Introduction
Over the last 5 years, the incidence of breast
cancer has increased by 0.3% per year (1), although the mortality rate has
decreased by 40% between 1989 and 2017 in the United States
(1). However, the clinical
prognosis from TNBC remains poor (2). Compared with other subtypes of breast
cancer, TNBC are relatively more common in younger patients, the
clinical characteristics of which tend to be more aggressive
(2). In addition, patients with
TNBC frequently present with larger tumour masses, where lesions
with higher grades are more likely to be detected (3–5). In
total ~33% patients with TNBC will develop metastatic disease
(6,7). The lack of effective therapeutic
strategies for TNBC, such as endocrine therapy or targeted therapy,
is one of the key reasons for the poor prognosis of TNBC (8). Therefore, further exploration of
novel targets for the treatment of TNBC is warranted to improve the
prognosis from this disease.
Transient receptor potential channels 5 (TRPC5) has
been previously reported to regulate various biological processes
such as oxidative stress, constitutively active TRPs, and neuronal
cell death (9). Accumulating
evidence suggests that TRPC5 can serve an important role in
promoting resistance to chemotherapy in breast cancer (10–12).
As such, increased TRPC5 expression has been reported to induce the
expression of P-glycoprotein in MCF-7 cells (12). Subsequently, P-glycoprotein then
functions to remove cytotoxic drugs from the cells to mediate
chemotherapy resistance (13).
TRPC5 opposite strand (OS) is the antisense strand
of TRPC5 and is also known as TRPC5 antisense RNA1 (TRPC5-AS1)
(14). TRPC5OS has been previously
found to encode a microprotein that consists of 111 amino acids and
is highly expressed particularly in the testis (15). Genes that are specifically
expressed in normal testicular tissues may serve an important role
in liver cancers, such as the OY-TES-1 gene. Under physiological
conditions, OY-TES-1 mRNA is only expressed in the testis (13). However, its expression has been
reported to be upregulated in several types of cancer, such as
bladder cancer and breast cancer, where it has been shown to be a
novel member of the cancer/testicular antigen family (14). In addition, the OSs of other
important genes, such as long non-coding RNA FOXD2 adjacent
opposite strand RNA 1 (FOXD2-AS1) and the antisense strand of
HOXA11 (HOXA11-AS) may also be associated with the occurrence and
development of breast cancer. There is evidence that interfering
with HOXA11-AS and FOXD2-AS1 function can attenuate the invasive
and migratory capabilities of breast cancer cells (16,17).
Therefore, it could be hypothesised that TRPC5OS may affect the
physiology of tumours by regulating tumour progression, in turn
affecting patient prognosis. However, little is known regarding the
possible role of TRPC5OS in breast cancer. Therefore, in the
present study, the aim is to elucidate the possible function and
mechanism of TRPC5OS in TNBC, to explore its potential value as a
novel therapeutic target.
Materials and methods
Cell lines and culture
The human breast cancer cell lines ZR-75-1,
MDA-MB-453, SK-BR-3, JIMT-1, BT474, HCC1937 and SUM-1315, in
addition to the normal human breast cancer cell line MCF-10A, were
gifts from Professor Ziyi Fu (Breast Disease Laboratory, Women
& Children Central Laboratory, First Affiliated Hospital,
Nanjing Medical University). The human breast cancer cell lines
MDA-MB-231 and MCF-7 were purchased from The Cell Bank of Type
Culture Collection of the Chinese Academy of Sciences (https://www.cellbank.org.cn/). MCF-10A, ZR-75-1,
MDA-MB-453, SK-BR-3, JIMT-1, BT474, HCC1937, MDA-MB-231, SUM-1315
and MCF-7 cells were cultured in high-glucose DMEM (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% (v/v) FBS (Gibco;
Thermo Fisher Scientific, Inc.) 100 U/ml penicillin (Gibco; Thermo
Fisher Scientific, Inc.) and 100 U/ml streptomycin (Gibco; Thermo
Fisher Scientific, Inc.) in a humidified atmosphere with 5%
CO2 at 37°C. MCF-10A cells are normal mammary epithelial
cells (18), whereas BT474, MCF-7
and ZR-75-1 cells are luminal-type breast cancer cells (18). HCC1937, MDA-MB-231 and SUM-1315 are
TNBC cells, whilst JIMT-1, SK-BR-3 and MDA-MB-453 are oestrogen
receptor (ER)-, progesterone receptor (PR)-, human epidermal growth
factor receptor 2 (HER)+ breast cancer cells (18). All experiments were independently
reproduced three times.
Patient tissue samples
A total of 30 pairs of breast cancer tissues and
matching adjacent normal tissues (3–5 cm from the edge of the
breast cancer tissues) were obtained from Jiangsu Province Hospital
(Table SI). All tissues were
collected from 2015–2018 and tumour size was obtained from
postoperative pathological reports. All patients were female and
mean ± SD age was 53.17±11.94. Written informed consent was
obtained from all patients recruited in the present study. The
present study was approved by the Ethics Committee of Jiangsu
Province Hospital (The First Hospital Affiliated Hospital with
Nanjing Medical University; approval no. 2010-SR-091). The
inclusion criteria were: i) Patients diagnosed with TNBC by
immunohistochemical method after ultrasound-guided core-needle
biopsy and ii) no active therapy prior to operative treatment.
Patients undergoing neoadjuvant chemotherapy or with multiple
neoplasm were excluded. Tissues were obtained by surgical resection
and stored in a −80°C refrigerator for utilization.
Lentiviral transfection of MDA-MB-231
and MCF-7 cells
MDA-MB-231 and MCF-7 cells were incubated in
six-well plates. After confluency reached 50%, cells were
transfected with 5 µg/ml LV-TRPC5OS, LV5 (NC; the overexpression
control; EF-1Af/GFP&Puro), LV-193 (sh-TRPC5OS,
GGGAGAATTTGTCTCTGAAGT) and LV3 (NC; the knockdown control;
H1/GFP&Puro) for 6 h. The generation system used was 3rd, the
temporary cell line used was 293 cells (Chinese Academy of Medical
Sciences Tumor Cell Bank). MOI=-In (0.01)=4.6 pfu/cell. The ratio
of the lentiviral plasmid: packaging vector: envelope=1:2:1. All
agents and products were obtained from Shanghai GenePharma, Co.,
Ltd. In total, 3 µg/ml puromycin (VWR, Avantor, Inc.) was used to
screen cells that had been successfully transfected for 2 weeks at
37°C. Transfection efficiency was confirmed using fluorescence
microscopy following screening (magnification of fluorescence
microscopy was ×40).
Cell Counting Kit-8 (CCK-8) assay
A total of 2×103 MDA-MB-231 or MCF-7
cells in 100 µl high-glucose DMEM culture solution per well were
added into a 96-well plate and incubated overnight. Subsequently,
it was tested daily for ≤6 days. Prior to testing, 90 µl serum-free
medium with 10 µl CCK-8 labelling reagent (Dojindo, Molecular
Technologies, Inc.) was added into each well and incubated for 2 h
in the dark at 37°C. The viability of the cells was then analysed
by measuring their absorbance at 450 nm using an enzyme–labelled
meter (Thermo Fisher Scientific, A33978, Inc.).
5-Ethynyl-2′-deoxyuridine (EdU)
assay
The EdU assay (Guangzhou RiboBio, C10310-1 Co, Ltd.)
was performed according to the manufacturer's protocols. Briefly,
1×104 cells were incubated in 24-well plates for 24 h at
37°C. A total of 2×103 MDA-MB-231 or MCF-7 cells in 100 µl
high-glucose DMEM culture solution per were treated with 50 µM EdU
for 2 h at 37°C. After fixing for 15 min with 4% paraformaldehyde
at 37°C, the cell membranes were permeabilised using 0.5% Triton
X-100 for 15 min at 37°C. A total of 2×103 MDA-MB-231 or MCF-7
cells in 100 µl high-glucose DMEM culture solution per were then
treated with 100 µl 100:1 diluted Hoechst 33342 for 30 min at room
temperature and imaged using a fluorescence microscope (Nikon
Corporation, ×40). The images were analysed using ImageJ 1.8.0
(National Institutes of Health).
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
According to the manufacturer's protocol,
TRIzol® (Invitrogen, 15596018) was added to the
different cell lines to extract total RNA. All RNA samples were
dissolved in DEPC water (Beyotime Institute of Biotechnology). The
quality and concentration of total RNA was detected using
NanoDrop® ND-1000 (Thermo Fisher Scientific, Inc.). The
total RNA was then reverse transcribed using the PrimeScript™ RT
reagent kit (37°C 15 min; 85°C 5 sec; 4°C for 5 min, Takara Bio,
Inc.). SYBR Green qPCR Master Mix (Takara Bio, Code No. RR430S
RR430A, Inc.) was added to the generated cDNAs alongside the
specific primers (obtained from TsingKe Biotechnology, Co., Ltd.),
which were all dissolved in DEPC to a final volume of 10 µl (95°C
30 sec; 95°C 5 sec; 60°C 30 sec, number of cycles: 40; 95°C 15 sec,
60°C 60 sec, 95°C 15 sec). This system was then added to a 96-well
plate and tested using a LightCycler 480® Real Time PCR
System (Roche Diagnostics). Each reaction was tested ≥ three times.
Non-specific amplification was detected using a dissolution curve.
The sequences of the primers used were: TRPC5OS forward,
5′-TCATTGATGGACTTGTTGCTTG-3′ and reserve,
5′-AAGTCTGAGAGATCAGGGAGAT-3′ and GAPDH (glyceraldehyde-3-phosphate
dehydrogenase) forward, 5′-GCTGCGAAGTGGAAACCTAC-3′ and reverse,
5′-CCTCCTTCTGCACACATTTGAA-3′. The quantification method used for
mRNA expression was comparative Cq method (19).
High-throughput sequencing
The RNA was extracted as aforementioned following
TRPC5OS overexpression. Agarose gel electrophoresis was used for
assessing the integrity of the extracted RNAs, whereas
quantification and further quality tests were performed using a
NanoDrop® ND-1000 (Thermo Fisher Scientific, Inc.).
Subsequently, an NEB Next® Poly (A) mRNA Magnetic
Isolation Module (New England BioLabs, E7490L, Inc.) was used for
mRNA enrichment by adding to the total RNA extract. A library of
the processed RNA product was established using the KAPA Stranded
RNA-Seq Library Prep kit (KK8401, Roche Diagnostics). The loading
concentration of the final library was ≥200 ng/µl and total RNA was
≥2.5 µg. The sequence was generally greater than 200 nt, the
direction of sequencing is 5′-3′. The established library was
sequenced using SeqPlex RNA amplification Kit (SEQR-50RXN, Merck,
Inc) on the Illumina HiSeq 4000 platform (Illumina, Inc.). The data
was analysed by SUPPA2.0, Cytoscape 3.6.1 and cd-hit-v4.8.1.
Bioinformatics analysis of
differentially expressed genes (DEGs), screening the top 10 Gene
Ontology (GO) and Kyoto Encyclopaedia of Genes and Genomes
(KEGG)
To explore the possible mechanism by which TRPC5OS
exerts its effects, bioinformatics analysis was performed.
Principal component analysis (PCA) was performed with princomp
function of R (http://www.r-project.org/) in this experience. Scale
function was used to perform z-score processing on expression
spectrum before PCA dimensionality reduction. PC1 and PC2 are
principal components 1 and 2 respectively, which are the two
principal components with the highest proportion after
dimensionality reduction and clustering by PCA algorithm. The GO
database was used to perform GO functional enrichment analysis for
the DEGs (www.geneontology.org). GO terms were sorted by their
enrichment scores [-log10 (P-value)] before the top 10
terms were selected for further analysis (‘P-value’ stands for the
fisher exact test value of the term and was calculated by DAVID
databases after DEGs were inputted into). KEGG analysis was used to
identify the pathways in which the DEGs were enriched (https://www.kegg.jp/), where the top 10 KEGG terms
were sorted and selected for further analysis. We input DEGs that
exhibited >1.5-fold change in expression into DAVID databases
for GO and KEGG analysis.
In addition, protein-protein interactions (PPIs)
were analysed to elucidate the interactions among the targeted
genes using Search Tool for the Retrieval of Interacting
Genes/Proteins (STRING; www.string-db.org/; active interaction sources:
Textmining, Experiments, Databases, Co-expression, Neighborhood,
Gene Fusion, Co-occurrence; required interaction score: 0.40). The
network nodes represented proteins, whereas the edges between nodes
represented PPIs. PPI enrichment P-value <0.05 means that
proteins were defined as interactions among themselves. GEPIA 2
(www.gepia2.cancer-pku.cn/) was used
to analyse the expression of enolase 1 (ENO1) in tumour and normal
tissue samples(from the Jiangsu Province Hospital). Kaplan-Meier
curves followed by a log-rank test of the overall survival of
patients with breast cancer were analysed using KMplot
(kmplot.com/analysis/). The patient survival data of KMplot came
from GEO, EGA, and TCGA.
Western blotting
After cells were transfected with the TRPC5OS
lentivirus, protein extraction was performed using RIPA Lysis
Buffer (Beyotime Institute of Biotechnology). Total protein
concentration in the supernatant was determined with Bicinchoninic
Acid assay (Beyotime biotechnology, China). SDS-PAGE Sample Loading
Buffer (Beyotime Institute of Biotechnology) was added to the
protein lysates and boiled to 95°C for 5 min. These protein samples
(10 ul/well) were resolved using 10% SDS-PAGE and transferred onto
PVDF. Subsequently, the membranes were blocked in QuickBlock™
Blocking Buffer for Western Blotting (Beyotime Institute of
Biotechnology) for 15 min at room temperature. These membranes were
then incubated with an anti-β-actin (Abcam, ab179467, 1:5,000) or
anti-TRPC5OS antibody (Dia-an Biological Technology Incorporation;
NP_001182505, 1:5,000) at 4°C for 12–14 h. Subsequently, the
membranes were washed three times and then incubated with
HRP-conjugated Affinipure Goat Anti-Mouse and Anti-Rabbit IgG
antibodies (H+L; SA00001-2; SA00001-1; ProteinTech Group, Inc) for
2 h. The bands were observed by ECL chemiluminescence substrate
(Biosharp, BL520A). Densitometry analysis was performed using
ImageJ 1.8.0.
Co-immunoprecipitation assay
(Co-IP)
Co-IP was performed using anti-TRPC5OS (Dia-an
Biological Technology Incorporation; catalog number: NP_001182505)
or rabbit IgG (control) (Thermo Fisher Scientific, Inc. catalog
number: 88804) antibodies using a classic magnetic Co-IP kit
according to the manufacturer's protocol (Thermo Fisher Scientific,
Inc. catalog number: 88804). First, 10 µg antibody was incubated
with the 1,000 ug protein in the cell lysate in a final volume of
500 µl. This mixture was incubated on a wheel at 4°C overnight. A
total of 250 µg magnetic beads (IP antibody binds to protein A/G
magnetic microbeads) conjugated to anti-TRPC5OS or rabbit IgG
(control) antibodies were added into a 1.5-ml centrifuge tube, Wash
Buffer was used to clean the magnetic beads three times.
Subsequently, the protein-antibody mixture was added this a
centrifuge tube and mixed with the magnetic beads. This mixture was
then incubated at room temperature for 1 h on rotation. Place the
tubes on a magnetic separator (i.e. Millipore Cat. # 20–400) and
discard the supernatant after bead aggregation. Remove the tubes
from the magnet. Add 0.5 ml of Wash Buffer to each tube and vortex
briefly for three times. In total, 100 µl Low pH Elution Buffer was
then added to the centrifuge tube and incubated for 10 min at room
temperature. The supernatant was collected by centrifugation at
4°C, 14,000 × g for 1 min, and magnetic beads were thrown away. The
proteins eluted using the anti-TRPC5OS or rabbit IgG (control)
antibodies were then analysed using the Fast Silver Stain Kit
(Fig. S1; Beyotime Institute of
Biotechnology; catalog number: P0017S). These protein samples were
then loaded into 10% SDS-PAGE gels with 30 ul per well, and
staining gels at 4°C for 3 min. Liquid chromatography (LC)-mass
spectrometry (MS)/MS was performed using these last eluted
supernatant immunoprecipitated proteins.
LC-MS/MS
The protein was extracted using RIPA Lysis Buffer
and the protein concentration of the samples were determined by
using BCA Protein Assay Kit (Beyotime Institute of Biotechnology).
Next, the samples underwent enzyme hydrolysis in solution and gel
using formic acid (Fisher Scientific; A117-50); acetonitrile
(Fisher Scientific; A955-4); trypsin (Promega; V528A); sep-Pak C18
Desalination column (Waters; WAT200685); Peptides capture column
Acclaim PepMap C18, 100 µm ×20 mm (Thermo Scientific; cat. no.
164946); Peptides analytical column Acclaim PepMap C18, 75 µm ×250
mm (Thermo Scientific; 164569). Appropriate volume 10 mmol/l DTT
was added and placed in 56°C water bath for 60 min. After cooling
to room temperature, all liquid was absorbed, and then appropriate
volume 55 mmol/l IAA was added and placed away from light for the
45 min at room temperature. Pancreatic enzyme solution was added
and placed at 4°C for 30 min. Whereafter, 25 mmol/l NH4HCO3 was
added and placed in 37°C water bath overnight. After enzymatic
hydrolysis, solid phase extraction enrichment and purification were
performed. In LC-MS/MS (Q-Exactive liquid chromatograph- mass
spectrometer (Thermo Fisher Scientific, Inc.); Ion transport tube
temperature was 320°C), the samples were separated on a Poroshell
120 C18 column (2.7 µm; 2.1×30 mm; Agilent Technologies, Inc,
689975-302). The mobile phase consisted of the A phase (0.1% formic
acid/pure water) and the B phase (100% acetonitrile). The flow rate
of the mobile phase was 0.3 ml/min and the concentration gradient
was 10% (0 min), 10% (1 min), 90% (4 min), 90% (8 min) and 10% (9
min) of the B phase. A total of 5 µl were injected each time. The
mass spectrometer was connected to an ion source and the positive
ionisation mode of the multiple reaction monitoring mode (MRM) was
selected. Both Q1 and Q3 were set at Unit resolution. The flow rate
of the drying gas (nitrogen) was 10 l/min and its temperature was
maintained at 350°C. The optimum capillary voltage was 5,500 V. The
atomiser pressure was set to 35 psi. Data were collected and
processed using an AB SCIEX Workstation Software (pAnalyst 1.62,
Shanghai AB SCIEX Analytical Instrument Trading Co.). The peptides
were monitored in the MRM.
Statistical analysis
Data from clinical tissue samples of 30 patients
were compared using a paired t-test. One-way ANOVA was used for
comparing among multiple groups and the Tukey's post hoc test was
used. Numerical data are presented as the mean ± standard
deviation. P<0.05 was considered to indicate a statistically
significant difference. Statistical analysis was performed using
SPSS version 20 (IBM Corp.). Each experiment was independently
repeated three times except for RNA sequencing.
Results
TRPC5OS expression in the TNBC tissue
samples and breast cancer cell lines
To measure the expression of TRPC5OS in tissue
samples of patients with TNBC and in a panel of different breast
cancer cell lines, mRNA expression levels of TRPC5OS were detected
using RT-qPCR. As shown in Fig.
1A, the mRNA expression levels of TRPC5OS in TNBC tumour
tissues were significantly higher compared with those in the
adjacent normal tissues. In addition, TRPC5OS expression was found
to be significantly higher in tissue samples from larger masses of
tumours (≥3 cm; Fig. 1B). However,
there was no difference in TRPC5OS expression between tissues from
different histological grades (II and III; Fig. 1C). Among the different breast
cancer cell lines, the mRNA expression levels of TRPC5OS were
generally higher compared with those in the normal breast cell line
MCF-10A. However, compared with other breast cancer cells such as
ZR-75-1, MDA-MB-453, SK-BR-3, JIMT-1, BT474, the mRNA expression
levels of TRPC5OS were significantly higher in TNBC cell lines,
such as SUM-1315 and MDA-MB-231 (Fig.
1D).
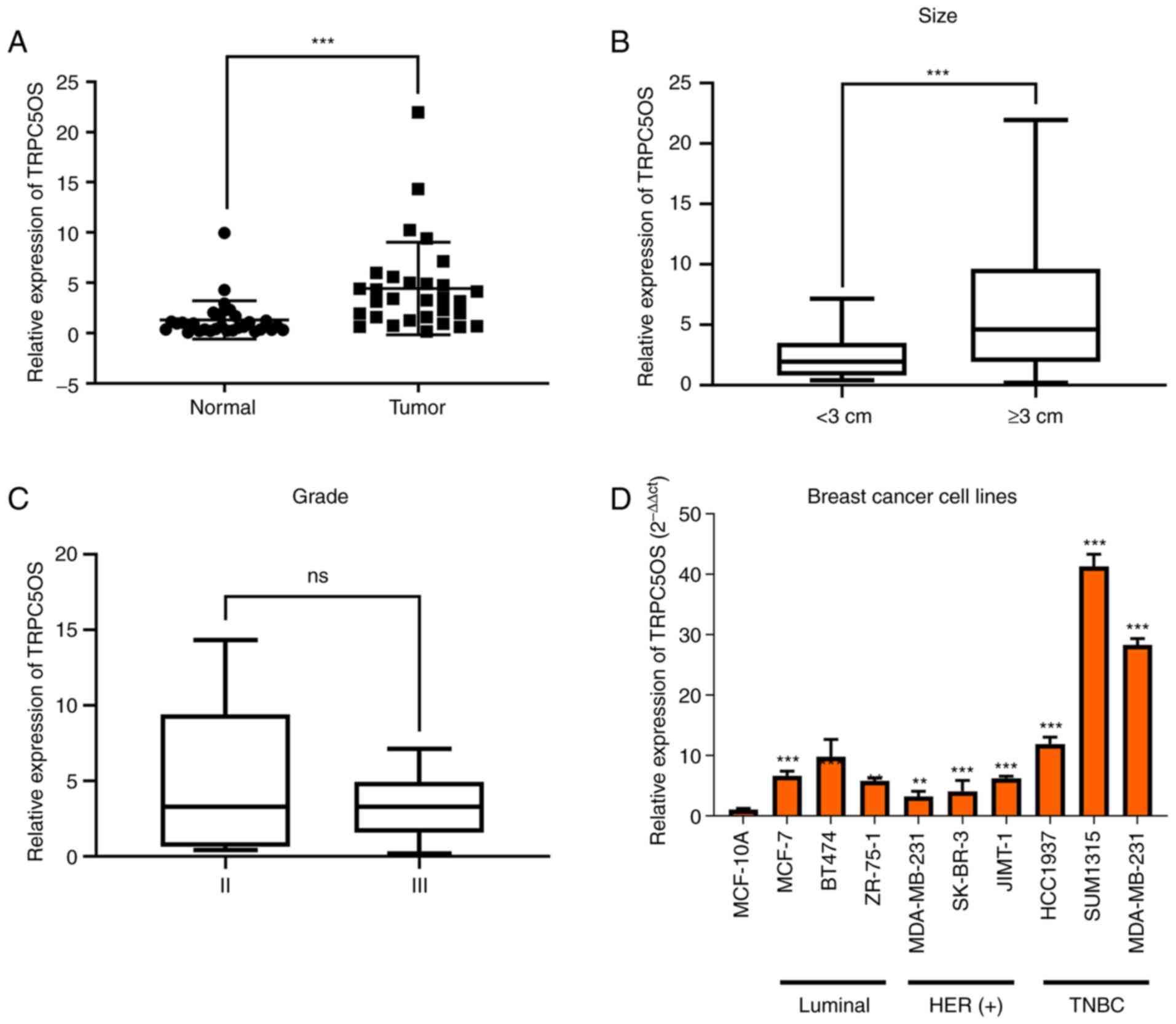 | Figure 1.TRPC5OS expression in the tissue
samples of patients with TNBC and in a panel of breast cancer cell
lines. (A) mRNA expression levels of TRPC5OS were detected in the
tissues of patients with TNBC using reverse
transcription-quantitative PCR. (B) The relationship between tumour
size and TRPC5OS expression levels was calculated, where a tumour
size cut-off value of 3 cm was used. (C) Relationship between
histological grade and TRPC5OS expression levels following
stratification. (D) mRNA expression levels of TRPC5OS in MCF-10A
normal mammary epithelial cells compared with nine different breast
cancer cell lines. HER-2(−), ER(+) cell lines were MCF-7, BT-474,
ZR-75-1, where the HER-2(+) cell lines were MDA-MB-453, SK-BR-3,
JIMT-1. TNBC cell lines were HCC1937, SUM1315 and MDA-MB-231. Each
experiment was repeated ≥ three times. **P<0.01 and
***P<0.001 vs. MCF-10A. TNBC, triple-negative breast cancer;
TRPC5OS, Transient receptor potential channel 5 opposite
strand. |
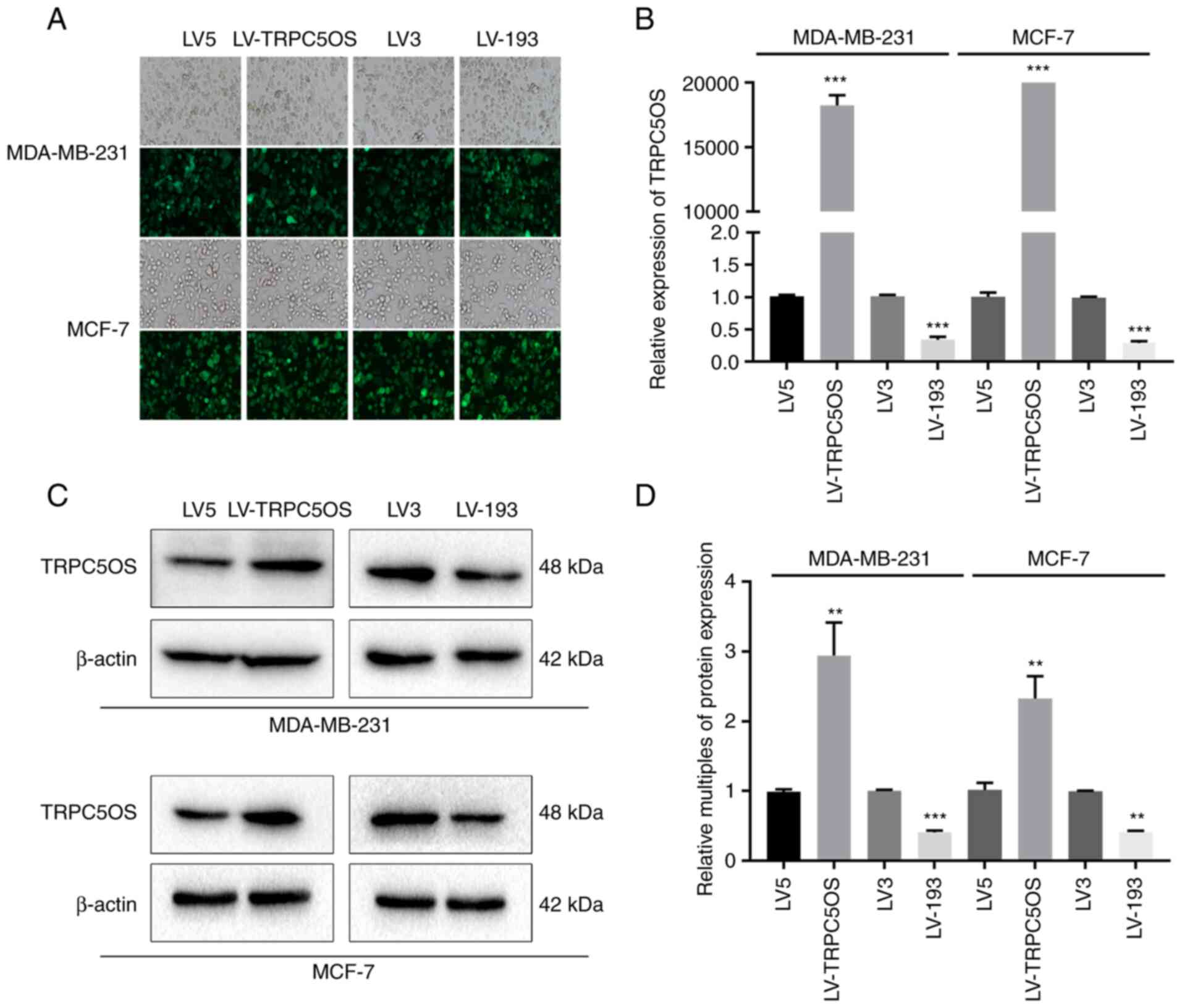
Transfection efficiency of lentiviral
constructs in MDA-MB-231 and MCF-7 cells
To verify the transfection efficiency of the
lentiviral constructs, fluorescence microscopy, western blotting
and RT-qPCR were performed following transfection and treatment
with puromycin for 2 weeks. Green fluorescence protein expression
was observed in >90% transfected cells (Fig. 2A). The green fluorescence comes
from a fluorescent gene carried by the lentivirus itself. RT-qPCR
analysis demonstrated that both in MDA-MB-231 and MCF-7 cells, the
mRNA expression levels of TRPC5OS were significantly higher in
TRPC5OS-overexpressing cells compared with the control cells
(transfection of empty virus), whereas its expression was
significantly decreased in cells with TRPC5OS expression knocked
down (Fig. 2B). Western blotting
subsequently showed that in MDA-MB-231 and MCF-7 cells, the protein
expression level of TRPC5OS was significantly increased by
LV-TRPC5OS transfection compared with that in its corresponding LV5
control (Fig. 2C and D). By
contrast, it was significantly decreased by LV-193 transfection
compared with that in its corresponding LV3 control (Fig. 2C and D). These results suggest that
lentiviral transfection was effective in MDA-MB-231 and MCF-7
cells.
TRPC5OS promotes proliferation of
breast cancer cells
CCK-8 and EdU assays were next performed to detect
the biological function of TRPC5OS in MDA-MB-231 and MCF-7 cells.
CCK-8 assay results revealed that the proliferative ability was
markedly increased following TRPC5OS overexpression but it was
decreased following TRPC5OS knockdown (Fig. 3A and B) in MDA-MB-231 cells
compared with those in their corresponding negative controls. The
results of the CCK-8 assay in MCF-7 cells were consistent with
those of MDA-MB-231 cells (Fig. 3C and
D). EdU results also showed that cell viability was
significantly increased in TRPC5OS-overexpressing cells, whereas it
was significantly decreased following TRPC5OS knockdown (Fig. 3C), compared with those in their
corresponding negative controls. Therefore, these results suggest
that the viability and proliferative abilities of MDA-MB-231 cells
were promoted by TRPC5OS overexpression.
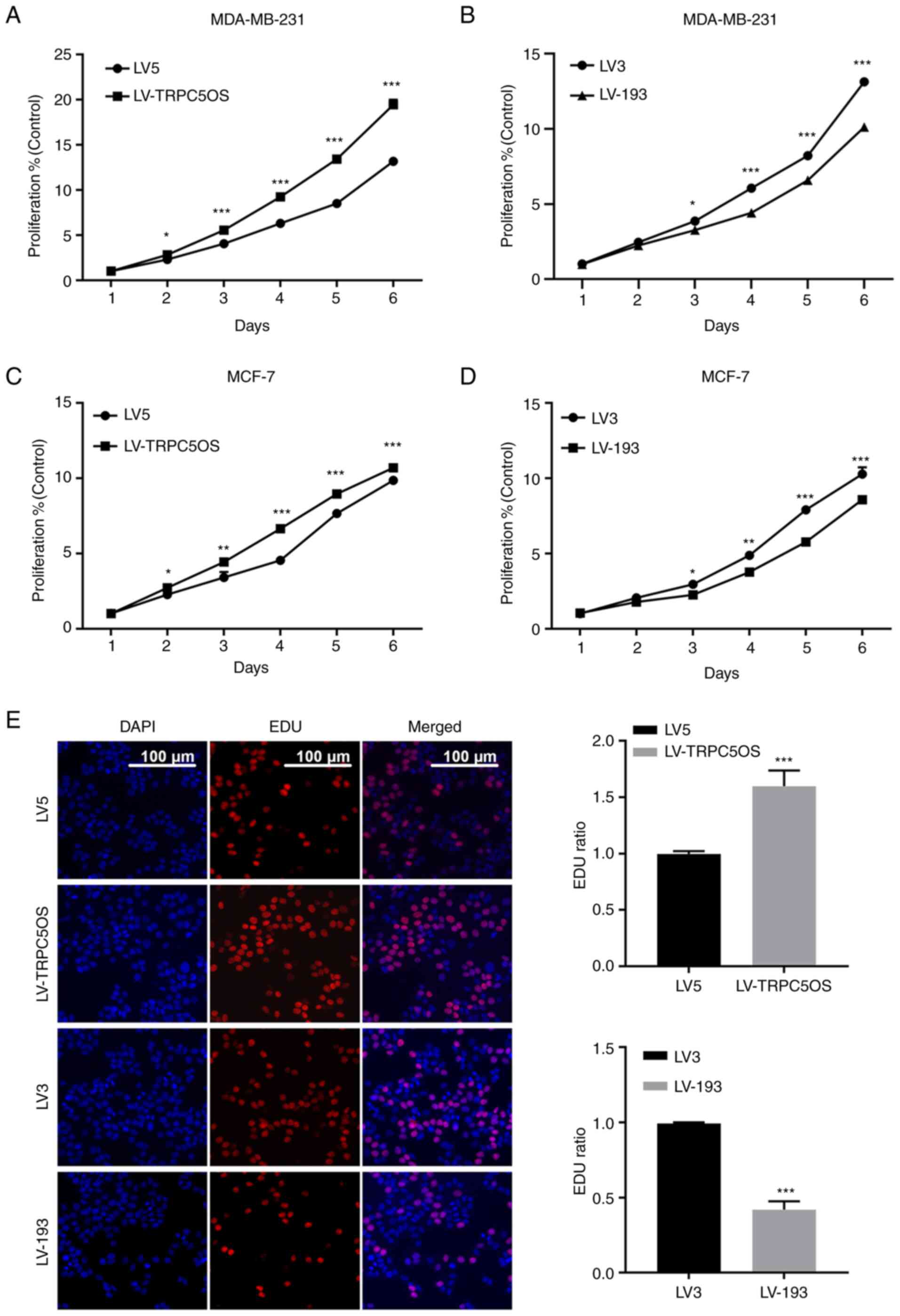 | Figure 3.TRPC5OS overexpression increases the
proliferative ability of breast cancer cells. The proliferative
ability of MDA-MB-231 cells was assessed using a Cell Counting
Kit-8 assay following (A) LV5, LV-TRPC5OS, (B) LV3 or LV-193
transfection. The proliferative ability of MCF-7 cells was assessed
using a Cell Counting Kit-8 assay following (C) LV5, LV-TRPC5OS,
(D) LV3 or LV-193 transfection. (E) An EdU-incorporation assay was
used to detect the proliferative ability of MDA-MB-231 cells
following LV5, LV5-TRPC5OS, LV3 or LV-193 transfection. Scale bar,
100 µm. Each experiment was repeated at least three times.
*P<0.05, **P<0.01 and ***P<0.001 (LV5 vs LV-TRPC5OS; LV3
vs LV-193). TRPC5OS, Transient receptor potential channel 5
opposite strand; LV, lentivirus. |
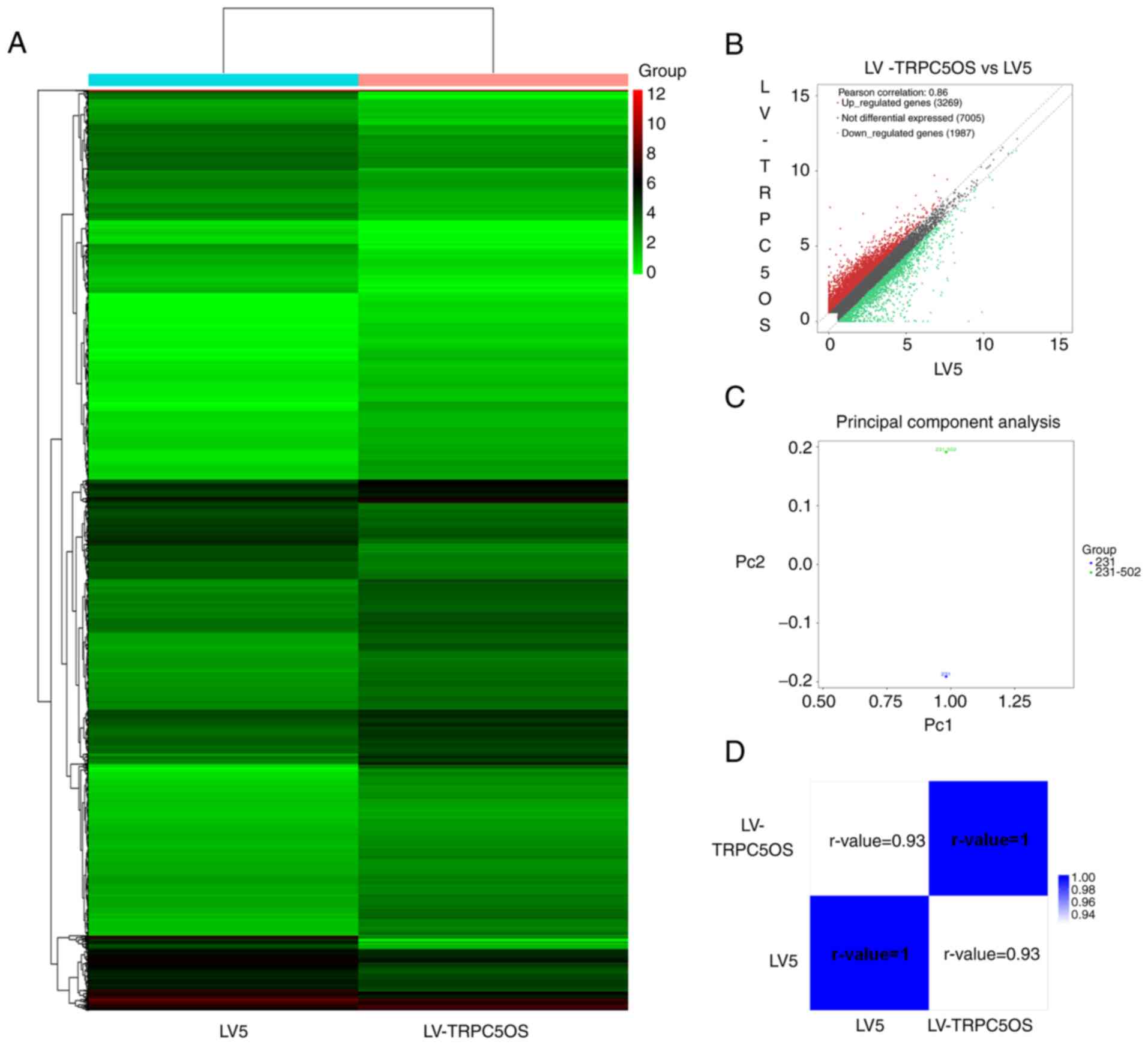
Identification of DEGs following
TRPC5OS overexpression
To further investigate the effects of TRPC5OS
overexpression, high-throughput sequencing was used to screen for
the DEGs following TRPC5OS overexpression in MDA-MB-231 cells. The
top 10 of upregulated and downregulated DEGs are shown in the
supplemental material (Table
SII). Inter-group comparison and cluster analysis were then
performed according to the fragments per kilobase of exon per
million values of significant DEGs, where heat map was generated
showing the gene expression pattern in the two different samples.
There were significant differences between control (LV5) and
TRPC5OS overexpressed MDA-MB-231 cell line (Fig. 4A). A scatter plot also described
the overall distribution trend in the two samples. The results
showed that a total of 5,256 genes were differentially expressed,
including 3,269 upregulated genes and 1,987 downregulated genes
(fold change ≥1.5; Fig. 4B). The
principal component analysis results showed the difference between
control (LV5) and TRPC5OS overexpressed (LV-TRPC5OS) MDA-MB-231
cell line. Significant differences in the expression data were
shown in Fig. 4C between control
(LV5) and TRPC5OS overexpressed (LV-TRPC5OS) MDA-MB-231 cell line.
Correlation between the two samples can be seen in the heatmap
diagrams of the sample correlation coefficients and indicated that
the similarity of the two samples was high, the experiment was
reliable, and the sample selection was reasonable (r-value
>0.92; Fig. 4D).
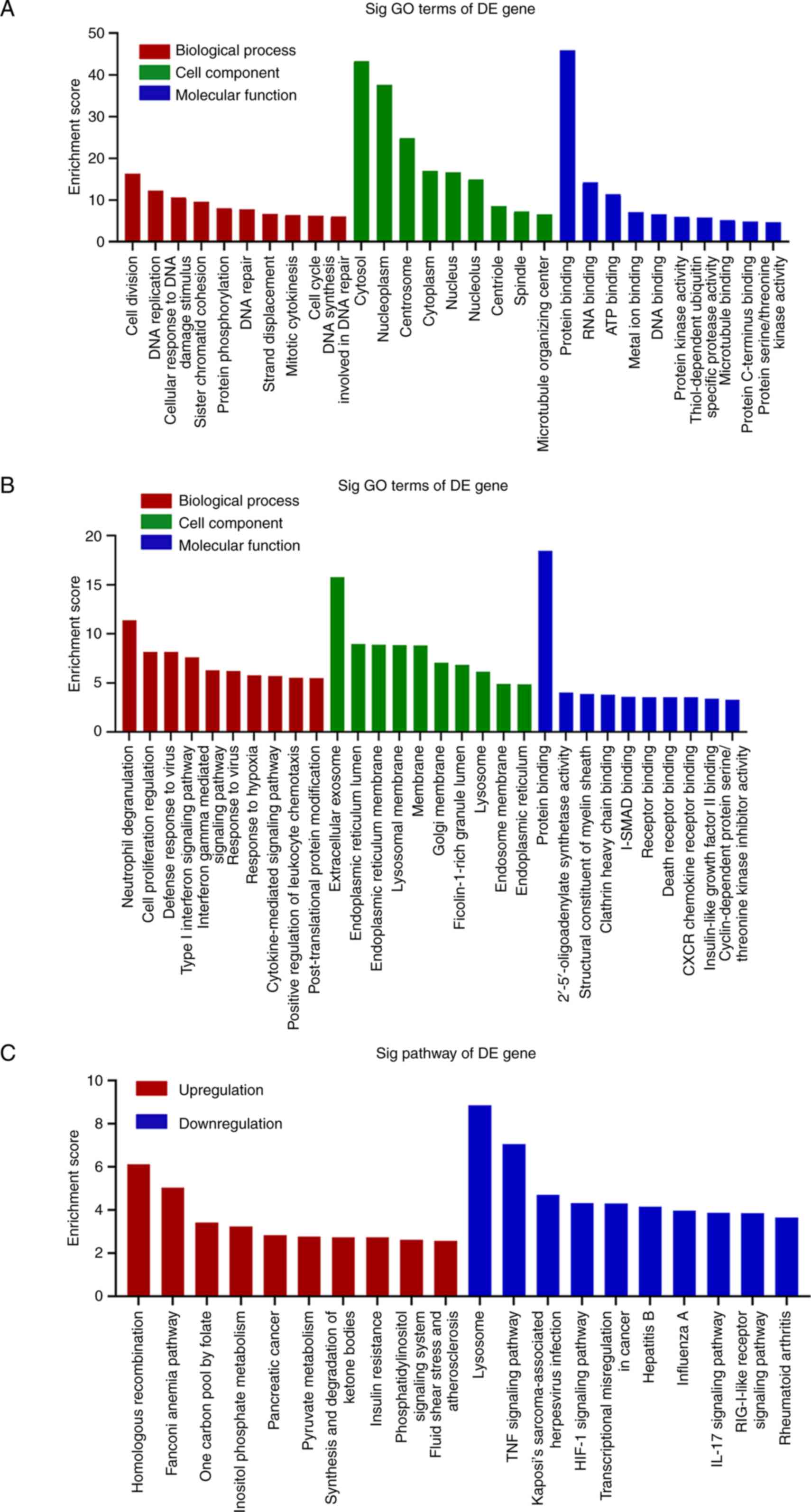
GO and KEGG analysis of DEGs
To further investigate whether these differential
genes could serve a role in cancer proliferation, GO and KEGG
analyses were performed to identify the biological functions and
pathways associated with these aberrantly expressed genes. GO
analysis revealed that the biological processes most commonly
associated with the upregulated genes generally took part in the
‘cell division’ and ‘DNA replication’ processes (Figs. 5A and S1A). By contrast, the biological
processes most commonly associated with the downregulated genes
were involved in ‘neutrophil degranulation’ and ‘cell proliferation
regulation’ (Figs. 5B and S1C). The upregulated genes were
primarily associated with the ‘cytosol’, ‘nucleoplasm’ and
‘centrosome’ compartments (Fig.
5A), whereas the downregulated genes were primarily associated
with the ‘extracellular exosome’ and ‘endoplasmic reticulum lumen’
compartments (Fig. 5B). The
molecular functions of the upregulated genes were primarily
associated with ‘protein binding’, ‘RNA binding’ and ‘ATP binding’
(Fig. 5A), whilst those associated
with the downregulated genes included ‘protein binding’ and
‘2′-5′-oligoadenylate synthetase activity’ (Fig. 5B). The pathways of these aberrantly
expression genes were then explored using KEGG analysis. The
pathways of the upregulated genes included ‘homologous
recombination’, the ‘Fanconi anaemia pathway’, ‘one carbon pool by
folate’, ‘inositol phosphate metabolism’, ‘pancreatic cancer’ and
‘pyruvate metabolism’ (Figs. 5C
and S1B). The biological pathways
of the downregulated genes included ‘lysosome’, the ‘TNF signalling
pathway’, the ‘HIF-1 signalling pathway’, ‘transcriptional
misregulation in cancer’ and ‘hepatitis B’ (Figs. 5C and S1D).
Prediction of target proteins
following TRPC5OS overexpression
To identify the potential target proteins following
TRPC5OS overexpression, Co-IP was performed. There was an obvious
band near 40 kDA in TRPC5OS overexpression group which indicated
that the molecular weight of the protein interacted with TRPC5OS
was close to 40 kDA (Fig. S2)
before the targeted proteins were predicted based on LC-MS/MS
analysis. Differential proteins that were identified at least twice
out of the three runs were selected. The results revealed that 17
potential interacting targets of TRPC5OS were identified, including
Annexin A1 (ANXA1), peroxiredoxin 1 (PRDX1), enolase 1 (ENO1), LDHB
(lactate dehydrogenase B), YWHAQ (tyrosine
3-monooxygenase/tryptophan 5–monooxygenase activation protein
theta), FLNC (filamin C), VDAC1 (voltage dependent anion channel
1), eukaryotic translation elongation factor 1 γ (EEF1G), ribosomal
protein L7a), RPS3 (ribosomal protein S3), PCBP2 [poly(rC) binding
protein 2], CCT6A (chaperonin containing TCP1 subunit 6A), CS
(citrate synthase), HNRNPD (heterogeneous nuclear ribonucleoprotein
D), AKR7A2 (aldo-keto reductase family 7 member A2), CALR
(calreticulin) and LCN1 (lipocalin 1) (Table SIII). Subsequently, PPI network
analysis was performed to analyse the interactome of these 17
differentially-expressed proteins. Proteins with the most
interactions were selected, including ENO1, PRDX1 and EEF1G
(Fig. 6A).
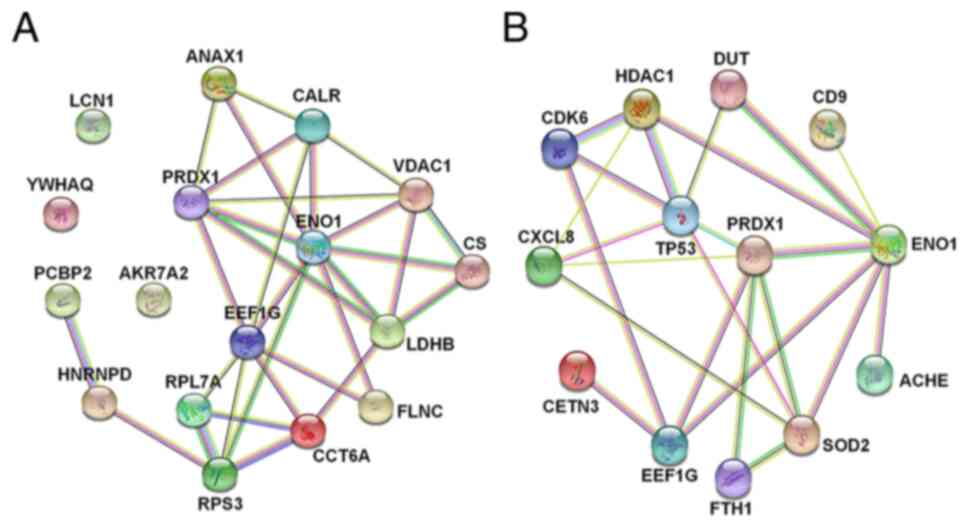 | Figure 6.PPI network analysis. (A) PPI network
of the targeted proteins was established (ANXA1, PRDX1, ENO1, LDHB,
YWHAQ, FLNC, VDAC1, EEF1G, RPL7A, RPS3, PCBP2, CCT6A, CS, HNRNPD,
AKR7A2, CALR and LCN1). (B) Genes in the biological processes of
Gene Ontology analysis that interacted closely with the targeted
proteins were subjected to PPI analysis. PPI, protein-protein
interaction; ANXA1, Annexin A1; peroxiredoxin 1 (PRDX1), enolase 1
(ENO1), LDHB (lactate dehydrogenase B), YWHAQ (tyrosine
3-monooxygenase/tryptophan 5-monooxygenase activation protein
theta), FLNC, filamin C; VDAC1 (voltage dependent anion channel 1),
EEF1G, eukaryotic translation elongation factor 1 γ; RP, ribosomal
protein; PCBP2 [poly(rC) binding protein 2], CCT6A (chaperonin
containing TCP1 subunit 6A), CS (citrate synthase), HNRNPD
(heterogeneous nuclear ribonucleoprotein D), AKR7A2 (aldo-keto
reductase family 7 member A2), CALR (calreticulin) and LCN1
(lipocalin 1). |
Interactions between the DEGs and
proteins
Subsequently, three biological processes associated
with proliferation from GO analysis were selected, namely cell
division, RNA replication and negative regulation of cell
proliferation. The genes enriched in these biological processes
were then subjected to PPI analysis with the target proteins. The
genes that interacted with the target proteins are shown in
Fig. 6B. The results showed that
the interaction relationship among tumour protein p53, histone
deacetylase 1 (HDAC1) and superoxide dismutase 2 (SOD2), all of
which are associated with proliferation, were the most significant.
Deletion of TP53 has been found to promote glycolysis and tumour
cell proliferation (20). A
previous study showed that HDAC1 can mediate the acetylation of
ENO1, the upregulation of which could promote the biological
activity of ENO1 (21) to in turn
increase the proliferation of MDA-MB-231 and MCF-7 cells. SOD2 is a
mitochondrial enzyme that decreases the levels of reactive oxygen
species (ROS) in the mitochondria (22). Low levels of ROS exerts a negative
regulatory role on proliferation (23). To conclude, these DEGs
aforementioned were all associated with cancer proliferation.
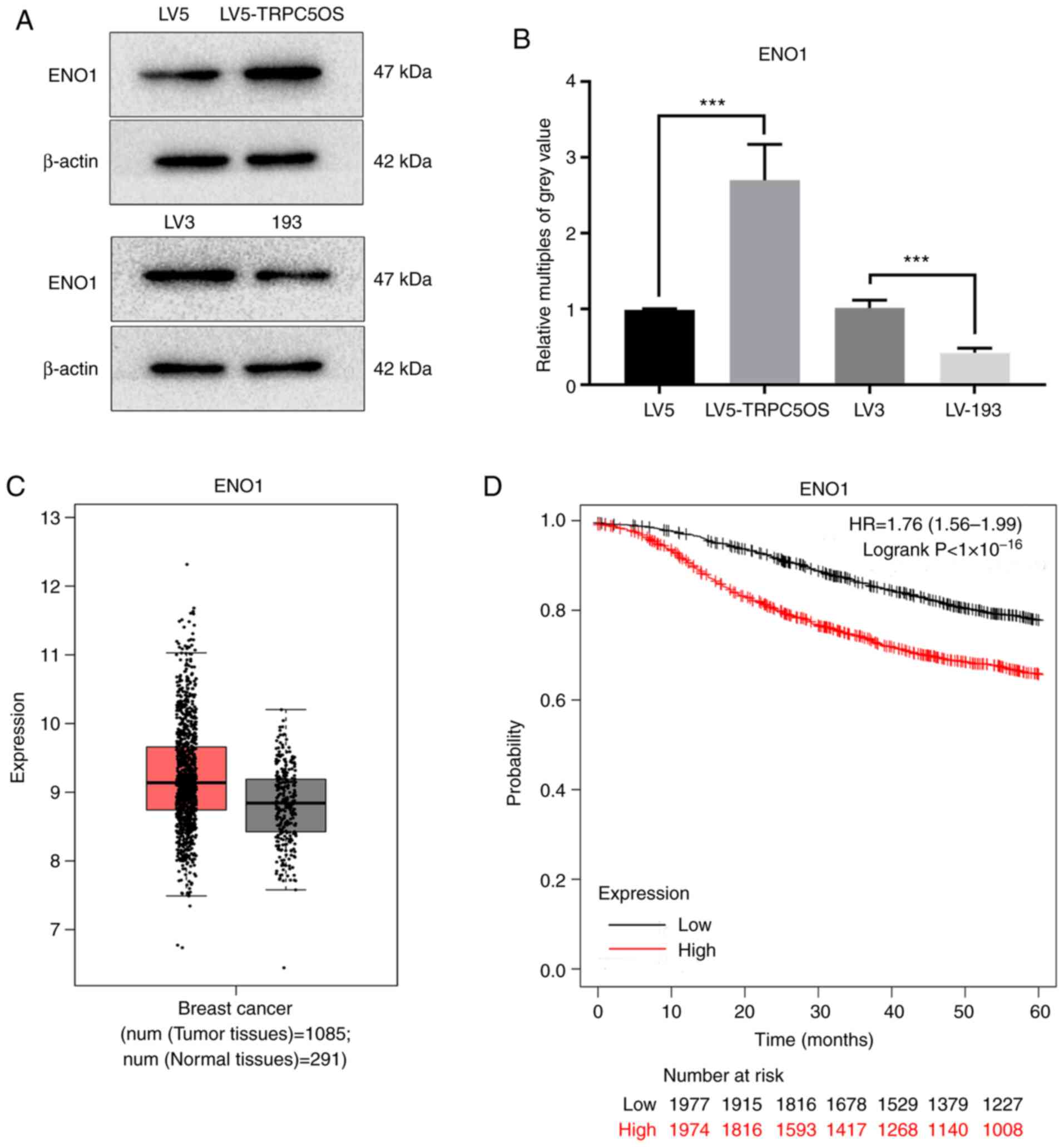
ENO1 may be a potential target protein
mediated by TRPC5OS
Due to it exhibiting the most interactions with
other target genes and proteins, ENO1 was chosen fo examination as
a potential target protein of TRPC5OS. Western blotting revealed
that the protein expression of ENO1 was significantly increased
following TRPC5OS overexpression, but was significantly decreased
following TRPC5OS knockdown compared with that in their
corresponding negative controls (Fig.
7A and B). Expression of ENO1 was markedly higher in tumour
tissues compared with that in the normal tissues based on GEPIA 2
analysis (Fig. 7C). Kaplan-Meier
curves and log-rank tests of the overall survival of patients with
breast cancer revealed that high expression levels of ENO1 were
associated with poorer prognoses (Fig.
7D). Therefore, these data suggest that TRPC5OS may regulate
the expression of ENO1, which may in turn be associated with less
favourable outcomes in patients with breast cancer.
Discussion
At present, anthracyclines and taxanes are the
primary treatment methods for breast cancer patients (24). However, the prognosis of patients
with TNBC remains poor (25).
Therefore, it remains necessary to identify novel targets for the
treatment of TNBC. In a previous study, it was found that the
levels of TRPC5OS expression were the highest in MDA-MB-231 cells,
where overexpression of TRPC5OS could promote proliferation and
viability. Therefore, the present study investigated the potential
function mechanism of TRPC5OS by overexpressing it or knocking down
its expression in breast cancer cells.
The proliferative ability of MDA-MB-231 and MCF-7
cells was first measured after overexpressing TRPC5OS. The results
revealed that TRPC5OS overexpression promoted the proliferation of
the breast cancer cells and TRPC5OS knockdown inhibited the
proliferation of breast cancer cells. Next, high-throughput
sequencing of samples from TRPC5OS-overexpressing MDA-MB-231 cells
and cells from the LV5 control group was performed. MDA-MB-231
cells are TNBC cells (26).
Current treatment methods for breast cancer include surgery,
chemotherapy, radiotherapy, endocrine therapy and targeted therapy
(27,28). However, due to the lack of ER, PR
and HER-2 expression (29), TNBC
is characterized by a unique molecular profile, metastatic pattern,
aggressive behavior and lack of an effective targeted therapy, lead
to poor prognosis (3–5). Targeting TRPC5OS for therapy may
markedly improve the outcome of patients with TNBC. Therefore,
MDA-MB-231 cells was used for RNA sequencing. Sequencing with only
one cell line has been reported in other studies (30,31).
In addition, it was found that TRPC5OS also served as a
cancer-promoting role in MCF-7 cells, which is non-TNBC by using
EdU) assay. However, in future studies, the interactions between
TRPC5OS and target proteins in MCF-7 cells will need to be
assessed.
The present study found that there were 5,256 DEGs
between the two groups. Among these, 3,269 genes were upregulated
and 1,987 genes were downregulated (fold change threshold >1.5).
Subsequently, these DEGs were analysed using GO and KEGG. GO
analysis revealed that the main biological processes involving
these upregulated genes were ‘cell division’ and ‘DNA replication’,
whereas the main biological processes involving the downregulated
genes were ‘neutrophil degranulation’ and ‘cell proliferation
regulation’. Cell division and DNA replication are closely
associated with cell cycle progression and proliferation (32–34),
where dysregulation can lead to carcinogensis (35,36).
Before DNA replication in the cell cycle, cells need to enter the
G1 phase and release a large number of intracellular
signals to regulate cell division (37). During this process, in cases of
dysregulation, such as insufficient or excessive chromosomal DNA
replication, then cell division and proliferation would not be
tightly regulated, resulting in the development of cancer (36).
Subsequent KEGG results showed that the main
pathways these upregulated genes were associated with primarily
took part in ‘homologous recombination’, whereas for the
downregulated genes, they primarily took part in ‘lysosome’ and
‘TNF signalling pathways’. Gross chromosome rearrangement (GCR) and
copy-number variations (CNVs) are the result of incorrect DNA
replication and are frequently observed features of cancer
(38). Homologous recombination
can cause replication restarts, where such recombination-restarted
forks have high probabilities of error, leading to DNA replication
errors, production of GCRs and gene amplification in cancer
(28). In addition, homologous
recombination can result in the production of non-recurrent CNVs in
genomic diseases. HR proteins associate with the nascent strand
behind the collapsed fork and subsequent strand invasion at the
collapse site facilitates accurate HR-dependent fork restart with
the correct template (39). There
is evidence that the lysosome pathway can regulate cancer
proliferation (40). The
proliferation of cancer cells leads to energy depletion, which in
turn affects their proliferation (41). To overcome this obstacle, cancer
cells typically use lysosomes to degrade macromolecules obtained
from the microenvironment to produce ATP and synthesise metabolic
substrates (42).
In the present study, GO and KEGG analyses revealed
that biological processes and pathways are closely associated with
proliferation. Therefore, the potential target proteins of TRPC5OS
were next explored. Co-IP followed by LC-MS/MS analysis was thereby
performed, following which proteins that were detected at least
twice were used to establish a PPI network. From the analysis of
the PPI network, three target proteins were selected (ENO1, PRDX1
and EEF1G) that had the most interactions and therefore were the
most likely to be associated with cancer proliferation. ENO1
functions as a glycolytic enzyme in glycolysis (43,44).
Accumulating evidence has reported that ENO1 is closely associated
with tumour development (45–47).
ENO1 can regulate the proliferation, migration and invasion of
glioma cells through the PI3K/Akt pathway (48). PRDX1 is a member of the
peroxiredoxin family of antioxidant enzymes (49). The biological function of PRDX1
primarily depends on the proteins that interact with PRDX1,
including macrophage migration inhibitory factor (MIF) and
peptidylprolyl isomerase A (PPIA) (50). MIF can inhibit the peroxidase
activity of PRDX1, whereas PPIA binds to all isoforms of PRDXs to
enhance antioxidant activity, leading to tumorigenesis (50). EEF1G is a member of the EEF1
family, which transfers aminoacyl tRNAs to the ribosome (51). Accumulating evidence has shown that
EEF1G serves a key role in cancer development (52,53).
EEF1G has also been associated with oncogene-induced aging, which
is an important mechanism that inhibits tumour formation, to
prevent the uncontrolled proliferation of tumour cells under the
action of abnormal carcinogenic signals (53). Taken together, it was suggested
that ENO1, PRDX1 and EEF1G are all associated with tumorigenesis
through the PI3K/AKT pathway, antioxidant activity alterations and
OIS, respectively. In addition, interactions between differential
genes and proteins were analysed based on the PPIs. Amongst ENO1,
PRDX1 and EEF1G, given that ENO1 was closely associated with
proliferation and had the most interactions with other target genes
and proteins, it was considered to be a potential target protein
downstream of TRPC5OS.
In conclusion, the function, biological processes
and signalling pathways associated with TRPC5OS in TNBC were
investigated in the present study, In addition, potential target
proteins and interactions between differential genes and target
proteins were analysed, which provided guidance and direction for
future studies experiments. However, the lack of analysis in >
one sample per cell line for RNA sequencing is a limitation to the
present study. Additional samples should be analyzed to rule out
any false positive. Results from the present study suggest that
TRPC5OS may serve as a novel therapeutic target for the treatment
of breast cancer. Therefore, exploring the mechanisms by which
TRPC5OS regulates TNBC cell proliferation may provide novel
insights for the development of clinically viable therapeutic
strategies.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was financially supported by the National
Natural Science Foundation of China (grant nos. 81672612 and
81903653), Project of Invigorating Health Care through Science,
Technology and Education (Jiangsu Provincial Medical Youth Talent;
grant nos. QNRC2016095 and FRC201758), the Project of Nanjing
Medical and Science Development (grant nos. 201803014 and YK17179)
and the Beijing Medical Award Foundation (grant no.
YXJL-2021-0028-0279).
Availability of data and materials
The data generated in the present study may be found
in the Gene Expression Omnibus under accession number GSE181330 or
at the following URL: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE181330.
Authors' contributions
SW, and ZYF participated in the design of the study.
XH provided the idea of the project and participated in the design
of the experimental scheme. JHP, YH, SBP, YQX, MJZ, WBZ and YYC
performed the experiments. MMW, XWW, HX, LLY, XH and HTF
contributed to data collection and analysis. HX and ZYF confirm the
authenticity of all the raw data. All authors were involved in the
writing of the article. All authors have read and approved the
final manuscript.
Ethics approval and consent to
participate
The protocols involving patients were approved by
the Ethics Committees of the First Hospital Affiliated Hospital
with Nanjing Medical University (approval no. 2010-SR-091). The
patients recruited in this research provided written informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
DeSantis CE, Ma J, Gaudet MM, Newman LA,
Miller KD, Sauer AG, Jemal A and Siegel RL: Breast cancer
statistics, 2019. CA Cancer J Clin. 69:438–451. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bauer KR, Brown M, Cress RD, Parise CA and
Caggiano V: Descriptive analysis of estrogen receptor
(ER)-negative, progesterone receptor (PR)-negative, and
HER2-negative invasive breast cancer, the so-called triple-negative
phenotype: A population-based study from the California cancer
registry. Cancer. 109:1721–1728. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kaplan HG, Malmgren JA and Atwood M: T1N0
triple negative breast cancer: Risk of recurrence and adjuvant
chemotherapy. Breast J. 15:454–460. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mersin H, Yildirim E, Berberoglu U and
Gülben K: The prognostic importance of triple negative breast
carcinoma. Breast. 17:341–346. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tan AR and Swain SM: Therapeutic
strategies for triple-negative breast cancer. Cancer J. 14:343–351.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dent R, Trudeau M, Pritchard KI, Hanna WM,
Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P and Narod SA:
Triple-negative breast cancer: Clinical features and patterns of
recurrence. Clin Cancer Res. 13:4429–4434. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cheang MC, Voduc D, Bajdik C, Leung S,
McKinney S, Chia SK, Perou CM and Nielsen TO: Basal-like breast
cancer defined by five biomarkers has superior prognostic value
than triple-negative phenotype. Clin Cancer Res. 14:1368–1376.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Foulkes WD, Smith IE and Reis-Filho JS:
Triple-negative breast cancer. N Engl J Med. 363:1938–1948. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Venkatachalam K and Montell C: TRP
channels. Annu Rev Biochem. 76:387–417. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ma X, Chen Z, Hua D, He D, Wang L, Zhang
P, Wang J, Cai Y, Gao C, Zhang X, et al: Essential role for
TrpC5-containing extracellular vesicles in breast cancer with
chemotherapeutic resistance. Proc Natl Acad Sci USA. 111:6389–6394.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang P, Liu X, Li H, Chen Z, Yao X, Jin J
and Ma X: TRPC5-induced autophagy promotes drug resistance in
breast carcinoma via CaMKKβ/AMPKα/mTOR pathway. Sci Rep.
7:31582017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dong Y, Pan Q, Jiang L, Chen Z, Zhang F,
Liu Y, Xing H, Shi M, Li J, Li XY, et al: Tumor endothelial
expression of P-glycoprotein upon microvesicular transfer of TrpC5
derived from adriamycin-resistant breast cancer cells. Biochem
Biophys Res Commun. 446:85–90. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma X, Cai Y, He D, Zou C, Zhang P, Lo CY,
Xu Z, Chan FL, Yu S, Chen Y, et al: Transient receptor potential
channel TRPC5 is essential for P-glycoprotein induction in
drug-resistant cancer cells. Proc Natl Acad Sci USA.
109:16282–16287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fagerberg L, Hallström BM, Oksvold P,
Kampf C, Djureinovic D, Odeberg J, Habuka M, Tahmasebpoor S,
Danielsson A, Edlund K, et al: Analysis of the human
tissue-specific expression by genome-wide integration of
transcriptomics and antibody-based proteomics. Mol Cell Proteomics.
13:397–406. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wichman L, Somasundaram S, Breindel C,
Valerio DM, McCarrey JR, Hodges CA and Khalil AM: Dynamic
expression of long noncoding RNAs reveals their potential roles in
spermatogenesis and fertility. Biol Reprod. 97:313–323. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu CW, Zhou DD, Xie T, Hao JL, Pant OP, Lu
CB and Liu XF: HOXA11 antisense long noncoding RNA (HOXA11-AS): A
promising lncRNA in human cancers. Cancer Med. 7:3792–3799. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiang M, Qiu N, Xia H, Liang H, Li H and
Ao X: Long noncoding RNA FOXD2AS1/miR1505p/PFN2 axis regulates
breast cancer malignancy and tumorigenesis. Int J Oncol.
54:1043–1052. 2019.PubMed/NCBI
|
|
18
|
Breast Cancer Association Consortium, .
Mavaddat N, Dorling L, Carvalho S, Allen J, González-Neira N,
Keeman R, Bolla MK, Dennis J, Wang Q, et al: Pathology of tumors
associated with pathogenic germline variants in 9 breast cancer
susceptibility genes. JAMA Oncol. 8:e2167442022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hitosugi T, Zhou L, Elf S, Fan J, Kang HB,
Seo JH, Shan C, Dai Q, Zhang L, Xie J, et al: Phosphoglycerate
mutase 1 coordinates glycolysis and biosynthesis to promote tumor
growth. Cancer Cell. 22:585–600. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Arito M, Nagai K, Ooka S, Sato T, Takakuwa
Y, Kurokawa MS, Sase T, Okamoto K, Suematsu N, Kato T, et al:
Altered acetylation of proteins in patients with rheumatoid
arthritis revealed by acetyl-proteomics. Clin Exp Rheumatol.
33:877–886. 2015.PubMed/NCBI
|
|
22
|
Yuan L, Mishra R, Patel H, Abdulsalam S,
Greis KD, Kadekaro AL, Merino EJ and Garrett JT: Utilization of
reactive oxygen species targeted therapy to prolong the efficacy of
BRAF inhibitors in melanoma. J Cancer. 9:4665–4676. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Prasad S, Gupta SC and Tyagi AK: Reactive
oxygen species (ROS) and cancer: Role of antioxidative
nutraceuticals. Cancer Lett. 387:95–105. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
McDonald ES, Clark AS, Tchou J, Zhang P
and Freedman GM: Clinical diagnosis and management of breast
cancer. J Nucl Med. 57 (Suppl 1):9S–16S. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yin L, Duan JJ, Bian XW and Yu SC:
Triple-negative breast cancer molecular subtyping and treatment
progress. Breast Cancer Res. 22:612020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Abdelmalek CM, Hu Z, Kronenberger T,
Küblbeck J, Kinnen FJM, Hesse SS, Malik A, Kudolo M, Niess R,
Gehringer M, et al: Gefitinib-tamoxifen hybrid ligands as potent
agents against triple-negative breast cancer. J Med Chem.
65:4616–4632. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Paskins Z, Bromley K, Lewis M, Hughes G,
Hughes E, Hennings S, Cherrington A, Hall A, Holden MA, Stevenson
K, et al: Clinical effectiveness of one ultrasound guided
intra-articular corticosteroid and local anaesthetic injection in
addition to advice and education for hip osteoarthritis (HIT
trial): Single blind, parallel group, three arm, randomised
controlled trial. BMJ. 377:e0684462022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen P, Ning X, Li W, Pan Y, Wang L, Li H,
Fan X, Zhang J, Luo T, Wu Y, et al: Fabrication of
Tbeta4-exosome-releasing artificial stem cells for myocardial
infarction therapy by improving coronary collateralization. Bioact
Mater. 14:416–429. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schneider BP, Winer EP, Foulkes WD, Garber
J, Perou CM, Richardson A, Sledge GW and Carey LA: Triple-negative
breast cancer: Risk factors to potential targets. Clin Cancer Res.
14:8010–8018. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang P, Li J, Peng C, Tan Y, Chen R, Peng
W, Gu Q, Zhou J, Wang L, Tang J, et al: TCONS_00012883 promotes
proliferation and metastasis via DDX3/YY1/MMP1/PI3K-AKT axis in
colorectal cancer. Clin Transl Med. 10:e2112020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yu J, Wang F, Zhang J, Li J, Chen X and
Han G: LINC00667/miR-449b-5p/YY1 axis promotes cell proliferation
and migration in colorectal cancer. Cancer Cell Int. 20:3222020.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mohamed TMA, Ang YS, Radzinsky E, Zhou P,
Huang Y, Elfenbein A, Foley A, Magnitsky S and Srivastava D:
Regulation of cell cycle to stimulate adult cardiomyocyte
proliferation and cardiac regeneration. Cell. 173:104–116.e12.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pike MC, Spicer DV, Dahmoush L and Press
MF: Estrogens, progestogens, normal breast cell proliferation, and
breast cancer risk. Epidemiol Rev. 15:17–35. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Matson JP and Cook JG: Cell cycle
proliferation decisions: The impact of single cell analyses. FEBS
J. 284:362–375. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shostak A: Circadian clock, cell division,
and cancer: From molecules to organism. Int J Mol Sci. 18:8732017.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Massague J: G1 cell-cycle control and
cancer. Nature. 432:298–306. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bartek J and Lukas J: Pathways governing
G1/S transition and their response to DNA damage. FEBS Lett.
490:117–122. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lupski JR: Genomic disorders: Structural
features of the genome can lead to DNA rearrangements and human
disease traits. Trends Genet. 14:417–422. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mizuno K, Miyabe I, Schalbetter SA, Carr
AM and Murray JM: Recombination-restarted replication makes
inverted chromosome fusions at inverted repeats. Nature.
493:246–249. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nowosad A and Besson A: Lysosomes at the
crossroads of cell metabolism, cell cycle, and stemness. Int J Mol
Sci. 23:22902022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Umeda S, Kanda M, Shimizu D, Nakamura S,
Sawaki K, Inokawa Y, Hattori N, Hayashi M, Tanaka C, Nakayama G and
Kodera Y: Lysosomal-associated membrane protein family member 5
promotes the metastatic potential of gastric cancer cells. Gastric
Cancer. 25:558–572. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Finicle BT, Jayashankar V and Edinger AL:
Nutrient scavenging in cancer. Nat Rev Cancer. 18:619–633. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kang HJ, Jung SK, Kim SJ and Chung SJ:
Structure of human alpha-enolase (hENO1), a multifunctional
glycolytic enzyme. Acta Crystallogr D Biol Crystallogr. 64:651–657.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cappello P, Principe M, Bulfamante S and
Novelli F: Alpha-enolase (ENO1), a potential target in novel
immunotherapies. Front Biosci (Landmark Ed). 22:944–959. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Principe M, Borgoni S, Cascione M,
Chattaragada MS, Ferri-Borgogno S, Capello M, Bulfamante S,
Chapelle J, Modugno FD, Defilippi P, et al: Alpha-enolase (ENO1)
controls alpha v/beta 3 integrin expression and regulates
pancreatic cancer adhesion, invasion, and metastasis. J Hematol
Oncol. 10:162017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ji M, Wang Z, Chen J, Gu L, Chen M, Ding Y
and Liu T: Up-regulated ENO1 promotes the bladder cancer cell
growth and proliferation via regulating beta-catenin. Biosci Rep.
39:BSR201905032019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang J, Li H, Miao L and Ding J:
Silencing of ENO1 inhibits the proliferation, migration and
invasion of human breast cancer cells. J BUON. 25:696–701.
2020.PubMed/NCBI
|
|
48
|
Song Y, Luo Q, Long H, Hu Z, Que T, Zhang
X, Li Z, Wang G, Yi L, Liu Z, et al: Alpha-enolase as a potential
cancer prognostic marker promotes cell growth, migration, and
invasion in glioma. Mol Cancer. 13:652014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Min Y, Kim MJ, Lee S, Chun E and Lee KY:
Inhibition of TRAF6 ubiquitin-ligase activity by PRDX1 leads to
inhibition of NFKB activation and autophagy activation. Autophagy.
14:1347–1358. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Coumans JV, Gau D, Poljak A, Wasinger V,
Roy P and Moens PD: Profilin-1 overexpression in MDA-MB-231 breast
cancer cells is associated with alterations in proteomics
biomarkers of cell proliferation, survival, and motility as
revealed by global proteomics analyses. OMICS. 18:778–791. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Biterge-Sut B: Alterations in eukaryotic
elongation factor complex proteins (EEF1s) in cancer and their
implications in epigenetic regulation. Life Sci. 238:1169772019.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Palacios G, Shaw TI, Li Y, Singh RK,
Valentine M, Sandlund JT, Lim MS, Mullighan CG and Leventaki V:
Novel ALK fusion in anaplastic large cell lymphoma involving EEF1G,
a subunit of the eukaryotic elongation factor-1 complex. Leukemia.
31:743–747. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Bengsch F, Tu Z, Tang HY, Zhu H, Speicher
DW and Zhang R: Comprehensive analysis of the ubiquitinome during
oncogene–induced senescence in human fibroblasts. Cell Cycle.
14:1540–1547. 2015. View Article : Google Scholar : PubMed/NCBI
|