Introduction
Testicular germ cell tumors (GCTs) account for only
1% of all solid tumors; however, they are the most common solid
malignant tumors in males aged between 15 and 35 years (1,2).
GCTs are considered a model for curable malignancies, as these
tumors are exceedingly sensitive to cisplatin-based chemotherapy
(3). However, chemotherapy
regimens may induce non-negligible adverse effects (e.g.,
haematological, renal and gastrointestinal toxicity). Prevention
and the correct management of treatment-related side effects are
critical for minimizing morbidity and mortality, thus enhancing the
quality of life of patients (4–6).
Febrile neutropenia (FN) is a life-threatening
complication of cisplatin-dependent chemotherapy (7,8).
Previous studies have demonstrated that the incidence of FN in
patients with GCT varies greatly (9–12).
The risk of developing FN depends on numerous factors associated
with the patient and the treatment regimen. Previous studies have
specified the potential risk factors associated with FN development
in patients with GCT (9,12,13).
Increased age has been recognized as a crucial risk factor for FN
development, as Feldman et al (13) demonstrated a 44% incidence of FN in
patients aged >50 years. Alternate risk factors observed by
Terbuch et al (12)
included a poor performance status [odds ratio (OR), 2.73; 95%
confidence interval (CI), 1.47-5.06; P=0.001] and a poor-risk class
according to the International Germ Cell Cancer Collaboration Group
(IGCCCG) classification (14,15)
(OR, 4.20; 95% CI, 1.71-10.33; P=0.002).
The use of prophylactic granulocyte-colony
stimulating factor (G-CSF) following treatment with
myelosuppressive chemotherapy has reduced the incidence of FN in
various cancer types, including breast and small cell lung cancer
(16). Fossa et al
(17) reported that prophylactic
use of filgrastim in high-risk patients with GCT was associated
with a decreased incidence of FN events. However, further studies
assessing the effects of G-CSF prophylaxis on FN incidence in
patients with GCT are required.
Therefore, the objective of the present
retrospective study was to evaluate the effects of primary G-CSF
prophylaxis on the incidence and outcomes of FN in patients with
GCT.
Patients and methods
Study patients
The present retrospective study was conducted using
the National Cancer Institute (Bratislava, Slovakia) medical record
database of hospitalized patients with GCTs. The Institutional
Review Board of the National Cancer Institute (Bratislava,
Slovakia) approved the present study, and granted a waiver of
consent (approval no. IZLO1). The subjects included patients
diagnosed with GCTs, who were treated with first-line/adjuvant
chemotherapy at the National Cancer Institute between January 2000
and December 2017. The present study excluded patients who had any
concurrent malignancy, other than non-melanoma skin cancer, in the
last 5 years. Patients who had undergone previous chemotherapy were
also excluded. The study population consisted of male patients
between 17 and 63 years of age (median, 31 years). Patients who did
not receive G-CSF prophylaxis were aged between 17 and 63 years
(median, 30 years). Patients who received G-CSF prophylaxis were
aged between 18 and 61 years (median, 32 years). All patients
received platinum-based chemotherapy regimens.
Definition of FN events
The European Society of Medical Oncology Clinical
Practice Guidelines describe FN as a single oral temperature
reading of >38.5°C, or two consecutive readings of >38.0°C
for 2 h, combined with an absolute neutrophil count of
<0.5×109/l, or a level anticipated to fall below
0.5×109/l (18). While
the baseline absolute neutrophil count is relatively stable in an
individual, there is large interindividual variability. Therefore,
the ‘normal’ range of absolute neutrophil count is very wide and is
considered between 1.5×109/l and 7×109/l
(19–21).
All FN episodes occurring during first-line
chemotherapy were documented. Notably, only the first FN episodes
that occurred in patients were deemed as events on the grounds that
the aim of the present study was the assessment of the effects of
primary G-CSF prophylaxis. Some patients who suffered FN events and
did not receive primary G-CSF prophylaxis received secondary
prophylaxis in subsequent chemotherapy cycles.
Baseline data
Patients underwent chest, abdominal and pelvic
computed tomography scans during the initial staging. The baseline
data included age, primary tumor location, tumor histology, TNM
stage (22), IGCCCG risk class and
first-line chemotherapy regimen.
FN prophylaxis
Only 1 of the patients treated before January 2006
received primary G-CSF prophylaxis. In January 2006, a decision was
made by the National Cancer Institute to administer G-CSF
prophylaxis (filgrastim or pegfilgrastim) to male GCT patients
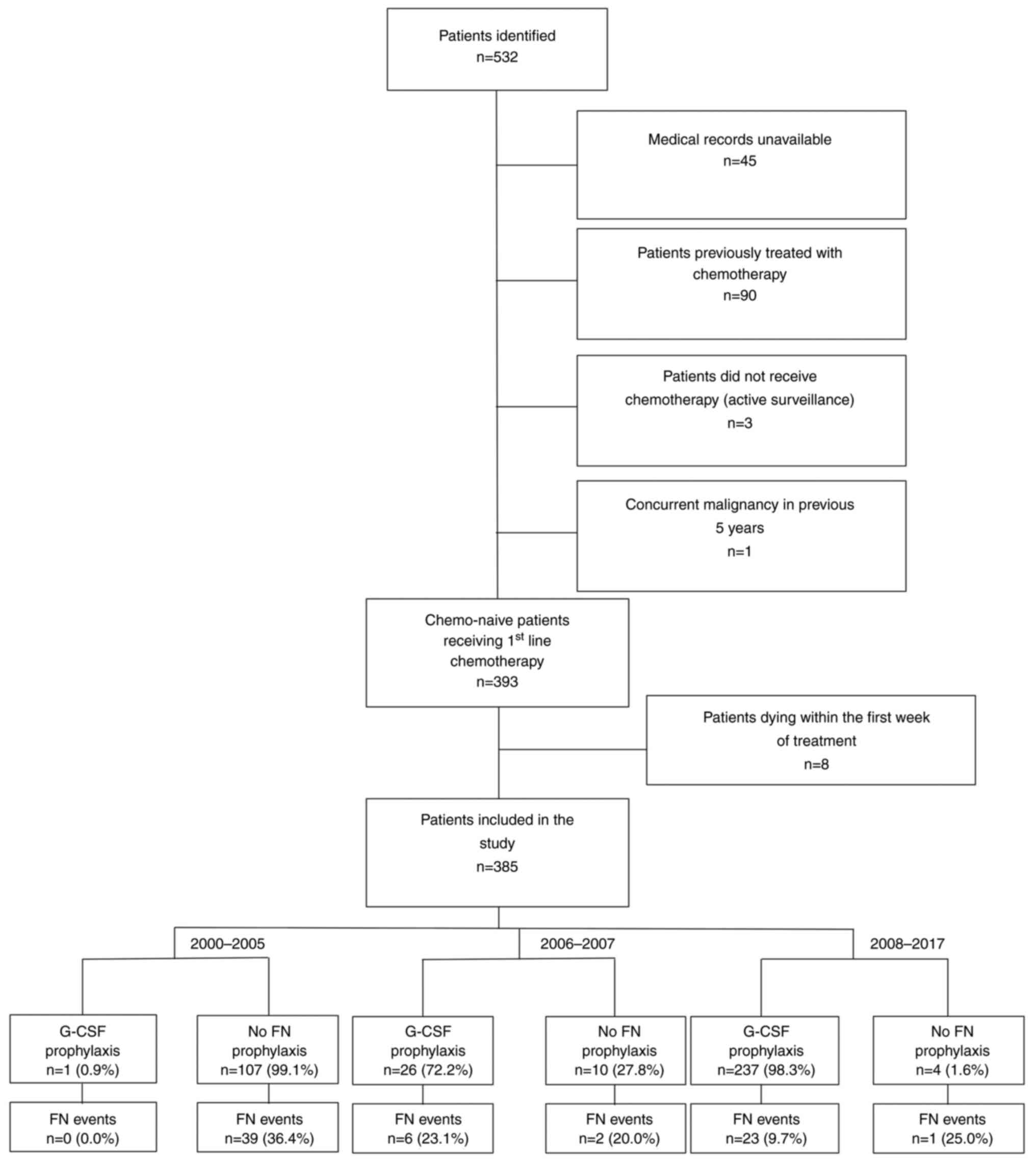
after every cycle of chemotherapy. Fig. 1 shows a flow diagram outlining the
selection process of the study population and a brief timeline of
G-CSF prophylaxis implementation. There was only 1 patient (0.9%)
before January 2006, who received primary G-CSF prophylaxis. During
the transition period in the years 2006 and 2007, 26 patients
(72.2%) received primary G-CSF prophylaxis. From 2008, 237 patients
(98.3%) received primary G-CSF prophylaxis.
Filgrastim (480 µg, subcutaneous) was administered
for 10 days, on days 6, 7, 9–14, 16 and 17 in the bleomycin,
etoposide and cisplatin (BEP) regimen, and for 10 consecutive days
beginning on day 6 in all other regimens. Pegfilgrastim (6 mg/0.6
ml subcutaneous) was administered on day 6. Chemotherapy dosing
schedules are shown in Table
SI.
When FN was observed following the first
chemotherapy cycle, and the patient did not receive primary G-CSF
prophylaxis, G-CSF was administered in subsequent cycles.
Statistical analysis
The present study performed a retrospective review
of the medical records of the patients. All first episodes of FN
were categorized as events. Characteristics of patients are
presented as the median (range) values for continuous variables and
frequency (percentage) for categorical variables. Using Fisher's
exact test for statistical analysis, FN events were compared
between groups with and without prophylaxis. P≤0.05 was considered
to indicate a statistically significant difference.
The primary outcome was the overall incidence of FN
events in the first-line chemotherapy. The secondary outcomes
included incidences of FN events in the numerous subgroups and
overall survival (OS).
Median follow-up was defined as the median
observation time of the patients. OS was determined using the
chemotherapy start date, and the last follow-up date or death. The
median follow-up time of all patients was 68 months (range, 0–224
months), while the median follow-up time of living patients was 82
months (range, 6–224 months) (Table
I). The last follow-up date was May 15, 2019. Follow-up
included history and physical examination, measurement of serum
tumor markers and imaging studies of the chest, abdomen and pelvis.
The follow-up frequency was guided by the initial tumor histology
and clinical stage as recommended by the European Society of
Medical Oncology clinical practice guidelines (23). Kaplan-Meier analysis was performed
to calculate OS. The differences in survival between patients with
and without prophylaxis were calculated using the log-rank test. A
multivariate Cox proportional hazards model for OS revealed the
differences in outcomes following G-CSF prophylaxis and prognosis,
depending on age and IGCCCG risk score. NCSS 2019 statistical
software was utilized for all statistical analyses (24).
 | Table I.Patient characteristics (n=385). |
Table I.
Patient characteristics (n=385).
|
Characteristics | Value |
|---|
| Histology, n
(%) |
|
|
Seminoma | 87 (22.6) |
|
NSGCT | 292 (75.8) |
|
Unknowna | 6 (1.6) |
| Primary tumor, n
(%) |
|
|
Gonadal | 344 (89.4) |
|
Extragonadal | 41 (10.6) |
|
Retroperitoneum | 25 (6.5) |
|
Mediastinum | 12 (3.1) |
|
CNS | 2 (0.5) |
|
Unknown | 2 (0.5) |
| Stage, n (%) |
|
|
I.A-B | 39 (10.1) |
|
I.S | 22 (5.7) |
|
II.A | 31 (8.1) |
|
II.B | 48 (12.5) |
|
II.C | 47 (12.2) |
|
III.A | 49 (12.7) |
|
III.B | 50 (13.0) |
|
III.C | 99 (25.7) |
| IGCCCG risk group,
n (%) |
|
|
Good | 206 (53.5) |
|
Intermediate | 51 (13.2) |
|
Poor | 89 (23.1) |
| Treatment regimen,
n (%) |
|
|
BEP | 273 (70.9) |
| EP | 51 (13.2) |
| Other
regimen | 61 (15.8) |
| Follow-up status, n
(%) |
|
|
Alive | 332 (86.2) |
|
Dead | 53 (13.8) |
| Median follow-up
time (range), months | 68 (0–224) |
| Median follow-up
time for alive patients (range), months | 82 (6–224) |
| Estimated 2-year OS
rate, % | 88.6 |
| Estimated 5-year OS
rate, % | 84.8 |
| Primary G-CSF
prophylaxis, n (%) |
|
| No prophylaxis | 121 |
| G-CSF
prophylaxis | 264 |
| Filgrastim | 39 |
| Pegfligrastim | 225 |
Results
Patient characteristics
The cohort described in the present study included
385 chemotherapy-naive patients with GCTs, treated with first-line
chemotherapy (Fig. 1). A summary
of patient characteristics is shown in Table I. The median age of patients at the
time of enrollment was 31 years (range, 17–63 years). The majority
of patients (75.8%) exhibited non-seminomatous germ cell tumors
(NSGCTs). All patients received platinum-based chemotherapy. A
total of 121 patients (31.4%) did not receive primary G-CSF
prophylaxis, and 264 (68.6%) received primary prophylaxis. A total
of 39 of these patients (14.8%) received filgrastim, while 225
(85.2%) were administered pegfilgrastim. Out of 272 patients
treated with BEP, 176 (64.7%) were administered pegfilgrastim
(Table I).
FN events
During the study period, 71 patients (18.4%)
suffered FN events. Of the 121 patients who did not receive primary
prophylaxis, 42 (34.7%) suffered from FN events. Of these 121
patients, 31 patients (25.6%) had only 1 FN episode, and 11
patients (9.1%) experienced >1. Of the 264 patients receiving
prophylaxis, 29 (11.0%) had FN events, 25 (9.5%) experienced only 1
FN episode and 4 patients (1.5%) had >1 episode (Table II).
 | Table II.FN events. |
Table II.
FN events.
| Variables | Overall incidence,
n (%) | Incidence in the no
prophylaxis group, n (%) | Incidence in the
G-CSF prophylaxis group, n (%) | P-value |
|---|
| All FN events | 71/385 (18.4) | 42/121 (34.7) | 29/264 (11.0) | ≤0.0001 |
| Histology |
|
|
|
|
|
Seminoma | 10/87 (11.5) | 4/22 (18.2) | 6/65 (9.2) | 0.2552 |
|
NSGCT | 61/292 (20.9) | 38/99 (38.4) | 23/193 (11.9) | ≤0.0001 |
| Primary tumor
location |
|
|
|
|
|
Gonadal | 59/344 (17.2) | 32/103 (31.1) | 27/241 (11.2) | ≤0.0001 |
|
Extragonadal | 12/41 (29.3) | 10/18 (55.6) | 2/23 (8.7) | 0.0011 |
| Stage
IA/B | 2/39 (5.1) | 0/12 (0.0) | 2/27 (7.4) | 0.3330 |
| IGCCCG risk
group |
|
|
|
|
|
Good | 22/206 (10.7) | 12/55 (21.8) | 10/151 (6.6) | 0.0017 |
|
Intermediate | 10/51 (19.6) | 5/14 (35.7) | 5/37 (13.5) | 0.0747 |
|
Poor | 37/89 (41.6) | 25/40 (62.5) | 12/49 (24.5) | 0.0003 |
| Chemotherapy
regimen |
|
|
|
|
|
BEP | 40/273 (14.7) | 16/69 (23.2) | 24/204 (11.8) | 0.0204 |
| EP | 5/51 (9.8) | 3/19 (15.8) | 2/32 (6.3) | 0.2680 |
|
Other | 26/61 (42.6) | 23/33 (69.7) | 3/28 (10.7) | ≤0.0001 |
| FN episodes per
patient |
|
|
|
|
| 1 | 56/385 (14.5) | 31/121 (25.6) | 25/264 (9.50) |
|
|
>1 | 15/385 (3.90) | 11/121 (9.09) | 4/264 (1.52) |
|
The majority of FN episodes (69.1%) occurred in the
first chemotherapy cycle. Overall, FN incidence after the first
chemotherapy cycle was 16.8%. In patients receiving prophylaxis, FN
incidence after the first chemotherapy cycle was 9.8%, while FN
incidence was 32.3% in those without prophylaxis (P<0.0001; data
not shown). The FN incidence in patients administered filgrastim
and pegfilgrastim was 20.5 and 9.3%, respectively (P=0.0393).
Patients that received G-CSF prophylaxis experienced a prolonged
period before experiencing FN compared with patients without
prophylaxis [hazard ratio (HR), 0.30; 95% CI, 0.18-0.50;
P=0.00000001]. A total of 11 (2.9%) patients died during the
chemotherapy. Of these, 8 patients experienced FN events, with 4
receiving primary G-CSF prophylaxis and 4 patients not.
Association between FN prophylaxis and
patient/tumor characteristics
The highest FN incidence (42.6%) occurred in
patients that received a chemotherapy regimen other than BEP, or
etoposide and cisplatin (EP). The two regimens most frequently
associated with FN development were the paclitaxel, bleomycin,
etoposide and cisplatin (T-BEP; 69.0%) regimen, and the etoposide,
iphosphamide and cisplatin (VIP; 50.0%) regimen. A high FN
incidence was also observed in patients with poor-risk disease
according to the IGCCCG classification (41.6%), and in those with
extragonadal tumors (29.3%).
A total of 61 FN events (20.9%) occurred in patients
with NSGCTs and 10 (11.5%) in patients with seminoma. While there
was a significantly lower (11.9 vs. 38.4%; P<0.0001) FN
incidence in patients with NSGCT receiving prophylaxis compared
with that in patients without prophylaxis, the difference in
incidence rates in patients with seminoma was not statistically
significant (9.2 vs. 18.2%; P=0.2552).
A total of 59 FN events (17.2%) were observed in
patients with gonadal tumors, and 12 (29.3%) were recorded in
patients with extragonadal tumors. There was a significantly lower
FN incidence in patients receiving prophylaxis for both primary
tumor locations (P<0.0001 and P=0.0011 for patients with gonadal
and extragonadal tumors respectively).
A total of 22 (10.7%) FN events occurred in patients
with good-risk disease, according to the IGCCCG classification, a
total of 10 events (19.6%) occurred in those with intermediate-risk
disease and 37 events (41.6%) occurred in patients with poor-risk
disease. There was a significantly lower FN incidence in patients
receiving prophylaxis with either good (P=0.0017) or poor-risk
disease (P=0.0003). A lower incidence rate was observed in patients
receiving G-CSF prophylaxis with intermediate-risk disease, but
this difference was not statistically significant (P=0.0747). These
data are summarized in Table
II.
FN events occurred in 40 patients (14.7%) receiving
BEP, 20 patients (69.0%) receiving T-BEP, 5 patients (9.8%)
receiving EP, 4 patients (50.0%) receiving VIP and 2 patients
(22.2%) receiving the paclitaxel, iphosphamide and cisplatin
regimen. No FN events occurred in patients who received GETUG 13
(25) or other regimens (Table III).
 | Table III.FN event incidence based on
chemotherapy regimen. |
Table III.
FN event incidence based on
chemotherapy regimen.
| Regimen | Overall incidence,
n (%) | Incidence in the no
prophylaxis group, n (%) | Incidence in the
G-CSF prophylaxis group, n (%) |
|---|
| BEP | 40/273 (14.7) | 16/69 (23.2) | 24/204 (11.8) |
| EP | 5/51 (9.8) | 3/19 (15.8) | 2/32 (6.3) |
| T-BEP | 20/29 (69.0) | 19/27 (70.4) | 1/2 (50.0) |
| GETUG 13 | 0/11 (0.0) | 0/0 (0.0) | 0/11 (0.0) |
| TIP | 2/9 (22.2) | 0/0 (0.0) | 2/9 (22.2) |
| VIP | 4/8 (50.0) | 4/6 (66.7) | 0/2 (0.0) |
| Othera | 0/4 (0.0) | 0/0 (0.0) | 0/4 (0.0) |
A significantly lower (P=0.0296) FN incidence
occurred in patients receiving G-CSF prophylaxis, according to a
subgroup analysis of patients subjected to BEP chemotherapy
compared with patients without prophylaxis. The incidence was also
significantly lower in the BEP subgroup for patients receiving
prophylaxis with NSGCT histology (P=0.0250) or good-risk disease
(P=0.0061) in comparison with patients without prophylaxis. A lower
(P=0.0578) FN incidence occurred in patients with extragonadal
tumors treated with BEP who received G-CSF prophylaxis compared
with that in patients without prophylaxis. These results are shown
in Table IV.
 | Table IV.FN events in patients receiving the
bleomycin, etoposide and cisplatin regimen. |
Table IV.
FN events in patients receiving the
bleomycin, etoposide and cisplatin regimen.
| Variables | Overall incidence,
n (%) | Incidence in the no
prophylaxis group, n (%) | Incidence in the
G-CSF prophylaxis group, n (%) | P-value |
|---|
| All FN events | 40/272 (14.7) | 16/69 (23.2) | 24/203 (11.8) | 0.0296 |
|
Histologya |
|
|
|
|
|
Seminoma | 4/34 (11.8) | 0/3 (0.0) | 4/31 (12.9) | >0.999 |
|
NSGCT | 36/237 (15.2) | 16/66 (24.2) | 20/171 (11.7) | 0.0250 |
| Primary tumor
location |
|
|
|
|
|
Gonadal | 35/252 (13.9) | 12/61 (19.7) | 23/191 (12.0) | 0.1407 |
|
Extragonadal | 5/20 (25.0) | 4/8 (50.0) | 1/12 (8.3) | 0.0578 |
| Stage
IA/B | 2/37 (5.4) | 0/12 (0.0) | 2/25 (8.0) | 0.5495 |
| IGCCCG risk
group |
|
|
|
|
|
Good | 17/154 (11.0) | 9/37 (24.3) | 8/117 (6.8) | 0.0061 |
|
Intermediate | 7/44 (15.9) | 2/10 (20.0) | 5/34 (14.7) | 0.6490 |
|
Poor | 14/37 (37.8) | 5/10 (50.0) | 9/27 (33.3) | 0.4537 |
Association between OS and G-CSF
prophylaxis
The median follow-up time of all patients was 68
months (range, 0–224 months), while the median follow-up time of
living patients was 82 months (range, 6–224 months) (Table I). A total of 53 deaths (13.8%)
occurred in the present study population (Table V). The estimated 2- and 5-year OS
rates of the patients were 88.6 and 84.8%, respectively (Table VI).
 | Table V.Cause of death. |
Table V.
Cause of death.
| Cause of death | Patients, n
(%) | No prophylaxis, n
(%) | G-CSF prophylaxis,
n (%) |
P-valuea |
|---|
| Death during
treatment | 11 (2.9) | 6 (5.0) | 5 (1.9) | 0.1071 |
| Disease
progression | 39 (10.1) | 19 (15.7) | 20 (7.6) | 0.0180 |
| Unknown | 1 (0.3) | 0 (0.0) | 1 (0.4) | NA |
| Second primary
malignancy | 1 (0.3) | 0 (0.0) | 1 (0.4) | NA |
| Death unrelated to
cancer | 1 (0.3) | 1 (0.8) | 0 (0.0) | NA |
 | Table VI.Overall survival. |
Table VI.
Overall survival.
| Variables | G-CSF prophylaxis,
n | No G-CSF
prophylaxis, n | HR | Lower 95% CI | Upper 95% CI | P-value |
|---|
| All patients | 264 | 121 | 0.54 | 0.31 | 0.96 | 0.0235 |
| Stage IA/B | 27 | 12 | NA | NA | NA | NA |
| IGCCCG risk
group |
|
|
|
|
|
|
|
Good | 151 | 55 | 0.28 | 0.04 | 1.92 | 0.1390 |
|
Intermediate | 37 | 14 | 0.85 | 0.07 | 10.11 | 0.8968 |
|
Poor | 49 | 40 | 0.88 | 0.48 | 1.61 | 0.6783 |
| Chemotherapy
regimen |
|
|
|
|
|
|
|
BEP | 204 | 69 | 0.63 | 0.27 | 1.46 | 0.2341 |
| EP | 32 | 19 | NA | NA | NA | NA |
|
Other | 28 | 33 | 1.11 | 0.50 | 2.49 | 0.7964 |
| Tumor
histology |
|
|
|
|
|
|
|
Seminoma | 65 | 22 | NA | NA | NA | NA |
|
NSGCT | 193 | 99 | 0.52 | 0.29 | 0.93 | 0.0172 |
| Primary tumor
location |
|
|
|
|
|
|
|
Gonadal | 241 | 103 | 0.56 | 0.28 | 1.09 | 0.0607 |
|
Extragonadal | 23 | 18 | 0.70 | 0.24 | 2.10 | 0.5178 |
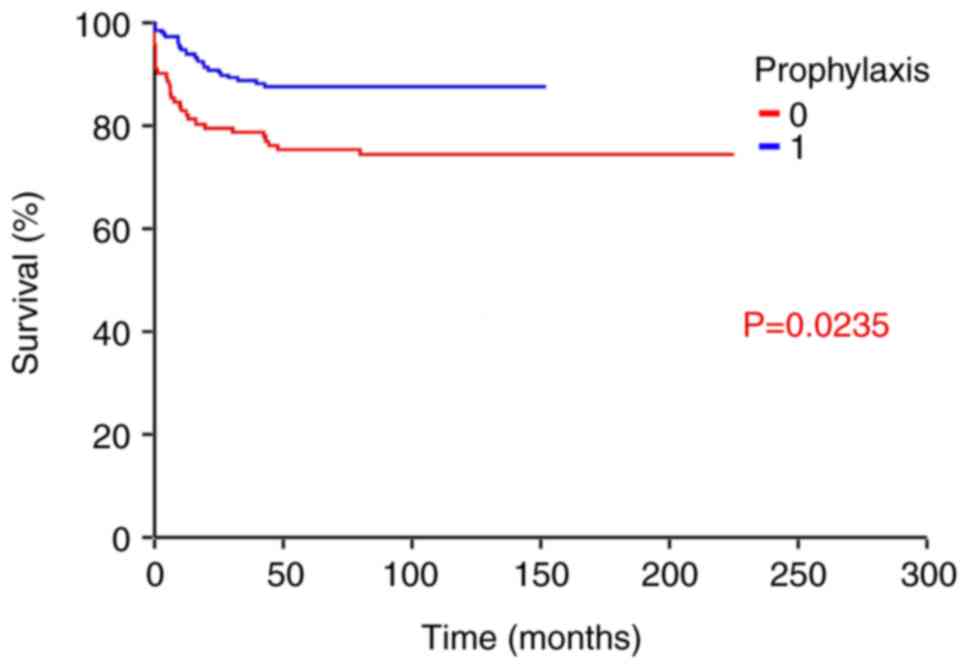
Patients receiving G-CSF prophylaxis exhibited a
significantly prolonged OS rate (HR, 0.54; 95% CI, 0.31-0.96;
P=0.0235) compared with patients without it (Fig. 2). These results are shown in
Table VI. Patients with NSGCT
histology that received G-CSF prophylaxis exhibited a significantly
prolonged OS time (HR, 0.52; 95% CI, 0.29-0.93; P=0.0172) compared
with those without it. An increased OS rate (HR, 0.56; 95% CI,
0.28-1.09; P=0.0607) was observed in patients that received G-CSF
prophylaxis with a gonadal tumor, compared with those without
prophylaxis, although the difference was not significant. An
increased OS rate (HR=0.28; 95% CI, 0.04-1.92; P=0.1390) occurred
in patients taking prophylaxis who were also categorized as
good-risk, although the difference was not significant (P=0.1390;
Fig. 3A). No statistically
significant difference in OS rate was observed in intermediate risk
(P=0.8968; Fig. 3B) or poor risk
(P=0.6783; Fig. 3C) groups when
comparing patients with and without prophylaxis. The results of the
multivariate analysis demonstrated that only the IGCCCG risk class
was associated with survival, while the remaining results,
including those for patients who received G-CSF were not
statistically significant (P=0.06; Table VII). Notably, patients who
received G-CSF prophylaxis exhibited a reduced death rate due to
disease progression (7.6 vs. 15.7%; P=0.0180) compared with
patients without prophylaxis (Table
V).
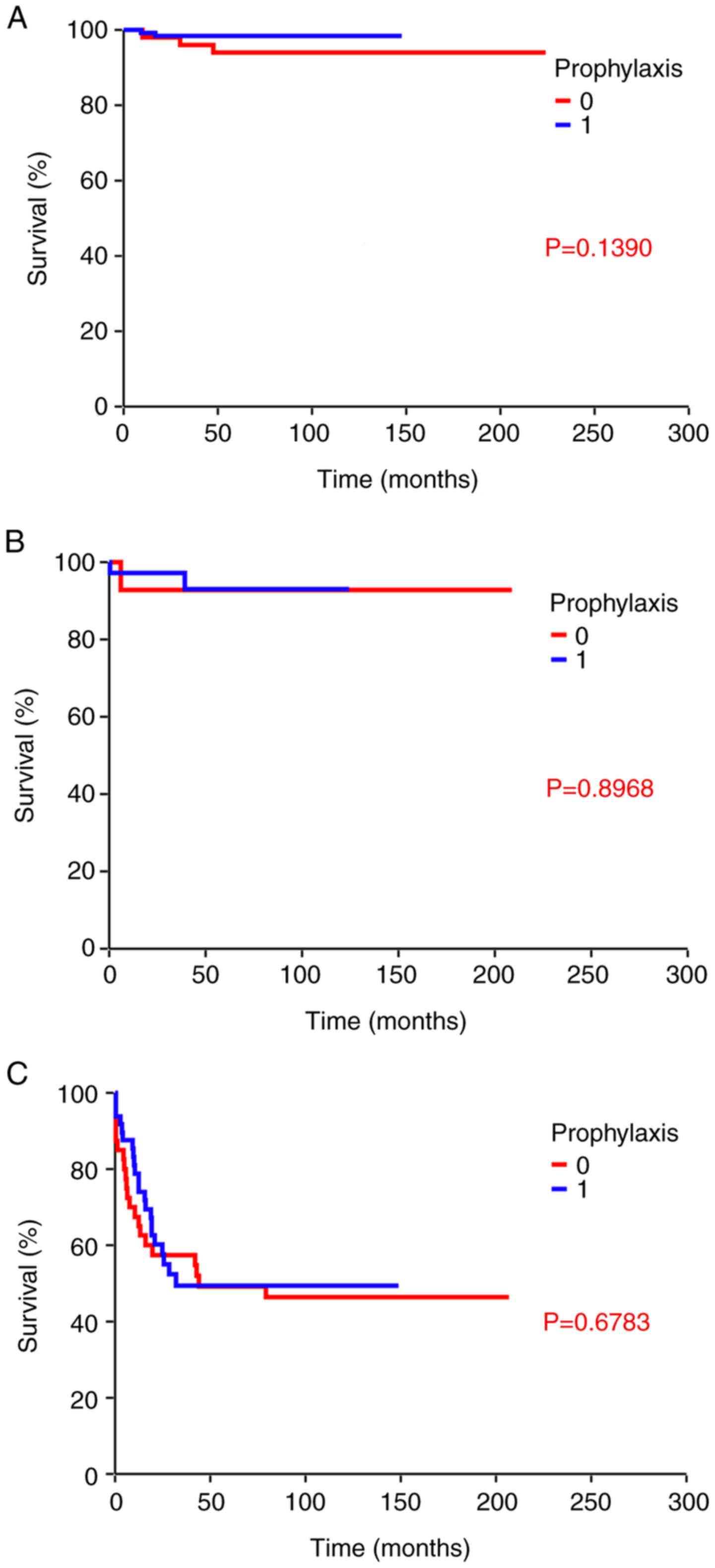 | Figure 3.G-CSF prophylaxis and OS by
International Germ Cell Cancer Collaboration Group risk group. (A)
Kaplan-Meier analysis determined estimates of probabilities of OS,
according to primary G-CSF prophylaxis in patients with a good
prognosis (n=206) (HR, 0.28; 95% CI, 0.04-1.92; P=0.1390). (B)
Kaplan-Meier analysis determined estimates of probabilities of OS,
according to primary G-CSF prophylaxis in patients with an
intermediate prognosis (n=51) (HR, 0.85; 95% CI, 0.07-10.11;
P=0.8968). (C) Kaplan-Meier analysis determined estimates of
probabilities of OS, according to primary G-CSF prophylaxis in
patients with a poor prognosis (n=89) (HR, 0.88; 95% CI, 0.48-1.61;
P=0.6783). 0, no primary G-CSF prophylaxis; 1, primary G-CSF
prophylaxis with filgrastim/pegfilgrastim; CI, confidence interval;
G-CSF, granulocyte-colony stimulating factor; HR, hazard ratio; OS,
overall survival. |
 | Table VII.Multivariate Cox regression analysis
of the potential prognostic value of G-CSF. |
Table VII.
Multivariate Cox regression analysis
of the potential prognostic value of G-CSF.
| Variable | HR (95% CI) | P-value |
|---|
| Age
(continuous) | 1.02
(0.99-1.05) | 0.2500 |
| G-CSF (present vs.
absent) | 0.58
(0.33-1.03) | 0.0600 |
| IGCCCG
(poor/intermediate vs. good risk) | 16.15
(6.37-40.94) | <0.0001 |
No statistically significant difference was observed
between the OS of patients with or without G-CSF prophylaxis,
following a subgroup analysis of patients receiving a BEP
chemotherapy regimen (Table
VIII).
 | Table VIII.Overall survival in patients
receiving the BEP regimen. |
Table VIII.
Overall survival in patients
receiving the BEP regimen.
| Variables | G-CSF prophylaxis,
n | No G-CSF
prophylaxis, n | HR | Lower 95% CI | Upper 95% CI | P-value |
|---|
| Patients receiving
BEP | 204 | 69 | 0.63 | 0.27 | 1.46 | 0.2341 |
| Stage IA/B | 26 | 12 | NA | NA | NA | NA |
| IGCCCG risk
group |
|
|
|
|
|
|
|
Good | 117 | 37 | 0.25 | 0.04 | 1.79 | 0.1011 |
|
Intermediate | 34 | 10 | 0.66 | 0.05 | 9.07 | 0.7334 |
|
Poor | 27 | 10 | 0.69 | 0.24 | 2.00 | 0.4609 |
| Tumor
histology |
|
|
|
|
|
|
|
Seminoma | 31 | 3 | NA | NA | NA | NA |
|
NSGCT | 171 | 66 | 0.56 | 0.23 | 1.36 | 0.1573 |
| Primary tumor
location |
|
|
|
|
|
|
|
Gonadal | 191 | 61 | 0.66 | 0.26 | 1.67 | 0.3321 |
|
Extragonadal | 12 | 8 | 0.71 | 0.10 | 5.17 | 0.7269 |
Discussion
FN is a complication that often occurs during the
course of chemotherapy, leading to prolonged hospital stays, often
increasing morbidity and mortality rates (26,27).
In the present study, patients receiving primary G-CSF prophylaxis
exhibited markedly lower FN incidence rates than patients without
it. A decreased FN incidence occurred in patients receiving
pegfilgrastim, compared with those receiving filgrastim. These
results may suggest that patients have lower compliance to
filgrastim than pegfilgrastim. Filgrastim was selected as the main
prophylaxis in the inpatient setting for hospitalized patients with
a poor performance status. Researchers have previously identified
poor performance status as an FN risk factor (12). In the present study, the highest FN
incidence was observed in the first chemotherapy cycle, similar to
the results of previous studies (12,28).
The highest FN incidence in the first chemotherapy cycle may also
be associated with the use of G-CSF in subsequent cycles, if the
patient developed FN in the first cycle, and/or following dose
reductions in patients that previously experienced FN.
Hematological toxicity is also more pronounced in patients with
lower baseline neutrophil and lymphocyte counts, which may be
associated with tumor-induced immunosuppression (29,30).
In the present study, an FN incidence rate of 34.7%
was observed in patients without G-CSF prophylaxis, which was
notably higher than the results reported in previous studies
(11,12,31).
However, Nishikawa et al (9) reported a high incidence rate of
39.5%. In the present study, FN incidence was particularly high in
patients treated with T-BEP and VIP. Notably, primary prophylaxis
was not mandatory in the phase II trial by Mardiak et al
(32) using the T-BEP regimen. One
factor accounting for the discrepancy may be the difference in
study populations. Terbuch et al (12) reported a 17% FN incidence rate.
However, patients with poor-risk disease accounted for only 12% of
all metastatic patients in the previous study, compared with 23.1%
observed in the present study. Furthermore, the results of the
present study demonstrated that FN incidence was positively
associated with IGCCCG risk. While patients with good-risk disease
exhibited an FN incidence of 21.8%, the FN incidence was 62.5% in
poor-risk disease patients without primary G-CSF prophylaxis. These
results are comparable with those of previous studies (16,17),
stating that poor-risk disease is a risk factor for FN. Therefore,
the study population structure must be considered while comparing
FN incidence rates. Furthermore, even patients with good-risk
disease exhibited higher levels of FN incidence (21.8%) in the
present study, compared with the results of previous studies
(12,33). Culine et al (33) reported an FN incidence of 7% in
patients with good-risk NSGCTs receiving the BEP chemotherapy
regimen, and 5% in patients receiving the EP regimen. In addition,
Terbuch et al (12)
revealed an incidence of 17.7% in patients with good-risk
disease.
Primary G-CSF prophylaxis reduced FN incidence in
patients with NSGCTs and seminomas. However, the difference
observed in the seminoma subgroup was not statistically
significant. The differences in sample size (seminomas represented
only 22.6% of the study population) and lower FN risk in seminomas
without prophylaxis may explain these inconclusive results.
The chemotherapy regimen influences the FN incidence
(32,34,35).
Patients treated with T-BEP or VIP exhibit markedly higher
hematological toxicity rates than those subjected to the BEP
regimen (32,35–37).
This may account for the higher incidence of FN in patients with
extragonadal GCTs in the present study, as they were treated with
VIP or T-BEP regimens more frequently than patients with gonadal
tumors. While 16.7% of patients with extragonadal GCT were treated
with a VIP chemotherapy regimen, only 2.3% with gonadal tumors
received a VIP regimen. T-BEP chemotherapy was also a more frequent
treatment choice in patients with extragonadal GCTs (27.7 vs.
21.4%) compared with that in patients with a gonadal primary tumor
location. In addition, a higher proportion of patients with
extragonadal GCTs had poor-risk disease, compared with those with
gonadal GCTs (55.6 vs. 29.1%) (data not shown).
The present data suggested that G-CSF prophylaxis
reduced FN incidence in all subgroups, except for patients with
stage I disease. The clinical practice guidelines issued in 2010
for the management of FN suggested using an individualized
approach, if the expected FN incidence was between 10 and 20%
(34). In the present study, two
subgroups of patients were in this category: Patients with seminoma
and patients receiving EP chemotherapy. Although a numerically
lower FN incidence was present in patients receiving primary G-CSF
prophylaxis than in patients without prophylaxis, the difference
was not statistically significant.
The results of the univariate analysis revealed a
statistically significant increase in OS in patients receiving
primary G-CSF prophylaxis; however, the results of the multivariate
analysis did not verify this finding. Furthermore, when only the
subgroup of patients treated with BEP was analyzed, primary G-CSF
did not affect OS. Therefore, the observed effect may have been
mediated in patients treated with a different regimen than BEP. In
addition, cause of death analysis indicated that primary G-CSF
reduced deaths during the treatment and was also associated with a
lower risk of disease progression. This may be associated with dose
modification of chemotherapy and treatment delays, due to FN
occurrence in patients without primary prophylaxis. However, other
immune-related mechanisms may also contribute to this observation.
Previous studies have demonstrated that immune-related factors,
such as programmed death-ligand 1 expression, systemic inflammatory
index and serum cytokines, are associated with GCT prognosis
(38–40). Therefore, administration of G-CSF
may have a pleiotropic effect on the immune system beyond
neutrophil count. Consequently, an increased OS rate in patients
receiving G-CSF prophylaxis may also be explained by this mechanism
(41). Notably, G-CSF mediates the
differentiation of granulocyte progenitors into mature
granulocytes, including neutrophils, eosinophils and basophils
(42,43).
The pro-tumor role of G-CSF has been described in a
preclinical study (44). While no
detrimental effects have been observed in patients receiving
prophylactic G-CSF to prevent chemotherapy-induced neutropenia,
further research is required to understand the role of G-CSF in
tumor growth, progression, metastasis and treatment outcomes
(45).
To the best of our knowledge, the present study is
the most expansive study evaluating the effects of primary G-CSF
prophylaxis in patients with GCT, reflecting routine clinical
practice in a tertiary cancer center. The present study has
limitations due to its retrospective, non-randomized and
single-center design. The majority of patients received BEP or EP
chemotherapy. The number of patients receiving other chemotherapy
regimens was not high and was not uniformly distributed among
subgroups. Therefore, the results predominantly apply to patients
with GCT treated with BEP or EP regimens. The number of patients in
several subgroups was also limited. Implementation of primary G-CSF
prophylaxis into practice was progressive and there were some
patients during the transition period (2006–2007) that did not
receive it. It cannot be excluded that during this period the
decision for administration of G-CSF prophylaxis was also driven by
the perception of the physician of the risk of FN and this could be
a potential source of bias; however, from 2008, almost all patients
received prophylaxis. In addition, patients that did not receive
primary G-CSF prophylaxis received G-CSF prophylaxis in subsequent
cycles, secondary to the neutropenia or FN in the previous
cycle.
In conclusion, the present retrospective study
demonstrated the prophylactic effects of G-CSF on FN incidence in
patients with GCT who were treated with first-line chemotherapy for
metastatic disease. This effect was most pronounced in more
aggressive chemotherapy regimens other than EP or BEP (such as
T-BEP and VIP). According to the results of the present study,
G-CSF prophylaxis should be considered in daily clinical practice
for patients with metastatic GCTs that have been subjected to
first-line chemotherapy, particularly in the first cycle in
patients with high-risk features.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to acknowledge Mrs Veronika
Remenarova (Second Department of Oncology, Comenius University,
Faculty of Medicine, National Cancer Institute, Bratislava, Slovak
Republic) for providing technical help. The abstract was presented
at the 2020 ASCO Annual Meeting I (May 29–31, 2020) and was
published as abstract no. e17056 in J Clin Oncol 38 (issue 15
suppl).
Funding
The present study was funded by the Slovak Research and
Development Agency (APVV; grant no. APVV-15-0086), the Ministry of
Health (grant no. 2018/39-LFUK-13) and Scientific Grant Agency
(VEGA) contracts (grant nos. 1/0349/21, 1/0327/19 and
1/0043/18).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JM and MM were responsible for conceptualization.
NH, MC, KR, KK, JO, PP, VDA, DS, ZSM, JM and MM were responsible
for data collection. NH and MM were responsible for the formal
analysis. NH, JM and MM were responsible for the methodology. NH
and MM were responsible for visualization. NH and MM confirm the
authenticity of all the raw data. MM and NH were responsible for
writing the original draft, and NH, MC, KR, KK, JO, PP, VDA, DS,
ZSM, JM and MM were responsible for writing, reviewing and editing.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The Institutional Review Board of the National
Cancer Institute (Bratislava, Slovakia) approved the present study,
and granted a waiver of consent (approval no. IZLO1) for the
collection, analysis and publication of the retrospectively
obtained and anonymized data for this non-interventional study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
FN
|
febrile neutropenia
|
|
G-CSF
|
granulocyte–colony stimulating
factor
|
|
GCTs
|
testicular germ cell tumors
|
|
IGCCCG
|
International Germ Cell Cancer
Collaboration Group
|
|
NSGCT
|
non-seminomatous germ cell tumor
|
|
OS
|
overall survival
|
|
T-BEP
|
paclitaxel, bleomycin, etoposide and
cisplatin
|
|
VIP
|
etoposide, iphosphamide and
cisplatin
|
References
|
1
|
Nigam M, Aschebrook-Kilfoy B, Shikanov S
and Eggener S: Increasing incidence of testicular cancer in the
United States and Europe between 1992 and 2009. World J Urol.
33:623–631. 2015. View Article : Google Scholar
|
|
2
|
Heidenreich A, Paffenholz P, Nestler T and
Pfister D: European Association of Urology Guidelines on Testis
Cancer: Important Take Home Messages. Eur Urol Focus. 5:742–744.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Einhorn LH: Treatment of testicular
cancer: A new and improved model. J Clin Oncol. 8:1777–1781. 1990.
View Article : Google Scholar
|
|
4
|
Beyer J, Albers P, Altena R, Aparicio J,
Bokemeyer C, Busch J, Cathomas R, Cavallin-Stahl E, Clarke NW,
Claßen J, et al: Maintaining success, reducing treatment burden,
focusing on survivorship: Highlights from the third European
consensus conference on diagnosis and treatment of germ-cell
cancer. Ann Oncol. 24:878–888. 2013. View Article : Google Scholar
|
|
5
|
Travis LB, Beard C, Allan JM, Dahl AA,
Feldman DR, Oldenburg J, Daugaard G, Kelly JL, Dolan ME, Hannigan
R, et al: Testicular cancer survivorship: Research strategies and
recommendations. J Natl Cancer Inst. 102:1114–1130. 2010.
View Article : Google Scholar
|
|
6
|
Chovanec M, Vasilkova L, Setteyova L,
Obertova J, Palacka P, Rejlekova K, Sycova-Mila Z, Kalavska K,
Svetlovska D, Cingelova S, et al: Long-term cognitive functioning
in testicular germ-cell tumor survivors. Oncologist. 23:617–623.
2018. View Article : Google Scholar
|
|
7
|
Oun R, Moussa YE and Wheate NJ: The side
effects of platinum-based chemotherapy drugs: A review for
chemists. Dalton Trans. 47:6645–6653. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Crawford J, Becker PS, Armitage JO,
Blayney DW, Chavez J, Curtin P, Dinner S, Fynan T, Gojo I,
Griffiths EA, et al: Myeloid growth factors, version 2.2017, NCCN
clinical practice guidelines in oncology. J Natl Compr Canc Netw.
15:1520–1541. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nishikawa M, Miyake H and Fujisawa M:
Identification of risk factors predicting febrile neutropenia in
patients with metastatic germ cell tumors receiving cisplatin-based
combination chemotherapy. Int J Urol. 24:449–453. 2017. View Article : Google Scholar
|
|
10
|
Cullen M and Baijal S: Prevention of
febrile neutropenia: Use of prophylactic antibiotics. Br J Cancer.
101 (Suppl 1):S11–S14. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Counsell R, Pratt J and Williams MV:
Chemotherapy for germ cell tumours: Prophylactic ciprofloxacin
reduces the incidence of neutropenic fever. Clin Oncol (R Coll
Radiol). 6:232–236. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Terbuch A, Posch F, Partl R, Zurl B,
Bauernhofer T, Pichler M, Szkandera J, Hutterer GC, Pummer K, Kapp
KS, et al: Risk stratification for febrile neutropenia in patients
with testicular germ cell tumors. Cancer Med. 7:508–514. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Feldman DR, Voss MH, Jacobsen EP, Jia X,
Suarez JA, Turkula S, Sheinfeld J, Bosl GJ, Motzer RJ and Patil S:
Clinical features, presentation, and tolerance of platinum-based
chemotherapy in germ cell tumor patients 50 years of age and older.
Cancer. 119:2574–2581. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Beyer J, Collette L, Sauvé N, Daugaard G,
Feldman DR, Tandstad T, Tryakin A, Stahl O, Gonzalez-Billalabeitia
E, De Giorgi U, et al: Survival and new prognosticators in
metastatic seminoma: Results from the IGCCCG-update consortium. J
Clin Oncol. 39:1553–1562. 2021. View Article : Google Scholar
|
|
15
|
Gillessen S, Sauvé N, Collette L, Daugaard
G, de Wit R, Albany C, Tryakin A, Fizazi K, Stahl O, Gietema JA, et
al: Predicting outcomes in men with metastatic nonseminomatous germ
cell tumors (NSGCT): Results from the IGCCCG update consortium. J
Clin Oncol. 39:1563–1574. 2021. View Article : Google Scholar
|
|
16
|
Wang L, Baser O, Kutikova L, Page JH and
Barron R: The impact of primary prophylaxis with granulocyte
colony-stimulating factors on febrile neutropenia during
chemotherapy: A systematic review and meta-analysis of randomized
controlled trials. Support Care Cancer. 23:3131–3140. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fosså SD, Kaye SB, Mead GM, Cullen M, de
Wit R, Bodrogi I, van Groeningen CJ, De Mulder PH, Stenning S,
Lallemand E, et al: Filgrastim during combination chemotherapy of
patients with poor-prognosis metastatic germ cell malignancy.
European organization for research and treatment of cancer,
genito-urinary group, and the medical research council testicular
cancer working party, Cambridge, United Kingdom. J Clin Oncol.
16:716–724. 1998. View Article : Google Scholar
|
|
18
|
de Naurois J, Novitzky-Basso I, Gill MJ,
Gill MJ, Marti FM, Cullen MH and Roila F; ESMO Guidelines Working
Group, : Management of febrile neutropenia: ESMO clinical practice
guidelines. Ann Oncol. 21 (Suppl 5):v252–v256. 2010. View Article : Google Scholar
|
|
19
|
von Vietinghoff S and Ley K: Homeostatic
regulation of blood neutrophil counts. J Immunol. 181:5183–5188.
2008. View Article : Google Scholar
|
|
20
|
Foucar K, Chabot-Richards D, Czuchlewski
DR, Karner KH, Reichard KK, Vasef MA, Wilson CS, Zhang QY and
Culbreath K: Neutropenia. Diagnostic pathology: Blood and bone
marrow. 2nd edition. Elsevier; Netherlands: pp. 180–187. 2018
|
|
21
|
Foucar K, Chabot-Richards D, Czuchlewski
DR, Karner KH, Reichard KK, Vasef MA, Wilson CS, Zhang QY and
Culbreath K: Neutrophilia. Diagnostic pathology: Blood and bone
marrow. 2nd edition. Elsevier; Netherlands: pp. 188–193. 2018
|
|
22
|
Amin MB, Greene FL, Edge SB, Compton CC,
Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR and
Winchester DP: The eighth edition AJCC cancer staging manual:
Continuing to build a bridge from a population-based to a more
‘personalized’ approach to cancer staging. CA Cancer J Clin.
67:93–99. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Honecker F, Aparicio J, Berney D, Beyer J,
Bokemeyer C, Cathomas R, Clarke N, Cohn-Cedermark G, Daugaard G,
Dieckmann KP, et al: ESMO consensus conference on testicular germ
cell cancer: Diagnosis, treatment and follow-up. Ann Oncol.
29:1658–1686. 2018. View Article : Google Scholar
|
|
24
|
NCSS 2019 statistical software., . NCSS,
LLC. Kaysville; Utah, USA: Version 19.0.3. 2019, https://www.ncss.com/software/ncss
|
|
25
|
Fizazi K, Pagliaro L, Laplanche A, Fléchon
A, Mardiak J, Geoffrois L, Kerbrat P, Chevreau C, Delva R, Rolland
F, et al: Personalised chemotherapy based on tumour marker decline
in poor prognosis germ-cell tumours (GETUG 13): A phase 3,
multicentre, randomised trial. Lancet Oncol. 15:1442–1450. 2014.
View Article : Google Scholar
|
|
26
|
Kuderer NM, Dale DC, Crawford J, Cosler LE
and Lyman GH: Mortality, morbidity, and cost associated with
febrile neutropenia in adult cancer patients. Cancer.
106:2258–2266. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Caggiano V, Weiss RV, Rickert TS and
Linde-Zwirble WT: Incidence, cost, and mortality of neutropenia
hospitalization associated with chemotherapy. Cancer.
103:1916–1924. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Crawford J, Dale DC, Kuderer NM, Culakova
E, Poniewierski MS, Wolff D and Lyman GH: Risk and timing of
neutropenic events in adult cancer patients receiving chemotherapy:
The results of a prospective nationwide study of oncology practice.
J Natl Compr Canc Netw. 6:109–118. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ray-Coquard I, Borg C, Bachelot T, Sebban
C, Philip I, Clapisson G, Le Cesne A, Biron P, Chauvin F and Blay
JY; ELYPSE study group, : Baseline and early lymphopenia predict
for the risk of febrile neutropenia after chemotherapy. Br J
Cancer. 88:181–186. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee RK, Soyemi SA, Chen M, Kanis MJ and
Lee YC: Pretreatment absolute neutrophil counts predict
neutropenia-related events in patients undergoing first line
chemotherapy in gynecologic malignancies. Gynecol Oncol. 154 (Suppl
1):S1262019. View Article : Google Scholar
|
|
31
|
Aagaard T, Roen A, Reekie J, Daugaard G,
Brown PN, Specht L, Sengeløv H, Mocroft A, Lundgren J and Helleberg
M: Development and validation of a risk score for febrile
neutropenia after chemotherapy in patients with cancer: The FENCE
score. JNCI Cancer Spectr. 2:pky0532018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mardiak J, Sálek T, Sycová-Milá Z,
Obertová J, Recková M, Mego M, Hlavatá Z, Brozmanová K, Risnyovzská
Z, Svetlovská D and Koza I: Paclitaxel, bleomycin, etoposide, and
cisplatin (T-BEP) as initial treatment in patients with
poor-prognosis germ cell tumors (GCT): A phase II study. Neoplasma.
54:240–245. 2007.
|
|
33
|
Culine S, Kerbrat P, Kramar A, Théodore C,
Chevreau C, Geoffrois L, Bui NB, Pény J, Caty A, Delva R, et al:
Refining the optimal chemotherapy regimen for good-risk metastatic
nonseminomatous germ-cell tumors: A randomized trial of the
genito-urinary group of the french federation of cancer centers
(GETUG T93BP). Ann Oncol. 18:917–924. 2007. View Article : Google Scholar
|
|
34
|
Aapro MS, Bohlius J, Cameron DA, Dal Lago
L, Donnelly JP, Kearney N, Lyman GH, Pettengell R, Tjan-Heijnen VC,
Walewski J, et al: 2010 Update of EORTC guidelines for the use of
granulocyte-colony stimulating factor to reduce the incidence of
chemotherapy-induced febrile neutropenia in adult patients with
lymphoproliferative disorders and solid tumours. Eur J Cancer.
47:8–32. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fujiwara M, Tanaka H, Yuasa T, Komai Y,
Oguchi T, Fujiwara R, Numao N, Yamamoto S, Fujii Y, Fukui I and
Yonese J: First-line combination chemotherapy with etoposide,
ifosfamide and cisplatin for the treatment of disseminated germ
cell cancer: Efficacy and feasibility in current clinical practice.
Int J Urol. 28:920–926. 2021. View Article : Google Scholar
|
|
36
|
de Wit R, Stoter G, Sleijfer DT, Neijt JP,
ten Bokkel Huinink WW, de Prijck L, Collette L and Sylvester R:
Four cycles of BEP vs four cycles of VIP in patients with
intermediate-prognosis metastatic testicular non-seminoma: A
randomized study of the EORTC genitourinary tract cancer
cooperative group. European organization for research and treatment
of cancer. Br J Cancer. 78:828–832. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wit R, Skoneczna I, Daugaard G, De Santis
M, Garin A, Aass N, Witjes AJ, Albers P, White JD, Germa-Lluch JR,
et al: Randomized phase III study comparing paclitaxel-bleomycin,
etoposide, and cisplatin (BEP) to standard BEP in
intermediate-prognosis germ-cell cancer: Intergroup study EORTC
30983. J Clin Oncol. 30:792–799. 2012. View Article : Google Scholar
|
|
38
|
Cierna Z, Mego M, Miskovska V, Machalekova
K, Chovanec M, Svetlovska D, Hainova K, Rejlekova K, Macak D,
Spanik S, et al: Prognostic value of programmed-death-1 receptor
(PD-1) and its ligand 1 (PD-L1) in testicular germ cell tumors. Ann
Oncol. 27:300–305. 2016. View Article : Google Scholar
|
|
39
|
Chovanec M, Cierna Z, Miskovska V,
Machalekova K, Kalavska K, Rejlekova K, Svetlovska D, Macak D,
Spanik S, Kajo K, et al: Systemic immune-inflammation index in
germ-cell tumours. Br J Cancer. 118:831–838. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Svetlovska D, Miskovska V, Cholujova D,
Gronesova P, Cingelova S, Chovanec M, Sycova-Mila Z, Obertova J,
Palacka P, Rajec J, et al: Plasma cytokines correlated with disease
characteristics, progression-free survival, and overall survival in
testicular germ-cell tumor patients. Clin Genitourin Cancer.
15:411–416.e2. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xiao BG, Lu CZ and Link H: Cell biology
and clinical promise of G-CSF: Immunomodulation and
neuroprotection. J Cell Mol Med. 11:1272–1290. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Barreda DR, Hanington PC and Belosevic M:
Regulation of myeloid development and function by colony
stimulating factors. Dev Comp Immunol. 28:509–554. 2004. View Article : Google Scholar
|
|
43
|
Weston BR, Li L and Tyson JJ: Mathematical
analysis of cytokine-induced differentiation of
granulocyte-monocyte progenitor cells. Front Immunol. 9:20482018.
View Article : Google Scholar
|
|
44
|
Karagiannidis I, Salataj E, Said Abu Egal
E and Beswick EJ: G-CSF in tumors: Aggressiveness, tumor
microenvironment and immune cell regulation. Cytokine.
142:1554792021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mouchemore KA and Anderson RL:
Immunomodulatory effects of G-CSF in cancer: Therapeutic
implications. Semin Immunol. 54:1015122021. View Article : Google Scholar
|