Introduction
Prostate cancer (PCa) is the second most common
cancer in men, only behind lung cancer. It has been reported that
~1.6 million patients are diagnosed with PCa worldwide every year,
and 366,000 individuals succumb to PCa (1) and these numbers are increasing year
by year. Previous studies found that ~5% of diagnosed PCa patients
developed local spread, and 10% developed distant metastasis and
ultimately developed metastatic castration-resistant prostate
cancer (mCRPC) (2,3). mCRPC is an incurable PCa that
bypasses the normal pathway of androgen-dependent growth and
survival (4). Löffeler et
al (5) identified that the
median survival time of mCRPC patients without treatment was only
12.3 months. Even after treatment, the prognosis of mCRPC patients
is poor, and the expected survival time is less than 19 months
(6). This PCa type puzzles
clinicians. Compared with western countries, the incidence of PCa
in China is relatively low, but since 2008, PCa has become the most
prevalent tumour among urinary system diseases, ranking sixth in
incidence and ninth in mortality among male malignant tumours in
China (7). Therefore, identifying
optimal therapeutic means and therapeutic targets of mCRPC has been
a crucial scientific problem in PCa research in recent years. A
previous study found that the 14-3-3 protein family can be used as
a new target for PCa diagnosis and treatment (8). The 14-3-3 proteins are a family of
proteins that are conservatively expressed in eukaryotic cells and
have regulatory functions. They can regulate physiological
processes such as signal transduction, protein transport (9), cell proliferation (10) and apoptosis through
serine/threonine motif phosphorylation of target proteins. A
previous study found that the 14-3-3 protein family members 14-3-3ζ
and 14-3-3ε play roles as proto-oncogenes in PCa and can be used as
new targets for PCa therapy (8).
MicroRNAs (miR/miRNAs), conserved and endogenous
single-stranded noncoding small RNA molecules, widely participate
in physiological processes such as biological development, cell
proliferation (11), apoptosis and
differentiation (12), immune
inflammation and tumourigenesis (13,14)
and they play roles similar to tumour suppressor genes or
proto-oncogenes in tumourigenesis. miR-29b-3p is a member of the
miR-29 family, is located on chromosome 7q32 and plays distinct
roles in different types of cancer (15). miR-29b-3p is positively correlated
with MDA-MB-231 human breast cancer cells, and its overexpression
promotes MDA-MB-231 cell proliferation and migration ability
(16). Whereas miR-29b-3p was
negatively correlated with cancer cell proliferation in colon
cancer (17) and multiple myeloma
(18), Mao et al (19) found that miR-29b-3p can improve
radiosensitivity by regulating WISP1-mediated mitochondrial
apoptosis in PCa LNCaP cells. A study in PCa extracellular vesicles
suggested that miR-29b-3p can be used as a marker for PCa
extracellular vesicle detection (20). In addition, it was also revealed
that miR-29b-3p combined with miR-424-5p and miR-27a-3p had
potential diagnostic value and favourable specificity in PCa
(21). miR-29b-3p plays an
important role in the occurrence and development of PCa.
In the present study, online software was used for
predictions and it was found that miR-29b-3p is a vital candidate
miRNA for regulating expression of tyrosine
3-monooxygenase/tryptophan 5-monooxygenase activation protein
epsilon (YWHAE). However, the molecular mechanism by which
miR-29b-3p targets YWHAE to regulate PCa proliferation is unclear.
Therefore, the aim of the present study was to explore whether
miR-29b-3p controls PCa cell proliferation and apoptosis via YWHAE.
The results revealed that miR-29b-3p acts as a tumour suppressor
and is upregulated in PCa 22Rv1 cells, significantly decreases the
expression level of YWHAE, and reduces the ratio of p-BAD/BAD,
BCL-2/Bax and full-length caspase 3/cleaved caspase 3. Furthermore,
miR-29b-3p inhibits PCa 22Rv1 cell proliferation through the
YWHAE/BCL-2 regulatory axis. The present results may provide a
theoretical basis for the diagnosis and targeted therapy of
PCa.
Materials and methods
Bioinformatics analysis
The co-expression relationship between miR-29b-3p
and YWHAE in prostate adenocarcinoma tissues was analysed using the
ENCORI online website (https://starbase.sysu.edu.cn/index.php). In addition,
the expression levels of YWHAE in prostate adenocarcinoma tissues
and normal tissues were analysed using the UALCAN-The Cancer Genome
Atlas (TCGA) online database (http://ualcan.path.uab.edu/analysis.html).
Cells and antibodies
The human PCa cell lines LNCaP, PC3 and
22Rv1 and the human prostate stromal immortalized cell line WPMY-1
were purchased from Shanghai Zhongqiao Xinzhou Biotechnology Co.,
Ltd. LNCaP cells were cultured in RPMI-1640 complete medium
containing 10% fetal bovine serum (both from Gibco; Thermo Fisher
Scientific, Inc.), 1% penicillin-streptomycin, 2% HEPES, 1%
glutamine and 1% sodium pyruvate (Invitrogen; Thermo Fisher
Scientific, Inc.). PC3, 22Rv1, and WPMY-1 cells were cultured in
DMEM/F12, RPMI-1640, and high-glucose DMEM (Gibco; Thermo Fisher
Scientific, Inc.) containing 10% fetal bovine serum and 1%
streptomycin, respectively. All cells were cultured in a constant
temperature incubator at 37°C and 5% CO2. Anti-YWHAE
(cat. no. 66946-1-Ig), anti-GAPDH (cat. no. 60004-1-Ig), anti-CCND1
(cat. no. 60186-1-Ig) mouse monoclonal antibodies and BAD (cat. no.
10435-1-ap) and BAX (cat. no. 50599-2-Ig) rabbit polyclonal
antibodies were purchased from ProteinTech Group, Inc. The rabbit
monoclonal antibodies anti-BCL2 (cat. no. ab182858) and anti-BAD
(phospho S112) (cat. no. ab129192) were purchased from Abcam. The
mouse monoclonal antibody anti-caspase 3 (cat. no. 9668) was
purchased from Cell Signaling Technology, Inc. HRP-conjugated goat
anti-rabbit IgG, HRP-conjugated goat anti-mouse IgG, and ECL
ultrasensitive colour development kits were purchased from
Biyuntian Biotechnology Co., Ltd.
miRNA screening, sequence synthesis,
and vector construction
miRNAsystem (http://mirsystem.cgm.ntu.edu.tw/) online software
predicts, screens, and regulates the potential miRNAs of YWHAE.
Microrna.org (http://www.microrna.org/microrna/home.do) online
software analysis was used to predict the possible binding sites
between miR-29b-3p and YWHAE, and primer mutation was performed at
the YWHAE/3′untranslated region (UTR) interaction site to construct
the mutant (MT) vector. The miR-29b-3p mimic sequence and NC mimic
sequence of the negative control group were biosynthesized by
Shanghai GenePharma Co., Ltd. The expression vector pCI YWHAE in
the CD region of the YWHAE gene and the wild-type (WT) vector
pGL-WT-YWHAE/3′UTR of the YWHAE gene were constructed and preserved
by the Key Laboratory of genetics, breeding, and reproduction of
plateau mountain animals of the Ministry of Education. The 3′UTR MT
vector of the YWHAE gene was sent to Beijing Qingke Biotechnology
Co., Ltd. to synthesize the MT sequence and was then re-ligated
with the pmirGLO vector to construct the pGL-MT-YWHAE/3′UTR MT
vector.
Reverse transcription-quantitative
(RT-q) PCR
Total RNA was extracted from cells according to the
protocol for the TRIzol® RNA Extraction kit (Invitrogen;
Thermo Fisher Scientific, Inc.). Then, 2,000 ng of RNA was
extracted and reverse transcribed using the RevertAid first-strand
cDNA synthesis kit (Thermo Fisher Scientific, Inc.) following the
manufacturer's protocol. Reaction conditions were as follows:
Initial denaturation at 95°C for 5 min, followed by denaturation at
95°C for 10 sec, annealing at 55°C for 25 sec and extension at 72°C
for 10 sec, for 40 cycles. The reverse transcription primer
sequence of miR-29b-3p was
5′-GGTCGTATGCAAAGCAGGGTCCGAGGTATCCATCGCACGCATCGCACTGCATACGACCAACACTGA-3′,
and the reverse transcription primer sequence of U6 was
5′-AACGCTTCACGAATTTGCGT-3′. The expression levels of miR-29b-3p in
LNCaP, PC3, 22Rv1, and WPMY-1 cells were detected using a Bio-Rad
C1000 fluorescence quantitative PCR instrument according to the
protocol of the Quanti Fast® SYBR® Green PCR
kit (Bio-Rad Laboratories, Inc.). U6 was used as an internal
reference, and the 2−ΔΔCq method was used for data
analysis (22). The primer
sequences were as follows: miR-29b-3p forward,
5′-TCGGCTAGCACCATTTGAAAT-3′ and reverse,
5′-CAAAGCAGGGTCCGAGGTATC-3′; and U6 (internal reference) forward,
5′-CTCGCTTCGGCAGCACA-3′ and reverse,
5′-AACGCTTCACGAATTTGCGT-3′.
Cell transfection and luciferase
test
To detect the effect of miR-29b-3p on YWHAE
expression, the experiment was divided into four groups: miR-29b-3p
+ pGL-WT-YWHAE/3′UTR, miR-29b-3p + pGL-MT-YWHAE/3′UTR, NC +
pGL-MT-YWHAE/3′UTR, and NC + pGL-MT-YWHAE/3′UTR, with 6 replicates
in each group. 22Rv1 cells were co-transfected with
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) in 96-well plates. The transfection system of the
96-well plate was as follows: 6 µl OPTI-MEM® + 100 ng
plasmid + 30 nM miRNA mimics + 0.6 µl transfection reagent, mixed
by vortex oscillation and incubated at room temperature for 10 min.
After 24 of transfection, the Dual-Glo® Luciferase Assay
System (Promega Corporation) operation instructions were combined
with a multifunctional enzyme labelling instrument (BioTek
Instruments, Inc.), and firefly luciferase and Renilla
luciferase activity levels were measured. The regulatory activity
of miR-29b-3p on the 3′-UTR of YWHAE was expressed by the ratio of
firefly to Renilla luciferase activity (22).
Cell proliferation assay
22Rv1 cells were seeded at a density of
2×103 cells/well into 96-well plates. miR-29b-3p and
negative control groups were transfected with 6 replicates in each
group. Cell activity was detected at 0, 24, 48 and 72 h after
transfection. Each test point had the following steps: first, the
old medium was discarded, and then 100 µl of fresh medium and 10 µl
of Cell Counting Kit-8 (Shanghai Shangbao Biotechnology Co., Ltd.)
mixed solution was added and continuously cultured in a cell
incubator at 37°C for 3 h. Then, the OD value at 490 nm was
detected by a multifunctional microplate reader. A cell growth
curve was drawn, and the effect of miR-29b-3p on 22Rv1 cell
proliferation was analysed (22).
Transwell assay
Transwell assays are generally used to detect cell
migration and invasion. In the present study, the effect of
miR-29b-3p on the invasive ability of 22Rv1 cells was detected
using this test. The specific steps were as follows: the matrix
glue was diluted at a ratio of 1:8, and 100 µl was added to each
well, followed by a Transwell chamber with a diameter of 8 µm. The
plates were incubated in a 5% CO2 incubator at 37°C for
3 h. Then, 600 µl of complete medium containing 20% foetal bovine
serum was added to the lower chamber, and 150 µl of serum-free
medium was added in each upper chamber, cell suspension at
2×105 cells/well was added to the upper chamber and
cultured in a CO2 incubator for 24 h. Then, the
Transwell chamber was removed, the culture medium in the well was
discarded, and the Transwell was moved to a well with 800 µl of
methanol and fixed at room temperature for 20 min. The upper
chamber was then removed, the fixing liquid was discarded, and the
Transwell was moved into a well with 800 µl of Giemsa dye solution
(Beijing Solarbio Science & Technology Co., Ltd.) (1:9 ratio)
and stained at room temperature for 30 min. The Transwell was then
gently washed with ddH2O 4 times, removed, gently wiped
with a cotton swab to absorb the residual liquid in the upper
chamber, and images were captured under an inverted fluorescence
microscope (magnification, ×200). The number of cells in five
random regions was calculated to evaluate their invasive ability
(22).
Cell scratch assay
22Rv1 cells were seeded in six-well plates and
cultured at 37°C overnight. When the cell abundance reached 70%,
the cells were transfected with antibiotic-free medium with 3%
serum. After transfection for 6 h, a 100-µl sterile pipette tip was
used to scratch the culture plate, PBS was used to wash the cells
twice, 2 ml of complete medium was added, images were captured
under an inverted fluorescent microscope at the same position at 0
and 24 h after scratching, and the migration distance was
calculated. Then, the effects of different treatments on the
migration ability of 22Rv1 cells were analysed (22).
Flow cytometry
22Rv1 cells in the logarithmic growth stage at a
density of 2×105 cells per well were inoculated in a
six-cm petri dish. After the cells covered 70% of the cell plate,
miR-29b-3p, pCI-YWHAE, and miR-29b-3p + pCI-YWHAE were transfected.
A total of 3 double wells were transfected in each group, and the
experiment was repeated three times independently. After 24 h of
transfection, the cells were collected and washed twice with 1 ml
of precooled PBS and then resuspended in PBS. Then, precooled
absolute ethanol was added to a final concentration of 70%. After
gentle mixing, the cells were fixed at 4°C overnight. The next day,
the cells were collected by centrifugation. According to the
instructions of the cell cycle and apoptosis detection kit (cat.
no. c1052) from Biyuntian Biotechnology Co., Ltd., 0.5 ml of
propidium iodide staining solution was added to each tube, the
cells were resuspended and incubated at 37°C in the dark for 30
min, and the cell cycle and early apoptosis were detected by flow
cytometry (Model C6; BD Biosciences) (11). The results were analysed using
FlowJo software (FlowJo LLC).
Western blot analysis
After 48 h of cell transfection, the total protein
was extracted with RIPA lysis buffer (Beyotime Institute of
Biotechnology, Inc.) preapplied with PMSF. Protein quantification
was performed with a BCA assay kit (Beijing Solarbio Science &
Technology Co., Ltd.). Total protein (20 µg) was separated by
SDS-PAGE on gels containing 30% acrylamide and transferred onto
PVDF membranes. Subsequently, 5% skimmed milk powder was used for
blocking at room temperature for 2 h. After blocking, membranes
were incubated overnight at 4°C with a primary antibody (1:2,000).
The next day, the membrane was rinsed with TBST buffer containing
0.1% Tween-20 3 times for 10 min each time. Then, HRP-labelled
secondary antibody (1:1,000) was added and incubated at room
temperature for 1 h. Finally, the images were obtained using a
supersensitive ECL chemiluminescence liquid and colour imaging
system, and ImageJ V1.8.0 software (National Institutes of Health)
was used for densitometric analysis (22).
Tumour formation experiment in nude
mice
All animal experiments were approved (approval no.
EAE-GZU-2020-T037) by the animal Ethics Committee of Guizhou
University (Guiyang, China). Nude mice were used for in vivo
studies and were cared for in accordance with the Guide for the
Care and Use of Laboratory Animals published by the National
Institutes of Health. The housing conditions of the nude mice was
as follows: Temperature, 28°C; relative humidity, 50%; ventilation,
12 times per h; 10-h light and 14-h dark cycle per day. Food was
autoclaved and drinking water was sterile water containing a
mixture of vitamins, and both were available ad libitum. A
total of 15 male BALB/c-nude mice (5-weeks-old; weight, 18±1.5 g)
were randomly divided into 3 groups (5 mice in each group). 22Rv1
cells transfected with pCI-YWHAE, miR-29b-3p, and miR-29b-3p +
YWHAE (3×106) suspended in 100 µl of PBS were
subcutaneously injected into the right scapula of nude mice once
every other day for 7 days. When the tumour size reached 12–20 mm
and the total volume of each tumour did not exceed 4,400
mm3, the mice were euthanized and tumour were resected
and weighed. The length (a, cm) and width (b, cm) of the tumour
were measured with Vernier callipers at 7, 10, 13, 16, 19, 22, 25
and 28 days after inoculation. The formula v=0.5 × a ×
b2 was used to calculate the tumour volume (14).
Statistical analysis
All experiments were repeated more than 3 times and
the results are expressed as the mean ± standard deviation. ImageJ
(National Institutes of Health), SPSS 19.0 data analysis software
(IBM Corp.), and GraphPad Prism 5 graphics analysis software
(GraphPad Software, Inc.) were used for statistical analysis and
chart processing, respectively. P<0.05 was considered to
indicate a statistically significant difference. When multiple
comparisons were performed, different lowercase letters indicate a
significant difference (P<0.01) and the same letter indicates no
significant difference.
Results
miR-29b-3p is expressed at low levels
and is negatively associated with YWHAE in PCa
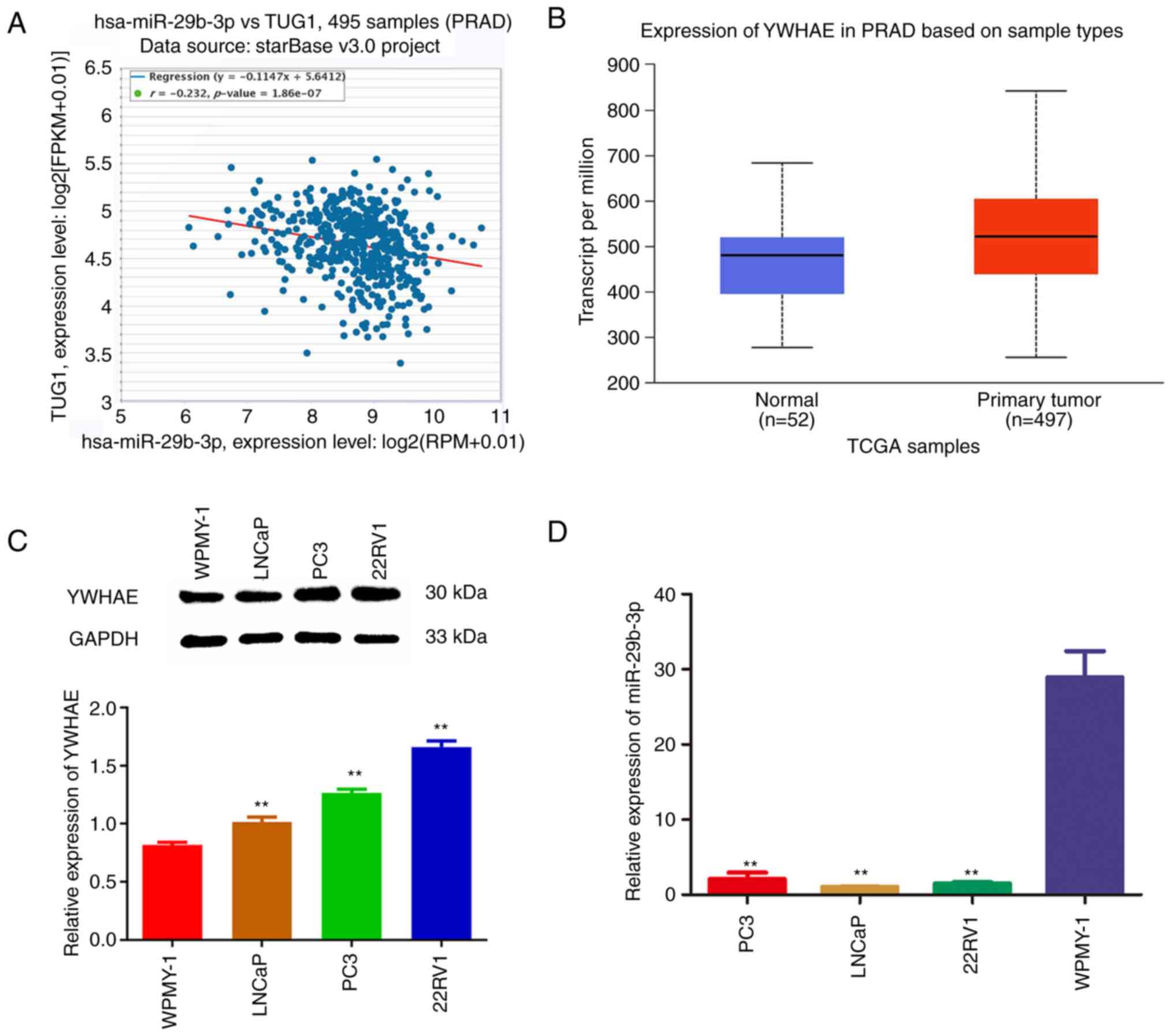
To determine the expression relationship between
miR-29b-3p and YWHAE, the co-expression relationship between
miR-29b-3p and YWHAE was first analysed in 495 PCa samples using
the ENCORI online website (https://starbase.sysu.edu.cn/index.php). The results
showed that there was a negative association between them (Fig. 1A). In addition, the expression
levels of YWHAE in prostate adenocarcinoma tissues (n=497) and
normal tissues (n=52) were analysed using the UALCAN-TCGA online
database (http://ualcan.path.uab.edu/analysis.html). The
expression of YWHAE was upregulated in PCa tissues compared with
normal tissues (Fig. 1B). To
further verify the prediction results of YWHAE in the TCGA
database, the expression levels of YWHAE were detected in different
PCa cell lines using western blotting. The results revealed that
the expression levels of YWHAE in LNCaP, PC3 and 22Rv1 cells were
significantly higher than that in WPMY-1 cells (P<0.01; Fig. 1C). These results revealed that
YWHAE is significantly upregulated in PCa tissues and cells and may
play a role as a proto-oncogene in PCa. Concurrently, to verify the
negative association between miR-29b-3p and YWHAE, the expression
levels of miR-29b-3p in human PCa LNCaP, PC3, 22Rv1 and WPMY-1 were
investigated using RT-qPCR. The results demonstrated that the
expression levels of miR-29b-3p in PC3 cell lines were
significantly lower than that in WPMY-1 cells (P<0.01; Fig. 1D), which indicated that miR-29b-3p
plays a tumour suppressor role and that its upregulation may affect
the proliferation of cancer cells.
YWHAE is the candidate target gene of
miR-29b-3p
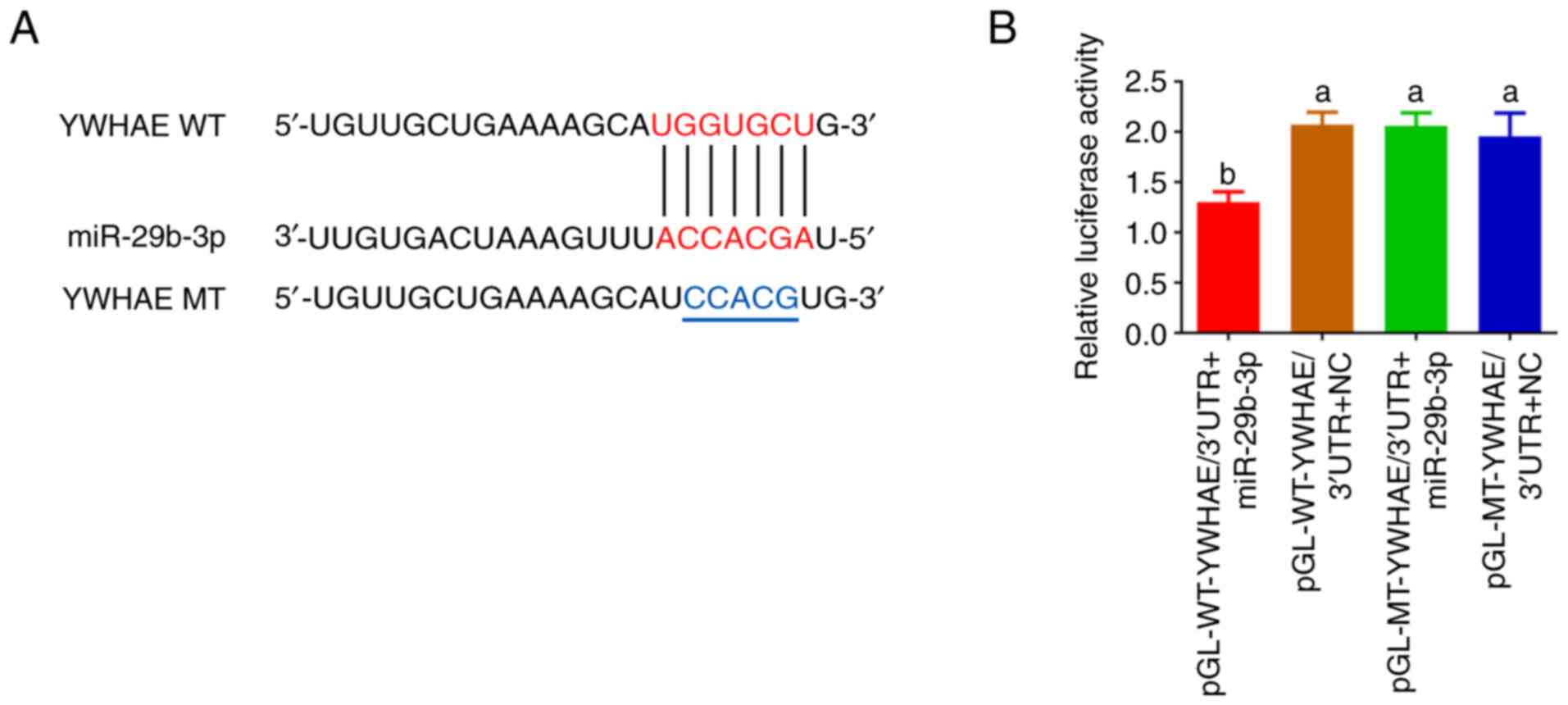
To identify the interaction site between miR-29b-3p
and YWHAE, YWHAE/3′UTR WT and MT double luciferase reporter vectors
were constructed and a mutation of 5 bases was introduced at the
interaction site (Fig. 2A). It was
identified that the luciferase activity of the transfected
pGL-WT-YWHAE/3′UTR + miR-29b-3p group was significantly lower than
that of the transfected pGL-WT-YWHAE/3′UTR + NC group (P<0.01).
However, there was no significant difference between the
transfected pGL-MT-YWHAE/3′UTR + miR-29b-3p group and the
pGL-MT-YWHAE/3′UTR + NC group (P>0.05; Fig. 2B), indicating that the mutation of
the YWHAE/3′UTR interaction site significantly affected the
association between miR-29b-3p and YWHAE. The aforementioned
results suggested that YWHAE may be a direct target gene of
miR-29b-3p and ‘ACCACGA’ may be a direct target for miR-29b-3p to
regulate YWHAE expression.
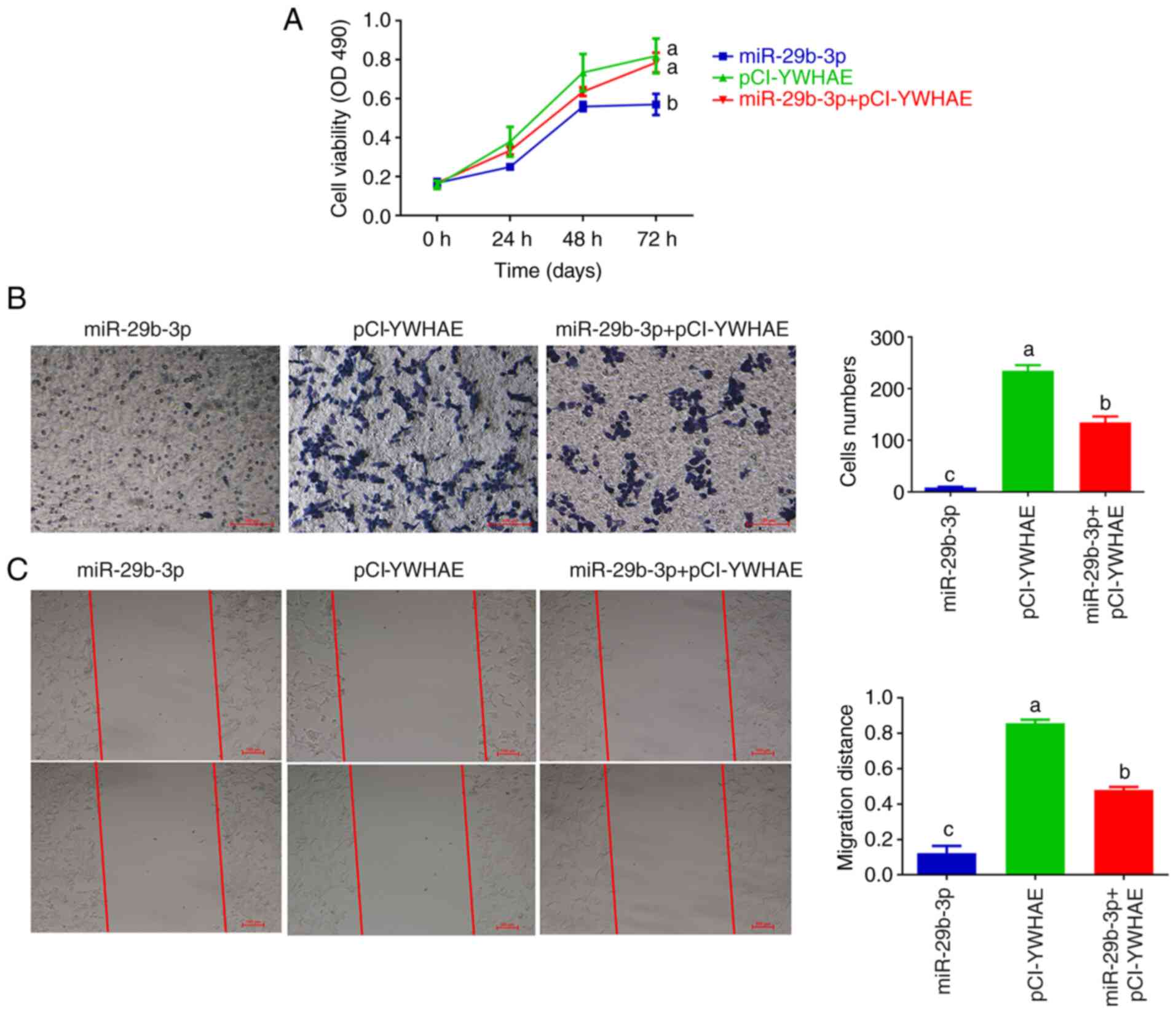
Overexpression of miR-29b-3p inhibits
22Rv1 cell proliferation by downregulating YWHAE expression
A previous study demonstrated that YWHAE knockout
significantly decreased the proliferation of 22Rv1 cells (21). Therefore, it was inferred that the
tumour inhibitory effect of miR-29b-3p in 22Rv1 cells may be
achieved by inhibiting the expression of YWHAE. To examine this
hypothesis, 22Rv1 cells were transfected with miR-29b-3p,
pCI-YWHAE, or miR-29b-3p + pCI-YWHAE. The effects of miR-29b-3p and
YWHAE on the proliferation, migration and invasion of 22Rv1 cells
were observed by compensation experiments. The results revealed
that in the miR-29b-3p transfection group, the proliferation
(Fig. 3A), invasion (Fig. 3B), and migration ability (Fig. 3C) of 22Rv1 cells decreased
significantly (P<0.01) and increased considerably in the
pCI-YWHAE transfection group. In the miR-29b-3p + pCI-YWHAE
co-transfection group, the tumour inhibitory effect of miR-29b-3p
on 22Rv1 cells could be partially reversed after pCI-YWHAE
introduction. The aforementioned results suggested that miR-29b-3p
plays a tumour inhibitory role in 22Rv1 cells by suppressing the
expression of YWHAE.
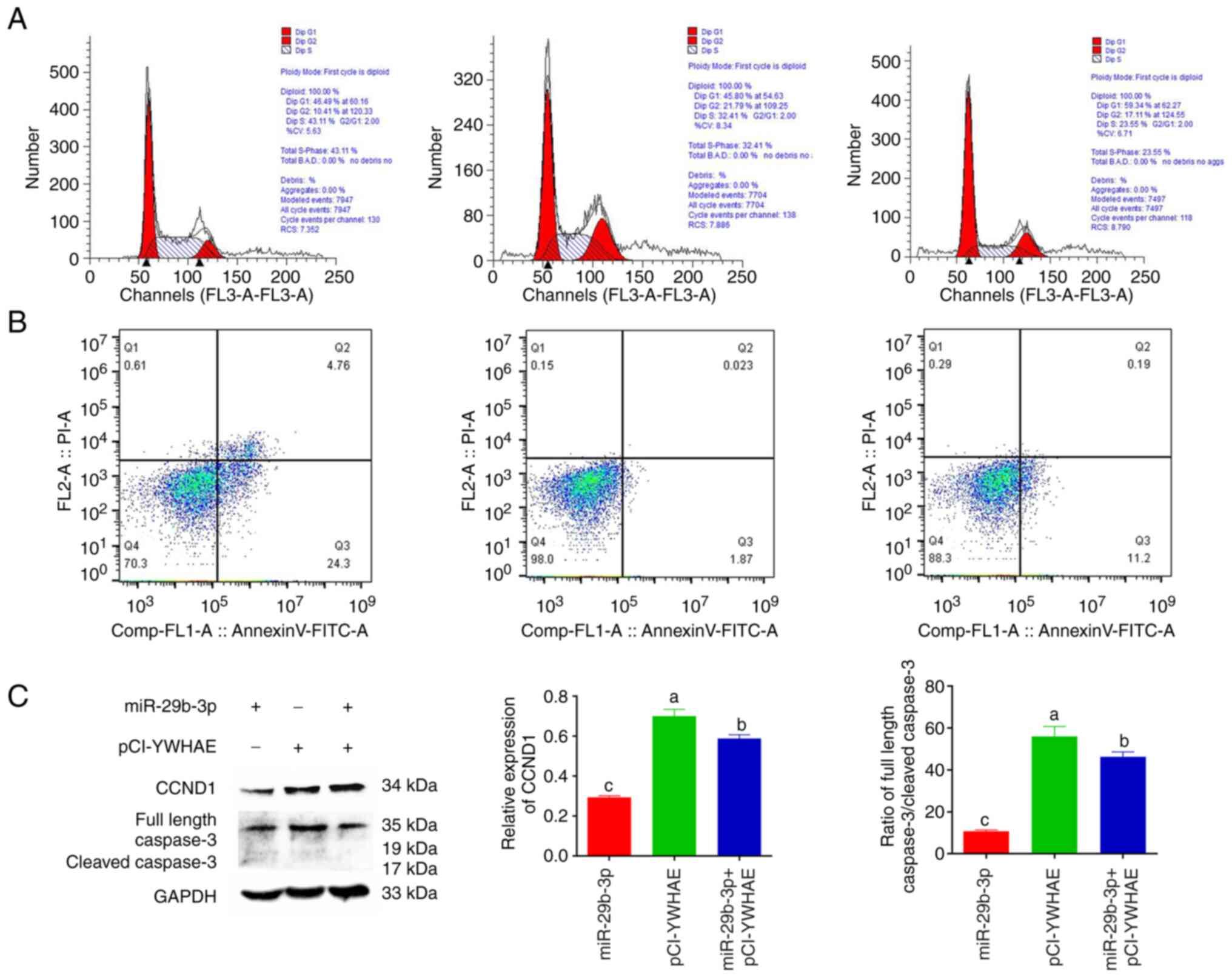
miR-29b-3p positively regulates the
cycle and apoptosis of 22Rv1 cells by inhibiting the expression of
YWHAE
To verify the effects of miR-29b-3p regulating YWHAE
on the 22Rv1 cell cycle and apoptosis, the effects of transfection
of miR-29b-3p, pCI-YWHAE, miR-29b-3p + pCI-YWHAE on the 22Rv1 cell
cycle and apoptosis were assessed by flow cytometry. The expression
changes of cyclin CCND1 and the apoptotic protein caspase 3 were
detected using western blotting. Cell cycle results revealed that
YWHAE overexpression promoted the development of 22Rv1 cells from
G1 phase to G2/M phase, which was conducive to cell proliferation.
By contrast, the overexpression of miR-29b-3p seriously blocked the
progression from G1 phase to G2/M phase, resulting in a longer
duration of cells remaining in G1 phase, thus inhibiting cell
proliferation. When miR-29b-3p and YWHAE were co-transfected into
22Rv1 cells, the inhibitory effect of miR-29b-3p on the 22Rv1 cell
cycle was partially reversed (Fig.
4A). Apoptosis results showed that compared with the YWHAE
transfection group, the miR-29b-3p transfection group promoted the
early apoptosis of 22Rv1 cells. Similarly, after co-transfection of
the two cell lines, YWHAE partially reversed the early apoptosis
ability of miR-29b-3p in 22Rv1 cells (Fig. 4B). In addition, to further prove
the aforementioned results, the expression of the cell cycle marker
protein CCND1 and the apoptosis marker protein caspase 3 were
verified in different transfection groups by western blotting. The
results showed that the expression of CCND1 and caspase 3 protein
was the lowest in the miR-29b-3p transfection group and had a very
significant difference (P<0.01) and was the highest in the
pCI-YWHAE transfection group (Fig.
4C). These results further confirmed that miR-29b-3p could
affect the 22Rv1 cell cycle and apoptosis by inhibiting the
expression of YWHAE.
miR-29b-3p suppresses 22Rv1 cell
proliferation through the YWHAE/BCL-2 regulatory axis
Min et al (23) found that a variety of
tumour-related mRNAs regulate the survival of dendritic cells by
targeting the YWHAZ and BCL-2 signalling pathways (23,24).
YWHAE and YWHAZ belong to the 14-3-3 protein family and play
essential roles in cell proliferation and apoptosis. The regulatory
effect of miR-29b-3p on YWHAE may also be mediated through the
BCL-2 pathway. To examine this hypothesis, western blotting was
used to study the changes in the protein expression levels of BAX,
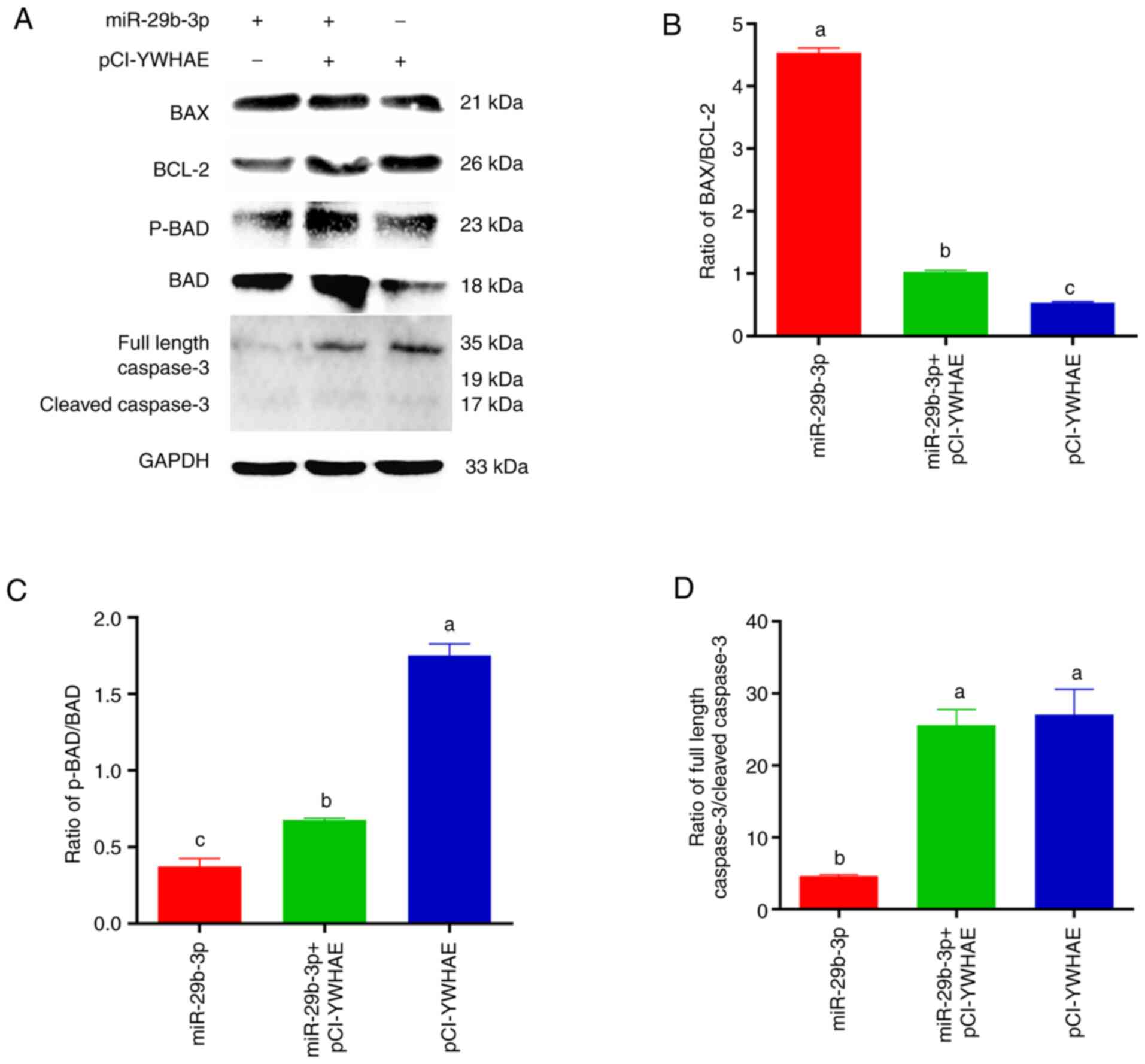
BCL-2, p-BAD, BAD and caspase 3 in the BCL-2 pathway (Fig. 5A). In the transfected miR-29b-3p
group, the ratios of p-BAD/BAD and full-length caspase 3/cleaved
caspase 3 decreased significantly (P<0.01), and the ratio of
BAX/BCL-2 was significantly upregulated (P<0.01). Notably, in
the transfected pCI-YWHAE group, the opposite results were
observed, and the aforementioned results were inhibited to varying
degrees in the co-transfected group (Fig. 4B-D). Due to the upregulation of
BAX/BCL-2 and the downregulation of p-BAD/BAD and full-length
caspase 3/cleaved caspase 3, the proliferation rate of cells
usually slows down, and cells gradually move towards apoptosis. In
conclusion, this result confirmed that miR-29b-3p plays a tumour
suppressor role in 22Rv1 cells, and the YWHAE/BCL-2 regulatory axis
plays an important role in miR-29b-3p regulating PCa 22Rv1 cell
proliferation and apoptosis.
miR-29b-3p inhibits xenograft tumour
growth by targeting YWHAE
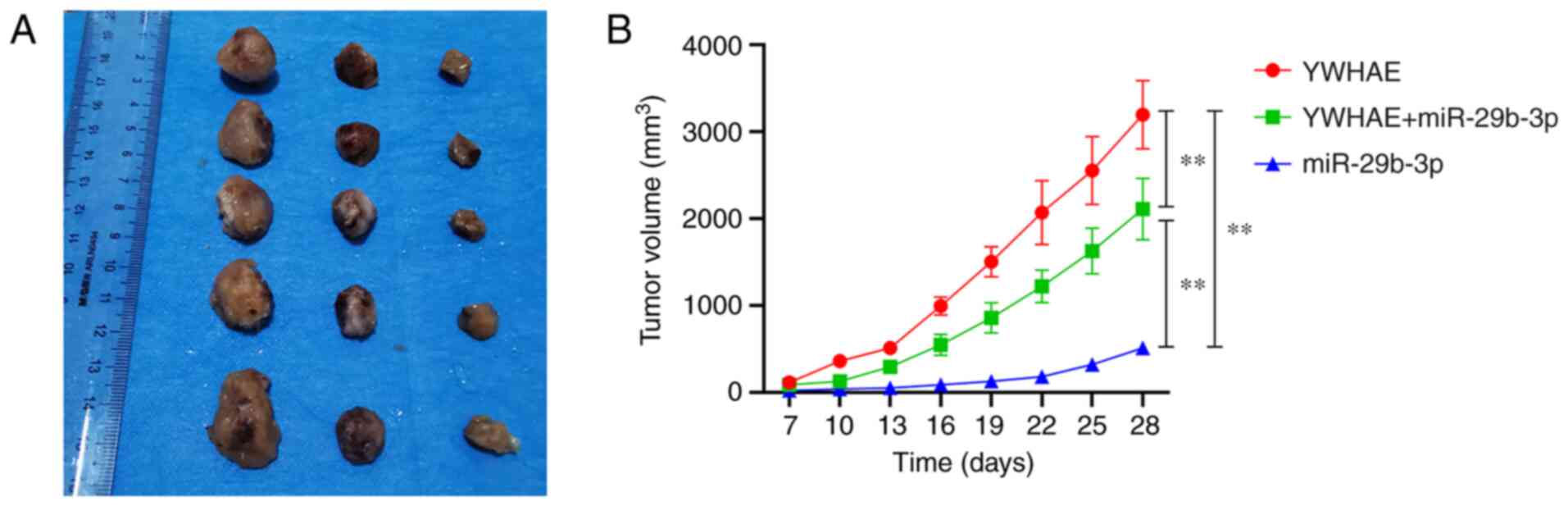
To confirm whether miR-29b-3p and YWHAE affect the
occurrence of PCa tumours in vivo, 22Rv1 cells transfected
with pCI-YWHAE, miR-29b-3p mimics, and miR-29b-3p + pCI-YWHAE for
36 h were injected subcutaneously into the left scapula of
BALB/c-nude mice. The volume of the subcutaneously transplanted
tumour was measured every 3 days from the 7th day after injection.
On day 28, mice were euthanized, and tumour images were captured
(Fig. 6A). The results revealed
that the tumour volume of mice injected with miR-29b-3p mimics was
significantly inhibited compared with those of the other two groups
(P<0.01), and the tumour volume of the YWHAE group was
significantly larger than those of the other two groups
(P<0.01). However, compared with the miR-29b-3p and pCI-YWHAE
groups, the tumour volume of mice in the combined injection of
miR-29b-3p and pCI-YWHAE group was between those of the miR-29b-3p
group and the YWHAE group, with a very significant difference
(P<0.01; Fig. 6B). These
results indicated that miR-29b-3p upregulation inhibits tumour
growth in vivo by targeting YWHAE.
Discussion
CRPC is a manifestation of advanced PCa. Almost all
PCa patients will develop CRPC after endocrine therapy. Therefore,
CRPC is also known as androgen-independent PCa (AIPC). AIPC is an
incurable PCa that bypasses the normal androgen-dependent growth
and survival pathway and is a PCa type that puzzles clinicians. An
increasing number of studies have shown that the expression levels
of miR-29b in various malignant and cancerous cell lines are lower
than those in normal tissues or cell lines (25), and its overexpression can inhibit
tumour occurrence and angiogenesis (26–28),
usually as a tumour suppressor in colorectal cancer (29–31),
breast cancer (32) and
osteosarcoma (33). In 2020, Mao
et al (19) identified that
overexpression of miR-29b-3p reduced cell viability, cell
proliferation and colony formation, resulting in increased
sensitivity of LNCaP cells to X-rays. In the present study, the
potential role of the miR-29b-3p, YWHAE and BCL-2 regulatory axis
in regulating the proliferation and apoptosis of advanced PCa cells
was investigated. The upregulation of miR-29b-3p inhibited the
expression of YWHAE and activated the mitochondrial apoptosis
pathway. By affecting the expression of BCL-2 apoptosis family
proteins, it inhibited the proliferation, migration and invasion of
22Rv1 cells and promoted cell apoptosis.
14-3-3ε, a member of the 14-3-3 family, is encoded
by the YWHAE gene and was first detected in mammalian brain tissue
by Moore et al in 1968 (34).
Studies have confirmed that YWHAE participates in cell
proliferation, apoptosis, invasion and metastasis in breast cancer
cells (35), the gastric cancer
cell line SGC7901 (36) and
hepatocellular carcinoma (37).
Similarly, a previous study on PCa also found that the 14-3-3
family plays an important role in the occurrence and development of
PCa and can be used as a potential drug target for the treatment of
PCa (8). Notably, it was
identified that YWHAE and miR-29b-3p had opposite expression
patterns in different PCa tissues and cells. At the same time, with
the help of miRNA online prediction software and a luciferase
reporting system, it was further confirmed that miR-29b-3p had a
targeted regulatory relationship with YWHAE. In addition, Sur et
al (14) revealed that the
expression of miR-29b-3p was reduced or absent in PCa tissues and
cell lines and the expression of miR-29b-3p was closely related to
the aetiology, classification, progression and prognosis of PCa
patients (38). In addition,
miR-29b has also been identified as a regulator of epithelial to
mesenchymal transition, which is involved in PCa metastasis
(39) and chemoresistance
(40,41). In the present study, cell
proliferation, invasion, migration, cycle and apoptosis and
tumourigenesis experiments in nude mice confirmed that YWHAE plays
a proto-oncogene role in PCa and that miR-29b-3p plays the role of
a tumour suppressor gene. This result is consistent with the
results of previous studies (14,22).
In addition, in the molecular mechanism by which
miR-29b-3p inhibits PCa proliferation, it was identified that
YWHAE, as a potential target gene of miR-29b-3p, was involved in
the regulation of PCa by miR-29b-3p. The study on the molecular
mechanism of the YWHAE protein showed that YWHAE, as a member of
the 14-3-3 family, has the same effect as YWHAZ and YWHAG. It can
bind to the BCL-2 family apoptosis-regulating protein BAX, prevent
BAX from entering mitochondria and terminate the regulation of
apoptosis by BAX (22–24). Furthermore, a study revealed that
the BAD and BCL-2 proteins play important roles in the effects of
14-3-3 proteins on cell proliferation and apoptosis (42). Moreover, it is generally considered
that high BAX and/or low BCL-2 and high BAX/BCL-2 ratios are
conducive to apoptosis (43). In
the present experiment, it was found that the overexpression of
miR-29b-3p promoted an increase in the BAX/BCL-2 ratio and a
decrease in the p-BAD/BAD and full-length caspase 3/cleaved caspase
3 ratios, while the addition of YWHAE reversed this phenomenon.
Therefore, the aforementioned results suggested that YWHAE and
BCL-2 play a vital role in inhibiting PCa cell proliferation by
miR-29b-3p. The upregulation of miR-29b-3p inhibits the expression
of YWHAE, indirectly affects the expression of apoptosis proteins
such as BCL-2, BAX and BAD, activates the expression of caspase 3
protein and then activates the mitochondrial apoptosis pathway.
In conclusion, it was demonstrated in the present
study that miR-29b-3p plays an anticancer role in PCa by targeting
YWHAE, and miR-29b-3p inhibits the proliferation of PCa 22Rv1
cells, which is likely to be realized by the YWHAE/BCL-2 regulatory
axis. The results provided a theoretical basis for further study of
miR-29b-3p and YWHAE as potential prognostic biomarkers of CRPC and
promising therapeutic targets of CRPC. However, the present study
still has some limitations. For example, in the experiment to
verify the targeted regulation of miR-29b-3p in YWHAE, only the
dual luciferase reporting system was adopted to verify the
targeting relationship between miR-29b-3p and YWHAE. If the results
can be verified through RNA immunoprecipitation experiments, this
will be more convincing.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural Science
Foundation of Guizhou Province [grant no. QKHJC (2020) 1Y085], the
Natural Science Foundation of China (grant no. 31860242) and the
Guizhou Province Science and Technology Plan Project [grant no.
Guizhou Branch Platform Talents (2019) 3336].
Availability of data and materials
All data generated or analysed during this study are
included in this published article.
Authors' contributions
JZ was responsible for data curation, statistical
analysis, writing the original draft and project administration. XM
was responsible for the investigation and data curation. HX was
responsible for study design/conception, study supervision,
writing/reviewing and editing the manuscript, and project
administration. JZ and HX confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were approved (approval no.
EAE-GZU-2020-T037) by the animal Ethics Committee of Guizhou
University (Guiyang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AIPC
|
androgen independent prostate
cancer
|
|
CCK-8
|
Cell Counting Kit-8
|
|
CRPC
|
castration-resistant prostate
cancer
|
|
ECL
|
electrochemiluminescence
|
|
EMT
|
epithelial-to-mesenchymal
transition
|
|
HRP
|
horse radish peroxidase
|
|
MT
|
mutant
|
|
NC
|
negative control
|
|
PBS
|
Dulbecco's phosphate medium
|
|
PCA
|
prostate cancer
|
|
PVDF
|
polyvinylidene fluoride
|
|
RT-qPCR
|
reverse transcription-quantitative
PCR
|
|
RIPA
|
radio immunoprecipitation assay
|
|
TCGA
|
The Cancer Genome Atlas
|
|
UTR
|
untranslated regions
|
|
WT
|
wild-type
|
|
YWHAE
|
tyrosine 3-monooxygenase/tryptophan
5-monooxygenase activation protein epsilon
|
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu M, Huang Y, Chen T, Wang W, Yang S, Ye
Z and Xi X: LncRNA MEG3 inhibits the progression of prostate cancer
by modulating miR-9-5p/QKI-5 axis. J Cell Mol Med. 23:29–38. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gillessen S, Omlin A, Attard G, de Bono
JS, Efstathiou E, Fizazi K, Halabi S, Nelson PS, Sartor O, Smith
MR, et al: Management of patients with advanced prostate cancer:
Recommendations of the St Gallen advanced prostate cancer consensus
conference (APCCC) 2015. Ann Oncol. 26:1589–1604. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Taghizadeh H, Marhold M, Tomasich E,
Udovica S, Merchant A and Krainer M: Immune checkpoint inhibitors
in mCRPC-rationales, challenges and perspectives. Oncoimmunology.
8:e16441092019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Löffeler S, Weedon-Fekjaer H, Wang-Hansen
MS, Sebakk K, Hamre H, Haug ES and Fosså SD: ‘Natural course’ of
disease in patients with metastatic castrate-resistant prostate
cancer: Survival and prognostic factors without life-prolonging
treatment. Scand J Urol. 49:440–445. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Heidenreich A, Pfister D, Merseburger A
and Bartsch G; German Working Group on Castration-Resistant
Prostate Cancer (GWG-CRPC), : Castration-resistant prostate cancer:
Where we stand in 2013 and what urologists should know. Eur Urol.
64:260–265. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Culp MB, Soerjomataram I, Efstathiou JA,
Bray F and Jemal A: Recent global patterns in prostate cancer
incidence and mortality rates. Eur Urol. 77:38–52. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Root A, Beizaei A and Ebhardt HA:
Structure-based assessment and network analysis of targeting 14-3-3
proteins in prostate cancer. Mol Cancer. 17:1562018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aghazadeh Y and Papadopoulos V: The role
of the 14-3-3 protein family in health, disease, and drug
development. Drug Discov Today. 21:278–287. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Neal CL, Xu J, Li P, Mori S, Yang J, Neal
NN, Zhou X, Wyszomierski SL and Yu D: Overexpression of 14-3-3ζ in
cancer cells activates PI3K via binding the p85 regulatory subunit.
Oncogene. 31:897–906. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Peng H, Wang L, Su Q, Yi K, Du J and Wang
Z: MiR-31-5p promotes the cell growth, migration and invasion of
colorectal cancer cells by targeting NUMB. Biomed Pharmacother.
109:208–216. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Arias N, Aguirre L, Fernández-Quintela A,
González M, Lasa A, Miranda J, Macarulla MT and Portillo MP:
Erratum to: MicroRNAs involved in the browning process of
adipocytes. J Physiol Biochem. 72:523–524. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ahn J, Lee H, Chung CH and Ha T: High fat
diet induced downregulation of microRNA-467b increased lipoprotein
lipase in hepatic steatosis. Biochem Biophys Res Commun.
414:664–669. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sur S, Steele R, Shi X and Ray RB:
miRNA-29b inhibits prostate tumor growth and induces apoptosis by
increasing bim expression. Cells. 8:14552019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kirimura S, Kurata M, Nakagawa Y, Onishi
I, Abe-Suzuki S, Abe S, Yamamoto K and Kitagawa M: Role of
microRNA-29b in myelodysplastic syndromes during transformation to
overt leukaemia. Pathology. 48:233–241. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang B, Shetti D, Fan C and Wei K:
miR-29b-3p promotes progression of MDA-MB-231 triple-negative
breast cancer cells through downregulating TRAF3. Biol Res.
52:382019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ding D, Li C, Zhao T, Li D, Yang L and
Zhang B: LncRNA H19/miR-29b-3p/PGRN axis promoted
epithelial-mesenchymal transition of colorectal cancer cells by
acting on wnt signaling. Mol Cells. 41:423–435. 2018.PubMed/NCBI
|
|
18
|
Liu D, Wang J and Liu M: Long noncoding
RNA TUG1 promotes proliferation and inhibits apoptosis in multiple
myeloma by inhibiting miR-29b-3p. Biosci Rep. 39:BSR201824892019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mao A, Tang J, Tang D, Wang F, Liao S,
Yuan H, Tian C, Sun C, Si J, Zhang H and Xia X: MicroRNA-29b-3p
enhances radiosensitivity through modulating WISP1-mediated
mitochondrial apoptosis in prostate cancer cells. J Cancer.
11:6356–6364. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Worst TS, Previti C, Nitschke K, Diessl N,
Gross JC, Hoffmann L, Frey L, Thomas V, Kahlert C, Bieback K, et
al: miR-10a-5p and miR-29b-3p as extracellular vesicle-associated
prostate cancer detection markers. Cancers (Basel). 12:432019.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lyu J, Zhao L, Wang F, Ji J, Cao Z, Xu H,
Shi X, Zhu Y, Zhang C, Guo F, et al: Discovery and validation of
serum MicroRNAs as early diagnostic biomarkers for prostate cancer
in chinese population. Biomed Res Int. 2019:93068032019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao J, Xu H, Duan Z, Chen X, Ao Z, Chen
Y, Ruan Y and Ni M: miR-31-5p regulates 14-3-3 ε to inhibit
prostate cancer 22RV1 cell survival and proliferation via
PI3K/AKT/Bcl-2 signaling pathway. Cancer Manag Res. 12:6679–6694.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Min S, Liang X, Zhang M, Zhang Y, Mei S,
Liu J, Liu J, Su X, Cao S, Zhong X, et al: Multiple
tumor-associated microRNAs modulate the survival and longevity of
dendritic cells by targeting YWHAZ and Bcl2 signaling pathways. J
Immunol. 190:2437–2446. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu D, Yi B, Liao Z, Tang L, Yin D, Zeng
S, Yao J and He M: 14-3-3γ protein attenuates
lipopolysaccharide-induced cardiomyocytes injury through the Bcl-2
family/mitochondria pathway. Int Immunopharmacol. 21:509–515. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiang H, Zhang G, Wu JH and Jiang CP:
Diverse roles of miR-29 in cancer (review). Oncol Rep.
31:1509–1516. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li Y, Cai B, Shen L, Dong Y, Lu Q, Sun S,
Liu S, Ma S, Ma PX and Chen J: MiRNA-29b suppresses tumor growth
through simultaneously inhibiting angiogenesis and tumorigenesis by
targeting Akt3. Cancer Lett. 397:111–119. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang H, Guan X, Tu Y, Zheng S, Long J, Li
S, Qi C, Xie X, Zhang H and Zhang Y: MicroRNA-29b attenuates
non-small cell lung cancer metastasis by targeting matrix
metalloproteinase 2 and PTEN. J Exp Clin Cancer Res. 34:592015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li Y, Zhang Z, Xiao Z, Ying L, Luo T, Zhou
Q and Zhang X: Chemotherapy-mediated miR-29b expression inhibits
the invasion and angiogenesis of cervical cancer. Oncotarget.
8:14655–14665. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Inoue A, Yamamoto H, Uemura M, Nishimura
J, Hata T, Takemasa I, Ikenaga M, Ikeda M, Murata K, Mizushima T,
et al: MicroRNA-29b is a novel prognostic marker in colorectal
cancer. Ann Surg Oncol. 22 (Suppl 3):S1410–S1418. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li L, Guo Y, Chen Y, Wang J, Zhen L, Guo
X, Liu J and Jing C: The diagnostic efficacy and biological effects
of microRNA-29b for colon cancer. Technol Cancer Res Treat.
15:772–779. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Basati G, Razavi AE, Pakzad I and Malayeri
FA: Circulating levels of the miRNAs, miR-194, and miR-29b, as
clinically useful biomarkers for colorectal cancer. Tumour Biol.
37:1781–1788. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Papachristopoulou G, Papadopoulos EI,
Nonni A, Rassidakis GZ and Scorilas A: Expression analysis of
miR-29b in malignant and benign breast tumors: A promising
prognostic biomarker for invasive ductal carcinoma with a possible
histotype-related expression status. Clin Breast Cancer.
18:305–312.e3. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hong Q, Fang J, Pang Y and Zheng J:
Prognostic value of the microRNA-29 family in patients with primary
osteosarcomas. Med Oncol. 31:372014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Moore BW, Perez VJ and Gehring M: Assay
and regional distribution of a soluble protein characteristic of
the nervous system. J Neurochem. 15:265–272. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang YF, Lee YC, Wang YY, Wang CH, Hou MF
and Yuan SF: YWHAE promotes proliferation, metastasis, and
chemoresistance in breast cancer cells. Kaohsiung J Med Sci.
35:408–416. 2019.PubMed/NCBI
|
|
36
|
Yan L, Gu H, Li J, Xu M, Liu T, Shen Y,
Chen B and Zhang G: RKIP and 14-3-3ε exert an opposite effect on
human gastric cancer cells SGC7901 by regulating the ERK/MAPK
pathway differently. Dig Dis Sci. 58:389–396. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu TA, Jan YJ, Ko BS, Liang SM, Chen SC,
Wang J, Hsu C, Wu YM and Liou JY: 14-3-3ε overexpression
contributes to epithelial-mesenchymal transition of hepatocellular
carcinoma. PLoS One. 8:e579682013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhu C, Hou X, Zhu J, Jiang C and Wei W:
Expression of miR-30c and miR-29b in prostate cancer and its
diagnostic significance. Oncol Lett. 16:3140–3144. 2018.PubMed/NCBI
|
|
39
|
Ru P, Steele R, Newhall P, Phillips NJ,
Toth K and Ray RB: miRNA-29b suppresses prostate cancer metastasis
by regulating epithelial-mesenchymal transition signaling. Mol
Cancer Ther. 11:1166–1173. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yan B, Guo Q, Fu FJ, Wang Z, Yin Z, Wei YB
and Yang JR: The role of miR-29b in cancer: Regulation, function,
and signaling. Onco Targets Ther. 8:539–548. 2015.PubMed/NCBI
|
|
41
|
Yan B, Guo Q, Nan XX, Wang Z, Yin Z, Yi L,
Wei YB, Gao YL, Zhou KQ and Yang JR: Micro-ribonucleic acid 29b
inhibits cell proliferation and invasion and enhances cell
apoptosis and chemotherapy effects of cisplatin via targeting of
DNMT3b and AKT3 in prostate cancer. Onco Targets Ther. 8:557–565.
2015.PubMed/NCBI
|
|
42
|
Mann J, Githaka JM, Buckland TW, Yang N,
Montpetit R, Patel N, Li L, Baksh S, Godbout R, Lemieux H and
Goping IS: Non-canonical BAD activity regulates breast cancer cell
and tumor growth via 14-3-3 binding and mitochondrial metabolism.
Oncogene. 38:3325–3339. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kulsoom B, Shamsi TS, Afsar NA, Memon Z,
Ahmed N and Hasnain SN: Bax, Bcl-2, and Bax/Bcl-2 as prognostic
markers in acute myeloid leukemia: Are we ready for Bcl-2-directed
therapy? Cancer Manag Res. 10:403–416. 2018. View Article : Google Scholar : PubMed/NCBI
|