Introduction
Solitary fibrous tumors (SFT) and
hemangiopericytomas (HPC) are solid tumors that originate from
mesenchymal tissue and typically occur in soft tissue (1). Although intracranial tumors are
possible, they are uncommon, accounting for only 0.4 percent of all
primary central nervous system (CNS) tumors (2). Repeated intracranial SFT/HPC recurs
locally and has a high rate of metastasis even long after the
initial treatment. At our department, a case with multiple
well-enhanced masses in the left upper lobe was encountered.
Initially, primary lung adenocarcinoma was suspected. The patient
had previously undergone two separate surgeries to remove two
intracranial meningiomas. The patient was eventually diagnosed with
an extracranial metastasis of a primary intracranial SFT/HPC, as
well as an intracranial recurrence and metastasis that had occurred
11 and 13 years after the first resection in 2004. Furthermore, the
differential diagnoses of intracranial masses and how to
distinguish them based on imaging characteristics and
immunohistochemistry were discussed.
Case report
A 50-year-old male presented at The First Affiliated
Hospital of Dali University (Dali, China) in September 2017 with
paroxysmal left-side chest discomfort that had become increasingly
severe over the preceding month. Routine biochemical and
hematological test results were within normal limits, but CT
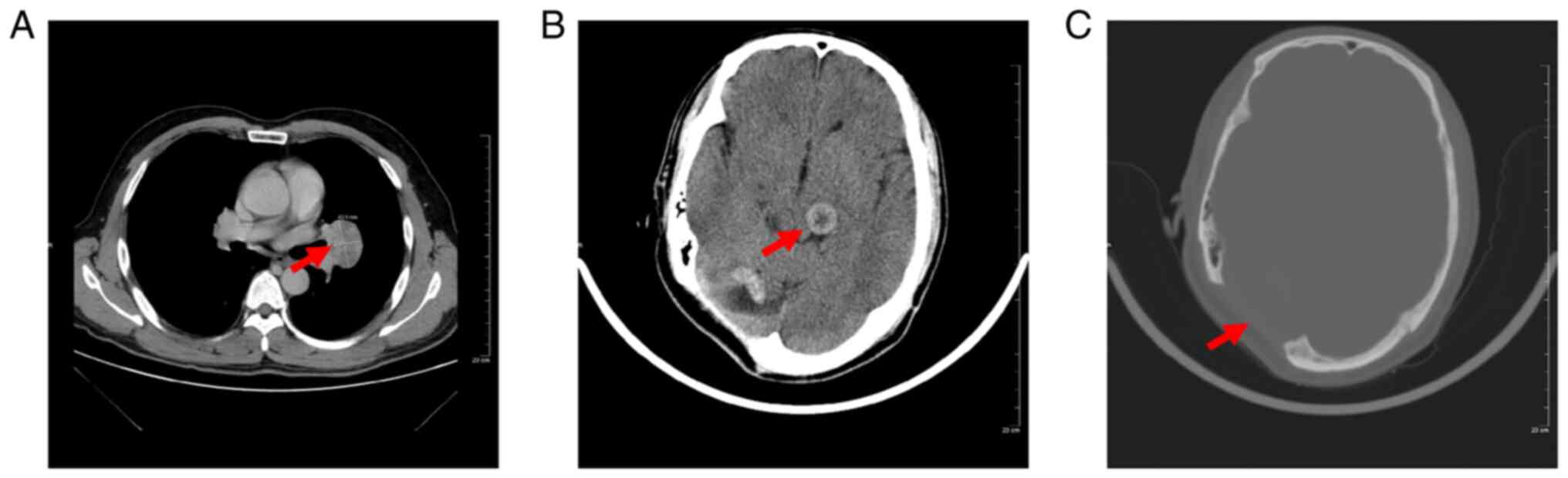
revealed a well-defined oval lung mass in the left upper lobe near
the hilum measuring 41×35 mm in size (Fig. 1A). Pre-operative CT indicated
heterogeneous enhancement of a round mass of up to 31×21 mm in the
left thalamus, and a significant cavity was observed on
post-operative review after resection of the brain tumor (Fig. 1B and C). MRI revealed well-defined
masses in the left thalamus, corpus callosum body, left frontal
lobe and right temporal lobe measuring 31×21 mm in size. The tumor
had a low signal on T1- and T2-weighted images and the tumor in the
bilateral cerebellum was isointense on T1- and T2-weighted images;
furthermore, the tumor had uneven enhancement. The patient had
previously undergone multiple intracranial tumor resections
(Table I). The first was an
excision of a right cerebellar tentorium tumor 17 years previously,
which had been identified as a fibrous meningioma. The second
procedure was the excision of a tumor that was diagnosed as
meningioma in the left cerebellopontine angle, World Health
Organization (WHO) grade I. According to the WHO 2016 revised
guidelines, meningiomas are classified as follows: Grade I, benign
meningiomas with <4+ mitoses per 10 consecutive high-power
fields (HPF; objective magnification, ×40); Grade II, atypical with
a mitotic rate of 4–19 per 10 HPF or brain invasion (if neither
feature is present, at least three of the following five histologic
criteria must be evident to arrive at a Grade II diagnosis:
Intratumoral micronecrosis not caused by presurgical thrombosis
therapy; patternless sheets of tumor cells; prominent nucleoli;
high cellularity; and tumor cells with scant cytoplasm relative to
nuclear size); Grade III, anaplastic (malignant) with >20+
mitoses per 10 consecutive HPF (2). Given that the tumor in the upper lobe
of the left lung was the largest, it was first assumed to be the
primary lesion but this was later disproven, as it was a pulmonary
metastasis from a primary intracranial tumor. The upper lobe of the
left lung tumor was subjected to a CT-guided biopsy. Based on these
findings, a definitive diagnosis of malignant SFT/HPC pulmonary
metastases was made.
 | Table I.Timeline of recurrence, metastasis
and treatment of the present case of malignant SFT/HPC. |
Table I.
Timeline of recurrence, metastasis
and treatment of the present case of malignant SFT/HPC.
| Year/month | Diagnosis | Location | Treatment |
|---|
| 2004 | Fibrous
meningiomas | Right cerebellar
tentorium | Gross total
resection |
| 2015 | Meningiomas (WHO
I) | Left
cerebellopontine angle | Gross total
resection |
| 2017.2 | Possibility of
meningioma recurrence | Upper lobe of left
lung near the hilum | Follow-up |
| 2017.9 | Central lung
malignant tumor | Upper lobe of left
lung near the hilum | Radiation |
| 2017.10 | Lung spindle cell
tumor | Upper lobe of left
lung near the hilum | Radiation |
| 2017.12 | SFT/HPC | Lung and bilateral
cerebral hemisphere | Radiation |
| 2018.1 | SFT/HPC | Lung and bilateral
cerebral hemisphere | Radiation |
| 2018.4 | SFT/HPC | Thoracic spine and
other parts of the body | Radiation |
In the present case, the patient had already
undergone two surgeries to remove intracranial meningiomas. The
specimens that had been removed in 2004 were also evaluated in
2015, since it was suspected that the excised tumors were indeed
SFT/HPC. A pathological examination on biopsy specimens from 2017
was also performed. The morphological appearance of the larger
intracranial mass was confirmed to be consistent with the biopsy.
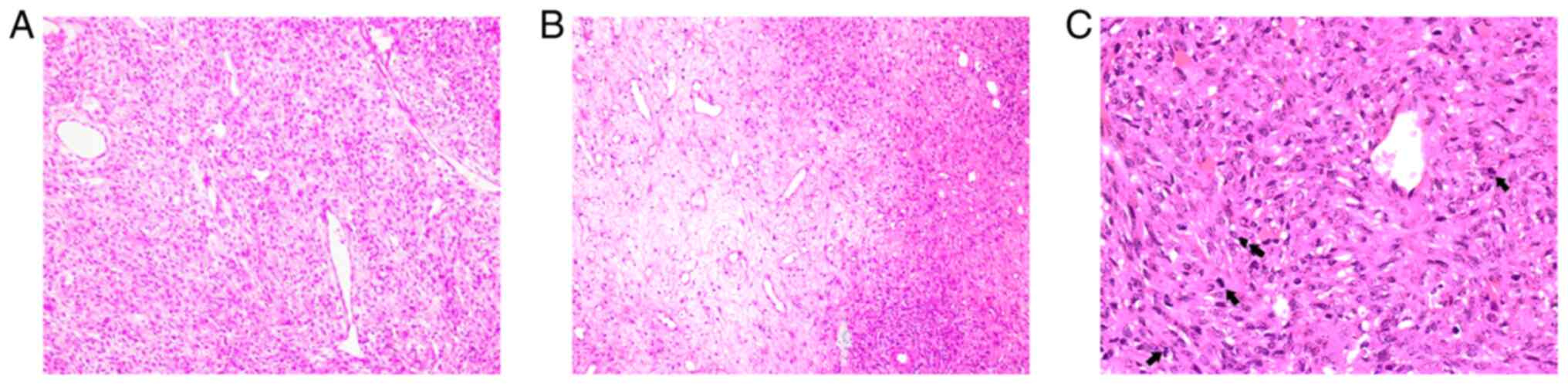
The tumor histopathology revealed an abundance of typical
‘staghorn’ vascularization in the tissue (Fig. 2A) and tumor cells grew around blood
vessels. A large number of spindle-shaped tumor cells arranged in
bundles and alternating between sparse and dense distribution was
observed between the vessels (Fig.
2B) and mitotic bodies were visible (≥5 mitoses per 10
high-power fields) (Fig. 2C).
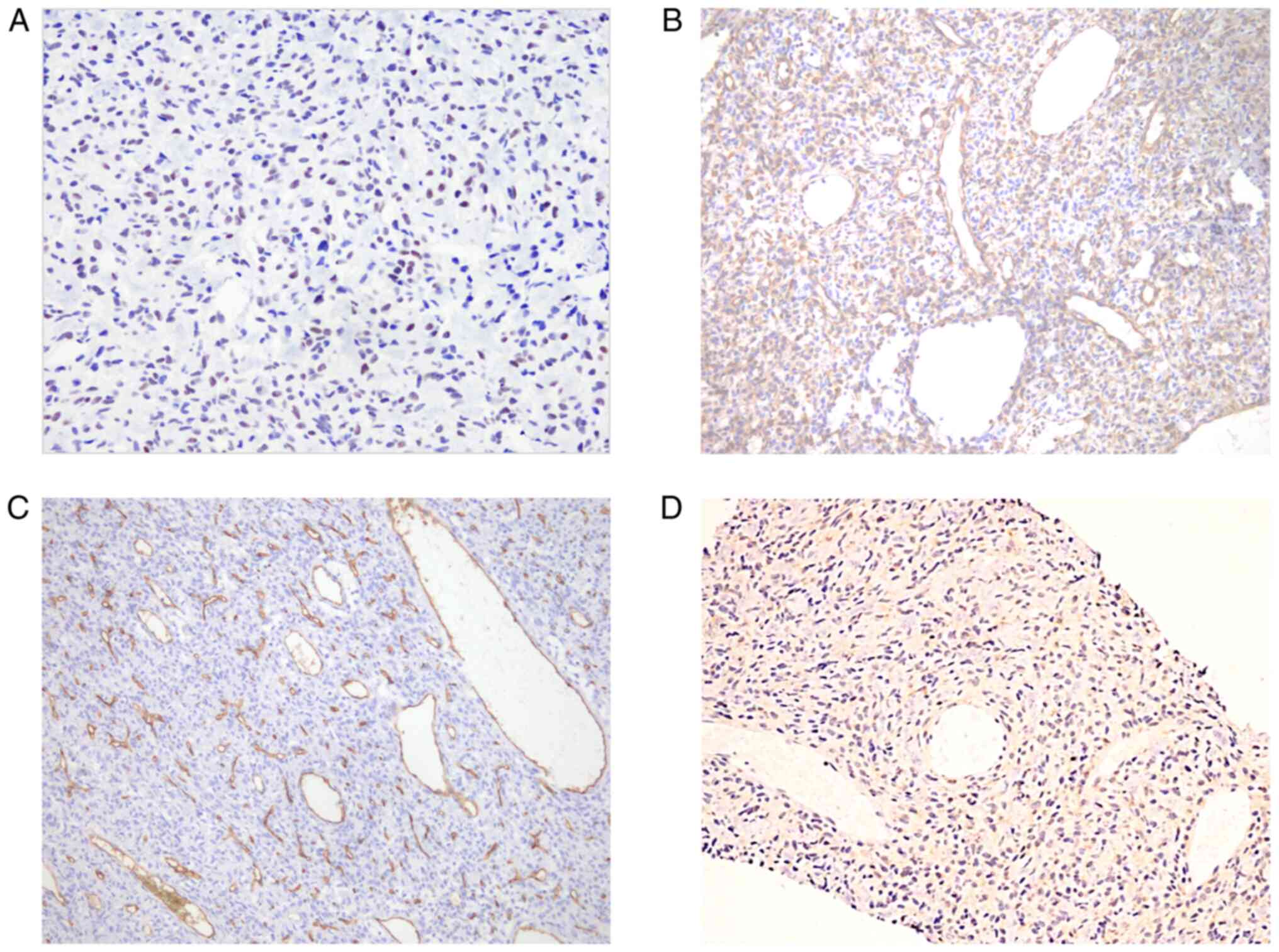
Histopathological and immunohistochemical findings of the tumor
from 6 years previously revealed spindle cells positive for STAT6
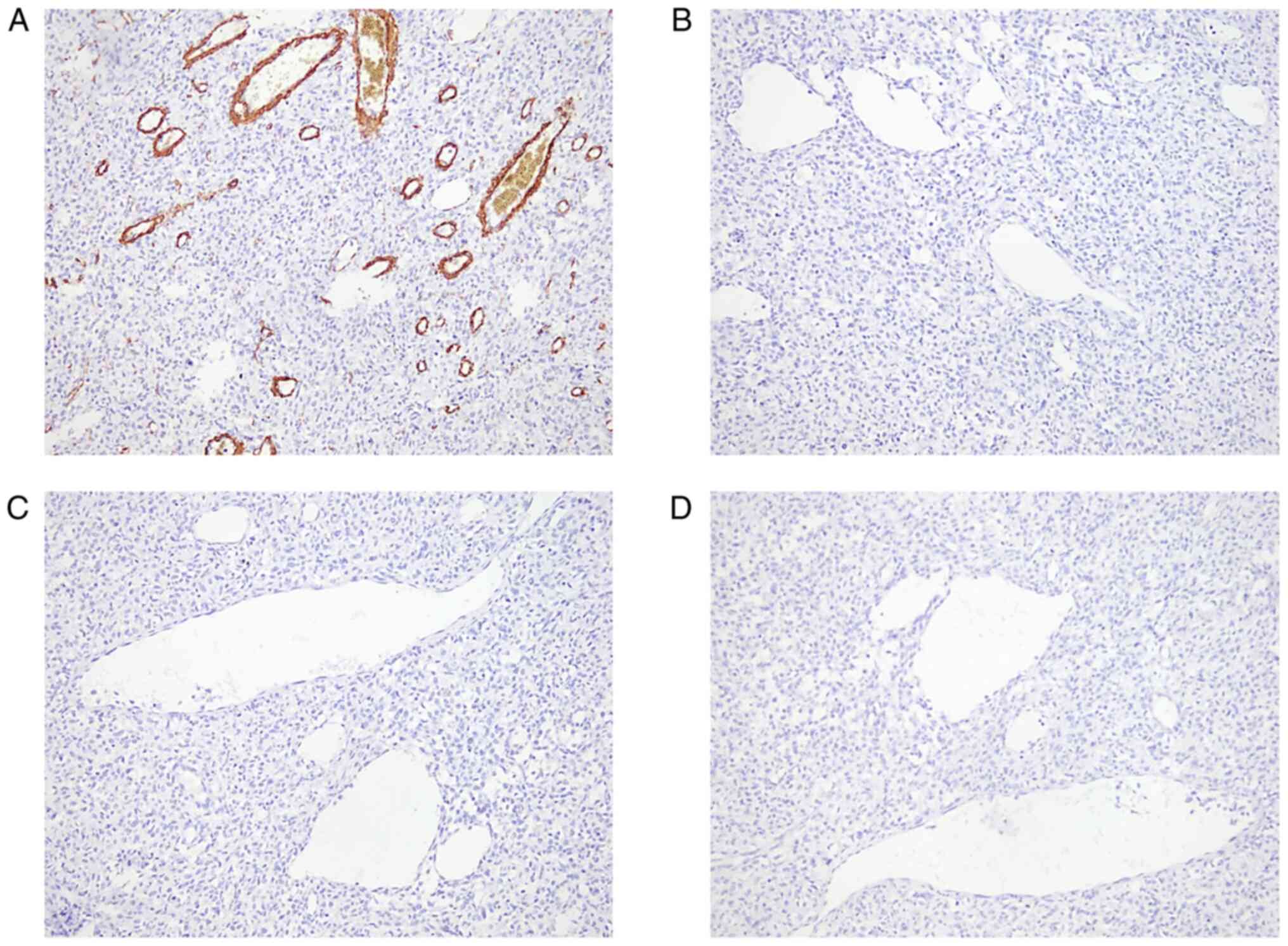
(Fig. 3A), vimentin (Fig. 3B), CD34 (Fig. 3C) and Bcl-2 (Fig. 3D), and negativity for smooth muscle
actin (Fig. 4A), S-100 (Fig. 4B), epithelial membrane antibody
(EMA) (Fig. 4C) and progesterone
receptor (PR) (Fig. 4D), findings
that were compatible with malignant SFT/HPC, WHO III. Therefore,
all intrapulmonary and intracranial lesions were considered
recurrence and metastasis from a primary intracranial SFT/HPC. The
patient was discharged after 15 days of radiotherapy. One year and
9 months after the onset of chest pain, the patient's systemic
condition worsened and CT indicated thoracolumbar metastasis. The
patient died two months after the detection of multiple metastases
throughout the body.
Discussion
SFT/HPC tumors are perivascular cell cancers.
Although such tumors may arise intracranially, they are infrequent,
accounting for <1% of all primary CNS tumors, with the majority
occurring in the fifth decade of life and with no apparent sex
differences (3). Solitary fibrous
tumors and hemangiopericytomas of the CNS exhibit overlapping
pathology and immunohistochemical characteristics, such as
occurrence in the neuraxis, inversions at 12q13 and overexpression
of the NGFI-A-binding protein 2 (NAB2)-STAT6 gene fusion; according
to recent research, quantitative PCR revealed high expression
levels of the 5′-end of NAB2 and the 3′-end of STAT6, which, on
deep sequencing of enriched DNA corresponded to NAB2/STAT6 fusions
(2,4). Their diagnoses inevitably overlap as
a result of this representation (5,6).
In the report for 2016, the WHO classification of
CNS tumors has created the combined term SFT/HPC (7,8).
Thus, as HPC and SFT have similar imaging features, their combined
diagnosis may decrease the incidence of presurgical misdiagnosis.
Until the tumor reaches a particular size or invades brain regions
that produces measurable effects or has functional implications,
there are no identifiable clinical symptoms.
Although radiologic characteristics may assist in
predicting and grading tumor pathology, pathological examination
remains the gold standard for diagnosis and pathological grading.
On T2-weighted MRI (T2WI), the majority of cases with WHO grade I
exhibit intermediate-low signal intensity (3). A minority of the cases have 2
different signal intensity areas on T2WI. T2 hyperintense regions
indicate fibrotic components with distinct difference enhancement,
whereas T2 iso- or hyperintense areas represent hypercellular
components with mild heterogeneous enhancement. The so-called
black-and-white or yin-yang signals are associated with
intracranial SFTs/HPCs when these two components are combined
(9–11). For WHO grades II and III, the
tumors generally have intermediate-high signal intensity on T2WI.
The existence of a tortuous flow-empty vascular shadow inside or on
the surface of these two grades of tumors is critical for
distinguishing WHO grade I from WHO grade II and III.
Preoperatively, SFTS/HPCs are frequently misdiagnosed as fibrous
meningiomas or nerve sheath tumors, which are difficult to
differentiate. SFTs/HPCs are more prone than meningiomas to develop
necrosis, cystic degeneration and areas of signal void, whereas
meningiomas usually present with dural caudal symptoms.
Owing to the diversity of histological patterns
exhibited by SFT, they frequently pose a diagnostic challenge and
integration of clinical, histomorphological, immunohistochemical
and molecular features is necessary for establishing a correct
diagnosis (12). However, the
final pathological and immunohistochemical findings remain the gold
standard for diagnosing intracranial SFTS/HPCs, which are
classified into three categories (I–III) based on a set of
characteristics (13). Grade I
SFT/HPC has more collagen and a relatively low cell density with
spindle-like cells. Grade II has more cells arranged in no specific
direction and less collagen, and staghorn-like vascular branches
were observed. There were at least 5 mitotic figures per 10
high-power microscopes at Grade III (2). In SFT, high mitosis, necrosis and
atypia are all crucial indicators of malignant and aggressive
behavior (14). Since intracranial
SFT/HPC is similar to meningioma in terms of clinical presentation
and pathological diagnosis, it is critical to distinguish between
them. The histopathological feature of SFT is the coexistence of
sparse and dense regions, which are separated by fibrous stroma and
have hemangiopericytoma branching vessels (15). Tumors with the SFT phenotype had a
patternless architecture or a short fascicular pattern, with
alternating hypocellular and hypercellular areas and thick collagen
bands on histopathology (2).
Tumors with an HPC phenotype have a high level of cellularity
across the entire area. Meningioma cells may be observed to be
arranged in nest-like clusters under the microscope, with abundant
cytoplasm and poorly defined cells (syncytium-like).
Pseudo-inclusions are common in the nucleus and the cells have
weakly defined cell borders (syncytial cell-like) (16). Within the meningioma, sand granule
formation is also seen. In SFT/HPCs, STAT6 is always positive, CD34
is positive to varying degrees, and most of them also express Bcl-2
and CD99. STAT6 immunohistochemistry is both a highly specific and
sensitive surrogate for NAB2-STAT6 gene fusions, and the
specificity and sensitivity of nuclear STAT6 for SFT/HPCs were 100
and 96.6%, respectively (17–19).
Detection of STAT6 nuclear expression, which is a molecular
hallmark of SFTs, is recommended to confirm the diagnosis of
SFT/HPC as per the 2021 WHO guidelines (20). SFT/HPCs are positive for CD34 and
STAT6 on immunohistochemistry and negative for EMA and PR. However,
for all forms of meningiomas, the opposite is true (21). When the histology results make it
difficult to distinguish between the two, immunohistochemical
examination of markers such as STAT6, CD34, EMA and PR may be a
valuable tool.
Most SFTs grow slowly, but low-grade SFTs/HPCs may
progress to higher-grade tumors (22). Of note, only a small number of
cases of malignant progression from lower-grade SFT/HPC tumors have
been reported in the literature, owing to the lack of a
comprehensive review of tumor recurrence. As in the present case, a
WHO grade II right cerebellar curtain mass had developed into a WHO
grade III left cerebellar horn mass. Surgical resection is the
treatment of choice for SFT/HPC and gross total resection is the
most important factor in tumor management (23). However, this is technically
difficult, as most higher-grade tumors invade important surrounding
anatomical structures, such as arteries, venous sinuses and nerves,
and because the tumor has a rich blood supply, intraoperative
bleeding frequently occurs. Preoperative embolization of the
tumor's blood supply artery has been documented in the literature
to reduce intraoperative bleeding and surgical difficulty (24). Complete excision of the mass is
superior to incomplete excision and sub-totally removed SFTs/HPCs
may recur or continue to grow. Subtotal resection carries a
recurrence risk of up to 54%, compared to the 14% recurrence rate
of total resection. As a result, the extent of resection is the
most important predictor of SFTS/HPC recurrence. RT positively
affects patient outcomes; in particular, patients undergoing gross
total tumor resection + radiotherapy treatment exhibited the best
survival advantage. Recurrence and metastasis of SFT/HPC are common
and cases may progress to advanced SFT (25). Patients with advanced SFT exhibit a
certain response to traditional chemotherapy drugs, but there are
not many options and alternative treatments for unresectable tumors
are urgently required.
In the present case, the patient had undergone
partial surgical resection of a meningioma diagnosed in 2004 and
2015, respectively. SFT/HPC lung metastases at our institution were
not surgically excised in the present case and the patient received
radiotherapy treatment in 2017. The patient died two months later
in 2018 after presenting with multiple metastases in the thoracic
spine and throughout the body. It is necessary to be aware that
recurrence and metastasis may occur even after a lengthy period of
resection treatment, up to 10 years (26–28).
The clinical course of patients with SFT/HPC is unpredictable, as
local recurrence occurs in 25–85% of cases and whole-body
metastases occur in 15–36% (29).
SFT is a malignant condition that exhibits different clinical
behaviors ranging from low to highly aggressive SFT. Malignant
progression may be just one of several mechanisms provoking
recurrence and metastasis (30).
High-grade SFT/HPCs are more likely to recur and have an
unfavorable overall survival rate. Higher histological grade and
subtotal resection were associated with recurrence, while higher
histological grade and recurrence were associated with metastasis
formation. Recurrence was also revealed to be a risk factor for the
establishment of metastases. The most prevalent locations of
distant metastasis are the bone, liver, lung and abdominal cavity
(31). Intraspinal spread of
metastases from an intracranial HPC, particularly thoracic
metastasis, is rare (32).
Intracranial SFT/HPC is a tumor with moderate to low malignancy and
a long survival period, and even if the tumor recurs or
metastasizes distantly, as long as it is discovered early and
treated immediately, it is possible to achieve a better outcome.
Thus, therapy for intracranial SFT/HPC should be based on surgical
resection with long-term vigilant monitoring (33). Follow-up of patients with SFT/HPC
would probably reveal new recurrences and histological progression.
The latest risk stratification model by Demicco et al
(34) is based on assessment of
patient age, mitoses/mm2, tumor size and percentage of
tumor necrosis to predict metastatic recurrences. It stratifies
SFTs into low, intermediate and high-risk categories and is more
accurate in predicting the prognosis. Therefore, it is appropriate
to monitor disease progression based on risk prediction
stratification model categories in combination with follow-up.
Intracranial SFT/HPCs are uncommon mesenchymal
neoplasms. SFT/HPCs may recur and metastasize even long after
initial treatment. In our group, a case of recurrence and pulmonary
metastasis 11 years after treatment with intracranial primary
SFT/HPC resection was encountered. It should also be noted that
SFT/HPC was previously thought to be a subtype of meningioma
(35). As the clinical features
and imaging presentation of SFT/HPC are similar to those of common
meningiomas, they are frequently difficult to recognize. Thus,
clinicians should depend on tissue biopsy and immunohistochemistry
to make a definitive diagnosis. Preoperative imaging helps to
clarify the diagnosis and determine the tumor grade. STAT6
immunohistochemistry is also a valuable and sensitive diagnostic
method. Adjuvant radiation therapy is effective for malignant
tumors that cannot be completely resected (36). In recent years, the molecular
genetics of soft tissue tumors have been developing rapidly and the
new generation of molecular tests, represented by second-generation
sequencing, may not only provide an accurate clinical diagnosis but
also assist the search for therapeutic targets in clinical
research, formulate treatment strategies, assist in determining
prognosis and provide relevant testing information for
individualized and precise treatment of patients with soft tissue
tumor (37,38).
Molecular target therapy is a promising approach for
unresectable or metastatic SFT and an improved knowledge of the
molecular biology of the neoplasm may support such therapy in the
near future. It was reported that certain growth factors and
kinases are overexpressed in SFTs, including platelet-derived
growth factor (PDGF) α, PDGFβ, PDGF receptor (PDGFR)-α, PDGFR-β,
insulin-like growth factor (IGF) 1 receptor, epidermal growth
factor receptor, vascular endothelial growth factor (VEGF), IGFII,
cellular-mesenchymal epithelial transition, c-kit, c-erbB2,
phosphatase and tensin homolog deleted on chromosome 10,
phosphorylated (p)AKT, pS6, phosphorylated 4E-binding protein,
ERBB2, FGFR1 and JAK2 (39,40).
Overexpression of these markers leads to activation of the Akt/mTOR
pathway and appears to be associated with tumor necrosis, targeted
therapies toward the IGF signaling pathway and the Akt/mTOR pathway
is considered a candidate therapeutic target, whereas it was not
possible to directly establish an association with the actual
clinical outcome (40). The 2021
National Comprehensive Cancer Network guidelines recommend the use
of four targeted agents, bevacizumab, sunitinib, pazopanib and
sorafenib, for the treatment of SFT/HPC, and all have activity
against VEGF receptor (VEGFR)-1, −2 and −3, whose broad spectrum of
targets may achieve in potential antitumor as well as
antiangiogenic effects in tumors (41–43).
Combination therapy with temozolomide and bevacizumab appears to
provide a clinical benefit (44).
Pazopanib is an anti-angiogenesis-based, small molecule,
multi-targeting agent that interferes with angiogenesis inhibitors
required for intractable tumor survival and growth, and has
activity against VEGFR-1, −2 and −3, as well as PDGFR and KIT
(45). Sorafenib inhibits the
tyrosine kinases VEGFR-1, −2 and −3, PDGFR, RET/PTC as well as the
Raf/Mek/Erk pathway (46). It is
suggested that the detection of molecular targets (such as VEGFR-1,
−2 and −3, BRAF, RET and PTC) is performed in patients with SFT,
which will help to screen the potential beneficiaries of targeted
therapy and extend the survival of the patient.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Yunnan Provincial
Education Department Scientific Research Fund Project (grant no.
2022Y837).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
QL was responsible for collecting clinical, imaging
and pathological data of the patient and drafting the manuscript.
and CZ analyzed the data and revised the manuscript. ZL
participated in making the pathological diagnosis. QL, CZ and YL
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The First Affiliated Hospital to Dali University
(approval no. 20200612).
Patient consent for publication
Written consent for publication of the case report
and any accompanying images, without any potentially identifying
information, was provided by the patient's family.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Stout AP and Murray MR:
Hemangiopericytoma: A vascular tumor featuring zimmermann's
pericytes. Ann Surg. 116:26–33. 1942. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 world health organization
classification of tumors of the central nervous system: A summary.
Acta Neuropathol. 131:803–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang ZY, Qiu K, Ma YH, Wang XT, Bao JJ,
Zhang ZF and Liu XZ: Intracranial solitary fibrous tumors: A report
of two cases and a review of the literature. Oncol Lett.
11:1057–1060. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yamashita D, Suehiro S, Kohno S, Ohue S,
Nakamura Y, Kouno D, Ohtsuka Y, Nishikawa M, Matsumoto S, Bernstock
JD, et al: Intracranial anaplastic solitary fibrous
tumor/hemangiopericytoma: Immunohistochemical markers for
definitive diagnosis. Neurosurg Rev. 44:1591–1600. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fritchie K, Jensch K, Moskalev EA, Caron
A, Jenkins S, Link M, Brown PD, Rodriguez FJ, Guajardo A, Brat D,
et al: The impact of histopathology and NAB2-STAT6 fusion subtype
in classification and grading of meningeal solitary fibrous
tumor/hemangiopericytoma. Acta Neuropathol. 137:307–319. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Georgiesh T, Namløs HM, Sharma N, Lorenz
S, Myklebost O, Bjerkehagen B, Meza-Zepeda LA and Boye K: Clinical
and molecular implications of NAB2-STAT6 fusion variants in
solitary fibrous tumour. Pathology. 53:713–719. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim BS, Kim Y, Kong DS, Nam DH, Lee JII,
Suh YL and Seol HJ: Clinical outcomes of intracranial solitary
fibrous tumor and hemangiopericytoma: Analysis according to the
2016 WHO classification of central nervous system tumors. J
Neurosurg. 129:1384–1396. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shin DW, Kim JH, Chong S, Song SW, Kim YH,
Cho YH, Hong SH and Nam SJ: Intracranial solitary fibrous
tumor/hemangiopericytoma: Tumor reclassification and assessment of
treatment outcome via the 2016 WHO classification. J Neuroncol.
154:171–178. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Frazier AA: The yin and yang of solitary
fibrous tumor. Radiographics. 34:2942014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang H, Zhang Y, Zheng T, Li C, Tang G and
Chen G: A solitary fibrous tumor/hemangiopericytoma of the fourth
ventricle: Case report and literature review. J Int Med Res.
47:6349–6355. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Clarençon F, Bonneville F, Rousseau A,
Galanaud D, Kujas M, Naggara O, Cornu P and Chiras J: Intracranial
solitary fibrous tumor: Imaging findings. Eur J Radiol. 80:387–394.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ronchi A, Cozzolino I, Marino FZ, Accardo
M, Montella M, Panarese I, Roccuzzo G, Toni G, Franco R and De
Chiara A: Extrapleural solitary fibrous tumor: A distinct entity
from pleural solitary fibrous tumor. An update on clinical,
molecular and diagnostic features. Ann Diagn Pathol. 34:142–150.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma L, Wang L, Fang X, Zhao CH and Sun L:
Diagnosis and treatment of solitary fibrous
tumor/hemangiopericytoma of central nervous system. Retrospective
report of 17 patients and literature review. Neuro Endocrinol Lett.
39:88–94. 2018.PubMed/NCBI
|
|
14
|
Kallen ME and Hornick JL: The 2020 WHO
classification: What's new in soft tissue tumor pathology? Am J
Surg Pathol. 45:e1–e23. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang SC and Huang HY: Solitary fibrous
tumor: An evolving and unifying entity with unsettled issues.
Histol Histopathol. 34:313–334. 2019.PubMed/NCBI
|
|
16
|
Buerki RA, Horbinski CM, Kruser T,
Horowitz PM, James CD and Lukas RV: An overview of meningiomas.
Future Oncol. 14:2161–2177. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Doyle LA, Vivero M, Fletcher CD, Mertens F
and Hornick JL: Nuclear expression of STAT6 distinguishes solitary
fibrous tumor from histologic mimics. Mod Pathol. 27:390–395. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schweizer L, Koelsche C, Sahm F, Piro RM,
Capper D, Reuss DE, Pusch S, Habel A, Meyer J, Göck T, et al:
Meningeal hemangiopericytoma and solitary fibrous tumors carry the
NAB2-STAT6 fusion and can be diagnosed by nuclear expression of
STAT6 protein. Acta Neuropathol. 125:651–658. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yoshida A, Tsuta K, Ohno M, Yoshida M,
Narita Y, Kawai A, Asamura H and Kushima R: STAT6
immunohistochemistry is helpful in the diagnosis of solitary
fibrous tumors. Am J Surg Pathol. 38:552–559. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Louis DN, Perry A, Wesseling P, Brat DJ,
Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM,
Reifenberger G, et al: The 2021 WHO classification of tumors of the
central nervous system: A summary. Neuro Oncol. 23:1231–1251. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Barresi V, Caffo M and Tuccari G:
Classification of human meningiomas: Lights, shadows, and future
perspectives. J Neurosci Res. 94:1604–1612. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Apra C, Mokhtari K, Cornu P, Peyre M and
Kalamarides M: Intracranial solitary fibrous
tumors/hemangiopericytomas: First report of malignant progression.
J Neurosurg. 128:1719–1724. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ramakrishna R, Rostomily R, Sekhar L,
Rockhill J and Ferreira M: Hemangiopericytoma: Radical resection
remains the cornerstone of therapy. J Clin Neurosci. 21:612–615.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Patel SR, Vachhani P and Moeslein F:
Embolic brain infarcts: A rare fatal complication of preoperative
embolization of a massive solitary fibrous tumor of the pleura.
Cardiovasc Intervent Radiol. 40:306–309. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sonabend AM, Zacharia BE, Goldstein H,
Bruce SS, Hershman D, Neugut AI and Bruce JN: The role for adjuvant
radiotherapy in the treatment of hemangiopericytoma: A
surveillance, epidemiology, and end results analysis. J Neurosurg.
120:300–308. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hashida S, Yokota H, Oyama Y, Kawakami M,
Murakami S and Kawakami H: Bulky cardiac metastasis of intracranial
solitary fibrous tumor/hemangiopericytoma: Delayed metastasis after
cranial tumor resection. Radiol Case Rep. 14:1175–1180. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nakada S, Minato H, Takegami T, Kurose N,
Ikeda H, Kobayashi M, Sasagawa Y, Akai T, Kato T, Yamamoto N and
Nojima T: NAB2-STAT6 fusion gene analysis in two cases of meningeal
solitary fibrous tumor/hemangiopericytoma with late distant
metastases. Brain Tumor Pathol. 32:268–274. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu Z, Yang H, Weng D and Ding Y: Rapid
recurrence and bilateral lungs, multiple bone metastasis of
malignant solitary fibrous tumor of the right occipital lobe:
Report of a case and review. Diagn Pathol. 10:912015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Damodaran O, Robbins P, Knuckey N,
Bynevelt M, Wong G and Lee G: Primary intracranial
haemangiopericytoma: Comparison of survival outcomes and metastatic
potential in WHO grade II and III variants. J Clin Neurosci.
21:1310–1314. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Giordan E, Marton E, Wennberg AM,
Guerriero A and Canova G: A review of solitary fibrous
tumor/hemangiopericytoma tumor and a comparison of risk factors for
recurrence, metastases and death among patients with spinal and
intracranial tumors. Neurosurg Rev. 44:1299–1312. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Patel AR, Flores BC, Ban VS, Hatanpaa KJ,
Mickey BE and Barnett SL: Intracranial hemangiopericytomas:
Recurrence, metastasis, and radiotherapy. J Neurol Surg B Skull
Base. 78:324–330. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ali HSM, Endo T, Endo H, Murakami K and
Tominaga T: Intraspinal dissemination of intracranial
hemangiopericytoma: Case report and literature review. Surg Neurol
Int. 7 (Suppl 40):S1016–S1020. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Demicco EG, Park MS, Araujo DM, Fox PS,
Bassett RL, Pollock RE, Lazar AJ and Wang WL: Solitary fibrous
tumor: A clinicopathological study of 110 cases and proposed risk
assessment model. Mod Pathol. 25:1298–1306. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Demicco EG, Wagner MJ, Maki RG, Gupta V,
Iofin I, Lazar AJ and Wang WL: Risk assessment in solitary fibrous
tumors: Validation and refinement of a risk stratification model.
Mod Pathol. 30:1433–1442. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schiariti M, Goetz P, El-Maghraby H,
Tailor J and Kitchen N: Hemangiopericytoma: Long-term outcome
revisited. Clinical article. J Neurosurg. 114:747–755. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen LF, Yang Y, Yu XG, Gui QP, Xu BN and
Zhou DB: Multimodal treatment and management strategies for
intracranial hemangiopericytoma. J Clin Neurosci. 22:718–725. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Anderson WJ and Doyle LA: Updates from the
2020 world health organization classification of soft tissue and
bone tumours. Histopathology. 78:644–657. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
von Mehren M, Kane JM, Bui MM, Choy E,
Connelly M, Dry S, Ganjoo KN, George S, Gonzalez RJ, Heslin MJ, et
al: NCCN guidelines insights: Soft tissue sarcoma, version 1.2021.
J Natl Compr Canc Netw. 18:1604–1612. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hajdu M, Singer S, Maki RG, Schwartz GK,
Keohan ML and Antonescu CR: IGF2 over-expression in solitary
fibrous tumours is independent of anatomical location and is
related to loss of imprinting. J Pathol. 221:300–307. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yamada Y, Kohashi K, Fushimi F, Takahashi
Y, Setsu N, Endo M, Yamamoto H, Tokunaga S, Iwamoto Y and Oda Y:
Activation of the Akt-mTOR pathway and receptor tyrosine kinase in
patients with solitary fibrous tumors. Cancer. 120:864–876. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Stacchiotti S, Negri T, Libertini M,
Palassini E, Marrari A, Troia BD, Gronchi A, Tos APD, Morosi C,
Messina A, et al: Sunitinib malate in solitary fibrous tumor (SFT).
Ann Oncol. 23:3171–3179. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Machado I, Nieto-Morales G, Cruz J,
Navarro S, Giner F, Ferrandez A, López-Soto MV, Lavernia J and
Llombart-Bosch A: Controversial issues in soft tissue solitary
fibrous tumors: A pathological and molecular review. Pathol Int.
70:129–139. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sawada N, Ishiwata T, Naito Z, Maeda S,
Sugisaki Y and Asano G: Immunohistochemical localization of
endothelial cell markers in solitary fibrous tumor. Pathol Int.
52:769–776. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Biermann JS, Chow W, Reed DR, Lucas D,
Adkins DR, Agulnik M, Benjamin RS, Brigman B, Budd GT, Curry WT, et
al: NCCN guidelines insights: Bone cancer, version 2.2017. J Natl
Compr Canc Netw. 15:155–167. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Maruzzo M, Martin-Liberal J, Messiou C,
Miah A, Thway K, Alvarado R, Judson I and Benson C: Pazopanib as
first line treatment for solitary fibrous tumours: The royal
marsden hospital experience. Clin Sarcoma Res. 5:52015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Park MS, Ravi V and Araujo DM: Inhibiting
the VEGF-VEGFR pathway in angiosarcoma, epithelioid
hemangioendothelioma, and hemangiopericytoma/solitary fibrous
tumor. Curr Opin Oncol. 22:351–355. 2010. View Article : Google Scholar : PubMed/NCBI
|