Center and maintained in
RPMI-1640 (Thermo Fisher Scientific, Inc.) supplemented with 10%
FBS and 1% penicillin-streptomycin. The human pancreatic cancer
cell lines PANC-1 and MIA PaCa-2 and human liver cancer cell lines
Huh-7 and HepG2 were from storage in our laboratory and cultured in
Dulbecco's Modified Eagle Medium [DMEM high glucose (4.5 g/l),
pyruvate (110 mg/l) (cat. no. 11995065; Thermo Fisher Scientific,
Inc.)] supplemented with 10% FBS and 1% penicillin-streptomycin.
Human intestinal fibroblasts (HIFs) were obtained from ScienCell
Research Laboratories (Cat. 2920) and cultured in a complete
fibroblast medium (Cat. 2301; ScienCell Research Laboratories). All
cell lines used in the present study from a commercial source were
aliquoted and frozen in liquid nitrogen immediately upon receipt,
and used for the present experiments within 6 months of thawing.
Cells were cultured at 37°C in 5% CO2 with normal oxygen
saturation and were selected for experiments during the logarithmic
growth phase under mycoplasma-free conditions. Sudachitin was
supplied by Ikeda Yakusou Co., Ltd.
Cell co-culture and collection of
conditioned medium (CM)
To generate CAFs, HIFs were co-cultured for 3 days
with HCT-116 or HT-29 cells at a ratio of 1:3 using Falcon
permeable supports for six-well plates with 0.4-µm pores (353090;
Corning, Inc.), which provided indirect contact between cell types
but used the same DMEM medium containing 10% FBS as we described in
our previous study (10). For
generating CAF (sudachitin), CAFs were further treated with 50 µM
sudachitin for 24 h. Total RNA samples for quantitative real-time
reverse transcription PCR (qRT-PCR) in each group were extracted in
this step. To generate CM, the medium was further changed by fresh
serum-free DMEM and continued to incubate cells for 48 h. Then the
supernatants were collected, centrifuged (500 × g) at room
temperature for 20 min, and filtered through 0.2-µm filter
membranes to remove cellular debris. The supernatants were named as
CAF-CM in CAFs group, CAF (sudachitin)-CM in CAFs (sudachitin)
group and HIF-CM in HIFs group, respectively. As shown via flow
charts (Fig. S1A-C). The CM from
each of the above groups was stored at −80°C and avoid repeated
freezing and thawing. For treatment of cancer cells in the
subsequent experiments, the CM was warmed to room temperature and
added to fresh DMEM medium at a ratio of 1:1, which will include
the soluble factors in the CM and supply enough nutrients to
support the cells for the next experiment. The final FBS
concentration were varies according to the different experiments
and details were mentioned in each of the experiments.
Cell proliferation and cytotoxicity
assay
Cancer cells were seeded at a density of
0.7×104 cells/well in 96-well plates. After cells
attachment, increasing concentrations of sudachitin ranged from 1
to 500 µM were added to treat the cells for 48 h. The medium in
each well was replaced with fresh medium containing a 10% (v/v)
Cell Counting Kit-8 (CCK-8) solution (Dojindo Molecular
Technologies), and the cells were incubated further for 2 h. Cell
proliferation was analyzed by measuring the absorbance values at
450 nm with a microplate reader (SpectraMax i3; Molecular Devices,
LLC) in accordance with the manufacturer's instructions. To
determine the effects of HIFs, CAFs and CAFs (sudachitin) on
proliferation, HCT-116 and HT-29 cells were incubated with the
indicated CM with 10% final FBS concentration for 48 h after
adhesion, and cell proliferation was analyzed using the above
method. To determine cytotoxicity of sudachitin against non-tumor
cells, HIFs were seeded in 96-well plates at a density of
0.5×104 cells/well and treated with increasing
concentrations of sudachitin ranged from 1 to 300 µM. The
proliferative status of the cells was measured every 24 h between
days 1 and 3 using the CCK-8 solution as described above. To
further compare the sensitivity of CAFs and HIFs on sudachitin.
HIFs after they were co-cultured with HIFs, HCT-116 cells and HT-29
cells were seeded in 96-well plates at a density of
0.5×104 cells/well and 10, 30, 50, 75, 100 µM sudachitin
were used to treat the cells for 24 and 48 h. And the proliferative
status of the cells was measured using the CCK-8 solution in the
same manner. The cell proliferation rate was measured by comparing
with control group.
Wound healing assay
HCT-116 or HT-29 cells were seeded at a density of
6×105 cells/well in six-well plates and incubated
overnight to form a 90% confluent monolayer. A 200-µl pipette tip
was used to scratch a wound through the entire center of each well.
After washing with PBS, the cells in each group were cultured with
the indicated CM in the absence of FBS for 48 h. The areas of the
wounds were observed at 0 and 48 h after scratching, and images
were captured using a light microscope (×40 magnification) equipped
with a DP22-CU digital camera (Olympus). The cell migration rates
were calculated using ImageJ v1.46r software (National Institutes
of Health) using the following equation: relative migration
rate=[width (0 h)-width (48 h)]/width (0 h) ×100%.
Migration assay
A 24-well Transwell system with 8.0-µm pores
(Corning) was used for the migration and invasion assays.
Serum-starved cancer cells (7×104/well) were seeded in
the upper chamber in a 100-µl suspension. After cell attachment,
the culture medium was replaced with the indicated CM in each
group. The final FBS concentration was 5% in the upper chambers and
10% in the lower chambers. After 36 h of incubation, the unattached
cells in the upper chambers were cleaned using cotton swabs. Then,
inserts were fixed by methanol for 20 min and stained with 1%
crystal violet (031-04852; Fujifilm Wako Pure Chemical Corporation)
for 20 min. Images were captured and the numbers of cells at the
bottom of the membrane in each chamber were calculated.
qRT-PCR
Total RNA from each group of cells was extracted
with an RNeasy Mini kit (Qiagen GmbH) in the stage mentioned above,
and concentrations were measured using a NanoDrop™ 2000
spectrophotometer (Thermo Fisher Scientific, Inc.). Subsequently,
2.5 µg RNA was reverse transcribed into cDNA in a total volume of
50 µl using a High-Capacity cDNA Reverse Transcription kit (Applied
Biosystems/Thermo Fisher) in accordance with the manufacturer's
instructions. A StepOnePlus™ Real-Time PCR System
(Applied Biosystems) was used to perform TaqMan qPCR with the
following reaction conditions: initial denaturation at 95°C for 3
min followed by 40 cycles of denaturation (95°C, 30 sec), annealing
(58°C, 30 sec), and extension (72°C, 45 sec) and a final extension
at 72°C for 10 min. The following TaqMan gene expression assays
were used: PFKP (assay ID, Hs00737347_m1, catalog, 4331182,
FAM-labeled, Thermo Fisher Scientific), and SLC16A3
(MCT4) (assay ID, Hs00358829_m1, catalog, 4331182,
FAM-labeled, Thermo Fisher Scientific). GAPDH (assay ID,
Hs99999905_m1, catalog, 4326317E, VIC-labeled, Thermo Fisher
Scientific) was used as the internal control to normalize the raw
data. The 2−∆∆Ct method was used for data analysis, and
the results were presented as the fold changes in the relative mRNA
expression for each experimental group compared with that in the
control group.
Lactate assay
To collect the sample for lactate assay, fresh
serum-free DMEM was replaced in all groups of cells. After
culturing cells for another 6 or 24 h, cell supernatants were
collected using the method described above for CM and cells were
fully digested and counted using a cell counter (model R1, Olympus)
after trypan blue staining. A lactate assay kit (MAK064;
Sigma-Aldrich) was used to detect the concentrations of lactate in
the supernatants. Briefly, supernatant and standard samples were
diluted with lactate assay buffer following the manufacturer's
instructions and added to a 96-well plate. After a 30 min
incubation with 50 µl master reaction mix at room temperature, the
absorbance values were measured at 570 nm with a microplate reader.
The lactate concentrations for each sample were calculated
according to a standard curve constructed using the standard
samples. The relative secretion of lactate was normalized to the
cell number.
Statistical analysis
All data are presented as the mean ± SD. GraphPad
Prism v7.0 software (GraphPad Software) and ImageJ v1.46r software
were used for the statistical analysis and construction of graphs.
The unpaired Student's t-test or Mann-Whitney U test was used for
comparisons between two groups. Differences between multiple groups
were analyzed using one-way analysis of variance followed by
Tukey's post hoc test. More than three biological replicates were
included for each experiment, and P<0.05 (two-sided) was
considered statistically significant.
Results
Sudachitin directly inhibited tumor
proliferation in vitro
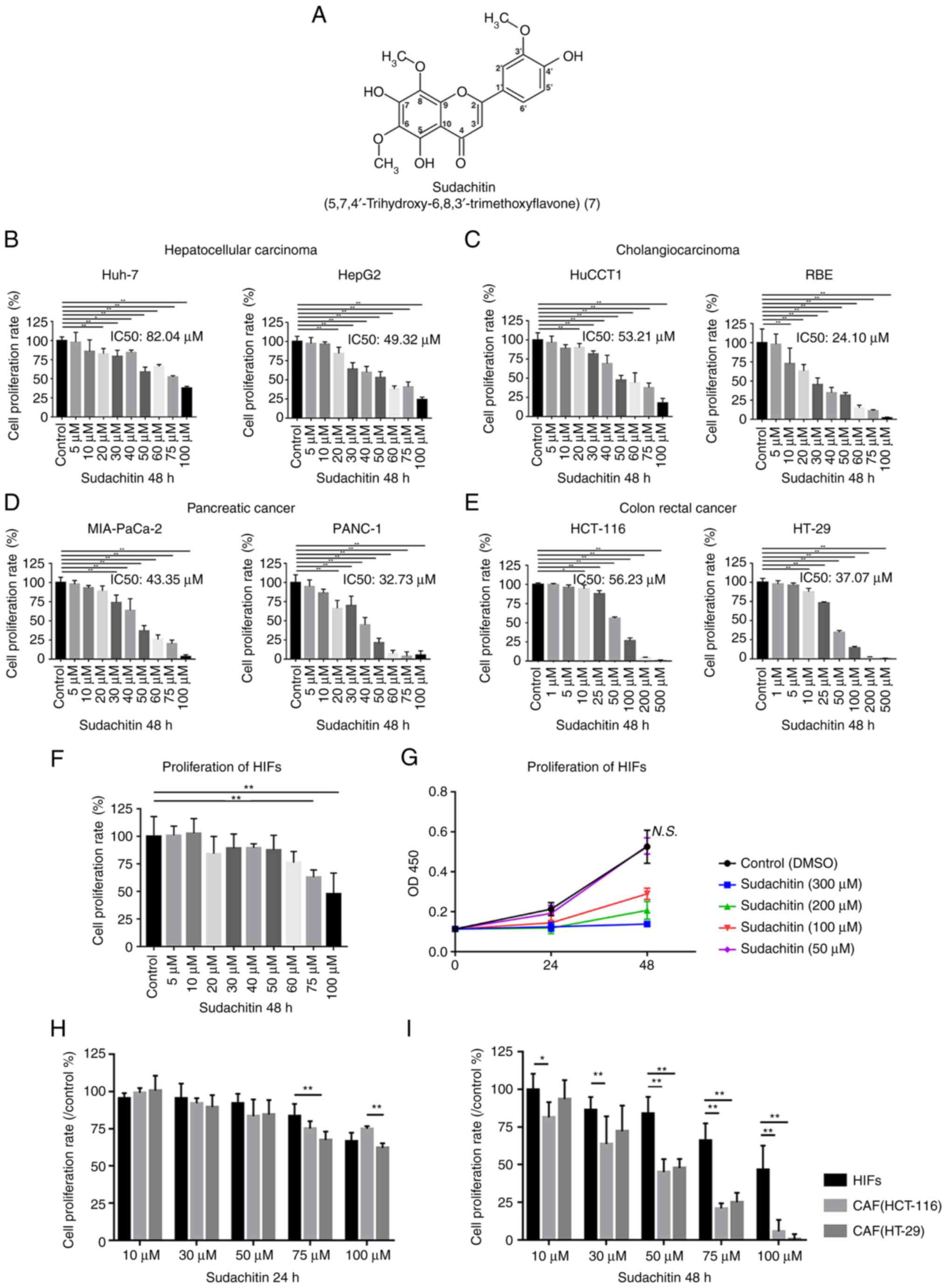
As a type of polymethoxylated flavone, the chemical
structure of sudachitin was shown in Fig. 1A (7). After 48 h treatments with different
concentrations of sudachitin, a dose-dependent inhibition of
proliferation was observed in four types of cancer cells (Fig. 1B-E). Our data demonstrated that the
efficacy of sudachitin-mediated antiproliferative activity varied
among different cell lines. For liver cancer lines, the
half-maximal inhibitory concentrations (IC50) of sudachitin were
82.04 µM for Huh-7 and 49.32 µM for HepG2 cells (Fig. 1B). For cholangiocarcinoma lines,
the IC50 values were 53.21 and 24.1 µM for HuCCT1 and RBE cells,
respectively (Fig. 1C). For
pancreatic cancer, the IC50 values were 43.35 µM for MIA PaCa-2
cells and 32.73 µM for PANC-1 (Fig.
1D). The IC50 values of sudachitin were 56.23 and 37.07 µM for
the colorectal cancer lines HCT-116 and HT-29, respectively
(Fig. 1E). We also investigated
the direct effects of sudachitin on the survival of HIFs to
determine cytotoxicity against normal cells. We demonstrated that a
48-h treatment with up to 50 µM sudachitin did not significantly
inhibit the proliferation of HIFs (Fig. 1F and G). Next, we further compared
the sensitivity of CAFs and HIFs on sudachitin. Although 24-h
treatment with up to 50 µM sudachitin did not significantly affect
the growth in both HIFs and CAFs (Fig.
1H). When treatment time was extended to 48 h, CAFs became more
sensitive to sudachitin compared with HIFs (Fig. 1I).
Sudachitin suppressed the
tumor-promoting capabilities of CAFs
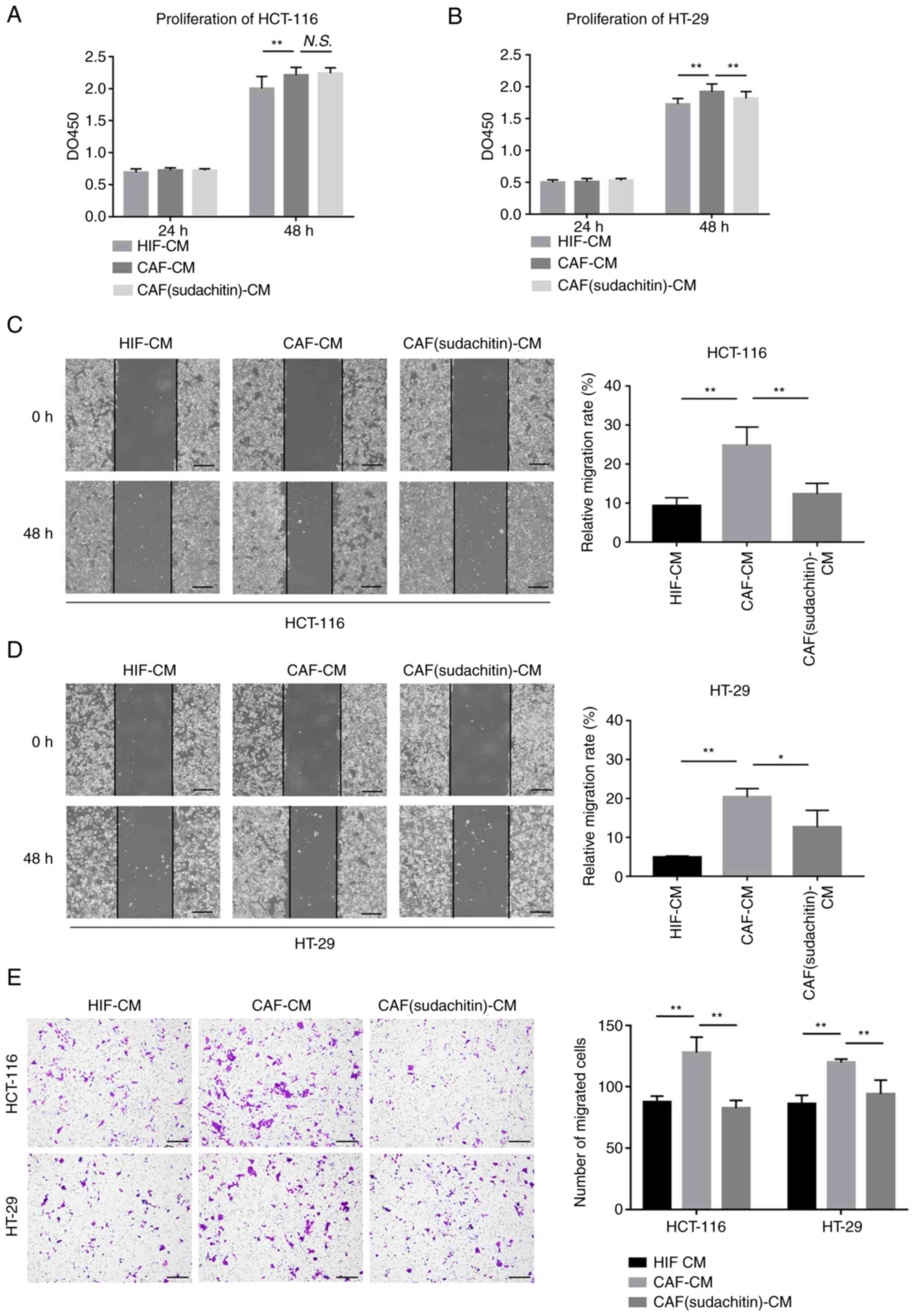
To investigate the effect of sudachitin on the
metabolic couplings between CAFs and tumor cells, we treated
colorectal cancer cells with CM collected from HIFs, CAFs and CAFs
(sudachitin). Proliferation assays illustrated that after
pretreatment with 50 µM sudachitin for 24 h, CAF-induced
stimulation of HT-29 colorectal cancer cell proliferation was
decreased, while no significant effect was observed for HCT-116
cells (Fig. 2A and B). As shown in
Fig. 2C-E, the ability of CAFs to
promote the migration and invasion of HCT-116 and HT-29 cells was
also suppressed after 50 µM sudachitin pretreatment for 24 h.
Sudachitin treatment inhibited the
glycolytic activity of CAFs
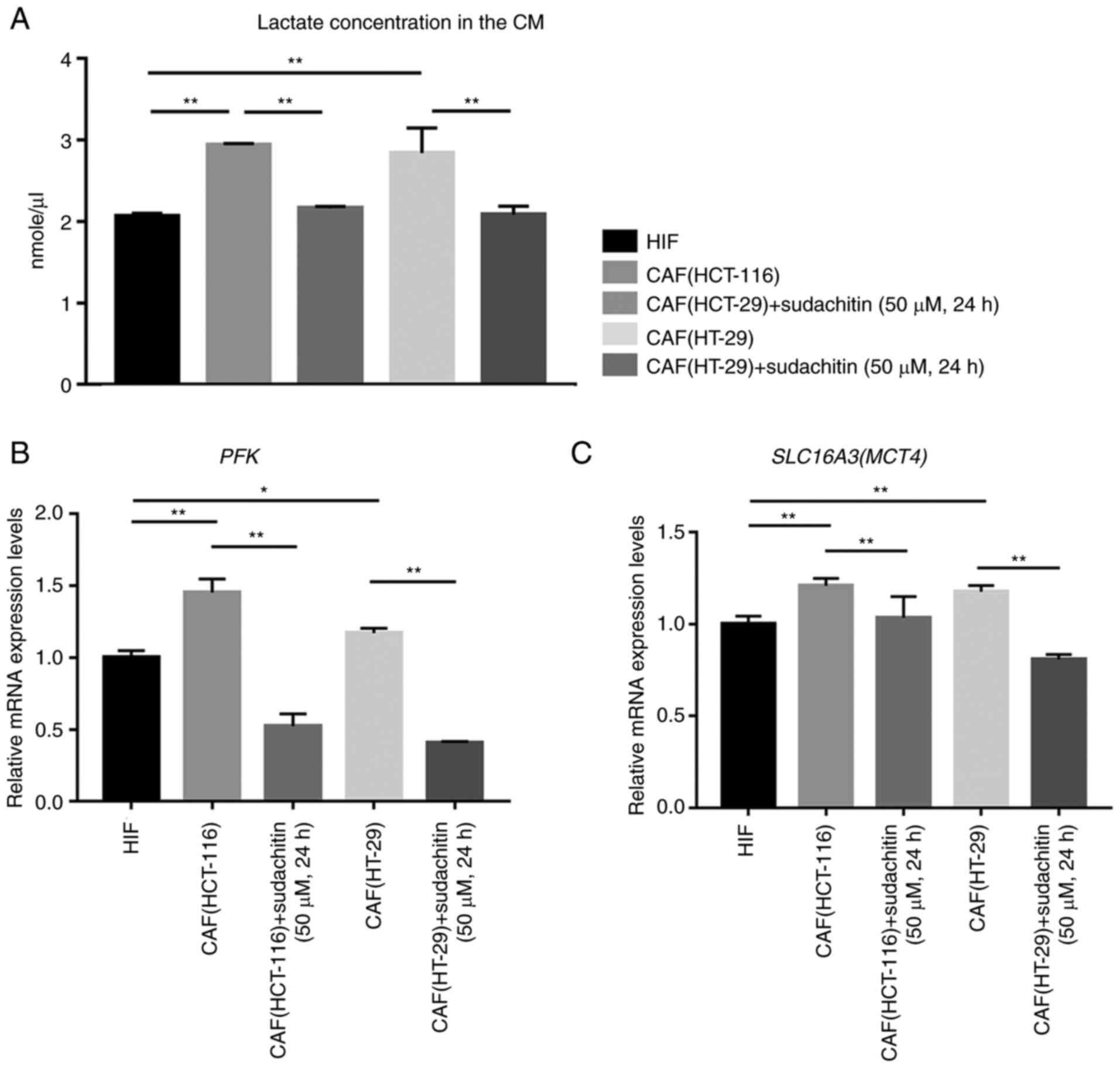
Next, we determined the glycolytic activity in CAFs
after treatment with 50 µM sudachitin for 24 h. CAFs presented
higher lactate productions and mRNA expressions of
phosphofructokinase (PFK) and monocarboxylate transporter 4
(MCT4) compared with HIFs as shown in Fig. 3A-C. However, 50 µM sudachitin
treatment for 24 h decreased lactate production by CAFs (Fig. 3A) as well as the mRNA expression of
PFK and MCT4 (Fig. 3B
and C). These data indicated that glycolytic activity of CAFs
was inhibited after treatment with sudachitin.
Discussion
In this study, we demonstrated that sudachitin
broadly and directly inhibited the proliferation of a wide range of
tumor cells in vitro. Furthermore, sudachitin reduced the
glycolytic activity of CAFs, thereby hindering the metabolic
coupling to colorectal cancer cells and reducing CAF-induced tumor
progression. Therefore, a relatively small dose of sudachitin can
exert antitumor effects by targeting the TME.
A previous study showed that polymethoxyflavones
induced apoptosis of gastric cancer cells by upregulating retinoic
acid receptor β both in vitro and in vivo (11). Nobiletin, a polymethoxyflavone
similar to sudachitin, was able to inhibit tumor cell proliferation
through various mechanisms, such as classical autophagy,
mitochondrial autophagy (mitophagy), apoptosis, and pyroptosis
(12). Moreover, sudachitin was
shown to induce apoptosis by regulating the MAPK pathway (13). Given that much has been published
regarding polymethoxyflavone-induced inhibition of tumor cell
proliferation, a direct mechanism of action of sudachitin on tumor
cells is theoretically supported. Because of its role in
metabolism, especially mitochondrial and glucose metabolism, we
investigated the effects of sudachitin on glucose metabolism in
CAFs (8,14). To illustrate the safety of the
sudachitin's treatment, we treated CAFs with a concentration of 50
µM and a treatment time of 24 h. This dose did not show significant
inhibition to the proliferation of HIFs according to our result. As
shown in Fig. 3, sudachitin
inhibited mRNA expression of PFK, which encodes a key enzyme
in glycolysis, and MCT4, which encodes a cellular membrane
transporter of lactate, thereby inhibiting glycolysis and lactate
export in CAFs. Recently, ‘the reverse Warburg effect’ has been
proposed as a new theory that CAFs also undergo a metabolic
transition similar to that in tumor cells. It makes the hypothesis
that in the normal oxygen saturation, CAFs undergo glycolysis and
produce a significant amount of lactate in the TME and support the
tumor malignancy (15,16). Inhibition of PFK and
MCT4 may diminish the promotion of tumor malignancy in
vitro by CAFs. As evidenced by our findings, sudachitin not
only acted directly against tumor cells, it also exerted antitumor
effects by targeting the glycolytic pathway of CAFs, which are
present in the TME. Moreover, the concentration required for these
antitumor effects was relatively low and safe for normal cells.
Interestingly, CAFs were more sensitive to sudachitin. We inferred
that the altered glucose metabolic profile might account for the
improved sensitivity to sudachitin of CAFs, which further suggested
that sudachitin was expected to play a unique antitumor role by
targeting the TME.
Currently, Ikeda Yakusou Co., Ltd. has achieved the
preparation of powdered extracts from Citrus sudachi peels
and further obtained high purity sudachitin powder. This allows
sudachitin to be easily taken by individuals via capsules. The
results of a 12-week randomized, double-blind, controlled trial by
Shikishima Y et al showed that the intake of Citrus
sudachi peel extract powder, containing a dose of 4.9 mg/day
sudachitin, significantly reduced the ratio of visceral fat to
subcutaneous fat and moderately reduced waist circumference, a
marker of metabolic syndrome, compared to placebo (17). According to information from Ikeda
Yakusou Co., Ltd., ~3.5 kg of Citrus sudachi peel extract
powder can be obtained from 100 kg of peel, which contains ~1.4% of
sudachitin. So it is estimated that the daily intake in the above
experiment is equivalent to ~10 g of Citrus sudachi peel. In
Japan, Citrus sudachi produces ~8,000 tons per year,
approximately half of which is extruded and processed into juice
and produces ~200 tons of peel residue, which is a very abundant
resource (8). Although we
performed the in vitro experiments with sudachitin, a
limitation is a lack of in vivo data, such as the
bioavailability, maximum blood concentration and metabolism
characteristics. Therefore, more studies in the future should focus
on in vivo studies involving the blood levels and safety of
sudachitin. In our study of sudachitin impaired glycolysis in CAFs,
the lack of measurements on pyruvate in conjunction with lactate
was another limitation. And although we found that sudachitin
reduced the expression of PFK and MCT4, the detailed
mechanism remains further explored. In conclusion, we investigated
for the first time the effects of sudachitin on tumor cells and
CAFs. Sudachitin directly inhibited tumor growth and indirectly
blocked CAF-mediated support of tumor cells by targeting glycolysis
in CAFs. This study extends the understanding of the biological
function of sudachitin and suggests that sudachitin is a
cost-effective, safe, and widely available anticancer agent.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by a Grants-in-Aid for Scientific
Research (grant no. 20K08957), Taiho Pharmaceutical Co., Ltd. and
Tsumura Pharmaceutical Co., Ltd.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SC, YM and MN designed the study. SC, KY, AN, TS and
TT performed the experiments. SC, HK, MS and CT collected and
analyzed the data. YW and TY interpreted the data. SC and MN wrote
the original draft. YM, AN, TS, and MS reviewed and edited the
manuscript. SC and MN confirm the authenticity of all the raw data.
SC and MN agree to be accountable for all aspects of the work in
ensuring that questions related to the accuracy or integrity of any
part of the work are appropriately investigated and resolved. All
the authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
This study was approved by The Ethics Committee of
Tokushima University Hospital (TOCMS approval no. 2901-2).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CAF
|
cancer-associated fibroblast
|
|
CCK-8
|
Cell Counting Kit-8
|
|
CM
|
conditioned medium
|
|
IC50
|
half-maximal inhibitory
concentrations
|
|
MCT4
|
monocarboxylate transporter 4
|
|
PFK
|
phosphofructokinase
|
|
TME
|
tumor microenvironment
|
References
|
1
|
Kumari S, Advani D, Sharma S, Ambasta RK
and Kumar P: Combinatorial therapy in tumor microenvironment: Where
do we stand? Biochim Biophys Acta Rev Cancer. 1876:1885852021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parker TM, Gupta K, Palma AM, Yekelchyk M,
Fisher PB, Grossman SR, Won KJ, Madan E, Moreno E and Gogna R: Cell
competition in intratumoral and tumor microenvironment
interactions. EMBO J. 40:e1072712021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wu F, Yang J, Liu J, Wang Y, Mu J, Zeng Q,
Deng S and Zhou H: Signaling pathways in cancer-associated
fibroblasts and targeted therapy for cancer. Signal Transduct
Target Ther. 6:2182021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Watanabe K, Shiga K, Maeda A, Harata S,
Yanagita T, Suzuki T, Ushigome H, Maeda Y, Hirokawa T, Ogawa R, et
al: Chitinase 3-like 1 secreted from cancer-associated fibroblasts
promotes tumor angiogenesis via interleukin-8 secretion in
colorectal cancer. Int J Oncol. 60:32022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Keller F, Bruch R, Schneider R,
Meier-Hubberten J, Hafner M and Rudolf R: A scaffold-free 3-D
co-culture mimics the major features of the reverse Warburg effect
in vitro. Cells. 9:19002020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Martinez-Outschoorn UE, Lisanti MP and
Sotgia F: Catabolic cancer-associated fibroblasts transfer energy
and biomass to anabolic cancer cells, fueling tumor growth. Semin
Cancer Biol. 25:47–60. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yuasa K, Tada K, Harita G, Fujimoto T,
Tsukayama M and Tsuji A: Sudachitin, a polymethoxyflavone from
Citrus sudachi, suppresses lipopolysaccharide-induced
inflammatory responses in mouse macrophage-like RAW264 cells.
Biosci Biotechnol Biochem. 76:598–600. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tsutsumi R, Yoshida T, Nii Y, Okahisa N,
Iwata S, Tsukayama M, Hashimoto R, Taniguchi Y, Sakaue H, Hosaka T,
et al: Sudachitin, a polymethoxylated flavone, improves glucose and
lipid metabolism by increasing mitochondrial biogenesis in skeletal
muscle. Nutr Metab (Lond). 11:322014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu N, Li X, Zhao P, Zhang X, Qiao O,
Huang L, Guo L and Gao W: A review of chemical constituents and
health-promoting effects of citrus peels. Food Chem.
365:1305852021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen SH, Nishi M, Morine Y, Shimada M,
Tokunaga T, Kashihara H, Takasu C, Yamada S and Wada Y:
Epigallocatechin-3-gallate hinders metabolic coupling to suppress
colorectal cancer malignancy through targeting aerobic glycolysis
in cancer-associated fibroblasts. Int J Oncol. 60:192022.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang Y, Chen Y, Zhang H, Chen J, Cao J,
Chen Q, Li X and Sun C: Polymethoxyflavones from citrus inhibited
gastric cancer cell proliferation through inducing apoptosis by
upregulating RARβ, both in vitro and in vivo. Food Chem Toxicol.
146:1118112020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang R, Chen J, Mao L, Guo Y, Hao Y, Deng
Y, Han X, Li Q, Liao W and Yuan M: Nobiletin triggers reactive
oxygen species-mediated pyroptosis through regulating autophagy in
ovarian cancer cells. J Agric Food Chem. 68:1326–1336. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Abe S and Yuasa K: Sudachitin, a
polymethoxyflavone from Citrus sudachi, induces apoptosis
via the regulation of MAPK pathways in human keratinocyte HaCaT
cells. Biochem Biophys Res Commun. 519:344–350. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gandhi GR, Vasconcelos ABS, Wu DT, Li HB,
Antony PJ, Li H, Geng F, Gurgel RQ, Narain N and Gan RY: Citrus
flavonoids as promising phytochemicals targeting diabetes and
related complications: A systematic review of in vitro and in vivo
studies. Nutrients. 12:29072020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pavlides S, Whitaker-Menezes D,
Castello-Cros R, Flomenberg N, Witkiewicz AK, Frank PG, Casimiro
MC, Wang C, Fortina P, Addya S, et al: The reverse Warburg effect:
Aerobic glycolysis in cancer associated fibroblasts and the tumor
stroma. Cell Cycle. 8:3984–4001. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Benny S, Mishra R, Manojkumar MK and
Aneesh TP: From Warburg effect to reverse Warburg effect; the new
horizons of anti-cancer therapy. Med Hypotheses. 144:1102162020.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shikishima Y, Tsutsumi R, Kawakami A,
Miura H, Nii Y and Sakaue H: Sudachi peel extract powder including
the polymethoxylated flavone sudachitin improves visceral fat
content in individuals at risk for developing diabetes. Food Sci
Nutr. 9:4076–4084. 2021. View Article : Google Scholar : PubMed/NCBI
|