Introduction
Osteosarcoma is a common, highly malignant bone
tumor which has high metastatic potential, occurring mainly in
children and adolescents (1).
Osteosarcoma mainly occurs in the proximal tibia or distal femur
and is highly malignant and prone to invasion and metastasis
(2,3). In spite of the use of neoadjuvant
chemotherapy (4), the 5-year
survival rate of patients osteosarcoma with metastasis or
recurrence is poor (5). Thus,
investigating the pathogenesis of osteosarcoma may lead to
development of novel therapeutic targets.
MicroRNAs (miRNAs or miRs) are a class of
single-stranded, non-coding small RNA that regulate gene expression
by binding to the 3′ untranslated region (3′-UTR) of target genes
(6,7). miRNAs have been reported to be
associated with biological processes including cell proliferation,
migration and inflammation, as well as apoptosis (8,9). In
addition, miRNAs serve as oncogenes or cancer suppressors, which
serves a key role in tumorigenesis (10–12).
Numerous studies have demonstrated that miRNAs modulate the
progression of osteosarcoma (13,14).
Studies have revealed the effect of miR-487a on cancer. For
example, Yang et al (15)
demonstrated that miR-487a promotes progression of gastric cancer
by targeting TIA1. Ma et al (16)
indicated that miR-487a enhances TGF-β1-induced
epithelial-mesenchymal transition, migration and invasion of breast
cancer cells via directly targeting membrane-associated guanylate
kinase, WW and PDZ domain-containing 2. Several studies have shown
that transcription factors and miRNAs are involved in the
development of cancer (17,18).
However, the precise mechanism of TF-miR-487a in the progression of
osteosarcoma remained to be fully explored.
The present study investigated candidate molecules
that may play important roles in the occurrence and development of
osteosarcoma through bioinformatics screening and experimental
verification, and finally identified that the KLF5/miR-487a/NKX3-1
axis plays an important role in the proliferation, invasion and
metastasis of osteosarcoma, which may offer aid to find new
therapeutic targets for osteosarcoma.
Materials and methods
Bioinformatics analysis
miRNA data and clinical information of osteosarcoma
were obtained from Gene Expression Omnibus (GEO;
ncbi.nlm.nih.gov/geo/) database (accession nos. GSE65071 and
GSE28423; Table SI). DESeq2
package (Version 3.12) (19) was
used to normalize raw osteosarcoma miRNAs and identify
differentially expressed miRNAs (DEMs) between the osteosarcoma and
non-osteosarcoma samples/cells. P<0.01 and logFC >4 were
considered to indicate a statistically significant difference.
Osteosarcoma DEMs were obtained by overlapping DEMs from GSE65071
with DEMs from GSE28423 dataset. Overlapped miRNAs were observed
using vennDiagram (Version: 1.6.20;
cran.r-project.org/src/contrib/Archive/VennDiagram/) package.
Volcano map was constructed using ggplot2 package (Version: 3.3.2;
cran.r-project.org/src/contrib/Archive/ggplot2/) to measure and
analyze DEMs.
Prediction of miR-487a target genes
and promoter binding sites
JASPAR database was used to determine candidate
transcription factors targeting promoters of miR-487a (20). The predicted binding ability was
ranked based on binding score. Online prediction software
miRWalk2.0 (21), TargetScan 6.2
(22), miRanda 1.0 (23) and RNA22 2.0 (24) were used to determine potential
target genes of miR-487a. Predicted target genes were overlapped
with DEGs of GSE12865 and TARGET-OS
(ocg.cancer.gov/programs/target/projects/osteosarcoma) sets
(Table SI). DEGs between
osteosarcoma tissue and non-osteosarcoma controls were identified
as previously described (19). PCR
and western blot assay were used to verify results.
Cell culture and transfection
Osteosarcoma cell lines (HOS and MG-63) and
osteoblasts (hFOB) were purchased from the American Type Culture
Collection. MG-63 and hFOB cells were cultured in DMEM (Gibco;
Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine
serum (FBS; PAN-Biotech GmbH) and 100 U/ml penicillin/streptomycin
in a humid atmosphere with 5% CO2 at 37°C. HOS cells
were maintained in Eagle's Minimum Essential Medium containing 15%
FBS (EMEM: Sigma-Aldrich; Merck KGaA) and 100 U/ml
penicillin/streptomycin with 5% CO2 at 37°C in a humid
atmosphere.
miR-487a mimic, mimic negative control (miR-NC),
miR-487a inhibitor (50 nM), inhibitor scramble control
(inhibitor-NC; all 50 nM), pcDNA (vector) and pcDNA-NKX3-1
overexpression (NKX3-1-OE) and pcDNA-KLF5 overexpression (KLF5-OE)
plasmids were purchased from Shanghai GenePharma Co., Ltd. All
transfections were performed using Lipofectamine® 3000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions for 6 h in 37°C. At 48 h
post-transfection, cells were harvested for evaluation of
transfection efficiency by reverse transcription-quantitative
(RT-q)PCR analysis.
Western blotting
Proteins were extracted from HOS and MG63 cells
using RIPA buffer (Beyotime Institute of Biotechnology; cat. no.
P0013B) and the concentration of extracted protein was assessed by
BCA kit (Thermo Fisher Scientific, Inc.). Protein was separated via
10% SDS-PAGE followed by transfer onto a polyvinylidene difluoride
membrane. Membranes were blocked with 5% skimmed milk for 1 h at
room temperature. The membranes were incubated with primary
antibodies (GAPDH, 1:10,000, cat. no. ab181603; NKX3-1, 1:1,000,
cat. no. ab196020; KLF5, 1:2,000, cat. no. ab137676; Abcam)
overnight at 4°C, followed by incubation with corresponding
secondary antibodies (1:5,000; cat. no. 31430, HRP-conjugated,
Thermo Fisher Scientific, Inc.) at room temperature for 2 h.
Proteins bands were visualized using electrochemiluminescence (ECL
Western Blotting Substrate; cat. no. 32106; Pierce; Thermo Fisher
Scientific, Inc.) and analyzed using the ChemiDoc™ XRS
Molecular Imager 3.0 system (Bio-Rad Laboratories, Inc.).
RT-qPCR
Total RNA was extracted from cells (HOS/MG63) using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.).
Extracted RNA was reverse transcribed to cDNA via cDNA RT kit
(Roche Diagnostics GmbH) according to the manufacturer's
instructions. RNA concentration was detected using Nanodrop
(Invitrogen; Thermo Fisher Scientific, Inc.). RT-qPCR was performed
using SYBR®Premix Ex Taq™ (Takara Bio, Inc.)
with an ABI Prism 7900 Sequence detection system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). Thermocycling
conditions of PCR cycling were as following: Activation of TaqMan
at 95°C for 10 min followed by 40 cycles of denaturation at 95°C
for 10 sec and annealing/extension at 60°C for 60 sec. Primer
sequences were synthesized by TsingKe Biological Technology
(Table I). The relative expression
of target genes was assessed via the 2−ΔΔCq method
(25). U6 and GAPDH were used as
the internal controls. Each sample was tested in triplicate.
 | Table I.Primer sequences for reverse
transcription-quantitative PCR and oligonucleotides for miRNA
mimic, inhibitor and negative control. |
Table I.
Primer sequences for reverse
transcription-quantitative PCR and oligonucleotides for miRNA
mimic, inhibitor and negative control.
| Primer | Sequence,
5′→3′ |
|---|
| miR-487a | Forward:
GCGGCGGAATCATACAGGGACATC |
|
| Reverse:
ATCCAGTGCAGGGTCCGAGG |
| U6 | Forward:
AACGCTTCACGAATTTGCGT |
|
| Reverse:
CTCGCTTCGGCAGCACA |
| GAPDH | Forward:
CGGATTTGGTCGTATTGGG |
|
| Reverse:
TCTCGCTCCTGGAAGATGG |
| miR-219-5p | Forward:
TGATTGTCCAAACGCAATTCT |
|
| Reverse:
ATCCAGTGCAGGGTCCGAGG |
| miR-331-5p | Forward:
CTAGGTATGGTCCCAGGGATCC |
|
| Reverse:
ATCCAGTGCAGGGTCCGAGG |
| NKX3-1 | Forward:
GCCAAGAACCTCAAGCTCAC |
|
| Reverse:
AGAAGGCCTCCTCTTTCAGG |
| CDK6 | Forward:
TGCCCACTGAAACCATAAA |
|
| Reverse:
TACCACAGCGTGACGACCA |
| AJUBA | Forward:
AGGCCAGGGAGGACTACTTCG |
|
| Reverse:
GCCTCCTGAAACCCTGAAA |
| DAPK1 | Forward:
AATCCTAGACGTGGTCCGGTAT |
|
| Reverse:
GTCCTCGGTGCGTATCCTTTCG |
| WNT5A | Forward:
GCGAAGACAGGCATCAAAG |
|
| Reverse:
GCAAAGCGGTAGCCATAGTC |
| KLF5 | Forward:
ACACCAGACCGCAGCTCCA |
|
| Reverse:
TCCATTGCTGCTGTCTGATTTGTAG |
| KLF15 | Forward:
CAGCGGCAGTAGCATTGGG |
|
| Reverse:
ACCTCCTGCACTGGCACCAC |
| SP1 | Forward:
TGGTGGGCAGTATGTTGT |
|
| Reverse:
GCTATTGGCATTGGTGAA |
| SP4 | Forward:
ATGGCTACAGAAGGAGGGAAAAC |
|
| Reverse:
TTGACCAGGGGTGGAAGAATTAC |
| miR-487a mimic | Forward:
GUGGUUAUCCCUGCUGUGUUCG |
|
| Reverse:
CGAACACAGCAGGGAUAACCAC |
| miR-NC | Forward:
UUUGUACUACACAAAAGUACUG |
|
| Reverse:
CAGUACUUUUGUGUAGUACAAA |
|
miR-487a-inhibitor |
CGAACACAGCAGGGAUAACCAC |
| Inhibitor-NC |
CAGUACUUUUGUGUAGUACAAA |
Luciferase reporter assay
StarBase 2.0 was used to predict the latent
targeting association between miR-487a and NKX3-1, which was
verified by luciferase reporter assay. HOS and MG-63 cells were
transfected with pMIR-REPORT luciferase vector with wild-type (WT)
or mutant (MUT)-NKX3-1-3′UTR (Promega) (1.6 ug per 12-well plate),
miR-487a mimic, miR-487a inhibitor (miR-487a-KD) or
miR-NC/inhibitor-NC using Lipofectamine 3000 (Invitrogen; Thermo
Fisher Scientific, Inc.) as aforementioned. At 48 h
post-transfection, luciferase activity was detected using
Dual-Luciferase Reporter Assay System (Promega Corporation)
according to the manufacturer's instructions. Renilla luciferase
activity was used for normalization. Each assay was performed in
triplicate.
Transwell assay
Cell invasion and migration were assessed using 6.5
mm Transwell® migration assay, with 8.0 µm Pore
Polycarbonate Membrane Insert, Sterile (Corning, Inc.; cat. no.
3422). For cell invasion, serum-free medium was mixed with the BD
Matrigel™ hESC-qualified Matrix (BD Biosciences; cat.
no. 354277) in a 1:10 ratio. This mixture (50 µl) was added to the
bottom of the insert. The Matrigel was then incubated at 37°C for 4
h to solidify. Then, 5×104 cells were transfected and at
24 h following transfection, cells were harvested by
trypsinization, washed with serum-free medium (HOS: EMEM; MG63:
DMEM) and placed in the upper chamber of the Transwell. The lower
chamber contained 500 µl medium supplemented with 10% FBS that was
used as chemo-attractant. After incubation at 37°C with 5%
CO2 for 48 h, the cells in the inner side of the chamber
were removed using cotton swabs. Invaded cells on the lower
membrane surface were fixed with methanol for 15 min at room
temperature and stained with 0.1% crystal violet for 10 min at room
temperature. Images of the invaded cells were captured using a
light microscope (Olympus IX71; ×200 magnification) and cells were
counted. Cell migration assay was performed in a similar way except
that 1×105 cells were added into the insert without
Matrigel pre-coating. Each experiment was conducted in triplicate
and repeated three times.
Cell viability assay
Viability of HOS and MG-63 cells was evaluated by
MTT assay according to the manufacturer's instructions. In brief,
cells were seeded into 96-well plates at a density of
2×103 cells/well. Then, cells were incubated in a humid
atmosphere with 5% CO2 at 37°C. MTT assay was performed
after days 1–5. A total of 20 µl MTT (5 mg/ml) was added into each
well followed by incubation for 4 h at 37°C. Then, 100 µl dimethyl
sulfoxide was added to dissolve the formazan crystals. Absorbance
at 490 nm was detected with a microplate reader.
Colony formation assay
Transfected HOS and MG63 cells (3×102
cells/well) were digested with 0.25% trypsin to form a cell
suspension, inoculated into 6-well plates and incubation at 37°C in
5% CO2 for 14 days. The cells were fixed with 70% ethanol at room
temperature (20–25°C) for 15 min and stained with 0.05% crystal
violet at 37°C for 20 min. The number of colonies formed was
counted (≥50 cells) manually using an Olympus BX40 light microscope
(Olympus Corporation).
Chromatin immunoprecipitation (ChIP)
assay
According to the manufacturer's protocol, total
genomic DNA was isolated using ChIP Assay kit (Beyotime Institute
of Biotechnology; cat. no. P2078). A total of 2×106
HOS/MG63 cells were transfected with pCDNA3.1 vectors and lysed
with 250 µl SDS Lysis Buffer (Beyotime Institute of Biotechnology).
Next, the HOS/MG63 cells were sonicated on ice and fragments of DNA
were then resolved using a 2% agarose gel. For ChIP, samples were
diluted in a 10X ChIP dilution buffer and pre-cleared with 60 µl
protein G-agarose beads mixed at 4°C for 1 h. During chromatin
separation, the chromatin was centrifuged at 15,000 × g at 4°C for
10 min to remove insoluble matter and then pre-cleared chromatin
was incubated with 1 µl antibodies against KLF5 (dilution, 1:100;
cat. no. ab137676; Abcam) at 4°C overnight. The precipitates were
washed with low-salt wash buffer, high-salt wash buffer and LiCI
wash buffer, and rinsed with TE buffer twice. Immunochromatin was
centrifuged at 15,000 × g for 10 min at 4°C, boiled, and then was
amplified via PCR as aforementioned.
Statistical analysis
The data are presented as the mean ± SD and were
assessed via GraphPad Prism 7 (GraphPad Software, Inc.).
Comparisons between two groups were performed by unpaired t test.
Comparison between >2 groups were performed by one-way ANOVA
followed by Tukey's post hoc test. All experiments were performed
three times. P<0.05 was considered to indicate a statistically
significant difference.
Results
miR-487a expression is increased in
osteosarcoma
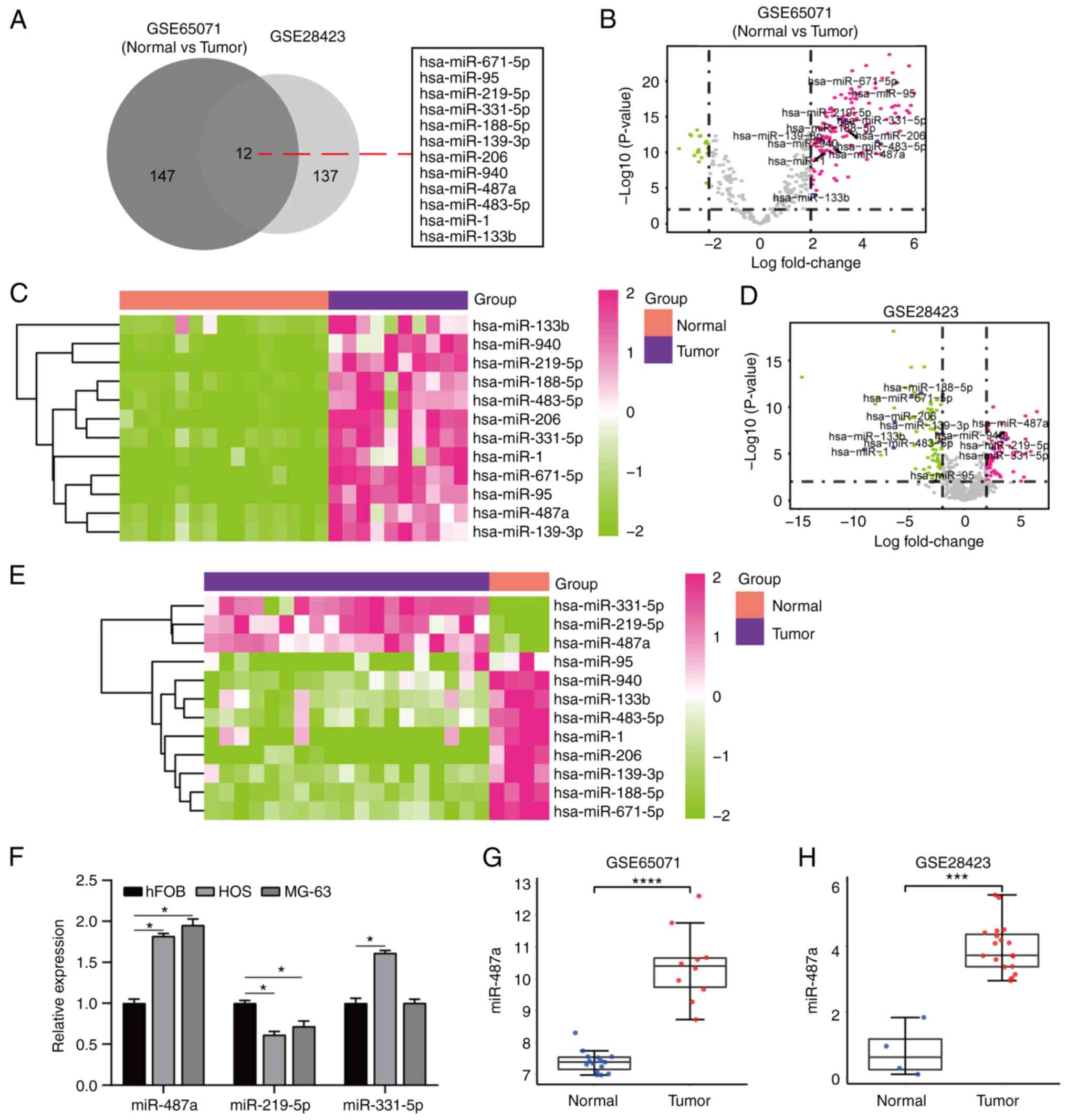
Osteosarcoma DEMs were identified via overlapped
analysis of osteosarcoma miRNAs in GSE65071 and GSE28423 datasets
from GEO database (Table SI). A
total of 12 DEMs was identified between osteosarcoma and
non-osteosarcoma tissue (Fig. 1A)
and volcano plot and heatmaps were constructed (Fig. 1B-E). RT-qPCR assay demonstrated
that miR-487a was highly expressed in osteosarcoma (HOS and MG-63)
compared with hFOB cells (Fig.
1F). miR-487a expression was significantly higher in tumor than
in normal samples in GSE65071 and GSE28423 datasets (Fig. 1G and H). These results indicate
that miR-487a was upregulated in osteosarcoma cells and tissue.
miR-143-5p promotes tumorigenesis of
osteosarcoma cells
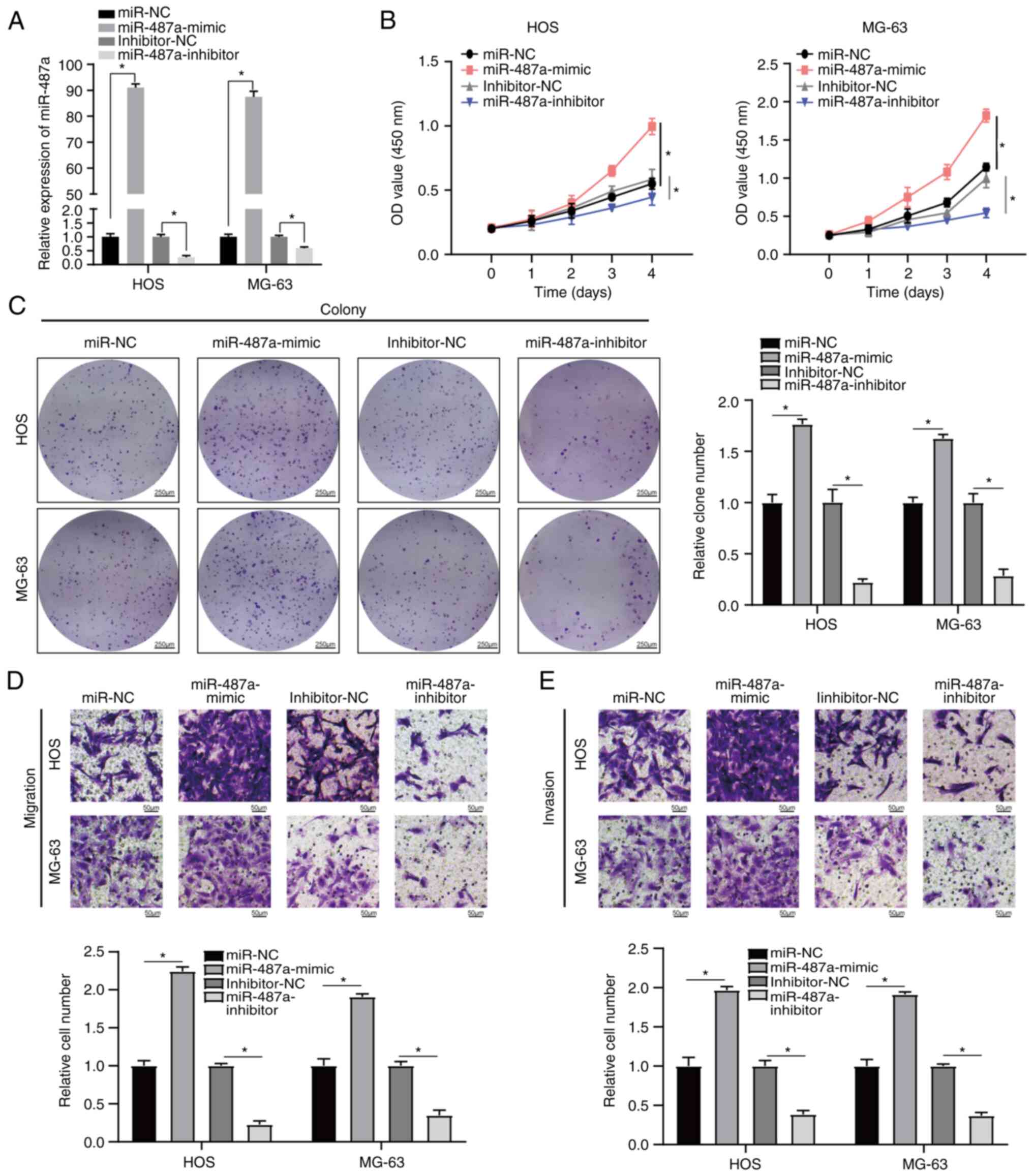
Osteosarcoma cells were transfected with miR-487a
mimic or inhibitor. RT-qPCR indicated that miR-487a was
significantly upregulated in osteosarcoma cells following
transfection with miR-487a mimic compared with miR-NC and
significantly downregulated following transfection with miR-487a
inhibitor compared with inhibitor-NC (Fig. 2A). Transwell assay was used to
assess the migratory and invasive capacity of osteosarcoma cells.
MTT assay was used to evaluate the proliferation ability of
osteosarcoma cells. Overexpression of miR-487a significantly
increased proliferation, invasion and migration of osteosarcoma
cells. However, inhibition of miR-487a significantly decreased the
proliferation, invasion and migration of osteosarcoma cells
(Fig. 2B-E). Taken together, these
data indicated that miR-487a promoted osteosarcoma progression via
increased cell proliferation, invasion and migration.
NKX3-1 is a direct target of miR-487a
in osteosarcoma cells and restoration of NKX3-1 rescues the effect
of miR-487a
To analyze the effect of miR-487a on osteosarcoma
cells, osteosarcoma DEGs between GSE12865 and TCGA database were
overlapped (Table SI) and target
genes were predicted using miRWalk database. A total of 13 common
target genes were identified (Fig.
3A) and volcano plot and heatmaps were constructed for GSE12865
and TCGA database (Fig. 3B-E).
Notably, five target genes (NKX3-1, CDK6, AJUBA, DAPK1, WNT5A) were
notably downregulated in both datasets (Fig. 3B-E). Of these five target genes,
RT-qPCR demonstrated that NKX3-1 exhibited the lowest expression in
osteosarcoma cells and was significantly decreased compared with
its expression in hFOB cells (Fig.
3F). Additionally, the effect of miR-487a on NKX3-1 expression
was assessed by RT-qPCR; expression of NKX3-1 was significantly
decreased by transfection with miR-487a mimic in HOS and MG-63
cells (Fig. 3G). The effect of
miR-487a on NKX3-1 was assessed via western blot analysis. miR-487a
overexpression notably decreased expression levels of NKX3-1 in HOS
and MG-63 cells (Fig. 3H). Online
bioinformatics tool Starbase was used to search potential binding
sites of miR-487a; NKX3-1 was predicted as a potential binding
target of miR-487a (Fig. 3I). In
addition, dual luciferase reporter assay indicated that luciferase
activity was significantly decreased in osteosarcoma cells
co-transfected with NKX3-1-WT-3′-UTR plasmid and miR-487a-mimic and
significantly increased by co-transfection with miR-487a inhibitor
(Fig. 3J), implying a direct
regulatory association between miR-487a and 3′-UTR of NKX3-1 mRNA.
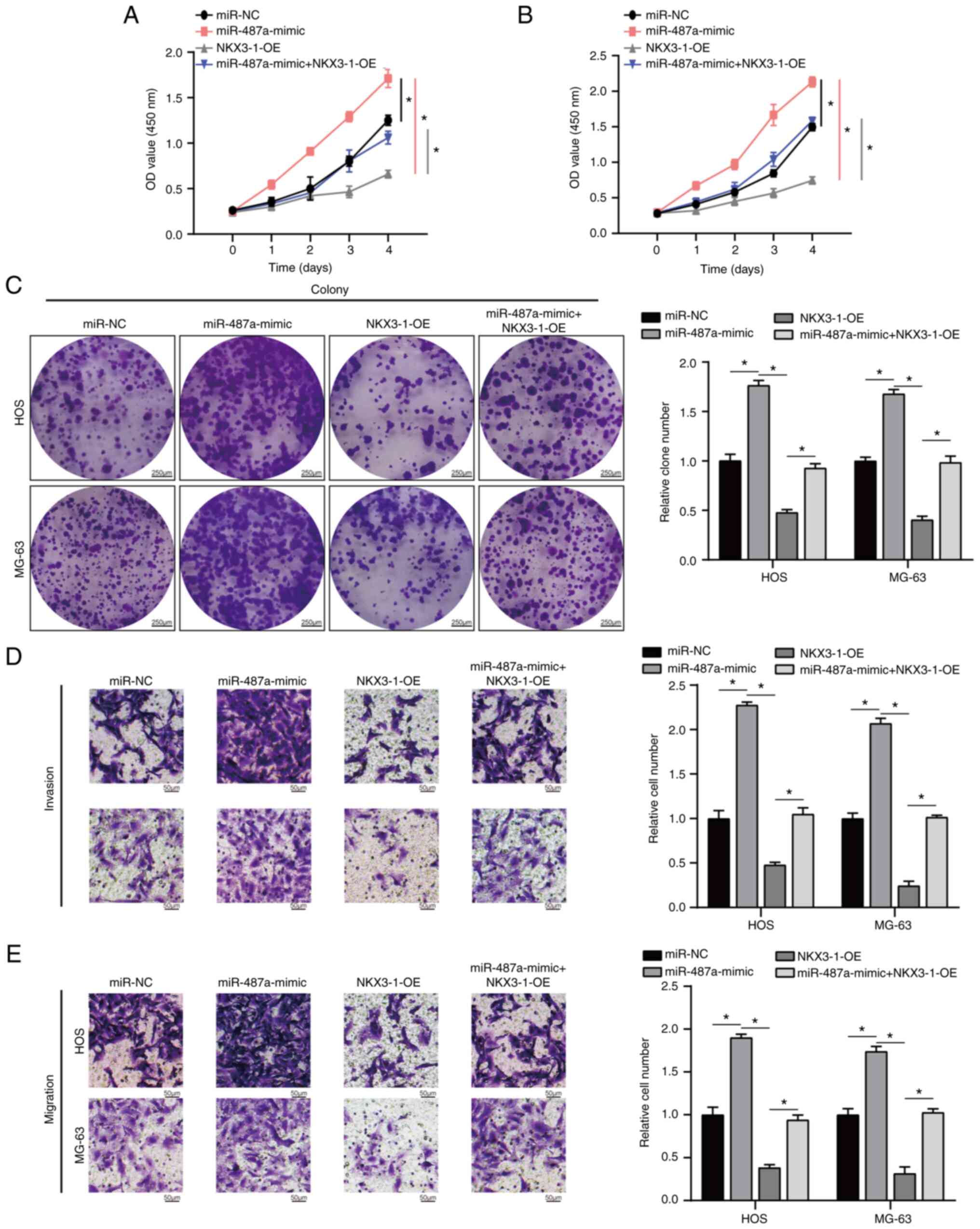
In addition, miR-487a overexpression significantly increased
proliferation, invasion and migration of HOS and MG-63 cells
(Fig. 4A-E); these effects were
significantly rescued by NKX3-1-OE. These data illustrated that
overexpression of miR-487a exerted its role in HOS and MG63 cells
by inhibiting NKX3-1.
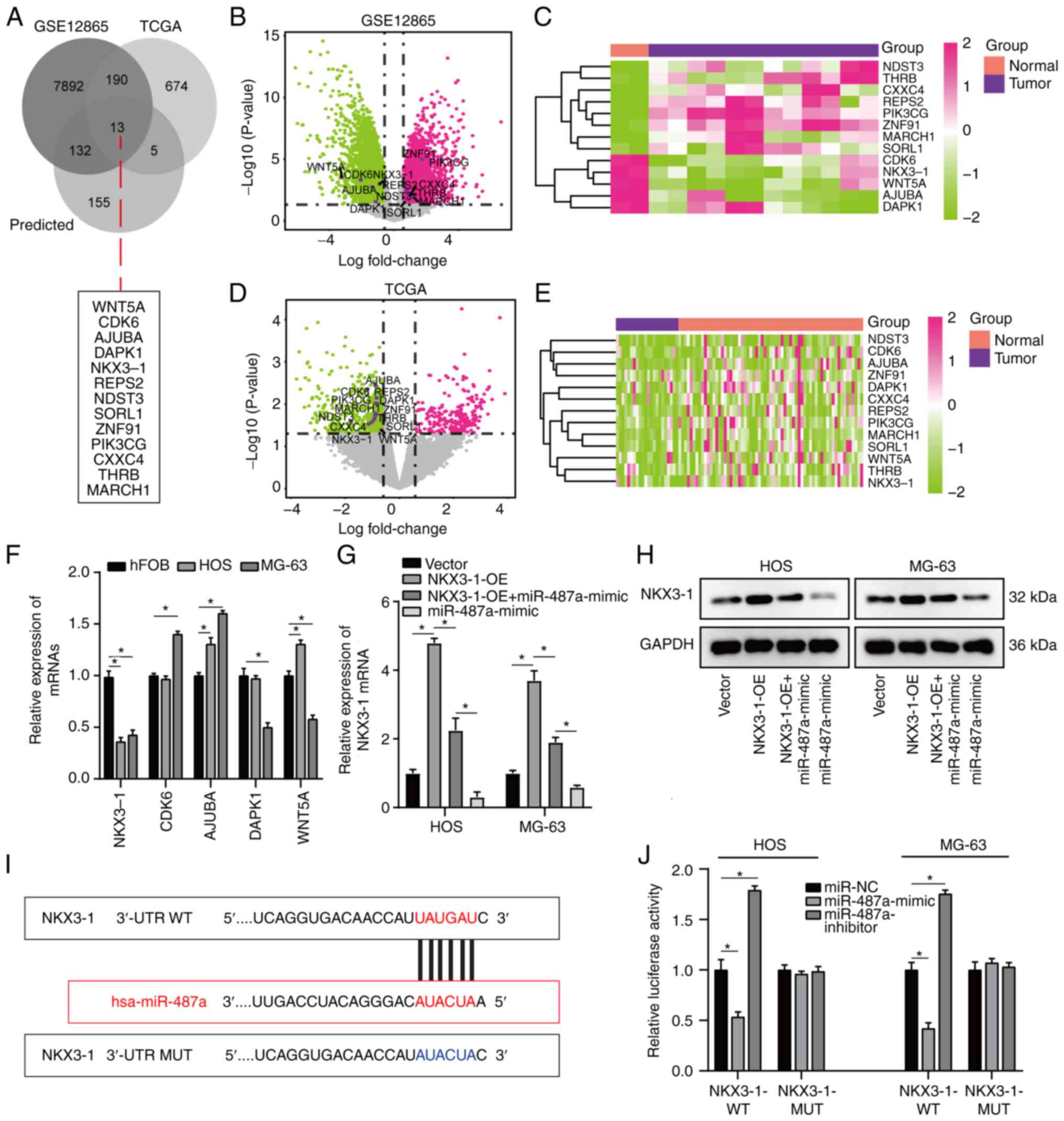 | Figure 3.NKX3-1 is a direct target of miR-487a
in osteosarcoma cells. (A) A total of 13 common target genes was
identified by overlapping osteosarcoma DEGs between GSE12865 and
TCGA and target genes predicted by miRWalk database. (B) Volcano
plot and (C) heatmap of 13 DEGs in GSE12865 dataset. (D) Volcano
plot and (E) heatmap of 13 DEGs in TCGA dataset. (F) RT-qPCR
analysis of NKX3-1, CDK6, AJUBA, DAPK1 and WNT5A in HOS, MG-63 and
hFOB cells. (G) Effect of miR-487a and NKX3-1-OE on NKX3-1
expression was assessed by RT-qPCR assay. (H) Western blot analysis
of NKX3-1 following transfection with miR-487a mimic or inhibitor.
(I) Binding site of miR-487a to NKX3-1 3′-UTR. (J) pMIR-REPORT
luciferase vector containing WT or MUT NKX3-1 3′-UTR was
co-transfected in HOS and MG-63 cells with miR-487a mimic,
inhibitor or NC. Firefly luciferase activity was compared with
Renilla luciferase activity. *P<0.05. NKX3-1, NK3 homeobox 1;
miR, microRNA; DEG, differentially expressed gene; TCGA, The Cancer
Genome Atlas; RT-q, reverse transcription-quantitative; AJUBA,
ajuba LIM protein; DAPK1, death-associated protein kinase 1; WT,
wild-type; MUT, mutant; UTR, untranslated region; NC, negative
control; OE, overexpression. |
KLF5 directly regulates miR-487a
expression in osteosarcoma cells
To determine the upstream regulators of miR-487a,
JASPAR database was used to determine the candidate transcription
factors targeting promoters of miR-487a. RT-qPCR was used to
measure the level of the top four transcription factors (KLF5,
KLF15, SP1, SP4) that may directly regulate miR-487a expression.
KLF5 was most highly expressed and was significantly upregulated in
HOS and MG-63 compared with hFOB cells (Fig. 5A). Western blot analysis was used
to assess KLF5 and NKX3-1 expression following transfection with
KLF5-OE in HOS and MG-63 cells. NKX3-1 expression levels were
significantly decreased following transfection with KLF5-OE in HOS
and MG-63 cells compared with Vector (Fig. 5B). As demonstrated by RT-qPCR
analysis, KLF5-OE significantly upregulated expression of miR-487a
in HOS and MG-63 cells (Fig. 5C).
KLF5-OE significantly promoted proliferation, invasion and
migration of osteosarcoma cells; this effect was notably reversed
by inhibition of miR-487a (Fig.
5D-F).
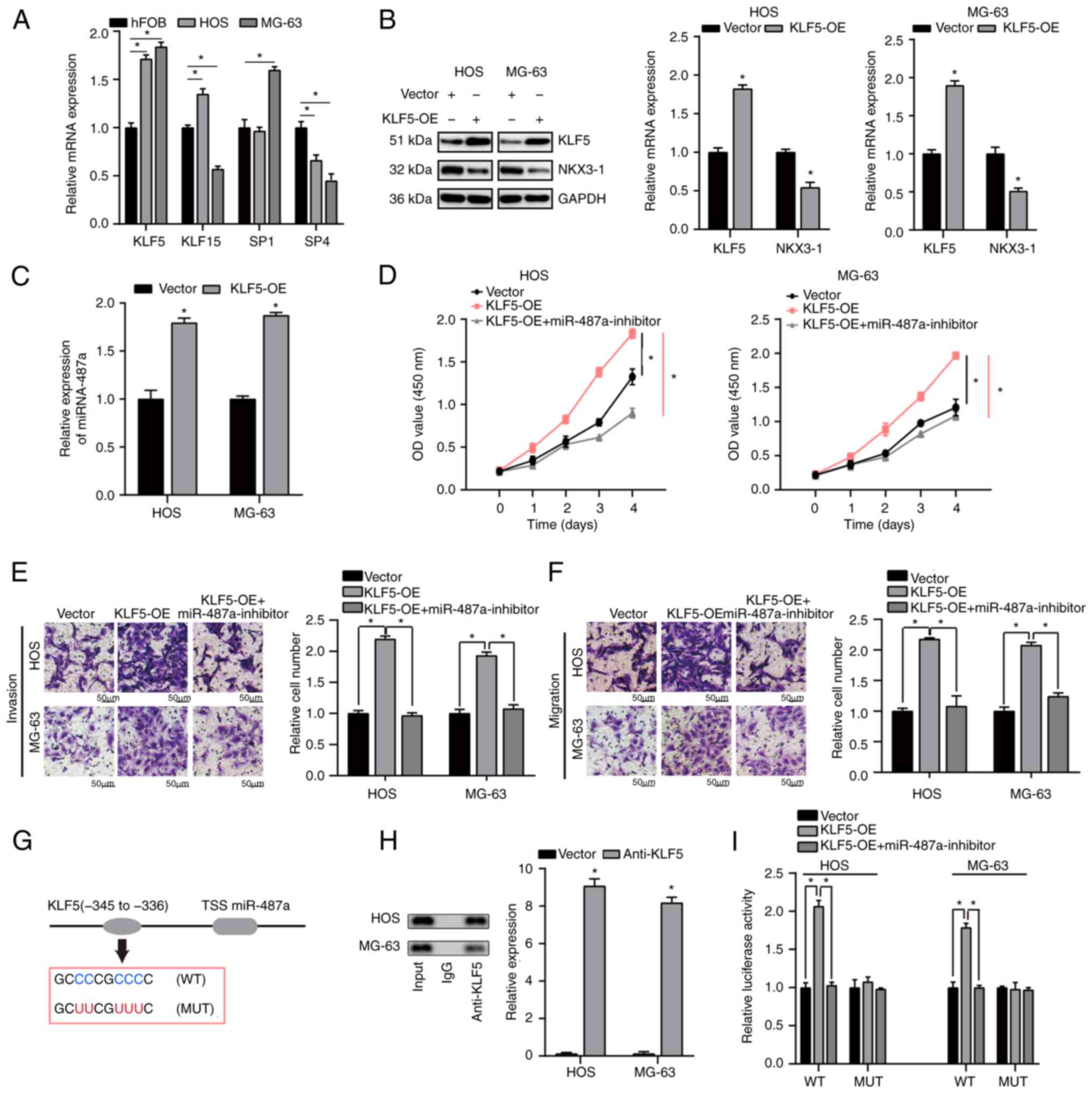 | Figure 5.KLF5 directly regulates miR-487a
expression in osteosarcoma cells. (A) RT-qPCR analysis of KLF5,
KLF15, SP1 and SP4 expression. Effect of KLF5-OE on (B) NKX3-1 and
(C) miR-487a in HOS and MG-63 cells was measured by western blot
and RT-qPCR assay. (D) MTT assay showed KLF5-OE increased
proliferative capacity of osteosarcoma cells; the effect was
reversed by inhibited miR-487a expression. Transwell assay
demonstrated that KLF5-OE significantly increased (E) invasion and
(F) migration of HOS and MG-63 cells; this was rescued by miR-487a
inhibition. (G) Proximal region of the miR-487a promoter. (H)
Chromatin immunoprecipitation assay was performed to verify the
binding site between KLF5 and miR-487a promoter in HOS and MG-63
cells. (I) Luciferase activity was increased following
co-transfection with WT miR-487a promoter and KLF5-OE; this was
significantly rescued by miR-487a inhibitor. No significant
difference in luciferase activity was observed when KLF5 target
sites at 336–345 bp were mutated. *P<0.05 KLF5, Kruppel-like
factor 5; miR, microRNA; RT-q, reverse transcription-quantitative;
SP, Sp transcription factor; OE, overexpression; NKX3-1, NK3
homeobox 1; WT, wild-type; MUT, mutant; TSS, transcription start
site; OD, optical density. |
ChIP assay was used to determine whether KLF5
transcriptionally regulated miR-487a expression. Binding site of
KLF5 in the miR-487a promoter region was identified using JASPAR
database. ChIP assay identified the region-345 to −336 bp upstream
of the pre-miR-487a promoter region as a target of KLF5 (Fig. 5G and H). Pre-miR-487a promoter
region was present in the KLF5 fraction, which revealed that KLF5
may bind to miR-487a promoter region (Fig. 5H). Furthermore, luciferase activity
of WT miR-487a promoter was significantly upregulated in HOS and
MG-63 cells transfected with KLF5-OE compared with vector; the
effect was significantly rescued by miR-487a inhibitor. No
significant difference was found following mutation of KLF5 binding
site 336–345 bp in upstream of the pre-miR-487a promoter region
(Fig. 5I). These data suggested
that KLF5-induced miR-487a promotes osteosarcoma progression via
targeting NKX3-1.
Discussion
Recent evidence has illustrated that numerous miRNAs
are dysregulated in osteosarcoma and serve as an oncogene or tumor
suppressor (13,26). Here, miR-487a was significantly
upregulated in osteosarcoma cells and tissue. However, the
mechanism of miR-487a regulation of proliferation and metastasis in
osteosarcoma remains unclear.
Here, miR-487a-OE markedly promoted proliferation,
invasion and migration of HOS and MG-63 cells. miR-487a
upregulation in cancer has been reported in multiple studies. For
example, Chang et al demonstrated that miRNA-487a promotes
proliferation and metastasis in hepatocellular carcinoma (27). Yang et al indicated that exosomal
miR-487a derived from M2 macrophages promotes progression of
gastric cancer (28); these data
are consistent with the present results. NKX3-1 was predicted to be
a direct target of miR-487a in osteosarcoma cells and confirmed by
dual luciferase assay. In addition, the suppressive effect of
NKX3-1 on proliferation, invasion and migration of HOS and MG-63
cells was confirmed. On the other hand, numerous studies have
revealed that NKX3-1 serves as a tumor suppressor. For example,
Jiang et al demonstrated that NKX3-1 increases forkhead box O1
expression in hepatocellular carcinoma, thereby suppressing tumor
proliferation and invasion (29).
Miyaguchi et al suggested that loss of NKX3-1 is a biomarker for
poor prognosis in oral squamous cell carcinoma, which were
consistent with the present study (30). The present results suggested that
overexpression of miR-487a exert its role in osteosarcoma cells by
inhibiting NKX3-1.
Other factors have been reported to be involved in
the progression of osteosarcoma. For example, Ren and Gu revealed
the prognostic implications of RB1 tumour suppressor gene
alterations in the clinical outcome of human osteosarcoma (31). Li et al indicated that LINC01133 is
an emerging tumor-associated long non-coding (lnc)RNA in tumor and
osteosarcoma (32). Chen et al
found that alkB homolog 5, RNA demethylase-mediated m(6)A
demethylation of lncRNA PVT1 serves an oncogenic role in
osteosarcoma (33). In addition, a
previous study revealed that methyl
2-cyano-3,11-dioxo-18b-olean-1,12-dien-30-oate serves an
antineoplastic role in bladder cancer cells by inducing ROS, which
decreases Sp and Sp-regulated protein levels (34). The aforementioned study suggested
that intracellular ROS serves an anti-tumor role in tumor. It was
hypothesized that ROS may also serve a crucial role in development
of osteosarcoma. Jia et al revealed that liensinine inhibits
osteosarcoma growth by ROS-mediated suppression of the JAK2/STAT3
signaling pathway (35) and Rawat
and Nayak (36) reported that
Piperlongumine induces ROS-mediated apoptosis by transcriptional
regulation of SMAD4/P21/P53 genes and synergizes with doxorubicin
in osteosarcoma cells.
A recent study suggested that miR-487a serves an
oncogenic role in osteosarcoma by targeting BTG2 mRNA (37). The present study also investigated
the effect of miR-487a on osteosarcoma progression but focused on
different target genes and upstream transcription factors of
miR-487a to identify novel drug targets for osteosarcoma. The
present study combined bioinformatics with in vitro assays to
identify up- and downstream regulatory molecules of miR-487a.
Numerous studies have revealed that transcription
factors are associated with different types of cancer. For example,
Zhao et al (38) found that
activating transcription factor 3 mediates radioresistance of
breast cancer. Zhu et al (39)
indicated that KLF4 modulates miR-106a to target SMAD7 in gastric
cancer. Huang et al (40) revealed
that YY1 modulates lung cancer progression by activating
lncRNA-PVT1. The present study predicted transcription factors that
targeted the promoter of miR-487a using JASPAR database. KLF5 was
identified as a key upstream regulator of miR-487a by RT-qPCR. The
present study investigated the effect of KLF5-OE on miR-487a and
NKX3-1 expression via RT-qPCR and western blot assay. The effect of
KLF5-OE on osteosarcoma cell proliferation, invasion and migration
was also investigated. A previous study revealed that KLF5 has a
key effect on the microenvironment of cancer, which showed that
high levels of M2 polarized macrophages is associated with
upregulated KLF in bladder cancer and promotes angiogenesis, tumor
grade and invasiveness (41). It
was hypothesized that KLF5 may have a key effect on the
microenvironment of osteosarcoma. Therefore, KLF5/miR-487a/NKX3-1
axis may serve a key role in the proliferation and progression of
osteosarcoma, which may be a promising therapeutic target for
osteosarcoma.
There are certain limitations to the present study.
Firstly, the mechanism of KLF5 upregulation remains to be analyzed.
In addition, other potential downstream target genes of miR-487a
need to be investigated in the future.
The present study found that the
KLF5/miR-487a/NKX3-1 axis played a significant role in
proliferation, invasion and migration in osteosarcoma. Precision
medicine may provide a more effective therapy strategy for patients
with cancer based on individual variability. The present study
suggested that the KLF5/miR-487a/NKX3-1 axis may serve as a
therapeutic target for osteosarcoma. Inhibition of KLF5 and
miR-487a and activation of NKX3-1 may exert a protective impact on
osteosarcoma, which requires further investigation in future.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AL and CH confirm the authenticity of all the raw
data. AL and HL collected data. AL and CH analyzed data and edited
the manuscript. HL and CH performed the experiments and wrote the
manuscript. All authors have read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lindsey BA, Markel JE and Kleinerman ES:
Osteosarcoma overview. Rheumatol Ther. 4:25–43. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang J and Zhang W: New molecular insights
into osteosarcoma targeted therapy. Curr Opin Oncol. 25:398–406.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ritter J and Bielack SS: Osteosarcoma. Ann
Oncol. 21 (Suppl 7):vii320–vii325. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bernthal NM, Federman N, Eilber FR, Nelson
SD, Eckardt JJ, Eilber FC and Tap WD: Long-term results (>25
years) of a randomized, prospective clinical trial evaluating
chemotherapy in patients with high-grade, operable osteosarcoma.
Cancer. 118:5888–5893. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Anderson ME: Update on survival in
osteosarcoma. Orthop Clin North Am. 47:283–292. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chu Y, Hu X, Wang G, Wang Z and Wang Y:
Downregulation of miR-136 promotes the progression of osteosarcoma
and is associated with the prognosis of patients with osteosarcoma.
Oncol Lett. 17:5210–5218. 2019.PubMed/NCBI
|
|
7
|
Wang H, Zhao F, Cai S and Pu Y: MiR-193a
regulates chemoresistance of human osteosarcoma cells via
repression of IRS2. J Bone Oncol. 17:1002412019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nishikawa R, Goto Y, Kurozumi A,
Matsushita R, Enokida H, Kojima S, Naya Y, Nakagawa M, Ichikawa T
and Seki N: MicroRNA-205 inhibits cancer cell migration and
invasion via modulation of centromere protein F regulating pathways
in prostate cancer. Int J Urol. 22:867–877. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen X and Zhang Y: BMP-2 and miR-29c in
osteosarcoma tissues on proliferation and invasion of osteosarcoma
cells. Oncol Lett. 17:5389–5394. 2019.PubMed/NCBI
|
|
10
|
Chen T, Xu C, Chen J, Ding C, Xu Z, Li C
and Zhao J: MicroRNA-203 inhibits cellular proliferation and
invasion by targeting Bmi1 in non-small cell lung cancer. Oncol
Lett. 9:2639–2646. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qin C, Zhao Y, Gong C and Yang Z:
MicroRNA-154/ADAM9 axis inhibits the proliferation, migration and
invasion of breast cancer cells. Oncol Lett. 14:6969–6975.
2017.PubMed/NCBI
|
|
12
|
Hannafon BN, Cai A, Calloway CL, Xu YF,
Zhang R, Fung KM and Ding WQ: miR-23b and miR-27b are oncogenic
microRNAs in breast cancer: Evidence from a CRISPR/Cas9 deletion
study. BMC Cancer. 19:6422019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xia P, Gu R, Zhang W, Shao L, Li F, Wu C
and Sun Y: MicroRNA-377 exerts a potent suppressive role in
osteosarcoma through the involvement of the histone
acetyltransferase 1-mediated Wnt axis. J Cell Physiol.
234:22787–22798. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang G, Zhu Y, Jin C, Shi Q, An X, Song
L, Gao F and Li S: CircRNA_0078767 promotes osteosarcoma
progression by increasing CDK14 expression through sponging
microRNA-330-3p. Chem Biol Interact. 360:1099032022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang X, Wang M, Lin B, Yao D, Li J, Tang
X, Li S, Liu Y, Xie R and Yu S: miR-487a promotes progression of
gastric cancer by targeting TIA1. Biochimie. 154:119–126. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ma M, He M, Jiang Q, Yan Y, Guan S, Zhang
J, Yu Z, Chen Q, Sun M, Yao W, et al: MiR-487a promotes
TGF-β1-induced EMT, the migration and invasion of breast cancer
cells by directly targeting MAGI2. Int J Biol Sci. 12:397–408.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li XL, Jones MF, Subramanian M and Lal A:
Mutant p53 exerts oncogenic effects through microRNAs and their
target gene networks. FEBS Lett. 588:2610–2615. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liao JM, Cao B, Zhou X and Lu H: New
insights into p53 functions through its target microRNAs. J Mol
Cell Biol. 6:206–213. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Varet H, Brillet-Guéguen L, Coppée JY and
Dillies MA: SARTools: A DESeq2- and EdgeR-based R pipeline for
comprehensive differential analysis of RNA-Seq data. PLoS One.
11:e01570222016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Castro-Mondragon JA, Riudavets-Puig R,
Rauluseviciute I, Lemma RB, Turchi L, Blanc-Mathieu R, Lucas J,
Boddie P, Khan A, Manosalva Pérez N, et al: JASPAR 2022: The 9th
release of the open-access database of transcription factor binding
profiles. Nucleic Acids Res. 50(D1): D165–D173. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dweep H, Gretz N and Sticht C: miRWalk
database for miRNA-target interactions. Methods Mol Biol.
1182:289–305. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Friedman RC, Farh KK, Burge CB and Bartel
DP: Most mammalian mRNAs are conserved targets of microRNAs. Genome
Res. 19:92–105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Betel D, Koppal A, Agius P, Sander C and
Leslie C: Comprehensive modeling of microRNA targets predicts
functional non-conserved and non-canonical sites. Genome Biol.
11:R902010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kertesz M, Iovino N, Unnerstall U, Gaul U
and Segal E: The role of site accessibility in microRNA target
recognition. Nat Genet. 39:1278–1284. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu X and Liu M: miR-522 stimulates
TGF-β/Smad signaling pathway and promotes osteosarcoma
tumorigenesis by targeting PPM1A. J Cell Biochem. 120:18425–18434.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chang RM, Xiao S, Lei X, Yang H, Fang F
and Yang LY: miRNA-487a promotes proliferation and metastasis in
hepatocellular carcinoma. Clin Cancer Res. 23:2593–2604. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang X, Cai S, Shu Y, Deng X, Zhang Y, He
N, Wan L, Chen X, Qu Y and Yu S: Exosomal miR-487a derived from m2
macrophage promotes the progression of gastric cancer. Cell Cycle.
20:434–444. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jiang J, Liu Z, Ge C, Chen C, Zhao F, Li
H, Chen T, Yao M and Li J: NK3 homeobox 1 (NKX3.1) up-regulates
forkhead box O1 expression in hepatocellular carcinoma and thereby
suppresses tumor proliferation and invasion. J Biol Chem.
292:19146–19159. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Miyaguchi K, Uzawa N, Mogushi K, Takahashi
K, Michikawa C, Nakata Y, Sumino J, Okada N, Mizushima H, Fukuoka Y
and Tanaka H: Loss of NKX3-1 as a potential marker for an increased
risk of occult lymph node metastasis and poor prognosis in oral
squamous cell carcinoma. Int J Oncol. 40:1907–1914. 2012.PubMed/NCBI
|
|
31
|
Ren W and Gu G: Prognostic implications of
RB1 tumour suppressor gene alterations in the clinical outcome of
human osteosarcoma: A meta-analysis. Eur J Cancer Care (Engl).
26:2017. View Article : Google Scholar
|
|
32
|
Li Z, Xu D, Chen X, Li S, Chan MTV and Wu
WKK: LINC01133: An emerging tumor-associated long non-coding RNA in
tumor and osteosarcoma. Environ Sci Pollut Res Int. 27:32467–32473.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen S, Zhou L and Wang Y: ALKBH5-mediated
m6A demethylation of lncRNA PVT1 plays an oncogenic role
in osteosarcoma. Cancer Cell Int. 20:342020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Takeuchi H, Taoka R, Mmeje CO, Jinesh GG,
Safe S and Kamat AM: CDODA-Me decreases specificity protein
transcription factors and induces apoptosis in bladder cancer cells
through induction of reactive oxygen species. Urol Oncol.
34:337.e11–e18. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jia F, Liu Y, Dou X, Du C, Mao T and Liu
X: Liensinine inhibits osteosarcoma growth by ROS-mediated
suppression of the JAK2/STAT3 signaling pathway. Oxid Med Cell
Longev. 2022:82456142022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rawat L and Nayak V: Piperlongumine
induces ROS mediated apoptosis by transcriptional regulation of
SMAD4/P21/P53 genes and synergizes with doxorubicin in osteosarcoma
cells. Chem Biol Interact. 354:1098322022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gu Z, Wu S, Xu G, Wu W, Mao B and Zhao S:
miR-487a performs oncogenic functions in osteosarcoma by targeting
BTG2 mRNA. Acta Biochim Biophys Sin (Shanghai). 52:631–637. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhao W, Sun M, Li S, Chen Z and Geng D:
Transcription factor ATF3 mediates the radioresistance of breast
cancer. J Cell Mol Med. 22:4664–4675. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhu M, Zhang N and He S: Transcription
factor KLF4 modulates microRNA-106a that targets Smad7 in gastric
cancer. Pathol Res Pract. 215:1524672019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huang T, Wang G, Yang L, Peng B, Wen Y,
Ding G and Wang Z: Transcription factor YY1 modulates lung cancer
progression by activating lncRNA-PVT1. DNA Cell Biol. 36:947–958.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Takeuchi H, Tanaka M, Tanaka A, Tsunemi A
and Yamamoto H: Predominance of M2-polarized macrophages in bladder
cancer affects angiogenesis, tumor grade and invasiveness. Oncol
Lett. 11:3403–3408. 2016. View Article : Google Scholar : PubMed/NCBI
|