The biogenesis process of exosomes distinguishes
them from apoptotic bodies and other extracellular vesicles.
Exosomes originate from the endosomal system and are formed from
early endosomes: Inward budding of cellular membranes leads to
formation of early endosomes (3,14,15).
Early endosomes mature to late endosomes with multiple intraluminal
vesicles (ILVs) and late endosomes form multivesicular bodies
(MVBs). After the fusion of MVBs with the plasma cell membrane,
exosomes are released from the cell into the extracellular
environment, carrying the biological information of secretory cells
and transmitting biological signals to recipient cells (3,14,15).
During the maturation of ILVs into MVBs, proteins
are sorted. Internalized proteins in early endosomes are
ubiquitinated and directed to late endosomes (3,14,15).
Multiprotein complexes are involved in the sorting and
ubiquitination of target proteins (3). The endosomal sorting complexes
required for transport (ESCRT) complex regulates endosomal
maturation (14). ESCRT complexes
are composed of four complexes (ESCRT-0, -I, -II and -III)
(14). Ubiquitylated proteins in
the endosomal membrane are sequestered by the ESCRT-0 complex
(15). The ESCRT-I and ESCRT-II
complexes are responsible for the budding of the membrane. For
instance, ESCRT induces MVB vesicles to sprout (15,16),
while Rab proteins regulate intracellular vesicle transport
(17). Furthermore, there are a
variety of proteins involved in this process, including Rab GTPases
(Rab11, Rab35, Rab27A and Rab 27b), which are involved in the
formation of exosomes (17,18).
Exosomes are known to contain a considerable number
of functional messenger RNAs (mRNAs) and miRNAs (24). Exosomes from breast cancer,
colorectal cancer and leukemia cells contain human telomerase
reverse transcriptase (hTERT) mRNA (25). When these exosomes are absorbed by
fibroblasts, they can promote the construction of a tumor
metastatic environment by promoting the proliferation and survival
of fibroblasts (25). Gutkin et
al (25) reported that human
tumor cells-derived exosomes can transfer hTERT transcriptional
mRNA to telomerase-negative fibroblasts. The delivered mRNA can be
translated into a protein, which renders the recipient cells
telomerase-positive. This was the first study to report that
exosomes can transfer the transcriptional mRNA of hTERT.
Exosomes from chronic lymphocytic leukemia (CLL)
cells promote the proliferation of stromal cells by secreting
miR-202-3p into human bone marrow cells (26). When CLL-exosomes, derived either
from CLL cell culture supernatants or plasma from patients with
CLL, were co-cultured with human stromal cells, the latter could
accept these exosomes, resulting in the promotion of their
proliferation associated with the induction of c-Fos expression
(26). These exosomes are rich in
small RNAs, particularly hsa-mir-202-3p, which can enhance the
Hedgehog signal transduction pathway, thus promoting the
proliferation of recipient cells (26). miR-9 transported by tumor
cell-derived exosomes can promote endothelial cell migration and
angiogenesis through the Janus kinase/signal transducer and
activator of the transcription signaling pathway (27).
Increasing evidence shows that exosomes serve an
important role in intercellular communication (28,29).
Exosomes transfer proteins, mRNAs and miRNAs to receptive cells,
resulting in the regulation of a variety of functions, including
immunoregulation, matrix remodeling, growth factor delivery and
oncoprotein transfer (28,30–32).
The tumor microenvironment (TME) is composed of tumor-associated
fibroblasts, osteoblasts and immune cells. The TME can promote the
proliferation of tumor cells and confers resistance to chemotherapy
(33). Exosomes can regulate
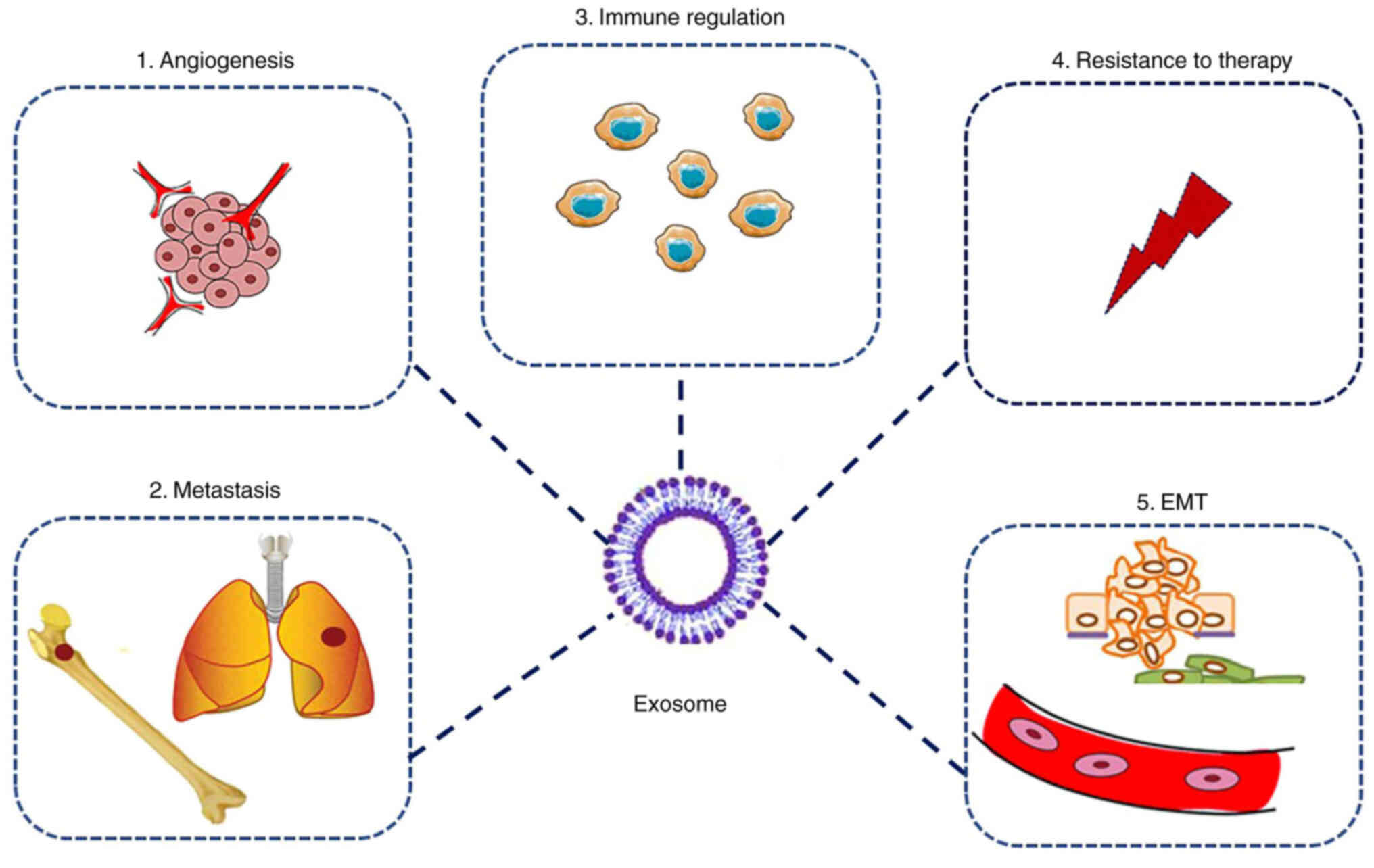
multiple biological behaviors of tumor cells, as shown in Fig. 1. Furthermore, exosomal miRNAs,
mRNA, proteins and other functional molecules are accepted by the
stromal cells in the TME where they exert regulatory effects
(34).
Exosomes from the breast cancer microenvironment can
promote breast cancer cell metastasis through the Wnt plane cell
polarity (PCP) signal (35).
During breast cancer cell metastasis, exosomes can promote tumor
metastasis via the Wnt PCP pathway, which is different from the
classical Wnt signaling pathway (35). Hood et al (30) found that exosomes secreted by
melanoma cells prepare sentinel lymph nodes for tumor cell
metastasis as tumor cells are more likely to metastasize to a site
with abundant melanoma exosomes. Bone marrow progenitor cells are
permanently ‘educated’ by exosomes from highly invasive melanoma
cells through the receptor tyrosine kinase, MET, to promote the
invasion and metastasis of primary tumor cells (36). The receptor tyrosine kinase MET
transforms bone marrow precursor cells into an
angiogenesis-promoting phenotype, which expresses c-kit, receptor
tyrosine kinase EGF-like domains 2 and Met (36). The inhibition of exosomal Met
expression can weaken the metastasis that is promoted by bone
marrow progenitor cells (36).
Exosomes derived from lung cancer cells can promote
epithelial-to-mesenchymal transition (EMT) (37). When serum-derived exosomes from
patients with advanced lung cancer are used to treat human
bronchial epithelium, they can promote the EMT of the recipient
cells (37). Exosomes from
pancreatic cancer cells can induce the formation of the
metastasis-promoting microenvironment in the liver (38). Exosomes derived from gastric cancer
cells can carry EGFR and regulate the microenvironment of the liver
to promote the metastasis of gastric cancer cells (39). Exosomes isolated from the serum of
patients with gastric cancer contain EGFR, while the exosomes
derived from the serum of normal individuals do not (39). This indicates that the levels of
the EGFR oncoprotein in the exosomes from the serum of patients
with gastric cancer are associated with gastric cancer metastatic
stages. The exosomes carrying the EGFR from the gastric cancer can
be taken up by liver cells, which will further activate liver
hepatocyte growth factor and prepare the premetastatic ‘soil’ for
the gastric cancer (39).
Exosomes derived from tumor cells can promote the
resistance of tumor cells to chemotherapeutic drugs via multiple
mechanisms, such as carrying multidrug resistance (MDR) proteins or
by secreting chemotherapeutic drugs (40). For example, exosomes can transfer
drug resistance by secreting MDR proteins into recipient cells
(41). Exosomes derived from 5T33
bone marrow stromal cells promote the proliferation of multiple
myeloma cells and induce their resistance to bortezomib (42). Exosomes can mediate drug resistance
by transporting drug resistance proteins. In prostate cancer,
docetaxel resistance is associated with an increased secretion of
exosomes (43). Exosomes secreted
from docetaxel-resistant prostate cancer cells can confer docetaxel
resistance to docetaxel-sensitive prostate cancer cells by
transporting P-glycoprotein in the exosomes (43). Exosomes can have countertherapeutic
effects by binding to chemotherapy drugs. In breast cancer,
HER-2-overexpressing exosomes can resist the therapeutic effect of
trastuzumab. Exosomes secreted by HER2-overexpressing SK-BR-3 and
br-474 breast cancer cells can bind to trastuzumab. In the early
stage of breast cancer, the binding level of exosomes isolated from
the patients is lower than that of patients at late stages of the
disease (44). In ovarian cancer
cisplatin-resistant cells, cisplatin can promote the development of
drug resistance through exosomes (45). When the cells were treated with
cisplatin, the exosomes from cisplatin-resistant ovarian cancer
cells contained 2.6 times more cisplatin compared with those from
cisplatin-sensitive ovarian cancer cells (45). The levels of the cisplatin
transporters, multidrug resistance-associated protein 2, ATPase
copper transporting α and ATPase copper transporting β, were also
higher in the exosomes from the resistant cells compared with those
from the sensitive cells (45).
Exosomes can induce drug resistance of tumor cells via the efflux
of chemotherapeutic drugs. Exosomes released from human ovarian
carcinoma cells can export cisplatin via exosomes (45). Exosomes can also mediate
chemotherapy resistance through priming cancer stem cells (CSCs)
(46). Hu et al (46) revealed that cancer-associated
fibroblast (CAF)-derived exosomes promote the chemoresistance of
colorectal cancer cells by priming CSCs. Drug-sensitive cells can
be transformed to drug resistant by acquiring exosomes from
chemoresistant cells. In breast cancer, drug resistance can be
transferred by glutathione S-transferase P1 (GSTP1)-containing
exosomes. GSTP1 can conjugate with glutathione and detoxify
chemotherapy drugs (47).
Chemosensitive breast cancer cells exhibit increased resistance to
anticancer drugs after acquiring the exosomes from chemoresistant
breast cancer cells (47).
Additionally, the expression levels of GSTP1 in exosomes are
associated with the clinical outcomes of patients with breast
cancer (47).
As the soil of tumor cell proliferation, the TME
regulates the proliferation, metastasis and vascular growth of
tumor cells (54). The TME is rich
in mesenchymal cells, fibroblasts, endothelial cells, extracellular
matrix components, CAFs, and immune cells, including T lymphocytes,
B lymphocytes, dendritic cells, macrophages and neutrophils
(48). Exosomes can also promote
tumor cell proliferation and angiogenesis (4). Vascular endothelial growth factor
(VEGF) (55), fibroblast growth
factor, TGF (56),
platelet-derived growth factor and IL-8 can be used as exosomal
proteins to promote the angiogenesis of target cells (57). The exosome-mediated interaction
between chronic myeloid leukemia cells and human bone marrow
stromal cells can promote the survival of IL-8-dependent leukemia
cells (57). Chronic myeloid
leukemia cells secrete exosomes to stimulate the secretion of IL-8
from bone marrow stromal cells, resulting in the regulation of
angiogenesis and lymphocyte survival (57). Zhu et al (58) reported that exosomes from human
bone marrow MSCs can promote the expression of tumor VEGF and
activate ERK1/2 in vivo, enhancing tumor cell
proliferation.
Exosomes from breast cancer cells can transform
adipose tissue-derived MSCs into fibroblast-like cells via the Smad
signaling pathway (51). This
process is associated with increased expression levels of α-SMA,
tumor-promoting factors TGF receptors I and II, stromal
cell-derived factor 1 (SDF-1), VEGF, C-C motif chemokine ligand 5,
and transglutaminases (51).
Exosomes derived from ovarian cancer cells can also induce adipose
tissue-derived MSCs to obtain cells with functional characteristics
of fibroblasts (52). During this
process, the expression levels of α-SMA, tumor-promoting factor
SDF-1 and TGF-β in the adipose tissue-derived MSCs also increase,
correlating to the increase in the expression levels of TGF-β
receptor I and II (52).
Exosomes from the phosphorous cell carcinoma TME can
increase the activity of the TGF-β signaling pathway in squamous
cell carcinoma (59). TGF-β can
activate Smad2 and Smad3 signaling pathways and regulate gene
transcription by binding to the TGF-β receptor (59). The exosomes from tumor stromal
fibroblasts contain TGF-β type II receptor (TβRII), a receptor
component of TGF-β (59). The
transferred receptor component increases the TβRII and Smad2
phosphorylation levels in squamous cell carcinoma cells (59).
Exosomes secreted by CLL cells can induce stromal
cells to transform into tumor-associated fibroblasts (57). Paggetti et al (57) revealed that when PKH67
fluorescence-labeled CLL cells were co-cultured with MSCs and
endothelial cells from the bone marrow, the exosomes from the CLL
cells were absorbed by the stromal cells. This event was also
associated with the transfer of miRNAs and proteins into these
recipient cells, resulting in the activation of their inflammatory
signals, such as the NF-κB signaling pathway, the transformation of
chronic lymphocytic leukemia stromal cells into tumor-related
fibroblasts, the upregulation of the expression levels of genes
encoding cytokines and chemokines (C-X-C motif chemokine ligand 1,
IL6, IL34 and C-C motif chemokine ligand 2) and migration-related
factors (intercellular adhesion molecule 1 and MMP1), and the
induction of the proliferation and migration of these stromal cells
(57). This study not only
provided valuable evidence for the regulatory effect of exosomes on
the TME but also indicated that tumor cells act on tumor stromal
cells and induce the formation of tumor-related fibroblasts
(57). Webber et al
(60) revealed that TGF-β delivery
by tumor exosomes was sufficient to transform fibroblasts into
myofibroblasts. Myofibroblasts are activated during the process of
wound healing and express α-SMA. Myofibroblasts can be activated in
the stroma of solid cancer and support the metastasis of cancer
cells (61,62).
Exosomes can deliver oncogenes to the receipt cells
to promote EMT and stem cell traits (65). For example, latent membrane protein
1 can be transported to the receipt cells by exosomes (65). Exosomes can also deliver miRNAs to
recipient cells (66). For
instance, exosomes can deliver miRNA to recipient cells to promote
liver cancer EMT and metastasis (66). CAFs can enhance the EMT of oral
cancer cells via delivery of miR-34a-5p by exosomes; miR-34a-5p in
the exosome will activate EMT by the AKT/GSK-3β/β-catenin signaling
pathway (67). By taking up the
exosomal miRNA that was secreted in the tumor environment, the oral
cancer cells increase their metastatic ability.
Hypoxic bone marrow MSC-derived exosomal miRNAs
promote metastasis of lung cancer cells via a STAT3-induced EMT
(68). Zhang et al
(68) reported that exosomes
derived from hypoxic bone marrow MSCs can be taken up by lung
cancer cells, which activates STAT3 signaling and induces EMT in
recipient cells, These plasma exosomes containing miRNA in the
patients with lung cancer also showed a diagnostic value (68).
Cancer-derived exosomes can transfer miRNAs to
macrophages to induce their M2 polarization and activate EMT in
recipient cells (69). In colon
cancer, exosomes derived from colon cancer cells can promote the
polarization of macrophages into an M2 phenotype (69). Colorectal cancer cells deliver
exosomes to macrophages and promote the upregulation of
miRNA-106b-5p (miR-106b) in macrophages (69). This process further activates the
PI3Kγ/AKT/mTOR signaling cascade in macrophages, prompting them to
polarize into an M2 phenotype (69). The polarized M2 macrophages will
further promote the EMT and metastasis of colorectal cancer cells
(69). CSCs of clear cell renal
cell carcinoma (CCRCC) contain integrin CD103, which can be
transported to cancer cells by activating EMT through PTEN
targeting (70). Bioactive
miR-19b-3p is transported to recipient cells by cancer stem
cell-derived exosomes, where miR-19b-3p activates CCRCC EMT by
targeting PTEN (70). miRNAs are
small non-coding single-stranded RNA molecules with a length of 22
nucleotides; miRNAs can regulate gene expression at the
post-transcriptional level (71).
Sun et al (72) reported
that exosomes derived from colorectal cancer cells express high
levels of miR-335-5p, which is transported to colorectal cells to
promote EMT and migration of the recipient cells by the RAS p21
protein activator 1.
Exosomes from the TME can serve immunoregulatory
roles in the communication of cancer cells (73) Exosomes can circumvent immune
defenses to cancer cells through multiple mechanisms (74). Exosomes can promote an
immunosuppressive environment through expanding the pool of
CD4+/CD25+ regulatory T cells (75). They can also inhibit the
proliferation of immune cells (53). For example, tumor-derived exosomes
can induce the apoptosis of tumor activated CD8+ T
lymphocytes (76). Exosomes
derived from dendritic cells can directly present antigen to T
cells (77,78). Exosomes derived from dendritic
cells contain functional major histocompatibility complex
(MHC)-peptide complexes, which can be presented on their surface
and transferred to recipient cells (79). Exosomes carrying MHC-peptide
complexes can be captured, processed and presented by
antigen-presenting cells (APCs) (79).
Tumor antigens can be transferred by exosomes to
antigen presenting cells, including macrophages and dendritic
cells, after phagocytosis of exosomes by APCs and release of the
exosomal antigens into the cytoplasm of APCs (80). For example, HSPs (HSP70-80) can be
transferred to dendritic cells by exosomes and induces
CD8+ T cell activation (81).
Exosomal antigen presentation to T cells can also
occur indirectly. For instance, ‘cross-dressing’ involves the
delivery of MHC-peptide complexes to antigen presenting cells
through the fusion of antigen-presenting cells with exosomes
derived from dendritic cells (82). This study has demonstrated the
activation of CD4+ T cells in vivo following
injection of exosomes carrying antigens (82). With the presence of MHC class
II-negative but T cell costimulatory molecules-positive dendritic
cells, antigen-bearing exosomes can activate T cells in
vitro (82). This indicates
that peptide-MHC complexes can be exchanged by exosomes, and
presented to T cells to initiate an adaptive immune response
(82).
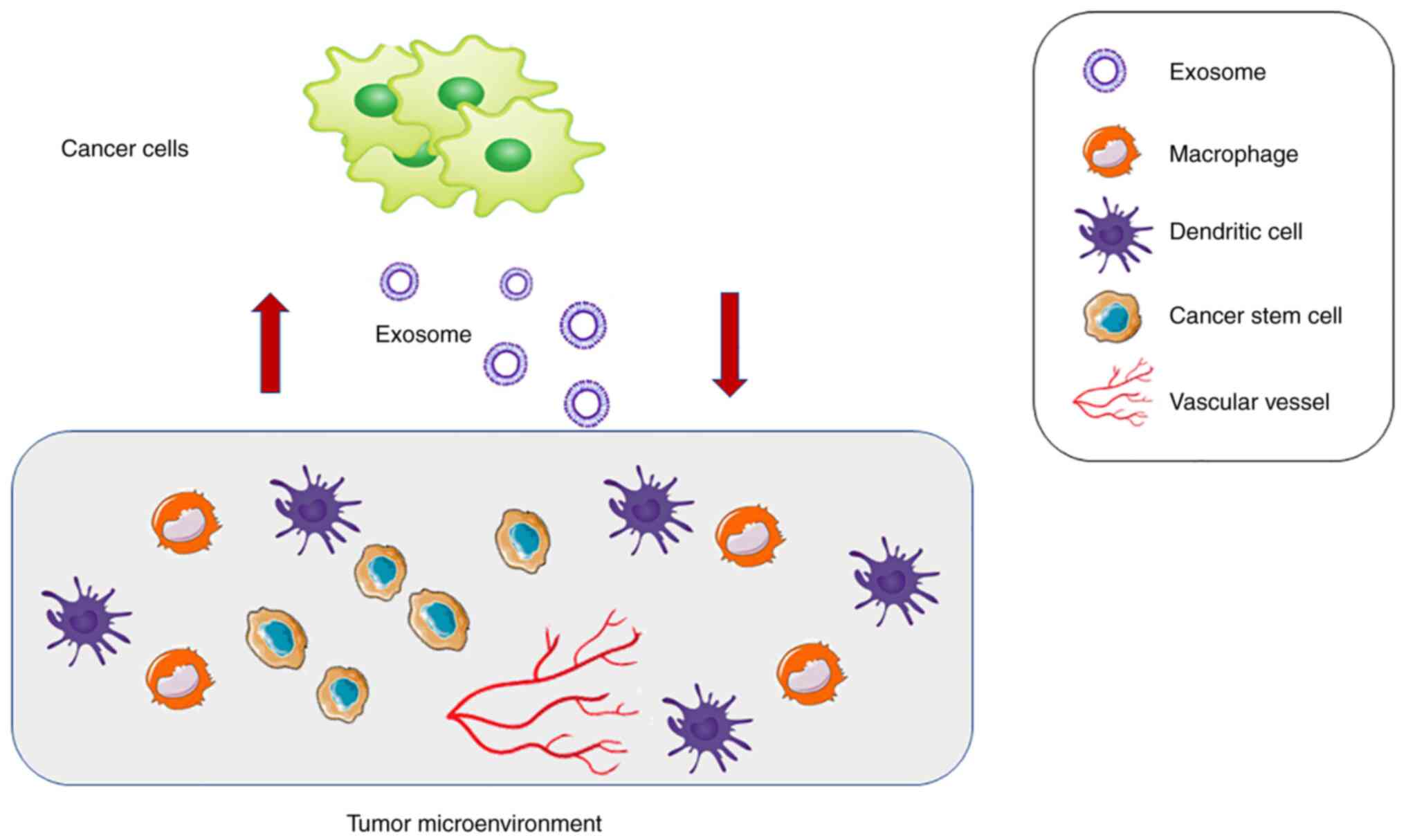
The interaction between tumor cells and the TME is
mediated by exosomes. The role of tumor cells and the TME is not a
one-way information transfer but a two-way information exchange
(29). Exosomes as communicators
between cancer cells and the tumor environment are shown in
Fig. 2. The targeted killing of
tumor cells is not sufficient for the treatment of tumors. The TME
is vital in tumor occurrence, and thus, has gained attention.
Exosomes are messengers between tumor cells and the TME. Therefore,
exosomes can be used as tools for the development of novel
therapeutic strategies (83).
At present, researchers can trace exosomes, for
example through the use of labeling tetraspanins, including CD63,
CD9 and CD82, which can also help visualize the interaction between
exosomes and tumor stromal cells. Researchers can use a technique
based on the Cre recombinase-locus of X (cross)-over in P1 system
to label exosome transportation by immunofluorescent signals
(84). When the exosomes are
released by cells expressing the Cre recombinase, they are absorbed
by Cre reporter cells, and the latter can display fluorescence
signals. Based on this technique, researchers can track exosomes
in vivo and in vitro (84).
The interaction between exosomes and the TME
regulates all aspects of tumor development, including the promotion
of tumor cell proliferation, the enhancement of stromal cell
transformation, the induction of tolerance of tumor cells to
chemotherapeutic drugs and the promotion of distant metastasis
(54). Participation of exosomes
in all aspects of tumor biological mechanisms makes them good
targets for antitumor treatment. Blocking the production and
release of exosomes and their uptake by receptor cells may be an
alternative treatment strategy for the development of an antitumor
treatment that targets exosomes.
Not applicable.
Funding: No funding was received.
Not applicable.
QL wrote, read and approved the final manuscript.
Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Johnstone RM, Adam M, Hammond JR, Orr L
and Turbide C: Vesicle formation during reticulocyte maturation.
Association of plasma membrane activities with released vesicles
(exosomes). J Biol Chem. 262:9412–9420. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Johnstone RM: Revisiting the road to the
discovery of exosomes. Blood Cells Mol Dis. 34:214–219. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Colombo M, Raposo G and Théry C:
Biogenesis, secretion, and intercellular interactions of exosomes
and other extracellular vesicles. Annu Rev Cell Dev Biol.
30:255–289. 2014. View Article : Google Scholar
|
|
4
|
Kalluri R: The biology and function of
exosomes in cancer. J Clin Invest. 126:1208–1215. 2016. View Article : Google Scholar
|
|
5
|
Kahlert C, Melo SA, Protopopov A, Tang J,
Seth S, Koch M, Zhang J, Weitz J, Chin L, Futreal A and Kalluri R:
Identification of double-stranded genomic DNA spanning all
chromosomes with mutated KRAS and p53 DNA in the serum exosomes of
patients with pancreatic cancer. J Biol Chem. 289:3869–3875. 2014.
View Article : Google Scholar
|
|
6
|
Escola JM, Kleijmeer MJ, Stoorvogel W,
Griffith JM, Yoshie O and Geuze HJ: Selective enrichment of
tetraspan proteins on the internal vesicles of multivesicular
endosomes and on exosomes secreted by human B-lymphocytes. J Biol
Chem. 273:20121–20127. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vlassov AV, Magdaleno S, Setterquist R and
Conrad R: Exosomes: Current knowledge of their composition,
biological functions, and diagnostic and therapeutic potentials.
Biochim Biophys Acta. 1820:940–948. 2012. View Article : Google Scholar
|
|
8
|
Bobrie A, Colombo M, Krumeich S, Raposo G
and Théry C: Diverse subpopulations of vesicles secreted by
different intracellular mechanisms are present in exosome
preparations obtained by differential ultracentrifugation. J
Extracell Vesicles. 1:2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Théry C, Boussac M, Véron P,
Ricciardi-Castagnoli P, Raposo G, Garin J and Amigorena S:
Proteomic analysis of dendritic cell-derived exosomes: A secreted
subcellular compartment distinct from apoptotic vesicles. J
Immunol. 166:7309–7318. 2001. View Article : Google Scholar
|
|
10
|
Chen H, Chengalvala V, Hu H and Sun D:
Tumor-derived exosomes: Nanovesicles made by cancer cells to
promote cancer metastasis. Acta Pharm Sin B. 11:2136–2149. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Raulf N, Lucarelli P, Thavaraj S, Brown S,
Vicencio JM, Sauter T and Tavassoli M: Annexin A1 regulates EGFR
activity and alters EGFR-containing tumour-derived exosomes in head
and neck cancers. Eur J Cancer. 102:52–68. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dai J, Su Y, Zhong S, Cong L, Liu B, Yang
J, Tao Y, He Z, Chen C and Jiang Y: Exosomes: Key players in cancer
and potential therapeutic strategy. Signal Transduct Target Ther.
5:1452020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun W, Luo JD, Jiang H and Duan DD: Tumor
exosomes: A double-edged sword in cancer therapy. Acta Pharmacol
Sin. 39:534–541. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wollert T and Hurley JH: Molecular
mechanism of multivesicular body biogenesis by ESCRT complexes.
Nature. 464:864–869. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Raiborg C and Stenmark H: The ESCRT
machinery in endosomal sorting of ubiquitylated membrane proteins.
Nature. 458:445–452. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Michelet X, Djeddi A and Legouis R:
Developmental and cellular functions of the ESCRT machinery in
pluricellular organisms. Biol Cell. 102:191–202. 2010. View Article : Google Scholar
|
|
17
|
Bhuin T and Roy JK: Rab proteins: The key
regulators of intracellular vesicle transport. Exp Cell Res.
328:1–19. 2014. View Article : Google Scholar
|
|
18
|
Ostrowski M, Carmo NB, Krumeich S, Fanget
I, Raposo G, Savina A, Moita CF, Schauer K, Hume AN, Freitas RP, et
al: Rab27a and Rab27b control different steps of the exosome
secretion pathway. Nat Cell Biol. 12:19–30. 1–13. 2010. View Article : Google Scholar
|
|
19
|
Chaput N, Flament C, Viaud S, Taieb J,
Roux S, Spatz A, André F, LePecq JB, Boussac M, Garin J, et al:
Dendritic cell derived-exosomes: Biology and clinical
implementations. J Leukoc Biol. 80:471–478. 2006. View Article : Google Scholar
|
|
20
|
Li W, Li C, Zhou T, Liu X, Liu X, Li X and
Chen D: Role of exosomal proteins in cancer diagnosis. Mol Cancer.
16:1452017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li Q, Shao Y, Zhang X, Zheng T, Miao M,
Qin L, Wang B, Ye G, Xiao B and Guo J: Plasma long noncoding RNA
protected by exosomes as a potential stable biomarker for gastric
cancer. Tumour Biol. 36:2007–2012. 2015. View Article : Google Scholar
|
|
22
|
Smolarz M and Widlak P: Serum exosomes and
their miRNA load-a potential biomarker of lung cancer. Cancers
(Basel). 13:13732021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lorenc T, Klimczyk K, Michalczewska I,
Słomka M, Kubiak-Tomaszewska G and Olejarz W: Exosomes in prostate
cancer diagnosis, prognosis and therapy. Int J Mol Sci.
21:21182020. View Article : Google Scholar
|
|
24
|
Zhang J, Li S, Li L, Li M, Guo C, Yao J
and Mi S: Exosome and exosomal microRNA: Trafficking, sorting, and
function. Genomics Proteomics Bioinformatics. 13:17–24. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gutkin A, Uziel O, Beery E, Nordenberg J,
Pinchasi M, Goldvaser H, Henick S, Goldberg M and Lahav M: Tumor
cells derived exosomes contain hTERT mRNA and transform
nonmalignant fibroblasts into telomerase positive cells.
Oncotarget. 7:59173–59188. 2016. View Article : Google Scholar
|
|
26
|
Farahani M, Rubbi C, Liu L, Slupsky JR and
Kalakonda N: CLL exosomes modulate the transcriptome and behaviour
of recipient stromal cells and are selectively enriched in
miR-202-3p. PLoS One. 10:e01414292015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhuang G, Wu X, Jiang Z, Kasman I, Yao J,
Guan Y, Oeh J, Modrusan Z, Bais C, Sampath D and Ferrara N:
Tumour-secreted miR-9 promotes endothelial cell migration and
angiogenesis by activating the JAK-STAT pathway. EMBO J.
31:3513–3523. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mathivanan S, Ji H and Simpson RJ:
Exosomes: Extracellular organelles important in intercellular
communication. J Proteomics. 73:1907–1920. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kharaziha P, Ceder S, Li Q and Panaretakis
T: Tumor cell-derived exosomes: A message in a bottle. Biochim
Biophys Acta. 1826:103–111. 2012.
|
|
30
|
Hood JL, San RS and Wickline SA: Exosomes
released by melanoma cells prepare sentinel lymph nodes for tumor
metastasis. Cancer Res. 71:3792–3801. 2011. View Article : Google Scholar
|
|
31
|
Luga V and Wrana JL: Tumor-stroma
interaction: Revealing fibroblast-secreted exosomes as potent
regulators of Wnt-planar cell polarity signaling in cancer
metastasis. Cancer Res. 73:6843–6847. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nogués L, Benito-Martin A,
Hergueta-Redondo M and Peinado H: The influence of tumour-derived
extracellular vesicles on local and distal metastatic
dissemination. Mol Aspects Med. 60:15–26. 2018. View Article : Google Scholar
|
|
33
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar
|
|
34
|
Maia J, Caja S, Strano Moraes MC, Couto N
and Costa-Silva B: Exosome-based cell-cell communication in the
tumor microenvironment. Front Cell Dev Biol. 6:182018. View Article : Google Scholar
|
|
35
|
Luga V, Zhang L, Viloria-Petit AM,
Ogunjimi AA, Inanlou MR, Chiu E, Buchanan M, Hosein AN, Basik M and
Wrana JL: Exosomes mediate stromal mobilization of autocrine
Wnt-PCP signaling in breast cancer cell migration. Cell.
151:1542–1556. 2012. View Article : Google Scholar
|
|
36
|
Peinado H, Alečković M, Lavotshkin S,
Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M,
Williams C, García-Santos G, Ghajar C, et al: Melanoma exosomes
educate bone marrow progenitor cells toward a pro-metastatic
phenotype through MET. Nat Med. 18:883–891. 2012. View Article : Google Scholar
|
|
37
|
Rahman MA, Barger JF, Lovat F, Gao M,
Otterson GA and Nana-Sinkam P: Lung cancer exosomes as drivers of
epithelial mesenchymal transition. Oncotarget. 7:54852–54866. 2016.
View Article : Google Scholar
|
|
38
|
Costa-Silva B, Aiello NM, Ocean AJ, Singh
S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, et
al: Pancreatic cancer exosomes initiate pre-metastatic niche
formation in the liver. Nat Cell Biol. 17:816–826. 2015. View Article : Google Scholar
|
|
39
|
Zhang H, Deng T, Liu R, Bai M, Zhou L,
Wang X, Li S, Wang X, Yang H, Li J, et al: Exosome-delivered EGFR
regulates liver microenvironment to promote gastric cancer liver
metastasis. Nat Commun. 8:150162017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mashouri L, Yousefi H, Aref AR, Ahadi AM,
Molaei F and Alahari SK: Exosomes: Composition, biogenesis, and
mechanisms in cancer metastasis and drug resistance. Mol Cancer.
18:752019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Torreggiani E, Roncuzzi L, Perut F, Zini N
and Baldini N: Multimodal transfer of MDR by exosomes in human
osteosarcoma. Int J Oncol. 49:189–196. 2016. View Article : Google Scholar
|
|
42
|
Wang J, Hendrix A, Hernot S, Lemaire M, De
Bruyne E, Van Valckenborgh E, Lahoutte T, De Wever O, Vanderkerken
K and Menu E: Bone marrow stromal cell-derived exosomes as
communicators in drug resistance in multiple myeloma cells. Blood.
124:555–566. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Corcoran C, Rani S, O'Brien K, O'Neill A,
Prencipe M, Sheikh R, Webb G, McDermott R, Watson W, Crown J and
O'Driscoll L: Docetaxel-resistance in prostate cancer: Evaluating
associated phenotypic changes and potential for resistance transfer
via exosomes. PLoS One. 7:e509992012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ciravolo V, Huber V, Ghedini GC,
Venturelli E, Bianchi F, Campiglio M, Morelli D, Villa A, Della
Mina P, Menard S, et al: Potential role of HER2-overexpressing
exosomes in countering trastuzumab-based therapy. J Cell Physiol.
227:658–567. 2012. View Article : Google Scholar
|
|
45
|
Safaei R, Larson BJ, Cheng TC, Gibson MA,
Otani S, Naerdemann W and Howell SB: Abnormal lysosomal trafficking
and enhanced exosomal export of cisplatin in drug-resistant human
ovarian carcinoma cells. Mol Cancer Ther. 4:1595–1604. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hu Y, Yan C, Mu L, Huang K, Li X, Tao D,
Wu Y and Qin J: Fibroblast-derived exosomes contribute to
chemoresistance through priming cancer stem cells in colorectal
cancer. PLoS One. 10:e01256252015. View Article : Google Scholar
|
|
47
|
Yang SJ, Wang DD, Li J, Xu HZ, Shen HY,
Chen X, Zhou SY, Zhong SL, Zhao JH and Tang JH: Predictive role of
GSTP1-containing exosomes in chemotherapy-resistant breast cancer.
Gene. 623:5–14. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kim J and Bae JS: Tumor-associated
macrophages and neutrophils in tumor microenvironment. Mediators
Inflamm. 2016:60581472016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Xouri G and Christian S: Origin and
function of tumor stroma fibroblasts. Semin Cell Dev Biol.
21:40–46. 2010. View Article : Google Scholar
|
|
50
|
Baroni S, Romero-Cordoba S, Plantamura I,
Dugo M, D'Ippolito E, Cataldo A, Cosentino G, Angeloni V, Rossini
A, Daidone MG and Iorio MV: Exosome-mediated delivery of miR-9
induces cancer-associated fibroblast-like properties in human
breast fibroblasts. Cell Death Dis. 7:e23122016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Cho JA, Park H, Lim EH and Lee KW:
Exosomes from breast cancer cells can convert adipose
tissue-derived mesenchymal stem cells into myofibroblast-like
cells. Int J Oncol. 40:130–138. 2012.
|
|
52
|
Cho JA, Park H, Lim EH, Kim KH, Choi JS,
Lee JH, Shin JW and Lee KW: Exosomes from ovarian cancer cells
induce adipose tissue-derived mesenchymal stem cells to acquire the
physical and functional characteristics of tumor-supporting
myofibroblasts. Gynecol Oncol. 123:379–386. 2011. View Article : Google Scholar
|
|
53
|
Gu J, Qian H, Shen L, Zhang X, Zhu W,
Huang L, Yan Y, Mao F, Zhao C, Shi Y and Xu W: Gastric cancer
exosomes trigger differentiation of umbilical cord derived
mesenchymal stem cells to carcinoma-associated fibroblasts through
TGF-β/Smad pathway. PLoS One. 7:e524652012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Milane L, Singh A, Mattheolabakis G,
Suresh M and Amiji MM: Exosome mediated communication within the
tumor microenvironment. J Control Release. 219:278–294. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Han Y, Ren J, Bai Y, Pei X and Han Y:
Exosomes from hypoxia-treated human adipose-derived mesenchymal
stem cells enhance angiogenesis through VEGF/VEGF-R. Int J Biochem
Cell Biol. 109:59–68. 2019. View Article : Google Scholar
|
|
56
|
Li Z, Zeng C, Nong Q, Long F, Liu J, Mu Z,
Chen B, Wu D and Wu H: Exosomal leucine-rich-alpha2-glycoprotein 1
derived from non-small-cell lung cancer cells promotes angiogenesis
via TGF-β signal pathway. Mol Ther Oncolytics. 14:313–322. 2019.
View Article : Google Scholar
|
|
57
|
Paggetti J, Haderk F, Seiffert M, Janji B,
Distler U, Ammerlaan W, Kim YJ, Adam J, Lichter P, Solary E, et al:
Exosomes released by chronic lymphocytic leukemia cells induce the
transition of stromal cells into cancer-associated fibroblasts.
Blood. 126:1106–1117. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhu W, Huang L, Li Y, Zhang X, Gu J, Yan
Y, Xu X, Wang M, Qian H and Xu W: Exosomes derived from human bone
marrow mesenchymal stem cells promote tumor growth in vivo. Cancer
Lett. 315:28–37. 2012. View Article : Google Scholar
|
|
59
|
Languino LR, Singh A, Prisco M, Inman GJ,
Luginbuhl A, Curry JM and South AP: Exosome-mediated transfer from
the tumor microenvironment increases TGFβ signaling in squamous
cell carcinoma. Am J Transl Res. 8:2432–2437. 2016.PubMed/NCBI
|
|
60
|
Webber J, Steadman R, Mason MD, Tabi Z and
Clayton A: Cancer exosomes trigger fibroblast to myofibroblast
differentiation. Cancer Res. 70:9621–9630. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Olumi AF, Grossfeld GD, Hayward SW,
Carroll PR, Tlsty TD and Cunha GR: Carcinoma-associated fibroblasts
direct tumor progression of initiated human prostatic epithelium.
Cancer Res. 59:5002–5011. 1999.PubMed/NCBI
|
|
62
|
Orimo A, Gupta PB, Sgroi DC,
Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL
and Weinberg RA: Stromal fibroblasts present in invasive human
breast carcinomas promote tumor growth and angiogenesis through
elevated SDF-1/CXCL12 secretion. Cell. 121:335–348. 2005.
View Article : Google Scholar
|
|
63
|
Bakir B, Chiarella AM, Pitarresi JR and
Rustgi AK: EMT, MET, plasticity, and tumor metastasis. Trends Cell
Biol. 30:764–776. 2020. View Article : Google Scholar
|
|
64
|
Kim H, Lee S, Shin E, Seong KM, Jin YW,
Youn H and Youn B: The emerging roles of exosomes as EMT regulators
in cancer. Cells. 9:8612020. View Article : Google Scholar
|
|
65
|
Yoshizaki T, Kondo S, Wakisaka N, Murono
S, Endo K, Sugimoto H, Nakanishi S, Tsuji A and Ito M: Pathogenic
role of Epstein-Barr virus latent membrane protein-1 in the
development of nasopharyngeal carcinoma. Cancer Lett. 337:1–7.
2013. View Article : Google Scholar
|
|
66
|
Lin Q, Zhou CR, Bai MJ, Zhu D, Chen JW,
Wang HF, Li MA, Wu C, Li ZR and Huang MS: Exosome-mediated miRNA
delivery promotes liver cancer EMT and metastasis. Am J Transl Res.
12:1080–1095. 2020.PubMed/NCBI
|
|
67
|
Li YY, Tao YW, Gao S, Li P, Zheng JM,
Zhang SE, Liang J and Zhang Y: Cancer-associated fibroblasts
contribute to oral cancer cells proliferation and metastasis via
exosome-mediated paracrine miR-34a-5p. EbioMedicine. 36:209–220.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zhang X, Sai B, Wang F, Wang L, Wang Y,
Zheng L, Li G, Tang J and Xiang J: Hypoxic BMSC-derived exosomal
miRNAs promote metastasis of lung cancer cells via STAT3-induced
EMT. Mol Cance. 18:402019. View Article : Google Scholar
|
|
69
|
Yang C, Dou R, Wei C, Liu K, Shi D, Zhang
C, Liu Q, Wang S and Xiong B: Tumor-derived exosomal
microRNA-106b-5p activates EMT-cancer cell and M2-subtype TAM
interaction to facilitate CRC metastasis. Mol Ther. 29:2088–2107.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Wang L, Yang G, Zhao D, Wang J, Bai Y,
Peng Q, Wang H, Fang R, Chen G, Wang Z, et al: CD103-positive CSC
exosome promotes EMT of clear cell renal cell carcinoma: Role of
remote MiR-19b-3p. Mol Cancer. 18:862019. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Mishra S, Yadav T and Rani V: Exploring
miRNA based approaches in cancer diagnostics and therapeutics. Crit
Rev Oncol Hematol. 98:12–23. 2016. View Article : Google Scholar
|
|
72
|
Sun X, Lin F, Sun W, Zhu W, Fang D, Luo L,
Li S, Zhang W and Jiang L: Exosome-transmitted miRNA-335-5p
promotes colorectal cancer invasion and metastasis by facilitating
EMT via targeting RASA1. Mol Ther Nucleic Acids. 24:164–174. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Olejarz W, Dominiak A, Żołnierzak A,
Kubiak-Tomaszewska G and Lorenc T: Tumor-derived exosomes in
immunosuppression and immunotherapy. J Immunol Res.
2020:62724982020. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Graner MW, Schnell S and Olin MR:
Tumor-derived exosomes, microRNAs, and cancer immune suppression.
Semin Immunopathol. 40:505–515. 2018. View Article : Google Scholar
|
|
75
|
Szajnik M, Czystowska M, Szczepanski MJ,
Mandapathil M and Whiteside TL: Tumor-derived microvesicles induce,
expand and up-regulate biological activities of human regulatory T
cells (Treg). PLoS One. 5:e114692010. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Wieckowski EU, Visus C, Szajnik M,
Szczepanski MJ, Storkus WJ and Whiteside TL: Tumor-derived
microvesicles promote regulatory T cell expansion and induce
apoptosis in tumor-reactive activated CD8+ T
lymphocytes. J Immunol. 183:3720–3730. 2009. View Article : Google Scholar
|
|
77
|
Pitt JM, André F, Amigorena S, Soria JC,
Eggermont A, Kroemer G and Zitvogel L: Dendritic cell-derived
exosomes for cancer therapy. J Clin Invest. 126:1224–1232. 2016.
View Article : Google Scholar
|
|
78
|
Tkach M, Kowal J, Zucchetti AE, Enserink
L, Jouve M, Lankar D, Saitakis M, Martin-Jaular L and Théry C:
Qualitative differences in T-cell activation by dendritic
cell-derived extracellular vesicle subtypes. EMBO J. 36:3012–3028.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Montecalvo A, Shufesky WJ, Stolz DB,
Sullivan MG, Wang Z, Divito SJ, Papworth GD, Watkins SC, Robbins
PD, Larregina AT and Morelli AE: Exosomes as a short-range
mechanism to spread alloantigen between dendritic cells during T
cell allorecognition. J Immunol. 180:3081–3090. 2008. View Article : Google Scholar
|
|
80
|
Whiteside TL: Exosomes and tumor-mediated
immune suppression. J Clin Invest. 126:1216–1223. 2016. View Article : Google Scholar
|
|
81
|
Wolfers J, Lozier A, Raposo G, Regnault A,
Théry C, Masurier C, Flament C, Pouzieux S, Faure F, Tursz T, et
al: Tumor-derived exosomes are a source of shared tumor rejection
antigens for CTL cross-priming. Nat Med. 7:297–303. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Théry C, Duban L, Segura E, Véron P, Lantz
O and Amigorena S: Indirect activation of naïve CD4+ T
cells by dendritic cell-derived exosomes. Nat Immunol. 3:1156–1162.
2002. View Article : Google Scholar
|
|
83
|
Xu Z, Zeng S, Gong Z and Yan Y:
Exosome-based immunotherapy: A promising approach for cancer
treatment. Mol Cancer. 19:1602020. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Mohr M, Tosun S, Arnold WH, Edenhofer F,
Zänker KS and Dittmar T: Quantification of cell fusion events human
breast cancer cells and breast epithelial cells using a
Cre-LoxP-based double fluorescence reporter system. Cell Mol Life
Sci. 72:3769–3782. 2015. View Article : Google Scholar
|