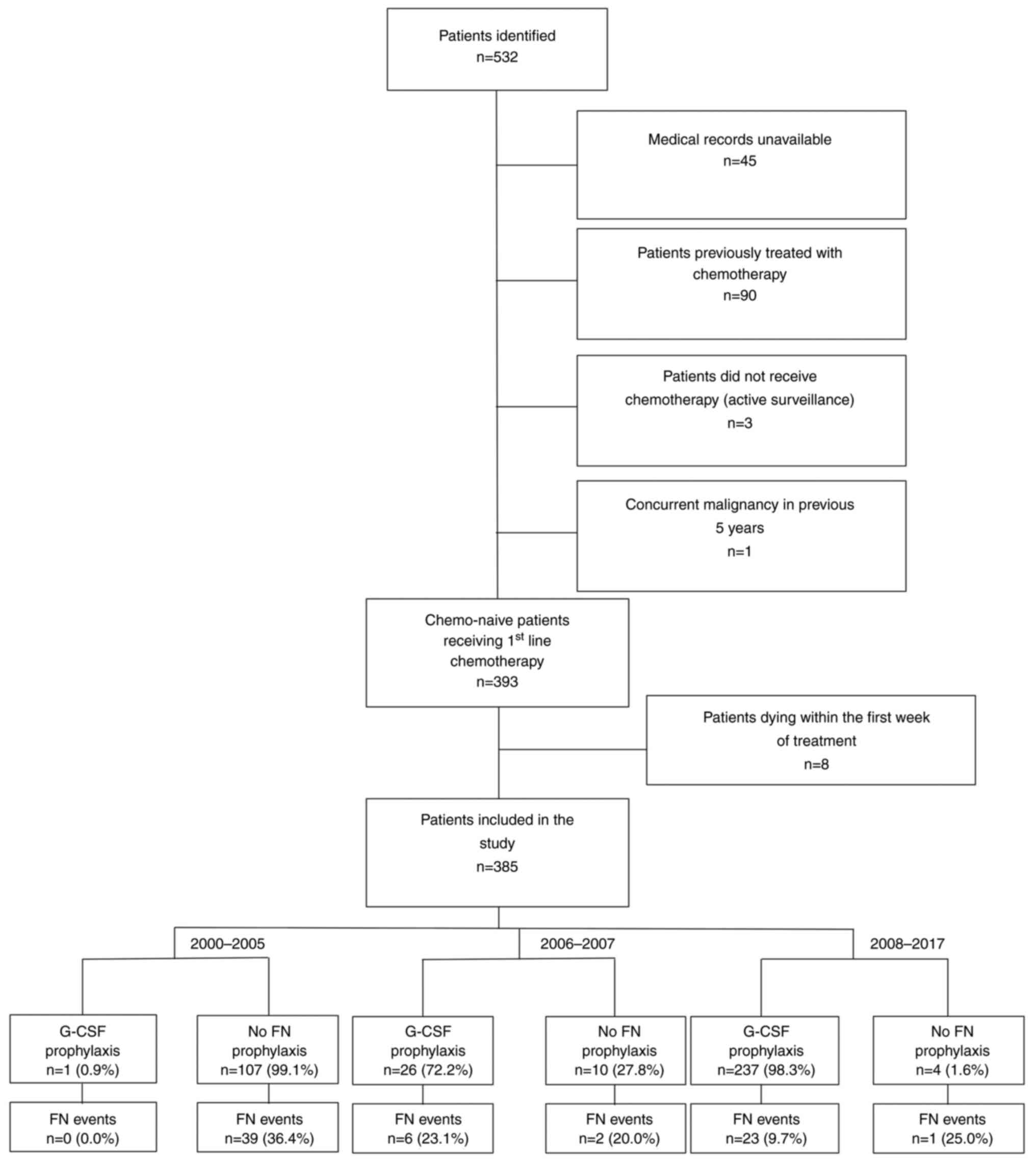
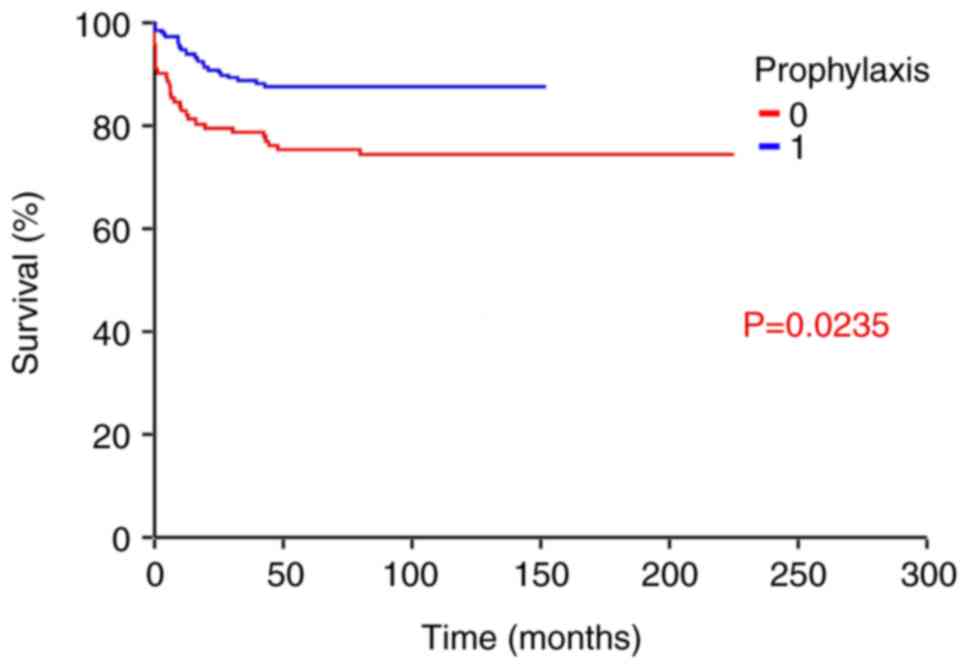
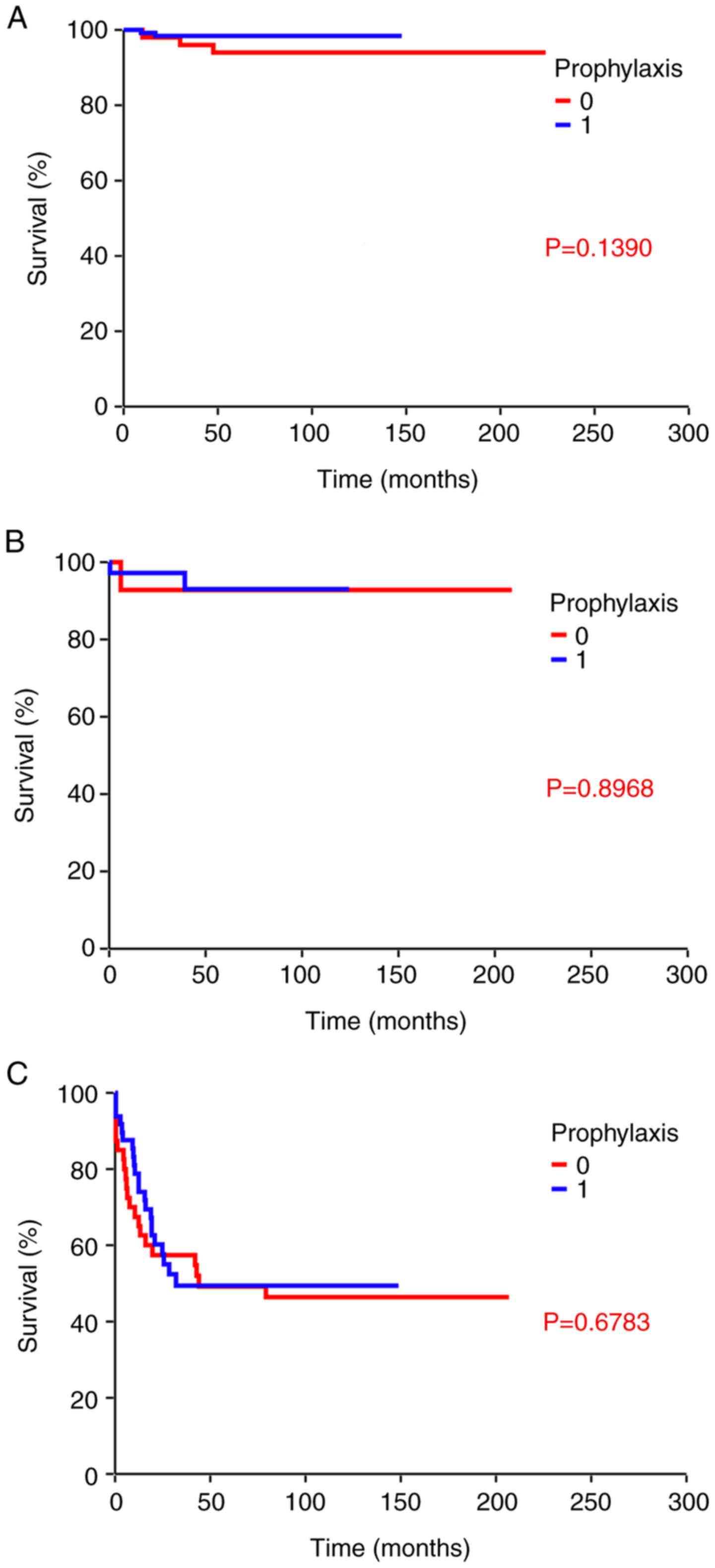
|
1
|
Nigam M, Aschebrook-Kilfoy B, Shikanov S
and Eggener S: Increasing incidence of testicular cancer in the
United States and Europe between 1992 and 2009. World J Urol.
33:623–631. 2015. View Article : Google Scholar
|
|
2
|
Heidenreich A, Paffenholz P, Nestler T and
Pfister D: European Association of Urology Guidelines on Testis
Cancer: Important Take Home Messages. Eur Urol Focus. 5:742–744.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Einhorn LH: Treatment of testicular
cancer: A new and improved model. J Clin Oncol. 8:1777–1781. 1990.
View Article : Google Scholar
|
|
4
|
Beyer J, Albers P, Altena R, Aparicio J,
Bokemeyer C, Busch J, Cathomas R, Cavallin-Stahl E, Clarke NW,
Claßen J, et al: Maintaining success, reducing treatment burden,
focusing on survivorship: Highlights from the third European
consensus conference on diagnosis and treatment of germ-cell
cancer. Ann Oncol. 24:878–888. 2013. View Article : Google Scholar
|
|
5
|
Travis LB, Beard C, Allan JM, Dahl AA,
Feldman DR, Oldenburg J, Daugaard G, Kelly JL, Dolan ME, Hannigan
R, et al: Testicular cancer survivorship: Research strategies and
recommendations. J Natl Cancer Inst. 102:1114–1130. 2010.
View Article : Google Scholar
|
|
6
|
Chovanec M, Vasilkova L, Setteyova L,
Obertova J, Palacka P, Rejlekova K, Sycova-Mila Z, Kalavska K,
Svetlovska D, Cingelova S, et al: Long-term cognitive functioning
in testicular germ-cell tumor survivors. Oncologist. 23:617–623.
2018. View Article : Google Scholar
|
|
7
|
Oun R, Moussa YE and Wheate NJ: The side
effects of platinum-based chemotherapy drugs: A review for
chemists. Dalton Trans. 47:6645–6653. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Crawford J, Becker PS, Armitage JO,
Blayney DW, Chavez J, Curtin P, Dinner S, Fynan T, Gojo I,
Griffiths EA, et al: Myeloid growth factors, version 2.2017, NCCN
clinical practice guidelines in oncology. J Natl Compr Canc Netw.
15:1520–1541. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nishikawa M, Miyake H and Fujisawa M:
Identification of risk factors predicting febrile neutropenia in
patients with metastatic germ cell tumors receiving cisplatin-based
combination chemotherapy. Int J Urol. 24:449–453. 2017. View Article : Google Scholar
|
|
10
|
Cullen M and Baijal S: Prevention of
febrile neutropenia: Use of prophylactic antibiotics. Br J Cancer.
101 (Suppl 1):S11–S14. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Counsell R, Pratt J and Williams MV:
Chemotherapy for germ cell tumours: Prophylactic ciprofloxacin
reduces the incidence of neutropenic fever. Clin Oncol (R Coll
Radiol). 6:232–236. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Terbuch A, Posch F, Partl R, Zurl B,
Bauernhofer T, Pichler M, Szkandera J, Hutterer GC, Pummer K, Kapp
KS, et al: Risk stratification for febrile neutropenia in patients
with testicular germ cell tumors. Cancer Med. 7:508–514. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Feldman DR, Voss MH, Jacobsen EP, Jia X,
Suarez JA, Turkula S, Sheinfeld J, Bosl GJ, Motzer RJ and Patil S:
Clinical features, presentation, and tolerance of platinum-based
chemotherapy in germ cell tumor patients 50 years of age and older.
Cancer. 119:2574–2581. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Beyer J, Collette L, Sauvé N, Daugaard G,
Feldman DR, Tandstad T, Tryakin A, Stahl O, Gonzalez-Billalabeitia
E, De Giorgi U, et al: Survival and new prognosticators in
metastatic seminoma: Results from the IGCCCG-update consortium. J
Clin Oncol. 39:1553–1562. 2021. View Article : Google Scholar
|
|
15
|
Gillessen S, Sauvé N, Collette L, Daugaard
G, de Wit R, Albany C, Tryakin A, Fizazi K, Stahl O, Gietema JA, et
al: Predicting outcomes in men with metastatic nonseminomatous germ
cell tumors (NSGCT): Results from the IGCCCG update consortium. J
Clin Oncol. 39:1563–1574. 2021. View Article : Google Scholar
|
|
16
|
Wang L, Baser O, Kutikova L, Page JH and
Barron R: The impact of primary prophylaxis with granulocyte
colony-stimulating factors on febrile neutropenia during
chemotherapy: A systematic review and meta-analysis of randomized
controlled trials. Support Care Cancer. 23:3131–3140. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fosså SD, Kaye SB, Mead GM, Cullen M, de
Wit R, Bodrogi I, van Groeningen CJ, De Mulder PH, Stenning S,
Lallemand E, et al: Filgrastim during combination chemotherapy of
patients with poor-prognosis metastatic germ cell malignancy.
European organization for research and treatment of cancer,
genito-urinary group, and the medical research council testicular
cancer working party, Cambridge, United Kingdom. J Clin Oncol.
16:716–724. 1998. View Article : Google Scholar
|
|
18
|
de Naurois J, Novitzky-Basso I, Gill MJ,
Gill MJ, Marti FM, Cullen MH and Roila F; ESMO Guidelines Working
Group, : Management of febrile neutropenia: ESMO clinical practice
guidelines. Ann Oncol. 21 (Suppl 5):v252–v256. 2010. View Article : Google Scholar
|
|
19
|
von Vietinghoff S and Ley K: Homeostatic
regulation of blood neutrophil counts. J Immunol. 181:5183–5188.
2008. View Article : Google Scholar
|
|
20
|
Foucar K, Chabot-Richards D, Czuchlewski
DR, Karner KH, Reichard KK, Vasef MA, Wilson CS, Zhang QY and
Culbreath K: Neutropenia. Diagnostic pathology: Blood and bone
marrow. 2nd edition. Elsevier; Netherlands: pp. 180–187. 2018
|
|
21
|
Foucar K, Chabot-Richards D, Czuchlewski
DR, Karner KH, Reichard KK, Vasef MA, Wilson CS, Zhang QY and
Culbreath K: Neutrophilia. Diagnostic pathology: Blood and bone
marrow. 2nd edition. Elsevier; Netherlands: pp. 188–193. 2018
|
|
22
|
Amin MB, Greene FL, Edge SB, Compton CC,
Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR and
Winchester DP: The eighth edition AJCC cancer staging manual:
Continuing to build a bridge from a population-based to a more
‘personalized’ approach to cancer staging. CA Cancer J Clin.
67:93–99. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Honecker F, Aparicio J, Berney D, Beyer J,
Bokemeyer C, Cathomas R, Clarke N, Cohn-Cedermark G, Daugaard G,
Dieckmann KP, et al: ESMO consensus conference on testicular germ
cell cancer: Diagnosis, treatment and follow-up. Ann Oncol.
29:1658–1686. 2018. View Article : Google Scholar
|
|
24
|
NCSS 2019 statistical software., . NCSS,
LLC. Kaysville; Utah, USA: Version 19.0.3. 2019, https://www.ncss.com/software/ncss
|
|
25
|
Fizazi K, Pagliaro L, Laplanche A, Fléchon
A, Mardiak J, Geoffrois L, Kerbrat P, Chevreau C, Delva R, Rolland
F, et al: Personalised chemotherapy based on tumour marker decline
in poor prognosis germ-cell tumours (GETUG 13): A phase 3,
multicentre, randomised trial. Lancet Oncol. 15:1442–1450. 2014.
View Article : Google Scholar
|
|
26
|
Kuderer NM, Dale DC, Crawford J, Cosler LE
and Lyman GH: Mortality, morbidity, and cost associated with
febrile neutropenia in adult cancer patients. Cancer.
106:2258–2266. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Caggiano V, Weiss RV, Rickert TS and
Linde-Zwirble WT: Incidence, cost, and mortality of neutropenia
hospitalization associated with chemotherapy. Cancer.
103:1916–1924. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Crawford J, Dale DC, Kuderer NM, Culakova
E, Poniewierski MS, Wolff D and Lyman GH: Risk and timing of
neutropenic events in adult cancer patients receiving chemotherapy:
The results of a prospective nationwide study of oncology practice.
J Natl Compr Canc Netw. 6:109–118. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ray-Coquard I, Borg C, Bachelot T, Sebban
C, Philip I, Clapisson G, Le Cesne A, Biron P, Chauvin F and Blay
JY; ELYPSE study group, : Baseline and early lymphopenia predict
for the risk of febrile neutropenia after chemotherapy. Br J
Cancer. 88:181–186. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee RK, Soyemi SA, Chen M, Kanis MJ and
Lee YC: Pretreatment absolute neutrophil counts predict
neutropenia-related events in patients undergoing first line
chemotherapy in gynecologic malignancies. Gynecol Oncol. 154 (Suppl
1):S1262019. View Article : Google Scholar
|
|
31
|
Aagaard T, Roen A, Reekie J, Daugaard G,
Brown PN, Specht L, Sengeløv H, Mocroft A, Lundgren J and Helleberg
M: Development and validation of a risk score for febrile
neutropenia after chemotherapy in patients with cancer: The FENCE
score. JNCI Cancer Spectr. 2:pky0532018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mardiak J, Sálek T, Sycová-Milá Z,
Obertová J, Recková M, Mego M, Hlavatá Z, Brozmanová K, Risnyovzská
Z, Svetlovská D and Koza I: Paclitaxel, bleomycin, etoposide, and
cisplatin (T-BEP) as initial treatment in patients with
poor-prognosis germ cell tumors (GCT): A phase II study. Neoplasma.
54:240–245. 2007.
|
|
33
|
Culine S, Kerbrat P, Kramar A, Théodore C,
Chevreau C, Geoffrois L, Bui NB, Pény J, Caty A, Delva R, et al:
Refining the optimal chemotherapy regimen for good-risk metastatic
nonseminomatous germ-cell tumors: A randomized trial of the
genito-urinary group of the french federation of cancer centers
(GETUG T93BP). Ann Oncol. 18:917–924. 2007. View Article : Google Scholar
|
|
34
|
Aapro MS, Bohlius J, Cameron DA, Dal Lago
L, Donnelly JP, Kearney N, Lyman GH, Pettengell R, Tjan-Heijnen VC,
Walewski J, et al: 2010 Update of EORTC guidelines for the use of
granulocyte-colony stimulating factor to reduce the incidence of
chemotherapy-induced febrile neutropenia in adult patients with
lymphoproliferative disorders and solid tumours. Eur J Cancer.
47:8–32. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fujiwara M, Tanaka H, Yuasa T, Komai Y,
Oguchi T, Fujiwara R, Numao N, Yamamoto S, Fujii Y, Fukui I and
Yonese J: First-line combination chemotherapy with etoposide,
ifosfamide and cisplatin for the treatment of disseminated germ
cell cancer: Efficacy and feasibility in current clinical practice.
Int J Urol. 28:920–926. 2021. View Article : Google Scholar
|
|
36
|
de Wit R, Stoter G, Sleijfer DT, Neijt JP,
ten Bokkel Huinink WW, de Prijck L, Collette L and Sylvester R:
Four cycles of BEP vs four cycles of VIP in patients with
intermediate-prognosis metastatic testicular non-seminoma: A
randomized study of the EORTC genitourinary tract cancer
cooperative group. European organization for research and treatment
of cancer. Br J Cancer. 78:828–832. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wit R, Skoneczna I, Daugaard G, De Santis
M, Garin A, Aass N, Witjes AJ, Albers P, White JD, Germa-Lluch JR,
et al: Randomized phase III study comparing paclitaxel-bleomycin,
etoposide, and cisplatin (BEP) to standard BEP in
intermediate-prognosis germ-cell cancer: Intergroup study EORTC
30983. J Clin Oncol. 30:792–799. 2012. View Article : Google Scholar
|
|
38
|
Cierna Z, Mego M, Miskovska V, Machalekova
K, Chovanec M, Svetlovska D, Hainova K, Rejlekova K, Macak D,
Spanik S, et al: Prognostic value of programmed-death-1 receptor
(PD-1) and its ligand 1 (PD-L1) in testicular germ cell tumors. Ann
Oncol. 27:300–305. 2016. View Article : Google Scholar
|
|
39
|
Chovanec M, Cierna Z, Miskovska V,
Machalekova K, Kalavska K, Rejlekova K, Svetlovska D, Macak D,
Spanik S, Kajo K, et al: Systemic immune-inflammation index in
germ-cell tumours. Br J Cancer. 118:831–838. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Svetlovska D, Miskovska V, Cholujova D,
Gronesova P, Cingelova S, Chovanec M, Sycova-Mila Z, Obertova J,
Palacka P, Rajec J, et al: Plasma cytokines correlated with disease
characteristics, progression-free survival, and overall survival in
testicular germ-cell tumor patients. Clin Genitourin Cancer.
15:411–416.e2. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xiao BG, Lu CZ and Link H: Cell biology
and clinical promise of G-CSF: Immunomodulation and
neuroprotection. J Cell Mol Med. 11:1272–1290. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Barreda DR, Hanington PC and Belosevic M:
Regulation of myeloid development and function by colony
stimulating factors. Dev Comp Immunol. 28:509–554. 2004. View Article : Google Scholar
|
|
43
|
Weston BR, Li L and Tyson JJ: Mathematical
analysis of cytokine-induced differentiation of
granulocyte-monocyte progenitor cells. Front Immunol. 9:20482018.
View Article : Google Scholar
|
|
44
|
Karagiannidis I, Salataj E, Said Abu Egal
E and Beswick EJ: G-CSF in tumors: Aggressiveness, tumor
microenvironment and immune cell regulation. Cytokine.
142:1554792021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mouchemore KA and Anderson RL:
Immunomodulatory effects of G-CSF in cancer: Therapeutic
implications. Semin Immunol. 54:1015122021. View Article : Google Scholar
|