Introduction
Global cancer statistics in 2020 revealed that there
were 2.22 million new diagnoses of lung cancer (LC), with a
corresponding death rate of ~1.8 million worldwide (1). Non-small cell LC (NSCLC) accounts for
up to 85% of all cases of LC and is a serious health risk; notably,
~60% of these patients are diagnosed with locally advanced NSCLC
and the current standard of care is radiotherapy-based combination
therapy (2). The survival rate of
patients following treatment with standard regimens has improved
but still remains unsatisfactory (3). Immune checkpoint inhibitors (ICIs)
are a relatively novel approach to the treatment of NSCLC. The
increased use of immunotherapy has elevated 5-year survival rates
in patients with NSCLC from 5 to 26% (4). Anti-programmed cell death protein-1
(PD-1) monotherapy is currently the most used immunotherapy for the
treatment of malignant tumors (5).
In addition, programmed death ligand-1 (PD-L1) is considered the
best predictive biomarker for anti-PD-1 treatment (6,7).
Nevertheless, PD-L1 assessments are associated with challenges that
include equipment that may yield inconsistent findings, high
fluctuation in detectable levels, tumor heterogeneity, puncture
biopsy limitations and high testing costs (8).
Tumor mutation burden (TMB) may predict
immunotherapy outcomes (9).
Results from phase III clinical trials that included patients with
advanced NSCLC with high TMB revealed a better objective response
rate (ORR; 47 vs. 28%) and median progression-free survival (PFS;
9.7 vs. 5.8 months) following treatment with nivolumab compared
with after chemotherapy (10). In
2017, microsatellite instability and mismatch repair defects were
proposed as immunomarkers associated with prognosis in colorectal
and endometrial cancer; however, their value in NSCLC remains
unclear due to their low expression levels in this disease
(11). Tumor-infiltrating
lymphocytes (TILs) at high density may recognize tumor cells and
increase their sensitivity to checkpoint inhibition. In patients
with advanced NSCLC (n=366) receiving nivolumab or pembrolizumab,
mesenchymal TILs had the greatest impact on long-term survival and
their levels were better predictors of outcomes than PD-L1 levels
(12). An increase in TIL levels
during treatment may help predict clinical and radiological
response; however, more clinical studies are needed to confirm
these findings. Patients with various genetic mutations respond
differently to immunotherapy (13). It has been shown that patients with
epidermal growth factor receptor (EGFR) mutations respond poorly to
immunotherapy (14). However,
patients with KRAS mutations tend to a have high (>50%)
probability of PD-L1 expression, high density of active TILs and
relatively high TMB values, which are associated with clinical
benefit (15). Although the use of
these immunomarkers is recommended by applicable clinical
guidelines, they are cumbersome and expensive to obtain, suggesting
a need for biomarkers that are straightforward and cost-effective,
and which may help improve outcomes by allowing for accurate
screening of patients most likely to benefit from a particular
treatment.
Recently, new host-related biomarkers have gained
attention, including lactic dehydrogenase levels (8), intestinal microecology profiles
(16,17) and peripheral serological indicators
(18), which may help
prognosticate multisystem malignancies, including NSCLC, and help
assess antitumor immune response. Peripheral serological indicators
are novel tumor markers associated with prognosis in multiple
malignant tumors, which have been used as biomarkers to predict the
efficacy of ICIs in gastric cancer (19) and malignant melanoma (20). However, to the best of our
knowledge, no studies have comprehensively investigated the role of
serologically based inflammatory indicators in patients with stage
IIIB-IV NSCLC undergoing PD-1 immunotherapy. Considering clinical
applicability, only two inflammatory indexes, PLR and NLR, were
examined in the present study.
Materials and methods
Patient selection
A total of 133 patients admitted to the Department
of Respiratory Medicine and Oncology of The First Affiliated
Hospital of Gannan Medical College (Ganzhou, China) between January
2019 and February 2021 were selected for the present study.
Inclusion criteria were as follows: i) Histologically or
cytologically confirmed diagnosis of NSCLC; ii) stage IIIB-IV
NSCLC, with at least one measurable lesion, based on imaging
findings and the 8th edition of the TNM staging criteria customized
by the International Association for the Study of Lung Cancer
(21); iii) treatment with a
first-line PD-1 monoclonal antibody; iv) complete serological
indicator data obtained within 1 week prior to receiving PD-1
monotherapy; v) receiving at least four cycles of PD-1 monotherapy;
vi) age ≥18 years; vii) complete clinicopathological and follow-up
information available. Patients with the following characteristics
were excluded: i) Infectious or inflammatory disease within 4 weeks
prior to admission; ii) recent history of antibiotic or hormone
treatment; iii) autoimmune disease or hematologic cancer; iv) bone
marrow suppression of grade II or higher 1 week prior to treatment
with a PD-1 monoclonal antibody.
The Institutional Research Board of The First
Affiliated Hospital of Gannan Medical College waived the
requirement for informed consent for the present study because it
involved only the analysis of an existing dataset and not the
collection of data related to the intervention.
Clinical features
Data on the following characteristics were extracted
from medical records: Sex, age, pathology type, disease stage,
distant metastasis site, brain metastasis status, bone metastasis
status, liver metastasis status, PD-L1 expression level, the
Eastern Cooperative Oncology Group Performance (ECOG) score
(22), driver gene mutation
status, immunotherapy type, PLR values, NLR values, immunotherapy
regimen (monotherapy or combination therapy) and immune-related
adverse event (irAE) incidence. Data on serological indicators were
obtained from routine blood tests performed within 1 week before
the start of anti-PD-1 immunotherapy.
Efficacy evaluation
After four to six cycles of treatment, curative
effect was evaluated by imaging examinations based on the
Immune-Modified Response Evaluation Criteria In Solid Tumors
(23). Complete remission (CR) was
confirmed when all lesions disappeared, tumor markers returned to
normal levels for 4 weeks and no new lesions appeared. Partial
response (PR) was confirmed when the sum of the maximum diameter of
the tumor was reduced by >30% from baseline and maintained at
this value for 4 weeks, while some non-target lesions remained, and
tumor markers did or did not return to normal levels. Immunity
unconfirmed progressive disease (PD) was defined as an increase in
the original lesion size of >20% or appearance of new lesions,
and its efficacy needed to be evaluated after at least 4 weeks of
treatment. Confirmed disease progression immunity confirmed PD was
performed to confirm progress at least 4 weeks later. Patients who
did not achieve PR and did not present with evidence indicative of
PD were classified as having stable disease (SD). ORR was
calculated as follows: ORR (%)=(CR + PR)/(CR +PR + SD + PD) × 100.
Disease control rate (DCR) was calculated as follows: DCR (%)=(CR +
PR + SD)/(CR +PR + SD + PD) × 100. PFS was measured from the start
of drug administration to the point of any signs of disease
progression, or death from any cause or the end of the observation
period, whichever occurred first. Overall survival (OS) was
measured from the start of immunotherapy to death or study end,
whichever occurred first. Side effects were evaluated according to
the National Cancer Institute Common Terminology Criteria for
Adverse Events 5.0 grading scale (score 1–5) (24).
Statistical analysis
Comparison of qualitative information between the
two groups was performed using the χ2 test or Fisher's
exact probability method. NLR and PLR values were used to draw
receiver operating characteristic (ROC) curves and calculate the
area under the curve (AUC) to evaluate the prognostic sensitivity
and specificity of parameters. Univariate and multivariate analyses
were performed using logistic regression models to identify
independent factors associated with irAEs. The Kaplan-Meier method
was used to evaluate any relationships between these parameters and
PFS and OS rates, and the log-rank method was chosen to test for
differences between groups. Univariate analysis was used to
identify prognostic factors associated with outcomes. Variables
that were statistically significant in univariate analyses were
included in multivariate Cox regression models. Finally, nomograms
were drawn based on the results of multivariate regression
analysis, and the predictive accuracy of the model was evaluated.
All analyses were performed in GraphPad Prism 8.0.1 (GraphPad
Software, Inc.) and R v.3.0.2 (R Project for Statistical Computing;
www.r-project.org). P<0.05 was considered to
indicate a statistically significant difference.
Results
Patient characteristics
The median follow-up time was 15.7 (range,
8.93-43.77) months. A total of 85.0% of the patients were male
(overall mean age, 58.80±0.896 years). A total of 97.0% of the
patients had an ECOG score of 0–1 points. The distribution of
genetic mutations was as follows: 79.7, and 12.8 and 7.5% patients
had no genetic mutations or lacked data on mutations, had EGFR
mutations or had ALK/ROS1 mutations, respectively. In addition,
13.6% of the patients were positive for PD-L1 expression. Moreover,
81.2% of the patients had distant metastases, including liver
(15.0%), brain (18.0%) and bone (44.4%) metastases (Table I).
 | Table I.Baseline clinical characteristics of
patients with NSCLC treated with PD-1 inhibitors. |
Table I.
Baseline clinical characteristics of
patients with NSCLC treated with PD-1 inhibitors.
| Clinical
characteristic | Overall number
(%) | H-PLR, n (%) | L-PLR, n (%) | P-value | H-NLR, n (%) | L-NLR, n (%) | P-value |
|---|
| Total | 133 | 67 | 66 |
| 65 | 68 |
|
| Sex |
|
|
| 0.971 |
|
| 0.707 |
|
Male | 113 (85.0) | 57 (85.1) | 56 (84.8) |
| 56 (86.2) | 57 (83.8) |
|
|
Female | 20 (15.0) | 10 (14.9) | 10 (15.2) |
| 9 (13.8) | 11 (16.2) |
|
| Age, years |
|
|
| 0.925 |
|
| 0.528 |
|
≤60 | 72 (54.1) | 36 (53.7) | 36 (54.5) |
| 37 (56.9) | 35 (51.5) |
|
|
>60 | 61 (45.9) | 31 (46.3) | 30 (45.5) |
| 28 (43.1) | 33 (48.5) |
|
| ECOG |
|
|
| >0.999 |
|
| 0.358 |
|
0-1 | 129 (97.0) | 65 (97.0) | 64 (97.0) |
| 62 (95.4) | 67 (98.5) |
|
| 2 | 4 (3.0) | 2 (3.0) | 2 (3.0) |
| 3 (4.6) | 1 (1.5) |
|
| History |
|
|
| 0.763 |
|
| 0.059 |
|
LUAD | 72 (54.1) | 36 (53.7) | 36 (54.5) |
| 38 (58.5) | 34 (50.0) |
|
|
LUSC | 57 (42.9) | 28 (41.8) | 29 (43.9) |
| 25 (38.5) | 32 (47.0) |
|
|
Others | 4 (3.0) | 3 (4.5) | 1 (1.6) |
| 2 (3.0) | 2 (3.0) |
|
| Stage |
|
|
| 0.479 |
|
| 0.923 |
|
IIIB | 25 (18.8) | 11 (16.4) | 14 (21.2) |
| 12 (18.5) | 13 (19.1) |
|
| IV | 108 (81.2) | 56 (85.6) | 52 (78.8) |
| 53 (81.5) | 55 (80.9) |
|
| Genetic
mutations |
|
|
| 0.586 |
|
| 0.063 |
|
Negative/not tested | 106 (79.7) | 51 (76.1) | 55 (83.3) |
| 47 (72.3) | 59 (86.8) |
|
|
EGFR(+) | 17 (12.8) | 10 (14.9) | 7 (10.6) |
| 10 (15.4) | 7 (10.3) |
|
|
ALK/ROS1(+) | 10 (7.5) | 6 (9.0) | 4 (6.1) |
| 8 (12.3) | 2 (2.9) |
|
| Numbers of
metastatic sites |
|
|
| 0.939 |
|
| 0.020a |
|
<3 | 75 (56.4) | 38 (56.7) | 37 (56.1) |
| 30 (46.2) | 45 (66.2) |
|
| ≥3 | 58 (43.6) | 29 (43.3) | 29 (43.9) |
| 35 (53.8) | 23 (33.8) |
|
| Liver
metastasis |
|
|
| 0.602 |
|
| 0.913 |
| No | 113 (85.0) | 58 (86.6) | 55 (83.3) |
| 55 (84.6) | 58 (85.3) |
|
|
Yes | 20 (15.0) | 9 (13.4) | 11 (16.7) |
| 10 (15.4) | 10 (14.7) |
|
| CNS metastasis |
|
|
| 0.682 |
|
| 0.140 |
| No | 109 (82.0) | 54 (80.6) | 55 (83.3) |
| 50 (76.9) | 59 (86.8) |
|
|
Yes | 24 (18.0) | 13 (19.4) | 11 (16.7) |
| 15 (23.1) | 9 (13.2) |
|
| Bone
metastasis |
|
|
| 0.426 |
|
| 0.071 |
| No | 74 (55.6) | 35 (52.2) | 39 (59.1) |
| 31 (47.7) | 43 (63.2) |
|
|
Yes | 59 (44.4) | 32 (47.8) | 27 (40.9) |
| 34 (52.3) | 25 (36.8) |
|
| Line of
therapy |
|
|
| 0.714 |
|
| 0.647 |
| 1 | 73 (54.8) | 36 (53.7) | 37 (56.1) |
| 33 (50.8) | 40 (58.8) |
|
| 2 | 30 (22.6) | 17 (25.4) | 13 (19.7) |
| 16 (24.6) | 14 (20.6) |
|
| ≥3 | 30 (22.6) | 14 (20.9) | 16 (24.2) |
| 16 (24.6) | 14 (20.6) |
|
| PD-L1 |
|
|
| 0.072 |
|
| 0.564 |
|
Negative/not tested | 115 (86.4) | 55 (82.1) | 60 (90.9) |
| 55 (84.6) | 60 (88.2) |
|
|
1–49% | 9 (6.8) | 4 (6.0) | 5 (7.6) |
| 4 (6.2) | 5 (7.4) |
|
|
≥50% | 9 (6.8) | 8 (11.9) | 1 (1.5) |
| 6 (9.2) | 3 (4.4) |
|
| Regimen |
|
|
| 0.800 |
|
| 0.727 |
|
Combination therapy | 102 (76.7) | 52 (77.6) | 50 (75.8) |
| 49 (75.4) | 53 (77.9) |
|
|
Monotherapy | 31 (23.3) | 15 (22.4) | 16 (24.2) |
| 16 (24.6) | 15 (22.1) |
|
| Immunotherapy
drug |
|
|
| 0.265 |
|
| 0.874 |
|
Pembrolizumab | 14 (10.5) | 11 (16.4) | 3 (4.6) |
| 9 (13.8) | 5 (7.4) |
|
|
Camrelizumab | 27 (20.3) | 14 (20.9) | 13 (19.7) |
| 13 (20.0) | 14 (20.6) |
|
|
Sintilimab | 63 (47.4) | 27 (40.3) | 36 (54.5) |
| 29 (44.6) | 34 (50.0) |
|
|
Tislelizumab | 19 (14.2) | 10 (14.9) | 9 (13.6) |
| 10 (15.4) | 9 (13.2) |
|
|
Toripalimab | 5 (3.8) | 3 (4.5) | 2 (3.0) |
| 2 (3.1) | 3 (4.4) |
|
|
Nivolumab | 5 (3.8) | 2 (3.0) | 3 (4.6) |
| 2 (3.1) | 3 (4.4) |
|
| irAEs |
|
|
| 0.619 |
|
| 0.726 |
| No | 111 (83.5) | 57 (85.1) | 54 (81.8) |
| 55 (84.6) | 56 (82.4) |
|
|
Yes | 22 (16.5) | 10 (14.9) | 12 (18.2) | 0.096 | 10 (15.4) | 12 (17.6) | 0.565 |
| Grade |
|
|
|
|
|
|
|
|
2-3 | 18 (81.8) | 10 (14.9) | 8 (12.1) |
| 9 (13.8) | 9 (13.2) |
|
| 4 | 4 (18.2) | 0 (0.0) | 4 (6.1) |
| 1 (1.5) | 3 (4.4) |
|
ROC curves for PLR, NLR
PLR and NLR values were calculated. Cut-off values
of 200.00 and 3.56 for PLR and NLR, respectively, were determined
based on the median values. PLR, NLR, and combined PLR and NLR all
revealed some prognostic value based on the ROC curve (Fig. 1). The AUC for NLR [0.77; 95%
confidence interval (CI), 0.70-0.85; P<0.0001] was higher than
that for PLR (0.68; 95% CI, 0.59-0.77; P=0.0004) and the combined
PLR and NLR (0.71; 95% CI, 0.62-0.80; P<0.0001).
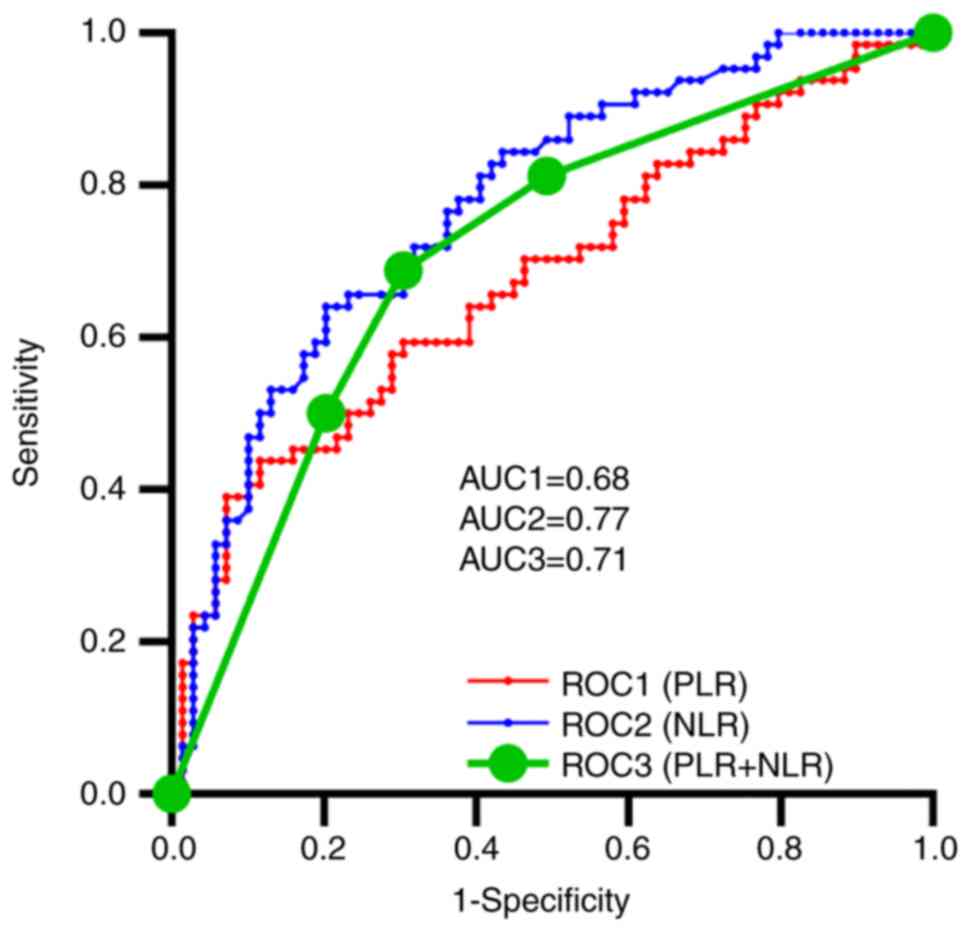 | Figure 1.ROC curves for PLR, NLR, and PLR
combined with NLR with AUC values of 0.68 (95% CI, 0.59-0.77;
P=0.0004), 0.77 (95% CI, 0.70-0.85; P<0.0001) and 0.71 (95% CI,
0.62-0.80; P<0.0001), respectively. AUC, area under the curve;
CI, confidence interval; NLR, neutrophil-lymphocyte ratio; PLR,
platelet-lymphocyte ratio; ROC, receiver operating
characteristic. |
Relationship between PLR, NLR and
recent outcomes
DCR values were 68.66 and 66.67% (P=0.76) and ORR
values were 23.88 and 21.21% in the high-PLR (H-PLR) and low-PLR
(L-PLR) groups, respectively (P=0.61). Disease control rates
(P>0.99) and ORR (P=0.17) were comparable in the high-NLR
(H-NLR) and low-NLR (L-NLR) groups (Table II). In conclusion, the levels of
PLR and NLR did not correlate with DCR and ORR.
 | Table II.Relationship between PLR, NLR and
recent outcomes. |
Table II.
Relationship between PLR, NLR and
recent outcomes.
|
| PLR |
| NLR |
|
|---|
|
|
|
|
|
|
|---|
| Groups | H-PLR | L-PLR | P-value | H-NLR | L-NLR | P-value |
|---|
| Efficacy
evaluation |
|
| 0.93 |
|
| 0.49 |
| CR | 0 (0.00) | 0 (0.00) |
| 0 (0.00) | 0 (0.00) |
|
| PR | 16 (23.88) | 14 (21.21) |
| 12 (18.46) | 18 (26.47) |
|
| SD | 30 (44.78) | 30 (45.45) |
| 32 (49.23) | 28 (41.18) |
|
| PD | 21 (31.34) | 22 (33.34) |
| 21 (32.31) | 22 (32.35) |
|
| ORR (CR + PR) |
|
| 0.61 |
|
| 0.17 |
|
Yes | 16 (23.88) | 14 (21.21) |
| 12 (18.46) | 18 (26.47) |
|
| No | 51 (76.12) | 52 (78.79) |
| 53 (81.46) | 50 (73.53) |
|
| DCR (CR + PR +
SD) |
|
| 0.76 |
|
| >0.99 |
|
Yes | 46 (68.66) | 44 (66.67) |
| 44 (67.69) | 46 (67.65) |
|
| No | 21 (31.34) | 22 (33.33) |
| 21 (32.31) | 22 (32.35) |
|
Clinicopathological factors associated
with irAEs
During the treatment period, 16.5% of the patients
experienced irAEs such as immune-associated pneumonia,
hypoadrenocorticism, immune-associated pituitary inflammation, drug
rash, immune-associated myocarditis and immune-related hepatitis.
The results of univariate analysis showed P<0.1 for age,
immunotherapy modality and PD-L1 expression, but were not
significant. The results of the calibrated multifactorial analysis
revealed a trend whereby outcomes were linked with age [adjusted OR
(aOR), 0.327; 95% CI, 0.105-1.016; P=0.053] and immunotherapy
modality (aOR, 0.348; 95% CI, 0.113-1.071; P=0.066) (Table III).
 | Table III.Logistic analysis of clinical factors
for immune-related adverse events in 133 patients with non-small
cell lung cancer. |
Table III.
Logistic analysis of clinical factors
for immune-related adverse events in 133 patients with non-small
cell lung cancer.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | OR (95% CI) | P-value | Adjusted OR (95%
CI) | P-value |
|---|
| Sex (male vs.
female) | 1.485
(0.314-7.020) | 0.618 |
|
|
| Age (≤65 vs. >65
years) | 0.276
(0.021-0.276) |
0.021a | 0.327
(0.105-1.016) | 0.053b |
| Stage (IIIB-IIIC
vs. IV) | 0.845
(0.225-3.177) | 0.804 |
|
|
| Genetic mutations
(yes vs. no) | 0.875
(0.263-2.909) | 0.828 |
|
|
| PD-L1 (yes vs.
no) | 0.371
(0.123-1.117) |
0.078b | 0.395
(0.119-1.307) | 0.128 |
| Number of
metastatic sites (<3 vs. ≥3) | 2.223
(0.744-6.641) | 0.153 |
|
|
| Liver metastasis
(yes vs. no) | 1.485
(0.314-7.020) | 0.618 |
|
|
| CNS metastasis (yes
vs. no) | 4.250
(0.537-33.610) | 0.170 |
|
|
| Bone metastasis
(yes vs. no) | 1.297
(0.469-3.584) | 0.616 |
|
|
| Line of therapy (1
vs. ≥2) | 1.770
(0.622-5.040) | 0.285 |
|
|
| Regimen
(combination therapy vs. monotherapy) | 0.393
(0.137-1.125) |
0.082b | 0.348
(0.113-1.071) | 0.066b |
| PLR (high vs.
low) | 1.018
(0.377-2.748) | 0.973 |
|
|
| NLR (high vs.
low) | 1.228
(0.452-3.336) | 0.686 |
|
|
Relationship between PLR, NLR and
long-term outcomes
The results of Kaplan-Meier survival analysis showed
a significant advantage of the L-PLR group over the H-PLR group for
both PFS and OS m[edian PFS (mPFS): 8.33 vs. 6.37 months, P=0.02;
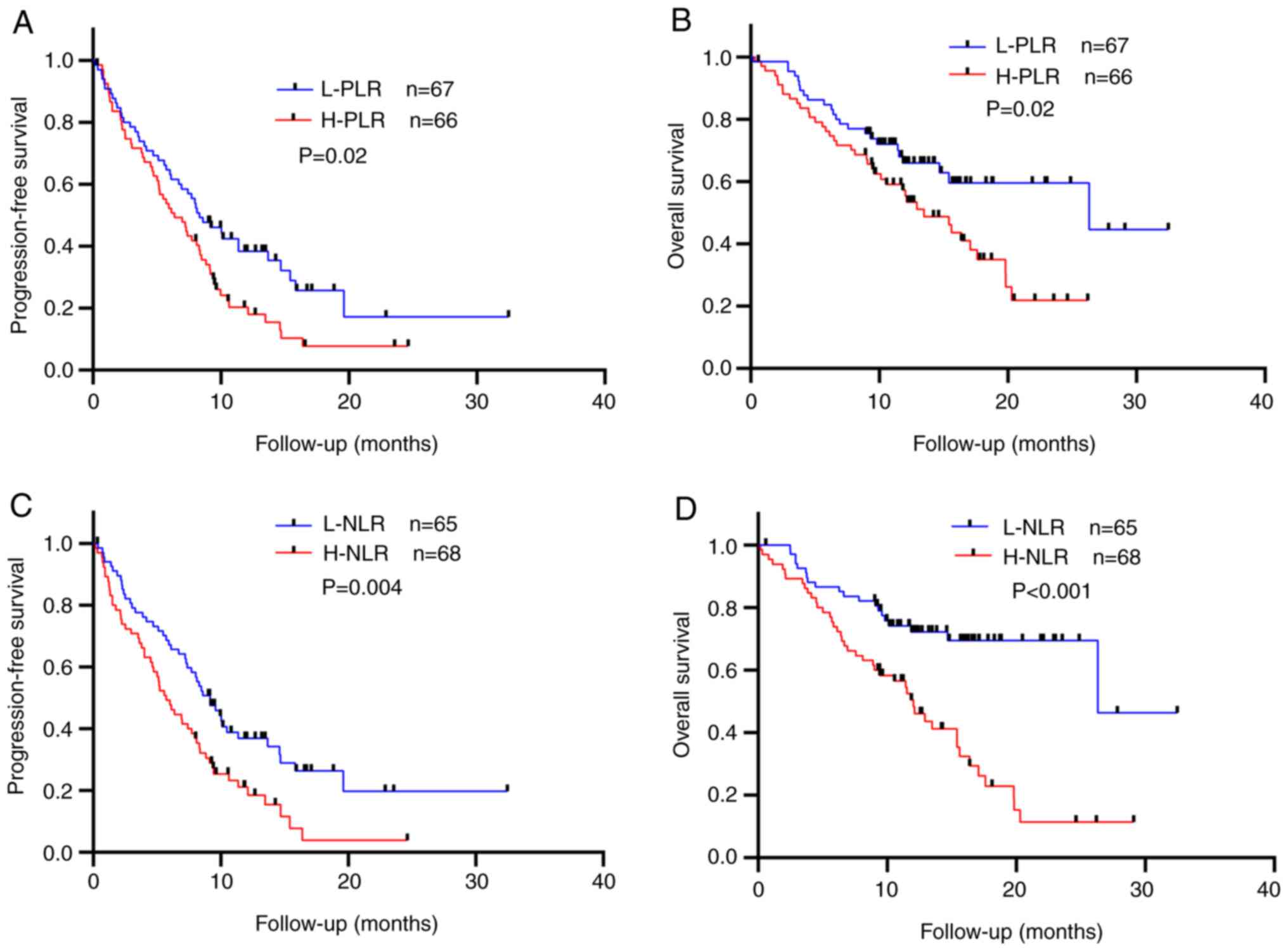
median OS (mOS): 26.33 vs. 13.47 months, P=0.02] (Fig. 2A and B). Similarly, PFS and OS
differed between the L-NLR and H-NLR groups (mPFS: 9.17 vs. 5.73
months, P=0.004; mOS: 26.33 vs. 12.03 months, P<0.001) (Fig. 2C and D). Overall, lower PLR and NLR
values at the start of immunotherapy were associated with improved
outcomes in this patient group.
From the start of the study until October 31, 2021,
a total of 101 patients experienced disease progression, and 64
patients died. The results of the univariate analyses suggested
that an ECOG score of 0–1 [hazard ratio (HR), 0.194; 95% CI,
0.060-0.628; P=0.006], absence of driver mutations (HR, 0.536; 95%
CI, 0.335-0.859; P=0.009), number of metastatic sites <3 (HR,
0.595; 95% CI, 0.401-0.883; P=0.010), L-PLR (HR, 0.632; 95% CI,
0.426-0.940; P=0.023) and L-NLR (HR, 0.565; 95% CI, 0.380-0.841;
P=0.005) reduced the risk of near-term progression in the patients
(Table IV). In univariate
analysis, the ECOG score did not affect OS, but the absence of
liver metastases (HR, 0.573; 95% CI, 0.310-1.059; P=0.076) reduced
the risk of death in the patients, although this was not
significant. Other protective factors for OS included negative
driver mutations (HR, 0.479; 95% CI, 0.279-0.821; P=0.007), number
of metastatic sites <3 (HR, 0.589; 95% CI, 0.360-0.964;
P=0.035), L-PLR (HR, 0.550; 95% CI, 0.329-0.919; P=0.022) and L-NLR
(HR, 0.336; 95% CI, 0.197-0.571; P<0.001) (Table V). A calibrated multifactorial
analysis showed that the ECOG score (HR, 0.613; 95% CI,
0.376-0.999; P=0.049) and number of metastatic sites (HR, 0.627;
95% CI, 0.418-0.940; P=0.024) were associated with the estimated
PFS (Table IV), whereas NLR was
an independent prognostic factor for PFS (HR, 0.201; 95% CI,
0.060-0.670; P=0.009) and OS (HR, 0.413; 95% CI, 0.226-0.754;
P=0.004) in patients with advanced NSCLC receiving immunotherapy
(Tables IV and V).
 | Table IV.Univariate and multivariate analyses
of progression-free survival in patients with non-small cell lung
cancer treated with PD-1 inhibitors. |
Table IV.
Univariate and multivariate analyses
of progression-free survival in patients with non-small cell lung
cancer treated with PD-1 inhibitors.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Sex (male vs.
female) | 0.782
(0.458-1.337) | 0.369 |
|
|
| Age (≤65 vs. >65
years) | 1.265
(0.853-1.877) | 0.243 |
|
|
| ECOG (0–1 vs.
2) | 0.194
(0.060-0.628) |
0.006a | 0.613
(0.376-0.999) | 0.049a |
| Stage (IIIB-IIIC
vs. IV) | 0.682
(0.404-1.151) | 0.378 |
|
|
| Genetic mutations
(yes vs. no) | 0.536
(0.335-0.859) |
0.009a | 0.770
(0.0.487-1.216) | 0.262 |
| PD-L1 (yes vs.
no) | 1.159
(0.860-2.509) | 0.159 |
|
|
| Number of
metastatic sites (<3 vs. ≥3) | 0.595
(0.401-0.883) |
0.010a | 0.627
(0.418-0.940) | 0.024a |
| Liver metastasis
(yes vs. no) | 0.746
(0.436-1.275) | 0.284 |
|
|
| CNS metastasis (yes
vs. no) | 0.890
(0.539-1.469) | 0.649 |
|
|
| Bone metastasis
(yes vs. no) | 0.808
(0.545-1.198) | 0.289 |
|
|
| Line of therapy (1
vs. ≥2) | 0.947
(0.639-1.403) | 0.786 |
|
|
| Regimen
(combination therapy vs. monotherapy) | 1.091
(0.683-1.744) | 0.716 |
|
|
| irAEs (yes vs.
no) | 0.997
(0.599-1.661) | 0.992 |
|
|
| PLR (high vs.
low) | 0.632
(0.426-0.940) |
0.023a | 0.781
(0.500-1.221) | 0.279 |
| NLR (high vs.
low) | 0.565
(0.380-0.841) |
0.005a | 0.201
(0.060-0.670) | 0.009a |
 | Table V.Univariate and multivariate analyses
of overall survival in patients with non-small cell lung cancer
treated with PD-1 inhibitors. |
Table V.
Univariate and multivariate analyses
of overall survival in patients with non-small cell lung cancer
treated with PD-1 inhibitors.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Sex (male vs.
female) | 0.613
(0.332–1.131) | 0.117 |
|
|
| Age (≤65 vs. >65
years) | 1.298
(0.786–2.145) | 0.308 |
|
|
| ECOG (0–1 vs.
2) | 0.865
(0.119–6.269) | 0.885 |
|
|
| Stage (IIIB–IIIC
vs. IV) | 0.559
(0.267–1.174) | 0.124 |
|
|
| Genetic mutations
(yes vs. no) | 0.479
(0.279–0.821) |
0.007a | 0.635
(0.363–1.111) | 0.112 |
| PD-L1 (yes vs.
no) | 1.081
(0.563–2.075) | 0.815 |
|
|
| Numbers of
metastatic sites (<3 vs. ≥3) | 0.589
(0.360–0.964) |
0.035a | 0.440
(0.467–1.392) | 0.440 |
| Liver metastasis
(yes vs. no) | 0.573
(0.310–1.059) |
0.076b | 0.638
(0.330–1.233) | 0.182 |
| CNS metastasis (yes
vs. no) | 0.946
(0.512–1.747) | 0.859 |
|
|
| Bone metastasis
(yes vs. no) | 0.708
(0.433–1.157) | 0.168 |
|
|
| Line of therapy (1
vs. ≥2) | 0.693
(0.424–1.131) | 0.142 |
|
|
| Regimen
(combination therapy vs. monotherapy) | 1.257
(0.689–2.294) | 0.456 |
|
|
| irAEs (yes vs.
no) | 1.718
(0.842–3.507) | 0.137 |
|
|
| PLR (high vs.
low) | 0.550
(0.329–0.919) |
0.022a | 0.566
(0.477–1.498) | 0.566 |
| NLR (high vs.
low) | 0.336
(0.197–0.571) |
<0.001a | 0.413
(0.226–0.754) | 0.004a |
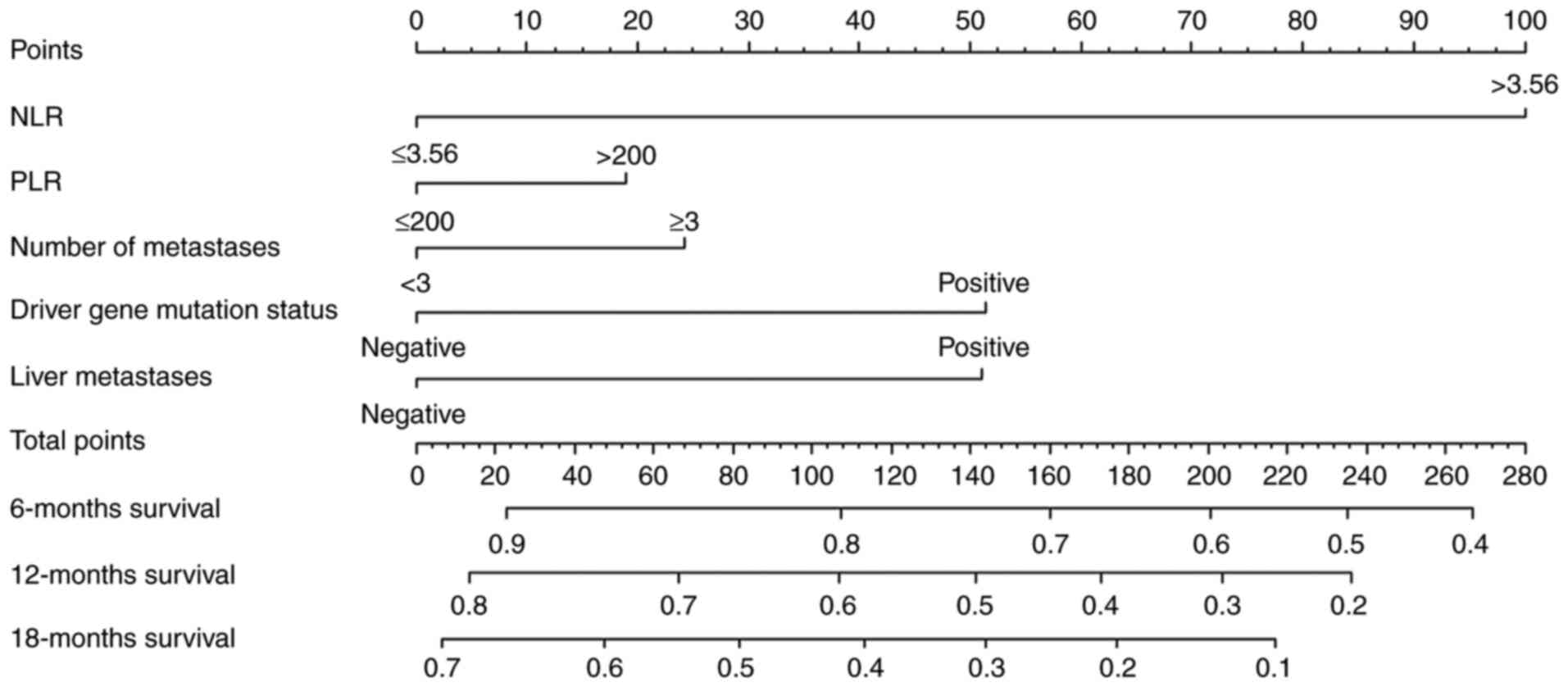
Nomogram for OS
The present study used variables that exhibited a
trend towards significant differences in the univariate analyses
(P<0.1) to construct a nomogram model for predicting patient
survival at 6, 12 and 18 months (Fig.
3). Nomograms can provide oncologists with a simple and
effective tool to predict the prognosis of their patients. To use
the column line plot, a straight line was plotted from the
variables NLR (≤3.56), PLR (≤200), number of metastatic sites (≥3),
driver gene mutation status (negative) and liver metastasis
(positive) to obtain the corresponding points 0, 0, 25, 0 and 50.
All values were then summed to obtain an overall score of 75. A
patient survival rate of 85% was derived at 6 months, the 12-month
survival rate was 68% and the 18-month survival rate was 55%. An
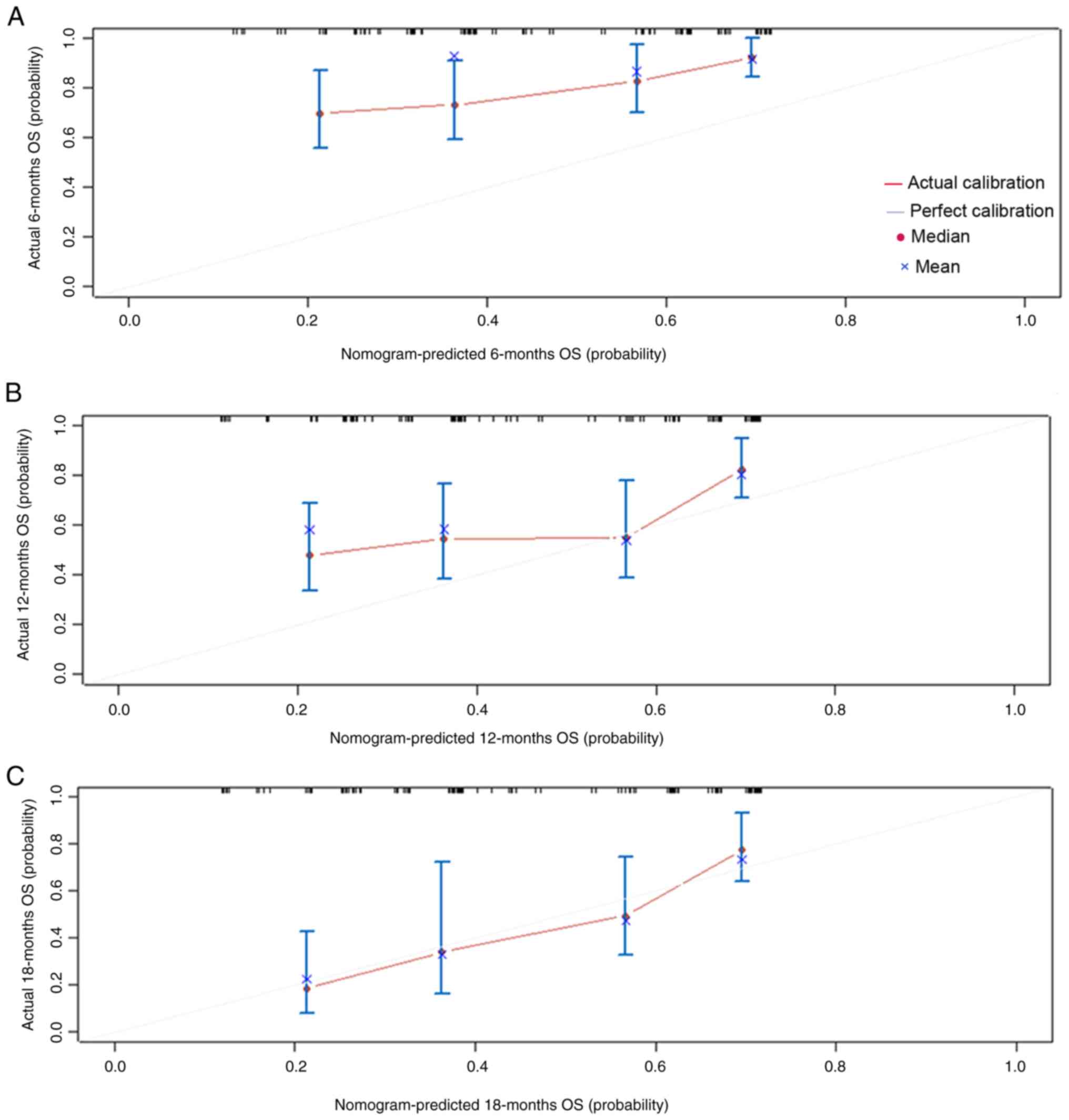
external calibration curve was then plotted, revealing the best
agreement between the predicted and observed survival probabilities
at 18 months (Fig. 4). The C-index
value (Dxy/2 + 0.5 using R) was 0.696 (95% CI, 0.602-0.790), which
indicated low predictive value of the model. Notably, when the
actual incidence of OS at 18 months in patients was between 30 and
70%, the prediction model underestimated the probability of its
occurrence; this is likely to result in lower patient survival
without the use of immunotherapy.
Discussion
LC is the leading type of cancer in terms of global
incidence and mortality (25). The
cause of LC remains unclear, and has been related to factors such
as smoking, air pollution, occupational carcinogenic factors, diet
and genetics. Multidisciplinary treatment is advocated for LC.
Between 30 and 40% of patients with NSCLC present with advanced
disease at the time of diagnosis, and treatment mainly involves a
combination of radiotherapy and chemotherapy (26). The decline in LC mortality in
recent years may be linked to the decline in smoking rates and
novel treatment options (27).
Although molecular therapy has improved outcomes in this patient
group, it has been associated with drug resistance. In 2015, the
United States Food and Drug Administration approved the first PD-1
inhibitor, nivolumab, as a second-line treatment for patients with
advanced NSCLC whose disease has progressed or who had previously
received chemotherapy. Subsequently, treatment of NSCLC has shifted
to immunotherapy, which has increased the 5-year survival rates of
patients from 5 to 26% globally (28). However, challenges associated with
immunotherapy remain, including effective screening of eligible
patients, in a manner that reduces the risk of immunotherapy
resistance, diagnosis and treatment of irAEs, and optimal timing of
immunotherapy. Further studies are required to establish an
evidence base that responds to these challenges.
PLR accounts for platelet and lymphocyte levels.
Once tumor cells enter the bloodstream, platelets aggregate on the
surface of tumor cells, forming platelet-tumor cell aggregates,
thus protecting tumor cells from the immune cells in the body.
Platelets also promote tumor endothelial cell blockage, protecting
the circulating tumor cells from the effects of stress (29). Lymphocytes, as important immune
cells, serve a role in the metastasis and infiltration of tumors.
Asher et al (30) reported
that PLR was an independent prognostic marker in patients with
ovarian cancer and revealed that higher PLR values were associated
with poorer prognosis. Qu et al (31) demonstrated that NLR and PLR could
be used as predictors of near-term outcome in patients with
advanced gastric cancer receiving immunotherapy. In addition, Song
et al (32) evaluated 389
patients receiving concurrent radiotherapy, and revealed that
higher NLR and PLR values were associated with poorer mOS estimates
(NLR: 14.13 vs. 23.8 months, P<0.001; PLR: 15.49 vs. 22.04
months, P<0.001). The present study demonstrated that H-PLR
values were associated with poorer PFS and OS estimates, with
median values of 6.37 and 8.33 months, and 13.47 and 23.66 months
in the H-PLR and L-PLR groups, respectively.
As determined by univariate analysis, PLR values
were associated with PFS and OS estimates; however, these
associations were not observed in multivariate analysis; this
finding is consistent with those of previous studies (30,32).
Notably, Raungkaewmanee et al (33) showed no correlation between PLR
values and ovarian cancer prognosis. Similarly, Zhao et al
(34) identified no correlation
between PLR values and OS estimates in ovarian cancer. These
discrepancies among studies may be due to the different cut-off
values used. The present study used the median to determine the
cut-off value of PLR. Future studies should aim to standardize the
approach to cut-off value determination when assessing the
prognostic role of various inflammatory markers. Other factors that
may account for among-study discrepancies include sample size,
treatment regimen and tumor heterogeneity.
NLR accounts for neutrophil and lymphocyte levels.
Circulating neutrophils promote tumor growth and metastasis through
tumor inflammatory mediators (arginine and nitric oxide). NLR
values have been shown to have prognostic relevance in various
types of cancer, including NSCLC (35), esophageal cancer (32) and pelvic malignancies (36). In the present study, higher NLR
values were associated with poorer PFS (mPFS, 5.73 vs. 9.17 months)
and OS (mOS, 12.03 vs. 26.33 months) in the H-NLR group compared
with in the L-NLR group. These estimates were higher than those
previously reported (37). These
discrepancies may be accounted for by patient age, as in the
present study, patients were younger than previous study patients
(mean age, 58.80±0.89 years); the proportion of patients with an
ECOG score of 0–1 points (97.0%); the proportion of patients that
used immunotherapy as the first-line treatment (54.8%); and the
proportion of patients treated with immune combination therapy
(76.7%). Previous studies (38,39)
have demonstrated that the use of immune combination therapies is
associated with clinical benefits in patients with advanced NSCLC.
Further evidence is required to determine optimum treatment
selection and timing; however, in the present study, NLR values
were independently associated with PFS (HR, 0.201; 95% CI,
0.060-0.670; P=0.009) and OS (HR, 0.413; 95% CI, 0.226-0.754;
P=0.004) estimates.
Predictive reference values for PLR and NLR remain
unclear. Yucel and Bilgin (14)
proposed an NLR value of 3 in patients with advanced NSCLC and EGFR
mutations. In a meta-analysis of 13 studies on ovarian cancer
(n=3467), Zhao et al (34)
proposed NLR values in the range of 2.6-5.03, and PLR values in the
range of 200–300. These values were mostly determined by ROC curve
analyses, as well as based on interquartile ranges, mean and median
values, equivalents of fixed ratio scores, and other software-based
methods that help identify cut-off values. In the present study,
PLR and NLR cut-off values were determined using the median,
yielding 200 and 3.56, respectively; these values are approximately
equivalent to those previously reported.
In contrast to a previous study (35), the present univariate analysis
suggested that number of metastases and mutation status were
associated with PFS and OS estimates. In clinical practice,
patients with multiple metastases tend to be in poor overall
health, have advanced disease and poor previous treatment response,
as well as high toxicity susceptibility.
In the present study on irAEs, a predictive value
could not be found for serological indicators, in contrast to
previous studies, which may be related to a retrospective bias
(40). However, the present study
revealed that patients aged <65 years and those receiving immune
monotherapy were less likely to experience irAEs.
In the univariate analysis of factors associated
with OS, the rates of liver metastases (HR, 0.573; 95% CI,
0.310-1.059; P=0.076) differed among the groups, although this
difference was not statistically significant. Nevertheless, this
finding suggested that liver metastases may affect prognosis.
Notably, the results of the ATLANTIC study revealed that the
presence of liver metastases was associated with poorer prognosis
compared with the absence of these metastases, with the median OS
of 5 and 10 months, respectively (HR, 1.83; 95% CI, 1.28-2.62;
P<0.005) (41).
The liver has a metabolic function and is closely
related to immune function. Non-parenchymal cells are present in
the liver, including hepatic sinusoidal endothelial cells, Kupffer
cells, hepatic stellate cells and dendritic cells, which may be
involved in the regulation of antigen expression, immune regulation
and immune tolerance; therefore, the liver may be considered a part
of a complex immune network (42).
Nevertheless, the impact of liver metastases tends to be
underestimated, specifically, relative to that of brain metastases.
Furthermore, under hypoxic conditions, hepatocytes may upregulate
the expression of several chemokines, such as CC motif chemokine
ligand (CCL)28 and CCL26, which recruit regulatory T cells to
promote the expression of vascular endothelial growth factor
(43). The results of the
IMPOWER150 study showed that in a subgroup of patients with liver
metastases, combination treatment with atezolizumab improved PFS
(8.2 vs. 5.4 months) and OS (13.3 vs. 9.4 months) estimates
(44). Consequently, combination
therapy may improve outcomes in patients with liver metastases.
In the present study, the AUC values of PLR, NLR,
and combined PLR and NLR were 0.68, 0.77 and 0.71, respectively,
indicating good prognostic sensitivity and specificity of these
parameters. The C-index was used to further evaluate the prognostic
value of this model. In a study of 80 patients with NSCLC, Deng
et al (45) revealed that
fibrinogen, PLR and NLR were significantly more effective in
diagnosing NSCLC than the individual indices. Further studies are
required to identify the most relevant combination of these
indices.
In the present study, nomograms were constructed for
133 patients at 6, 12 and 18 months, based on risk factors
associated with OS estimates, including number of metastatic sites,
liver metastases, driver gene mutation status, and PLR and NLR
values. Survival rates observed at 18 months were in good agreement
with the predicted survival rates. This finding was consistent with
those of previous studies (46,47).
However, the C-index of the model was 0.696, which was <0.7 (in
general, 0.50-0.70 indicates low accuracy, 0.71-0.90 indicates
moderate accuracy and >0.90 indicates high accuracy) (48), suggesting that the predictive value
of this model was poor. This finding may be accounted for by the
small sample size of the present study, and the NLR and PLR cut-off
values used.
The present study had some limitations. First, it
was a retrospective cohort study with a small and unrepresentative
sample, which may have affected the presented findings.
Retrospective studies are vulnerable to selection bias, which may
yield inaccurate results, including adverse event rates. Second,
the present study only included common inflammatory status
indicators. Third, there is no established cut-off value for NLR
and PLR. Fourth, the impact of unmeasured confounders could not be
controlled; in addition, different treatment regimens may have
affected the presented findings. Fifth, the follow-up period in the
present study was short. Future large multicenter prospective
cohort studies are required to validate the present findings.
Identifying biomarkers that are cost-effective and
straightforward to obtain is required to achieve good outcomes with
immunotherapy, specifically in the Chinese population, where lung
cancer rates are high and access to treatment is inconsistent
(49). Alongside PD-L1 expression,
serological indicators should be evaluated in patients on ICIs to
help predict outcomes. These serological parameters may be used in
combination with established parameters, such as PD-L1, TMB and
TILs.
In conclusion, in the present retrospective cohort
study of patients with stage IIIB-IV NSCLC treated with ICIs, NLR
values were associated with PFS and OS estimates. Our future work
aims to validate these findings. The present findings suggested
that the NLR indicator had good sensitivity and specificity;
however, the calibrated results show a poor predictive performance.
It is recommended that novel markers be used in combination with
established markers to build highly accurate prognostic models.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XL and HS designed the study, and wrote and edited
the manuscript. JW performed statistical analyses with GraphPad
Prism 8.0.1 and R-Studio. XL collected the data, including
follow-up data. XL and HS wrote the manuscript, and JW and HS
finalized the article. XL and HS confirm the authenticity of all
the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The informed consent requirement was waived by the
Scientific Research Ethics Committee of the First Affiliated
Hospital of Gannan Medical College due to the retrospective nature
of the present study, which involved secondary analysis of an
existing dataset.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
PLR
|
platelet-to-lymphocyte ratio
|
|
NLR
|
neutrophil-to-lymphocyte ratio
|
|
NSCLC
|
non-small cell lung cancer
|
|
PD-1
|
programmed death protein-1
|
|
PD-L1
|
programmed death-ligand 1
|
|
ECOG
|
Eastern Cooperative Oncology Group
|
|
TMB
|
tumor mutation burden
|
|
TILs
|
tumor-infiltrating lymphocytes
|
|
ROC
|
receiver operating characteristic
curve
|
|
AUC
|
area under the curve
|
|
CI
|
confidence interval
|
|
PFS
|
progression-free survival
|
|
OS
|
overall survival
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sui H, Ma N, Wang Y, Li H, Liu X, Su Y and
Yang J: Anti-PD-1/PD-L1 therapy for non-small-cell lung cancer:
Toward personalized medicine and combination strategies. J Immunol
Res. 2018:69849482018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
España S, Guasch I and Carcereny E:
Immunotherapy rechallenge in patients with non-small-cell lung
cancer. Pulmonology. 26:252–254. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Duan J, Cui L, Zhao X, Bai H, Cai S, Wang
G, Zhao Z, Zhao J, Chen S, Song J, et al: Use of immunotherapy with
programmed cell death 1 vs programmed cell death ligand 1
inhibitors in patients with cancer: A systematic review and
meta-analysis. JAMA Oncol. 6:375–384. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Califano R, Gomes F, Ackermann CJ, Rafee
S, Tsakonas G and Ekman S: Immune checkpoint blockade for non-small
cell lung cancer: What is the role in the special populations? Eur
J Cancer. 125:1–11. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen Y, Zhou Y, Tang L, Peng X, Jiang H,
Wang G and Zhuang W: Immune-checkpoint inhibitors as the first line
treatment of advanced non-small cell lung cancer: A meta-analysis
of randomized controlled trials. J Cancer. 10:6261–6268. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Prelaj A, Tay R, Ferrara R, Chaput N,
Besse B and Califano R: Predictive biomarkers of response for
immune checkpoint inhibitors in non-small-cell lung cancer. Eur J
Cancer. 106:144–159. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Choucair K, Morand S, Stanbery L, Edelman
G, Dworkin L and Nemunaitis J: TMB: A promising immune-response
biomarker, and potential spearhead in advancing targeted therapy
trials. Cancer Gene Ther. 27:841–853. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hellmann MD, Ciuleanu TE, Pluzanski A, Lee
JS, Otterson GA, Audigier-Valette C, Minenza E, Linardou H, Burgers
S, Salman P, et al: Nivolumab plus ipilimumab in lung cancer with a
high tumor mutational burden. N Engl J Med. 378:2093–2104. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rossi G, Russo A, Tagliamento M, Tuzi A,
Nigro O, Vallome G, Sini C, Grassi M, Dal Bello MG, Coco S, et al:
Precision medicine for NSCLC in the era of immunotherapy: New
biomarkers to select the most suitable treatment or the most
suitable patient. Cancers (Basel). 12:11252020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hashemi S, Fransen MF, Niemeijer A, Ben
Taleb N, Houda I, Veltman J, Becker-Commissaris A, Daniels H,
Crombag L, Radonic T, et al: Surprising impact of stromal TIL's on
immunotherapy efficacy in a real-world lung cancer study. Lung
Cancer. 153:81–89. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ying HQ, Liao YC, Luo YR, Xiong G, Huang
Y, Nie RW, Xiong CF and Cheng XX: Cancer-elicited inflammation
attenuates response and outcome in tyrosine kinase inhibitor naive
patients with advanced NSCLC. Pharmacol Res. 170:1057342021.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yucel S and Bilgin B: The prognostic
values of systemic immune-inflammation index and derived
neutrophil-lymphocyte ratio in EGFR-mutant advanced non-small cell
lung cancer. J Oncol Pharm Pract. 27:71–77. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Abdelhamed S, Ogura K, Yokoyama S, Saiki I
and Hayakawa Y: AKT-STAT3 pathway as a downstream target of EGFR
signaling to regulate PD-L1 expression on NSCLC cells. J Cancer.
7:1579–1586. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nagasaka M, Sexton R, Alhasan R, Rahman S,
Azmi AS and Sukari A: Gut microbiome and response to checkpoint
inhibitors in non-small cell lung cancer-a review. Crit Rev Oncol
Hematol. 145:1028412020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim K, Kwon O, Ryu TY, Jung CR, Kim J, Min
JK, Kim DS, Son MY and Cho HS: Propionate of a microbiota
metabolite induces cell apoptosis and cell cycle arrest in lung
cancer. Mol Med Rep. 20:1569–1574. 2019.PubMed/NCBI
|
|
18
|
Mountzios G, Samantas E, Senghas K, Zervas
E, Krisam J, Samitas K, Bozorgmehr F, Kuon J, Agelaki S, Baka S, et
al: P75.04 advanced lung cancer inflammation index (ALI),
neutrophil-to-lymphocyte ratio (NLR), and PD-(L)1 inhibitor
efficacy in NSCLC. J Thorac Oncol. 16 (Suppl):S573–S574. 2021.
View Article : Google Scholar
|
|
19
|
Zhuang H, Cheng L, Wang Y, Zhang YK, Zhao
MF, Liang GD, Zhang MC, Li YG, Zhao JB, Gao YN, et al: Dysbiosis of
the gut microbiome in lung cancer. Front Cell Infect Microbiol.
9:1122019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Capone M, Giannarelli D, Mallardo D,
Madonna G, Festino L, Grimaldi AM, Vanella V, Simeone E, Paone M,
Palmieri G, et al: Baseline neutrophil-to-lymphocyte ratio (NLR)
and derived NLR could predict overall survival in patients with
advanced melanoma treated with nivolumab. J Immunother Cancer.
6:742018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ettinger DS, Wood DE, Aisner DL, Akerley
W, Bauman JR, Bharat A, Bruno DS, Chang JY, Chirieac LR, D'Amico
AT, et al: NCCN Guidelines Insights: Non-Small Cell Lung Cancer,
Version 2.2021. J Natl Compr Canc Netw. 19:254–266. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Neeman E, Gresham G, Ovasapians N,
Hendifar A, Tuli R, Figlin R and Shinde A: Comparing physician and
nurse eastern cooperative oncology group performance status
(ECOG-PS) ratings as predictors of clinical outcomes in patients
with cancer. Oncologist. 24:e1460–e1466. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hodi FS, Ballinger M, Lyons B, Soria JC,
Nishino M, Tabernero J, Powles T, Smith D, Hoos A, McKenna C, et
al: Immune-modified response evaluation criteria in solid tumors
(imRECIST): Refining guidelines to assess the clinical benefit of
cancer immunotherapy. J Clin Oncol. 36:850–858. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
National Cancer Institute, National
Institutes of Health, U.S. Department of Health and Human Services,
. Common Terminology Criteria for Adverse Events (CTCAE) Version
4.0. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_8.5×11.pdfDecember
30–2010
|
|
25
|
Miller KD, Siegel RL, Lin CC, Mariotto AB,
Kramer JL, Rowland JH, Stein KD, Alteri R and Jemal A: Cancer
treatment and survivorship statistics, 2016. CA Cancer J Clin.
66:271–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Leonetti A, Wever B, Mazzaschi G, Assaraf
YG, Rolfo C, Quaini F, Tiseo M and Giovannetti E: Molecular basis
and rationale for combining immune checkpoint inhibitors with
chemotherapy in non-small cell lung cancer. Drug Resist Updat.
46:1006442019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shiraishi Y, Kishimoto J, Tanaka K,
Sugawara S, Daga H, Hirano K, Azuma K, Hataji O, Hayashi H,
Tachihara M, et al: Treatment rationale and design for APPLE
(WJOG11218L): A multicenter, open-label, randomized phase 3 study
of atezolizumab and platinum/pemetrexed with or without bevacizumab
for patients with advanced nonsquamous non-small-cell lung cancer.
Clin Lung Cancer. 21:472–476. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Low JL, Walsh RJ, Ang Y, Chan G and Soo
RA: The evolving immuno-oncology landscape in advanced lung cancer:
First-line treatment of non-small cell lung cancer. Ther Adv Med
Oncol. 11:17588359198703602019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Watanabe K, Noma D, Masuda H and Masuda M:
Preoperative inflammation-based scores predict early recurrence
after lung cancer resection. J Thorac Dis. 13:2812–2823. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Asher V, Lee J, Innamaa A and Bali A:
Preoperative platelet lymphocyte ratio as an independent prognostic
marker in ovarian cancer. Clin Transl Oncol. 13:499–503. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Qu Z, Wang Q, Wang H, Jiao Y, Li M, Wei W,
Lei Y, Zhao Z, Zhang T, Zhang Y and Gu K: The effect of
inflammatory markers on the survival of advanced gastric cancer
patients who underwent anti-programmed death 1 therapy. Front
Oncol. 12:7831972022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Song X, Chen D, Yuan M, Wang H and Wang Z:
Total lymphocyte count, neutrophil-lymphocyte ratio, and
platelet-lymphocyte ratio as prognostic factors in advanced
non-small cell lung cancer with chemoradiotherapy. Cancer Manag
Res. 10:6677–6683. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Raungkaewmanee S, Tangjitgamol S,
Manusirivithaya S, Srijaipracharoen S and Thavaramara T:
Platelet-to-lymphocyte ratio as a prognostic factor for epithelial
ovarian cancer. J Gynecol Oncol. 23:265–273. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhao Z, Zhao X, Lu J, Xue J, Liu P and Mao
H: Prognostic roles of neutrophil to lymphocyte ratio and platelet
to lymphocyte ratio in ovarian cancer: A meta-analysis of
retrospective studies. Arch Gynecol Obstet. 297:849–857. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yun NK, Rouhani SJ, Bestvina CM, Ritz EM,
Gilmore BA, Tarhoni I, Borgia JA, Batus M, Bonomi PD and Fidler MJ:
Neutrophil-to-lymphocyte ratio is a predictive biomarker in
patients with epidermal growth factor receptor (EGFR) mutated
advanced non-small cell lung cancer (NSCLC) treated with tyrosine
kinase inhibitor (TKI) therapy. Cancers (Basel). 13:1426–1441.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cao W, Yao X, Cen D, Zhi Y, Zhu N and Xu
L: Prognostic role of pretreatment thrombocytosis on survival in
patients with cervical cancer: A systematic review and
meta-analysis. World J Surg Oncol. 17:1322019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu N, Mao J, Tao P, Chi H, Jia W and Dong
C: The relationship between NLR/PLR/LMR levels and survival
prognosis in patients with non-small cell lung carcinoma treated
with immune checkpoint inhibitors. Medicine (Baltimore).
101:e286172022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Qiang H, Chang Q, Xu J, Qian J, Zhang Y,
Lei Y, Han B and Chu T: New advances in antiangiogenic combination
therapeutic strategies for advanced non-small cell lung cancer. J
Cancer Res Clin Oncol. 146:631–645. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
John T, Sakai H, Ikeda S, Cheng Y,
Kasahara K, Sato Y, Nakahara Y, Takeda M, Kaneda H, Zhang H, et al:
1311P First-line (1L) nivolumab (NIVO) + ipilimumab (IPI) +
chemotherapy (chemo) in Asian patients (pts) with advanced
non-small cell lung cancer (NSCLC) from CheckMate 9LA. Ann Oncol.
31 (Suppl 4):S847–S848. 2020. View Article : Google Scholar
|
|
40
|
Zhao L, Li Y, Jiang N, Song X, Xu J, Zhu
X, Chen C, Kong C, Wang X, Zong D, et al: Association of blood
biochemical indexes and antibiotic exposure with severe
immune-related adverse events in patients with advanced cancers
receiving PD-1 inhibitors. J Immunother. 45:210–216. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sridhar S, Paz-Ares L, Liu H, Shen K,
Morehouse C, Rizvi N, Segal NH, Jin X, Zheng Y, Narwal R, et al:
Prognostic significance of liver metastasis in Durvalumab-treated
lung cancer patients. Clin Lung Cancer. 20:e601–e608. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Carroll HK, Duffy AG and O'Farrelly C:
Liver immunology, immunotherapy, and liver cancers: Time for a
rethink? Semin Liver Dis. Mar 9–2022.(Epub ahead of print).
PubMed/NCBI
|
|
43
|
Chiu DK, Xu IM, Lai RK, Tse AP, Wei LL,
Koh HY, Li LL, Lee D, Lo RC, Wong CM, et al: Hypoxia induces
myeloid-derived suppressor cell recruitment to hepatocellular
carcinoma through chemokine (C-C motif) ligand 26. Hepatology.
64:797–813. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Reck M, Mok TSK, Nishio M, Jotte RM,
Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu
D, Moro-Sibilot D, et al: Atezolizumab plus bevacizumab and
chemotherapy in non-small-cell lung cancer (IMpower150): Key
subgroup analyses of patients with EGFR mutations or baseline liver
metastases in a randomised, open-label phase 3 trial. Lancet Respir
Med. 7:387–401. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Deng M, Ma X, Liang X, Zhu C and Wang M:
Are pretreatment neutrophil-lymphocyte ratio and
platelet-lymphocyte ratio useful in predicting the outcomes of
patients with small-cell lung cancer? Oncotarget. 8:37200–37207.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liu N, Jiang A, Zheng X, Fu X, Zheng H,
Gao H, Wang J, Liang X, Tian T, Ruan Z and Yao Y: Prognostic
nutritional index identifies risk of early progression and survival
outcomes in advanced non-small cell lung cancer patients treated
with PD-1 inhibitors. J Cancer. 12:2960–2967. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Xia J, Chen Y, Wen S, Du X and Shen B:
Peripheral blood inflammation indicators as predictive indicators
in
immunotherapy of advanced non-small cell lung cancer. Zhongguo
Fei Ai Za Zhi. 24:632–645. 2021.(In Chinese). PubMed/NCBI
|
|
48
|
Balachandran VP, Gonen M, Smith JJ and
DeMatteo RP: Nomograms in oncology: More than meets the eye. Lancet
Oncol. 16:e173–e180. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Feng J, Li Y, Wei B, Guo L, Li W, Xia Q,
Zhao C, Zheng J, Zhao J, Sun R, et al: Clinicopathologic
characteristics and diagnostic methods of RET rearrangement in
Chinese non-small cell lung cancer patients. Transl Lung Cancer
Res. 11:617–631. 2022. View Article : Google Scholar : PubMed/NCBI
|