Introduction
Colorectal cancer is the primary cause of
tumor-associated mortality and morbidity worldwide (1–3).
Notwithstanding the advantages of early high-quality detection,
screening, diagnosis, new chemotherapies, and surgery for enhanced
therapy and prognosis in patients with metastatic and advanced
colorectal cancer, the 5-year survival rate of these colorectal
cancer cases is still <10% (4,5).
Even though these therapeutic strategies attenuate cancer-related
growth and metastasis, they can result in severe toxic effects,
affecting healthy tissues, and interfering with therapeutic
progress and patient quality of life (6,7).
Consequently, the investigation and development of practical and
effective therapeutic agents, and strategies are urgently required
to improve the clinical outcomes of patients with colorectal
cancer.
Traditional Chinese natural compounds are a valuable
source of tumor therapeutic candidates due to their practical
effectiveness and low toxicity (8,9).
Alismatis rhizoma (zexie) serves as the rhizome of Alisma
Orientale, which is an oceanic herb belonging to the Alismataceae
family and is broadly distributed in Japan, Korea, and China
(10). It has been extensively
adopted as a hypolipidemic agent and folk diuretic for >1,000
years in China and has been used to treat urinary tract infections,
edema, hypertension, and dysuria (11). Modern research medicine has
confirmed the anti-atherosclerotic, hypoglycemic, antihypertensive,
diuretic and anti-cancer effects of Alismatis rhizoma (12). Alismatis rhizoma contains several
active chemical constituents, including essential oils, diterpenes,
sesquiterpenes, polysaccharides and triterpenoids (13). Alisol A serves as a tetracyclic
protostane-type triterpenoid and a major component of Alismatis
rhizoma (14). A study has
identified the antitumor effect of Alisol A on breast cancer cells
(15), but the function of Alisol
A in colorectal cancer development remains unknown.
In the present study, the effect of Alisol A on
proliferation, cell cycle, apoptosis, pyroptosis, migration and
invasion was investigated. The results confirmed that Alisol A
attenuated colorectal cancer by targeting PI3K/Akt signaling.
Materials and methods
Cell culture and cell treatment
Colorectal cancer cell lines (HCT-116 and HT-29)
were obtained from the Cell Bank of the Chinese Academy of
Sciences. The HCT-116 and HT-29 cells were cultured in RPMI-1640
medium with 10% FBS (both Biological Industries) and 1%
penicillin/streptomycin (Gibco; Thermo Fisher Scientific, Inc.).
Short tandem repeat confirmation of the HT-29 cell line (no.
VCPO20210816006STR01) was conducted by Shanghai VivaCell
Biosciences Ltd. Alisol A was obtained from the National
Pharmaceutical Engineering Research Center and its chemical
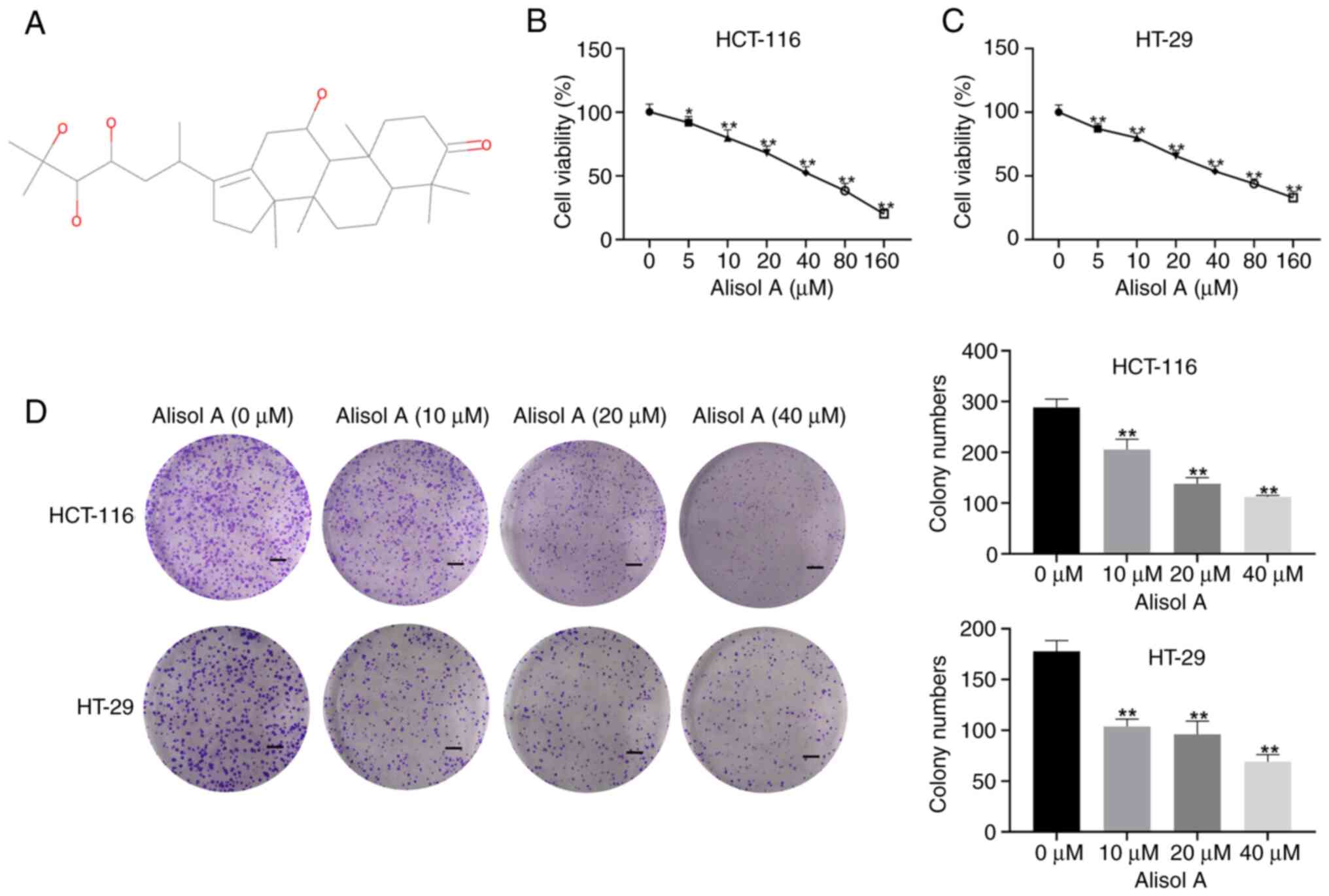
structure is shown in Fig. 1A. The
Akt inhibitor, SC79, and cisplatin (CIS) were purchased from
Sigma-Adrich (Merck KGaA). In rescue experiments, Alisol A and SC79
were simultaneously added to cells to validate the effect of
PI3K/Akt pathway in colorectal cancer cells.
MTT assays
MTT assays were performed to analyze the effect of
Alisol A at concentrations of 5, 10, 20, 40, 80, and 160 µM on the
cytotoxicity of HCT-116 and HT-29 cells. Approximately,
5×103/well cells were seeded into 96-well plates and
cultured for 24 h at 37°C. Then, MTT was added to the cells for 4 h
at 37°C. Following removal of MTT solution, DMSO (200 µl) was added
to each well. The optical density (490 nm) was determined using a
microplate reader (Bio-Rad Laboratories, Inc.).
Colony formation assays
A total of 500 HCT-116 and HT-29 cells were seeded
into 6-well plates and cultured for 14 days. The cells were then
fixed with 4% paraformaldehyde for 30 min at room temperature,
stained with 1% crystal violet (Beyotime Institute of
Biotechnology) for 20 min at room temperature, and washed three
times with PBS. Finally, the numbers of colonies containing >500
cells were assessed using a light microscope (magnification,
×100).
Apoptosis and cell cycle analysis
For cell apoptosis analysis, the HCT-116 and HT-29
cells (2×105 in each well) were resuspended in RNase
(Sigma-Aldrich), then stained with PI and Annexin V-fluorescein
isothiocyanate (Beyotime Institute of Biotechnology) for 20 min in
the dark at 37°C. The samples were then analyzed with a FACScan
flow cytometer (BD Biosciences), using CellQuest Pro
software(version 5.1, BD Biosciences).
For the cell cycle analysis, the HCT-116 and HT-29
cells were collected, washed with PBS, and then fixed for 24 h with
70% ice-cold ethanol at 4°C. Subsequently, RNase (MilliporeSigma)
was added to the cells and incubated for 30 min at 37°C. The cells
were then stained with PI (Beyotime Institute of Biotechnology) for
30 min at 4°C in the dark. Finally, cell cycle distribution was
then determined using FACSCalibur (BD Biosciences), using ModFit LT
software (version 3.1, Verity Software House).
Western blot analysis
RIPA (Beijing Solarbio Science & Technology Co.,
Ltd.) was used to prepare the cell lysate from HCT-116 and HT-29
cells. An enhanced BCA protein assay kit (Beyotime Institute of
Biotechnology) was used to quantify the protein concentration.
Then, the protein samples (50 µg) were separated using 10% SDS-PAGE
and transferred to PVDF membranes (0.45 µm; MilliporeSigma). The
membrane was incubated with antibodies against anti-Bcl-2 (1:1,000,
ab182858, Abcam), anti-Bax (1:1,000, ab32503, Abcam), anti-cleaved
caspase 3 (1:500, ab32042, Abcam), anti-caspase 3 (1:1,000,
ab32351, Abcam), anti-cleaved PARP (1:1,000, ab32064, Abcam),
anti-PARP (1:1,000, ab191217, Abcam), anti-caspase 1 (1:1,000,
#83383, Cell Signaling Technology, Inc.), anti-cleaved caspase 1
(1:500, #4199, Cell Signaling Technology, Inc.), anti-GSDMD
(1:1,000, ab210070, Abcam), anti-GSDME (1:1,000, ab215191, Abcam),
anti-E-cadherin (1:1,000, ab231303, Abcam), anti-N-cadherin
(1:1,000, ab76011, Abcam), anti-Vimentin (1:1,000, ab20346, Abcam),
anti-PI3K (1:1,000, #4255, Cell Signaling Technology, Inc.),
anti-Akt (1:1,000, #4691, Cell Signaling Technology, Inc.),
anti-mTOR (1:1,000, #2983, Cell Signaling Technology, Inc.),
anti-p-PI3K (1:1,000, #13857, Cell Signaling Technology, Inc.),
anti-p-Akt (1:1,000, #4060, Cell Signaling Technology, Inc.),
anti-p-mTOR (1:1,000, #2971, Cell Signaling Technology, Inc.), and
anti-GAPDH (1:1,000, ab125247, Abcam) (overnight at 4°C) after
blocking with 5% skimmed milk (2 h at 37°C). The results were
observed using an ECL kit (MilliporeSigma) after incubation with
horseradish peroxidase-labeled secondary antibody (1:5,000,
ab288151, Abcam) at room temperature for 2 h.
Lactate dehydrogenase(LDH)
analysis
LDH levels were measured using a LDH cytotoxicity
assay kit (BioVision, Inc.). Briefly, the cells were prepared using
Triton X-100 (0.2%; MilliporeSigma) and treated with LDH reaction
solution (100 µl; 30 min). The results were observed using a
microplate reader (490 nm; Bio-Rad Laboratories, Inc.).
Wound healing assay
The HCT-116 and HT-29 cells (3×105
cells/well) were seeded into 24-well plates. The cells were
cultured overnight at 37°C to 100% confluency, and wounds were
created in the cell monolayer using a 10-µl plastic pipette tip.
Subsequently, the cells were washed with PBS for three times, and
then serum-free medium was added into the wells for continuous
incubation. At 0 and 24 h after scratching, the images were
captured with a light microscope (magnification, ×100).
Evaluation of drug sensitivity
HCT-116 and HT-29 cells (2×104
cells/well) were added into 96-well plate. When the cells adhered
to the wall, CIS at different concentration was added into the
well. Subsequently, the cells were cultured in 5% CO2 at
37°C for 24 h. Then, Alisol A was added into the cells. Finally,
MTT assay and clone formation assay were performed to evaluate the
effect of Alisol A on the chemotherapeutic sensitivity of
colorectal cancer cells to CIS.
Bioinformatics
Signaling pathways involving Alisol A and colorectal
cancer were obtained via Bioinformatics Analysis Tool for Molecular
mechanism of Traditional Chinese Medicine (BATMAN;
bionet.ncpsb.org.cn/batman-tcm/) and Comparative Toxicgenomics
Database (CTD; ctdbase.org/). Then, the signaling pathways were
intersected using Venn analysis (version 2.1,
bioinfogp.cnb.csic.es/tools/venny/).
Statistical analysis
The experiments were performed three times
independently and the results are presented as the mean ± SEM. The
data were evaluated from multiple groups using one-way ANOVA with a
Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Alisol A represses colorectal cancer
cell proliferation in vitro
The function of Alisol A in modulating colorectal
cancer cell proliferation in vitro was assessed. MTT assays
showed that treatment with Alisol A dose-dependently decreased
cytotoxicity to HCT-116 and HT-29 cells at concentrations of 5, 10,
20, 40, 80, and 160 µM (Fig. 1B and
C), and 10, 20 and 40 µM Alisol A were used for subsequent
analysis. Similarly, the colony formation numbers of the HCT-116
and HT-29 cells were decreased by Alisol A (Fig. 1D and E), indicating that Alisol A
represses colorectal cancer cell proliferation in vitro.
Alisol A induces cell cycle arrest in
the colorectal cancer cells
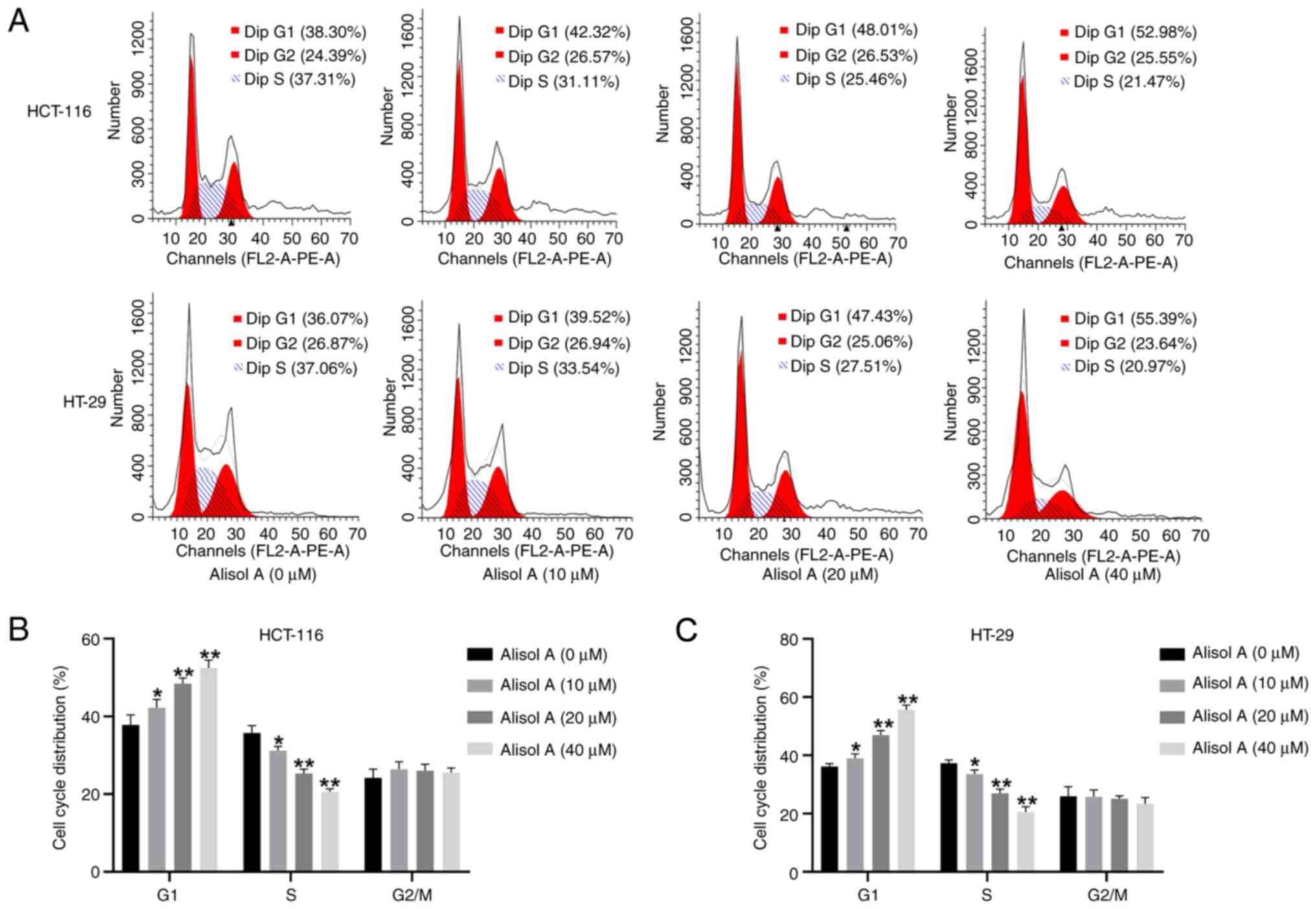
Then, the potential role of Alisol A in the cell
cycle of colorectal cancer cells was detected. For this purpose,
the HCT-116 and HT-29 cells were treated with Alisol A at
concentrations of 10, 20, and 40 µM and subjected to cell cycle
analysis using flow cytometry. These data showed that the number of
cells in G0/G1 phase was increased, while the
number of cells in S phase was decreased in Alisol A-treated
HCT-116 and HT-29 cells (Fig. 2A and
B), suggesting that Alisol A induces cell cycle arrest in
colorectal cancer cells.
Alisol A induces apoptosis and
pyroptosis of colorectal cancer cells
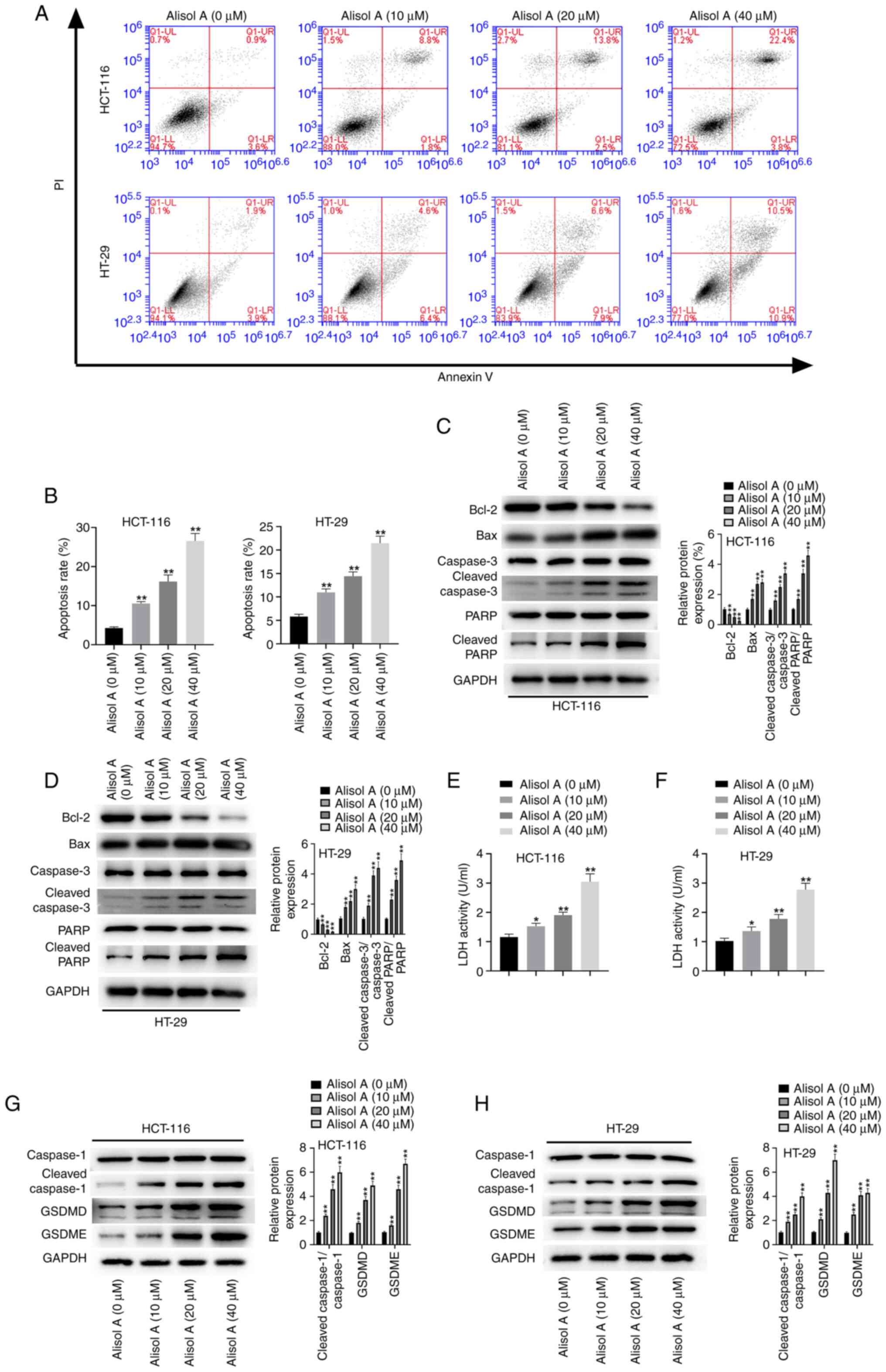
Next, the effect of Alisol A on apoptosis and
pyroptosis of colorectal cancer cells was evaluated. The results
demonstrated that HCT-116 and HT-29 cell apoptosis was stimulated
following treatment with Alisol A (Fig. 3A and B). The expression levels of
apoptosis-related proteins were detected and it was found that
Alisol A inhibited Bcl-2 protein expression level, but increased
Bax, cleaved caspase3, and cleaved PARP protein expression levels
in the HCT-116 and HT-29 cells (Fig.
3C and D). In addition, the levels of LDH were enhanced in
Alisol A-treated HCT-116 and HT-29 cells (Fig. 3E and F), suggesting that Alisol A
induces apoptosis in colorectal cancer cells. Meanwhile, treatment
with Alisol A upregulated the accumulation of pyroptosis-associated
factors, such as cleaved caspase 1, GSDMD and GSDME, in the HCT-116
and HT-29 cells (Fig. 3G and H),
indicating that Alisol A stimulates pyroptosis in colorectal cancer
cells.
Alisol A attenuates migration of
colorectal cancer cells
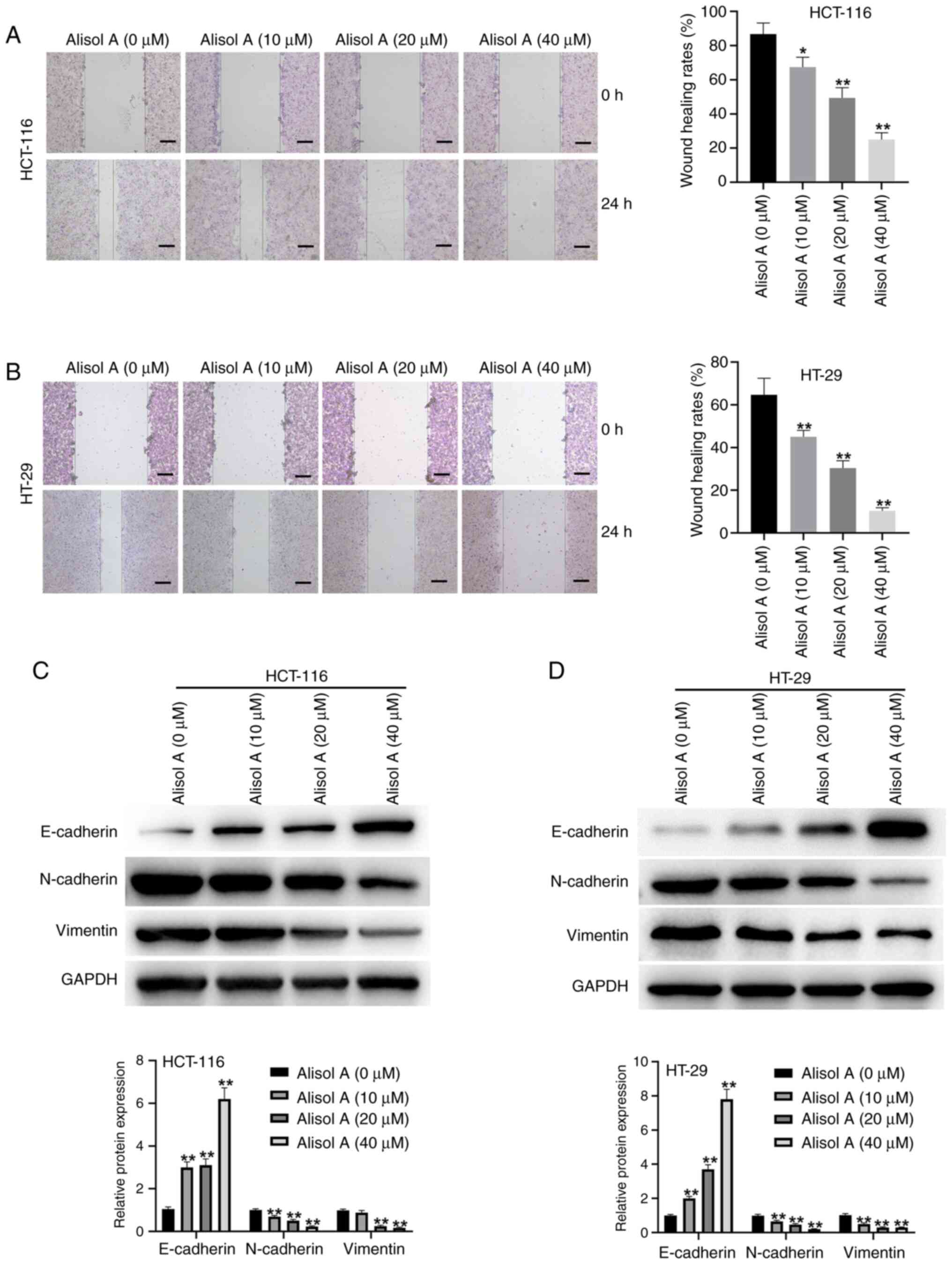
The association of Alisol A with the migration of
colorectal cancer cells was further assessed. The data showed that
Alisol A suppressed the migration ability of the HCT-116 and HT-29
cells, in a dose-dependent manner (Fig. 4A and B). Consistently, the effect
of Alisol A on epithelial-mesenchymal transition (EMT) markers,
including E-cadherin, N-cadherin and Vimentin, were also analyzed.
Western blot analysis demonstrated that Alisol A increased
E-cadherin protein expression levels, but decreased N-cadherin and
Vimentin protein expression levels in HCT-116 and HT-29 cells
(Fig. 4C and D).
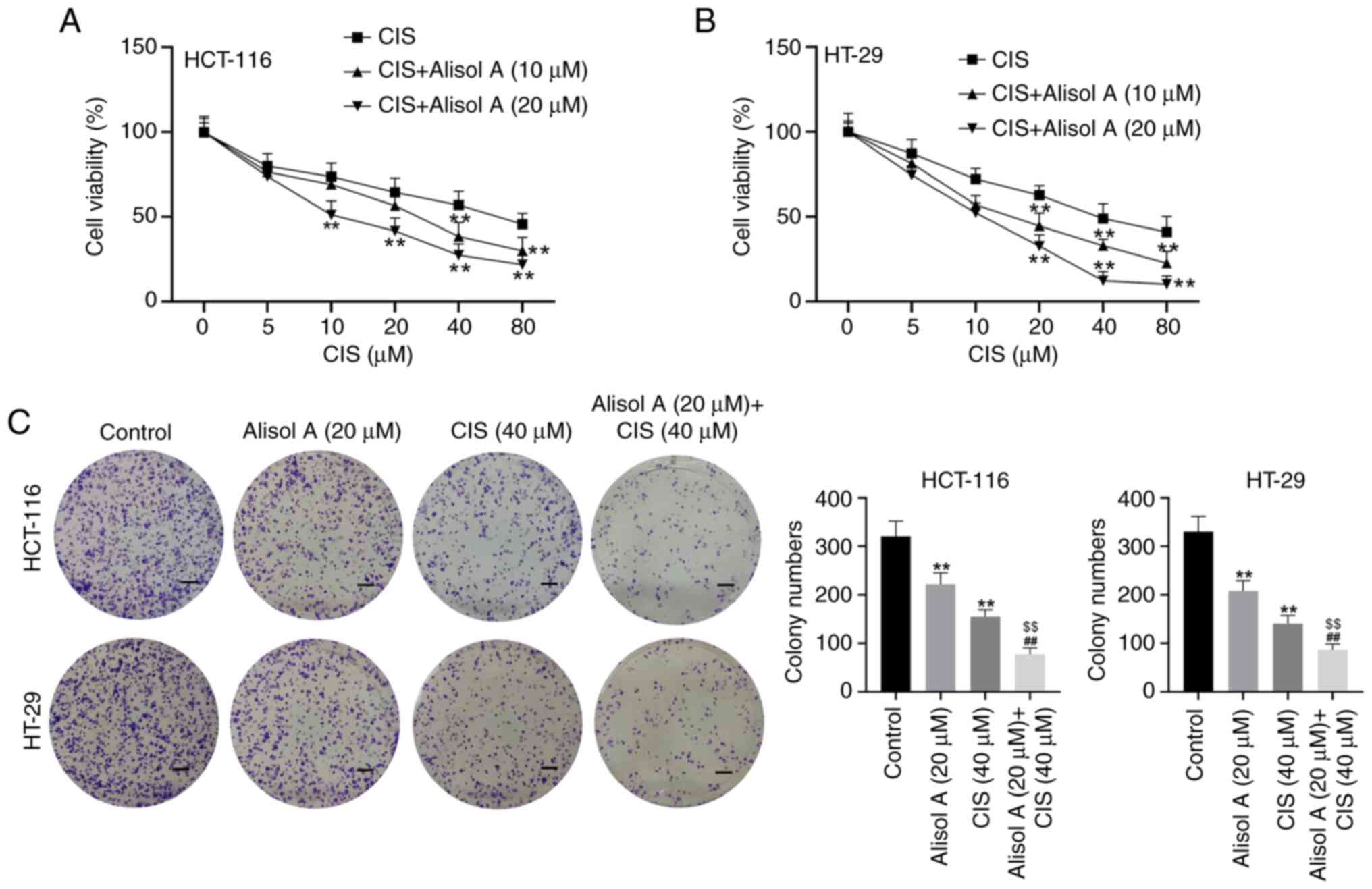
Alisol A increases the
chemotherapeutic sensitivity of colorectal cancer cells to CIS
MTT results showed that CIS reduced HCT-116 and
HT-29 cell viability, while co-treatment with CIS and Alisol A
reinforced this effect (Fig. 5A and
B). Moreover, the results from the colony formation assay also
confirmed that either CIS or Alisol reduced HCT-116 and HT-29
colony number, while co-treatment with CIS and Alisol A could
reinforce this effect (Fig. 5C).
These results suggest that Alisol A increases the chemotherapeutic
sensitivity of colorectal cancer cells to CIS.
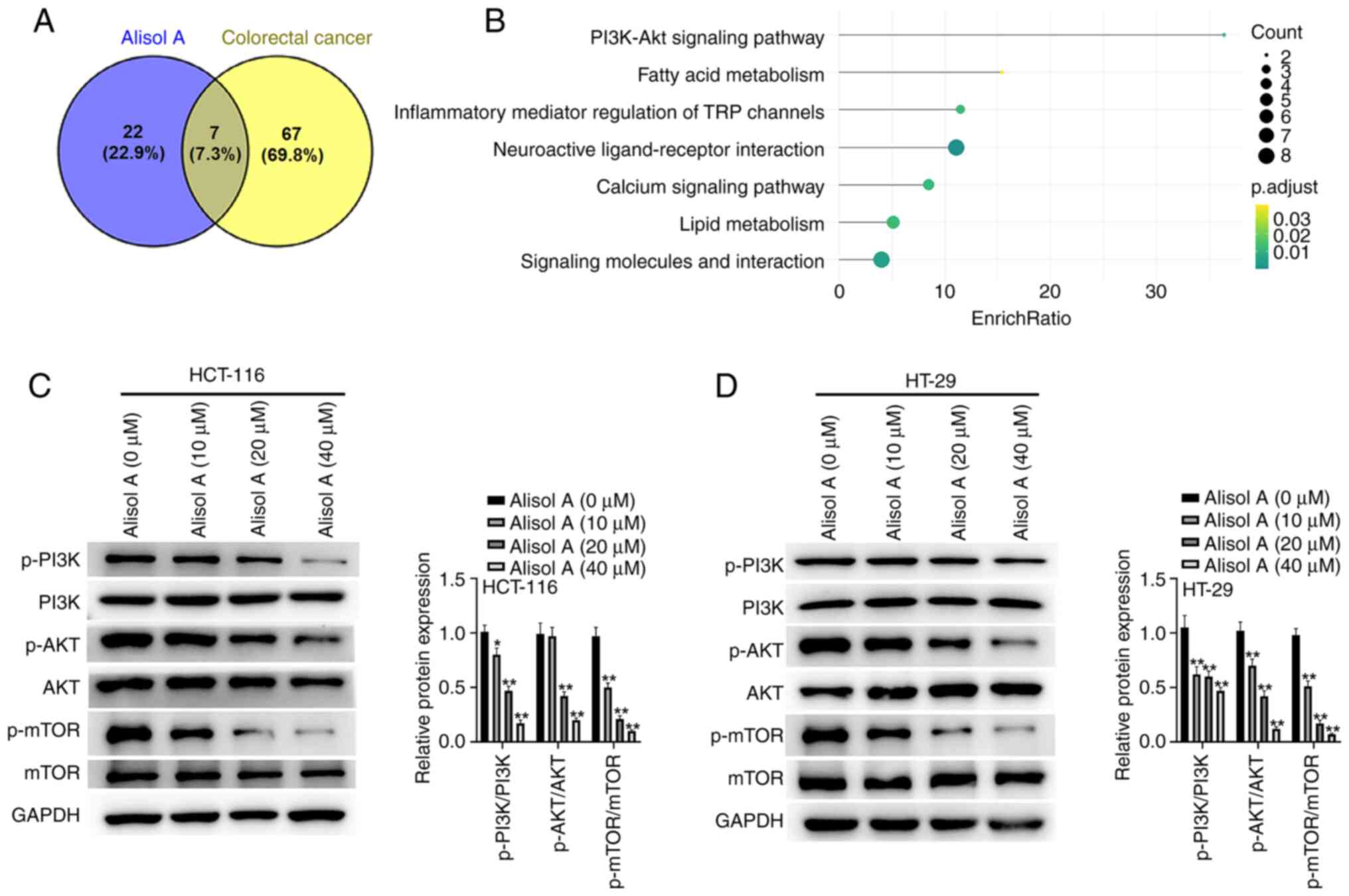
PI3K/Akt signaling is involved in
Alisol A-mediated colorectal cancer progression
Next, to investigate the potential mechanism
underlying Alisol A-mediated colorectal cancer progression,
bioinformatics analysis was performed using BATMAN and CTD. Venn
diagram of intersected candidate pathways was plotted (Fig. 6A), and the seven pathways were then
presented in Fig. 6B. Western blot
analysis confirmed that Alisol A repressed the phosphorylation
levels of PI3K, Akt, and mTOR in the HCT-116 and HT-29 cells
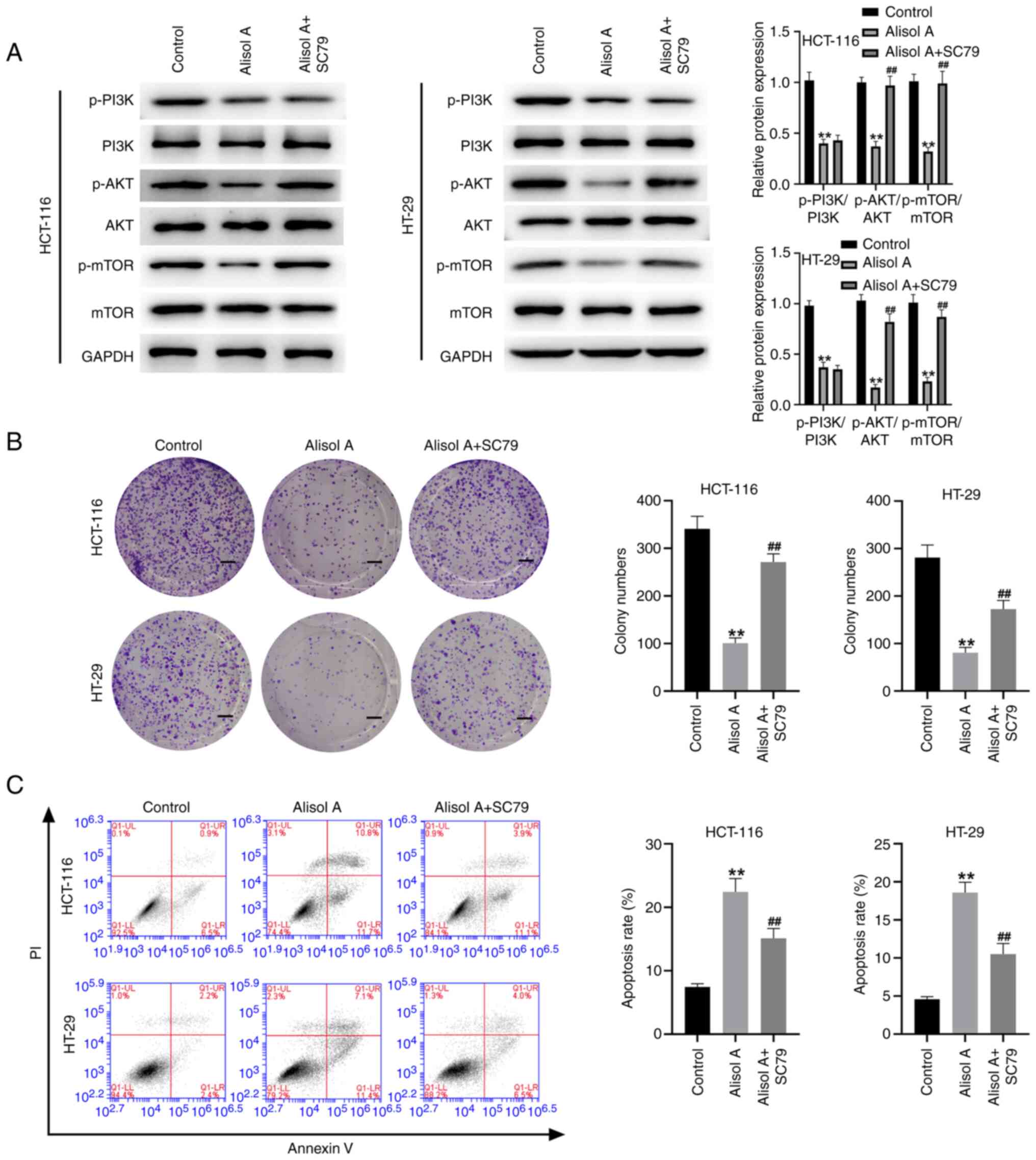
(Fig. 6C and D). The Akt activator
SC79 reversed the effect of Alisol A on the phosphorylation levels
of Akt and mTOR (Fig. 7A).
Moreover, treatment with Alisol A reduced viability and induced
apoptosis in the HCT-116 and HT-29 cells, in which the Akt
activator SC79 could reverse these phenotypes (Fig. 7B and C), indicating that PI3K/Akt
signaling could be associated with Alisol A-mediated colorectal
cancer progression.
Discussion
Colorectal cancer is one of the most prevalent
malignancies, leading to severe cancer-related mortality and
morbidity worldwide (2). Although
different strategies, such as chemotherapy and radiation therapy as
well as surgery, have been used for the treatment of colorectal
cancer, the overall survival rate of colorectal cancer patients is
relatively low (16,17). Therefore, the development of
innovative and practical treatment candidates is urgently required.
Alisol A is a natural agent from Alismatis rhizome and has shown
anti-cancer properties (15);
however, the role of Alisol A in colorectal cancer remains
unreported. In the present study, the effect and potential
mechanisms of Alisol A on proliferation, cell cycle, apoptosis,
pyroptosis, migration and invasion of colorectal cancer cells were
assessed.
Previous studies have identified several functions
of natural compounds in the treatment of colorectal cancer
(18,19). It has been reported that
resibufogenin represses metastasis and growth of colorectal cancer
by targeting RIP3-related necroptosis (20). Epigallocatechin-3-gallate
suppresses the stemness of colorectal cancer cells by inhibiting
the Wnt/β-catenin signaling pathway (21). Pancratistatin attenuates colorectal
cancer progression by initiating cell cycle arrest, autophagy and
apoptosis (22). Xanthohumol
decreases colorectal cancer cell proliferation by downregulating
hexokinases II-regulated glycolysis (23). Deoxypodophyllotoxin inhibits
colorectal cancer development by suppressing tumorigenesis and
inducing apoptosis (24).
Meanwhile, the constituents of Alismatis rhizoma (zexie) have shown
potential anti-cancer properties (12). The main protostane triterpenes of
Alismatis Rhizom are mainly composed of Alisol A, alisol B, alisol
B 23-acetate and Alisol A 24-acetate (25). In recent years, Alisol A is
reported to play an important role in numerous diseases. For
example, Alisol A represses metabolic disorders and high-fat
diet-related obesity via AMPK/ACC/SREBP-1c signaling (26). Alisol A relieves arterial plaque in
an apoE-knockout mouse model by enhancing AMPK-SIRT1 signaling
(14). Alisol A inhibits invasion,
migration and proliferation of breast cancer cells (27). Moreover, Chen and Liu (28) have reported that Alisol A inhibits
the proliferation, migration and invasion of nasopharyngeal
carcinoma cells by inhibiting the Hippo signaling pathway. However,
there has been no report regarding the effect of Alisol A on
colorectal cancer. In the present study, Alisol A repressed
proliferation, migration and induced cell cycle arrest, apoptosis,
and pyroptosis in colorectal cancer cells. Moreover, Alisol A
increased the chemotherapeutic sensitivity of colorectal cancer
cells to CIS. These data demonstrate an innovative anti-cancer
effect of Alisol A in colorectal cancer progression, presenting
valuable experimental basis for the function of Alisol A in the
treatment of colorectal cancer. Specifically, pyroptosis has been
reported to play critical roles in colorectal cancer development.
Pyroptosis has been associated with reducing the effect of FL118 on
the metastasis and growth of colorectal cancer cells (29). The present data showed that Alisol
A stimulated pyroptosis of colorectal cancer cells. This indicates
a new aspect of the anti-cancer function of Alisol A in colorectal
cancer tumorigenesis.
PI3K/Akt signaling is essential for the development
of colorectal cancer (30).
Furthermore, it has been reported that IMPDH2 induces the
progression of colorectal cancer by activating PI3K/AKT/FOXO1 and
PI3K/AKT/mTOR pathways (31).
DCLK1 enhances epithelial-mesenchymal transition in colorectal
cancer via PI3K/Akt/NF-κB signaling (32). Curcumol represses the proliferation
of colorectal cancer cells via miR-2/PTEN/PI3K/Akt signaling
(33). GLI1 increases the stemness
of colorectal cancer cells by PI3K/Akt/NF-κB signaling (34). FAT4 partially modulates autophagy
and EMT by affecting PI3K/AKT signaling pathway in colorectal
cancer cells (35). The present
results indicated that SC79 abolished the effect of Alisol A on the
phosphorylation levels of Akt and mTOR. In addition, SC79 could
also reverse the functions of Alisol A on the viability and
apoptosis in the colorectal cancer cells. This suggests that Alisol
A exerts its anti-cancer effect on colorectal cancer by targeting
PI3K/Akt signaling. However, the present study has some
limitations, including a lack of data on the effect of Alisol A in
other colorectal cancer cells and the effect of Alisol A in
vivo was not evaluated. These limitations will be investigated
further in future studies.
Alisol A induced an inhibitory effect on colorectal
cancer progression by inactivating PI3K/Akt signaling. Alisol A may
be used as a potential anticancer agent for the treatment of
colorectal cancer.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The dataset used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WH was involved in the conception and design of the
study. WX and KW were involved in performing the experiments. BW
and KB were involved in data analysis and interpretation. WH wrote
the manuscript. WH and KB confirm the authenticity of all the raw
data. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wrobel P and Ahmed S: Current status of
immunotherapy in metastatic colorectal cancer. Int J Colorectal
Dis. 34:13–25. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mármol I, Sánchez-de-Diego C, Pradilla
Dieste A, Cerrada E and Rodriguez Yoldi MJ: Colorectal carcinoma: A
general overview and future perspectives in colorectal cancer. Int
J Mol Sci. 18:1972017. View Article : Google Scholar
|
|
4
|
Boland PM, Yurgelun MB and Boland CR:
Recent progress in Lynch syndrome and other familial colorectal
cancer syndromes. CA Cancer J Clin. 68:217–231. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Das S, Ciombor KK, Haraldsdottir S and
Goldberg RM: Promising new agents for colorectal cancer. Curr Treat
Options Oncol. 19:292018. View Article : Google Scholar
|
|
6
|
Yu IS and Cheung WY: Metastatic colorectal
cancer in the era of personalized medicine: A more tailored
approach to systemic therapy. Can J Gastroenterol Hepatol.
2018:94507542018. View Article : Google Scholar
|
|
7
|
Zhai Z, Yu X, Yang B, Zhang Y, Zhang L, Li
X and Sun H: Colorectal cancer heterogeneity and targeted therapy:
Clinical implications, challenges and solutions for treatment
resistance. Semin Cell Dev Biol. 64:107–115. 2017. View Article : Google Scholar
|
|
8
|
Luo H, Vong CT, Chen H, Gao Y, Lyu P, Qiu
L, Zhao M, Liu Q, Cheng Z, Zou J, et al: Naturally occurring
anti-cancer compounds: Shining from Chinese herbal medicine. Chin
Med. 14:482019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yuan H, Ma Q, Ye L and Piao G: The
traditional medicine and modern medicine from natural products.
Molecules. 21:5592016. View Article : Google Scholar
|
|
10
|
Chen DQ, Feng YL, Tian T, Chen H, Yin L,
Zhao YY and Lin RC: Diuretic and anti-diuretic activities of
fractions of Alismatis rhizoma. J Ethnopharmacol. 157:114–118.
2014. View Article : Google Scholar
|
|
11
|
Li S, Wang L, Du Z, Jin S, Song C, Jia S,
Zhang Y and Jiang H: Identification of the lipid-lowering component
of triterpenes from Alismatis rhizoma based on the MRM-based
characteristic chemical profiles and support vector machine model.
Anal Bioanal Chem. 411:3257–3268. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang LL, Xu W, Xu YL, Chen X, Huang M and
Lu JJ: Therapeutic potential of rhizoma Alismatis: A review on
ethnomedicinal application, phytochemistry, pharmacology, and
toxicology. Ann N Y Acad Sci. 1401:90–101. 2017. View Article : Google Scholar
|
|
13
|
Liu SS, Sheng WL, Li Y, Zhang SS, Zhu JJ,
Gao HM, Yan LH, Wang ZM, Gao L and Zhang M: Chemical constituents
from Alismatis rhizoma and their anti-inflammatory activities in
vitro and in vivo. Bioorg Chem. 92:1032262019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang K, Zhang B, Song D, Xi J, Hao W, Yuan
J, Gao C, Cui Z and Cheng Z: Alisol A alleviates arterial plaque by
activating AMPK/SIRT1 signaling pathway in apoE-deficient mice.
Front Pharmacol. 11:5800732020. View Article : Google Scholar
|
|
15
|
Shi Y, Wang M, Wang P, Zhang T, Yu J, Shi
L, Li M, Wang H, Zhang Q and Zhao H: Alisol A is potentially
therapeutic in human breast cancer cells. Oncol Rep. 44:1266–1274.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen X, Zeng K, Xu M, Hu X, Liu X, Xu T,
He B, Pan Y, Sun H and Wang S: SP1-induced lncRNA-ZFAS1 contributes
to colorectal cancer progression via the miR-150-5p/VEGFA axis.
Cell Death Dis. 9:9822018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Haggar FA and Boushey RP: Colorectal
cancer epidemiology: Incidence, mortality, survival, and risk
factors. Clin Colon Rectal Surg. 22:191–197. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang XM, Yang ZJ, Xie Q, Zhang ZK, Zhang
H and Ma JY: Natural products for treating colorectal cancer: A
mechanistic review. Biomed Pharmacother. 117:1091422019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun X, Ng TTH, Sham KWY, Zhang L, Chan
MTV, Wu WKK and Cheng CHK: Bufalin, a traditional chinese medicine
compound, prevents tumor formation in two murine models of
colorectal cancer. Cancer Prev Res (Phila). 12:653–666. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Han Q, Ma Y, Wang H, Dai Y, Chen C, Liu Y,
Jing L and Sun X: Resibufogenin suppresses colorectal cancer growth
and metastasis through RIP3-mediated necroptosis. J Transl Med.
16:2012018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen Y, Wang XQ, Zhang Q, Zhu JY, Li Y,
Xie CF, Li XT, Wu JS, Geng SS, Zhong CY and Han HY:
(−)-Epigallocatechin-3-gallate inhibits colorectal cancer stem
cells by suppressing Wnt/β-catenin pathway. Nutrients. 9:5722017.
View Article : Google Scholar
|
|
22
|
Xiong Y, Xiong YJ, Liu DY and Shen RR:
Pancratistatin inhibits the growth of colorectal cancer cells by
inducing apoptosis, autophagy, and G2/M cell cycle arrest. Med Sci
Monit. 25:6015–6022. 2019. View Article : Google Scholar
|
|
23
|
Liu W, Li W, Liu H and Yu X: Xanthohumol
inhibits colorectal cancer cells via downregulation of hexokinases
II-mediated glycolysis. Int J Biol Sci. 15:2497–2508. 2019.
View Article : Google Scholar
|
|
24
|
Gamage CDB, Park SY, Yang Y, Zhou R, Taş
İ, Bae WK, Kim KK, Shim JH, Kim E, Yoon G and Kim H:
Deoxypodophyllotoxin exerts anti-cancer effects on colorectal
cancer cells through induction of apoptosis and suppression of
tumorigenesis. Int J Mol Sci. 20:26122019. View Article : Google Scholar
|
|
25
|
Xu W, Li X, Lin N, Zhang X, Huang X, Wu T,
Tai Y, Chen S, Wu CH, Huang M and Wu S: Pharmacokinetics and tissue
distribution of five major triterpenoids after oral administration
of rhizoma Alismatis extract to rats using ultra high-performance
liquid chromatography-tandem mass spectrometry. J Pharm Biomed
Anal. 146:314–323. 2017. View Article : Google Scholar
|
|
26
|
Ho C, Gao Y, Zheng D, Liu Y, Shan S, Fang
B, Zhao Y, Song D, Zhang Y and Li Q: Alisol A attenuates
high-fat-diet-induced obesity and metabolic disorders via the
AMPK/ACC/SREBP-1c pathway. J Cell Mol Med. 23:5108–5118. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lou C, Xu X, Chen Y and Zhao H: Alisol A
suppresses proliferation, migration, and invasion in human breast
cancer MDA-MB-231 cells. Molecules. 24:36512019. View Article : Google Scholar
|
|
28
|
Chen X and Liu H: Alisol A inhibited the
proliferation, migration, and invasion of nasopharyngeal carcinoma
cells by inhibiting the hippo signaling pathway. Yonsei Med J.
62:895–902. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tang Z, Ji L, Han M, Xie J, Zhong F, Zhang
X, Su Q, Yang Z, Liu Z, Gao H and Jiang G: Pyroptosis is involved
in the inhibitory effect of FL118 on growth and metastasis in
colorectal cancer. Life Sci. 257:1180652020. View Article : Google Scholar
|
|
30
|
Narayanankutty A: PI3K/Akt/mTOR pathway as
a therapeutic target for colorectal cancer: A review of preclinical
and clinical evidence. Curr Drug Targets. 20:1217–1226. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Duan S, Huang W, Liu X, Liu X, Chen N, Xu
Q, Hu Y, Song W and Zhou J: IMPDH2 promotes colorectal cancer
progression through activation of the PI3K/AKT/mTOR and
PI3K/AKT/FOXO1 signaling pathways. J Exp Clin Cancer Res.
37:3042018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu W, Wang S, Sun Q, Yang Z, Liu M and
Tang H: DCLK1 promotes epithelial-mesenchymal transition via the
PI3K/Akt/NF-κB pathway in colorectal cancer. Int J Cancer.
142:2068–2079. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu H, Wang J, Tao Y, Li X, Qin J, Bai Z,
Chi B, Yan W and Chen X: Curcumol inhibits colorectal cancer
proliferation by targeting miR-21 and modulated PTEN/PI3K/Akt
pathways. Life Sci. 221:354–361. 2019. View Article : Google Scholar
|
|
34
|
Yang Z, Zhang C, Qi W, Cui Y and Xuan Y:
GLI1 promotes cancer stemness through intracellular signaling
pathway PI3K/Akt/NFκB in colorectal adenocarcinoma. Exp Cell Res.
373:145–154. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wei R, Xiao Y, Song Y, Yuan H, Luo J and
Xu W: FAT4 regulates the EMT and autophagy in colorectal cancer
cells in part via the PI3K-AKT signaling axis. J Exp Clin Cancer
Res. 38:1122019. View Article : Google Scholar : PubMed/NCBI
|