Introduction
Gastric cancer has become the fifth most common and
the third most lethal malignant tumor type, with >1 million
cases diagnosed throughout the world in 2020 (1). Numerous patients had lymph node (LN)
or distant metastasis of cancer, leading to poor prognosis and
posing a major threat to their lives (2). Radical gastrectomy was the dominant
surgical therapy for gastric cancer and the extent of surgical
resection was mainly determined by tumor size, tumor location and
resection margin distance. Distal gastrectomy (DG) and total
gastrectomy (TG) were recommended as the standard methods for
radical resection of lower-third and upper-third gastric cancer,
respectively (3). However, the
optimal resection extent for middle-third gastric cancer (MTGC)
still remains controversial.
Studies suggested TG as the best choice for surgical
treatment of MTGC due to the possibility of a more thorough
lymphadenectomy and lower incidence of remnant gastric cancer
(4,5). Several other reports indicated that
DG was a reasonable procedure for MTGC with less weight loss,
better nutritional status and a lower post-operative complication
rate than TG (6–8). Further research discovered a similar
post-operative survival for DG and TG (9,10).
In addition, the study by Zheng et al (11) clarified that prophylactic clearance
of the no. 10 LN was not essential for MTGC. Therefore, it is still
being debated whether DG or TG is the more beneficial procedure for
MTGC.
Previous meta-analyses were performed to explore the
clinical efficacy and benefits of DG vs. TG, but the majority of
cases included were patients with lower-third gastric cancer, which
inevitably decreased the credibility of the analytical results for
MTGC (12–14). Hence, the present meta-analysis was
performed to compare the surgical and oncological outcomes between
DG and TG only in MTGC.
Materials and methods
Search strategy
Studies published in English and Chinese were
retrieved from the electronic databases PubMed (https://pubmed.ncbi.nlm.nih.gov/), Web of Science
(https://www.webofknowledge.com), Embase
(https://www.embase.com), Chinese National
Knowledge Infrastructure (CNKI; http://www.cnki.net) and Chinese BioMedical Literature
(http://www.sinomed.ac.cn) from inception
to February 2022. The key words for the search were as follows:
‘Gastric cancer or middle-third or gastrectomy’, ‘gastric carcinoma
or middle-third or gastrectomy’, ‘gastric cancer or distal
gastrectomy or total gastrectomy’ and ‘gastric cancer or subtotal
gastrectomy or total gastrectomy’. Papers unable to be directly
found on the internet were obtained via manually searching the
other publications of all of the authors of the studies already
retrieved.
Inclusion and exclusion criteria
All of the studies included were required to meet
the following criteria: i) Studies focused on the comparison of
short- or long-term outcomes between DG and TG; ii) patients in the
studies were diagnosed with MTGC; iii) at least one concerned
outcome was reported in the studies, such as operation time, blood
loss, retrieved LNs, hospital stay, post-operative complications
and 5-year overall survival (OS); and iv) studies with available
data.
Studies fulfilling the following criteria were
excluded from the present analysis: i) Studies not assessing the
clinical efficacy of DG and TG in patients with MTGC; ii)
non-case-control studies; iii) studies identified to be reviews,
case reports, brief communications or letters to editors; iv)
studies without extractable data of clinical outcomes; and v)
repeatedly published studies.
Data extraction
A total of two authors (YJ and FY) extracted the
data from each included study independently. In the case of any
discrepancy, a third author was involved in this process until a
final agreement was reached. The data for extraction were as
follows: Author, publication year, study design, study period,
sample size, median age, gender distribution, surgical procedure of
gastrectomy and lymphadenectomy, numbers of cases of each TNM
stage, median follow-up, blood loss, numbers of LNs, hospital stay,
post-operative morbidity (overall morbidity rate, anastomosis
leakage, anastomosis stenosis, duodenal stump fistula,
intro-abdominal infection, wound problems and post-operative
bleeding), 5-year OS, 5-year stage-specific OS and the 5-year OS
according to the width of the proximal resection margin (PRM).
Quality assessment
The quality of each selected study was determined
using the Newcastle-Ottawa-Scale and the scoring criteria contained
three aspects of selection of patients, comparability and exposure
(14). Studies with a score ≥6
were considered high-quality studies, while those with a score
<6 were considered low-quality studies.
Statistical analysis
The dichotomous variables and continuous variables
were described as the odds ratio (OR) and weighted mean difference
and the two types of variables were reported with the 95%
confidence interval (CI). P<0.05 was considered to indicated a
statistically significant difference. I2 statistics were
performed to calculate the heterogeneity among the studies; if
there was no significant heterogeneity (I2<50%,
P>0.1) observed in the results, the fixed-effects model was
used, while the random-effects model was used when significant
heterogeneities (I2>50%, P<0.1) were detected.
Funnel plots were generated to evaluate any possible publication
bias. All of the statistical analyses of the present meta-analysis
were performed by Review Manager version 5.3 software (Nordic
Cochrane Centre).
Results
Clinical characteristics
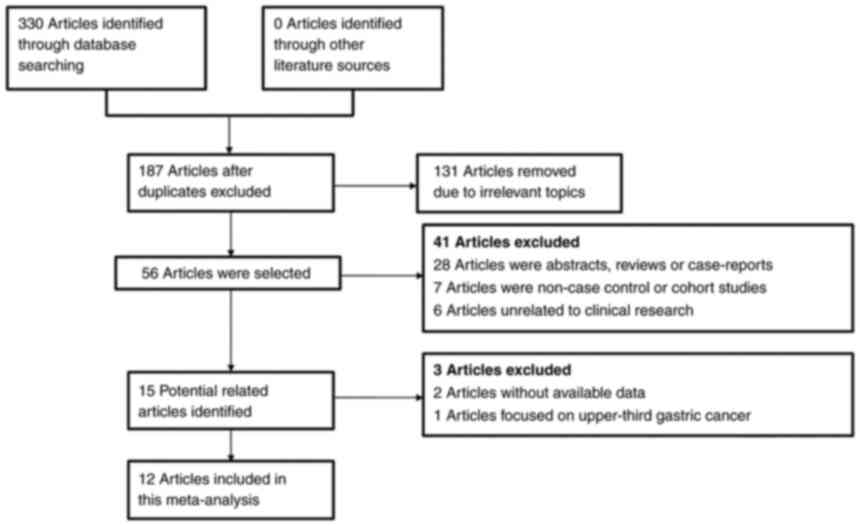
A total of 330 articles were retrieved in the
initial literature search. Subsequently, 143 papers were excluded
due to duplicated data. In the second screening, 131 papers with
irrelevant topics were excluded. Furthermore, 44 papers met the
exclusion criteria and were removed after the third screening.
Finally, 12 retrospective studies were included in this present
meta-analysis after rigorous literature screening (10,15–25).
The flowchart for the literature selection process is displayed in
Fig. 1. Among these studies, 9
pertained to conventional open gastrectomy and 3 to
laparoscopic-assisted gastrectomy (LAG). Of the enrolled patients,
1,077 underwent DG and 1,502 underwent TG. The clinical
characteristics were summarized in detail in Table I. The assessment process of the
methodological quality of selected studies is presented in Table II; each study reached a score
ranging from 6 to 8, which indicated that all of the included
papers were high-quality studies.
 | Table I.Detailed characteristics of patients
from the included studies. |
Table I.
Detailed characteristics of patients
from the included studies.
|
|
|
|
| Study period | Sample size | Age, years | Sex,
males/females | Surgical
procedure |
Lymphadenectomy | Tumor stage
(I/II/III/IV) | Median, follow-up
months | 5-year overall
survival, % |
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|---|
| First author | Year | Population | Study design | TG | DG | TG | DG | TG | DG | TG | DG | TG | DG | (Refs.) |
|---|
| Jang | 2010 | South Korea | Retrospective
study | 1993-2005 | 178 | 148 | 53.42±13.07 | 54.41±13.65 | 101/77 | 95/53 | OG | D1, D2 | 17/37/89/35 | 36/41/62/9 | - | 58.4 | 67.8 | (16) |
| Lee | 2010 | South Korea | Retrospective
study | 2000-2006 | 63 | 62 | 56.2 | 58 | 43/20 | 39/23 | OG | - | 5/13/40/5 | 29/13/19/1 | - | 38.1 | 69 | (15) |
| Wang | 2012 | China | Retrospective
study | 2001-2006 | 98 | 47 | - |
| 57/41 | 31/16 | OG | D1, D2, D2+ | 6/19/67/6 | 10/15/21/1 | 40 | 25.5 | 63.8 | (20) |
| Li | 2013 | China | Retrospective
study | 2010-2012 | 50 | 58 | 62.1±5.4 | 61.2±6.8 | 35/15 | 38/20 | OG | D1, D2, D3 | 16/14/13/7 | 10/15/28/5 | - | 49 | 59 | (24) |
| Tao | 2013 | China | Retrospective
study | 1998-2005 | 156 | 66 | 56.9±11.5 | 55.8±9.8 | 130/26 | 43/23 | OG | D2 | 15/19/122/0 | 18/13/35/0 | - | 49.8 | 63.9 | (21) |
| Lu | 2014 | China | Retrospective
study | 2000-2007 | 194 | 86 | 54.7±10.2 | 56.3±11.3 | 157/37 | 54/32 | OG | - | 24/32/138/0 | 27/25/34/0 | - | 47.6 | 64.3 | (25) |
| Zhou | 2014 | China | Retrospective
study | 2003-2008 | 85 | 32 | - |
| - |
| OG | - | 6/18/61/0 | 15/7/10/0 | - | 48.2 | 66.8 | (22) |
| Gao | 2015 | China | Retrospective
study | 2003-2008 | 104 | 53 | 58.87±11.45 | 59.56±10.97 | 60/44 | 34/19 | OG | D1, D2, D2+ | 8/20/69/7 | 12/17/23/1 | 48 | 24 | 64.2 | (23) |
| Ji | 2017 | China | Retrospective
study | 2005-2011 | 195 | 144 | - |
| 132/63 | 94/50 | OG | D1, D2 | 17/38/132/8 | 36/25/64/19 | 41.8 | 47 | 65 | (17) |
| Li | 2018 | China | Retrospective
study | 2005-2014 | 146 | 146 | 55.95±10.84 | 55.48±11.60 | 103/43 | 103/43 | LAG | D2 | 18/59/69/0 | 20/59/67/0 | 54 | 61 | 64.4 | (10) |
| Wang | 2018 | China | Retrospective
study | 2007-2013 | 188 | 188 | 57.9 ± 11.1 | 57.3 ± 11.4 | 151/37 | 146/42 | LAG | D2 | 49/40/99/0 | 45/40/103/0 | 44.8 | 41.8 | 55.6 | (18) |
| Liu | 2020 | China | Retrospective
study | 2013-2017 | 45 | 47 | 58.0 ± 9.9 | 57.0 ± 11.1 | 17/28 | 11/36 | LAG | D2 | 11/16/18/0 | 18/10/19/0 | 41 | - | - | (19) |
 | Table II.Results of quality assessment with
the Newcastle-Ottawa scale tool. |
Table II.
Results of quality assessment with
the Newcastle-Ottawa scale tool.
|
| Selection | Comparability | Exposure |
|
|
|---|
|
|
|
|
|
|
|
|---|
| Author (year) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Total score | (Refs.) |
|---|
| Jang (2010) | + | + | - | + | + | + | + | - | 6 | (16) |
| Lee (2010) | + | + | - | + | + | + | + | + | 7 | (15) |
| Wang (2012) | + | + | - | + | ++ | + | + | + | 8 | (20) |
| Li (2013) | + | + | - | + | + | + | + | + | 7 | (24) |
| Tao (2013) | + | + | - | + | + | + | + | - | 6 | (21) |
| Zhou (2014) | + | + | - | + | + | + | + | + | 7 | (22) |
| Lu (2014) | + | + | - | + | + | + | + | - | 6 | (25) |
| Gao (2015) | + | + | - | + | ++ | + | + | + | 8 | (23) |
| Ji (2017) | + | + | - | + | + | + | + | + | 7 | (17) |
| Li (2018) | + | + | - | + | ++ | + | + | + | 8 | (10) |
| Wang (2018) | + | + | - | + | ++ | + | + | + | 8 | (18) |
| Liu (2020) | + | + | - | + | ++ | + | + | + | 8 | (19) |
Surgical outcomes
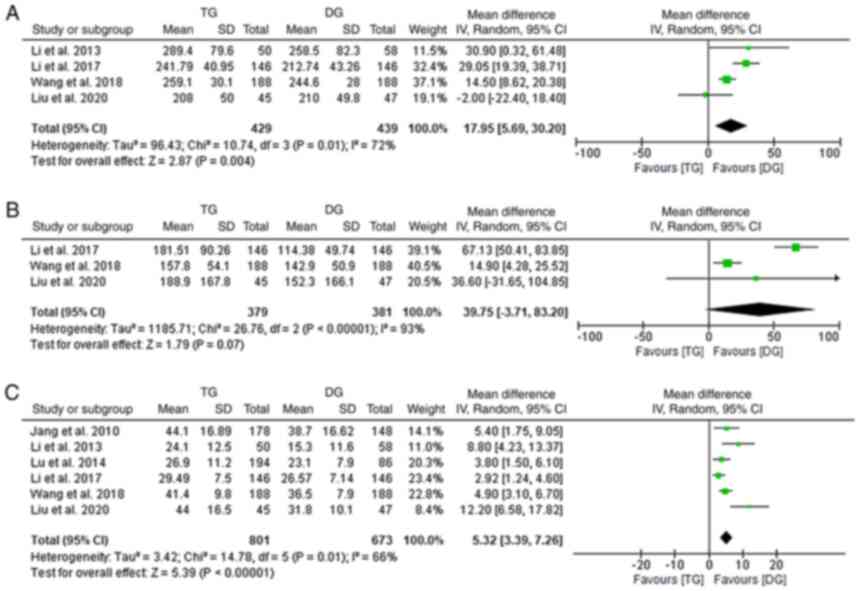
Comparisons of the duration of surgery were provided
by 4 of the studies included (10,18,19,24).
A significantly longer operative time was observed in the TG as
compared with that in the DG group (random-effects model;
I2: 72%; OR,17.95; 95% CI, 5.69-30.20; P=0.004;
Fig. 2A). A total of three studies
reported on the estimated blood loss and the analysis revealed
comparable results for the DG and TG groups (10,18,19)
(random-effects model; I2: 93%; OR, 39.75; 95%
CI, −3.71 to 83.20; P=0.07; Fig.
2B). Data of LN extraction were recorded in 6 studies and a
significant difference was detected with a higher number of LNs
extracted in the TG group (10,16,18,19,24,25)
(random-effects model; I2: 66%; OR, 5.32; 95% CI,
3.39-7.26; P<0.001; Fig. 2C).
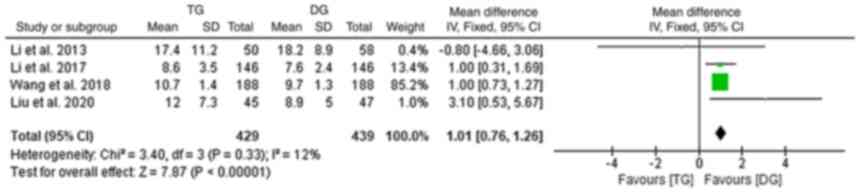
Furthermore, data on the post-operative hospital stay were provided
by 4 studies and the pooled analysis indicated that the TG group
had a longer hospital stay when compared with that of the DG group
(10,18,19,24)
(fixed-effects model; I2:12%; OR, 1.01; 95% CI,
0.76-1.26; P<0.001; Fig.
3).
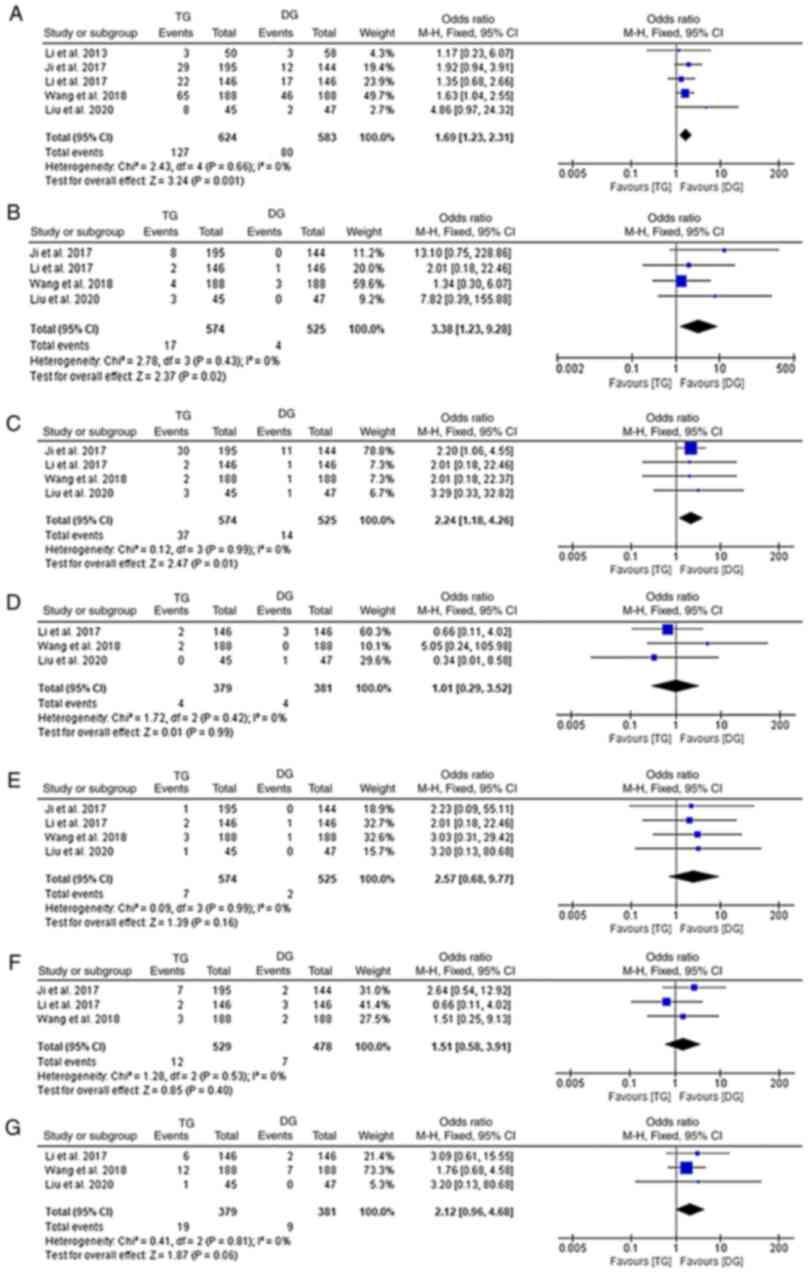
Post-operative complications
Post-operative complication rates were reported by 5
studies (10,17–19,24).
Regarding overall complications, DG was associated with a
significantly lower incidence rate than TG (fixed-effects model;
I2: 0%; OR, 1.69; 95% CI, 1.23-2.31; P=0.001;
Fig. 4A). When analyses were
stratified by the various types of complications, it was observed
that the incidence of duodenal stump fistula, anastomosis stenosis,
post-operative bleeding and wound problems were reported by 3, 4, 3
and 3 of the included studies, respectively (10,17–19).
The results did not indicate any significant differences in these
specific complications between the two groups (fixed-effects model;
I2: 0%; P>0.05; Fig. 4D-G). Of note, subgroup analyses
focusing on anastomosis leakage (fixed-effects model;
I2:0%; OR, 3.38; 95% CI, 1.23-9.28; P=0.02) and
intro-abdominal infection (fixed-effects model;
I2: 0%; OR, 2.24; 95% CI, 1.18-4.26; P=0.01)
suggested that TG was associated with a higher incidence of both of
the two complications (Fig. 4B and
C).
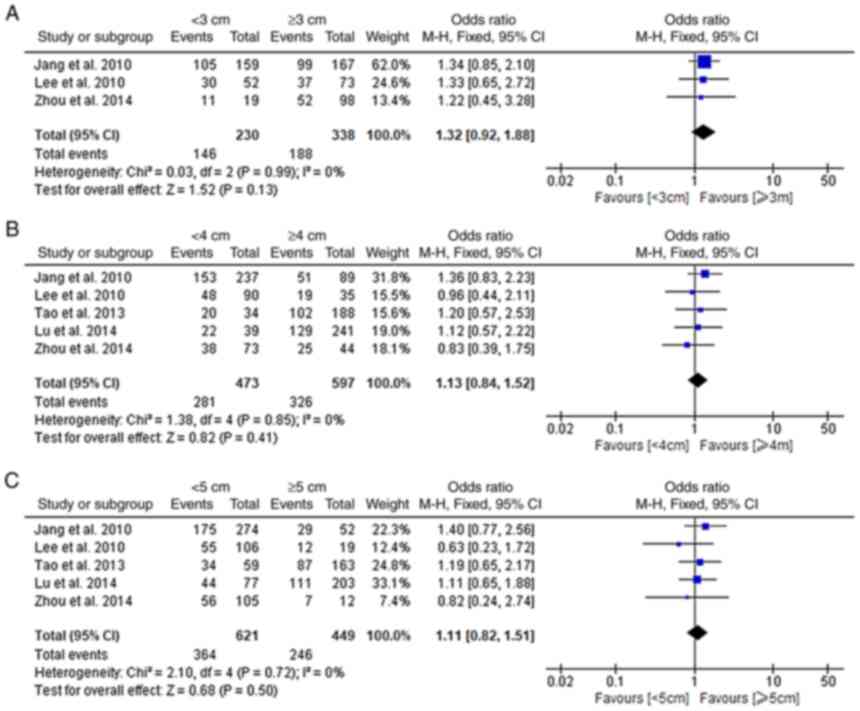
Impact of the distance to PRM on
prognosis
A total of five studies comprehensively explored the
associations of the distance to the PRM with the prognosis of
patients with MTGC (15,16,21,22,25).
Among these studies, 3 defined 3 cm as the standard and 5 defined 4
and 5 cm as the standards. No significant difference was discovered
in the 5-year OS between the groups if the standard for the PRM was
set as 3, 4 and 5 cm (fixed-effects model; I2:
0%; P>0.05; Fig. 5A-C).
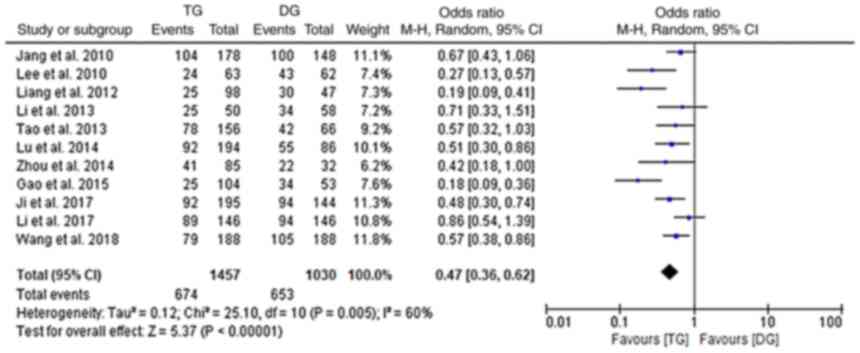
Post-operative survival
Data on post-operative survival were provided by 11
of the included studies (10,15–24).
A significantly lower 5-year OS was observed in the TG group as
compared with that in the DG group (random-effects model;
I2: 60%; OR, 0.47; 95% CI, 0.36-0.62; P<0.001;
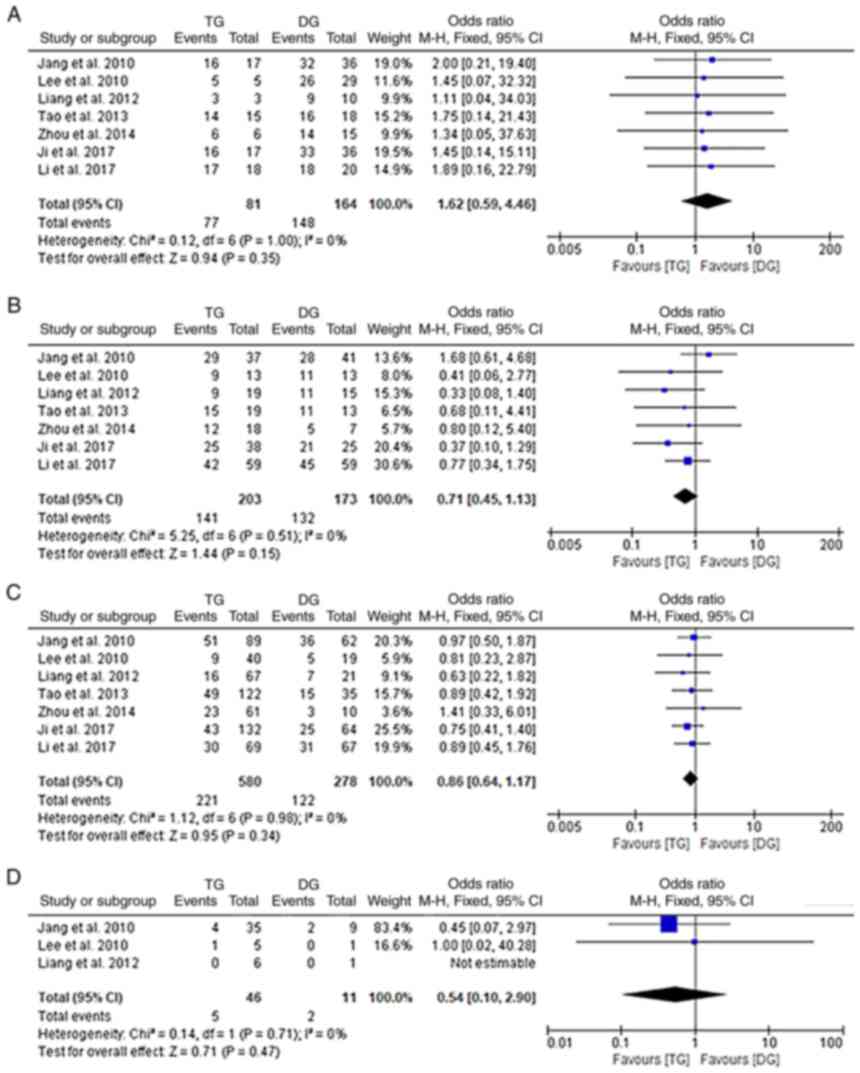
Fig. 6). However, when further
analyses with stratification by TNM stage (I, II, III or IV) were
performed, no significant differences were obtained between the TG
and DG groups (fixed-effects model; I2: 0%;
P>0.05; Fig. 7A-D).
Discussion
During the past two decades, the proportion of
patients diagnosed with upper or MTGC was gradually elevated
(26). Recently, an increasing
morbidity of advanced MTGC with poor survival was also reported by
statistics from the USA (27).
According to the 5th Japanese gastric cancer treatment guidelines
(JGCTG), pylorus-preserving distal gastrectomy is only suggested
for cases with cT1N0M0 stage
malignant tumor arising in the middle-third of the stomach if a
macroscopically negative distal margin of at least 4 cm was
feasible (28). TG and DG are the
two major surgical treatments for MTGC. However, the results
regarding short- and long-term outcomes of the two procedures in
the different studies were inconsistent. Certain studies
recommended TG for MTGC as a means of prevention for tumor
recurrence and gastric stump cancer. However, it was clarified that
DG was associated with better post-operative functional outcomes
with its lesser disruption of the digestive tract, which probably
enhanced the post-operative recovery (29). The present meta-analysis was
performed to determine the potential optimal surgical procedure for
MTGC.
Surgical performance data are important for the
assessment of post-operative short-term outcomes. In the present
study, TG was determined to be associated with a larger extent of
lymphadenectomy and a longer operative time. In the scenario of
standard lymphadenectomy in DG, an added stage of no. 2, 4sa and
11d LNs was necessary to be dissected in TG with D2 LN dissection
to meet the criteria of the 5th JGCTG (30), which may appropriately explain the
significantly larger number of retrieved LNs determined in the TG
group. The technical complexity may be another factor responsible
for the extended operative time in TG. Lee et al (31) detected a positive association
between longer operative time and higher morbidity rate and another
study reported a trend toward post-operative aspiration and
bacterial infection induced by prolonged anesthesia (32). Since DG was associated with a
shorter operative time in the present study, its possible benefit
in reducing the morbidity rate may raise the interest of
clinicians. A similar blood loss was detected between the DG and TG
groups; however, a significant heterogeneity existed in the
analysis and certain studies focused on the comparisons between TG
and DG in distal gastric cancer and obtained a different result
(33,34). As previously reported, when TG was
performed, the larger surgical region and more complex
reconstruction of the digestive tract increased the proneness to
bleeding (17). Thus, the
comparison of blood loss requires to be further estimated. A
significantly longer hospital stay in TG as compared to DG was also
determined in the present study. TG requires more stretching and
pulling of organs, possibly resulting in post-operative
inflammatory response and then extending the post-operative
intestinal recovery time (35).
The post-operative complication rate is a crucial
factor for judging the safety of a surgical procedure and closely
affects post-operative recovery and prognosis (36). In the present study, a higher
overall complication rate was present in the TG group and a similar
result was also obtained in a previous meta-analysis focusing on
distal gastric cancer (37). To
explore the specific origin of the significant difference, further
stratified analyses were performed and a higher morbidity for
anastomotic leakage was observed in TG. Oesophago-jejunal
anastomosis has been rarely performed in DG; however, it was a key
process in TG. As previously reported, in oesophago-jejunal
anastomosis, it was more difficult to maintain the integrity and
reduce the tension of anastomosis instead of gastro-jejunal
anastomosis, resulting in a possible fragile reconstruction of the
digestive tract in TG (38), which
may explain the significantly different anastomotic leakage rate
between the two procedures. In addition, since the application of
LAG was first reported in 1994 (39), although minimally invasive surgery
was widely used, its feasibility and safety were confirmed by
numerous studies. Of note, a Dutch study revealed a higher
anastomotic leakage risk in minimally invasive TG than conventional
open TG (40). It is well
recognized that anastomotic leakage is prone to causing secondary
abdominal infection and the present meta-analysis detected a higher
abdominal infection rate in TG (41). Besides the factor of anastomotic
leakage, it may be reasoned that the greater extent of resection
and longer operative time also contributed to the increased
occurrence of abdominal infection. Duodenal stump fistula is a
life-threatening complication, but no significant difference was
found in the comparison of this rate between the two groups.
However, the duodenal stump may be absent in certain
reconstructions for DG, such as Billroth-I anastomosis and results
pertaining to this aspect are expected to be provided by future
well-designed studies.
Another key factor for determining the surgical
procedure in gastric cancer was the PRM. Particularly in DG for
MTGC, an inadequate PRM not meeting the R0 resection probably
results in post-operative cancer recurrence. However, it is
difficult to warrant a completely clear PRM without remaining
cancer cells, even with the aid of intraoperative freezing
detection (17,19,20).
The 5th JGCTG from 2018 suggested that a PRM of >3 cm should be
ensured in gastrectomy for localized T2-T4b cancer and for tumors
of the infiltrative type, the criterion is a PRM of >5 cm
(30). Furthermore, a western
multicenter randomized controlled trial (RCT) recommended DG as an
alternative surgical therapy for MTGC when the free PRM was limited
to 3–6 cm (6). The results of the
present meta-analysis indicated no significant effect of the length
of the PRM on post-operative 5-year OS when the PRM ranged from 3
to 5 cm, which further confirmed similar findings from South Korea
(15,16).
Previous studies also compared the long-term
outcomes between TG and DG. The study by Bozzetti et al
(42) indicated a comparable
5-year OS for both the two procedures, while Chen et al
(43) reported a significantly
superior 5-year OS of patients with DG compared with that of
patients who underwent TG in the same period. Further multivariate
analyses considered the resection extent as an independent factor
for post-operative survival (44–46);
however, this was not supported by the evidence provided in certain
other studies (47,48). In the present study, it was
explored whether the 5-year OS of patients with MTGC differed
between those receiving TG and DG. A significantly higher 5-year OS
was determined for DG; however, when patients were stratified by
TNM stage, the benefit disappeared and the 5-year OS in the TG and
DG groups was similar for stages I, II, III and IV. Indeed, certain
surgical oncologists tend to perform TG in order to achieve a
curative PRM, particularly for more advanced-stage tumors (13), indicating that the factor most
likely to impact oncological outcomes for MTGC is the TNM stage
rather than resection extent.
Despite the rigorous design of the present study and
thorough analysis, several inevitable limitations should be
recognized. First, all of the studies included in the present
meta-analysis were retrospective studies and the absence of RCTs
may have affected the strength of the evidence of the results.
Furthermore, the included studies were only performed in East Asian
countries, such as China and Korea, while corresponding data from
other countries and ethnicities, particularly Japanese, Caucasian
and African populations, were not available. In addition, the
publication language was limited to English and Chinese at the step
of literature search and relevant studies published in other
languages may not be retrieved, resulting in a potential
publication bias. Finally, the sample size of the present
meta-analysis was relatively small and the findings require to be
confirmed in further studies with large samples.
In conclusion, the present meta-analysis indicated
that DG as a surgical treatment for MTGC resulted in a comparable
5-year OS, but a shorter hospital stay and a lower post-operative
complication rate compared to TG, which suggested that if a
negative PRM of >3 cm was ensured, DG was an effective, safe and
promising option for curative resection of MTGC.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
Study conception and design, interpretation of data
and critical revision: FY, GL and YJ. Literature review and data
analysis: JM, NZ, CZ, ZL, FY and YJ. Drafting of the manuscript:
FY, YJ and ZL. Revision of the manuscript: YJ. Revision of the
manuscript for important intellectual content: ZL. All authors read
and approved the final manuscript. YJ and FY checked and confirmed
the authenticity of the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
DG
|
distal gastrectomy
|
|
TG
|
total gastrectomy
|
|
MTGC
|
middle-third gastric cancer
|
|
CNKI
|
Chinese National Knowledge
Infrastructure
|
|
LN
|
lymph node
|
|
OS
|
overall survival
|
|
PRM
|
proximal resection margin
|
|
NOS
|
Newcastle-Ottawa Scale
|
|
OR
|
odds ratio
|
|
CI
|
confidence interval
|
|
OG
|
open gastrectomy
|
|
LAG
|
laparoscopic-assisted gastrectomy
|
|
JGCTG
|
Japanese gastric cancer treatment
guidelines
|
|
RCT
|
randomized controlled trial
|
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ito S, Masuda T, Noda M, Hu Q, Shimizu D,
Kuroda Y, Eguchi H, Tobo T, Utsunomiya T and Mimori K: Prognostic
significance of PD-1, PD-L1 and CD8 gene expression levels in
gastric cancer. Oncology. 98:501–511. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Japanese Gastric Cancer Association, .
Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric
Cancer. 20:1–19. 2017. View Article : Google Scholar
|
|
4
|
Clark CJ, Thirlby RC, Picozzi V Jr,
Schembre DB, Cummings FP and Lin E: Current problems in surgery:
Gastric cancer. Curr Probl Surg. 43:566–670. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stein HJ, Sendler A and Siewert JR:
Site-dependent resection techniques for gastric cancer. Surg Oncol
Clin N Am. 11:405–414. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Santoro R, Ettorre GM and Santoro E:
Subtotal gastrectomy for gastric cancer. World J Gastroenterol.
20:13667–13680. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xuan Y, Hur H, Byun CS, Han SU and Cho YK:
Efficacy of intraoperative gastroscopy for tumor localization in
totally laparoscopic distal gastrectomy for cancer in the middle
third of the stomach. Surg Endosc. 27:4364–4370. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Buhl K, Schlag P and Herfarth C: Quality
of life and functional results following different types of
resection for gastric carcinoma. Eur J Surg Oncol. 16:404–409.
1990.PubMed/NCBI
|
|
9
|
Tang T, Peng W, Zhang L, Zuo Z, Cao D,
Huang J and Duan L: Effectiveness and safety of total laparoscopic
distal gastrectomy versus laparoscopy-assisted distal gastrectomy
for gastric cancer: A retrospective cohort study. Am J Surg.
216:528–533. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Z, Ji G, Bai B, Yu D, Liu Y, Lian B and
Zhao Q: Laparoscopy-assisted distal gastrectomy versus
laparoscopy-assisted total gastrectomy with D2 lymph node
dissection for middle-third advanced gastric cancer. Surg Endosc.
32:2255–2262. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zheng G, Liu J, Guo Y, Wang F, Liu S, Xu
G, Guo M, Lian X, Zhang H and Feng F: Necessity of prophylactic
splenic hilum lymph node clearance for middle and upper third
gastric cancer: A network meta-analysis. BMC Cancer. 20:1492020.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kong L, Yang N, Shi L, Zhao G, Wang M and
Zhang Y: Total versus subtotal gastrectomy for distal gastric
cancer: Meta-analysis of randomized clinical trials. Onco Targets
Ther. 9:6795–6800. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Z, Bai B, Xie F and Zhao Q: Distal
versus total gastrectomy for middle and lower-third gastric cancer:
A systematic review and meta-analysis. Int J Surg. 53:163–170.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qi J, Zhang P, Wang Y, Chen H and Li Y:
Does total gastrectomy provide better outcomes than distal subtotal
gastrectomy for distal gastric cancer? A systematic review and
meta-analysis. PLoS One. 11:e01651792016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee JH and Kim YI: Which is the optimal
extent of resection in middle third gastric cancer between total
gastrectomy and subtotal gastrectomy? J Gastric Cancer. 10:226–233.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jang YJ, Park MS, Kim JH, Park SS, Park
SH, Kim SJ, Kim CS and Mok YJ: Advanced gastric cancer in the
middle one-third of the stomach: Should surgeons perform total
gastrectomy? J Surg Oncol. 101:451–456. 2010.PubMed/NCBI
|
|
17
|
Ji X, Yan Y, Bu ZD, Li ZY, Wu AW, Zhang
LH, Wu XJ, Zong XL, Li SX, Shan F, et al: The optimal extent of
gastrectomy for middle-third gastric cancer: Distal subtotal
gastrectomy is superior to total gastrectomy in short-term effect
without sacrificing long-term survival. BMC Cancer. 17:3452017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang WJ, Li HT, Chen P, Yu JP, Jiao ZY,
Han XP, Su L, Tao RY, Xu L, Kong YL, et al: A propensity
score-matched comparison of laparoscopic distal versus total
gastrectomy for middle-third advanced gastric cancer. Int J Surg.
60:194–203. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu H, Jin P, Ma FH, Ma S, Xie YB, Li Y,
Li WK, Kang WZ and Tian YT: Feasibility and nutritional impact of
laparoscopic assisted tailored subtotal gastrectomy for
middle-third gastric cancer. World J Gastroenterol. 26:6837–6852.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang L, Liang H, Wang XN, Ding XW, Wu LL
and Liu HG: The reasonable surgery for gastric body cancer and
prognostic analysis. Zhonghua Wai Ke Za Zhi. 50:966–970. 2012.(In
Chinese). PubMed/NCBI
|
|
21
|
Tao KL, Huang CM, Lin JX, Zheng CH, Li P,
Xie JW and Wang JB: Impact of the extent of gastric resection on
the prognosis of patients with middle one-third gastric cancer.
Zhonghua Wei Chang Wai Ke Za Zhi. 16:155–159. 2013.(In Chinese).
PubMed/NCBI
|
|
22
|
Zhou KK, Zhang JW and Huang RF: Analysis
of Gastric Resection Methods of Middle One-third Gastric Cancer of
117 cases. Med Innov China. 11:3–5. 2014.(In Chinese).
|
|
23
|
Gao JG, Du JQ, Zhang H, Zhang QY, Chen L,
Feng Y and Jiang HJ: Clinical analysis in effect of scope of
resection on prognosis of cancer in gastric body. Chin J Gen Surg.
24:554–559. 2015.(In Chinese).
|
|
24
|
Li SW and Tao KX: Influence of prognosis
on patients with central gastric cancer by different ways of
operation. China Med Herald. 13:39–41,47. 2013.
|
|
25
|
Lu WH, Cheng M and Ma YH: Analysis of
influence factors of the long-term prognosis of different schemes
on patients with gastric resection in cancer. Hainan Med J.
25:2042–2044. 2014.(In Chinese).
|
|
26
|
Cho BC, Jeung HC, Choi HJ, Rha SY, Hyung
WJ, Cheong JH, Noh SH and Chung HC: Prognostic impact of resection
margin involvement after extended (D2/D3) gastrectomy for advanced
gastric cancer: A 15-year experience at a single institute. J Surg
Oncol. 95:461–468. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rona KA, Schwameis K, Zehetner J, Samakar
K, Green K, Samaan J, Sandhu K, Bildzukewicz N, Katkhouda N and
Lipham JC: Gastric cancer in the young: An advanced disease with
poor prognostic features. J Surg Oncol. 115:371–375. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kosuga T, Tsujiura M, Nakashima S,
Masuyama M and Otsuji E: Current status of function-preserving
gastrectomy for gastric cancer. Ann Gastroenterol Surg. 5:278–286.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nomura E, Lee SW, Tokuhara T, Nitta T,
Kawai M and Uchiyama K: Functional outcomes according to the size
of the gastric remnant and the type of reconstruction following
distal gastrectomy for gastric cancer: An investigation including
total gastrectomy. Jpn J Clin Oncol. 43:1195–1202. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Japanese Gastric Cancer Association, .
Japanese gastric cancer treatment guidelines 2018 (5th edition).
Gastric Cancer. 24:1–21. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee J, Yun JH, Nam KH, Soh EY and Chung
WY: The learning curve for robotic thyroidectomy: A multicenter
study. Ann Surg Oncol. 18:226–232. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rosero EB, Kho KA, Joshi GP, Giesecke M
and Schaffer JI: Comparison of robotic and laparoscopic
hysterectomy for benign gynecologic disease. Obstet Gynecol.
122:778–786. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu Z, Feng F, Guo M, Liu S, Zheng G, Xu
G, Lian X, Fan D and Zhang H: Distal gastrectomy versus total
gastrectomy for distal gastric cancer. Medicine (Baltimore).
96:e60032017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Robertson CS, Chung SC, Woods SD, Griffin
SM, Raimes SA, Lau JT and Li AK: A prospective randomized trial
comparing R1 subtotal gastrectomy with R3 total gastrectomy for
antral cancer. Ann Surg. 220:176–182. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim MC, Kim KH, Kim HH and Jung GJ:
Comparison of laparoscopy-assisted by conventional open distal
gastrectomy and extraperigastric lymph node dissection in early
gastric cancer. J Surg Oncol. 91:90–94. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang LH, Zhu RF, Gao C, Wang SL and Shen
LZ: Application of enhanced recovery after gastric cancer surgery:
An updated meta-analysis. World J Gastroenterol. 24:1562–1578.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Durán Giménez-Rico H, Diéguez Aguirre L,
Ríos Pérez L, Cardinal-Fernández P, Caruso R, Ferri V, Quijano
Collazo Y and Vicente López E: Comparative study between total and
subtotal gastrectomy for distal gastric cancer: Meta-analysis of
prospective and retrospective studies. Cir Esp (Engl Ed).
98:582–590. 2020.(In English, Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang WJ, Li R, Guo CA, Li HT, Yu JP, Wang
J, Xu ZP, Chen WK, Ren ZJ, Tao PX, et al: Systematic assessment of
complications after robotic-assisted total versus distal
gastrectomy for advanced gastric cancer: A retrospective propensity
score-matched study using Clavien-Dindo classification. Int J Surg.
71:140–148. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kitano S, Iso Y, Moriyama M and Sugimachi
K: Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc
Endosc. 4:146–148. 1994.PubMed/NCBI
|
|
40
|
Gertsen EC, Brenkman HJF, Seesing MFJ,
Goense L, Ruurda JP and van Hillegersberg R; Dutch Upper
Gastrointestinal Cancer Audit (DUCA) group, : Introduction of
minimally invasive surgery for distal and total gastrectomy: A
population-based study. Eur J Surg Oncol. 45:403–409. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Guo Y, Guo X, Wang J, Li K, Xu G, Yan W,
Zhang J, Lian D, Fan Q, Han Z, et al: Abdominal infectious
complications associated with the dislocation of intraperitoneal
part of drainage tube and poor drainage after major surgeries. Int
Wound J. 17:1331–1336. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bozzetti F, Marubini E, Bonfanti G, Miceli
R, Piano C and Gennari L: Subtotal versus total gastrectomy for
gastric cancer: Five-year survival rates in a multicenter
randomized Italian trial. Italian gastrointestinal tumor study
group. Ann Surg. 230:170–178. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen GM, Yuan SQ, Nie RC, Luo TQ, Jiang
KM, Liang CC, Li YF, Zhang DY, Yu JH, Hou F, et al: Surgical
outcome and long-term survival of conversion surgery for advanced
gastric cancer. Ann Surg Oncol. 27:4250–4260. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang X, Liu F, Li Y, Tang S, Zhang Y, Chen
Y and Khan SA: Comparison on clinicopathological features,
treatments and prognosis between proximal gastric cancer and distal
gastric cancer: A national cancer data base analysis. J Cancer.
10:3145–3153. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ramos MFKP, Pereira MA, Yagi OK, Dias AR,
Charruf AZ, Oliveira RJ, Zaidan EP, Zilberstein B, Ribeiro-Júnior U
and Cecconello I: Surgical treatment of gastric cancer: A 10-year
experience in a high-volume university hospital. Clinics (Sao
Paulo). 73 (Suppl 1):e543s2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ramos MFKP, Pereira MA, Dias AR, Yagi OK,
Zaidan EP, Ribeiro-Júnior U, Zilberstein B and Cecconello I:
Surgical outcomes of gastrectomy with D1 lymph node dissection
performed for patients with unfavorable clinical conditions. Eur J
Surg Oncol. 45:460–465. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jiang Y, Zhao Y, Qian F, Shi Y, Hao Y,
Chen J, Li P and Yu P: The long-term clinical outcomes of robotic
gastrectomy for gastric cancer: A large-scale single institutional
retrospective study. Am J Transl Res. 10:3233–3242. 2018.PubMed/NCBI
|
|
48
|
Liang YX, Deng JY, Guo HH, Ding XW, Wang
XN, Wang BG, Zhang L and Liang H: Characteristics and prognosis of
gastric cancer in patients aged ≥70 years. World J Gastroenterol.
19:6568–6578. 2013. View Article : Google Scholar : PubMed/NCBI
|