Introduction
Papillary thyroid carcinoma (PTC), a common type of
differentiated thyroid cancer, exhibits an increasing global
incidence rate (1). PTC management
involves surgery followed by hormonal therapy, with or without
adjuvant radioiodine therapy (2).
Although the majority of patients with PTC present with a favorable
prognosis with a 5-year disease-free survival (DFS) rate ranging
from 80–97.4%, a small number of patients may develop
extrathyroidal invasion and recurrence, resulting in poor prognosis
(3–5). Of note, several biomarkers have been
identified to help monitor disease progression and predict the
recurrence risk for patients with PTC (6–8).
However, the discovery of additional biomarkers is required to
further improve the prognosis of PTC.
Kinesin family member 2A (KIF2A), a member of the
kinesin-13 family, is a microtubule depolymerase that regulates
microtubule assembly, spindle organization and chromosome
congression, to further mediate the cell cycle during cell division
(9–12). Results of previous studies have
demonstrated that KIF2A is an oncoprotein in different types of
cancer (13–15). For instance, KIF2A promotes
malignant behaviors of oral squamous carcinoma cells via activating
the PI3K/AKT signaling pathway (13). Furthermore, KIF2A induces cell
proliferation and invasion, while suppressing cell apoptosis in
nasopharyngeal carcinoma (14).
Within clinical practice, KIF2A exhibits key prognostic value for
the management of gastric cancer, colorectal cancer and
nasopharyngeal carcinoma (15–17).
However, to the best of our knowledge, there are currently no
studies focusing on the clinical role of KIF2A in patients with
PTC.
Thus, the present study aimed to detect the
expression levels of KIF2A in carcinoma and para-carcinoma tissues
obtained from patients with PTC, and explore the potential
association between KIF2A, clinical features and prognosis. The
present study aimed to provide a novel theoretical basis for the
management of PTC.
Materials and methods
Patients
A total of 200 patients with PTC who received
surgical resection at the Central Hospital of Wuhan (Wuhan, China)
between January 2014 and December 2020 were retrospectively
reviewed. Patients were eligible for inclusion in this study if
they met the following criteria: i) Pathological diagnosis of PTC;
ii) 18–80 years of age; iii) underwent surgical resection; iv)
available formalin-fixed paraffin-embedded (FFPE) samples of
carcinoma tissues and para-carcinoma tissues; and v) clinical
characteristics and follow-up data were available. The patients who
had a prior history of other malignancies/solid tumors were
excluded from the study. The present study was approved by the
Ethics Committee of The Central Hospital of Wuhan, Tongji Medical
College, Huazhong University of Science and Technology (Wuhan,
China) with approval number WHZXKYL2022-101.
Data collection
The clinical characteristics of all patients were
obtained from their medical records, including age, sex, tumor
size, extrathyroidal invasion, pathologic tumor-nodes-metastasis
(pTNM) stage and radioiodine treatment. The Eighth Edition of the
TNM staging criteria was used, based on which the patients treated
prior to 2016 were reclassified. In addition, follow-up data of all
patients were collected and the final date of recording was March
31, 2021. Subsequently, disease-free survival (DFS) and overall
survival (OS) were obtained for prognostic evaluation. DFS was
defined as the duration from surgical resection to disease relapse;
OS was defined as the duration from surgical resection to disease
relapse or patient death.
Sample collection and detection
FFPE samples of carcinoma and para-carcinoma tissues
were obtained from all patients to assess KIF2A protein expression
using immunohistochemistry (IHC) as previously described (18). Rabbit polyclonal anti-KIF2A
antibody (1:200 dilution; cat. no. ab197988; Abcam) was used as the
primary antibody and incubation was performed at room temperature
for 2 h. Goat anti-rabbit IgG (H&L; 1:1,000 dilution; cat. no.
ab150077; Abcam) was used as the secondary antibody and incubated
with the sample at room temperature for 1.5 h. Following staining,
IHC scores were determined using a light microscope based on the
intensity and density of stained cells (19). In detail, the intensity was scored
as four grades: 0 (negative), 1 (weak), 2 (moderate) and 3
(strong); the density was scored as five grades: 0 (0%), 1 (1–25%),
2 (26–50%), 3 (51–75%) and 4 (76–100%), representative examples are
provided in Fig. S1. The IHC
score was a product of the intensity score and the density score
with a maximum score of 12. The assessment of the IHC score was
performed by two independent pathologists who were blinded to the
clinical information of the patients. The final IHC score was the
average of two scores proposed by the two independent
pathologists.
Furthermore, carcinoma and para-carcinoma tissue
samples that were stored in liquid nitrogen were collected from 91
patients to detect KIF2A mRNA expression using reverse
transcription-quantitative (RT-q)PCR. After extraction of total RNA
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) from the specimens, RT-PCR was subsequently
performed using an iScript™ cDNA Synthesis kit (Bio-Rad
Laboratories, Inc.). qPCR was carried out using a KOD
SYBR® qPCR Mix with SYBR® Green I fluorophore
(Toyobo). The thermocycling conditions were 1 cycle of 98°C for 30
sec and 40 cycles of 98°C for 10 sec and 68°C for 30 sec. The
relative expression of KIF2A was calculated based on the
2−ΔΔCq method and β-actin was used as the internal
reference (20). The primers for
KIF2A and β-actin were designed as follows: KIF2A forward,
5′-GCCTTTGATGACTCAGCTCC-3′ and reverse, 5′-TTCCTGAAAAGTCACCACCC-3′;
β-actin forward, 5′-TGACGTGGACATCCGCAAAG-3′ and reverse,
5′-CTGGAAGGTGGACAGCGAGG-3′ (21).
Statistical analysis
SPSS version 24.0 (IBM Corp.) and R version 4.0.5
(TSHRC package, available at www.r-project.org) were used to analyze the clinical
data of patients and GraphPad Prism version 7.01 (GraphPad Software
Inc.) was used to construct the graphs. Comparisons of KIF2A
expression between samples of carcinoma and para-carcinoma tissues
were performed using the Wilcoxon signed-rank test or χ2
test. The ability of KIF2A expression to distinguish between
carcinoma and para-carcinoma tissue samples was evaluated using a
receiver operating characteristic (ROC) curve. Comparison of KIF2A
expression among patients with different clinical features was
performed using a Mann-Whitney U-test. The correlation between
KIF2A expression and clinical data was assessed by determining
Spearman's rank correlation coefficient. DFS and OS were presented
using Kaplan-Meier curves and were analyzed using the log-rank test
or two-stage test followed by post-hoc comparisons with
Bonferroni's test according to a previous study (22). Factors affecting DFS and OS were
determined using multivariate regression analysis with Cox's
proportional hazards model. In the survival analysis, KIF2A protein
expression was classified as high expression (final IHC score,
>3) or low expression (final IHC score, ≤3), and KIF2A mRNA
expression was based on the median expression value of carcinoma
tissues (2.630) and classified as high expression (≥2.630) and low
expression (<2.630). P<0.05 was considered to indicate a
statistically significant difference.
Results
Clinical features of patients with
PTC
Initially, 283 patients were enrolled. Subsequently,
83 patients who met the exclusion criteria or who disobeyed the
inclusion criteria were excluded. In detail, the FFPE samples of 46
patients were not available, the clinical data of 28 patients were
not available, 5 patients were aged below 18 years and 4 patients
had a previous cancer history. Thus, 200 patients were finally
enrolled. Among all recruited patients with PTC, the mean age was
45.2±11.7 years (Table I). The
cohort comprised 142 (71.0%) females and 58 (29.0%) males.
Furthermore, 78 (39.0%) patients presented with extrathyroidal
invasion, while the remaining 122 (61.0%) patients did not exhibit
any extrathyroidal invasion. In terms of pTNM stage, 139 (69.5%),
36 (18.0%), 19 (9.5%) and 6 (3.0%) patients had been diagnosed as
pTNM stage I, II, III and IV, respectively. A total of 98 (49.0%)
patients received adjuvant radioiodine therapy, while 102 (51.0%)
patients did not. The detailed clinical features of the patients
with PTC are presented in Table
I.
 | Table I.Characteristics of the patients
(n=200). |
Table I.
Characteristics of the patients
(n=200).
| Item | Value |
|---|
| Age, years | 45.2±11.7 |
| Sex |
|
|
Female | 142 (71.0) |
| Male | 58 (29.0) |
| Tumor size, cm | 3.6±1.7 |
| Extrathyroidal
invasion |
|
| No | 122 (61.0) |
| Yes | 78 (39.0) |
| pT stage |
|
| pT1 | 37 (18.5) |
| pT2 | 45 (22.5) |
| pT3 | 59 (29.5) |
| pT4a | 43 (21.5) |
| pT4b | 16 (8.0) |
| pN stage |
|
| pN0 | 67 (33.5) |
| pN1 | 133 (66.5) |
| pTNM stage |
|
| I | 139 (69.5) |
| II | 36 (18.0) |
|
III | 19 (9.5) |
| IV | 6 (3.0) |
| Radioiodine
therapy |
|
| No | 102 (51.0) |
|
Yes | 98 (49.0) |
KIF2A expression in patients with
PTC
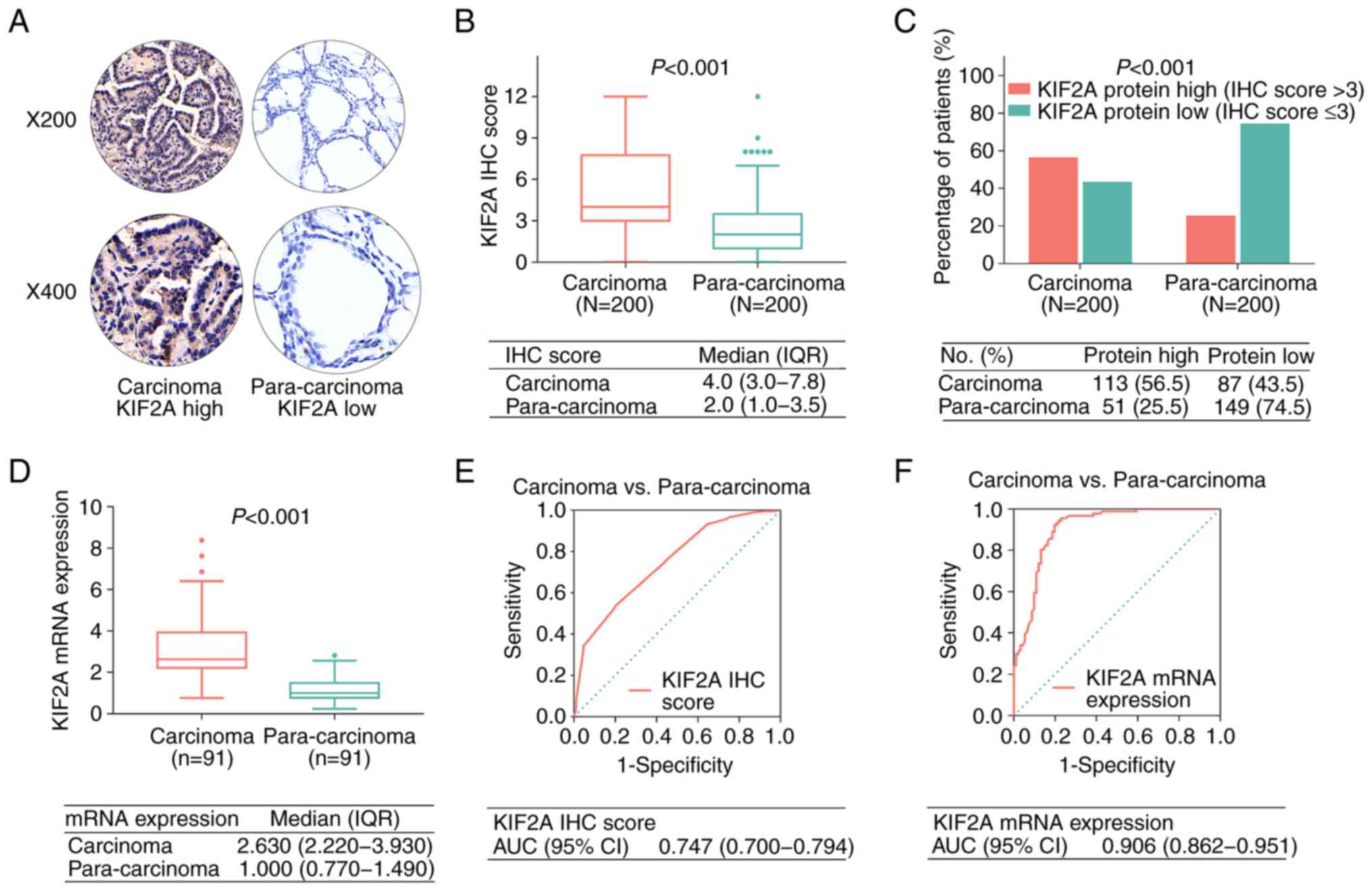
The IHC score for KIF2A was elevated in carcinoma
tissues compared with that in para-carcinoma tissues [median, 4.0
vs. 2.0; interquartile range (IQR), 3.0-7.8 vs. 1.0-3.5;
P<0.001; Fig. 1A and B]. In
addition, KIF2A was intracellularly located within the PTC cells
(both in the cytoplasm and nuclei). Furthermore, positive KIF2A
protein expression (defined as IHC score >3) was increased in
carcinoma tissues compared with para-carcinoma tissues (56.5 vs.
25.5%; P<0.001; Fig. 1C). KIF2A
mRNA expression levels were also increased in carcinoma tissues
compared with para-carcinoma tissues (median, 2.630 vs. 1.000; IQR,
2.220-3.930 vs. 0.770-1.490; P<0.001; Fig. 1D). The results of the ROC curve
analyses demonstrated that the KIF2A IHC score [area under ROC
curve (AUC), 0.747; 95% confidence interval (CI), 0.700-0.794] and
KIF2A mRNA expression (AUC, 0.906; 95% CI, 0.862-0.951) may be used
to differentiate carcinoma tissues from para-carcinoma tissues
(Fig. 1E and F). In addition, high
KIF2A expression in carcinoma tissues was related to high KIF2A
levels in para-carcinoma tissues from patients with PTC (P=0.007;
Table SI).
Association between KIF2A and clinical
features of patients with PTC
An increased KIF2A IHC score in carcinoma tissues
was associated with tumor size >4 cm (P=0.001) and advanced
pathological tumor (pT) stage (P=0.001), while there was no
significant association between the KIF2A IHC score in carcinoma
tissues and extrathyroidal invasion (P=0.077), pathological node
(pN) stage (P=0.053) or pTNM stage (P=0.173; Fig. 2A-E).
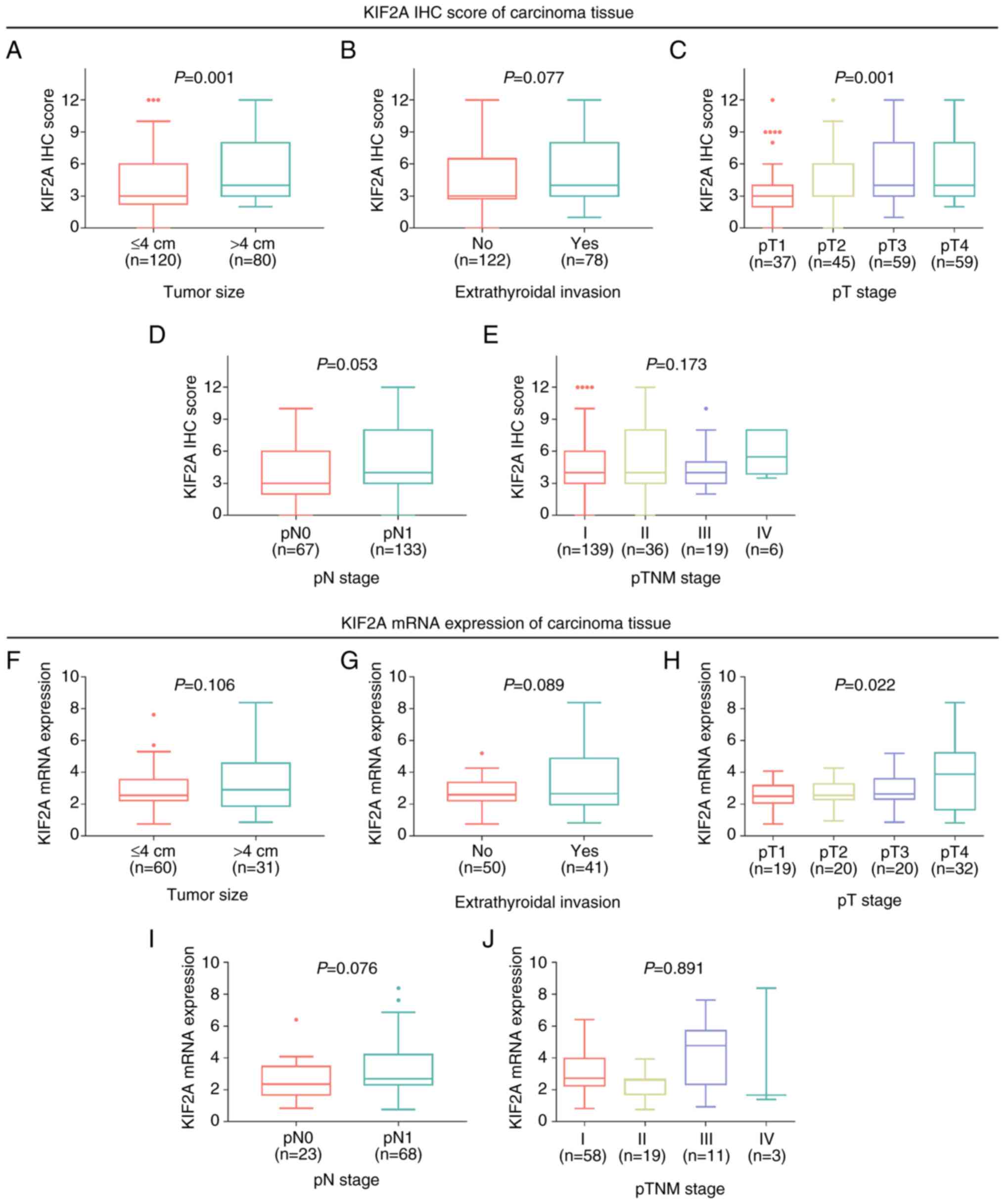 | Figure 2.Elevated KIF2A expression is
associated with larger tumor size and pT stage. Association of
KIF2A IHC score with (A) tumor size, (B) extrathyroidal invasion,
(C) pT stage, (D) pN stage and (E) pTNM stage in patients with PTC.
Association of KIF2A mRNA expression with (F) tumor size, (G)
extrathyroidal invasion, (H) pT stage, (I) pN stage and (J) pTNM
stage in patients with PTC. pT, pathological tumor; KIF2A, kinesin
family member 2A; IHC, immunohistochemistry; pN, pathological
nodal; pTNM, pathologic tumor-nodes-metastasis; PTC, papillary
thyroid carcinoma. |
Furthermore, KIF2A mRNA expression was only
associated with a higher pT stage (P=0.022), while no association
was observed between KIF2A mRNA expression and tumor size
(P=0.106), extrathyroidal invasion (P=0.089), pN stage (P=0.076) or
pTNM stage (P=0.891; Fig.
2F-J).
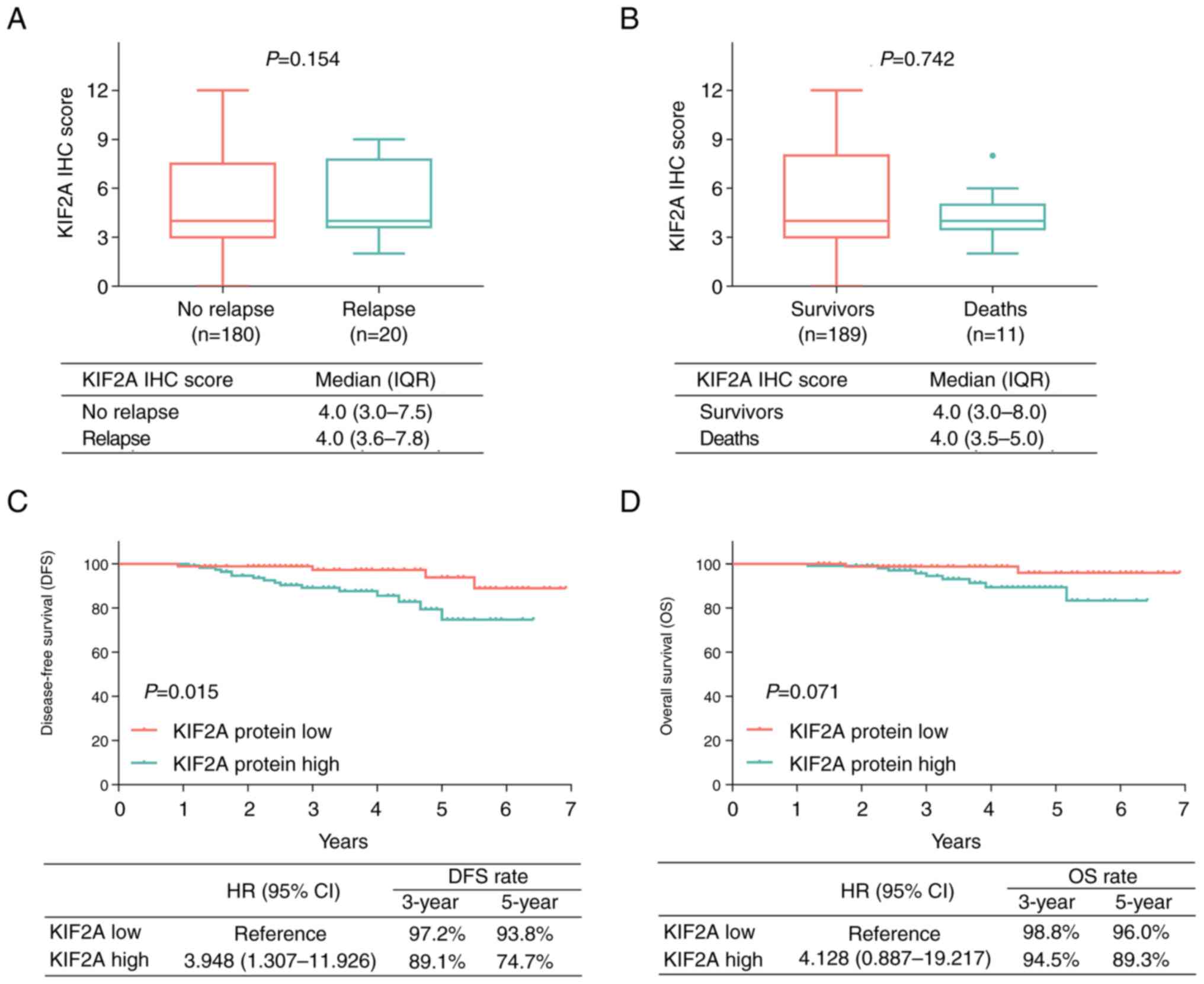
Association of KIF2A with relapse and
survival
At the last follow-up date, 20 (10.0%) patients had
relapsed and 11 (5.5%) patients had died. There was no significant
association between KIF2A IHC score and relapse (P=0.154; Fig. 3A) or between KIF2A IHC score and
death (P=0.742; Fig. 3B). Further
Kaplan-Meier curve and log-rank test analyses demonstrated that
high levels of KIF2A protein expression were associated with
shorter DFS (P=0.015; Fig. 3C),
but not significantly associated with OS (P=0.071; Fig. 3D). Due to the small sample size,
KIF2A mRNA levels were not significantly associated with DFS or OS
(Fig. S2A and B).
Subgroup analyses indicated that DFS was longer in
patients with PTC with low KIF2A in their carcinoma tissues and low
KIF2A in their para-carcinoma tissues compared to that in patients
with high KIF2A in their carcinoma tissues and low KIF2A in their
para-carcinoma tissues (P=0.033), as well as that in patients with
high KIF2A expression in both their and carcinoma and
para-carcinoma tissues (P=0.025; Fig.
S3A). Furthermore, OS was prolonged in patients with PTC with
low KIF2A in their carcinoma tissues and low KIF2A in their
para-carcinoma tissues compared to that in patients with low KIF2A
expression in their carcinoma tissues and high KIF2A in their
para-carcinoma tissues (P=0.011; Fig.
S3B).
Adjustment using multivariate Cox
regression analysis
After applying multivariate Cox regression for
adjustment, KIF2A protein expression [high vs. low; hazard ratio
(HR), 5.842; 95% CI, 1.178-28.983; P=0.031] was independently
associated with shorter DFS, but no significant influence of KIF2A
protein expression on OS was obtained (HR, 7.602; 95% CI,
0.732-78.939; P=0.089; Table II).
Furthermore, a higher pTNM stage was identified as an independent
factor for determining reduced DFS (HR, 4.403; 95% CI,
1.125-17.224; P=0.033).
 | Table II.Multivariate Cox proportional hazards
regression analysis for DFS and OS. |
Table II.
Multivariate Cox proportional hazards
regression analysis for DFS and OS.
|
| DFS | OS |
|---|
|
|
|
|
|---|
| Item | P-value | HR (95% CI) | P-value | HR (95% CI) |
|---|
| KIF2A protein
expression (high vs. low) | 0.031 | 5.842
(1.178-28.983) | 0.089 | 7.602
(0.732-78.939) |
| Age (≥55 vs. <55
years) | 0.528 | 0.397
(0.023-6.986) | 0.894 | 1.290
(0.031-54.402) |
| Sex (male vs.
female) | 0.625 | 1.405
(0.359-5.501) | 0.954 | 1.053
(0.185-6.004) |
| Higher pTNM
stage | 0.033 | 4.403
(1.125-17.224) | 0.211 | 3.020
(0.534-17.089) |
| Radioiodine therapy
(yes vs. no) | 0.617 | 1.615
(0.246-10.582) | 0.673 | 1.757
(0.128-24.122) |
Discussion
In the clinic, increased expression of KIF2A has
been reported in various types of solid tumor, such as gastric
cancer, colorectal cancer and laryngeal squamous cell carcinoma
(15–17). However, the expression of KIF2A in
patients with PTC had remained to be determined. The results of the
present study demonstrated that KI2FA expression (both mRNA and
protein) was increased in carcinoma tissues, compared with that in
para-carcinoma tissues of patients with PTC, which may be used to
distinguish between carcinoma and para-carcinoma tissues. This may
be due to KIF2A being able to regulate multiple oncogene-related
pathways, such as the PI3K/AKT pathway and the RhoA/rho-associated
coiled-coil containing protein kinase pathway, to initiate the
pathogenesis of PTC (13,15,23,24).
Thus, the results of the present study demonstrated that KIF2A was
increased in carcinoma tissues compared with that in para-carcinoma
tissues in patients with PTC.
In addition to its aberrant expression, the
association between KIF2A and the clinicopathological features of
patients with cancer is also of great interest. For instance,
upregulation of KIF2A has been associated with distal metastasis of
patients with gastric or ovarian cancer (15,25).
Furthermore, results of previous studies demonstrated that KIF2A
expression was positively associated with pTNM stage in patients
with either colorectal or gastric cancer (16,26).
The results of the present study demonstrated that increased KIF2A
protein expression was associated with tumor size >4 cm and
advanced pT stage, whereas increased KIF2A mRNA expression was only
associated with larger tumor size in patients with PTC. This may be
because an increase in the expression of KIF2A promoted cell
proliferation via mediating the cell cycle while inhibiting cell
apoptosis, thus leading to tumor growth and increased tumor size in
patients with PTC (10–12). Conversely, the sample size was
relatively small, which may have impacted the power of the
statistical analysis. Of note, there was a negative association
between KIF2A and extrathyroidal invasion in patients with PTC, but
this result was not statistically significant. However, increased
KIF2A expression was associated with increased tumor size and
extrathyroidal invasion to a certain extent, thus demonstrating an
association with pT stage in patients with PTC.
The results of previous studies indicated that
upregulated expression of KIF2A was associated with a poor survival
prognosis in patients with cancer (16,17,25).
For instance, upregulated KIF2A expression was associated with a
reduced 5-year OS rate in patients with ovarian cancer or gastric
cancer (16,25). A further study reported that high
expression of KIF2A was independently associated with declined OS
in patients with laryngeal squamous cell carcinoma (17). The results of the present study
demonstrated that high KIF2A protein expression was independently
associated with shorter DFS, but was not associated with OS. This
may be because KIF2A promotes PTC cell invasion and migration,
which further results in local invasion and distal metastasis,
thereby leading to an elevated risk of recurrence and shorter DFS
in patients with PTC (23). Of
note, the follow-up period was relatively short in the present
study and the survival profile of patients with PTC was frequently
favorable, as only a small number of deaths were recorded in
patients with PTC. This may have impacted the statistical
analysis.
Several further limitations exist in the present
study. For instance, it was a single-center study, which may lead
to regional selection bias, and further multi-center studies are
required to validate the results of the present study. Furthermore,
patient selection bias existed in the current study (i.e., only
those patients with available clinical and follow-up data were
enrolled), which may not be possible to avoid due to the
retrospective nature of the study. In addition, further in
vitro and in vivo studies are required to explore the
effects of KIF2A on PTC cell behaviors and the underlying
mechanisms. Furthermore, the present study only recruited patients
with PTC, while the prognostic value of KIF2A in patients with
other types of thyroid cancer, such as follicular thyroid cancer,
was not determined.
In conclusion, aberrant KIF2A expression may signify
tumor size and invasion and may help to predict an unfavorable
prognosis in patients with PTC.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XYZ and MW contributed to the study design. XYZ, MW,
GLP, WHL, ZG and HL reviewed the medical documents and collected
the clinical characteristics of the patients. XYZ, MW and MJ
contributed to the data acquisition and analysis. XYZ, MW, GLP and
WHL wrote the manuscript. XYZ and MW confirmed the authenticity of
all the raw data. XYZ and MJ revised the manuscript for important
intellectual content. All authors read and approved the final
version of the manuscript. MJ agrees to be accountable for all
aspects of the work in ensuring that questions associated with the
accuracy or integrity of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
The study was approved by The Ethics Committee of
The Central Hospital of Wuhan, Tongji Medical College, Huazhong
University of Science and Technology (Wuhan, China). All patients
or their families provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
PTC
|
papillary thyroid carcinoma
|
|
KIF2A
|
kinesin family member 2A
|
|
FFPE
|
formalin-fixed paraffin-embedded
|
|
pTNM
|
pathologic tumor-nodes-metastasis
|
|
DFS
|
disease-free survival
|
|
OS
|
overall survival
|
|
IHC
|
immunohistochemistry
|
|
RT-qPCR
|
reverse transcription-quantitative
PCR
|
|
ROC
|
receiver operating characteristic
|
|
IQR
|
interquartile range
|
|
HR
|
hazard ratio
|
References
|
1
|
Cabanillas ME, McFadden DG and Durante C:
Thyroid cancer. Lancet. 388:2783–2795. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schlumberger M and Leboulleux S: Current
practice in patients with differentiated thyroid cancer. Nat Rev
Endocrinol. 17:176–188. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lu Y, Jiang L, Chen C, Chen H and Yao Q:
Clinicopathologic characteristics and outcomes of papillary thyroid
carcinoma in younger patients. Medicine (Baltimore). 99:e197952020.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Arianpoor A, Asadi M, Amini E, Ziaeemehr
A, Ahmadi Simab S and Zakavi SR: Investigating the prevalence of
risk factors of papillary thyroid carcinoma recurrence and
disease-free survival after thyroidectomy and central neck
dissection in Iranian patients. Acta Chir Belg. 120:173–178. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Coca-Pelaz A, Shah JP, Hernandez-Prera JC,
Ghossein RA, Rodrigo JP, Hartl DM, Olsen KD, Shaha AR, Zafereo M,
Suarez C, et al: Papillary thyroid cancer-aggressive variants and
impact on management: A narrative review. Adv Ther. 37:3112–3128.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Abdullah MI, Junit SM, Ng KL, Jayapalan
JJ, Karikalan B and Hashim OH: Papillary thyroid cancer: Genetic
alterations and molecular biomarker investigations. Int J Med Sci.
16:450–460. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mastronikolis N, Tsiambas E, Roukas D,
Fotiades P, Chrysovergis A, Papanikolaou V, Kyrodimos E,
Mastronikoli S, Niotis A and Ragos V: Micro-RNAs signatures in
papillary thyroid carcinoma. J BUON. 25:2144–2146. 2020.PubMed/NCBI
|
|
8
|
Ruiz EML, Niu T, Zerfaoui M,
Kunnimalaiyaan M, Friedlander PL, Abdel-Mageed AB and Kandil E: A
novel gene panel for prediction of lymph-node metastasis and
recurrence in patients with thyroid cancer. Surgery. 167:73–79.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Drummond DR: Regulation of microtubule
dynamics by kinesins. Semin Cell Dev Biol. 22:927–934. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yi ZY, Ma XS, Liang QX, Zhang T, Xu ZY,
Meng TG, Ouyang YC, Hou Y, Schatten H, Sun QY and Quan S: Kif2a
regulates spindle organization and cell cycle progression in
meiotic oocytes. Sci Rep. 6:385742016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ali A, Veeranki SN, Chinchole A and Tyagi
S: MLL/WDR5 complex regulates Kif2A localization to ensure
chromosome congression and proper spindle assembly during mitosis.
Dev Cell. 41:605–622.e7. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bufe A, Garcia Del Arco A, Hennecke M, de
Jaime-Soguero A, Ostermaier M, Lin YC, Ciprianidis A, Hattemer J,
Engel U, Beli P, et al: Wnt signaling recruits KIF2A to the spindle
to ensure chromosome congression and alignment during mitosis. Proc
Natl Acad Sci USA. 118:e21081451182021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang K, Lin C, Wang C, Shao Q, Gao W, Song
B, Wang L, Song X, Qu X and Wei F: Silencing Kif2a induces
apoptosis in squamous cell carcinoma of the oral tongue through
inhibition of the PI3K/Akt signaling pathway. Mol Med Rep.
9:273–278. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Q, Lu D, Liu W, Ye S, Guo H, Liao T
and Chen C: Effects of KIF2A on the prognosis of nasopharyngeal
carcinoma and nasopharyngeal carcinoma cells. Oncol Lett.
18:2718–2723. 2019.PubMed/NCBI
|
|
15
|
Zhang X, Wang Y, Liu X, Zhao A, Yang Z,
Kong F, Sun L, Yu Y and Jiang L: KIF2A promotes the progression via
AKT signaling pathway and is upregulated by transcription factor
ETV4 in human gastric cancer. Biomed Pharmacother. 125:1098402020.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fan X, Wang X, Zhu H, Wang W, Zhang S and
Wang Z: KIF2A overexpression and its association with
clinicopathologic characteristics and unfavorable prognosis in
colorectal cancer. Tumour Biol. 36:8895–8902. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang Q, Zhang W, Zhang J, Xu H and You Y:
Aberrant Kif2a and Ki67 expression predicts poor survival in
laryngeal squamous cell carcinoma. Auris Nasus Larynx. 43:433–439.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang ZX, Ren SC, Chang ZS and Ren J:
Identification of kinesin family member 2A (KIF2A) as a promising
therapeutic target for osteosarcoma. Biomed Res Int.
2020:71027572020.PubMed/NCBI
|
|
19
|
Fu H, Jin C, Zhu Q, Liu T, Ke B, Li A and
Zhang T: Dysregulated expressions of PTEN, NF-κB, WWP2, p53 and
c-Myc in different subtypes of B cell lymphoma and reactive
follicular hyperplasia. Am J Transl Res. 11:1092–1101.
2019.PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li D, Sun H, Meng L and Li D: The
overexpression of kinesin superfamily protein 2A (KIF2A) was
associated with the proliferation and prognosis of esophageal
squamous cell carcinoma. Cancer Manag Res. 12:3731–3739. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li H, Han D, Hou Y, Chen H and Chen Z:
Statistical inference methods for two crossing survival curves: A
comparison of methods. PLoS One. 10:e01167742015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu L, Zhang X, Wang Z, Zhao X, Zhao L and
Hu Y: Kinesin family member 2A promotes cancer cell viability,
mobility, stemness, and chemoresistance to cisplatin by activating
the PI3K/AKT/VEGF signaling pathway in non-small cell lung cancer.
Am J Transl Res. 13:2060–2076. 2021.PubMed/NCBI
|
|
24
|
Liang X and Xia R: Kinesin family member
2A acts as a potential prognostic marker and treatment target via
interaction with PI3K/AKT and RhoA/ROCK pathways in acute myeloid
leukemia. Oncol Rep. 47:182022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sheng N, Xu YZ, Xi QH, Jiang HY, Wang CY,
Zhang Y and Ye Q: Overexpression of KIF2A is suppressed by miR-206
and associated with poor prognosis in ovarian cancer. Cell Physiol
Biochem. 50:810–822. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang S, Huang F, Wang Y, Song Q, Yang X
and Wu H: KIF2A overexpression and its association with
clinicopathologic characteristics and poor prognoses in patients
with gastric cancer. Dis Markers. 2016:74845162016. View Article : Google Scholar : PubMed/NCBI
|