Introduction
Lung cancer is a common malignancy worldwide and a
major cause of cancer-associated mortality (1,2). It
was estimated that lung cancer contributed to 2.1 million new
diagnoses and 1.8 million deaths worldwide (3). According to different histological
subtypes, lung cancer is classified into lung adenocarcinoma, lung
squamous carcinoma, large cell lung cancer and small cell lung
cancer (4). Of these, lung
adenocarcinoma accounts for >40% of lung cancer cases and is the
most prevalent histological subtype (5,6).
Thus, mechanistic studies are expected to be useful for the
prevention and treatment of lung adenocarcinoma.
Phospholipase C (PLC) is a critical enzyme in the
phosphatidylinositol metabolic system and is involved in various
physiological processes, such as cytoskeletal transformation,
tissue differentiation and tumorigenesis (7,8).
PLCδ1 (PLCD1), which is a member of the PLCδ subgroup, is
considered to be the basic isoform of the PLC family (9). PLCD1 is involved in energy
metabolism, calcium homeostasis and intracellular motility
(10,11). PLCD1 is frequently absent in a
variety of cancer types, including lung cancer (12–14).
However, to date, limited studies on PLCD1 in lung adenocarcinoma
have been reported. Origin recognition complex 1 (ORC1) is an
origin recognition complex gene that is regulated during the cell
division cycle and has an essential role in the initiation of DNA
replication (15). The ORC1 gene
has been indicated to be weakly expressed in quiescent cells, but
it was able to be upregulated by cell growth signals (16).
The present study was designed to discuss the role
of ORC1 in lung adenocarcinoma as well as its relationship with
PLCD1, aiming to find possible targeted-therapy for the prevention
and treatment of lung adenocarcinoma.
Materials and methods
Cell culture and transfection
The human bronchial epithelial cell line 16HBE and
the human lung cancer cell lines A549, PC9, H1975 and H3255 were
purchased from BeNa Culture Collection. Cells were cultured in
RPMI-1640 medium (Beijing Solarbio Science & Technology Co.,
Ltd.) containing 10% FBS and 1% penicillin/streptomycin (both
purchased from Beijing Solarbio Science & Technology Co.,
Ltd.), and were maintained at 37°C in the presence of 5%
CO2. PLCD1 overexpression plasmid (oe-PLCD1), ORC1
overexpression plasmid (pcDNA3.1-ORC1) and the corresponding
pcDNA3.1 empty vectors used as negative controls (NCs; oe-NC and
pcDNA3.1-NC) were obtained from Shanghai GenePharma Co., Ltd. The
sequences of the small hairpin (sh)RNAs used in the present study
were as follows: ORC1 (sh-ORC1-1; target sequence,
5′-AGCCTGGTGCACAGGAAATAT-3′; forward,
5′-CCGGAGCCTGGTGCACAGGAAATATCTCGAGATATTTCCTGTGCACCAGGCTTTTTTG-3′
reverse,
5′-AATTCAAAAAAGCCTGGTGCACAGGAAATATCTCGAGATATTTCCTGTGCACCAGGCT-3′;
sh-ORC1-2; target sequence, 5′-CTATTTGCGGAGTTGAATAAA-3′; forward,
5′-CCGGCTATTTGCGGAGTTGAATAAACTCGAGTTTATTCAACTCCGCAAATAGTTTTTG-3′
and reverse,
5′-AATTCAAAAACTATTTGCGGAGTTGAATAAACTCGAGTTTATTCAACTCCGCAAATAG-3′)
and NC (sh-NC; forward,
5′-CACCGCAATTTTTTTTTTTGATTCACGAATGAATCAAAAAAAAAAAAATGC-3′ and
reverse,
5′-AAAAGCAATTTTTTTTTTTGATTCATTCGTGAATCAAAAAAAAAAAAATGC-3′). The
above shRNAs were obtained from Shanghai GenePharma, Co., Ltd. A
total of 50 nM shRNAs against ORC1 (sh-ORC1) were transfected into
A549 cells at 37°C for 24 h with Lipofectamine® 2000
(Thermo Fisher Scientific, Inc.) transfection reagent according to
the manufacturer's instructions. After 24 h, the cells were adopted
for follow up experiments.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from A549 cells with
TRIzol® reagent (Thermo Fisher Scientific, Inc.). cDNA
was synthesized using a PrimeScript™ RT Reagent kit
(Takara Bio, Inc.). The iTaq Universal SYBR Green kit (Bio-Rad
Laboratories, Inc.) was then utilized to perform RT-qPCR according
to the manufacturer's protocol. The thermocycling conditions were
as follows: Initial denaturation at 95°C for 8 min; denaturation at
95°C for 25 sec, annealing at 60°C for 30 sec; extension at 72°C
for 30 sec; and final extension at 72°C for 10 min. GAPDH was used
as the internal reference gene. Relative expression levels were
measured using the 2−ΔΔCq method (17). The following primer pairs were
used: PLCD1 forward, 5′-ACCAGCGCAATACACTAGACC-3′ and reverse,
5′-GCCTGAGTGGTGGATGATCTT-3′; ORC1 forward,
5′-ACTACCCCACAAGGCTGAAGA-3′ and reverse,
5′-AGTGCAGTTTTCGATCCAACA-3′; and GAPDH forward,
5′-ACAACTTTGGTATCGTGGAAGG-3′ and reverse,
5′-GCCATCACGCCACAGTTTC-3′.
Western blot analysis
Total protein was collected from A549 cells with
RIPA lysis buffer (Beyotime Institute of Biotechnology) and the
protein concentration was determined using the BCA method. Proteins
(30 µg) were separated via 10% SDS-PAGE and transferred to PVDF
membranes, which were then blocked with 5% skimmed milk for 1 h at
room temperature and next incubated with primary antibodies against
PLCD1 (cat. no. ab134936; 1:1,000 dilution; Abcam), MMP2 (cat. no.
ab92536; 1:1,000 dilution; Abcam), MMP9 (cat. no. ab76003; 1:1,000
dilution; Abcam), E-cadherin (cat. no. ab40772; 1:10,000 dilution;
Abcam), N-cadherin (cat. no. ab76011; 1:5,000 dilution; Abcam),
vimentin (cat. no. ab92547; 1:1,000 dilution; Abcam), Snail (cat.
no. ab216347; 1:1,000 dilution; Abcam), ORC1 (cat. no. ab85830;
1:2,000 dilution; Abcam) or GAPDH (cat. no. ab9485; 1:2,500
dilution; Abcam) overnight at 4°C. The samples were then incubated
with a horseradish peroxidase-conjugated antibody (cat. no.
ab109489; 1:1,000 dilution; Abcam) for 2 h at room temperature.
Finally, protein signals were visualized with enhanced
chemiluminescence reagent (cat. no. P0018S; Beyotime) and
semi-quantitatively analyzed using Image-Pro Plus 6.0 software
(Media Cybernetics, Inc.).
Cell proliferation assay
Cell proliferation was analyzed using the MTT
method. Transfected A549 cells were inoculated in 96-well culture
plates (5×103 cells/well). Upon cell deposition, the
cells were incubated for 24, 48 and 72 h. Subsequently, 10 mg/ml
MTT (Beyotime Institute of Biotechnology) was added to the cell
culture plates. After incubation at 37°C for 4 h, DMSO was used to
dissolve the formazan and the absorbance was measured at 570 nm
using a microplate spectrophotometer (Thermo Fisher Scientific,
Inc.).
Colony formation assay
A549 cells were inoculated in 6-well plates
(1×103 cells/well) and incubated at 37°C for 12 days
until colonies had formed, which were observed under a light
microscope (magnification, ×100; Olympus Corporation).
Subsequently, cells were fixed in 4% paraformaldehyde for 15 min at
room temperature and stained with 0.5% crystal violet at room
temperature for 10 min. Cell colonies (≥50 cells) were visualized
under a light microscope (Olympus Corporation).
Immunofluorescence analysis
Transfected cells were inoculated into 24-well
plates (5×104 cells/well). Once the cells were 70–80%
confluent, they were fixed with 3.7% paraformaldehyde for 10 min at
room temperature, followed by permeabilization with 0.05% Triton
X-100 for 15 min at room temperature. Cells were incubated
sequentially with 5% normal goat serum (Beijing Solarbio Science
& Technology Co., Ltd.) for 1 h at room temperature, a primary
antibody against Ki67 (cat. no. ab243878; 1:100 dilution; Abcam)
overnight at 4°C and a DyLight® 488-conjugated secondary
antibody (cat. no. ab98571; 1:100 dilution; Abcam) for 2 h at 37°C.
Finally, nuclei were stained with DAPI for 10 min at room
temperature and cells were imaged under a fluorescence microscope
(magnification, ×200; Olympus Corporation).
Cell invasion assay
The cell invasion capacity was examined using a
Transwell assay. Transwell chambers (8-µm pore size) were
pre-treated with Matrigel® (Corning, Inc.) at 37°C for
30 min. Transfected cells (1×104 cells/well) were
inoculated into serum-free DMEM (Shanghai Yimiao Chemical
Technology Co., Ltd.) which was placed in the upper Transwell
chamber. Complete RPMI-1640 medium containing 5% FBS was added to
the bottom chamber and cells were incubated at 37°C for 12 h prior
to being fixed in 4% paraformaldehyde for 15 min at room
temperature. Cells were then stained with 0.1% crystal violet at
room temperature for 15 min. Next, cells were counted under a light
microscope (Olympus Corporation).
Cell migration assay
A wound-healing assay was used to determine the
migratory capacity of cells. Transfected cells were inoculated in
6-well culture plates (2×105 cells/well). Once cell
confluency reached 80–90%, the cells were scratched with a 200-µl
sterile pipette tip. To remove cell debris, A549 ells were washed
with PBS three times. After continued culture for 0 or 24 h in the
presence of serum-free medium, images of the wound areas were
acquired with a light microscope (Thermo Fisher Scientific, Inc.)
to measure the migration distance.
Database analysis
The GEPIA database (Ver.2, http://gepia2.cancer-pku.cn) is a newly developed
interactive web tool used for the analysis of RNA sequencing
expression data of tumors and normal samples from The Cancer Genome
Atlas (TCGA) database and the Genotype-Tissue Expression project
using a standard processing pipeline. The expression levels of ORC1
and PLCD1 in lung adenocarcinoma tissues were determined and an
association analysis was performed using the GEPIA database.
Survival rates were analyzed using the TCGA database (tcga.org/).
The Human Transcription Factor DataBase (TFDB, Ver.3.0) website
(http://bioinfo.life.hust.edu.cn/HumanTFDB#!/tfbs_predict)
was employed to predict the binding site between ORC1 and the PLCD1
promoter according to default parameters.
Luciferase reporter gene assay
The interaction of ORC1 with the PLCD1 promoter in
cells was confirmed with a luciferase reporter gene assay. PLCD1
wild-type (WT) and mutant (MUT) sequences obtained from Shanghai
GenePharma Co., Ltd. were cloned into the pGL3 luciferase vector
(Promega Corporation) and were named PLCD1-WT and PLCD1-MUT,
respectively. A total of 0.1 µg PLCD1-WT and PLCD1-MUT were
transfected into pcDNA3.1-negative control (NC) or
pcDNA3.1-ORC1-transfected cells, respectively, using Lipofectamine
2000 transfection reagent (Thermo Fisher Scientific, Inc.).
Following transfection at 37°C for 48 h, the relative luciferase
signals were analyzed with a Luciferase Reporter Gene Assay Kit
(cat. no. 11401ES60; Yeasen) according to the manufacturer's
protocol and the firefly luciferase activity was normalized to that
of Renilla luciferase.
Chromatin immunoprecipitation
(ChIP)
The ChIP Assay Kit (cat. no. P2078; Beyotime) was
used according to the manufacturer's protocol. In brief, cells were
incubated with 1% formaldehyde at 37°C for 10 min to crosslink DNA
with proteins, followed by the addition of glycine to interrupt the
crosslinking. The samples were centrifuged at 10,000 × g for 10 min
at 4°C to separate the insoluble material. Next, samples were
incubated overnight at 4°C with an anti-ORC1 antibody (cat. no.
ab85830; 2 µg/mg; Abcam) or with IgG (cat. no. ab6715; 2 µg/mg;
Abcam). The precipitated immune complexes were washed with elution
buffer. The complexes were incubated at 65°C overnight to reverse
the crosslinks and then treated with proteinase K (part of the kit)
at 45°C for 1.5 h. The purified DNA (Shanghai Xiangshu Industrial
Co., Ltd.) was subjected to PCR amplification as aforementioned and
its product was run on a 1% DNA agarose gel and visualized with
ethidium bromide. Analysis was performed semi-quantitatively using
Image-Pro Plus 6.0 software (Media Cybernetics, Inc.).
Statistical analysis
GraphPad Prism software (version 8.0; GraphPad
Software, Inc.) was used for all statistical analyses. Unpaired
Student's t-test was used to compare 2 groups, while one-way ANOVA
followed by Tukey's post-hoc test was used to compare >2 groups.
The two-stage procedure and the Neyman's smooth test were used for
survival analysis (18).
Correlation analysis was performed by Pearson's test. Values are
expressed as the mean ± standard deviation. Each experiment was
conducted ≥3 times. P<0.05 was considered to indicate a
statistically significant difference.
Results
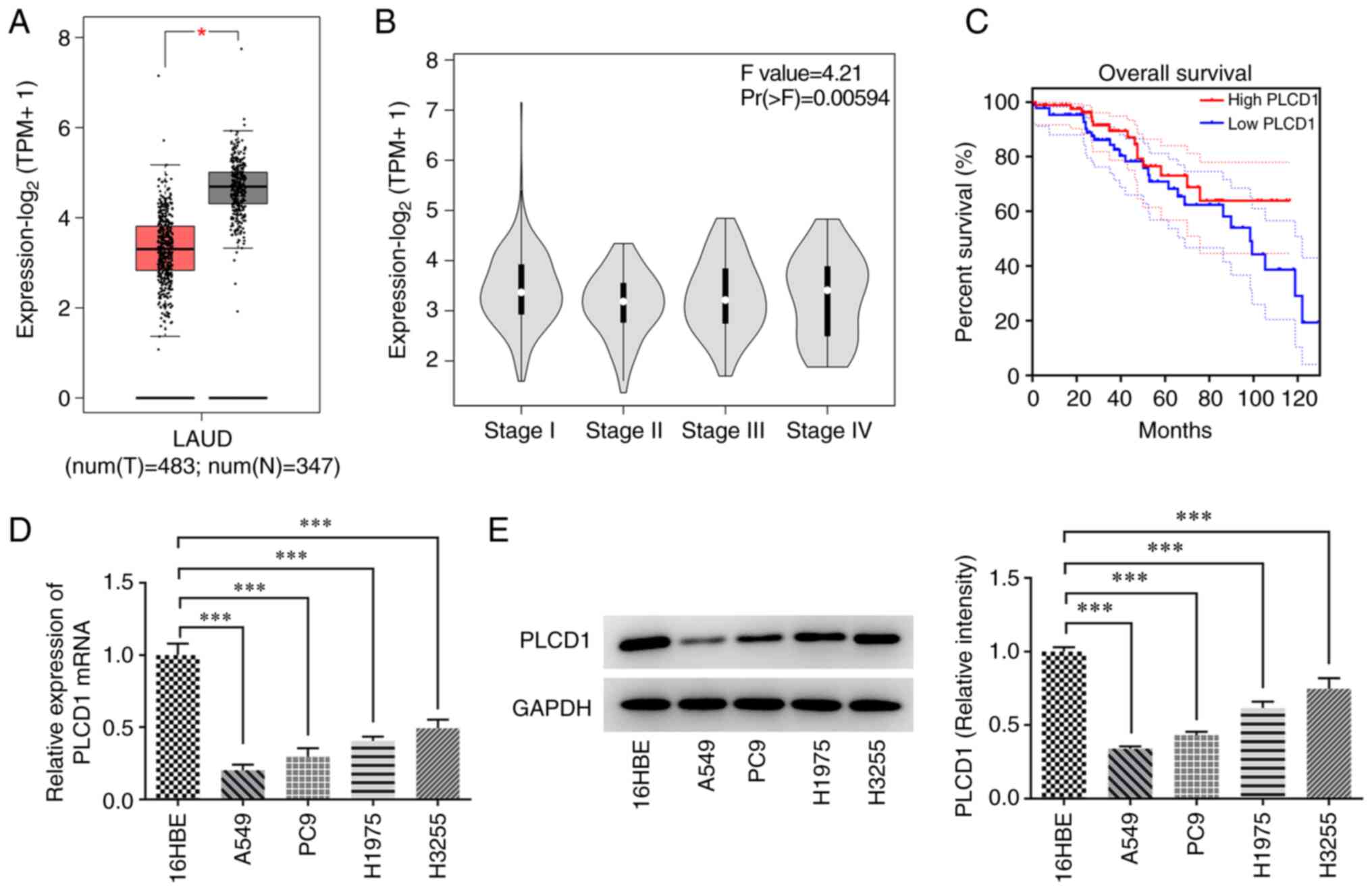
Low expression of PLCD1 in lung
adenocarcinoma tissues and cells
The expression of PLCD1 in tissues, as analyzed by
the GEPIA database, was low in lung adenocarcinoma compared with
that in normal tissues and was associated with low overall survival
in patients with lung adenocarcinoma (Fig. 1A and C). PLCD1 expression was not
associated with disease stage (Fig.
1B). The expression of PLCD1 in bronchial epithelial and lung
cancer cells was detected by RT-qPCR and western blot analysis
(Fig. 1D and E). The results
indicated that PLCD1 expression was significantly reduced in lung
cancer cells compared with that in bronchial epithelial cells. Of
note, PLCD1 had the lowest expression in A549 cells; therefore,
subsequent studies were performed in A549 cells.
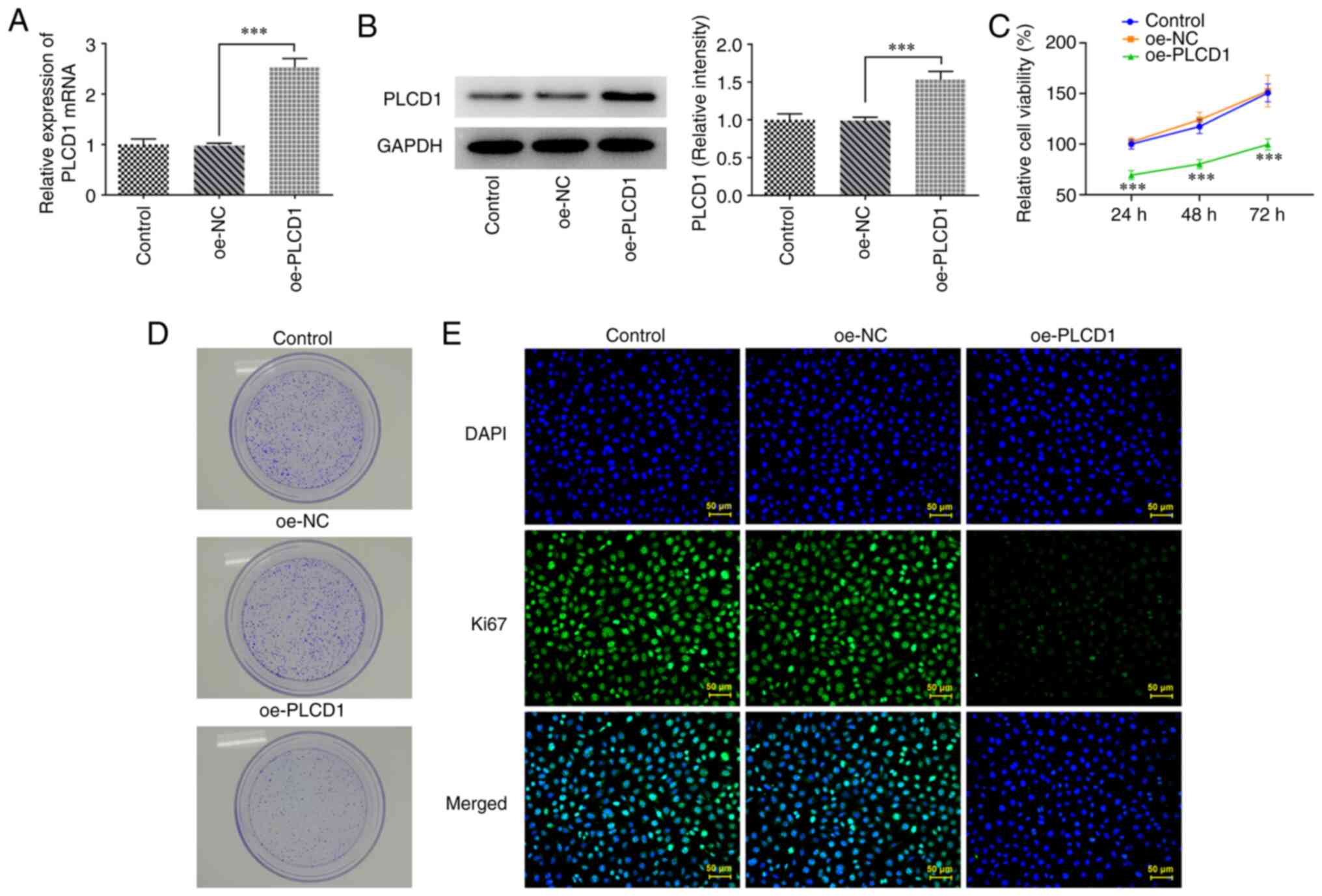
Overexpression of PLCD1 inhibits lung
adenocarcinoma cell proliferation
To investigate the role of PLCD1 in lung cancer,
PLCD1 overexpression was performed via cell transfection and the
overexpression efficacy was confirmed using RT-qPCR and western
blot analysis (Fig. 2A and B). The
results of the cell proliferation assay revealed that PLCD1
overexpression significantly inhibited the proliferation of lung
cancer cells compared with that of the oe-NC group (Fig. 2C). In addition, in the colony
formation assay, markedly slower growth of PLCD1-overexpressing
cells compared with that of the oe-NC group was observed (Fig. 2D). Similarly, detection of Ki67
expression levels in cells using immunofluorescence revealed that
Ki67 expression was markedly reduced in the oe-PLCD1 group
(Fig. 2E).
Overexpression of PLCD1 inhibits lung
adenocarcinoma cell invasion, migration and the
epithelial-mesenchymal transition (EMT) process
Transwell and wound-healing assays suggested that
cell invasion and migration were significantly inhibited in the
oe-PLCD1 group compared with the oe-NC group (Fig. 3A and B). The results of the western
blot analysis indicated that the protein expression levels of MMP2
and MMP9 were significantly reduced in the oe-PLCD1 group compared
with those in the oe-NC group (Fig.
3C). Western blotting was also employed for determining
EMT-related proteins, and it was observed that compared with that
in the oe-NC group, E-cadherin protein expression was significantly
increased in the oe-PLCD1 group, while the protein expression
levels of N-cadherin, vimentin and Snail were significantly
decreased in the oe-PLCD1 group (Fig.
3D).
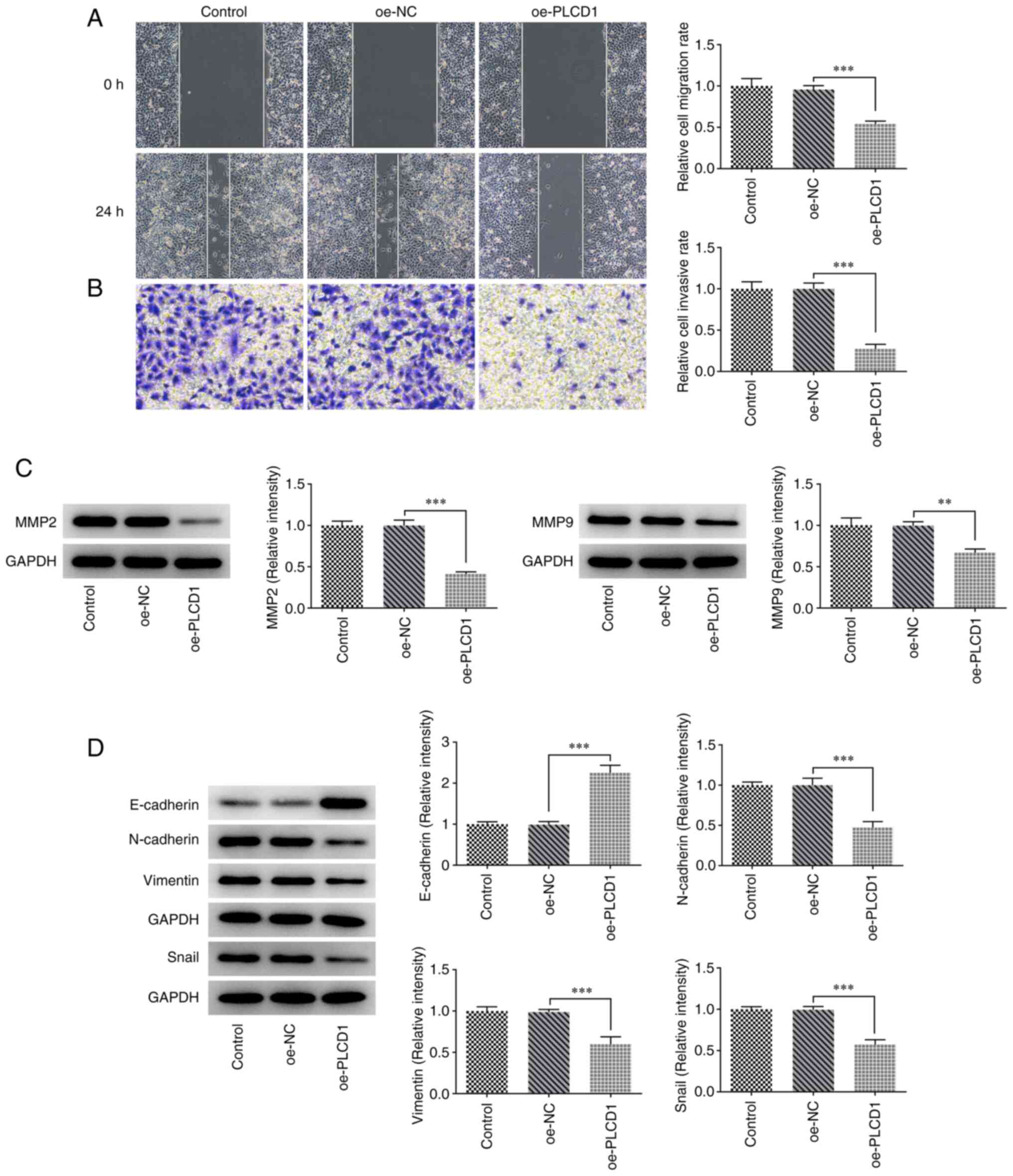 | Figure 3.Overexpression of PLCD1 inhibits lung
adenocarcinoma cell invasion, migration and EMT process. (A) Cell
migration was detected using a wound-healing assay (magnification,
×100). (B) Cell invasion was detected using a Transwell assay
(magnification, ×100). (C) The protein expression levels of MMP2
and MMP9 were detected via western blot analysis. (D) The
EMT-related protein expression levels, including E-cadherin,
N-cadherin, vimentin and Snail, were detected via western blot
analysis. **P<0.01 and ***P<0.001. PLCD1, phospholipase Cδ1;
EMT, epithelial to mesenchymal transition; oe, overexpression; NC,
negative control. |
High expression of ORC1 is observed in
lung adenocarcinoma tissues and is negatively regulated by
PLCD1
Analysis of the GEPIA database indicated that ORC1
was highly expressed in lung adenocarcinoma compared with that in
normal tissue, and ORC1 expression was associated with the
pathological stage of lung adenocarcinoma (Fig. 4A and B). Compared with Stage I,
ORC1 expression was higher in stage II, III and IV, especially in
Stage IV, indicating that higher expression of ORC1 was associated
with more advanced disease stages. Of note, ORC1 and PLCD1 were
indicated to be negatively correlated according to the data of the
GEPIA database (Fig. 4C). In
addition, ORC1 mRNA and protein expression levels were
significantly higher in A549 lung cancer cells than in 16HBE
bronchial epithelial cells (Fig. 4D
and E). Furthermore, the binding site of ORC1 with the PLCD1
promoter was predicted using the HumanTFDB website (Fig. 4F).
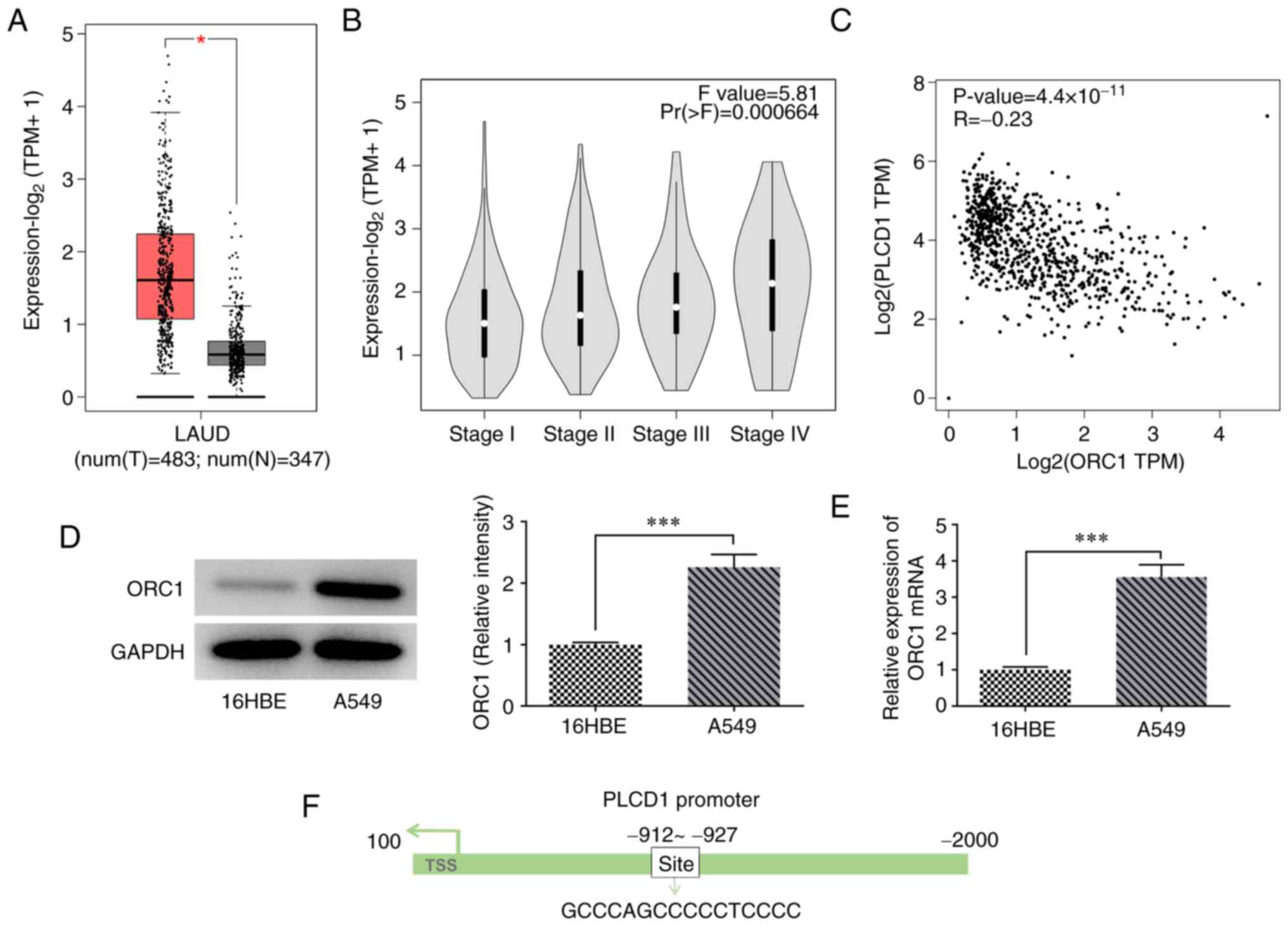 | Figure 4.High expression of ORC1 in LUAD
tissues and negative regulation of PLCD1. According to GEPIA
database, ORC1 expression was (A) upregulated in LUAD, (B)
associated with LUAD stage and (C) negatively correlated with PLCD1
expression in LUAD. The non-log scale was used for calculation and
the log-scale axis for visualization. (D) Protein and (E) mRNA
expression levels of ORC1 in cells. (F) Binding site of ORC1 to
PLCD1 promoter. *P<0.05 and ***P<0.001. ORC1, origin
recognition complex 1; PLCD1, phospholipase Cδ1; LUAD, lung
adenocarcinoma; TPM, transcripts per million; num, number; T,
tumor; N, normal; TSS, transcription start site. |
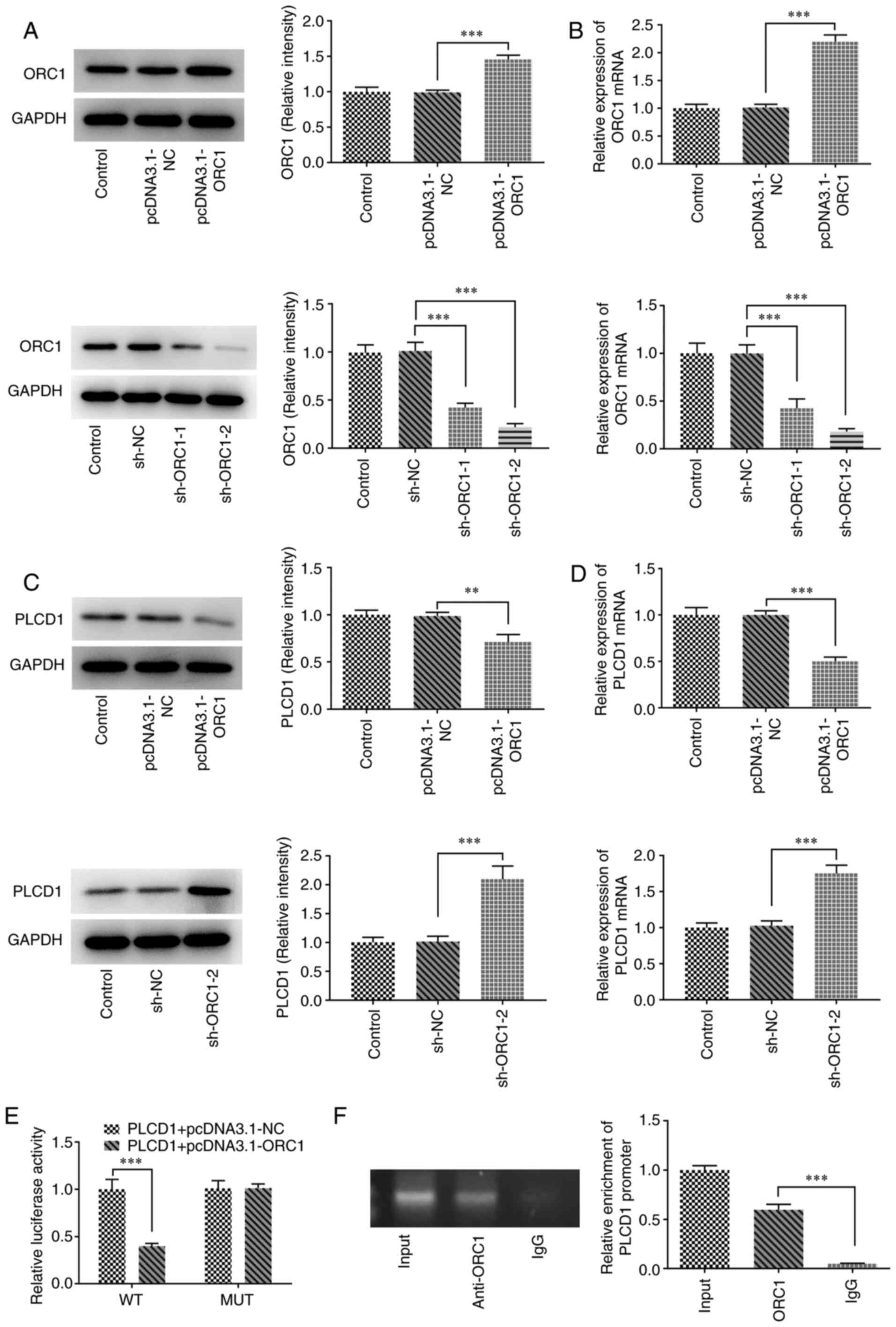
To further investigate the association between ORC1
and PLCD1, ORC1-overexpressing cells (pcDNA3.1-ORC1) and cells with
ORC1 knockdown (sh-ORC1) were established through transfection and
the expression levels of ORC1 and PLCD1 in such cells were detected
using western blot and RT-qPCR analyses. The results revealed that
ORC1 overexpressing and knockdown cells were successfully
established, since they exhibited significantly increased or
decreased ORC1 expression compared with the corresponding NC,
respectively, at the protein and mRNA levels (Fig. 5A and B). The protein and mRNA
expression levels of PLCD1 were significantly decreased in the ORC1
overexpression compared with those in the NC group (pcDNA3.1-ORC1
vs. pcDNA3.1-NC), while the levels of PLCD1 were significantly
increased in the ORC1 RNA interference group (sh-ORC1-2 vs. sh-NC;
Fig. 5C and D). ORC1
overexpression greatly reduced the activity of PLCD1. PLCD1 was
enriched in anti-ORC1, suggesting that ORC1 was able to directly
interact with the PLCD1 promoter (Fig.
5E and F).
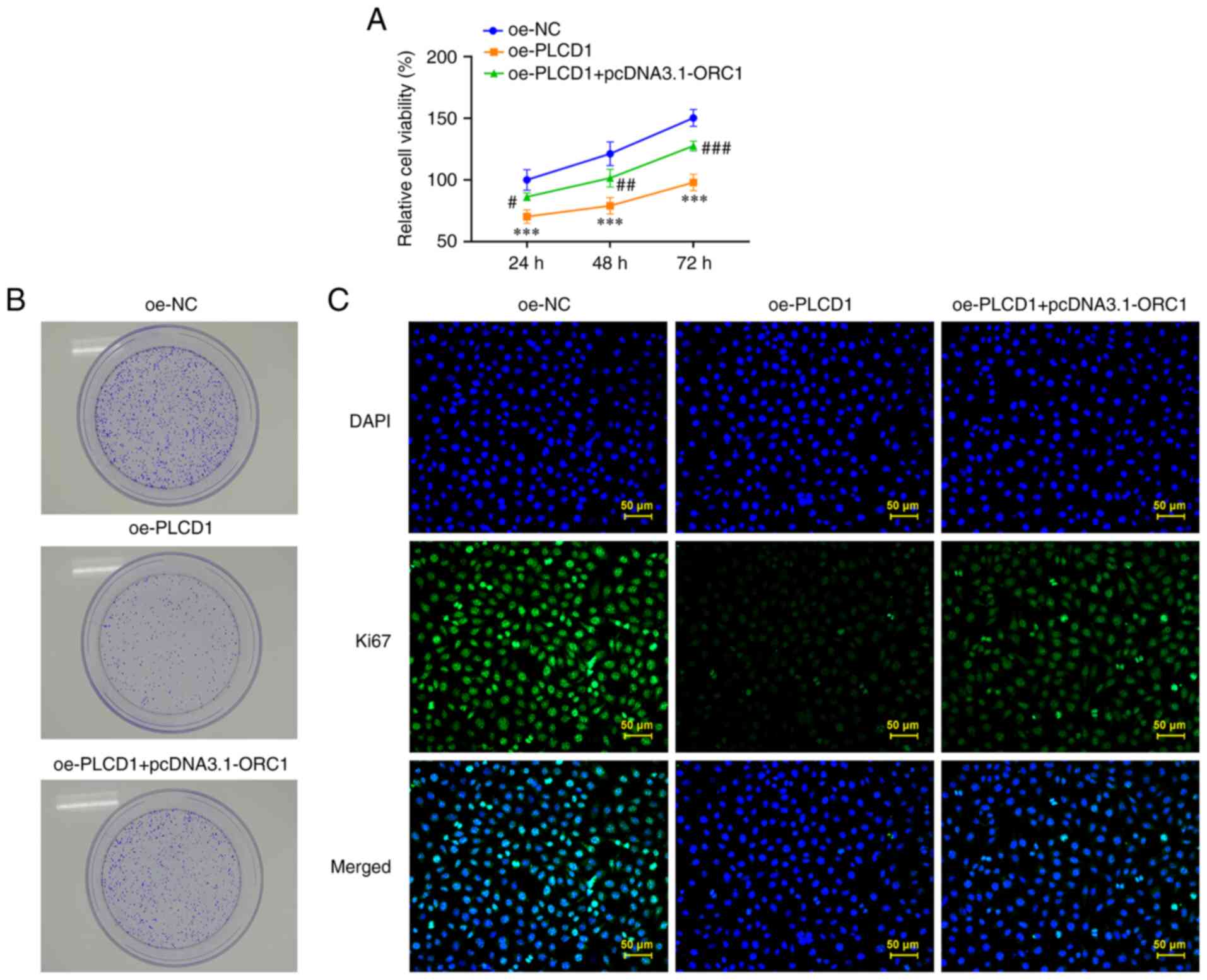
ORC1 overexpression partially
attenuates the inhibitory effects of PLCD1 overexpression on the
proliferation, invasion, migration and EMT of lung adenocarcinoma
cells
PLCD1 and ORC1 overexpression plasmids were
simultaneously transfected into A549 cells and the cells were then
evaluated. In terms of cell proliferation, PLCD1 overexpression
inhibited cell proliferation compared with that in the oe-NC group,
while ORC1 overexpression reduced the inhibitory effects of PLCD1
overexpression on cell proliferation compared with the oe-PLCD1
group (Fig. 6A). The same trend
was observed in the colony formation assays, with slower cell
growth noticed in the oe-PLCD1 group compared with that in the
oe-NC group and faster cell growth in the oe-PLCD1 + pcDNA3.1-ORC1
group compared with that in the oe-PLCD1 group (Fig. 6B).
Immunofluorescence staining revealed that Ki67
expression was markedly diminished in the oe-PLCD1 group compared
with that in the oe-NC group, whereas Ki67 expression was markedly
increased in the oe-PLCD1 + pcDNA3.1-ORC1 group compared with that
in the oe-PLCD1 group (Fig. 6C).
Of note, ORC1 overexpression attenuated the inhibitory effects of
PLCD1 overexpression on the invasion and migration of lung cancer
cells. The results suggested that cell migration and invasion were
promoted by ORC1 overexpression compared with that observed in the
oe-PLCD1 group (Fig. 7A and
B).
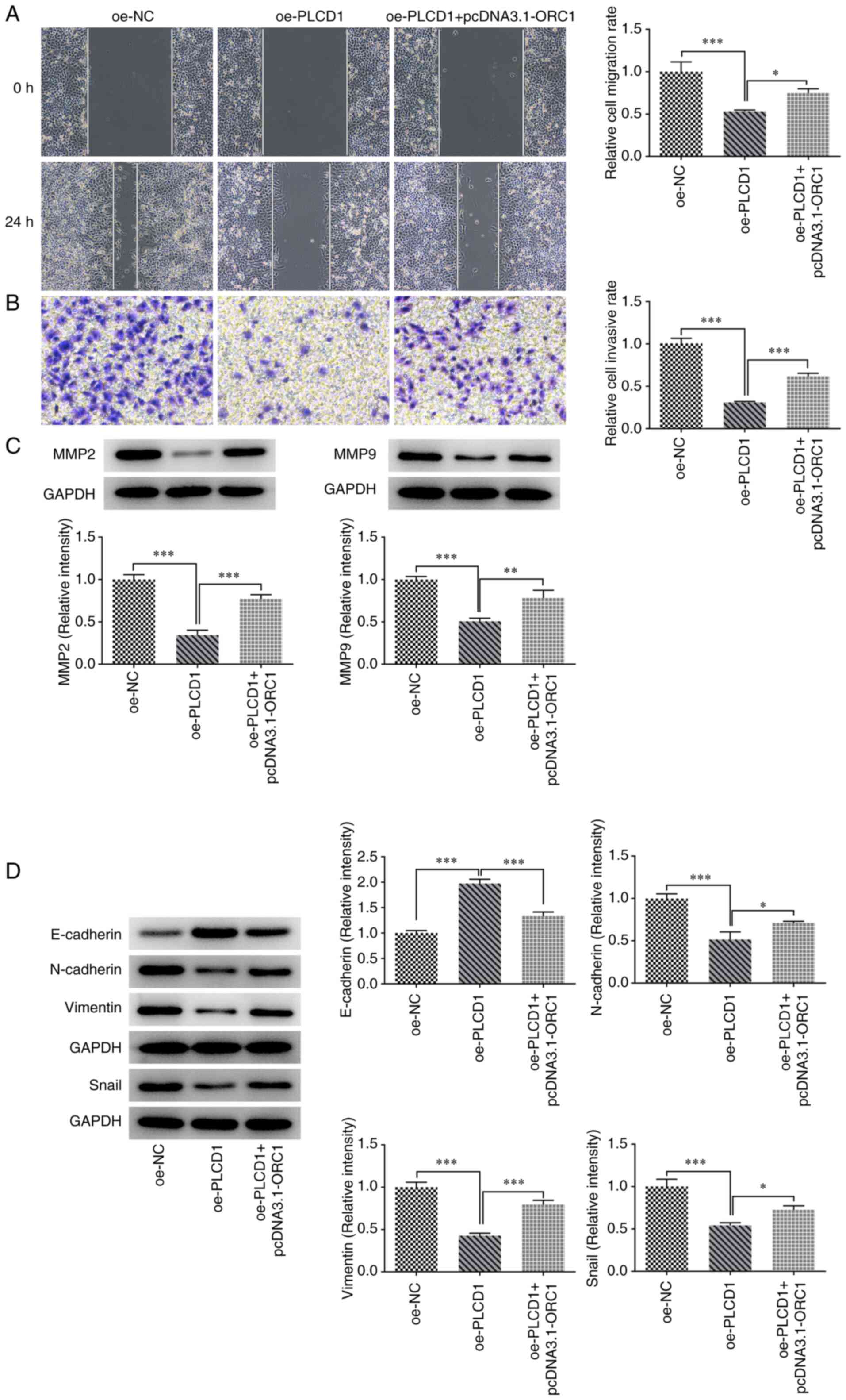 | Figure 7.ORC1 overexpression partially reduces
the inhibitory effect of PLCD1 overexpression on invasion,
migration and EMT of lung adenocarcinoma cells. (A) Cell migration
was detected using a wound-healing assay (magnification, ×100). (B)
Cell invasion was detected using a Transwell assay (magnification,
×100). (C) The protein expression levels of MMP2 and MMP9 were
detected by western blot analysis. (D) EMT-related protein
expression levels, including E-cadherin, N-cadherin, vimentin and
Snail, were detected by western blot analysis. *P<0.05,
**P<0.01 and ***P<0.001. ORC1, origin recognition complex 1;
PLCD1, phospholipase Cδ1; EMT, epithelial to mesenchymal
transition; oe, overexpression; NC, negative control. |
Analysis of the expression of MMPs revealed that
ORC1 overexpression significantly reversed the inhibitory effects
of PLCD1 overexpression on MMP2 and MMP9 (oe-PLCD1 + pcDNA3.1-ORC1
vs. oe-PLCD1) (Fig. 7C). In
addition, analysis of the expression levels of EMT-related proteins
indicated that ORC1 overexpression significantly reversed the
effects of PLCD1 overexpression on the expression levels of
EMT-related proteins (Fig. 7D).
Taken together, these results suggested that ORC1 exerts its
functions in lung adenocarcinoma by inhibiting PLCD1
expression.
Discussion
Lung cancer is the most common cancer worldwide and
the majority of patients with lung cancer are in advanced stages of
the disease at the time of diagnosis (19). PLCD1 is involved in energy
metabolism, calcium homeostasis and intracellular motility
(10). However, PLCD1 is
frequently downregulated in multiple types of cancer, such as
esophageal squamous cell carcinoma (20) and breast cancer (10). In a previous report, the genome
near the homozygous deletion of chromosome 3p in lung cancer cell
lines was sequenced and the gene encoding PLCD1 was observed to be
close to deletion region (13).
Furthermore, low PLCD1 expression in lung adenocarcinoma was
indicated in the GEPIA database analysis (data not shown) and this
low PLCD1 expression was associated with poor prognosis in patients
with lung adenocarcinoma. These results suggest that PLCD1 may have
the potential to affect the progression of lung cancer.
In the present study, the expression of PLCD1 was
analyzed in clinical patients using the GEPIA database, as well as
in bronchial epithelial and lung adenocarcinoma epithelial cells
using RT-qPCR and western blot analysis. Consistent with the
results of PLCD1 expression in clinical samples from the GEPIA
database, the PLCD1 mRNA and protein expression levels were
significantly decreased in the aforementioned four lung
adenocarcinoma cell lines compared with those in bronchial
epithelial cells.
A previous study has also demonstrated low
expression of PLCD1 in colorectal cancer cells and indicated that
restoring PLCD1 expression inhibited colorectal cancer cell
proliferation and induced apoptosis; furthermore, restoration of
PLCD1 inhibited cell proliferation, metastasis and tumorigenicity
in colorectal cancer (21). In
esophageal squamous carcinoma cells, PLCD1 was indicated to inhibit
cell proliferation, invasion and migration by suppressing the
Wnt/β-catenin signaling pathway (22). In pancreatic and breast cancer
tumors, PLCD1 expression also exerted similar regulatory effects
(23–25). Thus, it was hypothesized that the
activation of PLCD1 contributed to tumor suppression.
In the present study, the proliferation of lung
cancer cells was examined using MTT assay, colony formation assay
and immunofluorescence staining, and the results indicated that the
proliferation of cells overexpressing PLCD1 was significantly
inhibited compared with that in the control group. In addition, the
results of the cell invasion, migration and EMT assays revealed
that overexpression of PLCD1 had inhibitory effects on lung cancer
cells.
Of note, there are limited studies on the regulatory
factors located upstream of the PLCD1 gene. ORC1 is one of the ORCs
involved in the first step of DNA replication (26). Since ORC1 is synthesized in the
G1 phase and is degraded in the S phase of the cell
cycle, it has been previously speculated that ORC1 expression may
be closely associated with cell proliferation and the cell cycle,
and it may be involved in biological processes, such as cell
proliferation, apoptosis, invasion and migration (26,27).
In a study on glioma, overexpression of ORC1 promoted cancer cell
proliferation, while downregulation of ORC1 inhibited the
activation of the ERK/JNK signaling pathway, thereby suppressing
glioma invasion and migration (28).
In the present study, ORC1 was demonstrated to bind
to the PLCD1 promoter using ChIP and luciferase reporter gene
assays. The binding site of ORC1 to the PLCD1 promoter was
predicted using the HumanTFDB website. The results indicated that
ORC1 overexpression was able to significantly reduce PLCD1
expression. To further demonstrate the association between these
two genes, ORC1- and PLCD1-overexpressing cells were established.
However, this does not necessarily indicate that ORC1 protein has a
direct effect on the PLCD1 overexpression plasmid. The
downregulation of PLCD1 expression in cells transfected with ORC1
is different to the overexpression of PLCD1 caused by the
transfected plasmids. Compared with the changes of PLCD1 with
different treatment, it could be speculated that the effect of
endogenous ORC1 on PLCD1 expression in cells is transient, whereas
the effect of transfection with the pcDNA3.1-ORC1 plasmid on PLCD1
expression is stable (29). In
summary, PLCD1 expression in cancer cells was increased after
transfection with oe-PLCD1 plasmid. However, overexpression of ORC1
caused an upregulation of ORC1 expression, thereby suppressing
PLCD1 expression, which subsequently reduced the overall PLCD1
expression and ultimately affected the cellular activities. These
results demonstrated that the inhibitory effects of PLCD1
overexpression on lung adenocarcinoma cell proliferation, migration
and EMT were attenuated following overexpression of both ORC1 and
PLCD1 compared with those achieved by overexpression of PLCD1
alone. These findings indicated that ORC1 and PLCD1 have a negative
regulatory relationship.
In conclusion, to the best of our knowledge, the
present study was the first to demonstrate the association between
low PLCD1 expression in lung adenocarcinoma and ORC1, which may
downregulate PLCD1 and thus promote the proliferation, migration
and EMT process of lung adenocarcinoma cells. However, the absence
of animal models is a limitation of the present study. In future
studies, cells will be analyzed, and xenograft mice models will be
established to confirm the potential use of PLCD1 as a target
marker for the diagnosis and treatment of lung adenocarcinoma.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YJ, QQ, JT and XQ conceived and designed the study,
and acquired and interpreted the data. YJ and QQ were major
contributors in writing the manuscript. YJ and XQ confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Siegel RL and Jemal A: Lung
cancer statistics. Adv Exp Med Biol. 893:1–19. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hirsch FR, Scagliotti GV, Mulshine JL,
Kwon R, Curran WJ Jr, Wu YL and Paz-Ares L: Lung cancer: Current
therapies and new targeted treatments. Lancet. 389:299–311. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schabath MB and Cote ML: Cancer progress
and priorities: Lung cancer. Cancer Epidemiol Biomarkers Prev.
28:1563–1579. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ruiz-Cordero R and Devine WP: Targeted
therapy and checkpoint immunotherapy in lung cancer. Surg Pathol
Clin. 13:17–33. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Denisenko TV, Budkevich IN and Zhivotovsky
B: Cell death-based treatment of lung adenocarcinoma. Cell Death
Dis. 9:1172018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Abe Y and Tanaka N: The Hedgehog signaling
networks in lung cancer: The mechanisms and roles in tumor
progression and implications for cancer therapy. Biomed Res Int.
2016:79692862016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fukami K, Inanobe S, Kanemaru K and
Nakamura Y: Phospholipase C is a key enzyme regulating
intracellular calcium and modulating the phosphoinositide balance.
Prog Lipid Res. 49:429–437. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lattanzio R, Piantelli M and Falasca M:
Role of phospholipase C in cell invasion and metastasis. Adv Biol
Regul. 53:309–318. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fujii M, Yi KS, Kim MJ, Ha SH, Ryu SH, Suh
PG and Yagisawa H: Phosphorylation of phospholipase C-delta 1
regulates its enzymatic activity. J Cell Biochem. 108:638–650.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shao Q, Luo X, Yang D, Wang C, Cheng Q,
Xiang T and Ren G: Phospholipase Cδ1 suppresses cell migration and
invasion of breast cancer cells by modulating KIF3A-mediated
ERK1/2/β-catenin/MMP7 signalling. Oncotarget. 8:29056–29066. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Song JJ, Liu Q, Li Y, Yang ZS, Yang L,
Xiang TX, Ren GS and Chen JB: Epigenetic inactivation of PLCD1 in
chronic myeloid leukemia. Int J Mol Med. 30:179–184.
2012.PubMed/NCBI
|
|
12
|
Hu XT, Zhang FB, Fan YC, Shu XS, Wong AH,
Zhou W, Shi QL, Tang HM, Fu L, Guan XY, et al: Phospholipase C
delta 1 is a novel 3p22.3 tumor suppressor involved in cytoskeleton
organization, with its epigenetic silencing correlated with
high-stage gastric cancer. Oncogene. 28:2466–2475. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ishikawa S, Takahashi T, Ogawa M and
Nakamura Y: Genomic structure of the human PLCD1 (phospholipase C
delta 1) locus on 3p22–>p21.3. Cytogenet Cell Genet. 78:58–60.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Murata Y, Tamari M, Takahashi T, Horio Y,
Hibi K, Yokoyama S, Inazawa J, Yamakawa K, Ogawa A, Takahashi T, et
al: Characterization of an 800 kb region at 3p22-p21.3 that was
homozygously deleted in a lung cancer cell line. Hum Mol Genet.
3:1341–1344. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen X, Xiong D, Ye L, Wang K, Huang L,
Mei S, Wu J, Chen S, Lai X, Zheng L and Wang M: Up-regulated lncRNA
XIST contributes to progression of cervical cancer via regulating
miR-140-5p and ORC1. Cancer Cell Int. 19:452019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ohtani K, DeGregori J, Leone G, Herendeen
DR, Kelly TJ and Nevins JR: Expression of the HsOrc1 gene, a human
ORC1 homolog, is regulated by cell proliferation via the E2F
transcription factor. Mol Cell Biol. 16:6977–6984. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li H, Han D, Hou Y, Chen H and Chen Z:
Statistical inference methods for two crossing survival curves: A
comparison of methods. PLoS One. 10:e01167742015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jones GS and Baldwin DR: Recent advances
in the management of lung cancer. Clin Med (Lond). 18 (Suppl
2):S41–S46. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qin YR, Fu L, Sham PC, Kwong DL, Zhu CL,
Chu KK, Li Y and Guan XY: Single-nucleotide polymorphism-mass array
reveals commonly deleted regions at 3p22 and 3p14.2 associate with
poor clinical outcome in esophageal squamous cell carcinoma. Int J
Cancer. 123:826–830. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xiang Q, He X, Mu J, Mu H, Zhou D, Tang J,
Xiao Q, Jiang Y, Ren G, Xiang T and Peng W: The phosphoinositide
hydrolase phospholipase C delta1 inhibits epithelial-mesenchymal
transition and is silenced in colorectal cancer. J Cell Physiol.
234:13906–13916. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
He X, Meng F, Yu ZJ, Zhu XJ, Qin LY, Wu
XR, Liu ZL, Li Y and Zheng YF: PLCD1 suppressed cellular
proliferation, invasion, and migration via inhibition of
Wnt/β-catenin signaling pathway in esophageal squamous cell
carcinoma. Dig Dis Sci. 66:442–451. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mu H, Wang N, Zhao L, Li S, Li Q, Chen L,
Luo X, Qiu Z, Li L, Ren G, et al: Methylation of PLCD1 and
adenovirus-mediated PLCD1 overexpression elicits a gene therapy
effect on human breast cancer. Exp Cell Res. 332:179–189. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hu D and Jiang Z: Phospholipase C δ1
(PLCD1) inhibits the proliferation, invasion and migration of
CAPAN-1 and BXPC-3 pancreatic cancer cells. Xi Bao Yu Fen Zi Mian
Yi Xue Za Zhi. 32:739–745. 2016.(In Chinese). PubMed/NCBI
|
|
25
|
Zhou X, Liao X, Wang X, Huang K, Yang C,
Yu T, Han C, Zhu G, Su H, Han Q, et al: Noteworthy prognostic value
of phospholipase C delta genes in early stage pancreatic ductal
adenocarcinoma patients after pancreaticoduodenectomy and potential
molecular mechanisms. Cancer Med. 9:859–871. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang XK, Wang QQ, Huang JL, Zhang LB, Zhou
X, Liu JQ, Chen ZJ, Liao XW, Huang R, Yang CK, et al: Novel
candidate biomarkers of origin recognition complex 1, 5 and 6 for
survival surveillance in patients with hepatocellular carcinoma. J
Cancer. 11:1869–1882. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Park SY and Asano M: Function of the
origin recognition complex 1 (ORC1) outside DNA replication in
Drosophila. Cell Cycle. 10:3957–3963. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xiong W, Xie C, Qiu Y, Tu Z and Gong Q:
Origin recognition complex subunit 1 regulates cell growth and
metastasis in glioma by altering activation of ERK and JNK
signaling pathway. Mol Cell Probes. 49:1014962020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stepanenko AA and Heng HH: Transient and
stable vector transfection: Pitfalls, off-target effects,
artifacts. Mutat Res Rev Mutat Res. 773:91–103. 2017. View Article : Google Scholar : PubMed/NCBI
|