Introduction
Endometrial carcinoma, as a common gynecological
malignancy, displays increasing incidence rate globally (1,2). An
average annual increase of 11.3% for endometrial carcinoma
incidence was observed in South Africa between 2003 and 2012, and
an increase was also observed in Brazil, America, Japan and other
24 countries/regions (2).
Regarding the management of endometrial carcinoma, total
hysterectomy and bilateral salpingo-oophorectomy (with or without
adjuvant therapy) are recommended for affected patients who are
suitable for surgery, while external beam radiation therapy and/or
brachytherapy (with or without chemotherapy) is commonly applied
for those patients who are not suitable for surgery (3,4). For
those patients with endometrial carcinoma who meet the criteria for
consideration of fertility-sparing options, hormonal therapy
(continuous progestin-based therapy) may be considered as an
alternative therapy option (4).
Despite the appropriate management, patients with endometrial
carcinoma may experience multiple recurrences (locoregional
recurrence and extrapelvic recurrence), leading to a poor long-term
outcome (5,6). Thus, identifying potential biomarkers
to improve the prognosis for patients with endometrial carcinoma is
critical and urgent.
A-kinase-interacting protein 1 (AKIP1) has been
reported to serve as an oncogene in several solid tumors,
especially in gynecological malignancies (7,8). For
instance, AKIP1 may promote cervical cancer cell invasion and
migration through regulating nuclear factor (NF)-κB-induced
epithelial-to-mesenchymal transition (EMT) (7). Moreover, AKIP1 can enhance cervical
cancer cell proliferation and angiogenesis by upregulating
CXC-chemokines (such as CXCL1, CXCL2 and CXCL8) (8). In the clinical field, AKIP1 has been
identified as a potential prognostic biomarker for clear cell renal
cell carcinoma, non-small cell lung cancer, colorectal cancer,
hepatocellular carcinoma and cervical cancer (9–13).
To the best of our knowledge, no study has explored the clinical
role of AKIP1 in patients with endometrial carcinoma.
The present study collected 101 pairs of tumor
tissue and adjacent tissue from patients with endometrial carcinoma
in order to explore the associations between AKIP1 expression and
clinicopathological features and prognosis. In addition, the
present study further assessed the effect of AKIP1 on regulating
chemosensitivity in an endometrial carcinoma cell line.
Materials and methods
Patients
The present study retrospectively analyzed the cases
of 101 female patients with endometrial carcinoma who were treated
by surgical resection at Handan Central Hospital (Handan, China)
between January 2016 and January 2020. By reviewing their clinical
data, the eligible patients were enrolled based on the following
criteria: i) Histopathological diagnosis of endometrial carcinoma;
ii) age >18 years; iii) treated by surgical resection; iv)
surgically removed specimens, including tumor and tumor-adjacent
tissues, were available and accessible; v) main clinical data and
survival data were retrievable; and vi) no history of other
malignancies before the diagnosis of endometrial carcinoma.
Approval was acquired from the Institutional Review Board (IRB) of
Handan Central Hospital (approval number, HDZXYY-Ethics-2021015)
before the implementation of the study. The requirement for written
informed consent was absolved by the IRB, as the study was
performed based on post-operative patient samples and existing
clinical data, which involved no risk to the patients themselves
and did not affect the privacy of the patients.
Data acquisition
The main preoperative clinical features of the
patients were obtained from medical records or outpatient records,
and included age, menopausal status (pre-menopause or
post-menopause), comorbidities [diabetes mellitus (DM) and
hypertension], histological subtype (endometrioid carcinoma, serous
endometrial carcinoma and clear cell endometrial carcinoma),
myometrial invasion status, cervical invasion status,
lymphovascular invasion status and International Federation of
Gynecology and Obstetrics (FIGO) stage (14). Furthermore, the survival data of
the patients was collated from the follow-up documents, and the
overall survival (OS) time was determined.
Specimen acquisition
Tumor and tumor-adjacent tissue (2 cm from the tumor
tissue) specimens (formalin-fixed using 4% paraformaldehyde for
>24 h and paraffin-embedded and sliced into 4-µm sections) of
all patients were acquired from the specimen repository in order to
perform an immunohistochemistry (IHC) assay aimed at evaluating
AKIP1 protein expression. Meanwhile, 54 of the 101 patients had
fresh-frozen specimens that were immediately stored in liquid
nitrogen following surgical removal (stored at −80°C), which were
also collected to determine the mRNA expression levels of AKIP1 by
reverse transcription-quantitative PCR (RT-qPCR) assay.
IHC assay
The IHC staining was implemented as described in a
previous study (15), with the
AKIP1 Monoclonal Antibody as the primary antibody (cat. no.
MA5-26998) and Goat anti-Mouse IgG (H+L) as the secondary antibody
(cat. no. 31430) (both Invitrogen; Thermo Fisher Scientific, Inc.).
The primary antibody was diluted 1:150 and the secondary antibody
was diluted 1:20,000. The staining (10 min at room temperature) and
counterstaining (5 min at room temperature) were performed using
diaminobenzidine and hematoxylin, respectively. The IHC staining
result was viewed on a light microscope, and the AKIP1 expression
was evaluated using an IHC scoring method (16). In brief, five high-power fields
(×200 magnification) were randomly selected in each slide, then the
mean percentage of positively stained cells in five fields was
calculated and scored as 0 (0%), 1 (1–25%), 2 (26–50%), 3 (51–75%)
or 4 (76–100%). Meanwhile, the staining intensity was scored as 0
(negative), 1 (weak), 2 (moderate) or 3 (strong), and the mean
staining intensity score of five fields was calculated. The final
IHC score was obtained by multiplying the two scores. Using a
cut-off score of 3, the AKIP1 protein expression was classified as
low expression (IHC score ≤3) and high expression (IHC score
>3).
RT-qPCR assay
After extraction of total RNA from the tumor tissues
and adjacent tissues using TRIzol® (Invitrogen; Thermo
Fisher Scientific, Inc.), RT was conducted using iScript™ Reverse
Transcription Supermix (Bio-Rad Laboratories, Inc.) according to
the manufacturer's instructions. qPCR was performed using the
QuantiNova SYBR Green PCR Kit (Qiagen GmbH). The following
thermocycling conditions were applied: 95°C for 2 min, followed by
40 cycles of 95°C for 5 sec and 61°C for 30 sec. The relative
expression of AKIP1 was calculated using the 2−ΔΔCq
method, where GAPDH served as an internal reference (17). The forward and reserve primers were
designed in line with a previous study (11). According to the median expression
level in the tumor tissue, the AKIP1 mRNA expression was classified
as low expression (up to and including the median level) and high
expression (greater than the median level) for survival
analysis.
In vitro experiment
Human endometrial carcinoma Ishikawa cells (Shanghai
Enzyme Research Biotechnology Co., Ltd.) were cultured in DMEM
(Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine
serum and 1% penicillin/streptomycin (both Gibco; Thermo Fisher
Scientific, Inc.), in a 5% CO2 atmosphere at 37°C. The
small interfering (si)RNA targeting AKIP1 (50 nM) (sense,
5′-GGAGGCAGCTATCAAATATTT-3′ and antisense,
5′-ATATTTGATAGCTGCCTCCTT-3′) and the corresponding negative control
(NC) siRNA (50 nM) (sense, 5′-GAATTAATTAAAGATGGCCCGTTGTACT-3′ and
antisense, 5′-TCATCGAAGTTATAGGGATACATTACGTGATC-3′) were purchased
from Thermo Fisher Scientific, Inc., and separately transfected
into the 5×105 Ishikawa cells using
Lipofectamine® 3000 reagent (Thermo Fisher Scientific,
Inc.) at 37°C for 6 h. At 48 h post-transfection, the AKIP1 mRNA
expression levels were detected. Subsequently, the Ishikawa cells
were divided into three groups: Cells transfected with AKIP1 siRNA,
cells transfected with NC siRNA and cells without transfection
(used as a blank control), accordingly. Cisplatin and paclitaxel
(both MilliporeSigma; Merck KGaA) were used for the
chemosensitivity assay. The cisplatin was prepared at
concentrations of 0, 5, 10, 20, 40 and 80 µM, and the paclitaxel
was prepared at concentrations of 0, 0.5, 1, 2, 4 and 8 nM,
according to previous studies (18–20).
The cells in the three groups were respectively treated with the
cisplatin and paclitaxel at the prepared concentrations for 48 h at
37°C, and then the cell viability in each group was determined
using Cell Counting Kit-8 reagent (Beyotime Institute of
Biotechnology) for 2 h and detected at a wavelength of 450 nm.
Statistical analysis
SPSS 26.0 (IBM Corp.) was applied for data analysis,
and GraphPad Prism 7.02 (GraphPad Software Inc.) was used for graph
plotting. Quantitative data are presented as the mean ± standard
deviation or median (interquartile range). Qualitative data are
presented as n (%). The comparison of AKIP1 expression between
tumor and adjacent tissues was performed using Wilcoxon's signed
rank test. Receiver operating characteristic curve analysis was
applied to evaluate the accuracy of AKIP1 expression for
differentiating between different tissues. Associations between
AKIP1 expression and clinical features were analyzed using
Spearman's correlation (for ordered categorical variables) and
Wilcoxon's rank sum test (for unordered categorical variables). OS
was displayed in Kaplan-Meier curves and analyzed by log-rank test.
Prognostic implications of variables were estimated using Cox
proportional hazard model regression analysis. To further validate
the correlation of AKIP1 with OS, a search for AKIP1 was performed
in The Human Protein Atlas database (https://www.proteinatlas.org/ENSG00000130707-ASS1/pathology/endometrial+cancer),
from which, AKIP1-related survival data derived from The Cancer
Genome Atlas database (https://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga)
was obtained. In the in vitro experiment, AKIP1 mRNA
expression was compared using one-way analysis of variance followed
by Tukey's test. The cell viability between the AKIP1 siRNA group
and the NC siRNA group was determined by unpaired t-test. The half
maximal inhibitory concentration (IC50) was estimated by
Probit regression, and the mean IC50 between the AKIP1
siRNA group and the NC siRNA group was also determined by t-test.
The experiments were all performed in triplicate. P<0.05 was
used to indicate a statistically significant difference.
Results
Clinical features of patients with
endometrial carcinoma
The mean age of the analyzed patients with
endometrial carcinoma was 63.5±9.6 years (Table I). There were 15 (14.9%) and 86
(85.1%) patients with a pre-menopause and post-menopause status,
respectively. Moreover, 67 (66.3%), 11 (10.9%), 16 (15.8%) and 7
(6.9%) patients were diagnosed with endometrioid carcinoma G1/G2,
endometrioid carcinoma G3, serous endometrial carcinoma and clear
cell endometrial carcinoma, respectively. Furthermore, 61 (60.4%),
14 (13.9%), 20 (19.8%) and 6 (5.9%) patients were graded as FIGO
stage I, stage II, stage III and stage IV, respectively. The
detailed clinical characteristics of the patients with endometrial
carcinoma are listed in Table
I.
 | Table I.Characteristics of patients with
endometrial carcinoma (n=101). |
Table I.
Characteristics of patients with
endometrial carcinoma (n=101).
| Variables | Value |
|---|
| Mean age ± SD,
years | 63.5±9.6 |
| Menopausal status,
n (%) |
|
|
Pre-menopause | 15 (14.9) |
|
Post-menopause | 86 (85.1) |
| Diabetes mellitus,
n (%) |
|
| No | 73 (72.3) |
|
Yes | 28 (27.7) |
| Hypertension, n
(%) |
|
| No | 50 (49.5) |
|
Yes | 51 (50.5) |
| Histological
subtype, n (%) |
|
|
Endometrioid carcinoma
G1/G2 | 67 (66.3) |
|
Endometrioid carcinoma G3 | 11 (10.9) |
| Serous
endometrial carcinoma | 16 (15.8) |
| Clear
cell endometrial carcinoma | 7 (6.9) |
| Myometrial invasion
≥1/2, n (%) |
|
| No | 61 (60.4) |
|
Yes | 40 (39.6) |
| Cervical invasion,
n (%) |
|
| None or
epithelial | 76 (75.2) |
|
Stromal | 25 (24.8) |
| Lymphovascular
invasion, n (%) |
|
| No | 75 (74.3) |
|
Yes | 26 (25.7) |
| FIGO stage, n
(%) |
|
| I | 61 (60.4) |
| II | 14 (13.9) |
|
III | 20 (19.8) |
| IV | 6 (5.9) |
AKIP1 expression in patients with
endometrial carcinoma
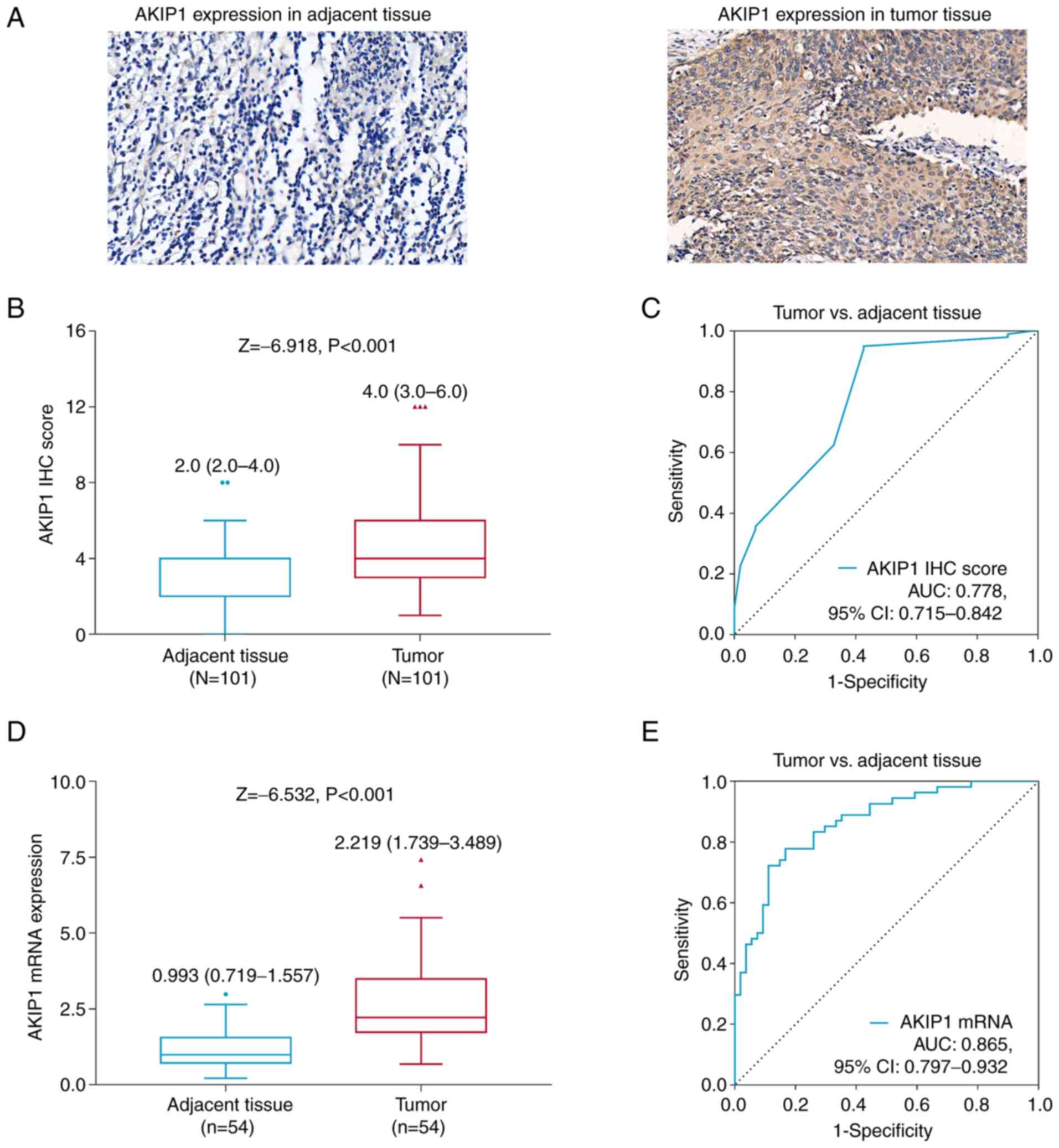
AKIP1 IHC score was increased in tumor tissue
compared with that in adjacent tissue in the patients with
endometrial carcinoma [median (IQR), 4.0 (3.0-6.0) vs. 2.0
(2.0-4.0); P<0.001; Fig. 1A and
B]. Moreover, AKIP1 IHC score was able to differentiate tumor
tissue from adjacent tissue, with an area under the curve (AUC) of
0.778 (95% CI, 0.715-0.842; Fig.
1C).
AKIP1 mRNA expression was also elevated in tumor
tissues compared with that in adjacent tissues in the patients with
endometrial carcinoma [median (IQR), 2.219 (1.739-3.489) vs. 0.993
(0.719-1.577); P<0.001; Fig.
1D]. Moreover, AKIP1 mRNA expression was also able to
differentiate between tumor tissue and adjacent tissue, with an AUC
of 0.865 (95% CI, 0.797-0.932; Fig.
1E).
Association between tumor AKIP1
expression and clinical features in patients with endometrial
carcinoma
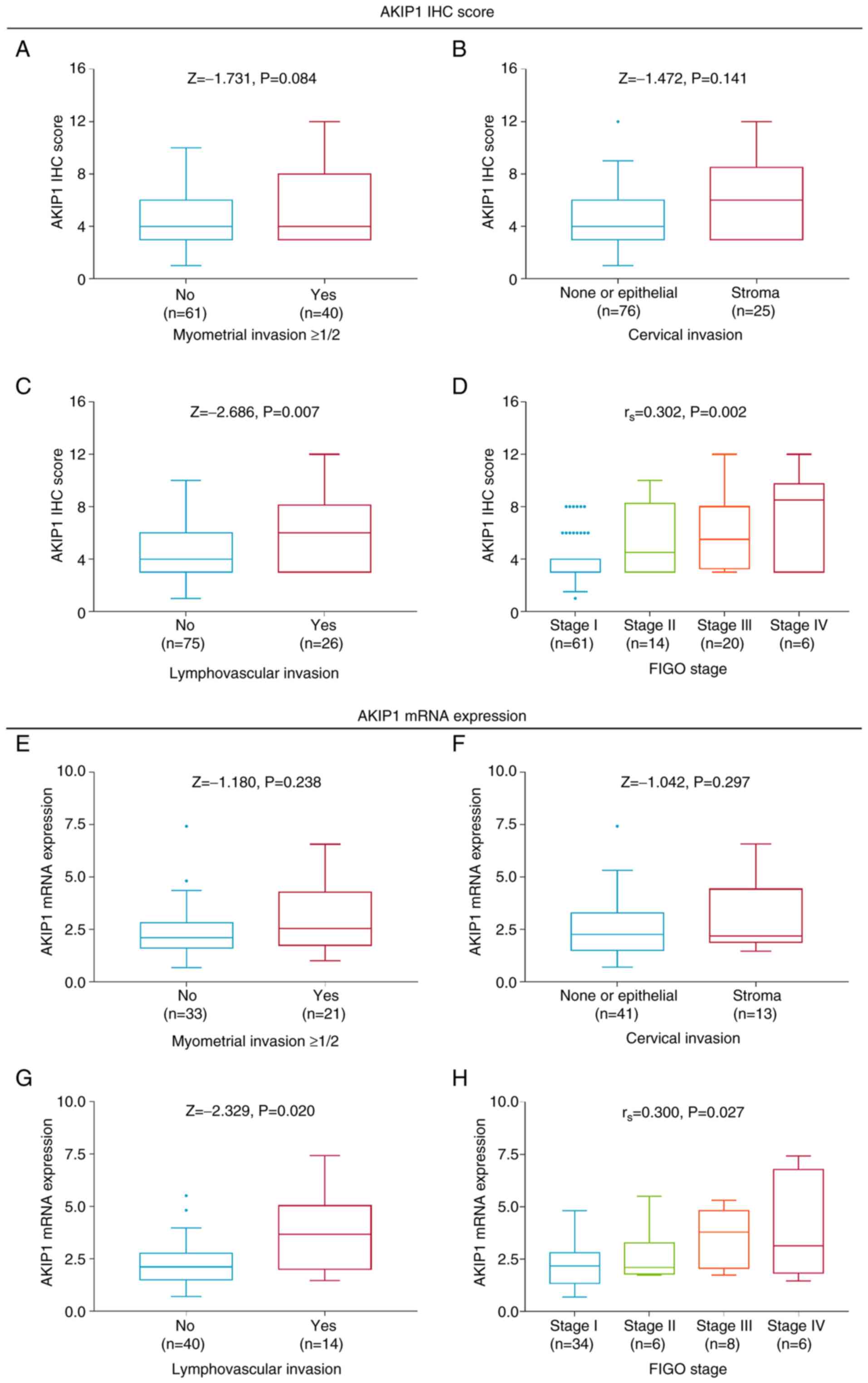
Even though AKIP1 IHC score was not associated with
myometrial invasion ≥1/2 (P=0.084; Fig. 2A) or cervical stroma invasion
(P=0.141; Fig. 2B), increased
AKIP1 IHC score was associated with lymphovascular invasion
(P=0.007; Fig. 2C) and advanced
FIGO stage (P=0.002; Fig. 2D) in
the patients with endometrial carcinoma.
Although there was no association between AKIP1 mRNA
expression and myometrial invasion ≥1/2 (P=0.238; Fig. 2E) or cervical invasion (P=0.297;
Fig. 2F), elevated AKIP1 mRNA
level was associated with lymphovascular invasion (P=0.020;
Fig. 2G) and higher FIGO stage
(P=0.027; Fig. 2H) in the patients
with endometrial carcinoma.
Association between tumor AKIP1
expression and OS in patients with endometrial carcinoma
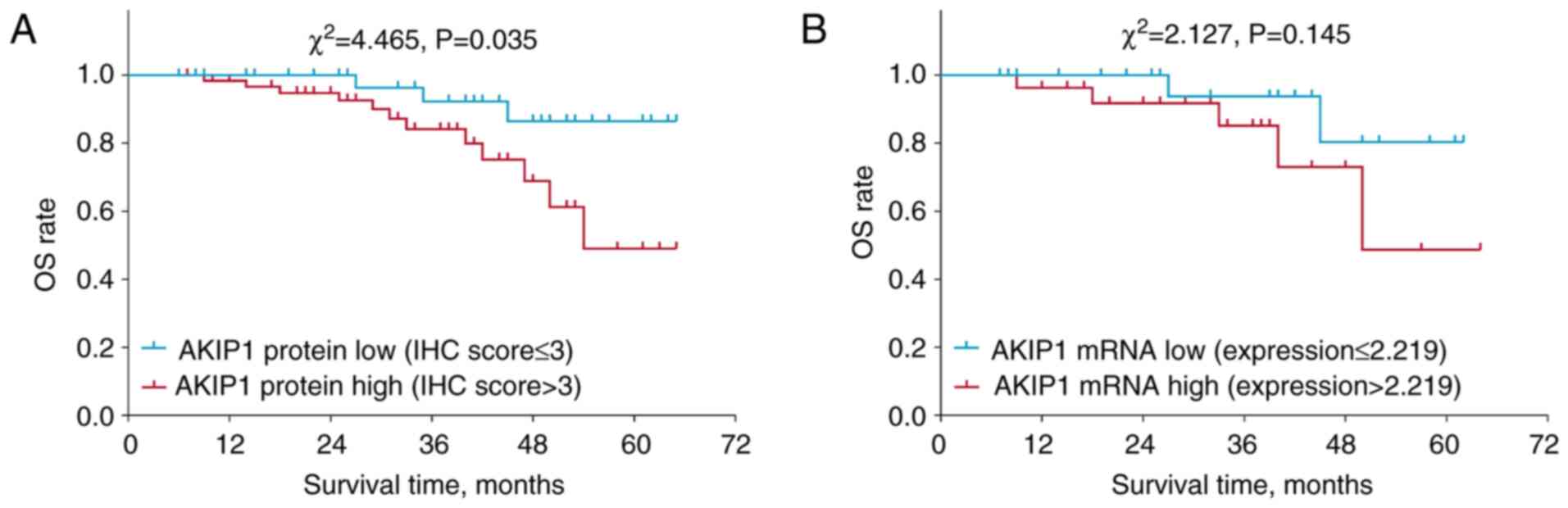
High AKIP1 protein expression (defined as an IHC
score >3) was associated with a shorter accumulating OS time in
the patients with endometrial carcinoma (P=0.035; Fig. 3A). However, high AKIP1 mRNA
expression (defined as expression >2.219) was not associated
with OS time (P=0.145; Fig. 3B).
Further validation using The Cancer Genome Atlas database showed
that high AKIP1 mRNA expression appeared to be associated with
shorter OS time in the patients with endometrial carcinoma,
although no statistical significance was found (P=0.052; Fig. S1).
Independent factors for OS in patients
with endometrial carcinoma
Univariate Cox regression analysis identified that
tumor AKIP1 protein expression (high vs. low) [hazard ratio (HR),
3.622; 95% confidence interval (CI), 1.013-12.946; P=0.048],
cervical invasion (stromal vs. none or epithelial) (HR, 3.821; 95%
CI, 1.382-10.565; P=0.010), lymphovascular invasion (yes vs. no)
(HR, 3.769; 95% CI, 1.363-10.419; P=0.011) and higher FIGO stage
(HR, 2.170; 95% CI, 1.382-3.406; P=0.001) were all associated with
decreased OS time in the patients with endometrial carcinoma
(Table II). Further multivariate
Cox regression analysis found that tumor AKIP1 protein expression
(high vs. low) (HR, 3.910; 95% CI, 1.095-13.965; P=0.036) and
cervical invasion (stromal vs. none or epithelial) (HR, 4.104; 95%
CI, 1.483-11.360; P=0.007) were independently associated with
shorter OS time.
 | Table II.Univariate and multivariate Cox
regression analyses on overall survival. |
Table II.
Univariate and multivariate Cox
regression analyses on overall survival.
| A, Univariate Cox
regression analysis |
|---|
|
|---|
|
|
|
| 95% CI |
|---|
|
|
|
|
|
|---|
| Variables | P-value | HR | Lower | Upper |
|---|
| Tumor AKIP1 protein
(high vs. low) | 0.048a | 3.622 | 1.013 | 12.946 |
| Age (≥60 vs. <60
years) | 0.294 | 1.848 | 0.587 | 5.817 |
| Menopausal status
(post-menopause vs. pre-menopause) | 0.652 | 1.408 | 0.317 | 6.248 |
| Diabetes mellitus
(yes vs. no) | 0.473 | 1.482 | 0.505 | 4.348 |
| Hypertension (yes
vs. no) | 0.343 | 0.609 | 0.219 | 1.696 |
| Myometrial invasion
≥1/2 (yes vs. no) | 0.277 | 1.757 | 0.636 | 4.855 |
| Cervical invasion
(stromal vs. none or epithelial) | 0.010a | 3.821 | 1.382 | 10.565 |
| Lymphovascular
invasion (yes vs. no) | 0.011a | 3.769 | 1.363 | 10.419 |
| FIGO stage (III/IV
vs. I/II) | 0.001a | 2.170 | 1.382 | 3.406 |
|
| B, Multivariate
Cox regression analysis |
|
|
|
|
| 95% CI |
|
|
|
|
|
|
Variables | P-value | HR | Lower | Upper |
|
| Tumor AKIP1 protein
(high vs. low) | 0.036a | 3.910 | 1.095 | 13.965 |
| Cervical invasion
(stromal vs. none or epithelial) | 0.007a | 4.104 | 1.483 | 11.360 |
Effect of AKIP1 knockdown on
chemosensitivity
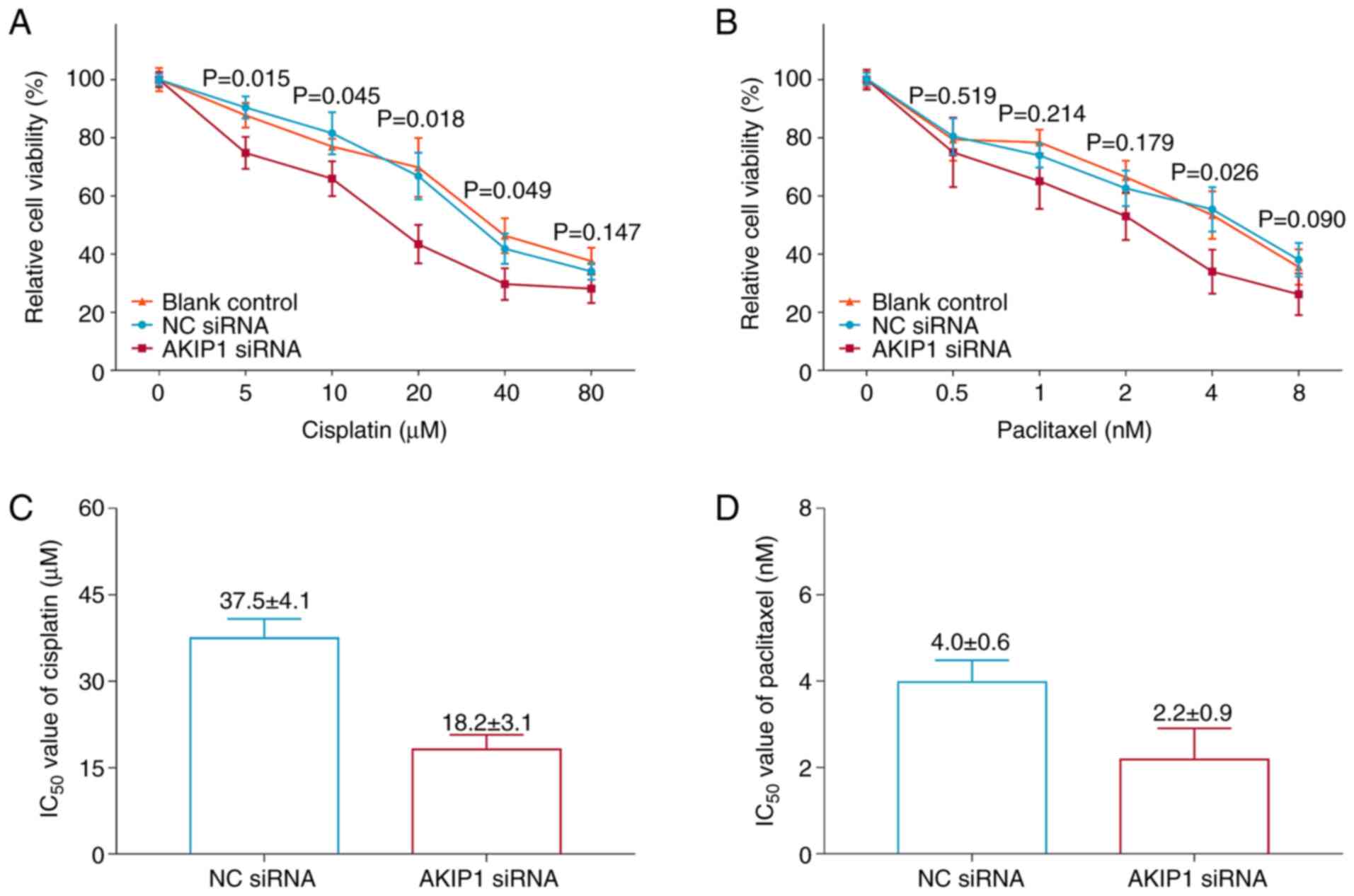
Silencing of AKIP1 decreased the mRNA expression
levels of AKIP1 in Ishikawa cells (P=0.002; Fig. S2), indicating successful
transfection. Moreover, silencing of AKIP1 significantly decreased
the relative cell viability of Ishikawa cells under treatment with
5 µM (P=0.015), 10 µM (P=0.045), 20 µM (P=0.018) and 40 µM
(P=0.049) cisplatin compared with the NC siRNA group (Fig. 4A). However, silencing of AKIP1 only
significantly decreased the relative cell viability of the Ishikawa
cells under treatment with 4 nM paclitaxel (P=0.026), but not at
other concentrations (all P>0.05) compared with the NC siRNA
group (Fig. 4B). Furthermore,
AKIP1 siRNA reduced the IC50 of paclitaxel and cisplatin
(Fig. 4C and D).
Discussion
AKIP1 has been identified as a new and potential
biomarker in several types of cancer in the clinical field
(9–12). Previous studies have indicated that
AKIP1 expression is upregulated in tumor tissue compared with that
in non-cancerous adjacent tissue in patients with clear cell renal
cell carcinoma, non-small cell lung cancer, colorectal cancer and
hepatocellular carcinoma (9–12);
however, to the best of our knowledge, there are no studies on the
clinical value of AKIP1 in patients with endometrial carcinoma.
Furthermore, most studies have detected AKIP1 protein expression by
evaluating the IHC score from surgical specimens, while the mRNA
expression levels of AKIP1 in these specimens have not been
determined. Therefore, in the present study, tumor tissues and
adjacent tissues were collected from patients with endometrial
carcinoma in order to detect AKIP1 protein levels by IHC assay and
AKIP1 mRNA expression levels by RT-qPCR, and to further explore the
clinical value of AKIP1 in patients with endometrial carcinoma. An
increase in AKIP1 expression was observed in the tumor tissues
compared with that in the adjacent tissues, which other studies
have suggested may be due to AKIP1 promoting angiogenesis through
the regulation of the NF-κB and AKT pathways. This would lead to
enhanced blood vessel formation, which is one of the hallmarks of
endometrial carcinoma (8,21,22).
In terms of the association between AKIP1 expression
and clinical features in patients with cancer, high AKIP1
expression has been reported to be associated with advanced TNM
stage in clear cell renal carcinoma, non-small cell lung cancer and
colorectal carcinoma (9–11). Moreover, high AKIP1 expression is
also correlated with poor pathological differentiation and lymph
node metastasis in patients with non-small cell lung cancer
(11). In the present study,
increased AKIP1 expression was associated with tumor invasion and
advanced FIGO grade in the patients with endometrial carcinoma. The
following reasons, suggested by other studies, could be applied to
explain these findings: i) AKIP1 promotes EMT, which further
enhances cancer invasion and metastasis by regulating the PI3K/Akt
pathway, as well as mediating the expression of transcription
factor zinc finger E-box binding homeobox 1 promoter and Slug,
which thereby leads to the presence of tumor invasion in patients
with endometrial carcinoma (7,23,24).
ii) Occurrence of lymphovascular invasion, myometrial invasion ≥1/2
and cervical stroma invasion are associated with advanced FIGO
grade in patients with endometrial carcinoma (25,26).
Therefore, elevated AKIP1 expression is associated with advanced
FIGO stage in patients with endometrial carcinoma.
The association between AKIP1 and prognosis in
patients with cancer is also noteworthy. For example, high AKIP1
protein expression has been shown to be associated with early
recurrence (defined as recurrence within 2 years) and is
independently correlated with shorter recurrence-free survival
times in patients with hepatocellular carcinoma (12). Moreover, high AKIP1 expression is
an independent factor for shorter OS time in patients with clear
cell renal carcinoma, non-small cell lung cancer and colorectal
carcinoma (9–11). In the present study, high tumor
AKIP1 protein expression was independently associated with
decreased OS time in the patients with endometrial carcinoma.
Possible reasons to explain these findings, as suggested by
previous studies, were: i) Elevated tumor AKIP1 expression
promoting endometrial carcinoma cell invasion and migration through
the mediation of multiple pathways (such as the Akt/GSK-3β and
PI3K/Akt pathways), thus leading to an enhanced risk of tumor
recurrence and an unfavorable prognosis in patients with
endometrial carcinoma (7,27,28).
ii) As illustrated in the present in vitro study, silencing
of AKIP1 enhanced the chemosensitivity to cisplatin and paclitaxel
in an endometrial carcinoma cell line, thus its overexpression may
decrease the postoperative chemosensitivity, thereby leading to an
unfavorable prognosis in patients with endometrial carcinoma.
Resistance to chemotherapy drugs is another factor
contributing to an unfavorable prognosis in patients with
endometrial carcinoma (29). A
previous study indicated that overexpression of AKIP1 promoted the
resistance to temozolomide by regulating CXCL1- and CXCL8-mediated
NF-κB and AKT pathways in glioblastoma cells (30). Moreover, AKIP1 was shown to be
associated with chemotherapy resistance in ovarian cancer using
transcriptomic data (31).
However, to the best of our knowledge, no relevant study has
investigated the effect of AKIP1 on chemosensitivity in endometrial
carcinoma. In the present study, it was shown that silencing of
AKIP1 could enhance the chemosensitivity to cisplatin and
paclitaxel (to some extent) in endometrial carcinoma cells, which
may be explained by the silencing of AKIP1 regulating multiple
chemosensitivity-related signaling pathways (such as NF-κB and AKT
pathways) to enhance cisplatin and paclitaxel chemosensitivity in
endometrial carcinoma (30,32,33).
The present findings suggested that AKIP1 was involved in
regulating the chemosensitivity of the endometrial carcinoma cell
line, which provided some evidence for further therapeutic
intervention.
The present study has several limitations. For
instance, the sample size was relatively small; therefore, further
studies with a larger sample size are needed to validate the
clinical value of AKIP1 in patients with endometrial carcinoma.
Furthermore, most patients in the current study did not live in the
local region and they did not receive long-term routine
management/follow-up after surgery in the same hospital; therefore,
the present study did not evaluate the disease relapse information
of the patients. Furthermore, the present study did not detect the
detailed molecular mechanism of AKIP1 with regard to its regulation
of chemosensitivity in endometrial carcinoma, which could be
explored in future studies.
In conclusion, the present study indicated that
elevated AKIP1 expression was associated with tumor invasion,
shorter survival times and reduced chemosensitivity in patients
with endometrial carcinoma.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FZ conceived and designed the study. ALL, TYZ, ZLM
and YLX were involved in performing the experiments and collected
the data. ALL, AJL, XPG and FZ analyzed the data. XPG prepared the
figures and tables. ALL and AJL confirm the authenticity of all the
raw data. ALL, AJL, TYZ, ZLM and YLX wrote the manuscript. XPG and
FZ revised the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Approval was acquired from the Institutional Review
Board (IRB) of Handan Central Hospital before the implementation of
the study (approval number HDZXYY-Ethics-2021015). The requirement
for written informed consent was waived by the IRB.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AKIP1
|
A-kinase-interacting protein 1
|
|
IHC
|
immunohistochemistry
|
|
FIGO
|
International Federation of Gynecology
and Obstetrics
|
|
OS
|
overall survival
|
|
NF
|
nuclear factor
|
|
EMT
|
epithelial-to-mesenchymal
transition
|
References
|
1
|
Lu KH and Broaddus RR: Endometrial cancer.
N Engl J Med. 383:2053–2064. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lortet-Tieulent J, Ferlay J, Bray F and
Jemal A: International patterns and trends in endometrial cancer
incidence, 1978-2013. J Natl Cancer Inst. 110:354–361. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee YC, Lheureux S and Oza AM: Treatment
strategies for endometrial cancer: Current practice and
perspective. Curr Opin Obstet Gynecol. 29:47–58. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Koh WJ, Abu-Rustum NR, Bean S, Bradley K,
Campos SM, Cho KR, Chon HS, Chu C, Cohn D, Crispens MA, et al:
Uterine neoplasms, version 1.2018, NCCN clinical practice
guidelines in oncology. J Natl Compr Canc Netw. 16:170–199. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Francis SR, Ager BJ, Do OA, Huang YJ,
Soisson AP, Dodson MK, Werner TL, Sause WT, Grant JD and Gaffney
DK: Recurrent early stage endometrial cancer: Patterns of
recurrence and results of salvage therapy. Gynecol Oncol.
154:38–44. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Connor EV and Rose PG: Management
Strategies for recurrent endometrial cancer. Expert Rev Anticancer
Ther. 18:873–885. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang X, Liu S and Zhu Y:
A-kinase-interacting protein 1 promotes EMT and metastasis via
PI3K/Akt/IKKβ pathway in cervical cancer. Cell Biochem Funct.
38:782–791. 2020. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang W, Wu Q, Wang C, Yang L, Liu P and
Ma C: AKIP1 promotes angiogenesis and tumor growth by upregulating
CXC-chemokines in cervical cancer cells. Mol Cell Biochem.
448:311–320. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peng H, Zhang R and Zhang H: A-kinase
interacting protein 1 high expression correlates with advanced
tumor stage and poor overall survival in surgical patients with
clear cell renal cell carcinoma. Medicine (Baltimore).
99:e207422020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu Y, Tian J, Qin D, Liu J and Xie Y:
AKIP1 expression in tumor tissue as a new biomarker for disease
monitoring and prognosis in non-small cell lung cancer: Results of
a retrospective study. J Clin Lab Anal. 34:e231282020.PubMed/NCBI
|
|
11
|
Jiang W, Yang W, Yuan L and Liu F:
Upregulation of AKIP1 contributes to metastasis and progression and
predicts poor prognosis of patients with colorectal cancer. Onco
Targets Ther. 11:6795–6801. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cui Y, Wu X, Lin C, Zhang X, Ye L, Ren L,
Chen M, Yang M, Li Y, Li M, et al: AKIP1 promotes early recurrence
of hepatocellular carcinoma through activating the
Wnt/β-catenin/CBP signaling pathway. Oncogene. 38:5516–5529. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wan X, Hong Z, Mao Y and Di W:
Correlations of AKIP1, CXCL1 and CXCL2 expressions with
clinicopathological features and survival profiles in cervical
cancer patients. Transl Cancer Res. 9:726–734. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Amant F, Mirza MR, Koskas M and Creutzberg
CL: Cancer of the corpus uteri. Int J Gynaecol Obstet. 143 (Suppl
2):S37–S50. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fang T and Lu Q: A-kinase interacting
protein 1 (AKIP1) associates with advanced overall disease
condition, tumor properties, and unfavorable prognosis in
hepatocellular carcinoma patients. J Clin Lab Anal. 34:e232132020.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu Z, Gu X, Zhong R and Zhong H:
Tumor-infiltrating CD45RO+ memory cells correlate with
favorable prognosis in patients with lung adenocarcinoma. J Thorac
Dis. 10:2089–2099. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gagnon V, Van Themsche C, Turner S,
Leblanc V and Asselin E: Akt and XIAP regulate the sensitivity of
human uterine cancer cells to cisplatin, doxorubicin and taxol.
Apoptosis. 13:259–271. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hoekstra AV, Ward EC, Hardt JL, Lurain JR,
Singh DK, Buttin BM, Schink JC and Kim JJ: Chemosensitization of
endometrial cancer cells through AKT inhibition involves FOXO1.
Gynecol Oncol. 108:609–618. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tang Y, He Y, Zhao N, Chen Y, Xing J and
Tang N: Sirtuin2 correlates with lymph node metastasis, increased
FIGO stage, worse overall survival, and reduced chemosensitivity to
cisplatin and paclitaxel in endometrial cancer. Ir J Med Sci.
191:147–154. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin C, Song L, Liu A, Gong H, Lin X, Wu J,
Li M and Li J: Overexpression of AKIP1 promotes angiogenesis and
lymphangiogenesis in human esophageal squamous cell carcinoma.
Oncogene. 34:384–393. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guo X, Zhao L, Cheng D, Mu Q, Kuang H and
Feng K: AKIP1 promoted epithelial-mesenchymal transition of
non-small-cell lung cancer via transactivating ZEB1. Am J Cancer
Res. 7:2234–2244. 2017.PubMed/NCBI
|
|
24
|
Chen D, Cao G and Liu Q:
A-kinase-interacting protein 1 facilitates growth and metastasis of
gastric cancer cells via Slug-induced epithelial-mesenchymal
transition. J Cell Mol Med. 23:4434–4442. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Grigsby PW, Massad LS, Mutch DG, Powell
MA, Thaker PH, McCourt C, Hagemann A, Fuh K, Kuroki L, Schwarz JK,
et al: FIGO 2018 staging criteria for cervical cancer: Impact on
stage migration and survival. Gynecol Oncol. 157:639–643. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Veade AE, Foote J, Ehrisman J, Broadwater
G, Davidson BA, Lee PS, Secord AA, Berchuck A and Havrilesky LJ:
Associations between lymphovascular space invasion, nodal
recurrence, and survival in patients with surgical stage I
endometrioid endometrial adenocarcinoma. World J Surg Oncol.
17:802019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mo D, Li X, Li C, Liang J, Zeng T, Su N,
Jiang Q and Huang J: Overexpression of AKIP1 predicts poor
prognosis of patients with breast carcinoma and promotes cancer
metastasis through Akt/GSK-3β/Snail pathway. Am J Transl Res.
8:4951–4959. 2016.PubMed/NCBI
|
|
28
|
Weyl A, Illac C, Lusque A, Leray H, Vaysse
C, Martinez A, Chantalat E and Motton S: Prognostic value of
lymphovascular space invasion in early-stage cervical cancer. Int J
Gynecol Cancer. 30:1493–1499. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Giannone G, Attademo L, Scotto G, Genta S,
Ghisoni E, Tuninetti V, Aglietta M, Pignata S and Valabrega G:
Endometrial cancer stem cells: Role, characterization and
therapeutic implications. Cancers (Basel). 11:18202019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Han D, Zhang N, Zhao S, Liu H, Wang X,
Yang M, Wang S, Li Y, Liu Z and Teng L: AKIP1 promotes glioblastoma
viability, mobility and chemoradiation resistance via regulating
CXCL1 and CXCL8 mediated NF-κB and AKT pathways. Am J Cancer Res.
11:1185–1205. 2021.PubMed/NCBI
|
|
31
|
Fekete JT, Ősz Á, Pete I, Nagy GR,
Vereczkey I and Győrffy B: Predictive biomarkers of platinum and
taxane resistance using the transcriptomic data of 1816 ovarian
cancer patients. Gynecol Oncol. 156:654–661. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu R, Chen Y, Liu G, Li C, Song Y, Cao Z,
Li W, Hu J, Lu C and Liu Y: PI3K/AKT pathway as a key link
modulates the multidrug resistance of cancers. Cell Death Dis.
11:7972020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Abdin SM, Tolba MF, Zaher DM and Omar HA:
Nuclear factor-κB signaling inhibitors revert multidrug-resistance
in breast cancer cells. Chem Biol Interact. 340:1094502021.
View Article : Google Scholar : PubMed/NCBI
|